Abstract
This work aimed to identify the chemical compounds of Cinnamomum burmannii leaf essential oil (CBLEO) and to unravel the antibacterial mechanism of CBLEO at the molecular level for developing antimicrobials. CBLEO had 37 volatile compounds with abundant borneol (28.40%) and showed good potential to control foodborne pathogens, of which Staphylococcus aureus had the greatest inhibition zone diameter (28.72 mm) with the lowest values of minimum inhibitory concentration (1.0 μg/mL) and bactericidal concentration (2.0 μg/mL). To unravel the antibacterial action of CBLEO on S. aureus, a dynamic exploration of antibacterial growth, material leakage, ROS formation, protein oxidation, cell morphology, and interaction with genome DNA was conducted on S. aureus exposed to CBLEO at different doses (1/2–2×MIC) and times (0–24 h), indicating that CBLEO acts as an inducer for ROS production and the oxidative stress of S. aureus. To highlight the antibacterial action of CBLEO on S. aureus at the molecular level, we performed a comparative association of ROS accumulation with some key virulence-related gene (sigB/agrA/sarA/icaA/cidA/rsbU) transcription, protease production, and biofilm formation in S. aureus subjected to CBLEO at different levels and times, revealing that CBLEO-induced oxidative stress caused transcript suppression of virulence regulators (RsbU and SigB) and its targeted genes, causing a protease level increase destined for the biofilm formation and growth inhibition of S. aureus, which may be a key bactericidal action. Our findings provide valuable information for studying the antibacterial mechanism of essential oil against pathogens.
1. Introduction
Food is rich in nutrients and suitable for the growth and reproduction of pathogens. Microbial food spoilage and foodborne disease remain major issues for public health worldwide [1]. Synthetic preservatives, such as tertiary butylhydroquinone (TBHQ), butylated hydroxytoluene (BHT), and several organic acids and salts, have been widely applied in the food industry to guarantee both food safety and security, but various adverse effects (immunity suppression, teratogenicity, carcinogenicity, hypersensitivity, allergic reactions, and acute toxicity) caused by prolonged use have greatly impacted public health, which has become an important topic worldwide [1,2]. Therefore, the development of novel and effective natural antimicrobial agents is of paramount importance to assure food safety and public health.
Plant essential oil has been identified to have high and extensive biological properties (such as antimicrobial, anticancer, antiviral, and antioxidant activities) [3,4,5,6,7,8]. In recent years, plant essential oils have been applied as a new natural source of antimicrobials, food preservatives, and packaging [1,9,10,11,12], and so developing and utilizing novel natural essential oils in the food industry have become one focus of future research. Several works have shown that essential oils can cause a range of damage to bacteria, including cell membrane damage and content leakage [5,7,8,13,14,15], respiratory metabolism depression [8,14,16], redox homeostasis disruption [7,17], DNA topological change, and RNA biosynthesis [8,15,18,19]. It is also noted that food poisoning and diseases caused by foodborne pathogens have become one major threat to human health and food safety due to the secretion of enterotoxins [20,21], but the growth and reproduction of pathogens are tightly dependent on biofilm formation, and thus the prevention of pathogen biofilm formation has become an effective way to ensure food safety [22]. The regulation of bacterial biofilm formation may be involved in the coordinated expression of several virulence genes [23,24,25]. In recent years, essential oils have been identified to have an anti-biofilm effect against fungi and bacteria [18,26,27,28,29,30,31] and effective inhibition of virulence-related gene expression [8,16,26,28]. Yet, few studies have studied the cellular response of bacteria to oxidative stress and ROS accumulation induced by essential oil. Therefore, the acting mechanism of essential oil involved in the virulence attenuation and biofilm formation inhibition remains enigmatic.
The genus Cinnamomum, a member of the family Lauraceae, is widely distributed in Southeast Asia with notable economic value owing to its rich essential oil and medicinal utilization [32,33,34]. The essential oils of Cinnamomum plants have been shown to have various biological effects (such as antimicrobial, anti-inflammatory, and antitumor) [32,33,34,35,36] and are widely used in the medicine, perfume, and chemical industries and especially in the food industry as natural preservatives, antimicrobial and antioxidant agents, and flavoring agents (such as brewing chocolate, chewing gum, and liquors) [37,38,39]. Of note, Cinnamomum burmannii is one of the most promising sources in the food, pharmaceutical, and cosmetic industries due to its unique compounds (such as borneol, α-terpineol, and α-pinene) [34,38]. Based on our studies on different C. burmannii germplasms, some accessions were selected with a high yield of essential oil (1.2–1.6%) and an average proportion (38.7%) of borneol, and notably, we established a standard system for the utilization of C. burmannii [40,41]. Yet, the antibacterial mechanism of C. burmannii leaf essential oil (CBLEO) is unknown, which has intercepted CBLEO application in modern industry.
The aim of this work was to unravel antibacterial action and to highlight the molecular mechanism that governs bacterial growth and biofilm inhibition for developing CBLEO as a potential source of natural antibacterial agent. To this end, one plus tree of C. burmannii (accession CB01) was used to detect the volatile profile of CBLEO and to assess antibacterial activity on seven representative foodborne pathogens, and the antibacterial action of CBLEO on Staphylococcus aureus (susceptible strain) was observed. As an initial step toward exploring the antibacterial action of CBLEO, we focused on the dynamic effects of CBLEO on bacterial growth, content release, electric conductivity, ROS and MDA formation, protein oxidation, and cell morphology in S. aureus subjected to different doses (1/2×MIC, 1×MIC, and 2×MIC) and times (0–8 h). Such an assay could help to unravel how CBLEO induces oxidative stress. To highlight the antibacterial acting mechanism of CBLEO on S. aureus at the molecular level, a comparative assay was conducted on the association of ROS accumulation with some key virulence-related gene transcription, protease level, biofilm formation, and the interaction with genome DNA in the response of S. aureus to CBLEO at different levels and times. This study presents the effect of essential-oil-mediated oxidative stress and ROS accumulation on the transcription of virulence-associated regulators as an attempt to elucidate the antibacterial mechanism of essential oil.
2. Results
2.1. Identification of Volatile Compounds in C. burmannii Leaf Essential Oil (CBLEO)
To develop C. burmannii leaf essential oil (CBLEO) as a potential source for application, we detected the chemical compounds of CBLEO by GC-MS. A total of 37 compositions were identified (Table 1), accounting for 99.56% of total oil. The main chemical components of CBLEO mainly included 14 monoterpene hydrocarbons, 29 sesquiterpene hydrocarbons, 6 monoterpene alcohols, 6 sesquiterpene alcohols, and 5 sesquiterpene hydrocarbons, of which monoterpene (85.38%) was the dominant group of components. The richest compound was borneol (28.40%), followed by bornyl acetate (9.43%), eucalyptol (9.22%), D-limonene (7.44%), α-pinene (3.96%), β-cymene (3.96%), β-caryophyllene (3.71%), α-terpineol (3.15%), α-phellandren (2.67%), sabinene (2.53%), β-myrcene (2.41%), β-pinene (2.38%), and camphor (2.14%). These results revealed a complex of chemical components with rich borneol in CBLEO.

Table 1.
Compounds and contents of essential oils from Cinnamomum burmami leaves by GC-MS analysis.
2.2. Assay of Antibacterial Activity of CBLEO
The values of DIZ, MIC, and MBC were detected to determine the antibacterial activity of CBLEO. In the case of CBLEO, the DIZ values ranged from 7.51 to 28.72 mm across all tested strains, and the MIC and MBC values were in the range of 1.0−16.0 μg/mL and 2.0−32.0 μg/mL, respectively (Table 2), emphasizing that CBLEO had good inhibition effects on foodborne pathogens. Of note, the maximum value of DIZ (28.72 nm) and the minimum values of MIC (1.0 μg/mL) and MBC (2.0 μg/mL) were all marked in the response of S. aureus to CBLEO, indicating a strong bactericidal effect of CBLEO on S. aureus. Hence, our following work was focused on the exploration of the antibacterial action of CBLEO on S. aureus in order to develop CBLEO as a potential antibacterial agent.

Table 2.
Assay of antibacterial activity of C. burmannii leaf essential oil (CBLEO) against seven representative foodborne pathogens.
2.3. Effect of CBLEO on Bacterial Growth of S. aureus
To unravel the antibacterial action of CBLEO against S. aureus, the assay of the antibacterial kinetics curve was conducted on the CBLEO treatments with five different concentrations (1/8×MIC, 1/4×MIC, 1/2×MIC, 1×MIC, and 2×MIC) and times (0–24 h), all of which showed a dose/time-dependent inhibition manner for bacterial growth (Figure 1). The greatest inhibitory effect was recorded at 2×MIC for 24 h with the number of viable cells reduced by 96.86% from 7.0 to 0.2 lg CFU/mL, followed by a 93.57% decrease at 1×MIC, but a 38.29%, 20.28%, and 6.86% decline was marked for 1/2×MIC, 1/4×MIC, and 1/8×MIC, respectively. A notable inhibition of bacterial growth was identified within the 1 h incubation of 1×MIC and 2×MIC, and a complete inhibition occurred within the first 8 h and 12 h at 2×MIC and 1×MIC, respectively. Yet, the control cells showed a normal growth status (Figure 1). These results revealed a great bactericidal potential of CBLEO toward S. aureus.
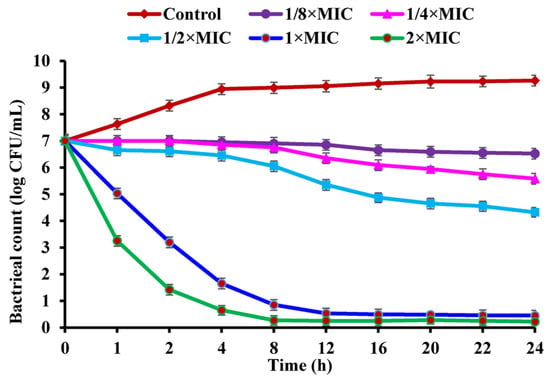
Figure 1.
