Abstract
Rice effective panicle is a major trait for grain yield and is affected by both the genetic tiller numbers and the early tillering vigor (ETV) traits to survive environmental adversities. The mechanism behind tiller bud formation has been well described, while the genes and the molecular mechanism underlying rice-regulating ETV traits are unclear. In this study, the candidate genes in regulating ETV traits have been sought by quantitative trait locus (QTL) mapping and bulk-segregation analysis by resequencing method (BSA-seq) conjoint analysis using rice backcross inbred line (BIL) populations, which were cultivated as late-season rice of double-cropping rice systems. By QTL mapping, seven QTLs were detected on chromosomes 1, 3, 4, and 9, with the logarithm of the odds (LOD) values ranging from 3.52 to 7.57 and explained 3.23% to 12.98% of the observed phenotypic variance. By BSA-seq analysis, seven QTLs on chromosomes 1, 2, 4, 5, 7, and 9 were identified using single-nucleotide polymorphism (SNP) and insertions/deletions (InDel) index algorithm and Euclidean distance (ED) algorithm. The overlapping QTL resulting from QTL mapping and BSA-seq analysis was shown in a 1.39 Mb interval on chromosome 4. In the overlap interval, six genes, including the functional unknown genes Os04g0455650, Os04g0470901, Os04g0500600, and ethylene-insensitive 3 (Os04g0456900), sialyltransferase family domain containing protein (Os04g0506800), and ATOZI1 (Os04g0497300), showed the differential expression between ETV rice lines and late tillering vigor (LTV) rice lines and have a missense base mutation in the genomic DNA sequences of the parents. We speculate that the six genes are the candidate genes regulating the ETV trait in rice, which provides a research basis for revealing the molecular mechanism behind the ETV traits in rice.
1. Introduction
Rice is one of the staple cereal crops that has contributed, is contributing, and will continue to contribute to global food security [1]. Rice yield is mostly affected by the factors of panicle number, grain number, and grain weight. The variation in panicle number per plant is mainly caused by the tiller number per plant, while the panicle number and genetic tiller number per plant do not have a simple linear relationship [2,3].
Rice tillering is a two-stage process: the formation of a tiller bud at each leaf axil and its subsequent outgrowth [4,5,6]. The cytoplasm-dense cells first formed a bulge at the axillary meristem in the axil of the leaf primordium, and then the bulge developed into a cone-like structure and formed into the tiller bud. During rice tiller formation, the adverse environments, including high-temperature stress, are harmful to the tiller bud growth [6,7,8], and only buds with early tillering vigor (ETV) traits could grow into rice tillers and become the final effective panicles of rice.
High temperatures in late-season rice in double-cropping systems are major adverse environmental impacts for obtaining rice tiller bud information [9]. Japonica rice originated from high latitude zones, and high-temperature climates are more harmful to its tiller occurrence than indica rice [10,11]. Recently, the cultivation pattern ‘indica rice to japonica rice’ has been implemented in a double rice cropping system in South China to ensure food security [12,13]. Recently, the cultivation pattern ‘indica rice to japonica rice’ refers to indica–japonica hybrid rice, pure japonica hybrid rice, and japonica inbred rice cultivars instead of indica rice cultivars planted as late-season rice in double-cropping systems [14]. Compared to indica rice, the grain yields of japonica rice are significantly higher because of the high canopy light capture capability and full use of solar radiation in late-season japonica rice [14,15]. However, late-season japonica rice at the seedling stage was just met with a high-temperature climate in the summer season when the paddy field water temperature may exceed 45 °C and sometimes reached 50 °C [9]. Under this cultivation pattern, the tiller bud growth of japonica rice is restrained by the paddy field hot water, which is the bottleneck for the application of the cultivation pattern in double-cropping rice systems in southern China [9,14].
Several genes and transcriptional co-regulators critical for the tiller bud normal formation in rice have been identified by genetic studies of induced mutants [16,17,18,19,20], and the molecular mechanisms behind the tiller bud normal formation were gradually clear [21,22,23,24,25]. MONOCULM 1 (MOC1) is the first cloned gene and characterized as a key regulator in controlling tiller bud normal formation in rice; MOC1 encodes a transcriptional regulator, belonging to the GRAS family nuclear protein, and MOC1-overexpressing plants showed higher-order tiller buds, while the loss-of-function mutant exhibits only one main culm without any tillers [4]. Two genes, TAD1 and TE working upstream of MOC1, encode a rice homolog of Cdh1 that functions as a co-activator or activator of APC/C (Anaphase promoting complex/cyclosome), which is a multi-subunit ubiquitin ligase; TAD1 functions as a co-activator of APC/C to target MOC1 for degradation in a cell-cycle-dependent manner, and TE recognizes and binds the APC/C to form a APC/C TE complex interacting with MOC1 and OsCDC2 to mediate the degradation of MOC1 by the ubiquitin-26S proteasome pathway [21,22]. The degradation of MOC1 with APC/C and TAD1/TE in a cell-cycle-dependent manner regulates rice tillering pattern [22]. On the other hand, MOC1 is protected from degradation by binding to the DELLA protein SLENDER RICE 1 (SLR1), and gibberellins trigger the degradation of SLR1, leading to the degradation of MOC1 and, hence, a decrease in tiller number [23]. Recently, studies have revealed that NUMBER PER PANICLE 6 (GNP6) was a novel allele of MOC1, and both GNP6 and MOC1 could not directly bind the FLORAL ORGAN NUMBER1 (FON1) promoter, and functions as a co-activator of MOC3 to activate FON1 expression in the presence of MOC3 [5,25]. The identified genes and the clear molecular mechanism behind tiller bud normal formation enrich the genetic resources available for improving tiller numbers in rice. However, few genes regulating the rice tiller bud to survive adversities, including hot environments and the paddy field hot water caused by high-temperature climates in the summer season, are reported.
