Abstract
Gestational diabetes mellitus (GDM) is considered the most common metabolic disorder of the pregnancy period. It is characterized by pancreatic beta-cell dysfunction in the setting of chronic insulin resistance. Zinc is a nutrient involved in numerous metabolic processes and shows a relationship with glycometabolic disorders and GDM. The latest data have demonstrated the association of zinc with insulin sensitivity and resistance. The exact role of zinc in the connection with indexes of insulin resistance and insulin sensitivity is still not fully clarified. The aim of the study is to analyze the newly calculated indexes Glu/Zn, Ins/Zn, and HOMA-IR/Zn as surrogate markers to explore the correlation between serum zinc status and some indexes of insulin sensitivity and insulin resistance. The possible role of these indexes as markers of insulin resistance in pregnant women was analyzed too. An ROC analysis demonstrated that HOMA-IR/Zn with AUC 0.989, p < 0.001 (95% CI 0.967–1.000) and Ins/Zn with AUC 0.947, p < 0.001 (95% CI 0.889–1.000) in the GDM group, and only HOMA-IR/Zn index with AUC 0.953, p < 0.001 (95% CI 0.877–1.000) in healthy pregnant women, have good power as markers of insulin resistance in both groups. We speculate that these new ratios could be suitable for the assessment of pregnant women at high risk of insulin resistance development and, probably, for the evaluation of the specific pathophysiologic characteristics of women with GDM.
1. Introduction
Gestational diabetes is defined as a glucose intolerance of variable severity with onset during pregnancy [1]. It is a common disease during pregnancy and reflects a set of endocrine complications arising from the failure in the adaptive physiologic capability of the pancreas. GDM is considered the most common metabolic disorder of the pregnancy period with negative short- and long-term effects on both maternal and fetal health [2,3]. According to the International Association of Diabetes and Pregnancy Study Group (IADPSG) criteria, the global prevalence of GDM was 14.0% (95% confidence interval, CI: 13.97–14.04%) in 2021 and regional prevalence ranges from 7.1–27.6% [4]. Gestational diabetes mellitus is a result of pancreatic beta-cell dysfunction in the setting of chronic insulin resistance during pregnancy and thus both beta-cell impairment and tissue insulin resistance represent the main mechanisms for GDM development [5].
Trace elements support numerous biochemical reactions [6] including those related to insulin and glucose metabolism. Disturbances in the levels of essential elements may lead to the development of GDM by causing disorders in insulin sensitivity, insulin resistance and glucose intolerance [7,8].
Zinc is an essential micronutrient involved in many cellular processes such as protein and DNA synthesis, nucleic acid metabolism, gene transcription [9], cellular replication and differentiation, and hormone regulation [10]. Zinc mediates its metabolic actions via the action of several zinc transporters that regulate zinc homeostasis and control its cellular compartmentalization [11]. This determines that intracellular zinc homeostasis as a whole, and in particular the intracellular presence of zinc-binding proteins like metallothioneins (MTs), are important for normal cell function in various tissues [12].
Zinc plays an important role in the endocrine system. It has been previously shown that there is an association between zinc and both insulin sensitivity and resistance and that zinc has the ability to regulate insulin receptors and directly modulate insulin activity and glucose homeostasis. [13]. Several studies have investigated the relationship of zinc with Type 2 Diabetes Mellitus (T2DM) and glycometabolic disorders, but the conclusions are inconsistent [14,15]. A meta-analysis conducted by Yang et al. detected that zinc supplementation could significantly improve fasting plasma glucose (FPG) and improve HOMA-IR, glycated hemoglobin, and 2 h-plasma glucose [16].
Zinc plays a crucial role in the healthy development of the embryo during pregnancy. Adequate maternal zinc status is very important for the transfer of zinc to the fetus [17]. In the first weeks of pregnancy, zinc is required for cell multiplication and differentiation during the processes of fetal organ formation. The deficiency of zinc, mainly in later pregnancy, adversely impacts normal neuronal replication, migration, synaptogenesis, and gene expressions [18].
There has been recent interest in the complex relationship between trace elements and GDM. Evidence showed the associations between serum zinc, glucose levels, and indexes of insulin resistance and insulin sensitivity during GDM pregnancy using zinc supplements [19,20] and revealed the beneficial effect of zinc on FPG and markers of insulin resistance.
There are few studies examining the potential association between zinc levels and GDM to date. Mishu et al. [21] reported that the maternal serum zinc concentration during pregnancy is associated with GDM incidence. A meta-analysis and systematic review by Fan et al. [22] found that the measurement of zinc and other trace elements during the second trimester of the gestation period helps to distinguish high-risk cases for GDM.
The newly calculated indexes Glu/Zn, Ins/Zn, and HOMA-IR/Zn are presented as surrogate markers of the relationship between serum zinc status, the secretory function of pancreatic beta cells, and the homeostasis model assessment of insulin resistance (HOMA-IR) [23].
The aim of the study is to analyze the correlation between the aforementioned indexes of zinc status, indexes of insulin resistance, and insulin sensitivity in pregnant women with GDM and normal glucose tolerance (NGT) and to assess their possible role as markers of insulin resistance in pregnant women.
2. Results
2.1. Characteristics and Comparison of Pregnant Women with and Without GDM
One hundred and eight pregnant women were included in the study. Diagnosis of GDM was made based on an abnormal 75 g oral glucose tolerance test at 23–28 gestational weeks (gw). Fifty-four pregnant women were diagnosed with GDM and 54 did not have GDM. The general characteristics and comparison of variables for the two groups included in the study are presented in Table 1.

Table 1.
Comparison of characteristics of the study participants.
Compared to women without GDM, pregnant women with GDM were statistically significantly more likely (p < 0.05) to have a family history of diabetes, higher pre-pregnancy BMI at the GDM diagnosis, and higher concentrations of FPG and glucose levels post-1 h and 2h OGTT. Pregnant women with GDM presented significantly higher FSI, HOMA-IR; HOMA-B, HOMA-S, and QUICKI indexes (all p < 0.05). No relevant differences were shown in age, gestational age between groups, and zinc level.