The growth kinetics curve of Staphylococcus aureus affected by CBLEO. The data represent the mean value ± SD of three parallel replicates.
2.4. Impact of CBLEO on Cell Structure of S. aureus
The damage to cell structure (cell wall and membrane) in CBLEO-treated S. aureus was evaluated as part of an attempt to understand the antibacterial action of CBLEO. Firstly, the effect of CBLEO on cell wall damage was tested by the leakage assay of AKP (cell-wall-damage-biomarker enzyme). In the presence of CBLEO, AKP activity in S. aureus suspensions increased by a dose/time-dependent pattern (Figure 2A). After 8 h of exposure to CBLEO, AKP activity at 2×MIC was 1.7- and 0.8-fold greater than that at 1/2×MIC and 1×MIC, respectively, and notably increased activity was recorded within the first 2 h at both 1×MIC and 2×MIC, but the control exhibited no change in AKP activity (Figure 2A), so it was concluded that CBLEO could cause the destruction of the cell wall of S. aureus.
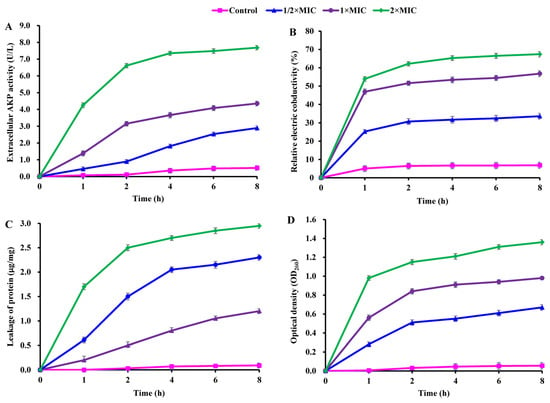
Figure 2.
Effect of CBLEO on cell wall and cell membrane of S. aureus. (A) Extracellular activity of alkaline phosphatase (AKP); (B) relative electric conductivity; (C) leakage of protein; (D) release of 260 nm absorbing material. Data represent the value ± SD of three replicates.
Next, CBLEO-mediated damage to the permeability of the cell membrane was assayed by detecting electric conductivity. During exposure to CBLEO, a dose/time-dependently increased profile for electric conductivity was marked in S. aureus suspensions, of which a rapid increase was detected within the first 1 h (Figure 2B). After the 8 h incubation of CBLEO, the values of electric conductivity at 1/2×MIC, 1×MIC, and 2×MIC were 4.0-, 7.3-, and 8.9-fold greater than those of the control (Figure 2B), indicating that CBLEO could effectively cause the cell membrane damage and permeability increase of S. aureus with the intracellular electrolyte release.
As for cell membrane integrity, the releases of intracellular protein and nucleic acid were tested. After the incubation of S. aureus with different doses of CBLEO (1/2×MIC, 1×MIC, and 2×MIC), the released amount of protein showed a dose/time-dependent increase, in which the increased degree of protein release at 2×MIC for 8 h was 2.0- and 0.7-fold greater than at 1/2×MIC and 1×MIC, respectively (Figure 2C). A similar dose/time-dependent increase pattern was also noted for nucleic acid leakage (Figure 2D). Yet, those in the control showed no significant change (Figure 2C,D). These results indicated that CBLEO could result in irreversible damage to the cytoplasmic membrane integrity of S. aureus with a loss of cellular materials.
To highlight the antibacterial action of CBLEO on S. aureus, we further performed SEM analysis to explore the influence of CBLEO on the cell morphology of S. aureus. The untreated S. aureus cells retained a normal and complete appearance with a smooth surface, intact cell membrane, and cell wall structure (Figure 3A), whereas the CBLEO-treated S. aureus cells became irregular, and cell surface collapsed or shriveled (Figure 3B). We thus considered that CBLEO could change cell morphology and damage the cell membrane of S. aureus. This further confirmed the above assayed results of CBLEO-caused damage to the permeability and integrity of the cell membrane of S. aureus (Figure 2).
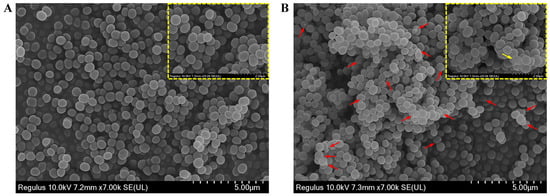
Figure 3.
Effect of CBLEO on cell morphologically of S. aureus by scanning electron microscope (SEM) assay. (A) SEM image of untreated S. aureus; (B) SEM image of S. aureus treated with CBLEO (1×MIC) for 2 h. Red arrows represent cell morphology change and cell membrane damage. Yellow boxes represent the details of cell morhology, bar = 2 μm. Yellow arrow represents the severely damaged cell.
2.5. Effect of CBLEO on Cellular MDA and ROS Generation and Protein Oxidation of S. aureus
Considering that CBLEO could damage cell structure and control the bacterial growth of S. aureus (Figure 1, Figure 2 and Figure 3), it was vital to determine the potential of CBLEO to induce oxidative stress destined for the cell membrane damage and growth inhibition of S. aureus. Firstly, MDA was selected as a lipid peroxidation biomarker to evaluate the temporal amount change in S. aureus cells during exposure to CBLEO. A dose/time-dependent increase pattern was identified for intracellular MDA (Figure 4A), of which MDA content was much higher at 2×MIC than at both 1×MIC and 1/2×MIC after 8 h exposure, while the control showed almost no change, implying that CBBLEO could induce the cell lipid peroxidation of S. aureus. This allowed us to explore the intracellular ROS generation and protein oxidation of S. aureus cells as an oxidative stress indicator. The amounts of intracellular ROS and protein carbonyl formation increased in S. aureus cells in a dose/time-dependent manner during exposure to CBLEO, both of which showed the maximum value after 8 h of exposure to CBLEO at 2×MIC, but no notable change was detected for the control (Figure 4B,C), emphasizing that CBLEO-induced oxidative stress could result in an accumulation of ROS and protein oxidation product in S. aureus cells.

Figure 4.
Effect of CBLEO on cell lipid peroxidation and oxidative stress in response of S. aureus to different doses and times. (A) Intracellular mallondialdehyde (MDA) level; (B) intracellular ROS generation; (C) protein carbonyl content; (D) total cellular protein. Data represent mean value ± SD of three parallel replicates, and different letters denote significant differences (p < 0.05).
2.6. Effect of CBLEO on Cellular Total Protein Concentration of S. aureus
Given the effect of CBLEO-induced oxidative stress on the cellular lipid peroxidation and protein oxidation of S. aureus (Figure 4B,C), it was essential for us to investigate whether CBLEO-induced oxidative stress affected the total protein of S. aureus cells. Hence, a dynamic analysis of cellular total protein was performed in S. aureus exposed to CBLEO. Compared with the control, the amount of cellular total protein exhibited a dose/time-dependently decreased profile in S. aureus exposed to CBLEO, and a 25.79%, 34.37%, and 53.01% decline was observed at 1/2×MIC, 1×MIC, and 2×MIC after the 8 h incubation of CBLEO, respectively (Figure 4D), suggesting the induction of bacterial protein fragmentation or its biosynthesis disturbance by CBLEO.
2.7. Effect of CBLEO on Cellular Biofilm Development and Protease Activity of S. aureus
Another concern was whether CBLEO shows anti-biofilm activity against S. aureus. A dose/time-dependent decline pattern was noted for biofilm biomass in S. aureus cells exposed to CBLEO, of which the reduction in biofilm biomass was 43.64%, 63.75%, and 85.42% after 8 h of exposure to 1/2×MIC, 1×MIC, and 2×MIC, respectively, but the increased biofilm formation was observed for the control (Figure 5A), emphasizing that CBLEO had a good anti-biofilm effect on S. aureus. It was also noted that the biofilm formation decline was highly associated with protease production [42]. Compared with biofilm biomass (Figure 5A), the protease activity exhibited a dose/time-dependent increase in S. aureus subjected to CBLEO, while a decrease in protease activity was detected for the control (Figure 5B), indicating that the CBLEO-induced low capacity of biofilm formation may be associated with an increase in protease activity.
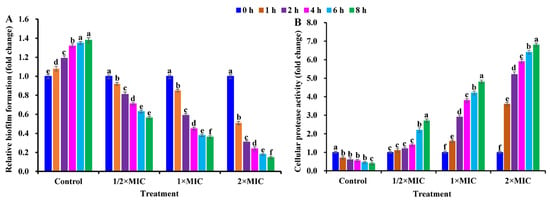
Figure 5.
Effect of CBLEO on biofilm formation and protease activity in response of S. aureus cells to different doses and times. (A) Assessment of inhibitory capacity of CBLEO on biofilm formation by microtiter plate assay; (B) change in cellular protease activity. Values of biofilm formation and protease production in S. aureus cells from control and CBLEO-treated samples with different doses at 0 h were arbitrarily set to 1.00 for standardization. Data represent mean value ± SD of six parallel replicates, and different letters denote significant differences (p < 0.05).
2.8. Effect of CBLEO on Genome DNA of S. aureus
It was reported that carvacrol or citral from essential oils could be chimeric with bacterial DNA and break the DNA structure of S. aureus and E. coli [8,14,16]. The same single electrophoretic band of genomic DNA was marked for S. aureus from all CBLEO-treated and control samples (Figure S1), suggesting no direct effect of CBLEO on the genome DNA of S. aureus.