In the present study, we identified the main QTLs and their candidate genes regulating the bud to grow into rice tillers in the summer season by carrying out QTL mapping and bulked-segregant analysis by resequencing method (BSA-seq) conjoint analysis using a rice backcross inbred line (BIL) population that generated the crossing of an indica rice ‘T461′ and a japonica rice ‘Wuyungeng24′ (WYG). T461 was derived from the hybrid progeny of Lunhui 22/Crip, and WYG was bred by the Wujin Rice Research Institute of Jiangsu Province, China.
2. Results
2.1. Phenotypic Variation in Rice Tillering Vigor
According to the investigation results, the tiller number (TN) and increased tiller number (ITN) showed significant differences between the parents T461 and WYG (Table 1), and T461 showed an ETV trait, which was earlier in emergency tillers and faster in tillering rate than those of WYG (Figure 1). The mean values of the traits TN and ITN for the BIL populations were greater than those of the parents at each time of investigation (Table 1), which suggested that the traits exhibited transgressive inheritance in the BIL populations. The absolute values of skewness and kurtosis for the TN and ITN traits in the BIL populations were less than 1, except for TN in the fourth-time investigation (T4) and ITN in the third period (P3) from the third-time investigation (T3) to the fourth-time investigation (T4).

Table 1.
Statistical description for tillering traits of the BIL populations and their parents.
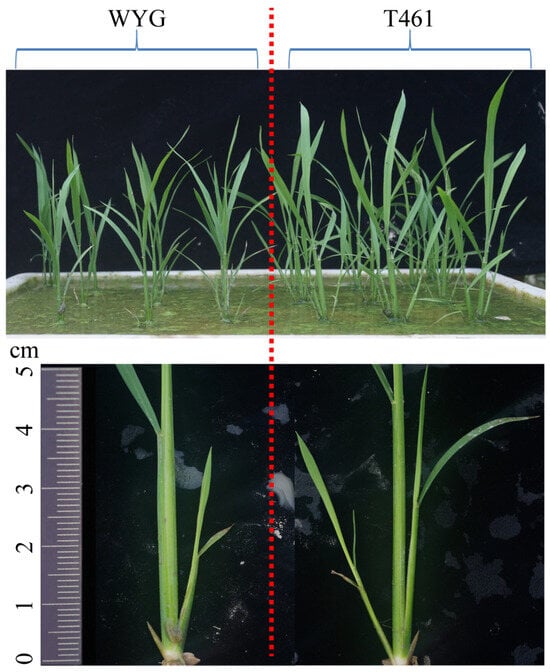
Figure 1.
The difference in tiller numbers of the parents T461 and WYG for the rice backcross inbred line (BIL) populations. T461 and WYG are the parents of the BIL populations, T461 is the indica rice cultivar, and WYG is the japonica rice cultivar ‘Wuyungeng 24’. T461 and WYG were cultivated as late-season rice in double-cropping systems in Nanchang city, Jiangxi province, China.
The individual distribution among the BIL populations in TN and ITN for each time of investigation showed continuous near-normal distribution (Figure 2), which suggested that the ETV traits of rice may considered as quantitative traits and controlled by multiple genes.
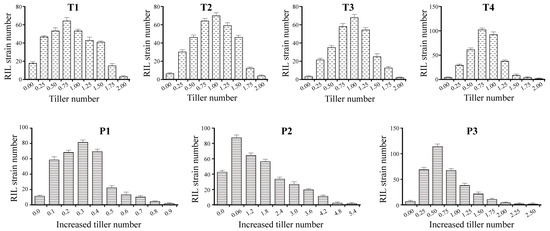
Figure 2.
The individual distribution among the BIL populations in TN and ITN. BIL indicates the recombinant inbred line of rice. TN and ITN indicate tiller number and increased tiller number, respectively. T1, T2, T3 and T4 indicate the 1st-, 2nd-, 3rd-, and 4th-time investigation for TN, respectively. P1 indicates the ITN from the 1st-time to the 2nd-time investigation for TN, P2 indicates the ITN from the 2nd-time to the 3rd-time investigation for TN, and P3 indicates the ITN from the 3rd-time to the 4th-time investigation for TN.
