Abstract
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract, with proto-oncogene, receptor tyrosine kinase (c-kit), or PDGFRα mutations detected in around 85% of cases. GISTs without c-kit or platelet-derived growth factor receptor alpha (PDGFRα) mutations are considered wild-type (WT), and their diverse molecular alterations and biological behaviors remain uncertain. They are usually not sensitive to tyrosine kinase inhibitors (TKIs). Recently, some molecular alterations, including neurotrophic tyrosine receptor kinase (NTRK) fusions, have been reported in very few cases of WT GISTs. This novel finding opens the window for the use of tropomyosin receptor kinase (TRK) inhibitor therapy in these subtypes of GIST. Herein, we report a new case of NTRK-fused WT high-risk GIST in a female patient with a large pelvic mass (large dimension of 20 cm). The tumor was removed, and the histopathology displayed spindle-predominant morphology with focal epithelioid areas, myxoid stromal tissue, and notable lymphoid infiltration with tertiary lymphoid structures. Ten mitoses were quantified in 50 high-power fields without nuclear pleomorphism. DOG1 showed strong and diffuse positivity, and CD117 showed moderate positivity. Succinate dehydrogenase subunit B (SDHB) was retained, Pan-TRK was focal positive (nuclear pattern), and the proliferation index Ki-67 was 7%. Next-generation sequencing (NGS) detected an ETV6::NTRK3 fusion, and this finding was confirmed by fluorescence in situ hybridization (FISH), which showed NTRK3 rearrangement. In addition, an RB1 mutation was found by NGS. The follow-up CT scan revealed peritoneal nodules suggestive of peritoneal dissemination, and Entrectinib (a TRK inhibitor) was administered. After 3 months of follow-up, a new CT scan showed a complete response. Based on our results and the cases from the literature, GISTs with NTRK fusions are very uncommon so far; hence, further screening studies, including more WT GIST cases, may increase the possibility of finding additional cases. The present case may offer new insights into the potential introduction of TRK inhibitors as treatments for GISTs with NTRK fusions. Additionally, the presence of abundant lymphoid infiltration in the present case may prompt further research into immunotherapy as a possible additional therapeutic option.
1. Introduction
Gastrointestinal stromal tumors (GISTs) are the most frequent mesenchymal neoplasm of the gastrointestinal tract, and the majority occur in the stomach and the small intestine [,,,]. Receptor tyrosine kinase (c-kit) or platelet-derived growth factor receptor alpha (PDGFRα) mutations have been detected in around 85% of cases, and these cases without both mutations are considered wild-type (WT) GISTs [,,,,,,]. WT GISTs are commonly not sensitive to tyrosine kinase inhibitors (TKIs) []. A small proportion of GISTs either reveal succinate dehydrogenase (SDH) deficiency or display murine sarcoma viral oncogene homolog B (BRAF) or RAS mutations [,,,,,,]. The other GISTs without mutations in any of the previous genes have been classified as ‘quadruple WT’ GISTs [,,,,,,], and additional molecular alteration, including the phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha (PIK3CA) mutation, fibroblast growth factor receptor 1 (FGFR1), BRAF or anaplastic lymphoma kinase (ALK) gene fusions, and neurotrophic tyrosine receptor kinase (NTRK) fusions (ETV6::NTRK3 and LMNA::NTRK1), have been reported in quadruple WT GISTs [,,,,,,].
The NTRK family consists of NTRK1, NTRK2, and NTRK3, which encode tropomyosin receptor kinase (TRK) [,]. NTRK fusion has been reported in many unrelated neoplasms, including infantile fibrosarcoma [], mesoblastic nephroma [], secretory breast carcinoma [], mammary analog secretory carcinoma of the salivary gland [], thyroid carcinoma [], glioblastoma [], cholangiocarcinoma [], myofibroblast tumors [], and WT GISTs [,,,,,]. Herein, we report a new case of quadruple WT with ETV6::NTRK3 gene fusion, a rare genetic event with only a few cases previously reported. In addition, the present case displays an extensive lymphoid infiltration (tumor infiltrate lymphocytes as well as tertiary lymphoid structures) that offers new therapeutic options based on immunotherapy. Although NTRK fusions are rarely detected in WT GISTs at a low frequency, patients with GIST presenting NTRK fusions had a good chance of responding to treatment with TRK inhibitors, particularly patients with unresectable neoplasms, disseminate disease, or recurrent tumors that are resistant to TKIs. In such cases, TRK inhibitors, such as Larotrectinib and Entrectinib, may be a new therapeutic option [,,,,,,]. Based on the present case, we further explored the clinicopathological and genetic features of previously reported GISTs with ETV6::NTRK3 fusion.