2.9. Effect of CBLEO on Transcript of Virulence-Related Genes and Regulatory Proteins in S. aureus
The above findings that oxidative stress could effectively induce ROS accumulation (Figure 4B) and biofilm formation reduction (Figure 5A) with no direct effect on bacterial genome DNA (Figure S1) in the response of S. aureus cells to CBLEO prompted us to highlight the mechanism for how CBLEO causes virulence attenuation and biofilm formation inhibition at the molecular level. Biofilm formation, one key virulence determinant, is controlled by several virulence genes in response to ROS. To ascertain the anti-biofilm mechanism of CBLEO at the molecular level, some vital virulence-associated genes responsible for biofilm formation, including agrA (accessory gene regulator A), sigB (sigma factor B, involved in biofilm formation and stress response), sarA (staphylococcal accessory regulator A), cidA (murein hydrolase regulator, involved in cell lysis and extracellular DNA release), icaA (intercellular adhesin A, involved in cell wall and biofilm formation), and rsbU (involved in the regulation of sigB and biofilm formation), were selected as potential antibacterial targets to analyze dynamic transcription changes in S. aureus cells during exposure to CBLEO by qRT-PCR detection.
As shown in Figure 6, the transcriptional levels of sigB/rsbU/agrA/sarA/icaA/cidA in CBLEO-treated S. aureus cells were all down-regulated in a dose/time-dependent pattern, of which the lowest transcript level was recorded for 8 h at 2×MIC, but an increase in them was marked in the control, as also noted for biofilm formation in CBLEO-treated S. aureus cells (Figure 5A), indicating that CBLEO could inhibit the transcription of virulence-related genes destined for the reduction in S. aureus biofilm formation. Of note, the down-regulated degree of the transcriptional level of sigB (88.02%) and rsbU (92.01%) was much higher than that of agrA/sarA/icaA/cidA (68.89−73.97%) after 8 h of exposure at 2×MIC (Figure 6), implying that SigB and RsbU may be a crucial regulator to control biofilm formation in CBLEO-treated S. aureus cells.
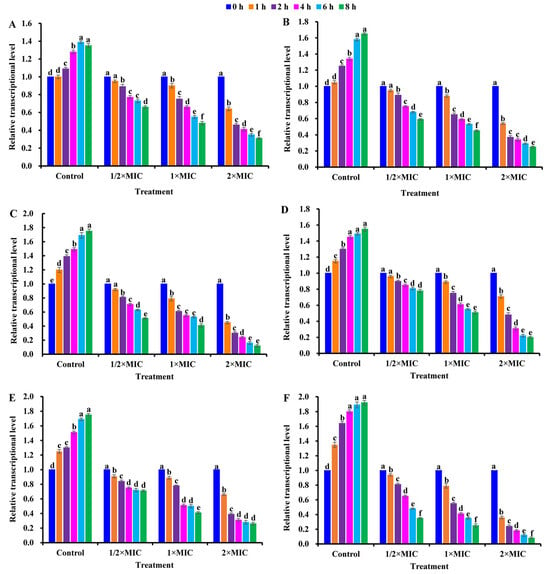
Figure 6.
Effect of CBLEO on transcriptions of virulence-associated regulators of S. aureus under exposure to different concentrations and times by qRT-PCR detection. (A) Relative transcription of agrA (accessory gene regulator A); (B) relative transcription of sarA gene (staphylococcal accessory regulator A); (C) relative transcription of sigB (sigma factor B); (D) relative transcription of icaA (intercellular adhesin A); (E) relative transcription of cidA gene (encoding for holin); (F) relative transcription of rsbU (SigB activator). Relative expression values were counted as 2−ΔΔCt, and 16S RNA was used as internal control. Transcription level in S. aureus cells from control and CBLEO-treated samples with different concentrations at 0 h was arbitrarily set to 1.00 for standardization. Data represent mean value ± SD of three parallel replicates, and different letters denote significant differences (p < 0.05).
Together, the CBLEO-induced collaborative transcription repression of virulence-related genes was mainly responsible for the inhibition of biofilm formation, of which both RsbU and SigB may be the antibacterial targets of CBLEO against S. aureus (Figure 7).
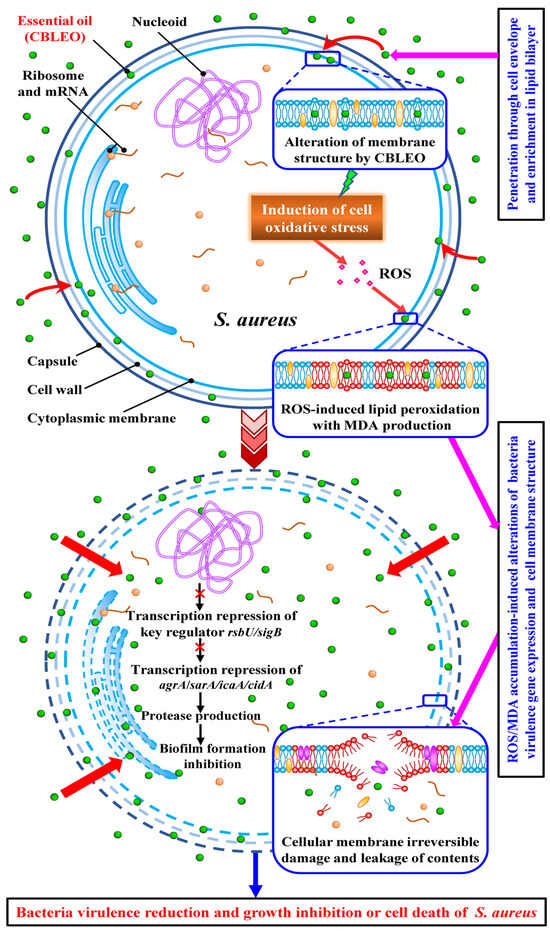
Figure 7.
The antibacterial acting mode of C. burmami leaf essential oil (CBLEO) on S. aureus. The identified antibacterial mechanism of CBLEO against S. aureus is based on the present work and is summarized from the two perspectives of virulence-related gene transcription regulation and cellular structure destruction. The abbreviations are shown as follows: agrA, accessory gene regulator A; sarA, staphylococcal accessory regulator A; sigB, sigma factor B; icaA, intercellular adhesin A; ROS, reactive oxygen species; MDA, mallondialdehyde.
3. Discussion
3.1. Rich Volatile Profiling with High Borneol Amount and Good Antibacterial Activity of CBLEO
Plant-derived essential oils have been widely used as natural antimicrobials in the food industry [9,11,12]. In this work, 37 volatile compositions were identified in C. burmannii leaf essential oil (CBLEO) (Table 1), of which many compounds and their contents were different from those reported for other Cinnamomum species (such as C. pauciflorum, C. zeylanicum, and C. camphora) [26,37,43], indicating a difference in volatile profiling and its contents among different Cinnamomum species. It was also noted that most of our detected volatile compounds have been shown to have high antimicrobial activity, especially some major constituents, such as borneol (28.31%) [44,45,46], D-limonene (7.44%) [5,6,47,48], α-pinene (3.96%) [49], β-Caryophyllene (3.71%) [4,48,50], and α-terpineol (3.15%) [36]. Combined with the good inhibitory effect of CBLEO on all tested foodborne pathogens (Table 2), it seems certain that these chemical compositions may be a potential of CBLEO to control pathogens. Several studies have indicated that borneol shows several pharmacological activities including analgesic, anti-inflammatory, and antioxidant properties [45,46]. Our finding of borneol (28.31%) as the richest compound of CBLEO (Table 1) that was higher than that for C. burmannii, C. zeylanicum, C. camphora, C. pauciflorum, and C. tamala (0.81–11.95%) [34,37,43,51,52] revealed that it may be the most promising antibacterial agent. Altogether, the advantage of CBLEO over other essential oils from different Cinnamomum species was its rich volatile components with a high proportion of borneol, emphasizing that CBLEO may be a novel source for utilization. Of note, S. aureus was marked as the most susceptible pathogen (Table 2), and thus the following work focused on highlighting the antibacterial action of CBLEO against S. aureus for the development of CBLEO as a natural antibacterial agent for potential utilization.
3.2. ROS-Generation-Mediated Oxidative Stress and Cell Membrane Damage Involved in Antibacterial Action of CBLEO
During the exposure of bacteria to the essential oil, lipophilic components could bind to the bacterial cell surface and penetrate the outer membrane, and then its lipid bilayer made contact with the hydrophobic part of the cell membrane, subsequently causing a toxic effect, leading to cell wall and membrane damage, function destruction, material release, ROS generation, and cell death [5,7,8,14,16]. Yet, the essential-oil-mediated mechanism of bacterial cell membrane damage is still unclear. Accumulating evidence showed that intracellular material leakage may be a good biomarker of irreversible damage to the cell membrane [5,7,13]. Here, a close negative correlation was established between the inhibitory effect of bacterial growth (Figure 1) and a significant increase in material release, extracellular AKP activity (cell-wall-damage-marker enzyme), and electric conductivity in S. aureus during exposure to CBLEO (Figure 2), implying that the destruction of cell membrane structure induced by CBLEO may be one pivotal cause for the growth inhibition of S. aureus. This was in line with the previously found antibacterial effect of essential oil on several foodborne pathogens (such as Bacillus subtilis, B. cereus, Escherichia coli, E. faecalis, Listeria monocytogenes, Pseudomonas aeruginosa, Shigella dysenteries, S. aureus, Salmonella typhimurium, and Shigella flexneri) [5,7,8,14,15].