The levels of Glu/Zn, Ins/Zn, and HOMA-IR/Zn were found to be significantly higher for the GDM group in comparison to the NGT group (p < 0.05) as shown in Table 2.

Table 2.
Comparison of ratios in GDM and NGT pregnant groups.
The correlations between Glu/Zn, HOMA-IR/Zn, Ins/Zn indexes, and selected variables of insulin resistance and insulin sensitivity in both studied groups of pregnant women are presented in Table 3 and Table 4.

Table 3.
Correlations of Glu/Zn, HOMA-IR/Zn, Ins/Zn with insulin resistance and sensitivity in GDM pregnant group.

Table 4.
Correlations of Glu/Zn, HOMA-IR/Zn, Ins/Zn with selected indexes of insulin resistance and sensitivity in NGT pregnant group.
In the NGT group, there was a weak negative significant correlation between Glu/Zn and HOMA-S for the GDM cohort. There was a weak negative correlation between Glu/Zn and QUICKI in both groups but with no significance for GDM.
The correlation analyses of HOMA-IR/Zn and Ins/Zn with selected markers of insulin resistance and sensitivity are presented in Figure 1 and Figure 2 separately.
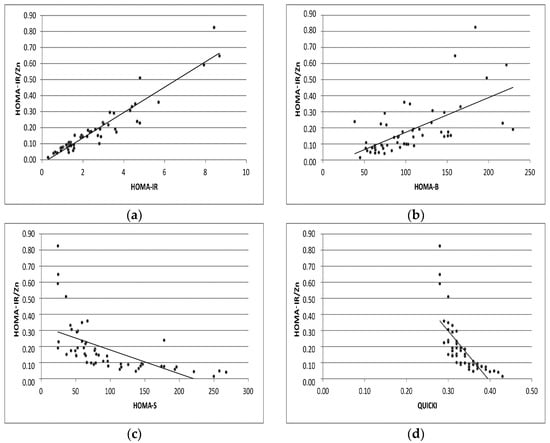
Figure 1.
Correlation analyses of HOMA-IR/Zn with levels of HOMA-IR, HOMA-B, HOMA-S, QUICKI indexes (a–d) in GDM pregnant group.
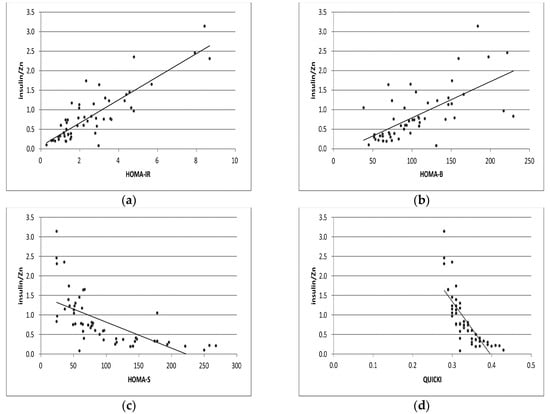
Figure 2.
Correlation analyses of Ins/Zn with levels of HOMA-IR, HOMA-B, HOMA-S, and QUICKI indexes (a–d) in GDM pregnant group, respectively.
2.2. Assessment of the Usefulness of the Examined Indexes Glu/Zn, HOMA-IR/Zn, Ins/Zn as Markers of Insulin Resistance Using ROC Curves
An ROC analysis was carried out to define the potential of Glu/Zn, HOMA-IR/Zn, and Ins/Zn as biomarkers of insulin resistance among pregnant women. This study has shown that HOMA-IR/Zn with AUC 0.989, p < 0.001 (95% CI 0.967–1.000) and Ins/Zn with AUC 0.947, p < 0.001 (95% CI 0.889–1.000) were the only indexes that were statistically significant biomarkers of insulin resistance in the GDM group (Figure 3). Additionally, the differences in AUC values between the two indexes were insignificant (p = 0.4) according to the comparison model suggested by DeLong [24].
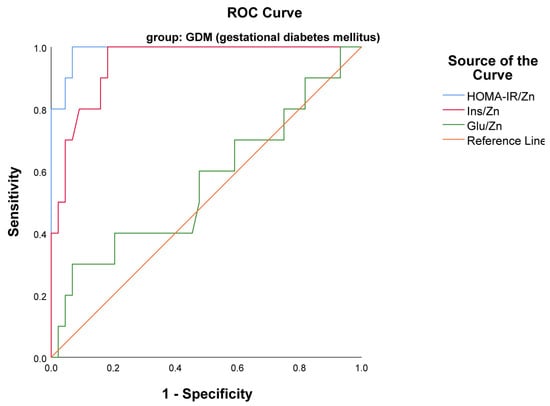
Figure 3.
ROC curves presenting the usefulness of the examined indexes HOMA-IR/Zn and Ins/Zn as markers of insulin resistance in GDM group.
An ROC curve of examined indexes in the NGT group revealed that only the HOMA-IR/Zn index with AUC 0.953, p < 0.001 (95% CI 0.877–1.000) was a statistically significant biomarker of insulin resistance in the NGT group (Figure 4). However, the diagnostic potential of the Glu/Zn index in both groups of pregnant women was not statistically significant (p > 0.05).
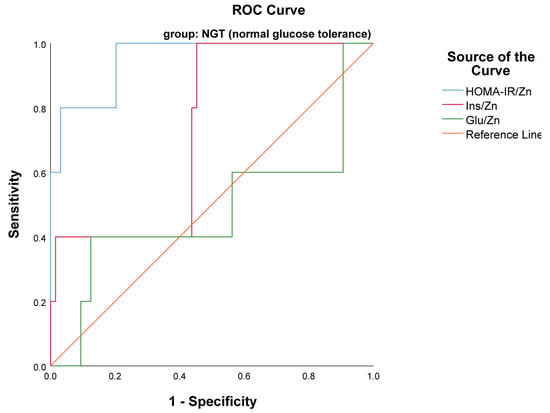
Figure 4.