2.2. Genetic Linkage Map Construction
After the genome polymorphism survey, 188 (13.32%) of 1276 SSR markers were almost uniformly distributed on the 12 chromosomes of rice and showed polymorphic stripes between two parents, T461 and WYG. The polymorphic SSR markers were then used and constructed a genetic linkage map (Figure S1), and the constructed genetic linkage map contains a total map distance of 2297.51cM with an average map distance of 14.01cM, covering 89.20% of the rice genome.
2.3. QTLs of the ETV Traits
Based on the constructed genetic linkage map and the phenotypic data generated from 537 BILs, a total of seven QTLs of the ETV traits were detected on chromosomes 1, 3, 4, and 9, with the logarithm of the odds (LOD) values ranging from 3.52 to 7.57, and explained 3.23% to 12.98% of the observed phenotypic variance with QTL IciMapping 4.2 software with the Kosambi mapping function (Table 2). Among those detected QTLs, qETV1 was repeatedly detected twice by the phenotypic data of TN and ITN on the region of RM11797-RM11873 on chromosome 1, qETV4-3 was detected three times on the region of RM17303-RM17445 on chromosome 4, and the other QTLs, including qETV3-1, qETV3-2, qETV4-1, qETV4-2, and qETV9, were detected once. The positive alleles for almost all detected QTLs were contributed by T461 to increase the ETV traits, while the QTLs qETV3-1 and qETV3-2 on chromosome 3 were contributed by WYG.

Table 2.
QTLs of ETV traits detected by composite interval mapping.
2.4. SNP/InDel Calling Via BSA-seq Method
A total of 1,159,492 confident SNPs and 149,736 confident InDels were identified and applied to determine the probable QTL region by SNP-index algorithm and Euclidean Distance (ED) algorithm, and 7 QTLs were identified by the SNP-index algorithm (Figure S2A), and 5 QTLs were respectively located on chromosome 1, 2, 5, 7, and 9, and the other two QTLs were located on chromosome 4; while 13 QTLs were identified by the ED algorithm, and these QTLs were mapped to all 12 chromosomes except for chromosome 6, 8, and 10 (Figure S2B). The same QTLs between the SNP-index algorithm and the ED algorithm were exactly the QTLs identified by the SNP-index algorithm, which suggested that the QTLs identified by the SNP-index algorithm were reliable, and these QTLs were named bETV1, bETV2, bETV4-1, bETV4-2, bETV5, bETV7, and bETV9 (Table 3).

Table 3.
The QTLs identified by the SNP-index algorithm and ED algorithm.
2.5. The Overlapping Regions
Based on the identified QTLs by QTL mapping and the BSA-seq method, we found an overlapping region with a length of 1.39 Mb at positions 22,634,622 to 24,020,000 bp on chromosome 4 based on qETV4-2 generated from QTL mapping and bETV4-1 from BSA-seq method (Figure 3A), and 211 unigenes were located on the region in the reference genome.
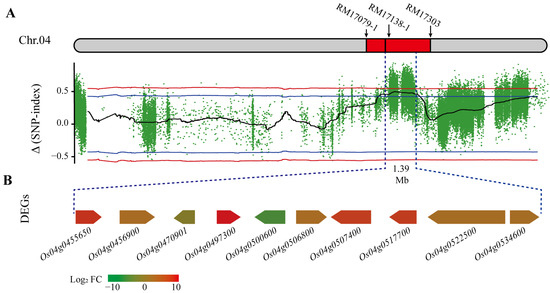
Figure 3.
The overlapping region on chromosome 4 and the differential expressed genes (DEGs) in the region. (A) The overlapping region 1.39 Mb among the makers RM17079-1, RM17138-1, and RM17303 was identified by QTL mapping and the BSA-seq method. The colored points indicate the calculated ∆(SNP-index) values. The red and blue lines indicate thresholds with confidence levels of 0.99 and 0.95, respectively. Chr.04 indicates the rice chromosome 4. (B) DEGs indicate the differential expressed genes between the E-pool constructed by 21 rice lines with early tillering vigor traits and L-pool constructed by 18 rice lines with late tillering vigor traits. FC indicates fold change in the gene expression, and different colors indicate different fold changes in Log2FC values in gene expression.
2.6. The Candidate Genes Revealed by RNA-seq
A total of 381.28 million raw reads generated from RNA-seq and 361.93 million clean reads were obtained after raw read filtration (Table S1), and about 96% of high-quality reads were successfully mapped to the rice reference genome International Rice Genome Sequencing Project (IRGSP-1.0). After the unigene expression levels were normalized by the fragments per kilo bases of exon per million fragments (FPKM) value, and gene expression patterns were verified by qPCR (Figure S3), we found that only 10 unigenes of the 211 unigenes in the overlapping region showed the differential expression between the E-pool constructed by 21 rice lines with early tillering vigor traits and L-pool constructed by 18 rice lines with late tillering vigor (LTV) traits (Figure 3B).
Among the 10 differentially expressed genes (DEGs), 8 DEGs showed more than 2 fold-change upregulation, and 2 DEGs showed more than 2 fold-change downregulation in the E-pool compared to the L-pool. Gene functional annotations show that seven DEGs encode functional known proteins and three DEGs in functional unknown genes (Table S2).