ROS-induced lipid peroxidation during oxidative stress is known as one key initiator that causes cell membrane damage. MDA, the most abundant product of lipid peroxidation, can induce cellular protein oxidation [53] and thus widely serves as an indicator of oxidative stress for studying microbial growth, cell death, and disease incidence [7,54]. However, the association of oxidative stress with MDA variation in bacterial cells caused by essential oil remains unclear. Given that a similar accumulative pattern of MDA (Figure 4A) and ROS (Figure 4B) in CBLEO-treated S. aureus cells was positively associated with the degree of bacterial growth inhibition, material release, and cell membrane damage (Figure 1, Figure 2 and Figure 3), it seems certain that CBLEO-induced oxidative stress may be one crucial bactericidal effect. Our results were consistent with the effect of the essential oils from different plants and natural compounds (such as carvacrol, citral, flavonoid, anthocyanin, dihydromyricetin, and eugenol) on S. flexneri, S. aureus, E. coli, and P. digitatum [7,8,14,16,17,19,28,55,56] and also evidenced by previous studies showing that the effects of antibacterial agents (such as fluconazole, cerulein, catechin, chitosan, miconazole, indomethacin, and hypocrellin A) could cause oxidative stress with the consequence of ROS accumulation and membranous damage destined for bacterial cell death [44,57,58,59,60].
In general, many microbes possess a range of defensive systems to detoxify ROS [61]. The thioredoxin (Trx) and glutaredoxin (Grx) systems are the two major thiol-dependent antioxidant systems in the defense against the oxidative stress of bacteria cells [62]. The Trx system, which is composed of NADPH, thioredoxin reductase (TrxR), and Trx, can provide the electron to thiol-dependent peroxidases (known as peroxiredoxins, Prx) to remove reactive oxygen species such as glutathione peroxidase (GPx) and contribute to the redox state of methionine sulfoxide reductases (Msr) for the repair of oxidized proteins [62,63]. The Grx system, containing NADPH, glutathione reductase (GR), GSH, and Grx, is involved in the defense against oxidative stress via the efficient removal of various ROS by GPx [64]. It is also noted that catalase (CAT), superoxide dismutase (SOD), and TrxR homolog alkyl hydroperoxide peroxidase subunit C/F (Ahpc/f) participate in the antioxidant process in bacteria [62]. Several works have shown that the mutation of grx1/2/5, trx1/2, msr1/2, trxR, grl, ahpC, or gpx3 in some bacteria (such as E. coli, Helicobacter pylori, Mycobacter tuberculosis, Streptococcus pyogenes, S. cerevisiae, or S. aureus) was sensitive to oxidative stress with a decreased survival [62,65,66,67,68,69,70]. In combination with our findings of cell membrane damage, bacterial growth inhibition, and MDA and ROS accumulation in CBLEO-treated S. aureus (Figure 1, Figure 2 and Figure 4A,B), it was considered that the CBLEO-induced deficiency of enzymatic antioxidant systems may be one critical antibacterial factor. This could be verified by our recent results that essential-oil-mediated decrease in ROS-detoxified enzymes (SOD, CAT, Prx, GPx, and GST) may likely contribute to the cell membrane damage and growth inhibition of S. flexneri [7] and that exogenous ROS scavenger (such as SOD and CAT) treatment could alleviate ROS formation and the membrane damage of P. digitatum and S. aureus cells [17,60]. Therefore, CBLEO may act as an inhibitor of the enzyme systems participating in antioxidant responses in S. aureus.
Also noteworthy was the impact of ROS accumulation on cellular protein oxidation [71]. Here, the increased amount of cellular protein carbonyl in CBLEO-treated S. aureus (Figure 4C) was positively associated with the accumulated amount of ROS and MDA (Figure 4A,B), both of which showed a negative correlation with the declined content of total cellular protein (Figure 4D). Thus, we concluded that CBLEO-induced oxidative stress could cause cellular protein oxidation and biosynthesis disturbance, as also noted for the effect of essential oil on P. digitatum and S. aureus [14,17].
Together, CBLEO-mediated oxidative stress and ROS accumulation may be the primary driver of the cell membrane damage of S. aureus. Yet, little attention has been paid to the cross-talk between oxidative stress and virulence-associated factor expression required for biofilm formation during the exposure of bacteria to the essential oil.
3.3. CBLEO-Induced Transcription Repression of Virulence-Associated Genes in S. aureus as Pivotal Antibacterial Action
Foodborne disease has become a serious issue affecting human health and food safety. S. aureus, one of the most common foodborne pathogens, can grow in various foods and cause food poisoning by secreting enterotoxins that cause various disease symptoms (such as nausea, vomiting, and diarrhea) [20,21] and thus poses a serious threat to human health [22]. S. aureus enterotoxins, one superfamily of secreted virulence factors, are generally regulated by a quorum-sensing agr system via the autoinducer peptide (AIP) and two divergent transcripts (called RNAII and RNAIII), of which the RNAII transcript is an operon of agr genes (agrBDCA) that encode key factors for agr regulatory activation [72]. Of these, AIP is produced from the AgrD precursor and then processed and exported as a quorum signal by AgrB to activate sensor kinase AgrC and response regulator AgrA, subsequently leading to the induction of the agr system and the upregulation of RNAII/RNAIII transcription essential for virulence production [72,73,74]. Also, AgrA is known as a key virulence regulator, but SarA has been identified as a positive regulator of agr activity in S. aureus [23,24,25]. Yet, it is unclear whether essential oil can affect bacterial AgrA or SarA activity. Here, the coordinately repressed transcriptions of both sarA and agrA in the response of S. aureus cells to CBLEO (Figure 6A,B) were temporally and positively correlated with bacterial growth inhibition (Figure 1), indicating that the CBLEO-induced repression of the transcriptions of sarA and its targeted agrA may contribute to the growth inhibition of S. aureus, which was the case for the effect of essential oil on C. albicans, C. violaceum, P. aeruginosa, P. arotovorum, P. aroidearumor, and S. aureus [16,18,26,27,28]. AgrA and SarA, two key global regulators of virulence genes, were tightly controlled by transcription regulator SigB [24,75], which may in turn regulate the transcriptions of several virulence-related factors crucial for the cell processes (such as stress response and biofilm formation) of B. subtilis, L. monocytogenes, P. aeruginosa, and S. aureus [25,75,76]. Considering that a similar dose/time-dependently repressed transcription of sigB/agrA/sarA/icaA/cidA in CBLEO-treated S. aureus cells (Figure 6) was temporally and positively correlated to the inhibition of bacterial growth (Figure 1) and biofilm formation (Figure 5A) but concomitantly with an increase in protease activity (Figure 5B), it seems likely that the CBLEO-mediated coordinate repression of the sigB/agrA/sarA/icaA/cidA transcript could activate the expression of the protease gene destined to increase its production in S. aureus cells during exposure to CBLEO, which may contribute to the inhibition of biofilm formation and bacterial growth. This was consistent with the antibacterial effect of essential oil on E. coli, A. baumannii, C. violaceum, C. albicans, P. aroidearumor, P. aeruginosa, and P. arotovorum [8,18,26,27,28,29,31]. In support of our results, sigB, sarA, agrA, icaA, or cidA mutation in S. aureus could increase protease amount with a significant decline of biofilm formation [42,77,78,79,80,81,82,83], and the exogenous addition of protease notably limited the biofilm formation of S. aureus [84,85].
Also of note was the role of RsbU in SigB activity activation [86,87]. The mutation of rsbU could repress the transcription of sigB and its targeted downstream gene (ica/sarA/agr) related to bacterial biofilm formation [76,88]. In this work, the reduced biofilm formation (Figure 5A) was positively associated with the coordinately repressed transcription of rsbU/sigB/agrA/sarA/icaA/cidA during the exposure of S. aureus cells to CBLEO (Figure 6), and notably, the transcriptions of rsbU and sigB were significantly down-regulated (Figure 6C,F) and thus revealed that both RsbU and SigB may be key regulators in controlling the biofilm formation of S. aureus cells exposed to CBLEO. This fact was supported by previous results that rsbU or sigB mutation could increase the expression of the protease gene and decrease biofilm formation [85,87]. Therefore, it seems that CBLEO-mediated transcriptional repressions of RsbU and SigB may be pivotal antibacterial targets against S. aureus. Yet, the actual mechanism by which essential oil repressed the transcription of virulence regulators destined for biofilm formation inhibition remains unknown.
ROS-mediated oxidative stress could cause AgrA oxidation to loss regulatory activity [23,25], and the mutation of rsbU, sigB, agrA, or sarA in S. aureus increased susceptibility to oxidative stress and inhibited biofilm formation [23,85]. In this work, a close correlation was established between the massive accumulation of ROS and protein oxidation (Figure 4B,C), the low transcript of rsbU/sigB/agrA/sarA/icaA/cidA (Figure 6), the less formation of biofilm (Figure 5A), and the notable inhibition of bacterial growth (Figure 1) in S. aureus exposed to CBLEO, indicating that the CBLEO-induced growth inhibition of S. aureus may be attributed mostly to the limitation of biofilm formation via the depression of the transcription of the virulence-related regulator caused by ROS accumulation, which may confer the key acting mechanism of CBLEO on S. aureus. This finding was compatible with the antibacterial effect of essential oil on P. aeruginosa, C. violaceum, and E. coli [8,18] and was also consistent with previous results that antibacterial agents (linezolid, benzimidazole, and vancomycin) could cause oxidative stress, leading to the transcription repression of the virulence-related gene destined for protease activity increase and biofilm formation inhibition [85,89,90]. All of this indicated that ROS-mediated oxidative stress could be a critical initiator of the transcriptional repression of key regulators (RsbU and SigB) and their targeted genes in the response of S. aureus to CBLEO, leading to a biofilm formation decrease, which eventually causes bacterial growth.
Altogether, the collaborative transcription repression of virulence-related genes and the effective increase in protease activity, as well as the notable inhibition of biofilm formation, may respond specifically to an increase in ROS-induced oxidative stress in S. aureus by CBLEO. Of these, the RsbU/SigB-mediated transcription regulatory system was responsible for the bactericidal effect of CBLEO against S. aureus.
4. Materials and Methods
4.1. Plant Materials
The leaves of C. burmannii were collected from one 10-year-old plus tree with high borneol content (germplasm accession CB01) planted in our research base (Guangdong Huaqingyuan Biotechnology Co., Ltd.) in Meizhou City, Guangdong Province (E115°50′1″, N24°28′28″), China [40].