ROC curve presented HOMA-IR/Zn as a marker of insulin resistance in NGT group.
The optimal cutoff for HOMA-IR/Zn was >0.22 with 93.2% specificity and 100% sensitivity; for Ins/Zn a cutoff of >0.9 yielded 81.8% specificity and 100% sensitivity, respectively, in the GDM group. No threshold of HOMA-IR/Zn was found in the NGT group.
3. Discussion
It is now well known that trace elements may play specific roles in the pathogenesis and progression of diabetes. An increasing number of animal and human studies have demonstrated that several essential elements, including zinc (Zn), iron (Fe), manganese (Mg), copper (Cu), and calcium (Ca), can affect glucose metabolism and insulin sensitivity with downstream effects on hyperglycemia and hence GDM [25,26].
In the current study, the values of serum zinc in GDM pregnant women were not found to be significantly different when compared to the NGT group (p = 0.872). Earlier work by Hamdan et al. [27] and Wilson et al. [28] demonstrated no significant differences in serum zinc levels between pregnant women with GDM and NGT in late pregnancy. Our previous study did not identify statistically significant differences between healthy pregnant women and those with GDM in the levels of plasma and hemolyzate zinc [29]. Both pregnant groups (second trimester of pregnancy) had higher levels of intracellular erythrocyte zinc in comparison to non-pregnant [29]. Various studies have examined serum levels of zinc in GDM pregnant women with inconsistent results. A study by Deng et al. has shown that serum zinc concentrations in GDM pregnant were markedly higher than in the non-GDM group [30]. The authors pointed out that some [21,31] but not all [32] cross-sectional studies were in agreement with their results, in that patients with T2DM or GDM have lower serum zinc levels due to increased urinary excretion. In a systemic review and meta-analysis by Fun et al., the zinc content demonstrated a significant difference between GDM patients and healthy controls in subgroup analysis in the second trimester. The subgroup analysis stratified based on geographical position, showed that circulating zinc levels amongst the Asia population are lower in the GDM cases without significant difference among the Caucasians and Africans [22]. For the second trimester, Liu et al. reported that the zinc level declined with the progression of pregnancy, suggesting that such reduced zinc content was related to the disproportional elevation of plasma volume and the transfer of zinc from the mother to the fetus [33]. Zinc levels are mainly dependent on dietary intake. Moreover, zinc required for multiple general metabolic functions is physiologically provided not by body stores but by the rate of removing zinc pools. It has no body stores. The zinc level is also a result of the existence of slow and rapid removable zinc pools [34]. Not surprisingly, pregnant women in developed countries normally present better zinc status due to dietary zinc consumption as a result of various national dietary interventions [35]. Liu XB et al. [36] found that serum zinc concentration in healthy pregnant women is higher in comparison to the value reported in previous Chinese studies [37], but slightly lower than the results of American [38], European [39,40], and Korean studies [41]. Other factors, such as infection, inflammation, exercise, stress or trauma, circadian variation, and fasting status could affect serum zinc levels [42].
A healthy pancreas contains relatively high levels of zinc with the predominant concentration within the beta cells, where it is co-released with insulin [43]. Zinc has insulin-mimicking properties, promotes glucose uptake in insulin-dependent tissues [44], and stimulates phosphorylation of the β-subunit of the insulin receptor tyrosine kinase, increasing insulin sensitivity [45]. Furthermore, this trace element is mandatory for induction of the translocation of glucose transporter 4 (GLUT4) [46,47,48]. Apart from this, zinc has the function of modulating the transcription of the insulin receptor gene. The process is mediated by zinc finger proteins containing zinc in their conformation. In particular, zinc finger 407 regulates glucose uptake by improving GLUT4 messenger RNA levels and stimulates its transcription [49].
It is now well known that zinc is involved in blood glucose homeostasis and is associated with insulin resistance, and insulin sensitivity through different mechanisms which are not fully understood and elucidated. This study demonstrated the relationship between zinc status and glycemic biomarkers and found that optimal zinc intake may play a role as a modifiable factor in glycemic control and diabetes prevention. However, this needs to be tested and confirmed in a larger study.
A significant association was observed between serum zinc levels, insulin sensitivity, beta-cell function, and insulin resistance in pediatric patients with no significant association of HOMA parameters in a normoglycemia state [50].
Zinc supplementation significantly reduces the levels of FPG, HOMA-IR, insulin, and glycated hemoglobin as established by umbrella systematic review and meta-analysis [51]. The published results of meta-analyses in the literature are controversial. Some display the beneficial effects of zinc on glycemic biomarkers such as FPG, HOMA-IR, and insulin [16,52], but others report a lack of a significant effect on FPG, glycated hemoglobin, HOMA-IR [53], and insulin [54,55,56].
Zinc supplementation can alter glycemic markers in patients with diabetes and in patients at a high risk of developing diabetes. Zinc supplement intake is associated with diminished blood glucose levels and nonenzymatic glycation, thus raising insulin sensitivity [56].
A comparison between normoglycemia, prediabetes, and diabetes groups indicates that higher serum zinc concentration is associated with lower insulin secretion and lower insulin resistance in prediabetic individuals. The higher serum zinc level is associated with ameliorated pancreatic function in the normoglycemic state [57]. Zinc supplementation has a positive effect on FPG in patients with T2DM [58] and significantly lower FPG, HOMA-IR, glycated hemoglobin, and OGTT-2h in overweight and obese population groups [16]. A notable correlation between FPG and OGTT-2h and serum zinc is indicated in T2DM [59] in contrast to other observations about a significant negative correlation between zinc and FPG [60].
