Abstract
The kallikrein–kinin system is a versatile regulatory network implicated in various biological processes encompassing inflammation, nociception, blood pressure control, and central nervous system functions. Its physiological impact is mediated through G-protein-coupled transmembrane receptors, specifically the B1 and B2 receptors. Dopamine, a key catecholamine neurotransmitter widely distributed in the CNS, plays a crucial role in diverse physiological functions including motricity, reward, anxiety, fear, feeding, sleep, and arousal. Notably, the potential physical interaction between bradykinin and dopaminergic receptors has been previously documented. In this study, we aimed to explore whether B2R modulation in catecholaminergic neurons influences the dopaminergic pathway, impacting behavioral, metabolic, and motor aspects in both male and female mice. B2R ablation in tyrosine hydroxylase cells reduced the body weight and lean mass without affecting body adiposity, substrate oxidation, locomotor activity, glucose tolerance, or insulin sensitivity in mice. Moreover, a B2R deficiency in TH cells did not alter anxiety levels, exercise performance, or motor coordination in female and male mice. The concentrations of monoamines and their metabolites in the substantia nigra and cortex region were not affected in knockout mice. In essence, B2R deletion in TH cells selectively influenced the body weight and composition, leaving the behavioral and motor aspects largely unaffected.
1. Introduction
Several biological processes, such as the inflammatory response [1], nociception, pain transmission [2], blood pressure control [3], cardiac hypertrophy, local blood flow, electrolyte and glucose transport, and cell proliferation [1] are modulated by the kallikrein–kinin system (KKS). Additionally, KKS is actively involved in gastrointestinal functions [4]. However, important for this work, this system regulates various brain functions [5].
Briefly, this KKS is composed of glycoprotein precursor substrates (kininogens); proteolytic enzymes from the serine protease family (tissue and plasma kallikrein), present in glandular cells, neutrophils, and biological fluids; and vasoactive peptides (kinins), derived from the hydrolysis of kininogens by kallikreins. The actions of tissue and plasma kallikreins in humans release the vasoactive peptides kallidin or Lys-BK (LBK) and bradykinin (BK). LBK is generated by the action of kallikreins on low-molecular-weight kininogen (LMWK), while BK is generated by the activity of kallikreins on a high-molecular-weight kininogen (HMWK) [6].
The physiological effects of kinins are mediated by two G-protein-coupled transmembrane receptors known as the B1 and B2 receptors (B1Rs and B2Rs, respectively). The B1R is normally poorly expressed but highly regulated in the presence of inflammatory stimuli or by des-Arg-kinins [7]. In contrast, the B2R is constitutively expressed under physiological conditions and is responsible for most of the effects of kinins [8]. The dysfunction of this receptor is implicated in cancer development and cardiovascular and neurological diseases [9]. Consequently, both the structure and pharmacology of B2Rs have become focal points in medical research [10]. Both kinin receptors are positively regulated under pathological conditions involved in proinflammatory effects [11]; for example, in temporal lobe epilepsy [12] or ischemic stroke [13]. Regarding the central nervous system (CNS), the expression of the B2R is well described in the hippocampal and olfactory bulb neurons of rats [14,15].
For almost five decades, researchers have provided evidence of the KKS’s involvement in pathological and physiological conditions in the brain [16]. The interest in the involvement of kinin B2Rs in pathophysiological conditions has evolved more rapidly than that of B1Rs, probably due to the B1R’s inflammatory profile [17]. Thus, it is considered that the B1R contributes to the pathogenesis of neurodegenerative diseases, while the B2R has been considered a neuroprotective factor [7,18]. Notably, the B2R plays a pivotal role in facilitating long-term neurogenesis [19,20], influencing the differentiation of neuronal cells, particularly in the hippocampus [21], and assuming a protective function against cell death [22].
Recently, our research group provided evidence that animals lacking the B1R and expressing only the B2R showed an increase in cell proliferation in the dentate gyrus after running, thus showing the importance of the B2R in mediating exercise-induced neuroprotective effects. Additionally, data also show that the absence of B2Rs alters exercise performance in mice [21]. Considering the pivotal role of the mesolimbic dopaminergic pathway in regulating locomotor activity in animal models [23,24], and given the fact that the B2R is able to physically interact with the D2 dopamine receptor (DrD2), with both being members of G protein-coupled receptors superfamily [25], we wonder if kinin signaling in the dopaminergic pathway could be one of the possible mechanisms involved in the altered locomotor activity of B2R knockout (KO) animals.
Dopamine is synthesized from tyrosine by tyrosine hydroxylase (TH) and then transported inside secretory vesicles for storage and release. A well-established model to study the catecholaminergic/dopaminergic pathway is through modulating gene expression from neurons expressing TH [26], the rate-limiting enzyme of catecholamine biosynthesis. The central aim of this study was to evaluate the effects of selective ablation of the B2R in catecholaminergic neurons, with a particular focus on neurons located in the substantia nigra (SN), given their crucial role in dopamine production and their significant abundance in this region. Investigating the effects of this ablation on the metabolic, behavioral, and motor aspects, we aim to provide a comprehensive overview of this animal model, contributing to the understanding of B2R implications in this process. Focusing our analysis on catecholaminergic neurons, especially those located in the SN, we can comprehensively clarify the role played by the B2R in this specific context.
2. Results
2.1. Generation of Mice Carrying Ablation of B2R in TH Neurons
To explore the consequences of B2R ablation in TH cells, control and THΔB2R mice were generated. Considering the critical physiological importance of the mesolimbic dopaminergic pathway and the high number of TH neurons in the substantia nigra (SN), micropunches comprising the SN were obtained from 12-week-old mice. Initially, the B2R mRNA levels were quantified. We observed a significant reduction in B2R mRNA in the SN of THΔB2R animals compared to the control group (Figure 1C). This result confirms the genetic ablation of the B2R in areas of the brain containing TH neurons. Importantly, B1R expression was not affected in THΔB2R mice (Figure 1D), indicating the absence of compensatory adaptations of the KKS. The TH mRNA levels in the SN were similar between groups (Figure 1E). Since TH cells are also found outside in peripheral tissues, B2R mRNA was analyzed in the adrenal gland, and in this case, no significant reduction was observed compared to the control mice (Figure 1F). Together, these findings indicate a robust ablation of the B2R in major catecholaminergic neuronal populations without compensatory adaptations.