2. Materials and Methods
2.1. Histopathology and Immunohistochemistry (IHC)
Histopathology was performed with the conventional hematoxylin and eosin method. IHC was performed on formalin-fixed, paraffin-embedded (FFPE) tissue sections with a thickness of 4 µm using an automated staining instrument (DAKO). Appropriated negative and positive controls were included. The testing and assessment were performed according to the manufacturer’s instructions for every biomarker. For Pan-TRK, nuclear, cytoplasmic, or membranous staining in more than 5% of tumor cells was considered positive [,,,]. All IHC-stained sections were interpreted blinded by two experienced pathologists (IM and ALLB).
2.2. Next-Generation Target Sequencing (DNA and RNA)
An Oncomine™ Comprehensive v3 panel (OCAv3) from Thermo Fisher Scientific (Based in Waltham, MA, USA) was used for sequencing. A list of the genes include in the panel is summarized in (https://assets.thermofisher.com/TFS-Assets/LSG/brochures/oncomine-comprehensive-assay-v3-flyer.pdf (accessed on 13 March 2024)).
Nucleic acids were extracted and quantified using the Genexus™Purification System with a Genexus™ FFPE DNA/RNA Purification Combo Kit (Thermo Fisher Scientific). Detection of genomic alterations was then performed using a Genexus™Integrated Sequencer. The OCAv3 is an amplicon-based, targeted assay that enables the detection of relevant single-nucleotide variants, amplifications, gene fusions, and indels from 161 unique genes. Genomic data were analyzed, and alterations were detected using the Ion Torrent Genexus Software 6.8.1.1. (Thermo Fisher Scientific). We also manually reviewed the variant call format file and integrated the Genomic Viewer. Only variants in coding regions, promoter regions, or splice variants were retained.
The threshold for positive detection was defined at ≥25 RNA reads, originating from at least two unique PCR products, as determined by molecular barcoding.
2.3. Fluorescence In Situ Hybridization
Formalin-fixed paraffin-embedded (FFPE) section tissue was used to perform fluorescence in situ hybridization (FISH). We utilized a commercial kit, the FISH Pretreatment kit (Vitro, Master Diagnostica®, Granada, Spain), to prepare the histological specimens according to a standardized protocol. This kit has been specifically designed for manual use to maintain a consistent procedure.
For the hybridization step, we employed the NTRK3 Break Apart FISH Probe Kit by Cytotest® (Rockville, MD, 208050, USA). This probe is tailored to detect rearrangements in the human NTRK3 gene located on chromosome band 15q25.3. Apart from identifying breaks that may result in gene translocation, inversion, or fusion with other genes, the probe set can also detect other NTRK3 aberrations, such as deletions or amplifications. The NTRK3 Break Apart FISH Probe Kit spans approximately 500 Kb and covers the 5’ (start) portion of the ABL2 locus, and adjacent genomic sequences marked with CytoGreen fluorochrome (Figure 1 and Figure 2) show NTRK and ETV gene details and the probe design. The LSP NTRK3 3‘ FISH Probe spans approximately 720 Kb and covers sequences at the 3’ (end) of the gene marked with CytoOrange fluorochrome. These two probes flank sequences across the NTRK3 locus where variable breakpoints have been observed.
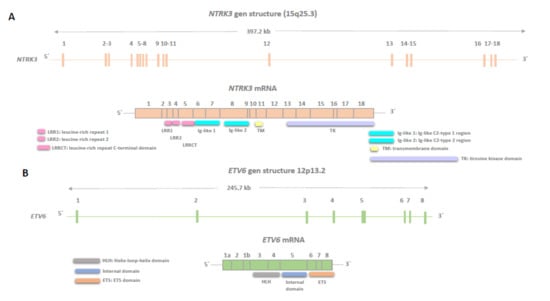
Figure 1.
Genomic structures of neurotrophic tyrosine receptor kinase (NTRK3) (A) and ETS Variant Transcription Factor 6 (ETV6) (B) are shown, illustrating exons encoding the canonical isoforms as described in the Genome Browser v461 software. The regions of the corresponding mRNAs encoding functional domains are marked. NTRK3 stands for Neurotrophic Tyrosine Kinase Receptor Type 3 (NCBI Gene ID: 4916), and ETV6 stands for ETS Variant Transcription Factor 6 (NCBI Gene ID: 2120).
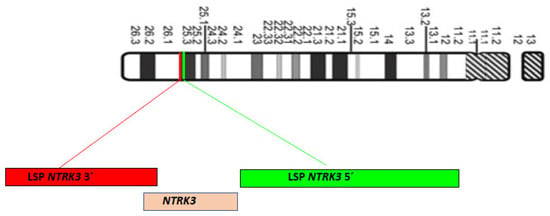
Figure 2.
Chromosome 15 diagram, ISCN 2009, and localization of LSP neurotrophic tyrosine receptor kinase NTRK3 5′ (Green) and LSP NTRK3 3′ (Red) FISH probes on 15q25.3 chromosome position.