A few studies have examined the potential effects of serum zinc levels on glucometabolic parameters, particularly with respect to pregnant women with GDM. A meta-analysis conducted by Li et al. suggests that zinc supplementation results in significantly decreased FPG, insulin, HOMA-IR, and an increased QUICKI index in GDM [52]. Significant negative associations between plasma zinc concentration and OGTT-1h and OGTT-2 h glucose levels in pregnant women with GDM [61] were established as consistent with growing evidence supporting the relation between hyperglycemia and serum zinc status. In contrast, insufficient evidence was accumulated for improved outcomes by zinc supplementation for mothers and newborns [62].
To date, only limited studies have examined the correlation between markers of insulin resistance and insulin sensitivity (HOMA-IR, HOMA-B, QUCKI index), some glycemic markers (glucose and insulin), and serum zinc levels in GDM, with no statistically significant correlations between zinc and parameters, as mentioned above. Notably, the correlations are not adjusted for confounder factors [63,64]. In addition, plasma zinc is not correlated with insulin and HOMA-IR even after controlling for race and age in healthy early-adolescent girls [65].
In this study, we hypothesized that the relationship between serum zinc level and some glucose metabolic indexes will be more significant if this nutrient element is included in new ratios. We also attempted to ‘normalize’ the generally accepted indexes of be-ta-cells secretory function and markers of insulin resistance to serum zinc and found new surrogate biomarkers, namely Glu/Zn, Ins/ Zn, and HOMA-IR/Zn ratios. Furthermore, we speculated that these ratios may act as useful markers of insulin resistance.
Our results have shown a positive correlation between zinc surrogate indexes HOMA-IR/Zn, Ins/Zn, and HOMA-IR and HOMA-B markers, and a significant negative correlation between these indexes and HOMA-S and the QUICKI index (for all p < 0.001) in GDM pregnant women. The outcomes in healthy pregnant women are similar due to the presence of insulin resistance, and slightly lower in comparison to results in the GDM group.
Grâdinaru et al. suggested these new ratios were helpful in the development of preventive and therapeutic strategies among elderly diabetic patients based on the evaluation of serum zinc levels [23]. In the current study, we found that these new, zinc-containing surrogate biomarkers are involved in glucose homeostasis in pregnant women with and without GDM.
4. Materials and Methods
This study is a case–control, single-center study, conducted on pregnant women in the second trimester, between May 2018 and October 2020. All participants were included with signed informed consent. The study is in agreement with the principles expressed in the Declaration of Helsinki [66]. The study was performed with ethical approval from the Medical University Ethics Review Board (Survey code: BK-324/06.03.2018, Ethics approval date: N12/21.03.2018).
The inclusion criteria were as follows: gestational age 23–28 gestational week, singleton pregnancy, and pregnant women older than 18 years. Women were excluded from the survey in cases of pre-pregnancy diabetes; some endocrine, metabolic, and cardiovascular diseases including pre-gestational and gestational hypertension; other cases of chronic diseases (chronic kidney and liver diseases); cases of inflammatory, mental, and infectious diseases; and patients treated with drugs affecting glucose levels. Likewise, women with data for preeclampsia, eclampsia, fetoplacental abnormalities, and a history of previous GDM were excluded. Furthermore, all participants, selected from a cohort of pregnant women, declared a lack of anamnestic data about polycystic ovary syndrome (PCOS). The research study included a group of 54 newly diagnosed pregnant with GDM and a control group of 54 healthy pregnant women after a 2-h OGTT. The mothers’ socio-demographic data, medical history, habits, and behavior factors were recorded in detail, directly employing a structured questionnaire through personal interviews. Variables such as maternal age at recruitment, smoking, and drinking habits, pre-pregnancy height and weight and weight of pregnant women at the diagnosis of GDM, and the gestational week at enrollment were collected by means of a structured questionnaire. The information about all participants in the study was anonymized to preserve their identity.
4.1. Diagnosis of GDM
The total group of 108 pregnant women was required to conduct a 2-h 75 g oral glucose tolerance test with fasting. The interpretation of the OGTT results was in accordance with the criteria of the International Association of Diabetes and Pregnancy Study Groups. Typically, GDM was diagnosed if any of the OGTT results fulfilled at least one of the following criteria: FPG ≥ 5.1 mmol/L; OGTT-1h ≥10.0 mmol/L, and OGTT-2h ≥ 8.5 mmol/L (American Diabetes Association 2021) [2].
4.2. Sample Collection and Measurement
The blood collection was drawn in the morning, between 7 and 9 am in a fasting state. About 5 mL of fasting venous blood was drawn by experienced phlebotomists with a vacutainer with SST gel and 4 mL venous blood with a vacutainer with K2EDTA from each enrolled woman. After that, all participants were given a 300 mL solution of water containing 75 g of glucose and instructed to drink the solution within 5 minutes for the OGTT. Venous blood samples were collected at 60 and 120 minutes. Fasting venous blood samples were centrifuged for 10 min at 3000 rpm to obtain blood serum. The separated serum samples were transferred into metal-free plastic microcentrifuge tubes and stored at −20 °C for about 7 days until zinc analysis. All blood glucose samples were analyzed using the amperometric method for glucose (Analyzer Biosen C-line, Barleben, Germany). FSI concentration in the serum was analyzed by electrochemiluminescence immunoassay (ECLIA). For this purpose, we used a commercially available kit (Roche Diagnostics, Mannheim, Germany) with the automatic analyzer Elecsys 2010 (Roche Diagnostics, Mannheim, Germany). Serum zinc levels were analyzed by flame atomic absorption spectrophotometry (Perkin Elmer AAnalyst 300, Norwalk, CT, USA); CV% day-to-day variation was 5.2% and bias < 1%.
All laboratory analyses fulfill the demands of the standardization and certification program.
4.3. Calculation of Indexes of Interest
Updated HOMA-IR (HOMA2): estimated insulin resistance, pancreatic β cell function (%B), and insulin sensitivity (%S) were obtained using the HOMA2 calculator available at http://www.dtu.ox.ac.uk/homacalculator/index.php (accessed on 8 January 2013) [67].