4.2. Extraction of Essential Oil and Analysis of Chemical Components of CBLEO
Essential oil was extracted by steam distillation, and the fresh leaves (about 50 g) were powdered and subjected to hygro-distillation using a modified Clevenger-type apparatus for 5 h [91]. The obtained oils were collected, measured, and dried with anhydrous Na2SO4 and then stored in a sealed tube at −20 °C for further use. The extracted essential oils were weighted, and their yield (1.61%) was calculated and expressed as the percentage (%, w/w) of fresh leaf [7].
The chemical constituents of CBLEO were detected by GC-MS method. The obtained oil sample was performed on GCMS-QP2020W/O gas chromatograph (Shimadzu, Kyoto, Japan), equipped with SH-R×ITM-5SIL MS column (30 m × 0.25 mm, 0.25 μm) [91]. The temperature program was as follows: from 70 °C to 160 °C at 2 °C/min and hold for 2 min, then increased to 220 °C at 10 °C/min and kept for 5 min. The carrier gas was nitrogen at 1.19 mL/min. The GC inlet was set in a splitting mode with split ratio of 1:20 and at 230 °C, and 1.0 μL of diluted samples (1/10, v/v, in hexane) was injected. The quadrupole MS operating parameters: interface temperature 200 °C; electron impact ionization at 70 eV with scan mass range of 45−450 m/z. The volatile compounds were identified according to the Mass Spectral Library database and retention indices of authentic reference standards.
4.3. Bacterial Strains and Culture
Seven representative foodborne pathogens used for antibacterial activity assay were purchased from the China Center of Industrial Culture Collection (CICC), including four Gram-negative bacteria of Pseudomonas aeruginosa (CICC 21636), Escherichia coli (CICC 10389), Salmonella enterica subsp. enterica (CICC 10982), and Enterobacter aerogenes (CICC 10293) and three Gram-positive bacteria of Staphylococcus aureus (CICC 10384), Bacillus subtilis (CICC 10275), and Listeria monocytogenes (CICC 21633).
To assess the antibacterial activity of CBLEO on foodborne pathogens, the tested bacterial suspensions were prepared in nutrient broth (NB) (Difco 234000, Becton, Dickinson and Company, Franklin Lake, NJ, USA), except for L. monocytogenes which was prepared in brain heart infusion (BHI) broth (Difco 237500, USA), and incubated at 37 °C for 24 h. Each strain inoculum was suspended in 0.85% of sterile saline to obtain a standard microbial density (about 107 CFU/mL).
4.4. Assessment of Antibacterial Activity of CBLEO
4.4.1. Detection of Diameter of Inhibition Zone (DIZ)
The detection of DIZ by agar disc diffusion was used to screen antimicrobial activity of CBLEO [7]. The prepared sterile media of BHI agar for L. monocytogenes and NB agar for other bacteria were cooled to 50 °C and solidified in the sterilized plate, and each strain inoculum (100 µL, 107 CFU/mL) was streaked over on the surface of culture medium using commercial bacteriological loops, and then the sterile 6 mm paper disc impregnated with CBLEO (10 μL, 50 mg/mL) was placed on medium surface. After the incubation at 37 °C for 24 h, antimicrobial activity was determined by a clear zone around the disc, and the DIZ was detected. Ampicillin (10 μg/disc, 10 mg/mL) and sterile distilled water (10 μL) were used as the positive and negative controls, respectively.
4.4.2. Determination of Minimum Inhibitory (MIC) and Bactericidal Concentration (MBC)
The values of MIC and MBC of CBLEO were detected by microdilution method [7]. A series of two-fold dilutions of CBLEO (0.125−32 μg/mL) was added into the bacteria suspension (1 × 107 CFU/mL) in the wells of a sterile microplate and cultured overnight at 37 °C. The lowest concentration of CBLEO that showed no visible bacteria growth was defined as the MIC, and the MBC was expressed as the lowest concentration of CBLEO required to kill bacteria. Ampicillin was applied as reference antibacterial agent.
4.5. Analysis of Bacterial Growth Kinetics of S. aureus
Antibacterial kinetics assay was used to assess antibacterial mechanism of CBLEO on S. aureus (the most susceptible strain) [5]. In order to increase CBLEO solubility, the stock CBLEO was prepared in 4% dimethyl sulphoxide (DMSO) [50] to obtain five different doses (1/8×MIC, 1/4×MIC, 1/2×MIC, 1×MIC, and 2×MIC) and then was added into 100 mL of bacterial suspension (107 CFU/mL). After incubating for 3, 6, 9, 12, 15, 18, 21, and 24 h, the collected suspension was used to detect optical density (OD) at 600 nm by ultraviolet spectrophotometer (Agilent Cary 3500, Agilent, Santa Clara, CA, USA). The growth kinetic curve of S. aureus was elaborated by drawing the lg number of CFU/mL versus incubated time [7].
4.6. Analysis of Antibacterial Mechanism of CBLEO on S. aureus
4.6.1. Cell Membrane Permeability
The impact of CBLEO on cell membrane permeability of S. aureus was evaluated by detecting electric conductivity using electrical conductivity meter (DDS-11D, Shanghai, China) [7]. The obtained S. aureus cells by centrifugation (10,000 rpm) at 4 °C for 10 min were washed with 5% glucose until electric conductivity was close to that of 5% glucose and defined as isotonic bacteria for electric conductivity detection, and then different doses of CBLEO were respectively added. After incubation at 37 °C for 0, 1, 2, 4, 6, 8, 10, 12, and 24 h, the electric conductivity of the mixtures was detected and marked as EC2. The bacteria in 5% glucose treated in boiling water for 5 min and 5% glucose containing different doses of CBLEO were used as positive and negative controls, respectively, and their electric conductivities were measured and marked as EC0 and EC1, respectively. Cell membrane permeability was expressed as the relative electric conductivity and calculated by the following equation: Relative electric conductivity (%) = [(EC2 − EC1/EC0) × 100].
4.6.2. Integrity of Cell Membrane
The membrane integrity of S. aureus cells was evaluated by detecting the leakage of intracellular nucleic acid and protein [7]. S. aureus cells (1 × 107 CFU/mL) were incubated at 37 °C with CBLEO at different levels (1/2×MIC, 1×MIC, and 2×MIC) and times (0–8 h), and the samples were collected at different times. After centrifugation (10,000 rpm) at 4 °C for 10 min, the supernatants were used to detect the amounts of nucleic acid and protein by using ultraviolet spectrophotometer (Agilent Cary 3500, Agilent, Santa Clara, CA, USA). The detected result of nucleic acid was expressed as the absorbance value at 260 nm (OD260), and the amount of protein was standardized to the used amount of S. aureus cells (μg/mg).
4.6.3. Cell Wall Damage
The impact of CBBLEO on cell wall damage of S. aureus was analyzed by detecting release of alkaline phosphatase (AKP) [92]. Different doses (1/2×MIC, 1×MIC, and 2×MIC) of CBLEO were added into S. aureus cells (107 CFU/mL) and incubated at 37 °C, and then the supernatants collected at different times (0–8 h) were used for the detection of AKP activity by commercial kit (RS0904F, Redshineen Biotech, Guangdong, China). The AKP activity was standardized to the used amounts of S. aureus cells, and the result was expressed as U/L.
4.6.4. Scanning Electron Microscope (SEM) Analysis
The impact of CBLEO on cell morphology of S. aureus was tested by SEM assay [16]. The S. aureus suspension (107 CFU/mL) was added into CBLEO (1×MIC), and the group with equal amount of absolute ethanol was applied as the control. After 2 h of incubation, the collected cells were washed with phosphate-buffered saline buffer and then fixed in glutaraldehyde (2.5%) at 4 °C for 12 h, followed by dehydration under the different levels of ethanol gradient (30, 50, 80, 90, and 100%). The specimens were dried at critical point of CO2 and coated with gold-palladium by Polaron E5100 II (Polaron Instruments Inc., Hatfield, CA). Finally, the samples were observed with a scanning electron microscopy (SEM, JSM-7001F, JEOL, Tokyo, Japan) at voltage of 10 kV.
4.6.5. Analyses of Cellular Protein Oxidation and ROS and MDA Production
Oxidative stress and lipid peroxidation of S. aureus induced by CBLEO were assessed by detecting the amounts of ROS generation and protein oxidation (two key markers of oxidative stress) and MDA accumulation (one biomarker of lipid peroxidation) [7,71]. The S. aureus cells (107 CFU/mL) were incubated with different doses (1/2×MIC, 1×MIC, and 2×MIC) of CBLEO at 37 °C, and the samples were collected respectively at different times (0−8 h). After centrifuging at 10,000 rpm for 10 min under 4 °C, the obtained cell pellets were used to determine protein carbonyl, ROS, and MDA by using assay kits of ab126287, ab113851, and ab118970 (Abcam, Shanghai, China), respectively. The amount of ROS was given in fold of untreated controls, and the content of protein carbonyl (protein oxidation product) was standardized to the used amount of S. aureus cells and defined as nmol/mg. The MDA content was standardized to the used amounts of S. aureus cells, and the result was expressed as nmol/mg.
4.7. Analysis of Bacterial Total Protein
The S. aureus cells (107 CFU/mL) were incubated with CBLEO at 37 °C under different doses of 1/2×MIC, 1×MIC, and 2×MIC and times (0−8 h), and the samples were respectively collected to centrifugate at 10,000 rpm for 10 min under 4 °C. The obtained cell pellets were used for detection of total protein amount by using BCA Protein Assay Kit (102536, Abcam, Shanghai, China). The result was standardized to the used amounts of S. aureus cells and expressed as μg/mg.