Following hybridization with the FISH probes, we cleaned the sections with a post-hybridization buffer to remove nonspecific labeling. Subsequently, we used a dapi-antifade mounting medium, included in the kit, to observe and preserve the fluorescence. The analysis was performed using a Zeiss Axio Imager fluorescence microscope with a 63X magnification. We counted 100 nuclei with the assistance of two independent observers (RC and JALG). NTRK rearrangements were interpreted based on the presence of a predominant atypical signal pattern with extra-signal NTRK 3′ in more than 10-15% of tumor cell nuclei and an isolated break-apart pattern [,,,].
3. Results
Case Presentation
A 53-year-old woman presented with complaints of abdominal and pelvic discomfort and was admitted to the hospital. The physician’s examination revealed a large intra-abdominal mass. Computerized tomography (CT scan) and magnetic resonance imaging (MRI) confirmed a large intra-abdominal and pelvic mass measuring 22 cm (Figure 3A,B). Surgical resection was performed, including hysterectomy, intestinal resection, and tumor removal. Although the surgeon performed an apparently complete margin-negative (R0) resection, the tumor was fragmented during the surgical procedure and could not be removed in a single specimen. Macroscopically, a large solid and cystic neoplasm measuring 30 cm. or larger dimension was observed, attached to the large and small bowel; the tumor displayed a gray-yellow-tan color and hard texture. Histopathology examination with hematoxylin and eosin displayed a mesenchymal neoplasm with spindle-predominant morphology, with focal epithelioid shape, ill-defined eosinophilic cytoplasms (Figure 4), and myxoid stromal tissue. Notable lymphoid infiltration with intratumoral and focal tertiary lymphoid structures was detected (Figure 4D). Ten mitoses were quantified in 50 high-power fields without nuclear pleomorphism.
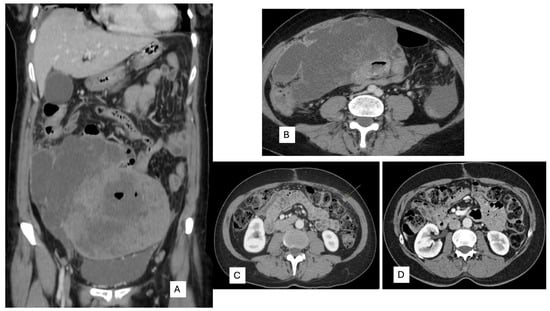
Figure 3.
(A) Coronal and (B) axial. Computerized tomography displays a large intra-abdominal and pelvic tumor with necrosis attached to the small bowel. (C) Peritoneal carcinomatosis (arrow) in CT scan of follow-up. (D) CT scan after 3 months with Entrectinic treatment, showing a complete response.
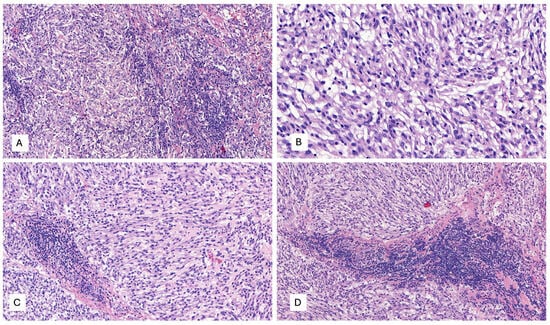
Figure 4.
(A–D) Microscopic examination with hematoxylin and eosin (H&E) displays a mesenchymal neoplasm with spindle-predominant morphology, with focal epithelioid shape, ill-defined eosinophilic cytoplasm, myxoid stromal tissue, and remarkable lymphoid infiltration with tumor-infiltrating lymphocytes (TILs) and focal tertiary lymphoid structures, H&E. (A) 40×, (B) 400×, and (C,D) 200×.
The IHC study revealed strong and diffuse DOG1 cytoplasmic positivity. CD117 showed moderate cytoplasmic immunoreactivity, and CD34 displayed focal positivity (Figure 5). SDHB was retained, and Pan-TRK showed focal and nuclear positivity (Figure 5D,E). The proliferation index Ki-67 was around 7%. S100, CK(AE1/AE3), Inhibin, estrogen receptors, calretinin, chromogranin, synaptophysin, smooth muscle actin, desmin, epithelial membrane antigen (EMA), CD31, anti-melanosome (HMB-45), and Melan A were all negative. CD3, CD4, and CD8 were positive in tumor infiltrate lymphocytes (TILs) (Figure 6A). CD20 highlighted the tertiary lymphoid structures (Figure 6B). CD138 was positive in plasma cells (Figure 6C). Programmed cell death 1 ligand (PDL1) displayed focal cytoplasmic/membranous positivity in tumor cells and PD-1 in lymphoid infiltration. CD163 showed diffuse positivity in the myeloid/histiocyte population (Figure 6D). Based on the histopathology and IHC results, the tumor was classified as a gastrointestinal stromal tumor (GIST) with a high risk of recurrence/metastasis. ETV6::NTRK3 fusion was identified by RNA NGS (OCAv3 panel) and 251.517 reads (Figure 7), and c-kit, PDGFRα, SDH, BRAF, and RAF were all wild-type/non-mutated. In addition, we found RB1 c.184C>T p.(Q62*), nonsense mutation, and an allelic frequency of 77.80%. An NTRK3 rearrangement was detected by FISH (Figure 8). The lymphoid infiltration was found to be polyclonal both by immunohistochemistry and through polymerase chain reaction (PCR) and fragment analysis by Sanger sequencing.