QUICKI index (quantitative insulin sensitivity check index) = 1/[log10 (FPG [mg/dL]) + log10 (FI [µU/mL])]
Pre-gestational BMI (kg/m2); BMI at the GDM diagnosis weight (kg)/height (m)2, where the data about weight and height were self-reported during the interview.
The indexes of glycemic control and zinc status were calculated as follows:
Glu/Zn ratio, using the FPG and Zn, expressed as mmol/µmol/L
Ins/Zn ratio, using FSI and Zn, expressed as µU/mL/µmol/L
HOMA-IR/Zn ratio, using HOMA-IR individual value and serum zinc level.
4.4. Statistical Analysis
All data were analyzed using IBM Statistical Package for Social Science (SPSS) software for Windows version 26. Results are expressed as frequencies or percentages for qualitative variables and mean (standard deviation) for normally distributed quantitative variables and median and interquartile range (IQR; both 25th and 75th percentile) in not normally distributed ones. For testing the normality of the distribution, the Kolmogorov–Smirnov test (sample size > 50) was used. Comparisons of variables with normal or skewed distribution between the GDM group and the group of healthy pregnant women were performed using the Mann–Whitney or Independent Samples t-test as well as the Pearson chi-squared test. The correlation between Glu/Zn, HOMA-IR/Zn, and Ins/Zn levels and HOMA-IR, HOMA-B, HOMA-S, and QUICKI were analyzed using the Spearman’s rho correlation coefficient. Receiver operating characteristic (ROC) curves and areas under the curve (AUC) were performed to determine the possibility of the indexes Glu/Zn, HOMA-IR/Zn, and Ins/Zn to be used as markers of IR. A p-value less than 0.05 is considered statistically significant. Cutoff values are selected at the maximum Youden index.
The sample size was calculated for two means based on the literature for zinc levels among second-trimester GDM and healthy controls [40]. It resulted in 46 pregnant women in each arm of the study, assuming 95% confidence and 80% power. We added some more cases to compensate for possible drop-outs.
5. Conclusions
Our pilot study was a single-center study conducted among two study groups. It has several limitations that should be mentioned. The major limitation of the current study is the small number of tested pregnant women. The pandemic of COVID-19 infection was a significant reason for the limitation of the participating number. As a result, subgroup analysis was not performed for this study. Moreover, a small sample size limits the statistical power of the study. Other confounding factors, such as age, pre-pregnancy BMI, BMI at GDM diagnosis, and gestational weeks, were not controlled in our study. Other limitations are no follow-up period and the absence of analysis of proteins involved in zinc metabolism such as zinc-α2 glycoprotein and zinc-induced metallothioneins.
Together with the limitations, we may mention that in this study, to our knowledge, we were the first to investigate these ratios in pregnant with GDM and healthy pregnant women.
Undoubtedly, the newly introduced ratios of Ins/Zn, HOMA-IR/Zn, and Glu/Zn need further research in larger research groups. However, as an initial survey, our results could serve as a useful starting point for future investigations, which may expand the benefit of the presented ratios in clinical practice for the diagnosis of insulin resistance. Additionally, they may be used routinely as numerous other markers to evaluate metabolic impairment and insulin resistance in pregnant women.
While these findings need to be confirmed through further larger and expanded studies, this investigation indicates a new potential role of zinc status and its significant associations with indexes of insulin sensitivity and insulin resistance. The results of this study show that these new surrogate biomarkers can be used as laboratory computational indexes in the diagnosis of insulin resistance in pregnant women. We speculate that these new ratios could be suitable for the assessment of pregnant women at high risk of insulin resistance development and, probably, for the evaluation of some specific pathophysiologic characteristics of women with GDM.
The suggested ratios are not definitive for the diagnosis, but they could be clinically beneficial for pregnancy management, especially in the complicated situation of GDM.
Author Contributions
Conceptualization, M.P.G. and B.A.; design of the study, M.P.G., B.A. and I.I.; software, statistical analyses, and data processing, E.N.; validation, accomplished the laboratory data quality control, B.A. and I.I.; clinical data collection; writing—original draft, M.P.G.; interpretation of results, M.P.G., B.A. and E.N.; writing-review editing M.P.G., B.A., I.I. and E.N.; supervision of the study B.A. and E.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Sofia, grant number D-63.
Institutional Review Board Statement
This study was conducted following the Declaration of Helsinki and approved by the Medical University Ethics Review Board of the Medical University of Sofia (Ethics approval number: 45/324/06.03.2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used to support the presented results of this survey are included in the article.
Acknowledgments
The authors acknowledge the kind assistance and support provided by the Central Clinical Laboratory Department, University Hospital “Alexander”; Central Clinical Laboratory Department, University Hospital “St. Ivan Rilski”, and all participants.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- International Diabetes Federation. IDF Diabetes ATLAS 9th Edition 2019. Available online: https://idf.org/about-diabetes/gestational-diabetes (accessed on 17 September 2024).
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2018. Diabetes Care 2018, 41 (Suppl. S1), S13–S27. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A clinical update on gestational diabetes mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Elise Powe, C.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas Committee Hyperglycaemia in Pregnancy Special Interest Group. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef] [PubMed]
- Homko, C.; Sivan, E.; Chen, X.; Reece, E.A.; Boden, G. Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 2001, 86, 568–573. [Google Scholar] [CrossRef]
- Ekmekcioglu, C.; Prohaska, C.; Pomazal, K.; Steffan, I.; Schernthaner, G.; Marktl, W. Concentrations of seven trace elements in different hematological matrices in patients with type 2 diabetes as compared to healthy controls. Biol. Trace Elem. Res. 2001, 79, 205–219. [Google Scholar] [CrossRef]
- Genova, M.; Atanasova, B.; Ivanova, I.; Todorova, K.; Svinarov, D. Trace elements and vitamin D in gestational diabetes. Acta Med. Bulg. 2018, 45, 45–49. [Google Scholar] [CrossRef]
- Li, P.; Yin, J.; Zhu, Y.; Li, S.; Chen, S.; Sun, T.; Shan, Z.; Wang, J.; Shang, Q.; Li, X.; et al. Association between plasma concentration of copper and gestational diabetes mellitus. Clin. Nutr. 2019, 38, 2922–2927. [Google Scholar] [CrossRef]
- Maxfield, L.; Shukla, S.; Crane, J.S. Zinc Deficiency; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK493231 (accessed on 17 September 2024).