2.7. Missense Base Mutation for the Candidate Genes
After missense base mutation analysis for the 10 DEGs, 6 DEGs, including Os04g0506800, Os04g0456900, Os04g0497300, Os04g0470901, Os04g0500600, and Os04g0455650, showed missense base mutation, while the other 4 DEGs showed synonymous base mutation between the genomes of T461 and WYG. Genes Os04g0506800, Os04g0497300, and Os04g0455650 had one site in single missense base mutation (Figure 4), and gene Os04g0500600 had two sites in single missense base mutation between the genomes of the two parents T461 and WYG. Compared to the WYG genome, gene Os04g0470901 in the T461 genome had two sites in single missense base mutation and one site in two missense base mutations, and gene Os04g0456900 in the T461 genome had CAGAGC insert bases, which led to two amino acids, alanine (A) and arginine (R) insert.
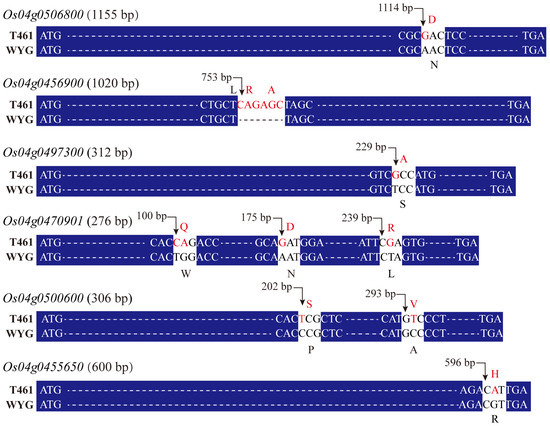
Figure 4.
The missense base mutation of DNA sequence for the DEGs. T461 indicates the DNA sequence of the indica rice cultivar ‘T461’, and WYG indicates the DNA sequence of the japonica rice cultivar ‘Wuyungeng 24’. Nucleotide base symbols in red font indicate the missense base mutation between the T461 and WYG genomes.
3. Discussion
3.1. The Candidate Genes Regulating Rice ETV Trait
The ETV is an important trait for high and stable rice yield by enhancing the effective tiller number and uniform panicle [3,26]. Therefore, identifying the genes and revealing the molecular mechanism behind the ETV trait is of great significance for high and stable yield varieties of breeding in rice. Although many QTLs and genes for tiller numbers were reported, such as the genes OsWUS [18], MOC1 and MOC3 [5], and WUSCHEL [27,28], few QTLs and no gene-regulating ETV traits were reported to be detected in the rice genome. In the present study, we identified an overlapping QTL which was located on chromosome 4 with a region length of 1.39 Mb by the joint analysis of the traditional QTL mapping and BSA-seq mapping methods. In the region, the functional unknown genes Os04g0455650, Os04g0470901, Os04g0500600, and ethylene-insensitive 3 (Os04g0456900), sialyltransferase family domain-containing protein (Os04g0506800) and ATOZI1 (Os04g0497300) are differentially expressed between the ETV and LTV lines of the BIL populations and have missense base mutations in the genomic DNA sequences between the parents T461 and WYG. We suggested that these six genes were the candidate genes regulating the ETV traits in rice. The results provided a research basis for revealing the molecular mechanism behind the ETV traits in rice.
3.2. Ethylene May Play an Important Role in ETV Traits of Rice
Plant hormones play an important role in plant growth and developmental processes [29,30,31]. Qi et al. reported that OsEATB restricts the ethylene-induced enhancement of gibberellin responsiveness and promotes the branching potential of both tillers and spikelets in rice [32,33]. In the present study, the ethylene signaling pathway activator (Os04g0456900) was also shown to be significantly upregulated based on the results of RNA-seq analysis in the rice lines with ETV traits, and the sequence alignment results showed two amino acids inserted in the T461 genome compared to WYG genome. We suggested that the mutation of gene DNA sequence in Os04g0456900 was the internal cause of high gene expression, which enhances the ethylene signal in the rice lines with ETV traits.
3.3. The ST-Like Protein Unigene OsSTLP3 Plays Important Roles in Plant ETV
Sialyltransferases (STs) are widely distributed among living creatures and play a key role in a variety of physiological processes, but little is known about the existence and functions of STs in plants [34,35]. However, previous studies have demonstrated that two of three ST-like proteins genes OsSTLP1 and OsSTLP3 from rice have ST activity in vitro assays, and the Arabidopsis T-DNA insertion mutants for all of the Arabidopsis CSTLP homologs exhibited a lethal phenotype, suggesting that these ST-like proteins play important roles in plant development [34]. The ST-like gene mgp2 was functional in Arabidopsis and required for normal pollen grain germination and pollen tube growth; meanwhile, its homolog SIA2 encodes an ST-like protein that may be involved in the machinery of building RG-II and thus have a dramatic effect on the integrity of the cell wall and correct pollen tube elongation [35]. Interestingly, the ST-like protein unigene OsSTLP3 (Os04g0506800) in the T461 genome in the present study had a non-synonymous mutation compared to the WYG genome and showed upregulation in the rice lines with ETV traits, which suggests that the non-synonymous mutation in OsSTLP3 sequence probably involved in the regulation of rice ETV traits.