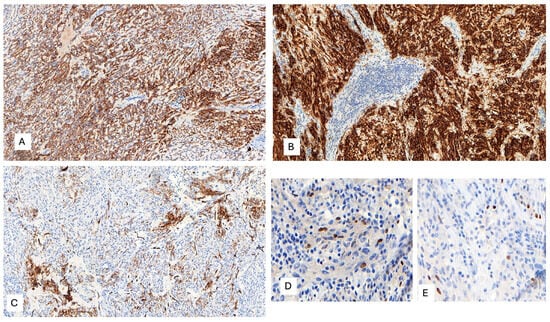
Figure 5.
(A) Diffuse and moderate CD117 cytoplasmic immunoreactivity, 100×. (B) Strong and diffuse DOG1 cytoplasmic positivity, 200×. (C) Patchy CD34 positivity, 100×. (D,E) Focal and nuclear Pan-TRK immunoreactivity, 400×.
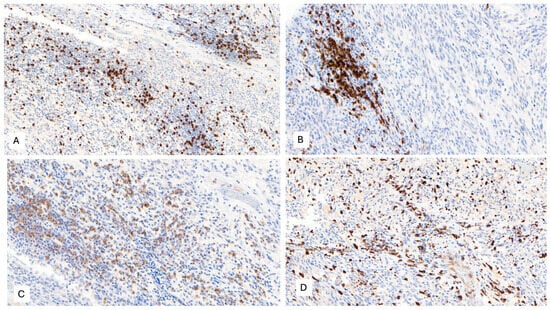
Figure 6.
(A) CD8 positivity in tumor-infiltrating lymphocytes (TILs), 100×. (B) CD20 immunoreactivity in B-cells from tertiary lymphoid structures, 200×. (C) CD138 positivity in plasma cells, 100×. (D) CD163 positivity in the myeloid/histiocyte population, 100×.
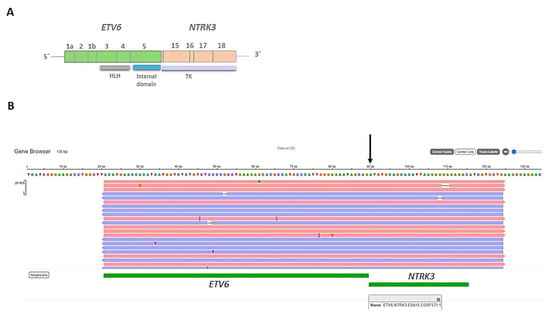
Figure 7.
(A) Representation of the fusion gene (ETV6::NTRK3) obtained by next-generation sequencing (NGS), showing the exons and domains involved in the resultant fusion gene. HLH: Helix-loop-helix domain, TK: tyrosine kinase domain. (B) Integrative Genomics Viewer (IGV) displaying the ETV6::NTRK3 fusion gene.
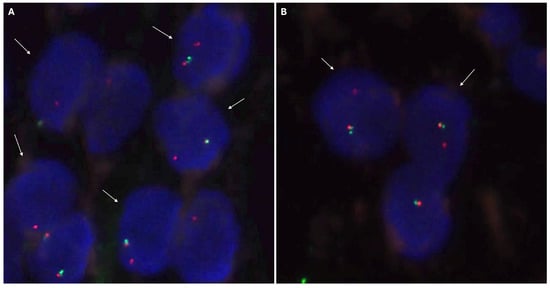
Figure 8.
Neurotrophic tyrosine receptor kinase type 3(NTRK3) FISH analysis with a break-apart probe. (A) Positive nuclei for the rearrangement of the NTRK3 gene, indicated by white arrows. The positive signal pattern corresponds to an atypical pattern with an extra NTRK3 3′ signal (red). Additionally, there are abnormal signal patterns with only one red signal and one normal nucleus with overlapping signals (NTRK3 5′ green and NTRK3 3′ red). (B) Two positive nuclei, indicated by white arrows, and one nucleus with a normal signal pattern. The positive nuclei present a typical positive signal pattern with separated NTRK3 3′ (red) and NTRK3 5′ (green) signals.