- Beyersmann, D.; Haase, H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 2001, 14, 331–341. [Google Scholar] [CrossRef]
- Banaszak, M.; Górna, I.; Przysławski, J. Zinc and the Innovative Zinc-α2-Glycoprotein Adipokine Play an Important Role in Lipid Metabolism: A Critical Review. Nutrients 2021, 13, 2023. [Google Scholar] [CrossRef]
- Tuncay, E.; Bitirim, V.C.; Durak, A.; Carrat, G.R.J.; Taylor, K.M.; Rutter, G.A.; Turan, B. Hyperglycemia-Induced Changes in ZIP7 and ZnT7 Expression Cause Zn2+ Release from the Sarco(endo)plasmic Reticulum and Mediate ER Stress in the Heart. Diabetes 2017, 66, 1346–1358. [Google Scholar] [CrossRef]
- Ortega, R.M.; Rodríguez-Rodríguez, E.; Aparicio, A.; Jiménez, A.I.; López-Sobaler, A.M.; González-Rodríguez, L.G.; Andrés, P. Poor zinc status is associated with increased risk of insulin resistance in Spanish children. Br. J. Nutr. 2012, 107, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cao, J.C.; Warthon-Medina, M.; Moran, H.V.; Arija, V.; Doepking, C.; Serra-Majem, L.; Lowe, N.M. Zinc intake and status and risk of type 2 diabetes mellitus: A systematic review and meta-analysis. Nutrients 2019, 11, 1027. [Google Scholar] [CrossRef] [PubMed]
- Yary, T.; Virtanen, J.K.; Ruusunen, A.; Tuomainen, T.P.; Voutilainen, S. Serum zinc and risk of type 2 diabetes incidence in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. J. Trace Elem. Med. Biol. 2016, 33, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Hung, K.C.; Chuang, M.H.; Chang, R.; Chen, R.Y.; Wang, F.W.; Wu, J.Y.; Chen, J.Y. Effect of zinc supplementation on blood sugar control in the overweight and obese population: A systematic review and meta-analysis of randomized controlled trials. Obes. Res. Clin. Pract. 2023, 17, 308–317. [Google Scholar] [CrossRef] [PubMed]
- King, J.C. Determinants of maternal zinc status during pregnancy. Am. J. Clin. Nutr. 2000, 71 (Suppl. S5), 1334S–1343S. [Google Scholar] [CrossRef]
- Sandstead, H.H. Zinc is essential for brain development and function. J. Trace Elem. Exp. Med. 2003, 16, 165–173. [Google Scholar] [CrossRef]
- Karamali, M.; Bahramimoghadam, S.; Sharifzadeh, F.; Asemi, Z. Magnesium-zinc-calcium-vitamin D co-supplementation improves glycemic control and markers of cardiometabolic risk in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Appl. Physiol. Nutr. Metab. 2018, 43, 565–570. [Google Scholar] [CrossRef]
- Li, D.; Cai, Z.; Pan, Z.; Yang, Y.; Zhang, J. The effects of vitamin and mineral supplementation on women with gestational diabetes mellitus. BMC Endocr. Disord. 2021, 21, 106. [Google Scholar] [CrossRef]
- Mishu, F.A.; Boral, N.; Ferdous, N.; Nahar, S.; Sultana, G.S.; Yesmin, M.S.; Khan, N.Z. Estimation of serum zinc, copper and magnesium levels in bangladeshi women with gestational diabetes mellitus attending in a tertiary care hospital. Mymensingh Med. J. 2019, 28, 157–162. [Google Scholar]
- Fan, J.; Zhang, T.; Yu, Y.; Zhang, B. Is serum zinc status related to gestational diabetes mellitus? A meta-analysis. Matern. Child. Nutr. 2021, 17, e13239. [Google Scholar] [CrossRef]
- Grădinaru, D.; Margină, D.; Ungurianu, A.; Nițulescu, G.; Pena, C.M.; Ionescu-Tîrgoviște, C.; Dănciulescu Miulescu, R. Zinc status, insulin resistance and glycoxidative stress in elderly subjects with type 2 diabetes mellitus. Exp. Ther. Med. 2021, 22, 1393. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of minerals and trace elements in diabetes and insulin resistance. Nutrients 2020, 12, 1864. [Google Scholar] [CrossRef]
- Khan, A.R.; Awan, F.R. Metals in the pathogenesis of type 2 diabetes. J. Diabetes Metab. Disord. 2014, 13, 16. [Google Scholar] [CrossRef]
- Hamdan, H.Z.; Elbashir, L.M.; Hamdan, S.Z.; Elhassan, E.M.; Adam, I. Zinc and selenium levels in women with gestational diabetes mellitus at Medani Hospital, Sudan. J. Obstet. Gynaecol. 2014, 34, 567–570. [Google Scholar] [CrossRef]
- Wilson, R.L.; Grieger, J.A.; Bianco-Miotto, T.; Roberts, C.T. Association between maternal zinc status, dietary zinc intake and pregnancy complications: A systematic review. Nutrients 2016, 8, 641. [Google Scholar] [CrossRef]
- Genova, M.P.; Atanasova, B.; Todorova-Ananieva, K.; Tzatchev, K. Plasma and Intracellular Erythocyte Zinc Levels during Pregnancy in Bulgarian Females with and without Gestational Diabetes. Int. J. Adv. Res. 2014, 2, 661–667. [Google Scholar]
- Deng, G.; Chen, H.; Liu, Y.; Zhou, Y.; Lin, X.; Wei, Y.; Sun, R.; Zhang, Z.; Huang, Z. Combined exposure to multiple essential elements and cadmium at early pregnancy on gestational diabetes mellitus: A prospective cohort study. Front. Nutr. 2023, 10, 1278617. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, M.; Huang, Z.; Sheng, L.; Ge, Y.; Zhang, H.; Jiang, M.; Zhang, G. Elemental contents in serum of pregnant women with gestational diabetes mellitus. Biol. Trace Elem. Res. 2002, 88, 113–118. [Google Scholar] [CrossRef]