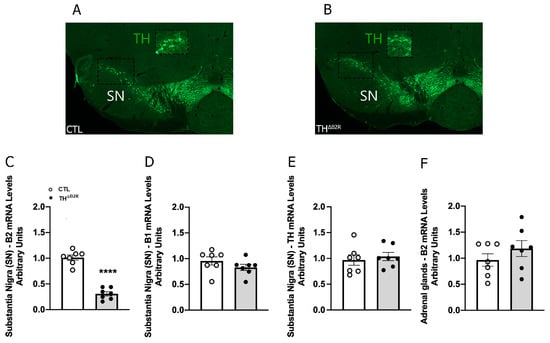
Figure 1.
Validation of the THΔB2R mouse model: B2R mRNA ablation in TH cells in the substantia nigra (SN). Localization of TH neurons in the substantia nigra (SN) and gene expression in control and THΔB2R animals. Photomicrographs indicating the locations of TH neurons (green) in control group (A) and THΔB2R group (B); TH immunoreactivity has been previously described by [26]. Bar graphs comparing the mRNA expression of the B2R (C), B1R (D), and TH (E) in the SN region. mRNA expression of the B2R in the adrenal gland (F) (control, n = 7, THΔB2R, n = 7). Data expressed as the mean ± SEM. **** p < 0.0001.
2.2. B2R Ablation in TH Cells Leads to a Transitory Reduction in Body Weight in Female and Male Mice
To verify the consequences of the B2R deletion in TH cells, the body weights and body compositions were assessed over time in both male and female mice. In the female group, a reduction in the body weight was observed at 5 and 10 weeks of age (Figure 2A). A reduction in the lean mass was noticed at 5 weeks of age (Figure 2B), while no change in fat mass was observed in the female mice (Figure 2C).
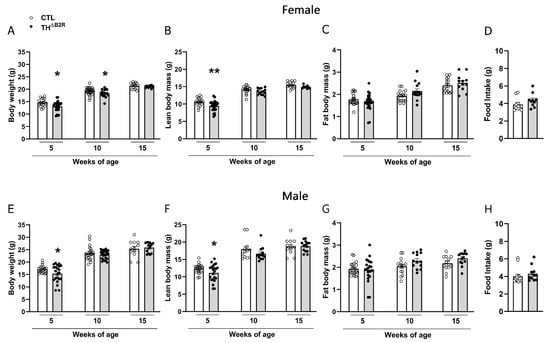
Figure 2.
Bradykinin B2 receptor (B2R) ablation from TH cells (THΔB2R) altered the body weights of female and male mice. Body weight (A,E); lean body mass (B,F); and fat body mass (C,G) at 5, 10, and 15 weeks of age in control (n = 17–22) and THΔB2R (n = 28–32) groups. Food consumption of female (D; n = 10) and male (H; n = 11–13) mice. Data expressed as the mean ± SEM. * p < 0.05; ** p < 0.01.
In the male group, we observed a reduction in the body weight (Figure 2E) and lean mass (Figure 2F) only in 5-week-old mice, while no change in the fat mass was observed (Figure 2G). No differences were observed at other ages compared to the control groups (Figure 2E–G). No changes in food consumption were verified in the THΔB2R mice (Figure 2D,H). Thus, B2R ablation in TH cells impairs body growth in pubertal mice but does not cause major consequences in adult animals.
2.3. Impact of B2R Ablation in TH Cells on Metabolic Parameters
Either the KKS or the mesolimbic dopaminergic system are involved in the control of metabolism [27,28,29,30,31]. To investigate the potential impact of B2R deletion in cells expressing TH on energy homeostasis, we conducted an analysis of O2 consumption and CO2 production in both female and male mice. We did not observe significant changes in the metabolic parameters, including VO2, RER, and ambulatory activity in both female (Figure 3A–C) and male (Figure 3D–F) THΔB2R mice. We also performed an area under the curve analysis for all parameters measured, and no change was found between the groups. Thus, B2R expression in TH neurons does not appear to be necessary for the maintenance of energy homeostasis.
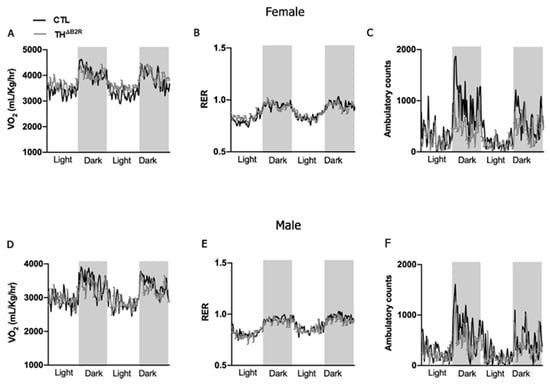
Figure 3.
Bradykinin B2 receptor (B2R) ablation from TH cells (THΔB2R) is not necessary for the maintenance of metabolic parameters in mice. Oxygen consumption (VO2; A); respiratory exchange ratio (RER; B), and ambulatory activity (C) in control (n = 7) and THΔB2R (n = 9) female mice. Oxygen consumption (VO2; D); respiratory exchange ratio (RER; E), and ambulatory activity (F) in control (n = 8) and THΔB2R (n = 10) male mice. Differences between groups were analyzed using repeated measures two-way ANOVA.
2.4. Glucose Homeostasis Is Unaltered by B2R Ablation from TH Cells
KO mice for the B1R and B2R [32] as well as alterations in the dopaminergic neurons [28] result in modifications in glucose homeostasis. Therefore, glucose homeostasis was assessed in THΔB2R mice. The Ablation of B2R in the TH cells did not affect the glucose tolerance test (Figure 4A,C) or insulin tolerance test, neither in the male or female mice (Figure 4B,D).
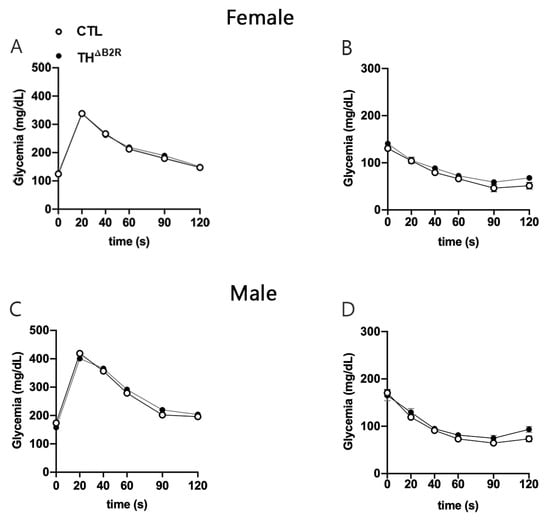
Figure 4.
Glucose homeostasis is unaltered by bradykinin B2 receptor (B2R) ablation from TH cells (THΔB2R) in female and male mice. Glucose tolerance test (GTT; A) and insulin tolerance test (ITT; B) in control (n = 9) and THΔB2R (n = 10) female mice. Glucose tolerance test (GTT; C) and insulin tolerance test (ITT; D) in control (n = 12) and THΔB2R (n = 9) male mice. Possible differences between groups were analyzed using repeated measures two-way ANOVA.