A new CT scan performed one month after surgery revealed peritoneal carcinomatosis (Figure 3C), and Entrectinib was administered. After 12 weeks of drug intake, she presented with mild peripheral sensory neuropathy, non-severe pneumonia treated with antibiotics, mild constipation, and hypotension. A subsequent CT scan performed 3 months later showed a complete response (Figure 3D).
4. Discussion
Kinase-driven alterations, such as NTRK or FGFR fusion, have been recently reported in some GISTs [,,,,,,,,,,,,]. The NTRK genes encode TRK proteins that exert oncogenic effects on tumors, and the activation of most TRK proteins is caused by NTRK fusions [,]. NTRK fusions occur in a variety of cancers with different incidences [,,,,,,,,,,,,,,,]. TRK inhibitors, such as Larotrectinib and Entrectinib, have shown encouraging antitumor efficacies in tumors with NTRK rearrangements and have been approved as treatments for multiple cancers harboring NTRK fusions [,,,,,,]. Although the prevalence of NTRK fusions in GIST is extremely low and only a few patients were enrolled in the clinical trials, the antitumor efficacies of TRK inhibitors were observed [,,,,,,]. Despite NTRK fusion being reported in some quadruple WT GISTs, only an ETV6::NTRK3 fusion has been reported in eight cases, as presented in Table 1 [,,,,], including the present case.

Table 1.
Clinicopathological features of GIST WT with NTRK3::ETV6 gene fusion.
The clinicopathological features of GISTs with ETV6::NTRK3 fusion are variable, and many of them have been reported in the fourth or fifth decade of life, predominantly in intestinal and pelvic locations, with variable dimensions but usually presenting as tumors with high dimensions [,,,,]. They may exhibit spindle or epithelioid morphology and typically have a high risk of recurrence and metastasis [,,,,]. The majority display DOG1 and CD117 immunohistochemical expression, with SDH retained and variable Pan-TRK immunoreactivity, often nuclear and focal [,,,,]. FISH analysis has confirmed NTRK3 rearrangement in five out of eight cases [,,,,]. In localized disease, the usual approach is surgical treatment, but in metastatic and/or recurrent settings, several drugs have been employed, including Sunitinib, Sorafenib, Nilotinib, Imatinib, Larotrectinib, and Entrectinib [,,,,]. The last two drugs seem to be very effective in tumors with NTRK rearrangement. The behavior depends on several factors, such as initial metastatic disease, tumor size, location, etc., but in the previously mentioned GISTs with ETV6::NTRK3, six patients were alive with or without the disease, and two died from the disease, with a median follow-up of 43 months [,,,,].
Although the prevalence of NTRK fusions in GIST is extremely low and only a few patients were enrolled in the clinical trials, the antitumor efficacies of TRK inhibitors were observed [,,,,,,]. In clinical trials (LOXO-TRK-14001, SCOUT, and NAVIGATE), 55 patients with tumors carrying NTRK rearrangements, including three GISTs, were enrolled to evaluate the efficacy of Larotrectinib [,,,,,,]. All three patients with GIST experienced tumor shrinkage >30%, and one had a pathological complete response with sufficient tumor shrinkage >90% [,,,,,,]. Furthermore, Larotrectinib has been recommended by the Belgian multidisciplinary expert panel as a first-line treatment for WT GIST with NTRK fusions []. These pieces of evidence [,,,,,,], as well as our results, suggest that NTRK fusions define a unique subgroup of GIST, and TRK inhibitors have the potential to benefit GISTs with NTRK fusions. Hence, it is clinically significant to screen for NTRK fusions in WT GIST.
One challenging issue is determining the best method for screening NTRK fusion in WT GIST [,,,]. FISH has been recommended as the gold standard for detecting gene rearrangements, including break-apart probes and fusion probes; however, it does not provide information about partner genes involved in the fusions [,,,]. As ETV6 is the most frequent partner in NTRK3 fusions, we presume that many of the previously reported GISTs with NTRK3 rearrangement harbor ETV6 as a partner, as reported by Castillon et al. []. Although FISH appears more suitable for verification than for screening gene fusions in tumors with a low incidence of the fusions, NGS may provide information on broad-spectrum molecular alterations in many genes, including mutations, amplifications/deletions, gene fusions, and SNPs [,,,,,]. However, NGS requires strict quality control and may not be available in all hospitals. Compared to FISH and NGS, IHC is a cheap and fast testing method and is available in many hospitals. IHC staining for Pan-TRK has been used to screen NTRK fusions in several cancers [,,,,,,]. However, both the sensitivity (50%) and specificity (16.7%) of Pan-TRK staining are low [], and the stain may be focal, as observed in our study. Therefore, the validity of Pan-TRK IHC staining in screening NTRK rearrangements is uncertain in GISTs. The detection of a specific fusion partner in NTRK rearrangement tumors would not be mandatory in cases where positive NTRK FISH results involve the tyrosine kinase domain. Additionally, targeted therapy may be administered independently of the NTRK-specific subtype (1, 2, or 3) and the fusion gene partner.