4.8. Total Protease Assay
Total protease activity was detected in the above-obtained cell pellets of S. aureus by analysis kit (ab111750) according to the manufacturer’s instructions, and the fluorescein isothiocyanate (FITC)-labeled casein was used as a general substrate. The protease activity was standardized to the used amounts of cell total protein and expressed as U/mg protein using BSA as standard. One unit (U) was defined as the amount of protease that cleaves substrate to yield an amount of fluorescence equivalent to 1.0 μmol of unquenched FITC per min at 25 °C.
4.9. Analysis of Virulence-Associated Gene Expression
Total RNA of S. aureus was extracted by RNAprep Cell/Bacterial kit (Tiangen, Beijing, China) and was reverse-transcribed by PrimeScriptTM RT reagent kit (Takara, Osaka, Japan). qRT-PCR was performed on BIO-RAD CF× ConnectTM Real-Time System using SYBR Green qPCR Mix (Biomarker, Beijing, China). All amplified primers used for the detection of virulence genes are listed in Table S1, and 16S rRNA was used as internal reference. The relative expression value of target genes in comparison with reference gene was counted by 2−ΔΔCt method [8], and the expression level in S. aureus from the treatments of different doses of CBLEO at 0 h was arbitrarily set to 1.00 for standardization.
4.10. Assay of Anti-biofilm Activity
The impact of CBLEO on biofilm formation of S. aureus was assessed by a microtiter plate assay [18]. An overnight culture of S. aureus was added into a 96-well dish containing 200 µL of LB broth supplemented with various levels of CBLEO. After incubation at 37 °C for 1−8 h, the bacterial cells were washed with PBS to remove all unattached cells and media components, and then 250 µL crystal violet staining solution (0.1%) was added and incubated for 20 min at 25 °C. After this, the plates were washed with PBS buffer 2−3 times and solubilized with ethanol. The absorbance at 570 nm (OD570) was detected in CBLEO-treated (different concentrations) or control sample at 0−8 h, of which the value of OD570 at 0 h was arbitrarily set to 1.00 for standardization, and the result was defined as fold change.
4.11. Assay of Binding Activity of CBLEO to Bacteria Genome DNA
The binding activity of CBLEO to genomic DNA of S. aureus was tested by agarose gel electrophoresis method [93]. Genomic DNA of S. aureus was isolated by TIANamp Bacteria DNA Kit (Tiangen, Beijing, China), and the value of OD260 was measured to calculate DNA concentration. An aliquot (100 ng) of genome DNA was incubated with CBLEO at different doses (1/2×MIC, 1×MIC, 2×MIC, 4×MIC, 8×MIC, and 16×MIC) at 37 °C for 0.5, 1, 3, and 5 h under darkness, and each incubated mixture (5.0 μL) was loaded to run agarose gel electrophoresis. The bacteria treated by PBS was used as the control.
4.12. Statistical Analysis
All the results were recorded as mean ± SD (standard deviation) three independent replicates and analyzed by ANOVA employing Student’s test at p < 0.05. All statistical assays were performed by IBM SPSS Statistics 25 software.
5. Conclusions
In this work, C. burmannii leaf essential oil (CBLEO) was identified to have diverse volatile compounds and a high amount of borneol, as well as good antibacterial activity, and S. aureus was the most susceptible pathogen. CBLEO could act as a strong inducer for ROS accumulation and the oxidative stress of S. aureus, causing cell structure damage and giving rise to the effective repression of virulence-related gene transcription with significant inhibition of biofilm formation destined for the growth inhibition of S. aureus. Notably, the comparative association among ROS accumulation, virulence-associated gene transcription, protease production, biofilm formation, and bacterial growth in S. aureus across different doses and times of CBLEO treatment led to the identification of RsbU and SigB as important transcriptional regulators crucial for bacterial biofilm formation and growth inhibition. The RsbU/SigB-mediated repression of biofilm formation caused by CBLEO-oxidative stress may be a key antibacterial target against S. aureus. Our findings should provide valuable information for those studying the acting mechanism of essential oil against pathogens. Further research should focus on the exploration of the functional attributes (especially for borneol) of essential oil from C. burmannii leaf in food practical utilization as a natural antibacterial agent.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25053078/s1.
Author Contributions
Investigation and writing—original draft preparation, L.S.; writing—reviewing and editing, W.L.; methodology, Y.C.; formal analysis, F.C.; investigation and validation, Q.Z.; data curation, D.L.; resources, Y.X.; project administration, B.H.; funding acquisition and conceptualization, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Specific Programs in Forestry Science and Technology Innovation of Guangdong (Grant No. 2020KJCX001) and the National Natural Science Foundation of China (Grant No. 31972952).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented in this study are included in the published article and Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gutiérrez-del-Río, I.; Fernández, J.; Lombó, F. Plant nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int. J. Antimicrob. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef]
- Erickson, M.C.; Doyle, M.P. The challenges of eliminating or substituting antimicrobial preservatives in foods. Annu Rev. Food Sci. Technol. 2017, 8, 371–390. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Akarca, G. Composition and antibacterial effect on food borne pathogens of Hibiscus surrattensis L. calyces essential oil. Ind. Crops Prod. 2019, 137, 285–289. [Google Scholar] [CrossRef]
- Bouyahya, A.; Assemian, I.C.C.; Mouzount, H.; Bourais, I.; Et-Touys, A.; Fellah, H.; Benjouad, A.; Dakka, N.; Bakri, Y. Could volatile compounds from leaves and fruits of Pistacia lentiscus constitute a novel source of anticancer, antioxidant, antiparasitic and antibacterial drugs? Ind. Crops Prod. 2019, 128, 62–69. [Google Scholar] [CrossRef]
- De Almeida, W.S.; de Lima, S.G.; Barreto, H.M.; Andrade, L.M.d.S.; Fonseca, L.; Athayde Sobrinho, C.; Santos, A.R.B.; Muratori, M.C.S. Chemical composition and antimicrobial activity of the essential oil of Lippia lasiocalycina Cham. (Verbenaceae). Ind. Crops Prod. 2018, 125, 236–240. [Google Scholar] [CrossRef]
- Chen, F.; Miao, X.; Lin, Z.; Xiu, Y.; Shi, L.; Zhang, Q.; Liang, D.; Lin, S.; He, B. Disruption of metabolic function and redox homeostasis as antibacterial mechanism of Lindera glauca fruit essential oil against Shigella flexneri. Food Control 2021, 130, 108282. [Google Scholar] [CrossRef]
- Dai, J.; Li, C.; Cui, H.; Lin, L. Unraveling the anti-bacterial mechanism of Litsea cubeba essential oil against E. coli O157:H7 and its application in vegetable juices. Int. J. Food Microbiol. 2021, 338, 108989. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef]
- Bondi, M.; Lauková, A.; de Niederhausern, S.; Messi, P.; Papadopoulou, C. Natural preservatives to improve food quality and safety. J. Food Qual. 2017, 2017, 1090932. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Ardjoum, N.; Chibani, N.; Shankar, S.; Salmieri, S.; Djidjelli, H.; Lacroix, M. Incorporation of Thymus vulgaris essential oil and ethanolic extract of propolis improved the antibacterial, barrier and mechanical properties of corn starch-based films. Int. J. Biol. Macromol. 2023, 224, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Sharma, A.; Baek, K.-H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Hu, W.; Li, C.; Dai, J.; Cui, H.; Lin, L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind. Crops Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- De Souza Moura, W.; de Souza, S.R.; Campos, F.S.; Sander Rodrigues Cangussu, A.; Macedo Sobrinho Santos, E.; Silva Andrade, B.; Borges Gomes, C.H.; Fernandes Viana, K.; Haddi, K.; Oliveira, E.E.; et al. Antibacterial activity of Siparuna guianensis essential oil mediated by impairment of membrane permeability and replication of pathogenic bacteria. Ind. Crops Prod. 2020, 146, 112142. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crops Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- OuYang, Q.; Tao, N.; Zhang, M. A damaged oxidative phosphorylation mechanism is involved in the antifungal activity of citral against Penicillium digitatum. Front. Microbiol. 2018, 9, 239. [Google Scholar] [CrossRef]