3.4. Functional Unknown Genes
After searching the databases of Rice Genome Annotation Project (RGAP; http://rice.plantbiology.msu.edu), the Rice Annotation Project Database (RAP-DB; https://rapdb.dna.affrc.go.jp/ (accessed on 17 April 2023)), and the Arabidopsis Information Resource (TAIR; https://www.arabidopsis.org/ (accessed on 17 April 2023)), genes Os04g0455650, Os04g0470901, and Os04g0500600 are showed to be functionally unknown. Three functional unknown genes exhibited not only significant differential expression patterns between the ETV and LTV lines but also missense base mutation in genomic DNA sequences between the parents T461 and WYG. Among these unigenes, Os04g0455650 showed a 9.06-fold upregulation in the rice lines with ETV traits compared to the rice lines with LTV traits, and a single-nucleotide substitution from G to A (G/A), resulting in Arg being replaced by His (Figure 4). Os04g0470901 and Os04g0500600, respectively, were downregulated by 2.03-fold and 4.21-fold in the ETV rice lines compared to the LTV lines among the BIL populations. The DNA sequence of the unigene Os04g0470901 showed three non-synonymous single-nucleotide substitutions; the DNA sequence of the unigene Os04g0500600 showed double non-synonymous single-nucleotide substitution. We suggested that these functional unknown genes, Os04g0455650, Os04g0470901, and Os04g0500600, play important roles in rice tillers to survive the adversities of high-temperature climates and become the final effective panicles.
4. Materials and Methods
4.1. Plant Materials
The BIL populations with 537 lines (BC2F13) was developed from a cross between the indica rice cultivar ‘T461′ and the japonica rice cultivar WYG, and the flow chart of the BIL group construction is shown in Figure S4. For the parents, T461 was generated from the crossing of Lunhui 22/Cript and showed strong ETV traits, and WYG showed LTV traits and bred by Wujin Rice Research Institute of Jiangsu Province, China, when T461 and WYG were planted as late-season rice in double-cropping systems and met with a high-temperature climate in the summer season at the rice seedling stage. T461 and WYG had almost the same effective panicle number (approximately 11 effective panicles) when rice was planted as single-cropping rice and met with a mild climate in the spring season at the seedling stage of rice.
4.2. Field Trial and Phenotypic Evaluation
The BIL populations were planted as late-season rice of double-cropping systems in the paddy field at Jiangxi Agricultural University (28°75′ N, 115°83′ E), China. Seeds were germinated in an incubator at 37 °C for 25 h after soaking in distilled water for 48 h. Germinated seeds with normal budding were selected for each line and set of parents. The selected seeds were directly sown one seed per hill to the horizontal surface paddy field in the plot with 6 rows and 8 hills for each line, and hill space of 16.8 cm × 23.1 cm apart. Three random plots were designed for each line of the BIL population. After sowing, the paddy field soil was kept moist and non-puddled until the two-leaf stage of rice. Then, the field was irrigated and kept a 3 cm water layer, and a slow-release fertilizer (N/P/K = 17:15:15) was applied in 150 kg/hm2.
The rice tiller number for each plot was manually counted when 95% of BIL lines were initiated to emerge tillers. TN of all the plants (24 plants), except the plants in border rows, were counted for each plot, and the rice tiller number was counted 4 times, 1 time every 72 h. The average tiller numbers per plot were used for further analysis for the ITN.
4.3. Linkage Map Construction and QTL Analysis
Fresh young leaves of parents and BIL populations were separately collected from 14-day-old rice seedlings for genomic DNA extraction using a cetyltrimethylammonium bromide (CTAB) method [36]. Parental polymorphism was surveyed with 1276 simple sequence repeat (SSR) markers from the Gramene database (www.gramene.org). The phenotypic and genotypic data of 537 BILs and parents were analyzed to construct the genetic linkage map using QTL IciMapping 4.2 software with the Kosambi mapping function [37]. The LOD threshold was calculated after the 1000-permutation test (p < 0.05) and used to claim a putative QTL. The proportion of phenotypic variance explained (PVE) and the corresponding additive effect were estimated for each QTL.
4.4. BSA-seq Mapping Analysis
To improve the reliability of identified QTLs, BSA-seq analysis by high-throughput sequencing (BSA-seq) and the SNP-index algorithm and ED algorithm were further employed to screen the significant genomic region related to the ETV traits. Fresh young leaves of the 21 individuals with ETV traits and 18 individuals with LTV traits were separately collected at the tillering stage of rice for BSA-seq analysis. For BSA-seq analysis, the dominant pool (namely the ETV traits pool, E-pool) was constructed by mixing an equal amount of leaf tissues from 21 individuals with ETV traits, while the recessive pool (namely the LTV traits pool, L-pool) was constructed by 18 individuals with LTV traits from the BIL populations, respectively. After library sequencing, high-quality read aligning and SNP calling, the SNP-index algorithm, and the ED algorithm were used to identify the candidate regions for the ETV traits, according to the previous method [38]. The same overlapping regions identified by the SNP-index algorithm and the ED algorithm were considered as BSA-seq mapping the candidate regions related to the ETV traits.