The present case also reveals an RB1 mutation, which seems to be associated with high-risk/malignant GISTs []. Our case was histologically classified as high-risk/malignant GIST.
Regarding the differential diagnosis, the relationship between mesenchymal tumors of the gastrointestinal tract with NTRK rearrangement and GIST is interesting []. Likely, the morphological differences are not quite evident, but the absence of CD117 and DOG1 immunohistochemical expression permits the distinction of NTRK-rearranged tumors from GISTs and highlights important phenotypic differences between these tumor types []. In fact, gastrointestinal mesenchymal tumors (CD117 and DOG1 negative) with NTRK rearrangements demonstrate substantial clinical and morphological heterogeneity and are generally unrelated to GIST [].
In the era of precision medicine, with the agnostic approval of Entrectinib and Larotrectinib for metastatic tumors with NTRK fusions, we consider either of these two drugs to be the treatment of choice for patients with metastatic GIST and NTRK fusion, as in this case. The extremely low frequency of GISTs with NTRK fusions means that we have little evidence of the benefit of NTRK inhibitors in these tumors, making it especially important to collect real-world data to confirm the long-term benefit of this treatment in this patient group. One limitation of the present study is the short period of follow-up for our patient treated with Entrectinib. However, despite a peritoneal lesion described in the first follow-up CT scan, a new CT scan conducted 3 months later revealed a complete response, providing evidence of the effectiveness of Entrectinib treatment.
Finally, an unreported histopathological and IHC finding in our case but not in previous GISTs with ETV6::NTRK3, to the best of our knowledge, was the prominent TILs and plasmacytic infiltration, as well as tertiary lymphoid structures throughout the tumor. These features would open the possibility of a new treatment approach for this patient with immunotherapy; however, these findings should be confirmed in additional GISTs with ETV6:NTRK3 gene fusion and GISTs or mesenchymal neoplasms of the gastrointestinal tract with NTRK rearrangement. This research should be conducted through multicenter studies with a large sample size. Nevertheless, these findings could be potentially used to guide personalized treatments for patients with GIST.
Future studies may further build on the genetic profile of so-called quadruple WT GISTs, which are likely not truly WT, and establish links between genomic kinase drivers (NTRK and FGFR1 rearrangement) and therapeutic regimens.
Author Contributions
Conceptualization, I.M. and J.L.; methodology, R.C.-A.; validation, all authors; formal analysis, I.M. and R.C.-A.; investigation, I.M., R.C.-A. and L.N.; resources, all authors; data curation, R.C.-A.; writing—original draft preparation, I.M. and R.C.-A.; writing—review and editing, I.M., J.L. and R.C.-A.; supervision, A.L.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Approved. Written informed consent was obtained from individual or guardian participants.
Informed Consent Statement
Informed consent was obtained from the patient involved in this study.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nannini, M.; Biasco, G.; Astolfi, A.; Pantaleo, M.A. An overview on molecular biology of KIT/PDGFRA wild type (WT) gastrointestinal stromal tumours (GIST). J. Med. Genet. 2013, 50, 653–661. [Google Scholar] [CrossRef]
- Nannini, M.; Astolfi, A.; Urbini, M.; Indio, V.; Santini, D.; Heinrich, M.C.; Corless, C.L.; Ceccarelli, C.; Saponara, M.; Mandrioli, A.; et al. Integrated genomic study of quadruple-WT GIST (KIT/PDGFRA/SDH/RAS pathway wild-type GIST). BMC Cancer 2014, 14, 685. [Google Scholar] [CrossRef] [PubMed]
- Huss, S.; Elges, S.; Trautmann, M.; Sperveslage, J.; Hartmann, W.; Wardelmann, E. Classification of KIT/PDGFRA wild-type gastrointestinal stromal tumors: Implications for therapy. Expert Rev. Anticancer Ther. 2015, 15, 623–628. [Google Scholar] [CrossRef]
- Li, J.; Ye, Y.; Wang, J.; Zhang, B.; Qin, S.; Shi, Y.; He, Y.; Liang, X.; Liu, X.; Zhou, Y.; et al. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin. J. Cancer Res. 2017, 29, 281–293. [Google Scholar] [CrossRef]