- Lewandowska, M.; Więckowska, B.; Sajdak, S.; Lubiński, J. First trimester microelements and their relationships with pregnancy outcomes and complications. Nutrients 2020, 12, 1108. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.; Shi, H.; Shen, C.; Zhou, W.; Dai, Q.; Jiang, Y. Blood copper, zinc, calcium, and magnesium levels during different duration of pregnancy in Chinese. Biol. Trace Elem. Res. 2010, 135, 31–37. [Google Scholar] [CrossRef] [PubMed]
- King, J.C. Zinc: An essential but elusive nutrient. Am. J. Clin. Nutr. 2011, 94, 679S–684S. [Google Scholar] [CrossRef] [PubMed]
- Grieger, J.A.; Grzeskowiak, L.E.; Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Jankovic-Karasoulos, T.; Perkins, A.V.; Norman, R.J.; Dekker, G.A.; Roberts, C.T. Maternal Selenium, Copper and Zinc Concentrations in Early Pregnancy, and the Association with Fertility. Nutrients 2019, 11, 1609. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.B.; Lu, J.X.; Wang, L.J.; Hu, Y.C.; Wang, R.; Mao, D.Q.; Huang, J.; Zhao, L.Y.; Yang, X.G.; Yang, L.C. Evaluation of Serum Zinc Status of Pregnant Women in the China Adult Chronic Disease and Nutrition Surveillance (CACDNS) 2015. Nutrients 2021, 13, 1375. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Piao, J.; Mao, D.; Li, Y.; Li, W.; Yang, L.; Yang, X. Reference Values of 14 Serum Trace Elements for Pregnant Chinese Women: A Cross-Sectional Study in the China Nutrition and Health Survey 2010–2012. Nutrients 2017, 9, 309. [Google Scholar] [CrossRef]
- Watson, C.V.; Lewin, M.; Ragin-Wilson, A.; Jones, R.; Jarrett, J.M.; Wallon, K.; Ward, C.; Hilliard, N.; Irvin-Barnwell, E. Characterization of trace elements exposure in pregnant women in the United States, NHANES 1999–2016. Environ. Res. 2020, 183, 109208. [Google Scholar] [CrossRef]
- Izquierdo Alvarez, S.; Castañón, S.G.; Ruata, M.L.; Aragüés, E.F.; Terraz, P.B.; Irazabal, Y.G.; González, E.G.; Rodríguez, B.G. Updating of normal levels of copper, zinc and selenium in serum of pregnant women. J. Trace Elem. Med. Biol. 2007, 21 (Suppl. S1), 49–52. [Google Scholar] [CrossRef]
- Pop, V.; Krabbe, J.; Maret, W.; Rayman, M. Plasma mineral (selenium, zinc or copper) concentrations in the general pregnant population, adjusted for supplement intake, in relation to thyroid function. Br. J. Nutr. 2021, 125, 71–78. [Google Scholar] [CrossRef]
- Choi, R.; Sun, J.; Yoo, H.; Kim, S.; Cho, Y.Y.; Kim, H.J.; Kim, S.W.; Chung, J.H.; Oh, S.Y.; Lee, S.Y. A prospective study of serum trace elements in healthy Korean pregnant women. Nutrients 2016, 8, 749. [Google Scholar] [CrossRef]
- Moran, V.H.; Skinner, A.L.; Medina, M.W.; Patel, S.; Dykes, F.; Souverein, O.W.; Dullemeijer, C.; Lowe, N.M. The relationship between zinc intake and serum/plasma zinc concentration in pregnant and lactating women: A systematic review with dose-response meta-analyses. J. Trace Elem. Med. Biol. 2012, 26, 74–79. [Google Scholar] [CrossRef]
- Chabosseau, P.; Rutter, G.A. Zinc and diabetes. Arch. Biochem. Biophys. 2016, 611, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Ruz, M.; Carrasco, F.; Rojas, P.; Basfi-Fer, K.; Hernández, M.C.; Pérez, A. Nutritional effects of zinc on metabolic syndrome and type 2 diabetes: Mechanisms and main findings in human studies. Biol. Trace Elem. Res. 2019, 188, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, S.; Adulcikas, J.; Sohal, S.S.; Myers, S. Zinc stimulates glucose oxidation and glycemic control by modulating the insulin signaling pathway in human and mouse skeletal muscle cell lines. PLoS ONE 2018, 13, e0191727. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Hashemipour, M.; Adeli, K.; Tavakoli, N.; Movahedian-Attar, A.; Shapouri, J.; Poursafa, P.; Rouzbahani, A. Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. Metab. Syndr. Relat. Disord. 2010, 8, 505–510. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Pigera, S.; Galappatthy, P.; Katulanda, P.; Constantine, G.R. Zinc and diabetes mellitus: Understanding molecular mechanisms and clinical implications. DARU J. Pharm. Sci. 2015, 23, 44. [Google Scholar] [CrossRef]
- Vardatsikos, G.; Pandey, N.R.; Srivastava, A.K. Insulino-mimetic and anti-diabetic effects of zinc. J. Inorg. Biochem. 2013, 120, 8–17. [Google Scholar] [CrossRef]
- Buchner, D.A.; Charrier, A.; Srinivasan, E.; Wang, L.; Paulsen, M.T.; Ljungman, M.; Bridges, D.; Saltiel, A.R. Zinc finger protein 407 (ZFP407) regulates insulin-stimulated glucose uptake and glucose transporter 4 (Glut4) mRNA. J. Biol. Chem. 2015, 290, 6376–6386. [Google Scholar] [CrossRef]