4.5. Analysis of the Overlapping Regions
The overlapping regions related to the ETV traits identified by QTL mapping and BSA-seq were manually obtained according to the intervals of the physical location on rice chromosomes, and the gene DNA sequence information for all the genes in the overlapping regions was retrieved from the database of IRGSP-1.0 (https://rgp.dna.affrc.go.jp/E/IRGSP/ (accessed on 6 May 2023)) for further candidate gene expression pattern and missense base mutation analysis.
4.6. Expression Pattern Analysis for the Genes in the Overlapping Regions
To further screen the candidate genes, transcriptome analysis by the RNA-sequencing method was employed to detect the gene expression patterns of all the genes in the overlapping regions. The BIL populations were cultivated as late-season rice in double-cropping systems. When 95% of BIL lines initiated to emerge tillers, tiller nodes of the 21 ETV and 18 LTV lines used to construct E-pool and L-pool in BSA-seq analysis were consecutively collected 4 times, 1 time every 72 h. Eight tiller nodes from each line were separately collected. Equal tillering nodes from each ETV rice line were mixed to form a T-Mix sample, and equal tillering nodes from each LTV rice line formed an S-Mix sample. Four biological replicates were carried out. After total RNA extraction, quality inspection, and concentration determination for each mixed sample, sequencing libraries were constructed using NEBNext® Ultra™ RNA Library Prep Kit for Illumina® and sequenced on the Illumina sequencing platform by Genedenovo Biotechnology, Co., Ltd. (Guangzhou, China).
The high-quality reads were then mapped to the rice reference genome IRGSP-1.0 after raw read filtration. The number of reads mapped to each gene was counted, and the unigene expression levels were normalized by FPKM value [39]. DEGs between T-Mix samples and S-Mix samples were screened using a preset threshold |log2fold change| ≥ 2 and false discovery rate (FDR) ≤ 0.05. The DEGs in the overlapping regions were DNA sequence retrieved and verified by qPCR in expression pattern among the parents T461 and WYG and functional annotated in the Rice Genome Annotation Project (RGAP; http://rice.plantbiology.msu.edu (accessed on 17 May 2023)), the Rice Annotation Project Database (RAP-DB; https://rapdb.dna.affrc.go.jp/ (accessed on 17 May 2023)), and the Arabidopsis Information Resource (TAIR; https://www.arabidopsis.org/ (accessed on 19 May 2023)).
4.7. Analysis of Missense Base Mutation for the DEGs
According to the genomic DNA sequences retrieved from the database IRGSP-1.0, the specific primers for the DEGs were, respectively, designed by Primer 5. Fresh young leaves of two parents, T461 and WYG, were separately collected from 14-day-old rice seedlings, and genomic DNA extraction was conducted using the CTAB method. The DNA for DEGs were, respectively, amplified by PCR from the genomes of two parents T461 and WYG, and the sequence message for the DEGs was obtained by Sanger method sequencing using PCR production. Analysis of missense base mutation in the genomic DNA sequences between the parents T461 and WYG was carried out for the DEGs by the software DNAMAN 9.0.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25031497/s1.
Author Contributions
J.-L.L. designed and supervised the experiments and edited the draft. W.W., T.-T.Z. and L.-L.Y. performed the experiments, prepared the original draft, and contributed equally to this paper. Z.-Y.W., S.-Q.D., H.-Y.S. and Z.-H.W. performed the tiller number investigation. Y.-J.H. guided and supervised the experiments. All authors contributed to this article and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the National Natural Science Foundation of China (31960062 and 32360481) and the Natural Science Foundation of Jiangxi Province (20212BAB205008 and 20224ACB205002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article/Supplementary Materials. The raw reads of BSA-seq and RNA-seq analysis could retrieved from the database in NCBI (https://www.ncbi.nlm.nih.gov/sra/PRJNA1018333 accessed on 18 September 2023). Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shi, J.; An, G.; Weber, A.P.M.; Zhang, D. Prospects for rice in 2050. Plant Cell Environ. 2023, 46, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, F.; Zhang, G. Difference in Grain Yield and Quality among Tillers in Rice Genotypes Differing in Tillering Capacity. Rice Sci. 2007, 14, 135–140. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J. Branching in rice. Curr. Opin. Plant Biol. 2011, 14, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qian, Q.; Fu, Z.; Wang, Y.; Xiong, G.; Zeng, D.; Wang, X.; Liu, X.; Teng, S.; Hiroshi, F.; et al. Control of tillering in rice. Nature 2003, 422, 618–621. [Google Scholar] [CrossRef]