2.5. B2R Ablation in TH Cells Does Not Affect Anxiety
Animal studies indicate that bradykinin may modulate anxiety [33,34,35]. Consequently, potential alterations in anxiety levels were scrutinized in THΔB2R mice through the utilization of the open field and elevated plus maze tests (Figure 5). In the open field test, both female and male mice from the control and THΔB2R groups exhibited a comparable frequency of entries into the center (Figure 5A,E). Additionally, no discernible disparity emerged in the duration spent in the center of the open field arena between the control and THΔB2R mice (Figure 5B,F). Similarly, there were no significant distinctions in the number of entries and time spent in the open arms during the elevated plus maze test for both female (Figure 5C,D) and male (Figure 5G,H) mice. It is worth noting that the distance travelled and the duration of movement in the open field and elevated plus maze tests remained similar between control and THΔB2R mice.
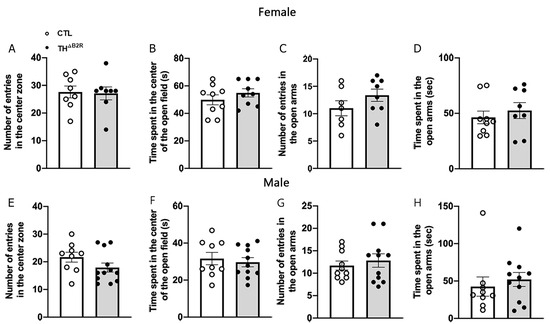
Figure 5.
Bradykinin B2 receptor (B2R) ablation from TH cells does not modulate anxiety. Number of entries (A) and time spent (B) in the center of the open field in control (n = 7–9) and THΔB2R (n = 7–9) female mice. Number of entries (C) and time spent (D) in the open arms during the elevated plus maze test in the control (n = 7–9) and THΔB2R (n = 7–9) female mice. Number of entries (E) and time spent (F) in the center of the open field in the control (n = 9–10) and THΔB2R (n = 11–12) male mice. Number of entries (G) and time spent (H) in the open arms during the elevated plus maze test in the control (n = 9–10) and THΔB2R (n = 11–12) male mice. Differences between groups were analyzed using an unpaired, two-tailed Student’s t-test.
2.6. B2R Ablation in TH Cells Does Not Impair Physical Exercise Performance and Motor Coordination
In a B2R KO model, the absence of B2R appears to enhance performance in aerobic exercise [31]. Therefore, we subjected THΔB2R mice to voluntary wheel tests, maximum effort tests on the treadmill, and motor coordination tests (rotarod; Figure 6). No differences were observed in the voluntary wheel tests for both female (Figure 6A) and male (Figure 6D) mice. Similarly, no differences were observed in the maximum effort tests on the treadmill for female (Figure 6B) and male (Figure 6E) mice, or in the motor coordination tests (Figure 6C,F).
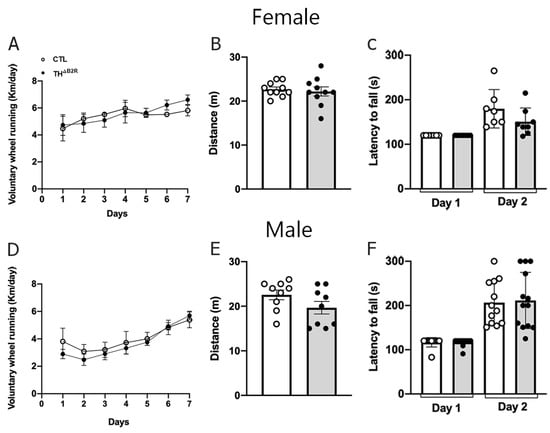
Figure 6.
Effect of bradykinin B2 receptor (B2R) ablation from TH cells (THΔB2R) in mice on exercise performance and motor coordination. Running distance in voluntary wheels (A), incremental treadmill maximal running (B), and retention time on the rod in the rotarod test (C) in control (n = 6–10) and THΔB2R (n = 6–11) female mice. Running distance in voluntary wheels (D), incremental treadmill maximal running (E), and retention time on the rod in the rotarod test (F) in control (n = 8–10) and THΔB2R (n = 8–11) male mice. Differences between groups were analyzed using an unpaired, two-tailed Student’s t-test.
2.7. Monoamine and Metabolite Concentrations Remain Unaltered Following B2R Ablation in TH Cells
The neuronal activity of monoamines is a pivotal indicator for various conditions encompassing depression, anxiety, and other neurodegenerative diseases that intricately influence the dopaminergic system [36]. In this study, we investigated the impact of B2R deletion in TH-expressing cells on the concentrations of monoamines and their metabolites in the SN and cerebral cortex of both female and male mice. Our findings revealed that B2R deletion in TH cells did not induce significant alterations in the concentrations of the monoamines and metabolites analyzed in the SN and cerebral cortex (Table 1).

Table 1.
Concentrations of monoamines and their metabolites in the substantia nigra (SN) and cortex region of THΔB2R and control animals. The concentrations are expressed as ng/g of wet tissue. Substantia nigra (SN); 4-hydroxy-3-methoxy mandelic acid (VMA); norepinephrine (NA); (3,4-dihydroxyphenyl)-L-alanine (L-Dopa); 3,4-dihydroxyphenylacetic acid (DOPAC); dopamine (DA); 4-hydroxy-3-methoxy-phenylacetic acid (HVA); 5-hydroxyindoleacetic acid (5HIAA); 5-hydroxytryptamine (5HT). Student’s t test was performed to compare the control (n = 10) and THΔB2R (n = 12) groups. Data are presented as the mean ± SEM.