- Huss, S.; Pasternack, H.; Ihle, M.A.; Merkelbach-Bruse, S.; Heitkötter, B.; Hartmann, W.; Trautmann, M.; Gevensleben, H.; Büttner, R.; Schildhaus, H.U.; et al. Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events. Hum. Pathol. 2017, 62, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Søreide, K.; Sandvik, O.M.; Søreide, J.A.; Giljaca, V.; Jureckova, A.; Bulusu, V.R. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016, 40, 39–46. [Google Scholar] [CrossRef]
- Pantaleo, M.A.; Nannini, M.; Corless, C.L.; Heinrich, M.C. Quadruple wild-type (WT) GIST: Defining the subset of GIST that lacks abnormalities of KIT, PDGFRA, SDH, or RAS signaling pathways. Cancer Med. 2015, 4, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Hechtman, J.F. NTRK insights: Best practices for pathologists. Mod. Pathol. 2022, 35, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Suurmeijer, A.J.; Dickson, B.C.; Swanson, D.; Zhang, L.; Sung, Y.S.; Huang, H.Y.; Fletcher, C.D.; Antonescu, C.R. The histologic spectrum of soft tissue spindle cell tumors with NTRK3 gene rearrangements. Genes Chromosomes Cancer 2019, 58, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Knezevich, S.R.; McFadden, D.E.; Tao, W.; Lim, J.F.; Sorensen, P.H. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat. Genet. 1998, 18, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.P.; Chen, C.J.; Morgan, T.W.; Xiao, S.; Grier, H.E.; Kozakewich, H.P.; Perez-Atayde, A.R.; Fletcher, J.A. Congenital mesoblastic nephroma t(12;15) is associated with ETV6-NTRK3 gene fusion: Cytogenetic and molecular relationship to congenital (infantile) fibrosarcoma. Am. J. Pathol. 1998, 153, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Tognon, C.; Knezevich, S.R.; Huntsman, D.; Roskelley, C.D.; Melnyk, N.; Mathers, J.A.; Becker, L.; Carneiro, F.; MacPherson, N.; Horsman, D.; et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2002, 2, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Skálová, A.; Vanecek, T.; Sima, R.; Laco, J.; Weinreb, I.; Perez-Ordonez, B.; Starek, I.; Geierova, M.; Simpson, R.H.; Passador-Santos, F.; et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: A hitherto undescribed salivary gland tumor entity. Am. J. Surg. Pathol. 2010, 34, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Leeman-Neill, R.J.; Kelly, L.M.; Liu, P.; Brenner, A.V.; Little, M.P.; Bogdanova, T.I.; Evdokimova, V.N.; Hatch, M.; Zurnadzy, L.Y.; Nikiforova, M.N.; et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer 2014, 120, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Long, P.; Wang, Y.; Ma, W. NTRK Fusions and TRK Inhibitors: Potential Targeted Therapies for Adult Glioblastoma. Front. Oncol. 2020, 10, 593578. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kurzrock, R.; Adashek, J.J. Evolution of the Targeted Therapy Landscape for Cholangiocarcinoma: Is Cholangiocarcinoma the ‘NSCLC’ of GI Oncology? Cancers 2023, 15, 1578. [Google Scholar] [CrossRef] [PubMed]
- Alassiri, A.H.; Ali, R.H.; Shen, Y.; Lum, A.; Strahlendorf, C.; Deyell, R.; Rassekh, R.; Sorensen, P.H.; Laskin, J.; Marra, M.; et al. ETV6-NTRK3 Is Expressed in a Subset of ALK-Negative Inflammatory Myofibroblastic Tumors. Am. J. Surg. Pathol. 2016, 40, 1051–1061. [Google Scholar] [CrossRef]
- Brenca, M.; Rossi, S.; Polano, M.; Gasparotto, D.; Zanatta, L.; Racanelli, D.; Valori, L.; Lamon, S.; Dei Tos, A.P.; Maestro, R. Transcriptome sequencing identifies ETV6-NTRK3 as a gene fusion involved in GIST. J. Pathol. 2016, 238, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Shi, E.; Chmielecki, J.; Tang, C.M.; Wang, K.; Heinrich, M.C.; Kang, G.; Corless, C.L.; Hong, D.; Fero, K.E.; Murphy, J.D.; et al. FGFR1 and NTRK3 actionable alterations in “Wild-Type” gastrointestinal stromal tumors. J. Transl. Med. 2016, 14, 339. [Google Scholar] [CrossRef]