- Vashum, K.P.; McEvoy, M.; Milton, A.H.; Islam, M.R.; Hancock, S.; Attia, J. Is Serum Zinc Associated with Pancreatic Beta Cell Function and Insulin Sensitivity in Pre-Diabetic and Normal Individuals? Findings from the Hunter Community Study. PLoS ONE 2014, 9, e83944. [Google Scholar] [CrossRef]
- Daneshvar, M.; Ghaheri, M.; Safarzadeh, D.; Karimi, F.; Adib-Hajbagheri, P.; Ahmadzade, M.; Haedi, A. Diabetology Metabolic Syndrome. Diabetol. Metab. Syndr. 2024, 16, 124. [Google Scholar] [CrossRef]
- Li, X.; Zhao, J. The influence of zinc supplementation on metabolic status in gestational diabetes: A meta-analysis of randomized controlled studies. J. Matern. -Fetal Neonatal Med. 2021, 34, 2140–2145. [Google Scholar] [CrossRef]
- Pompano, L.M.; Boy, E. Effects of dose and duration of zinc interventions on risk factors for type 2 diabetes and cardiovascular disease: A systematic review and meta-analysis. Adv. Nutr. 2021, 12, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Khazdouz, M.; Djalalinia, S.; Sarrafi Zadeh, S.; Hasani, M.; Shidfar, F.; Ataie-Jafari, A.; Asayesh, H.; Zarei, M.; Gorabi, A.M.; Noroozi, M.; et al. Effects of zinc supplementation on cardiometabolic risk factors: A systematic review and meta-analysis of randomized controlled trials. Biol. Trace Elem. Res. 2020, 195, 373–398. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Nikbaf-Shandiz, M.; Pashayee-Khamene, F.; Bagheri, R.; Goudarzi, K.; Hosseinnia, N.V.; Dolatshahi, S.; Omran, H.S.; Amirani, N.; Ashtary-Larky, D.; et al. Zinc supplementation in individuals with pre–diabetes and type 2 diabetes: A GRADE-assessed systematic review and dose-response meta-analysis. Biol. Trace Elem. Res. 2023, 202, 2966–2990. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ronsmans, C.; Woolf, B. Triangulating evidence for the causal impact of single-intervention zinc supplement on glycaemic control for type 2 diabetes: Systematic review and meta-analysis of randomised controlled trial and two-sample Mendelian randomisation. Br. J. Nutr. 2023, 129, 1929–1944. [Google Scholar] [CrossRef]
- Kant, R.; Verma, V.; Patel, S.; Chandra, R.; Chaudhary, R.; Shuldiner, A.R.; Munir, K.M. Effect of serum zinc and copper levels on insulin secretion, insulin resistance and pancreatic β cell dysfunction in US adults: Findings from the National Health and Nutrition Examination Survey (NHANES) 2011–2012. Diabetes Res. Clin. Pract. 2021, 172, 108627. [Google Scholar] [CrossRef]
- Wang, X.; Wu, W.; Zheng, W.; Fang, X.; Chen, L.; Rink, L.; Min, J.; Wang, F. Zinc supplementation improves glycemic control for diabetes prevention and management: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2019, 110, 76–90. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, C.; Yang, Z.; Zhang, W.; Niu, Y.; Li, X.; Qin, L.; Su, Q. Alterations of serum trace elements in patients with type 2 diabetes. J. Trace Elem. Med. Biol. 2017, 40, 91–96. [Google Scholar] [CrossRef]
- Eva, H.; Akter, Q.S.; Alam, M.K. Relationship of Serum Glycemic Status with Serum Zinc Level in Type 2 Diabetes Mellitus. Mymensingh Med. J. 2020, 29, 357–360. [Google Scholar]
- Zhu, G.; Zheng, T.; Xia, C.; Qi, L.; Papandonatos, G.D.; Ming, Y.; Zeng, Z.; Zhang, X.; Zhang, H.; Li, Y. Plasma levels of trace element status in early pregnancy and the risk of gestational diabetes mellitus: A nested case-control study. J. Trace Elem. Med. Biol. 2021, 68, 126829. [Google Scholar] [CrossRef]
- Carducci, B.; Keats, E.C.; Bhutta, Z.A. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst. Rev. 2021, 3, CD000230. [Google Scholar] [CrossRef]
- Genova, M.P.; Atanasova, B.; Todorova-Ananieva, K.; Velisarova, M.; Hristova, J.; Tzatchev, K. Zinc and insulin resistance in pregnancy complicated with gestational diabetes. Int. J. Health Sci. Res. 2016, 6, 191–197. [Google Scholar]
- Moghaddam, F.F.; Mehrzad, J.; Saeidi, J.; Ghasemi, A. A Study on the Association of Copper and Zinc Serum Levels with Insulin Resistance Indices in Gestational Diabetes. Int. J. Pharm. Phytopharm. Res. 2020, 10, 231–236. [Google Scholar]
- Lobene, A.J.; Kindler, J.M.; Jenkins, N.T.; Pollock, N.K.; Laing, E.M.; Grider, A.; Lewis, R.D. Zinc Supplementation Does Not Alter Indicators of Insulin Secretion and Sensitivity in Black and White Female Adolescents. J. Nutr. 2017, 147, 1296–1300. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Song, Y.S.; Hwang, Y.C.; Ahn, H.Y.; Park, C.Y. Comparison of the Usefulness of the Updated Homeostasis Model Assessment (HOMA2) with the Original HOMA1 in the Prediction of Type 2 Diabetes Mellitus in Koreans. Diabetes Metab. J. 2016, 40, 318–325. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).