3. Discussion
Kinins play a complex and important role in regulating several brain functions, including in pathological conditions. Niewiarowska-Sendo et al. (2017) described, in vitro, an intriguing physical interaction between the B2R and the DrD2, both members of the G protein-coupled receptor superfamily [25]. Although further studies are needed to fully understand this interaction in vivo, our study aimed to elucidate the impact of the B2R on the modulation of the dopaminergic pathway through its modulation in catecholaminergic neurons. Therefore, this investigation extended to assessing the subsequent impact on the metabolic, behavioral, and motor aspects in both male and female mice. To explore the potential functions of the B2R within this context, we used Cre–lox technology to selectively ablate the B2R from TH cells. Among other catecholamine-producing neurons, this approach targets the SN, a pivotal region for the dopaminergic neuron system, with crucial roles in various brain functions such as motor control, reward processing, and motivated behavior [37]. The B2R ablation in the SN was successfully confirmed by a reduction in B2R mRNA levels compared to the control mice. Thus, for the first time, we demonstrate the effects of the B2R on cells expressing TH in a specific deletion model. Importantly, B1R expression remained unchanged in THΔB2R mice, discarding possible compensatory mechanisms. It is worth mentioning that although our main focus was to study the importance of B2Rs in dopaminergic neurons of the SN/VTA complex, B2R ablation may have occurred in any catecholaminergic neuron. We did not detect B2R deletion in the adrenal gland, possibly because of the absence of B2R expression in the TH cells of the adrenal medulla. However, we cannot rule out that the B2R was not deleted in other dopamine-, noradrenaline- and adrenaline-producing cells, because they all express TH. In this way, we assessed other aspects to offer a comprehensive understanding of the consequences of the B2R deletion in this novel model.
Surprisingly, upon deletion of the B2R in cells of the SN expressing TH, the THΔB2R mice exhibited normal neurotransmitter levels in the brain. This suggests that the B2R does not play a direct role in dopamine synthesis, and consequently, the observed monoamine patterns in KO mice remained unaltered under physiologic conditions.
We noticed a decrease in the body weights and changes in the body compositions during the initial weeks of life in both male and female THΔB2R mice, with no discernible differences in food consumption or energy metabolism. Existing evidence underscores the involvement of bradykinin in neurons governing the secretion of gonadotropin-releasing hormone (GnRH) [38]. Another neuropeptide implicated in stimulating GnRH release and thereby modulating reproduction and growth is kisspeptin, encoded by the Kiss1 gene [39]. Studies in mice have shown that part of the Kiss1 neurons are dopaminergic [40,41,42]. Consequently, we hypothesize that in our study, the deletion of the B2R in TH-expressing cells might have influenced the activity of Kiss1 neurons in the anteroventral periventricular nucleus/preoptic area (AVPV/PeN) region, where a substantial proportion of neurons coexpress TH, and consequently, modulate GnRH functions in growth and reproduction [42]. This speculation could potentially elucidate the observed decrease in the body weights of THΔB2R mice. However, since the lower body weight in young THΔB2R mice was transitory and not sustained throughout age, we did not extensively investigate this hypothesis. Further research would be essential to unravel the role of the B2R in Kiss1 neurons, paving the way for future avenues of exploration.
The involvement of the B2R in glucose homeostasis has been thoroughly examined through both in vivo and in vitro studies. Existing research, as summarized in the review by Gregnani et al. [43], establishes that B2R signaling enhances the glucose uptake in cells, affecting both adipose tissue and skeletal muscle. Several studies have indicated that changes in the glucose metabolism may affect dopaminergic projections in the midbrain, particularly in relation to obesity and insulin resistance [44,45]. However, here, we did not observe any alterations in the glucose metabolism and energy expenditure in THΔB2R mice of both sexes. This suggests that B2R is not implicated, at least not in dopaminergic neurons and under normal conditions, in the modulation of energy metabolism, and indicates a peripheric role for the B2R in insulin secretion or sensitivity.
B2R is widely distributed in the CNS [14], and the dysfunction of this receptor is implicated in cancer development and cardiovascular and neurological diseases [9]. Consequently, both the structure and function of B2R have become focal points for future medicinal research [10]. Previous studies utilizing the Cre–lox technique have shown alterations independent of a Cre-mediated gene deletion [46,47,48,49]. In our study, the control group did not carry the Cre transgene, so we did not observe differences in motivation for exercise in animals expressing Cre, as all groups exhibited similar voluntary and forced activities.
The comprehension of how dopamine and its receptors modulate eating behaviors and physical activity has been clarified through investigations utilizing D2 receptor KO animal models. Generally, locomotor activity and anxiety-like behavior exhibit a positive correlation with dopamine levels [50]. Previous research has demonstrated that animals lacking D2 receptors, exposed to a high-fat diet and voluntary physical activity, manifested a substantial reduction in their activity levels and an increased susceptibility to obesity. This phenomenon underscores the pivotal role of D2 receptor inactivation in contributing to obesity through alterations in both energy expenditure and physical activity patterns [23]. In our investigation, we specifically examined cells expressing TH, the precursor of dopamine synthesis, with the ablation of the B2R under basal conditions. Our findings revealed no significant alterations in food consumption, and we observed no changes in voluntary and forced physical activities, or in motor coordination tests, across both female and male THΔB2R mice groups.
Dopamine plays a significant role in the control of anxiety by influencing various neurochemical processes and brain circuits, and abnormal dopamine levels can heighten reactivity to stressors, exacerbating anxiety symptoms [51,52]. For example, pharmacological activation/inhibition of dopamine D1 receptor in VTA neurons of mice can alter anxiety-like behavior [53]. The intracerebroventricular (i.c.v) injection of bradykinin into the brains of rats has been shown to elevate anxiety-like behavior while diminishing social interaction [33]. A more expansive investigation conducted in both mice and humans revealed that bradykinin exerts a diverse range of effects on stress responses through B2R-mediated mechanisms, demonstrating antagonistic effects in mice and humans [35].
In our study, we did not detect differences in the outcomes of the open field and elevated plus maze tests, both widely employed for assessing anxiety-related behaviors. Traditionally, an increase in the time spent in the center of the open field and in the open arms, or an augmentation in the number of entries into these areas, are indicators of reduced anxiety-like behavior [54,55]. However, following the deletion of the B2R in cells expressing TH in the SN, the THΔB2R mice exhibited anxiety levels comparable to those observed in the control group.
In summary, our study demonstrates that B2R ablation in cells expressing TH in both female and male mice resulted in transitory alterations in the body weights and body compositions. Importantly, these changes occurred without affecting the glucose metabolism and energy homeostasis. Additionally, there were no observed modifications in the voluntary physical activity, motor coordination, and anxiety levels, indicating a specific impact of B2R ablation in the catecholaminergic cells on body composition without significant systemic metabolic or behavioral alterations. One concern regarding our study is that we did not expose the control and THΔB2R animals to metabolic/behavioral challenges such as a high-fat diet or specific stressors like restraint or food restriction. Future investigations are warranted to comprehensively assess the potential role of B2R signaling in TH-expressing neurons under these conditions.
While previous studies in vitro suggest interactions between kinins, especially the B2R and dopaminergic receptors, further investigations are warranted to fully unravel the physiological significance of kinin actions on TH neurons. Additionally, the involvement of the B1R in the observed results remains unclear and represents an avenue for further exploration. Further research considering these factors will contribute to a more comprehensive understanding of the complex regulatory mechanisms involving the B2R and B1R in TH-expressing neurons.