- Mansuri, A.; Lokhande, K.; Kore, S.; Gaikwad, S.; Nawani, N.; Swamy, K.V.; Junnarkar, M.; Pawar, S. Antioxidant, anti-quorum sensing, biofilm inhibitory activities and chemical composition of Patchouli essential oil: In vitro and in silico approach. J. Biomol. Struct. Dyn. 2020, 40, 154–165. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Gao, H. A dual antibacterial mechanism involved in membrane disruption and DNA binding of 2R,3R-dihydromyricetin from pine needles of Cedrus deodara against Staphylococcus aureus. Food Chem. 2017, 218, 463–470. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Gu, H.; Hao, L.; Ye, H.; Gong, W.; Wang, Z. A review of the methods for detection of Staphylococcus aureus enterotoxins. Toxins 2016, 8, 176. [Google Scholar] [CrossRef]
- Fisher, E.L.; Otto, M.; Cheung, G.Y.C. Basis of virulence in enterotoxin-mediated Staphylococcal food poisoning. Front. Microbiol. 2018, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm formation and control strategies of foodborne pathogens: Food safety perspectives. RSC Adv. 2017, 7, 36670–36683. [Google Scholar] [CrossRef]
- Sun, F.; Liang, H.; Kong, X.; Xie, S.; Cho, H.; Deng, X.; Ji, Q.; Zhang, H.; Alvarez, S.; Hicks, L.M.; et al. Quorum-sensing agr mediates bacterial oxidation response via an intramolecular disulfide redox switch in the response regulator AgrA. Proc. Natl. Acad. Sci. USA 2012, 109, 9095–9100. [Google Scholar] [CrossRef]
- Cheung, A.L.; Nishina, K.A.; Trotonda, M.P.; Tamber, S. The SarA protein family of Staphylococcus aureus. Int. J. Biochem. Cell Biol. 2008, 40, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Jenul, C.; Horswill, A.R. Regulation of Staphylococcus aureus virulence. Microbiol. Spectr. 2019, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, D.; Huang, X.; Yang, H.; Qiu, Z.; Zou, L.; Liang, Q.; Shi, Y.; Wu, Y.; Wu, S.; et al. Study on antibacterial and quorum-sensing inhibition activities of Cinnamomum camphora leaf essential oil. Molecules 2019, 24, 3792. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, R.K.; Lee, J.-H.; Kim, Y.-G.; Kim, S.-I.; Lee, J. Inhibitory effects of the essential oils α-longipinene and linalool on biofilm formation and hyphal growth of Candida albicans. Biofouling 2017, 33, 143–155. [Google Scholar] [CrossRef]
- Joshi, J.R.; Khazanov, N.; Senderowitz, H.; Burdman, S.; Lipsky, A.; Yedidia, I. Plant phenolic volatiles inhibit quorum sensing in pectobacteria and reduce their virulence by potential binding to ExpI and ExpR proteins. Sci. Rep. 2016, 6, 38126. [Google Scholar] [CrossRef]
- Alves, S.; Duarte, A.; Sousa, S.; Domingues, F.C. Study of the major essential oil compounds of Coriandrum sativum against Acinetobacter baumannii and the effect of linalool on adhesion, biofilms and quorum sensing. Biofouling 2016, 32, 155–165. [Google Scholar] [CrossRef]
- Souza, C.M.C.; Pereira Junior, S.A.; Moraes, T.d.S.; Damasceno, J.L.; Amorim Mendes, S.; Dias, H.J.; Stefani, R.; Tavares, D.C.; Martins, C.H.G.; Crotti, A.E.M.; et al. Antifungal activity of plant-derived essential oils on Candida tropicalis planktonic and biofilms cells. Med. Mycol. 2016, 54, 515–523. [Google Scholar] [CrossRef]
- Lee, K.; Lee, J.-H.; Kim, S.-I.; Cho, M.H.; Lee, J. Anti-biofilm, anti-hemolysis, and anti-virulence activities of black pepper, cananga, myrrh oils, and nerolidol against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9447–9457. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Madella, D.; Martinazzo, A.P.; de Aquino Garcia Moura, L.; de Melo, N.R.; Sanches-Silva, A. Revisiting an ancient spice with medicinal purposes: Cinnamon. Trends Food Sci. Technol. 2017, 62, 154–169. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhubiab, B.E. Pharmaceutical applications and phytochemical profile of Cinnamomum burmannii. Pharmacogn. Rev. 2012, 6, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, Y.; Brooks, J.; Corke, H. Antibacterial properties and major bioactive components of cinnamon stick (Cinnamomum burmannii): Activity against foodborne pathogenic bacteria. J. Agric. Food Chem. 2007, 55, 5484–5490. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, L.; Zou, Y.; Luo, S.; Wang, X.; Liang, Y.; Du, Y.; Feng, R.; Wei, Q. Antibacterial activity and mechanism of three isomeric terpineols of Cinnamomum longepaniculatum leaf oil. Folia Microbiol. 2021, 66, 59–67. [Google Scholar] [CrossRef]
- Muhammad, D.R.A.; Lemarcq, V.; Alderweireldt, E.; Vanoverberghe, P.; Praseptiangga, D.; Juvinal, J.G.; Dewettinck, K. Antioxidant activity and quality attributes of white chocolate incorporated with Cinnamomum burmannii Blume essential oil. J Food Sci. Technol. 2020, 57, 1731–1739. [Google Scholar] [CrossRef]
- Muhammad, D.R.A.; Tuenter, E.; Patria, G.D.; Foubert, K.; Pieters, L.; Dewettinck, K. Phytochemical composition and antioxidant activity of Cinnamomum burmannii Blume extracts and their potential application in white chocolate. Food Chem. 2021, 340, 127983. [Google Scholar] [CrossRef]
- Muhammad, D.R.A.; Saputro, A.D.; Rottiers, H.; Van de Walle, D.; Dewettinck, K. Physicochemical properties and antioxidant activities of chocolates enriched with engineered cinnamon nanoparticles. Eur. Food Res. Technol. 2018, 244, 1185–1202. [Google Scholar] [CrossRef]
- Wu, G.; Lian, H.; Zhang, C.; Li, B.; Chen, J.; He, B.; Zhang, Q.; Wang, Y. Content variation and evaluation of essential oil and its main chemical components of Cinnamomum burmannii in Guangdong province. For. Environ. Sci. 2020, 36, 88–95. [Google Scholar] [CrossRef]
- Xie, P.; He, B.; Wang, Y.; Zhang, Q. Standardization of exploration and utilization of d-borneol type Cinnamomum camphora. For. Environ. Sci. 2019, 35, 94–100. [Google Scholar] [CrossRef]
- Zielinska, A.K.; Beenken, K.E.; Mrak, L.N.; Spencer, H.J.; Post, G.R.; Skinner, R.A.; Tackett, A.J.; Horswill, A.R.; Smeltzer, M.S. sarA-mediated repression of protease production plays a key role in the pathogenesis of Staphylococcus aureus USA300 isolates. Mol. Microbiol. 2012, 86, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ren, X.; Liu, Y.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Extraction of Cinnamomum camphora chvar. Borneol essential oil using neutral cellulase assisted-steam distillation: Optimization of extraction, and analysis of chemical constituents. Ind. Crops Prod. 2019, 141, 111794. [Google Scholar] [CrossRef]
- Bansod, S.; Chilvery, S.; Saifi, M.A.; Das, T.J.; Tag, H.; Godugu, C. Borneol protects against cerulein-induced oxidative stress and inflammation in acute pancreatitis mice model. Environ. Toxicol. 2021, 36, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wen, J.; Wang, Z.; Wang, J. Multiple regulation and targeting effects of borneol in the neurovascular unit in neurodegenerative diseases. Basic Clin. Pharmacol. Toxicol. 2022, 130, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Long, Y.; Yu, S.; Zhang, D.; Yang, Q.; Ci, Z.; Cui, M.; Zhang, Y.; Wan, J.; Li, D.; et al. Borneol in cardio-cerebrovascular diseases: Pharmacological actions, mechanisms, and therapeutics. Pharmacol. Res. 2021, 169, 105627. [Google Scholar] [CrossRef]
- Chebet, J.J.; Ehiri, J.E.; McClelland, D.J.; Taren, D.; Hakim, I.A. Effect of d-limonene and its derivatives on breast cancer in human trials: A scoping review and narrative synthesis. BMC Cancer 2021, 21, 902. [Google Scholar] [CrossRef] [PubMed]
- Mosbah, H.; Louati, H.; Boujbiha, M.A.; Chahdoura, H.; Snoussi, M.; Flamini, G.; Ascrizzi, R.; Bouslema, A.; Achour, L.; Selmi, B. Phytochemical characterization, antioxidant, antimicrobial and pharmacological activities of Feijoa sellowiana leaves growing in Tunisia. Ind. Crops Prod. 2018, 112, 521–531. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α-and β-Pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Mezrioui, N.; Setzer, W.; Abbad, A.; Hassani, L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Ind. Crops Prod. 2019, 137, 396–400. [Google Scholar] [CrossRef]
- Huang, H.; Lian, H.; He, B.; Wang, Y.; Chen, G.; Liang, D.; Chen, X.; Luo, W.; Lin, S.; Fengqing, L. Study on the dynamic change of essential oil content and chemical constituents in the leaves of Cinnamomum burmannii chvar. borneol. For. Environ. Sci. 2019, 35, 22–26. [Google Scholar] [CrossRef]
- Wang, R.; Wang, R.; Yang, B. Extraction of essential oils from five cinnamon leaves and identification of their volatile compound compositions. Innov. Food Sci. Emerg. 2009, 10, 289–292. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, M.; Su, G.; Cui, C.; Sun, W. Gelation of salted myofibrillar protein under malondialdehyde-induced oxidative stress. Food Hydrocoll. 2014, 40, 153–162. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Hsu, P.-F.; Chen, Y.-C.; Lin, H.-C.; Hung, C.-C.; Chen, P.-C.; Huang, Y.-L. Oxidative stress during bacterial growth characterized through microdialysis sampling coupled with HPLC/fluorescence detection of malondialdehyde. J. Chromatogr. B 2016, 1019, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Moon, S.-H.; Lee, J.-Y.; Kim, K.-T.; Park, Y.-S.; Paik, H.-D. Antibacterial activity of a novel flavonoid, 7-O-butyl naringenin, against methicillin-resistant Staphylococcus aureus (MRSA). Food Sci. Biotechnol. 2013, 22, 1725–1728. [Google Scholar] [CrossRef]