- Shao, G.; Lu, Z.; Xiong, J.; Wang, B.; Jing, Y.; Meng, X.; Liu, G.; Ma, H.; Liang, Y.; Chen, F.; et al. Tiller Bud Formation Regulators MOC1 and MOC3 Cooperatively Promote Tiller Bud Outgrowth by Activating FON1 Expression in Rice. Mol. Plant. 2019, 12, 1090–1102. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cho, L.; Lee, S.; Pasriga, R.; Tun, W.; Yang, J.; Yoon, H.; Jeong, H.J.; Jeon, J.; An, G. Chromatin Interacting Factor OsVIL2 Is Required for Outgrowth of Axillary Buds in Rice. Mol. Cells 2019, 42, 858–868. [Google Scholar] [PubMed]
- Tanaka, W.; Ohmori, Y.; Ushijima, T.; Matsusaka, H.; Matsushita, T.; Kumamaru, T.; Kawano, S.; Hirano, H.Y. Axillary Meristem Formation in Rice Requires the WUSCHEL Ortholog TILLERS ABSENT1. Plant Cell 2015, 27, 1173–1184. [Google Scholar] [CrossRef]
- Zha, M.; Zhao, Y.; Wang, Y.; Chen, B.; Tan, Z. Strigolactones and Cytokinin Interaction in Buds in the Control of Rice Tillering. Front. Plant Sci. 2022, 13, 837136. [Google Scholar] [CrossRef]
- Yan, Y.; Ding, C.; Zhang, G.; Hu, J.; Zhu, L.; Zeng, D.; Qian, Q.; Ren, D. Genetic and environmental control of rice tillering. Crop J. 2023, 11, 1287–1302. [Google Scholar] [CrossRef]
- Hu, Y.; Xue, J.; Li, L.; Cong, S.; Yu, E.; Xu, K.; Zhang, H. Influence of dynamic high temperature during grain filling on starch fine structure and functional properties of semi-waxy japonica rice. J. Cereal Sci. 2021, 101, 103319. [Google Scholar] [CrossRef]
- Tong, H.; Duan, H.; Wang, S.; Su, J.; Sun, Y.; Liu, Y.; Tang, L.; Liu, X.; Chen, W. Moderate drought alleviates the damage to grain quality at high temperatures by improving the starch synthesis of inferior grains in japonica rice. J. Integr. Agric. 2022, 21, 3094–3101. [Google Scholar] [CrossRef]
- Saud, S.; Wang, D.; Fahad, S.; Alharby, H.F.; Bamagoos, A.A.; Mjrashi, A.; Alabdallah, N.M.; AlZahrani, S.S.; AbdElgawad, H.; Adnan, M.; et al. Comprehensive Impacts of Climate Change on Rice Production and Adaptive Strategies in China. Front. Microbiol. 2022, 13, 926059. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, C.; Linderholm, H.W.; Fu, Y.; Cai, W.; Xu, J.; Zhuang, L.; Wu, M.; Shi, Y.; Wang, G.; et al. The negative impact of increasing temperatures on rice yields in southern China. Sci. Total Environ. 2022, 820, 153262. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Tan, X.; Huang, S.; Pan, X.; Zeng, Y.; Zhang, J.; Cheng, S.; Zeng, Y. Grain yield and quality performances of different late-season rice cultivars in response to experimental warming in subtropical China. Front. Plant Sci. 2023, 14, 1136564. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Liu, S.; Zheng, X.; Chu, G.; Xu, C.; Zhang, X.; Wang, D.; Chen, S. Solar radiation-use characteristics of indica/japonica hybrid rice (Oryza sativa L.) in the late season in southeast China. Crop J. 2021, 9, 427–439. [Google Scholar] [CrossRef]
- Wang, J.; Wu, B.; Lu, K.; Wei, Q.; Qian, J.; Chen, Y.; Fang, Z. The Amino Acid Permease 5 (OsAAP5) Regulates Tiller Number and Grain Yield in Rice. Plant Physiol. 2019, 180, 1031–1045. [Google Scholar] [CrossRef]
- Wen, X.; Sun, L.; Chen, Y.; Xue, P.; Yang, Q.; Wang, B.; Yu, N.; Cao, Y.; Zhang, Y.; Gong, K.; et al. Rice dwarf and low tillering 10 (OsDLT10) regulates tiller number by monitoring auxin homeostasis. Plant Sci. 2020, 297, 110502. [Google Scholar] [CrossRef]
- Xia, T.; Chen, H.; Dong, S.; Ma, Z.; Ren, H.; Zhu, X.; Fang, X.; Chen, F. OsWUS promotes tiller bud growth by establishing weak apical dominance in rice. Plant J. 2020, 104, 1635–1647. [Google Scholar] [CrossRef]
- Feng, F.; Guo, X.; Zhu, X.; Hu, Y.; Chen, Y.; Sun, H.; Li, J.; Zhao, C.; Sun, H.; Zhao, Q. OsPIN2 is involved in OsSPL14/17-inhibited tiller bud outgrowth in response to phosphate deficiency in rice. Environ. Exp. Bot. 2023, 209, 105297. [Google Scholar] [CrossRef]