4. Materials and Methods
4.1. Mice
The present study used 12–14-week-old male and female mice. To induce the ablation of the B2R specifically in catecholaminergic neurons, mice carrying loxP-flanked B2R alleles [13] (here called Bdkrb2flox/flox mice; The Jackson Laboratory, JAX stock #030446) were bred with THcre mice (stock #008601, JAX mice), [26]. THΔB2R mice were homozygous for the loxP-flanked B2R alleles and carried one copy of the Cre transgene, leading to the ablation of the B2R only in catecholaminergic neurons. The control mice were littermates, homozygous for the loxP-flanked B2R alleles without carrying the Cre gene. All mice used in the experiments were genotyped using the tail tip collected during weaning.
4.2. Evaluation of Energy and Glucose Homeostasis
The body weights and body compositions were recorded in mice at 5, 10, and 15 weeks of age. The body composition was analyzed using time-domain nuclear magnetic resonance (LF50 body composition mice analyzer; Bruker, Ettlingen, Germany). Subsequently, mice were individually housed for acclimation. Then, the daily food intake was recorded. For the glucose homeostasis evaluation, mice were fasted for 4 h by removing food from the cage before each test. Basal glucose levels were determined (time 0), mice received i.p. injections of 2 g/kg of glucose or 1 IU/kg of insulin, followed by serial determinations of blood glucose levels using a glucose meter through samples collected from the tail tip.
4.3. Metabolic Parameters
Female and male mice were individually housed in the Comprehensive Lab Animal Monitoring System (Columbus Instruments, Columbus, OH, USA). After a 48 h acclimation period, O2 consumption (VO2), CO2 production (VCO2), respiratory exchange ratio (RER; CO2 production/O2 consumption), and ambulatory activity (by infrared sensors) were determined for 48 h.
4.4. Behavioral Tests
All behavioral tests were performed between 12:00 and 4:00 p.m., except for the voluntary wheel running, which was monitored daily. Experiments were conducted in a sound-proof, temperature-controlled room, with a dimerized light maintained at 65 LUX. After each experiment, the apparatuses were cleaned with 70% ethanol and air dried between each trial to minimize potential confounding odors or residues. Each behavioral test was conducted with a 7-day interval between them. The data obtained were recorded and analyzed using ANY-Maze 7.3 software (Stoelting Co., Wood Dale, IL, USA).
4.4.1. Open Field (OFT) and Elevated Plus Maze Tests (EPM)
To assess anxiety patterns, we performed open field and elevated plus maze tests. For the open field test, mice were placed in an open field arena (40 cm [w] × 40 cm [d] × 30 cm [h]), and the number of entries and the time spent in the center of the arena were recorded for 5 min [56]. For the elevated plus maze test, mice were placed in the center of a maze that was elevated 40 cm from the ground and was composed of two opposite open arms measuring 35 cm × 4 cm, crossed by two closed arms of the same dimensions. The number of entries and the time spent in the open arms were measured for 5 min [56].
4.4.2. Voluntary Wheel Running and Incremental Treadmill Maximal Running Tests
To evaluate the motor activity and aerobic capacity, we submitted animals to voluntary wheel running and incremental treadmill maximal running tests. For the voluntary wheel running test, mice were single housed in standard cages equipped with a running wheel. The running wheels were made available to the mice for voluntary exercise, and access to the running wheel was unlimited throughout the experiment. An acclimation period was provided to the mice to familiarize themselves with the running wheel environment. The running distance and time were monitored daily using digital counters for 7 consecutive days. The amount of running was recorded to assess the voluntary physical activity of the mice.
For the incremental treadmill maximal running test, mice were adapted on the treadmill for 3 consecutive days at 5 m/min speed for 10 min. The speed was chosen to avoid a training effect. After the adaptation period, mice were submitted to an incremental treadmill maximal running test. Starting at 10 m/min, the speed was increased by 3 m/min every 3 min. The test stopped when the animal was incapable of maintaining the running speed for more than 5 s. The time required to reach fatigue and the mean velocity of the test were recorded. From these data, we calculated the average aerobic capacities of the animals [48].
4.4.3. Rotarod Test
The rotarod test assessed the animal’s motor coordination and balance (Insight Equipamentos Ltd., Ribeirão Preto, Brazil). Over a span of two days, mice were placed on a horizontal rod (30 cm [d]) that rotated around its long axis (rpm). On the second day, the duration that the mice spent walking forward on the rotarod until they fell was measured. Initially, the animals underwent 3 training attempts (each lasting 2 min) at 24 h intervals to establish the basal motor activity. Twenty-four hours after the last training attempt, the animals underwent 3 motor test attempts, with a maximum duration of 5 min each and a 30 min interval between attempts. The rotation speed increased in 8 stages (4–38 rpm). The average latency to fall was recorded, and the animals that remained on the rotarod until the maximum time of each attempt received a score of 120 (for training) or 300 (for the test) seconds. The fall latency observed with the rotational cylinder provides valuable insights into the animal’s motor behavior, with a shorter fall latency typically indicating a decrease in motor coordination [57].
4.5. HPLC-Electrochemical Detection and Sample Preparation
The content of monoamines was identified and quantified using an HPLC system with electrochemical detectors previously described by Castro Neto et al. [58]. The substantia nigra (SN) and cortex region were dissected bilaterally, placed on an ice-chilled plate, weighed, and stored at −80 °C until assay. Tissues were ultrasonically homogenized in a 0.1 mol/L solution of HClO4 containing 0.02% Na2S2O2 (15 µL of solution for each milligram of tissue) and dihydroxybenzyl-amine (DHBA, 146.5 ng/mL) as the internal standard for monoamines. The samples were centrifuged at 11,000× g at 4 °C for 40 min, and then the supernatant was filtered and injected into an HPLC system. The monoamines NA, DA, and 5-HT, as well as their metabolites, were quantified as previously described [58]. Briefly, a Shimadzu LC-10AD isocratic system was employed, with a 20 µL injection loop and a Spheri-5 RP-18 5 µm column (220 × 4.6 mm), using electrochemical detection at 0.75 V and a mobile phase composed of phosphate/citrate (pH 2.64, 0.02 mol/L), 0.12 mmol/L ethylene diamine tetraacetic acid, and 0.06% heptane sulphonic acid, in 10% methanol, at a flow rate of 1 mL/min. The concentrations of monoamines and metabolites were expressed as the mean ± SEM ng/mg of wet tissue. Standard concentrations of monoamines and metabolites were tested throughout the runs, and the retention time was verified for each substance to certify that there were no peaks overlapping on sample delivery and were expressed as the mean ± SEM (nmol/L per milligram) of wet tissue.