- Chen, F.; Wang, H.; Lin, Z.; Hu, J.; Wu, Y.; Shi, L.; Wang, J.; Xiu, Y.; Lin, S. Enzymatic and non-enzymatic bioactive compounds, and antioxidant and antimicrobial activities of the extract from one selected wild berry (Rubus coreanus) as novel natural agent for food preservation. LWT-Food Sci. Technol. 2022, 171, 114133. [Google Scholar] [CrossRef]
- Fathima, A.; Rao, J.R. Selective toxicity of Catechin-a natural flavonoid towards bacteria. Appl. Microbiol. Biotechnol. 2016, 100, 6395–6402. [Google Scholar] [CrossRef]
- Yan, L.; Li, M.; Cao, Y.; Gao, P.; Cao, Y.; Wang, Y.; Jiang, Y. The alternative oxidase of Candida albicans causes reduced fluconazole susceptibility. J. Antimicrob. Chemother. 2009, 64, 764–773. [Google Scholar] [CrossRef]
- Miura, T.; Muraoka, S.; Fujimoto, Y. Lipid peroxidation induced by indomethacin with horseradish peroxidase and hydrogen peroxide: Involvement of indomethacin radicals. Biochem. Pharmacol. 2002, 63, 2069–2074. [Google Scholar] [CrossRef]
- Du, W.; Sun, C.; Liang, Z.; Han, Y.; Yu, J. Antibacterial activity of hypocrellin A against Staphylococcus aureus. World J. Microbiol. Biotechnol. 2012, 28, 3151–3157. [Google Scholar] [CrossRef]
- Ayer, A.; Gourlay, C.W.; Dawes, I.W. Cellular redox homeostasis, reactive oxygen species and replicative ageing in Saccharomyces cerevisiae. FEMS Yeast Res. 2014, 14, 60–72. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Karpenko, I.L.; Valuev-Elliston, V.T.; Ivanova, O.N.; Smirnova, O.A.; Ivanov, A.V. Peroxiredoxins—The underrated actors during virus-induced oxidative stress. Antioxidants 2021, 10, 977. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.-C.; Mieyal, J.J. Glutathione and glutaredoxin-Key players in cellular Redox homeostasis and signaling. Antioxidants 2023, 12, 1553. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Lee, B.C.; Marino, S.M.; Zhang, Y.; Fomenko, D.E.; Kaya, A.; Hacioglu, E.; Kwak, G.-H.; Koc, A.; Kim, H.-Y.; et al. Functional analysis of free methionine-R-sulfoxide reductase from Saccharomyces cerevisiae. J. Biol. Chem. 2009, 284, 4354–4364. [Google Scholar] [CrossRef]
- Grant, C.M. Role of the glutathione/glutaredoxin and thioredoxin systems in yeast growth and response to stress conditions. Mol. Microbiol. 2001, 39, 533–541. [Google Scholar] [CrossRef]
- Inoue, Y.; Matsuda, T.; Sugiyama, K.-I.; Izawa, S.; Kimura, A. Genetic analysis of glutathione peroxidase in oxidative stress response of Saccharomyces cerevisiae. J. Biol. Chem. 1999, 274, 27002–27009. [Google Scholar] [CrossRef]
- Rodríguez-Manzaneque, M.T.; Ros, J.; Cabiscol, E.; Sorribas, A.; Herrero, E. Grx5 glutaredoxin plays a central role in protection against protein oxidative damage in Saccharomyces cerevisiae. Mol. Cell. Biol. 1999, 19, 8180–8190. [Google Scholar] [CrossRef]
- King, K.; Horenstein, J.; Caparon, M. Aerotolerance and peroxide resistance in peroxidase and PerR mutants of Streptococcus pyogenes. J. Bacteriol. 2000, 182, 5290–5299. [Google Scholar] [CrossRef] [PubMed]
- Uziel, O.; Borovok, I.; Schreiber, R.; Cohen, G.; Aharonowitz, Y. Transcriptional regulation of the Staphylococcus aureus thioredoxin and thioredoxin reductase genes in response to oxygen and disulfide stress. J. Bacteriol. 2004, 186, 326–334. [Google Scholar] [CrossRef]
- Qian, J.; Wang, C.; Zhuang, H.; Zhang, J.; Yan, W. Oxidative stress responses of pathogen bacteria in poultry to plasma-activated lactic acid solutions. Food Control 2020, 118, 107355. [Google Scholar] [CrossRef]
- Thoendel, M.; Kavanaugh, J.S.; Flack, C.E.; Horswill, A.R. Peptide signaling in the staphylococci. Chem. Rev. 2011, 111, 117–151. [Google Scholar] [CrossRef] [PubMed]
- Le, K.Y.; Otto, M. Quorum-sensing regulation in staphylococci—An overview. Front. Microbiol. 2015, 6, 1174. [Google Scholar] [CrossRef] [PubMed]
- Queck, S.Y.; Jameson-Lee, M.; Villaruz, A.E.; Bach, T.-H.L.; Khan, B.A.; Sturdevant, D.E.; Ricklefs, S.M.; Li, M.; Otto, M. RNAIII-independent target gene control by the agr quorum-sensing system: Insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell. 2008, 32, 150–158. [Google Scholar] [CrossRef]
- Bischoff, M.; Dunman, P.; Kormanec, J.; Macapagal, D.; Murphy, E.; Mounts, W.; Berger-Bächi, B.; Projan, S. Microarray-based analysis of the Staphylococcus aureus σB regulon. J. Bacteriol. 2004, 186, 4085–4099. [Google Scholar] [CrossRef] [PubMed]
- Guldimann, C.; Boor, K.J.; Wiedmann, M.; Guariglia-Oropeza, V. Resilience in the face of uncertainty: Sigma factor B fine-tunes gene expression to support homeostasis in gram-positive bacteria. Appl. Environ. Microbiol. 2016, 82, 4456–4469. [Google Scholar] [CrossRef]
- Atwood, D.N.; Loughran, A.J.; Courtney, A.P.; Anthony, A.C.; Meeker, D.G.; Spencer, H.J.; Gupta, R.K.; Lee, C.Y.; Beenken, K.E.; Smeltzer, M.S. Comparative impact of diverse regulatory loci on Staphylococcus aureus biofilm formation. MicrobiologyOpen 2015, 4, 436–451. [Google Scholar] [CrossRef]
- Harapanahalli, A.K.; Chen, Y.; Li, J.; Busscher, H.J.; van der Mei, H.C. Influence of adhesion force on icaA and cidA gene expression and production of matrix components in Staphylococcus aureus biofilms. Appl. Environ. Microbiol. 2015, 81, 3369–3378. [Google Scholar] [CrossRef]
- Savage, V.J.; Chopra, I.; O’Neill, A.J. Population diversification in Staphylococcus aureus biofilms may promote dissemination and persistence. PLoS ONE 2013, 8, e62513. [Google Scholar] [CrossRef]
- Lauderdale, K.J.; Boles, B.R.; Cheung, A.L.; Horswill, A.R. Interconnections between sigma B, agr, and proteolytic activity in Staphylococcus aureus biofilm maturation. Infect. Immun. 2009, 77, 1623–1635. [Google Scholar] [CrossRef]
- Rice, K.C.; Mann, E.E.; Endres, J.L.; Weiss, E.C.; Cassat, J.E.; Smeltzer, M.S.; Bayles, K.W. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2007, 104, 8113–8118. [Google Scholar] [CrossRef]
- Tsang, L.H.; Cassat, J.E.; Shaw, L.N.; Beenken, K.E.; Smeltzer, M.S. Factors contributing to the biofilm-deficient phenotype of Staphylococcus aureus sarA mutants. PLoS ONE 2008, 3, e3361. [Google Scholar] [CrossRef]
- Beenken, K.E.; Blevins, J.S.; Smeltzer, M.S. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect. Immun. 2003, 71, 4206–4211. [Google Scholar] [CrossRef]
- Loughran, A.J.; Atwood, D.N.; Anthony, A.C.; Harik, N.S.; Spencer, H.J.; Beenken, K.E.; Smeltzer, M.S. Impact of individual extracellular proteases on Staphylococcus aureus biofilm formation in diverse clinical isolates and their isogenic sarA mutants. MicrobiologyOpen 2014, 3, 897–909. [Google Scholar] [CrossRef]
- Mootz, J.M.; Malone, C.L.; Shaw, L.N.; Horswill, A.R. Staphopains modulate Staphylococcus aureus biofilm integrity. Infect. Immun. 2013, 81, 3227–3238. [Google Scholar] [CrossRef]
- Palma, M.; Cheung, A.L. σB activity in Staphylococcus aureus is controlled by RsbU and an additional factor (s) during bacterial growth. Infect. Immun. 2001, 69, 7858–7865. [Google Scholar] [CrossRef]
- Knobloch Johannes, K.M.; Jäger, S.; Horstkotte Matthias, A.; Rohde, H.; Mack, D. RsbU-dependent regulation of Staphylococcus epidermidis biofilm formation is mediated via the alternative sigma factor σB by repression of the negative regulator gene icaR. Infect. Immun. 2004, 72, 3838–3848. [Google Scholar] [CrossRef] [PubMed]
- Conlon, K.M.; Humphreys, H.; O’Gara, J.P. Inactivations of rsbU and sarA by IS256 represent novel mechanisms of biofilm phenotypic variation in Staphylococcus epidermidis. J. Bacteriol. 2004, 186, 6208–6219. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Chee, C.-F.; Richter, K.; Thomas, N.; Abd Rahman, N.; Nathan, S. Suppression of Staphylococcus aureus biofilm formation and virulence by a benzimidazole derivative, UM-C162. Sci. Rep. 2018, 8, 2758. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.; Kayama, S.; Sasaki, M.; Kato, F.; Hisatsune, J.; Tsuruda, K.; Koizumi, K.; Tatsukawa, N.; Yu, L.; Takeda, K. Inhibitory effects of antibiofilm compound 1 against Staphylococcus aureus biofilms. Microbiol. Immunol. 2016, 60, 148–159. [Google Scholar] [CrossRef]
- Zhu, B.; Hou, X.; Niu, J.; Li, P.; Fang, C.; Qiu, L.; Ha, D.; Zhang, Z.; Sun, J.; Li, Y. Volatile constituents from the fruits of Lindera glauca (Sieb. et Zucc.) with different maturities. J. Essent. Oil-Bear. Plants 2016, 19, 926–935. [Google Scholar] [CrossRef]
- Zhao, M.; Bai, J.; Bu, X.; Tang, Y.; Han, W.; Li, D.; Wang, L.; Yang, Y.; Xu, Y. Microwave-assisted aqueous two-phase extraction of phenolic compounds from Ribes nigrum L. and its antibacterial effect on foodborne pathogens. Food Control 2021, 119, 107449. [Google Scholar] [CrossRef]
- Pan, Y.; Zheng, L.-b.; Mao, Y.; Wang, J.; Lin, L.-s.; Su, Y.-q.; Li, Y. The antibacterial activity and mechanism analysis of piscidin 5 like from Larimichthys crocea. Dev. Comp. Immunol. 2019, 92, 43–49. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).