- Castillon, M.; Kammerer-Jacquet, S.F.; Cariou, M.; Costa, S.; Conq, G.; Samaison, L.; Douet-Guilbert, N.; Marcorelles, P.; Doucet, L.; Uguen, A. Fluorescent In Situ Hybridization Must be Preferred to pan-TRK Immunohistochemistry to Diagnose NTRK3-rearranged Gastrointestinal Stromal Tumors (GIST). Appl. Immunohistochem. Mol. Morphol. 2021, 29, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shin, S.J.; Choe, E.A.; Kim, J.; Hyung, W.J.; Kim, H.S.; Jung, M.; Beom, S.H.; Kim, T.I.; Ahn, J.B.; et al. Tropomyosin-Related Kinase Fusions in Gastrointestinal Stromal Tumors. Cancers 2022, 14, 2659. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Li, J.; Sun, L.; Xu, Z.; Ke, Y.; Shao, B.; Guo, Y.; Sun, Y. GISTs with NTRK Gene Fusions: A Clinicopathological, Immunophenotypic, and Molecular Study. Cancers 2022, 15, 105. [Google Scholar] [CrossRef] [PubMed]
- D’Alpino Peixoto, R.; Medeiros, B.A.; Cronemberger, E.H. Resected High-Risk Rectal GIST Harboring NTRK1 Fusion: A Case Report and Review of the Literature. J. Gastrointest. Cancer 2021, 52, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Al-Salama, Z.T.; Keam, S.J. Entrectinib: First Global Approval. Drugs 2019, 79, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Larotrectinib: First Global Approval. Drugs 2019, 79, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Laetsch, T.W.; DuBois, S.G.; Mascarenhas, L.; Turpin, B.; Federman, N.; Albert, C.M.; Nagasubramanian, R.; Davis, J.L.; Rudzinski, E.; Feraco, A.M.; et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: Phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018, 19, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Awada, A.; Berghmans, T.; Clement, P.M.; Cuppens, K.; De Wilde, B.; Machiels, J.P.; Pauwels, P.; Peeters, M.; Rottey, S.; Van Cutsem, E. Belgian expert consensus for tumor-agnostic treatment of NTRK gene fusion-driven solid tumors with larotrectinib. Crit. Rev. Oncol. Hematol. 2022, 169, 103564. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.P.; Hechtman, J.F. Detection of NTRK Fusions: Merits and Limitations of Current Diagnostic Platforms. Cancer Res. 2019, 79, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Ferrarotto, R.; Liang, L.; Goepfert, R.P.; Li, J.; Ning, J.; Broaddus, R.; Weber, R.S.; El-Naggar, A.K. Pan-Trk immunohistochemistry reliably identifies ETV6-NTRK3 fusion in secretory carcinoma of the salivary gland. Virchows Arch. 2020, 476, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Atiq, M.A.; Davis, J.L.; Hornick, J.L.; Dickson, B.C.; Fletcher, C.D.M.; Fletcher, J.A.; Folpe, A.L.; Mariño-Enríquez, A. Mesenchymal tumors of the gastrointestinal tract with NTRK rearrangements: A clinicopathological, immunophenotypic, and molecular study of eight cases, emphasizing their distinction from gastrointestinal stromal tumor (GIST). Mod. Pathol. 2021, 34, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Marchiò, C.; Scaltriti, M.; Ladanyi, M.; Iafrate, A.J.; Bibeau, F.; Dietel, M.; Hechtman, J.F.; Troiani, T.; López-Rios, F.; Douillard, J.Y.; et al. ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann. Oncol. 2019, 30, 1417–1427. [Google Scholar] [CrossRef]
- Hechtman, J.F.; Benayed, R.; Hyman, D.M.; Drilon, A.; Zehir, A.; Frosina, D.; Arcila, M.E.; Dogan, S.; Klimstra, D.S.; Ladanyi, M.; et al. Pan-Trk Immunohistochemistry Is an Efficient and Reliable Screen for the Detection of NTRK Fusions. Am. J. Surg. Pathol. 2017, 41, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Rudzinski, E.R.; Lockwood, C.M.; Stohr, B.A.; Vargas, S.O.; Sheridan, R.; Black, J.O.; Rajaram, V.; Laetsch, T.W.; Davis, J.L. Pan-Trk Immunohistochemistry Identifies NTRK Rearrangements in Pediatric Mesenchymal Tumors. Am. J. Surg. Pathol. 2018, 42, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Sasa, K.; Son, R.; Oguchi, A.; Ashizawa, K.; Hasegawa, N.; Kubota, D.; Suehara, Y.; Takagi, T.; Okubo, T.; Akaike, K.; et al. NTRK2 expression in gastrointestinal stromal tumors with a special emphasis on the clinicopathological and prognostic impacts. Sci. Rep. 2024, 14, 768. [Google Scholar] [CrossRef] [PubMed]
- Merten, L.; Agaimy, A.; Moskalev, E.A.; Giedl, J.; Kayser, C.; Geddert, H.; Schaefer, I.M.; Cameron, S.; Werner, M.; Ströbel, P.; et al. Inactivating Mutations of RB1 and TP53 Correlate with Sarcomatous Histomorphology and Metastasis/Recurrence in Gastrointestinal Stromal Tumors. Am. J. Clin. Pathol. 2016, 146, 718–726. [Google Scholar] [CrossRef] [PubMed][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).