4.6. qRT-PCR
For each experimental group (n = 6 per group), designated regions from the whole SN were dissected and pooled. Total RNA was isolated from the samples using TRIzol (Invitrogen, Waltham, MA, USA). The quantity and quality of the RNA were assessed using an Epoch microplate spectrophotometer (Biotek, Santa Clara, CA, USA). To remove any potential genomic DNA contamination, the total RNA was incubated with DNase I RNase-free (Roche Applied Science, Penzberg, Germany). For reverse transcription, 2 μg of total RNA was used with SuperScript II Reverse Transcriptase (Invitrogen) and random primers p(dN)6 (Roche Applied Science). Real-time PCR was performed on a 7500TM real-time PCR system (Applied Biosystems, Waltham, MA, USA) using Power SYBR Green (Applied Biosystems) as the fluorescent dye for detection. Relative quantification of mRNA expression was achieved using the 2−∆∆Ct method, with Actb (β-actin) and Ppia (cyclophilin A) as the reference genes. The following primer sets were used for amplification:
Actb (forward: gctccggcatgtgcaaag; reverse: catcacaccctggtgccta), Ppia (forward: cttcttgctggtcttgccattcc; reverse: tatctgcactgccaagactgagt), Bdkrb2 (forward: cctttctgggccatcaccat; reverse: ggtagcggtcgatactcacg), Bdkrb1 (forward: tggagttgaacgttttgggttt; reverse: gtgaggatcagccccatt), and Th (forward: tccaatacaagcagggtgagc; reverse: ggcaggcatgggtagcatag). This qRT-PCR protocol was used to assess the expression levels of specific genes in the SN, which is a crucial brain region involved in the dopaminergic system.
4.7. Statistical Analysis
For comparisons between the two groups, the unpaired, two-tailed Student’s t-test was used. Changes in the glucose and insulin tolerance tests and metabolic parameters were analyzed using repeated measures two-way ANOVA. A post hoc analysis was performed using Bonferroni’s multiple comparisons test to assess the significance of differences between specific groups. The statistical analyses were carried out using GraphPad Prism 8.0 software. A p-value of less than 0.05 was considered statistically significant. The results are presented as the mean ± SEM (standard error of the mean), which provides a measure of the precision of the mean estimate.
Author Contributions
Conceptualization, F.W.; methodology, F.W.; validation, T.M.F. and F.W.; formal analysis, M.R.T. and F.W.; investigation, T.M.F., L.S.N., J.E.P.-S. and J.D.J.; resources, F.W.; data curation, F.W.; writing—original draft preparation, M.R.T. and F.W.; writing—review and editing, T.M.F., M.R.T., L.S.N., C.D.M., J.E.P.-S., R.C.A., J.D.J. and M.B.; visualization, M.R.T. and F.W.; supervision, F.W.; project administration, F.W.; funding acquisition, F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP/Brazil, grant numbers 2019/07005-4 and 2023/01250-2 for F.W.; 2021/09559-7 for T.M.F.; 2023/09878-0 for M.R.T.; and 2020/01318-8 for J.D.J.) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil; finance code 151318/2023-9 for M.R.T.).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee on the Use of Animals of the Institute of Biomedical Sciences at the University of Sao Paulo (protocol number: 4261060721/21) and by the Ethics Committee on the Use of Animals (CEUA—UNIFESP number: 5501010623). The study was conducted according to the guidelines of the Declaration of Helsinki and the ethical guidelines adopted by the Brazilian College of Animal Experimentation.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Ana Maria P. Campos and Juliene Lima S. Silva for their technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bhoola, K.D.; Figueroa, C.D.; Worthy, K. Bioregulation of kinins: Kallikreins, kininogens, and kininases. Pharmacol. Rev. 1992, 44, 1–80. [Google Scholar]
- Dray, A.; Perkins, M. Bradykinin and inflammatory pain. Trends Neurosci. 1993, 16, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Schölkens, B.A. Kinins in the cardiovascular system. Immunopharmacology 1996, 33, 209–216. [Google Scholar] [CrossRef]
- Nsa Allogho, S.; Gobeil, F.; Pheng, L.H.; Nguyen-Le, X.K.; Neugebauer, W.; Regoli, D. Antagonists for kinin B1 and B2 receptors in the mouse. Can. J. Physiol. Pharmacol. 1997, 75, 558–562. [Google Scholar] [CrossRef]
- Asraf, K.; Torika, N.; Danon, A.; Fleisher-Berkovich, S. Involvement of the Bradykinin B(1) Receptor in Microglial Activation: In Vitro and In Vivo Studies. Front. Endocrinol. 2017, 8, 82. [Google Scholar] [CrossRef]
- Dutra, R.C. Kinin receptors: Key regulators of autoimmunity. Autoimmun. Rev. 2017, 16, 192–207. [Google Scholar] [CrossRef]
- Marceau, F.; Bachvarov, D.R. Kinin receptors. Clin. Rev. Allergy Immunol. 1998, 16, 385–401. [Google Scholar] [CrossRef]
- Regoli, D.; Barabé, J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980, 32, 1–46. [Google Scholar]
- Leeb-Lundberg, L.M.; Marceau, F.; Müller-Esterl, W.; Pettibone, D.J.; Zuraw, B.L. International union of pharmacology. XLV. Classification of the kinin receptor family: From molecular mechanisms to pathophysiological consequences. Pharmacol. Rev. 2005, 57, 27–77. [Google Scholar] [CrossRef]
- Shen, J.K.; Zhang, H.T. Function and structure of bradykinin receptor 2 for drug discovery. Acta Pharmacol. Sin. 2023, 44, 489–498. [Google Scholar] [CrossRef]