- Hang, J.; Wu, B.; Qiu, D.; Yang, G.; Fang, Z.; Zhang, M. OsNPF3.1, a nitrate, abscisic acid and gibberellin transporter gene, is essential for rice tillering and nitrogen utilization efficiency. J. Integr. Agric. 2023, in press. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, D.; Dong, H.; Gu, S.; Cheng, Z.; Gong, J.; Qin, R.; Jiang, L.; Li, G.; Wang, J.L.; et al. Rice APC/CTE controls tillering by mediating the degradation of MONOCULM 1. Nat. Commun. 2012, 3, 752. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, Y.; Yu, Y.; Duan, J.; Liao, Z.; Xiong, G.; Meng, X.; Liu, G.; Qian, Q.; Li, J. Degradation of MONOCULM 1 by APC/CTAD1 regulates rice tillering. Nat. Commun. 2012, 3, 750. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Yu, H.; Duan, J.; Yuan, K.; Yu, C.; Meng, X.; Kou, L.; Chen, M.; Jing, Y.; Liu, G.; et al. SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice. Nat. Commun. 2019, 10, 2738. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Han, T.; Song, Q.; Ye, W.; Song, X.; Chu, J.; Li, J.; Chen, Z.J. The Rice Circadian Clock Regulates Tiller Growth and Panicle Development Through Strigolactone Signaling and Sugar Sensing. Plant Cell 2020, 32, 3124–3138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, X.; Ma, X.; Xu, B.; Zhao, Y.; Ma, Z.; Li, G.; Khan, N.U.; Pan, Y.; Liang, Y.; et al. GNP6, a novel allele of MOC1, regulates panicle and tiller development in rice. Crop J. 2021, 9, 57–67. [Google Scholar] [CrossRef]
- Li, G.; Tang, J.; Zheng, J.; Chu, C. Exploration of rice yield potential: Decoding agronomic and physiological traits. Crop J. 2021, 9, 577–589. [Google Scholar] [CrossRef]
- Lu, Z.; Shao, G.; Xiong, J.; Jiao, Y.; Wang, J.; Liu, G.; Meng, X.; Liang, Y.; Xiong, G.; Wang, Y.; et al. MONOCULM 3, an Ortholog of WUSCHEL in Rice, Is Required for Tiller Bud Formation. J. Genet. Genom. 2015, 42, 71–78. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, S.; Luo, B.; Zhou, Z.; Bao, J.; Fang, R.; Wang, F.; Song, X.; Liao, Z.; Chen, G.; et al. Transcriptomic and Metabolomic Investigation on Leaf Necrosis Induced by ZmWus2 Transient Overexpression in Nicotiana benthamiana. Int. J. Mol. Sci. 2023, 24, 11190. [Google Scholar] [CrossRef]
- Huang, G.; Kilic, A.; Karady, M.; Zhang, J.; Mehra, P.; Song, X.; Sturrock, C.J.; Zhu, W.; Qin, H.; Hartman, S.; et al. Ethylene inhibits rice root elongation in compacted soil via ABA- and auxin-mediated mechanisms. Proc. Natl. Acad. Sci. USA 2022, 119, e2093895177. [Google Scholar] [CrossRef]
- Qin, H.; Pandey, B.K.; Li, Y.; Huang, G.; Wang, J.; Quan, R.; Zhou, J.; Zhou, Y.; Miao, Y.; Zhang, D.; et al. Orchestration of ethylene and gibberellin signals determines primary root elongation in rice. Plant Cell 2022, 34, 1273–1288. [Google Scholar] [CrossRef]
- Yin, C.C.; Huang, Y.H.; Zhang, X.; Zhou, Y.; Chen, S.Y.; Zhang, J.S. Ethylene-mediated regulation of coleoptile elongation in rice seedlings. Plant Cell Environ. 2023, 46, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Sun, F.; Wang, Q.; Chen, M.; Huang, Y.; Feng, Y.Q.; Luo, X.; Yang, J. Rice Ethylene-Response AP2/ERF Factor OsEATB Restricts Internode Elongation by Down-Regulating a Gibberellin Biosynthetic Gene. Plant Physiol. 2011, 157, 216–228. [Google Scholar] [CrossRef]
- Qi, H.; Liang, K.; Ke, Y.; Wang, J.; Yang, P.; Yu, F.; Qiu, F. Advances of Apetala2/Ethylene Response Factors in Regulating Development and Stress Response in Maize. Int. J. Mol. Sci. 2023, 24, 5416. [Google Scholar] [CrossRef] [PubMed]
- Takashima, S.; Abe, T.; Yoshida, S.; Kawahigashi, H.; Saito, T.; Tsuji, S.; Tsujimoto, M. Analysis of Sialyltransferase-Like Proteins from Oryza sativa. J Biochem. 2006, 139, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.; Lehner, A.; Bouton, S.; Kiefer-Meyer, M.C.; Voxeur, A.; Pelloux, J.; Lerouge, P.; Mollet, J.C. The cell wall pectic polymer rhamnogalacturonan-II is required for proper pollen tube elongation: Implications of a putative sialyltransferase-like protein. Ann. Bot. 2014, 114, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]
- Takagi, H.; Abe, A.; Yoshida, K.; Kosugi, S.; Natsume, S.; Mitsuoka, C.; Uemura, A.; Utsushi, H.; Tamiru, M.; Takuno, S.; et al. QTL-seq: Rapid mapping of quantitative trait loci in rice by whole genome resequencing of DNA from two bulked populations. Plant J. 2013, 74, 174–183. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).