- Gonçalves, E.C.D.; Vieira, G.; Gonçalves, T.R.; Simões, R.R.; Brusco, I.; Oliveira, S.M.; Calixto, J.B.; Cola, M.; Santos, A.R.S.; Dutra, R.C. Bradykinin Receptors Play a Critical Role in the Chronic Post-ischaemia Pain Model. Cell. Mol. Neurobiol. 2021, 41, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.G.; Gitaí, D.L.; Paçó-Larson, M.L.; Pesquero, J.B.; Garcia-Cairasco, N.; Costa-Neto, C.M. Modulation of B1 and B2 kinin receptors expression levels in the hippocampus of rats after audiogenic kindling and with limbic recruitment, a model of temporal lobe epilepsy. Int. Immunopharmacol. 2008, 8, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Howl, J.; Payne, S.J. Bradykinin receptors as a therapeutic target. Expert. Opin. Ther. Targets 2003, 7, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Emerich, D.F.; Bartus, R.T.; Kordower, J.H. B2 bradykinin receptor immunoreactivity in rat brain. J. Comp. Neurol. 2000, 427, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Shughrue, P.J.; Ky, B.; Austin, C.P. Localization of B1 bradykinin receptor mRNA in the primate brain and spinal cord: An in situ hybridization study. J. Comp. Neurol. 2003, 465, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Scicli, A.G.; Forbes, G.; Nolly, H.; Dujovny, M.; Carretero, O.A. Kallikrein-kinins in the central nervous system. Clin. Exp. Hypertens. A 1984, 6, 1731–1738. [Google Scholar] [CrossRef]
- Calixto, J.B.; Medeiros, R.; Fernandes, E.S.; Ferreira, J.; Cabrini, D.A.; Campos, M.M. Kinin B1 receptors: Key G-protein-coupled receptors and their role in inflammatory and painful processes. Br. J. Pharmacol. 2004, 143, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Lemos, M.T.; Amaral, F.A.; Dong, K.E.; Bittencourt, M.F.; Caetano, A.L.; Pesquero, J.B.; Viel, T.A.; Buck, H.S. Role of kinin B1 and B2 receptors in memory consolidation during the aging process of mice. Neuropeptides 2010, 44, 163–168. [Google Scholar] [CrossRef]
- Trujillo, C.A.; Negraes, P.D.; Schwindt, T.T.; Lameu, C.; Carromeu, C.; Muotri, A.R.; Pesquero, J.B.; Cerqueira, D.M.; Pillat, M.M.; de Souza, H.D.; et al. Kinin-B2 receptor activity determines the differentiation fate of neural stem cells. J. Biol. Chem. 2012, 287, 44046–44061. [Google Scholar] [CrossRef]
- Xia, C.F.; Yin, H.; Yao, Y.Y.; Borlongan, C.V.; Chao, L.; Chao, J. Kallikrein protects against ischemic stroke by inhibiting apoptosis and inflammation and promoting angiogenesis and neurogenesis. Hum. Gene Ther. 2006, 17, 206–219. [Google Scholar] [CrossRef]
- Wasinski, F.; Batista, R.O.; Bader, M.; Araujo, R.C.; Klempin, F. Bradykinin B2 receptor is essential to running-induced cell proliferation in the adult mouse hippocampus. Brain Struct. Funct. 2018, 223, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Cheng, B.; Pan, Y.; Wang, C.; Chen, J.; Bai, B. Neuroprotection of bradykinin/bradykinin B2 receptor system in cerebral ischemia. Biomed. Pharmacother. 2017, 94, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Beeler, J.A.; Faust, R.P.; Turkson, S.; Ye, H.; Zhuang, X. Low Dopamine D2 Receptor Increases Vulnerability to Obesity Via Reduced Physical Activity, Not Increased Appetitive Motivation. Biol. Psychiatry 2016, 79, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Knab, A.M.; Lightfoot, J.T. Does the difference between physically active and couch potato lie in the dopamine system? Int. J. Biol. Sci. 2010, 6, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Niewiarowska-Sendo, A.; Polit, A.; Piwowar, M.; Tworzydło, M.; Kozik, A.; Guevara-Lora, I. Bradykinin B2 and dopamine D2 receptors form a functional dimer. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Wasinski, F.; Pedroso, J.A.B.; Dos Santos, W.O.; Furigo, I.C.; Garcia-Galiano, D.; Elias, C.F.; List, E.O.; Kopchick, J.J.; Szawka, R.E.; Donato, J., Jr. Tyrosine Hydroxylase Neurons Regulate Growth Hormone Secretion via Short-Loop Negative Feedback. J. Neurosci. 2020, 40, 4309–4322. [Google Scholar] [CrossRef]
- Barros, C.C.; Haro, A.; Russo, F.J.; Schadock, I.; Almeida, S.S.; Ribeiro, R.A.; Vanzela, E.C.; Lanzoni, V.P.; Barros, F.C.; Moraes, M.R.; et al. Altered glucose homeostasis and hepatic function in obese mice deficient for both kinin receptor genes. PLoS ONE 2012, 7, e40573. [Google Scholar] [CrossRef]
- Doan, K.V.; Kinyua, A.W.; Yang, D.J.; Ko, C.M.; Moh, S.H.; Shong, K.E.; Kim, H.; Park, S.K.; Kim, D.H.; Kim, I.; et al. FoxO1 in dopaminergic neurons regulates energy homeostasis and targets tyrosine hydroxylase. Nat. Commun. 2016, 7, 12733. [Google Scholar] [CrossRef]
- Fonseca, R.G.; Sales, V.M.; Ropelle, E.; Barros, C.C.; Oyama, L.; Ihara, S.S.; Saad, M.J.; Araújo, R.C.; Pesquero, J.B. Lack of kinin B1 receptor potentiates leptin action in the liver. J. Mol. Med. 2013, 91, 851–860. [Google Scholar] [CrossRef]
- Murray, S.; Tulloch, A.; Gold, M.S.; Avena, N.M. Hormonal and neural mechanisms of food reward, eating behaviour and obesity. Nat. Rev. Endocrinol. 2014, 10, 540–552. [Google Scholar] [CrossRef]
- Reis, F.C.; Haro, A.S.; Bacurau, A.V.; Hirabara, S.M.; Wasinski, F.; Ormanji, M.S.; Moreira, J.B.; Kiyomoto, B.H.; Bertoncini, C.R.; Brum, P.C.; et al. Deletion of Kinin B2 Receptor Alters Muscle Metabolism and Exercise Performance. PLoS ONE 2015, 10, e0134844. [Google Scholar] [CrossRef] [PubMed]
- Morais, R.L.; Silva, E.D.; Sales, V.M.; Filippelli-Silva, R.; Mori, M.A.; Bader, M.; Pesquero, J.B. Kinin B1 and B2 receptor deficiency protects against obesity induced by a high-fat diet and improves glucose tolerance in mice. Diabetes Metab. Syndr. Obes. 2015, 8, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.K.; Mohan Rao, P.J.; Sen, A.P. Anxiogenic activity of intraventricularly administered bradykinin in rats. J. Psychopharmacol. 1995, 9, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Burdin, T.A.; Graeff, F.G.; Pelá, I.R. Opioid mediation of the antiaversive and hyperalgesic actions of bradykinin injected into the dorsal periaqueductal gray of the rat. Physiol. Behav. 1992, 52, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Rouhiainen, A.; Kulesskaya, N.; Mennesson, M.; Misiewicz, Z.; Sipilä, T.; Sokolowska, E.; Trontti, K.; Urpa, L.; McEntegart, W.; Saarnio, S.; et al. The bradykinin system in stress and anxiety in humans and mice. Sci. Rep. 2019, 9, 19437. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.E.; Cirincione, A.B.; Jimenez-Torres, A.C.; Zhu, J. The Impact of Neurotransmitters on the Neurobiology of Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 15340. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004, 5, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Mahesh, V.B.; Bhat, G.K.; Ping, L.; Brann, D.W. Evidence for a role of bradykinin neurons in the control of gonadotropin-releasing hormone secretion. Neuroendocrinology 1998, 67, 209–218. [Google Scholar] [CrossRef]
- Messager, S.; Chatzidaki, E.E.; Ma, D.; Hendrick, A.G.; Zahn, D.; Dixon, J.; Thresher, R.R.; Malinge, I.; Lomet, D.; Carlton, M.B.; et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl. Acad. Sci. USA 2005, 102, 1761–1766. [Google Scholar] [CrossRef]
- Kumar, D.; Candlish, M.; Periasamy, V.; Avcu, N.; Mayer, C.; Boehm, U. Specialized subpopulations of kisspeptin neurons communicate with GnRH neurons in female mice. Endocrinology 2015, 156, 32–38. [Google Scholar] [CrossRef]
- Semaan, S.J.; Murray, E.K.; Poling, M.C.; Dhamija, S.; Forger, N.G.; Kauffman, A.S. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology 2010, 151, 5807–5817. [Google Scholar] [CrossRef] [PubMed]
- Stephens, S.B.Z.; Rouse, M.L.; Tolson, K.P.; Liaw, R.B.; Parra, R.A.; Chahal, N.; Kauffman, A.S. Effects of Selective Deletion of Tyrosine Hydroxylase from Kisspeptin Cells on Puberty and Reproduction in Male and Female Mice. eNeuro 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Gregnani, M.F.; Hungaro, T.G.; Martins-Silva, L.; Bader, M.; Araujo, R.C. Bradykinin B2 Receptor Signaling Increases Glucose Uptake and Oxidation: Evidence and Open Questions. Front. Pharmacol. 2020, 11, 1162. [Google Scholar] [CrossRef] [PubMed]
- Beier, K.T.; Steinberg, E.E.; DeLoach, K.E.; Xie, S.; Miyamichi, K.; Schwarz, L.; Gao, X.J.; Kremer, E.J.; Malenka, R.C.; Luo, L. Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell 2015, 162, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Edwin Thanarajah, S.; Iglesias, S.; Kuzmanovic, B.; Rigoux, L.; Stephan, K.E.; Brüning, J.C.; Tittgemeyer, M. Modulation of midbrain neurocircuitry by intranasal insulin. Neuroimage 2019, 194, 120–127. [Google Scholar] [CrossRef]
- Declercq, J.; Brouwers, B.; Pruniau, V.P.; Stijnen, P.; de Faudeur, G.; Tuand, K.; Meulemans, S.; Serneels, L.; Schraenen, A.; Schuit, F.; et al. Metabolic and Behavioural Phenotypes in Nestin-Cre Mice Are Caused by Hypothalamic Expression of Human Growth Hormone. PLoS ONE 2015, 10, e0135502. [Google Scholar] [CrossRef]
- Morrison, C.D.; Münzberg, H. Capricious Cre: The devil is in the details. Endocrinology 2012, 153, 1005–1007. [Google Scholar] [CrossRef][Green Version]
- Pedroso, J.A.B.; Dos Santos, L.B.P.; Furigo, I.C.; Spagnol, A.R.; Wasinski, F.; List, E.O.; Kopchick, J.J.; Donato, J., Jr. Deletion of growth hormone receptor in hypothalamic neurons affects the adaptation capacity to aerobic exercise. Peptides 2021, 135, 170426. [Google Scholar] [CrossRef]
- Viollet, C.; Simon, A.; Tolle, V.; Labarthe, A.; Grouselle, D.; Loe-Mie, Y.; Simonneau, M.; Martel, G.; Epelbaum, J. Somatostatin-IRES-Cre Mice: Between Knockout and Wild-Type? Front. Endocrinol. 2017, 8, 131. [Google Scholar] [CrossRef]
- Yorgason, J.T.; España, R.A.; Konstantopoulos, J.K.; Weiner, J.L.; Jones, S.R. Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. Eur. J. Neurosci. 2013, 37, 1022–1031. [Google Scholar] [CrossRef]
- Qi, G.; Zhang, P.; Li, T.; Li, M.; Zhang, Q.; He, F.; Zhang, L.; Cai, H.; Lv, X.; Qiao, H.; et al. NAc-VTA circuit underlies emotional stress-induced anxiety-like behavior in the three-chamber vicarious social defeat stress mouse model. Nat. Commun. 2022, 13, 577. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, L.S.; Fadok, J.P.; Argilli, E.; Garelick, M.G.; Jones, G.L.; Dickerson, T.M.; Allen, J.M.; Mizumori, S.J.; Bonci, A.; Palmiter, R.D. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat. Neurosci. 2011, 14, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Cui, X.; Xu, H.; Zhang, X.; Hu, S.; Huang, F.; Xiao, L. D1 receptor-expressing neurons in ventral tegmental area alleviate mouse anxiety-like behaviors via glutamatergic projection to lateral septum. Mol. Psychiatry 2023, 28, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, e52434. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Dos Santos, W.O.; Gusmao, D.O.; Wasinski, F.; List, E.O.; Kopchick, J.J.; Donato, J., Jr. Effects of Growth Hormone Receptor Ablation in Corticotropin-Releasing Hormone Cells. Int. J. Mol. Sci. 2021, 22, 9908. [Google Scholar] [CrossRef]
- Nascimento, A.L.F.; Medeiros, P.O.S.; Pedrão, L.; Queiroz, V.C.; Oliveira, L.M.; Novaes, L.S.; Caetano, A.L.; Munhoz, C.D.; Takakura, A.C.; Falquetto, B. Oxidative Stress Inhibition Via Apocynin Prevents Medullary Respiratory Neurodegeneration and Respiratory Pattern Dysfunction in a 6-Hydroxydopamine Animal Model of Parkinson’s Disease. Neuroscience 2022, 502, 91–106. [Google Scholar] [CrossRef]
- de Castro-Neto, E.F.; da Cunha, R.H.; da Silveira, D.X.; Yonamine, M.; Gouveia, T.L.; Cavalheiro, E.A.; Amado, D.; Naffah-Mazzacoratti Mda, G. Changes in aminoacidergic and monoaminergic neurotransmission in the hippocampus and amygdala of rats after ayahuasca ingestion. World J. Biol. Chem. 2013, 4, 141–147. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).