Magnesium Bicarbonate–Walnut Shell Dual-Template Synthesis of Multifunctional Layered Porous Carbon for Enhanced Adsorption of Aqueous Chlorinated Organic Compounds
Abstract
1. Introduction
2. Results and Discussion
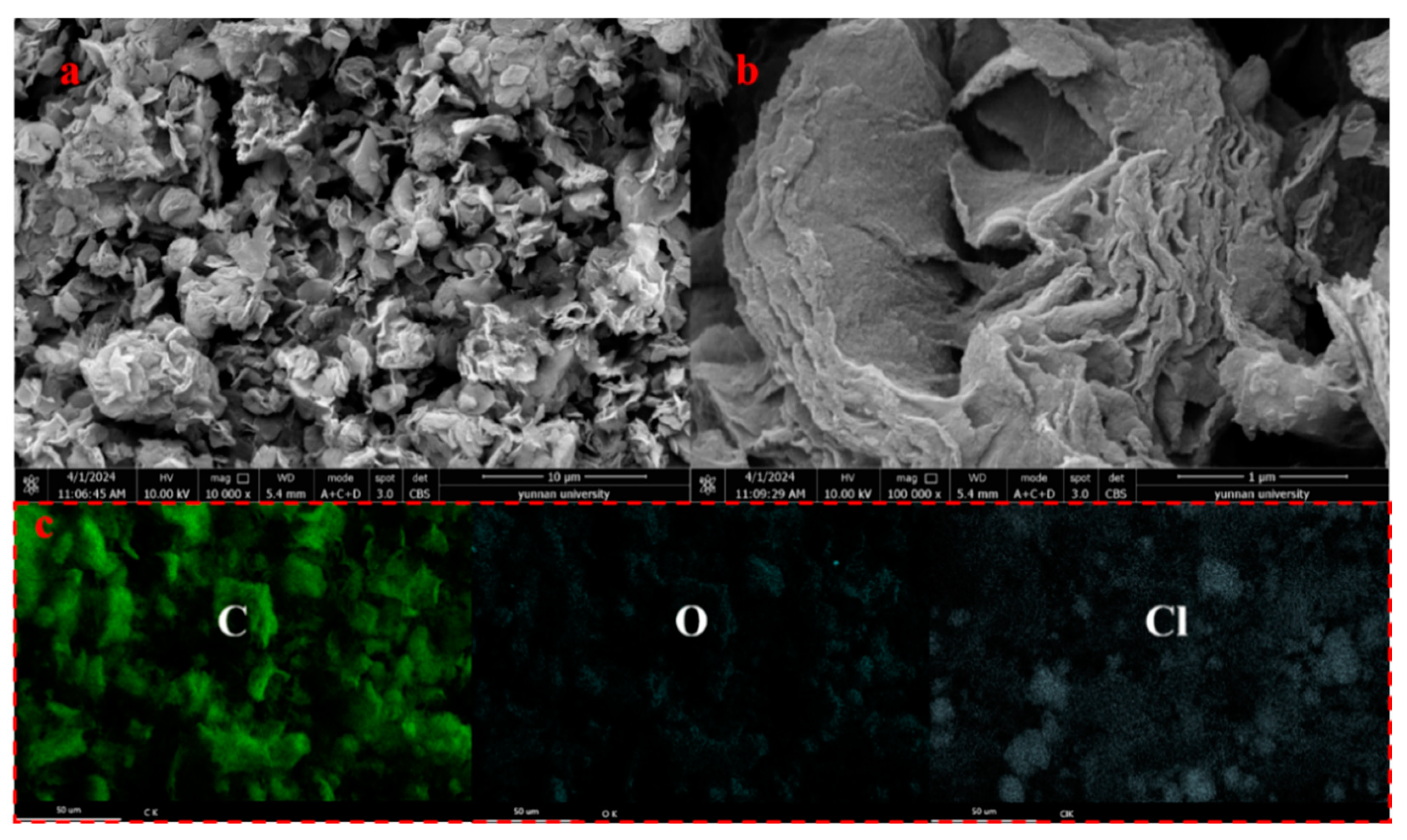
2.1. Characterization
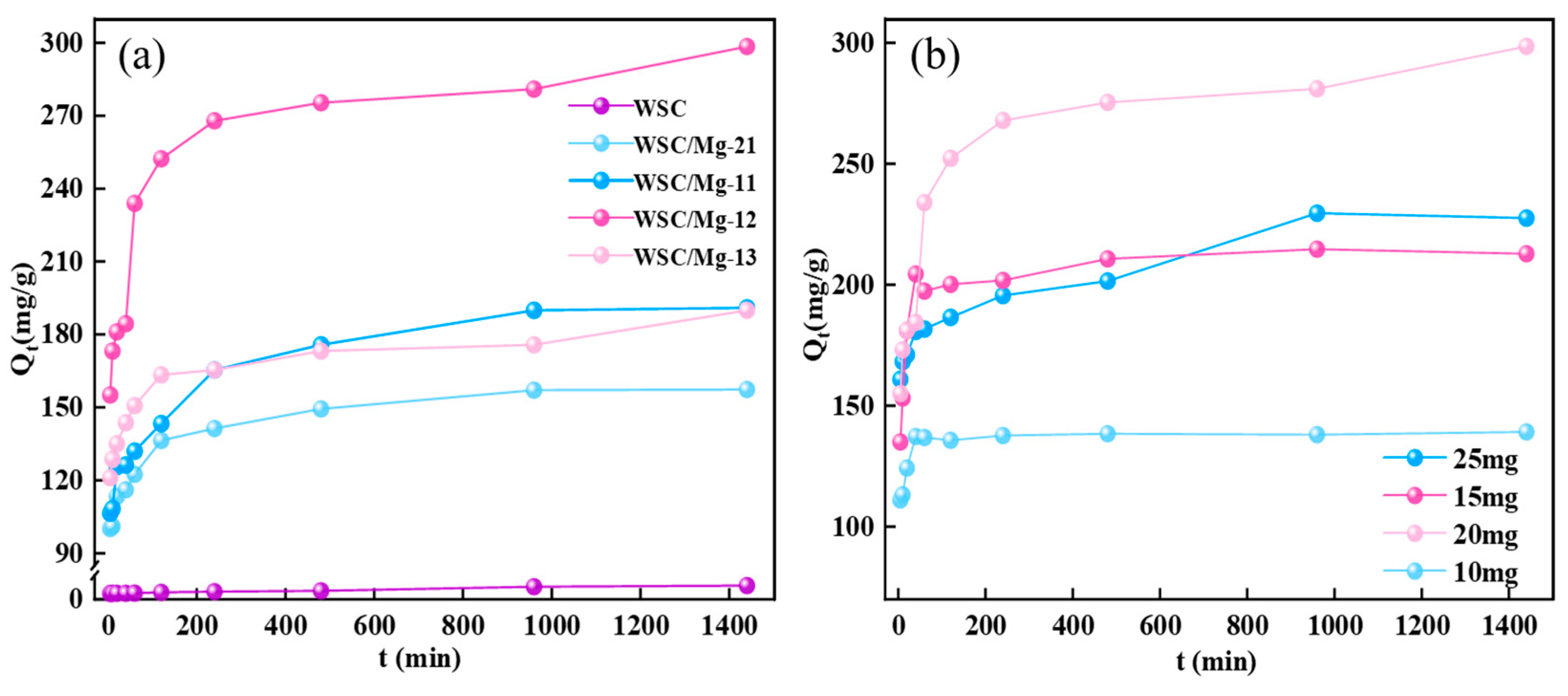
2.2. Effect of Different Materials on Adsorption Capacity
2.3. Adsorption Experiment
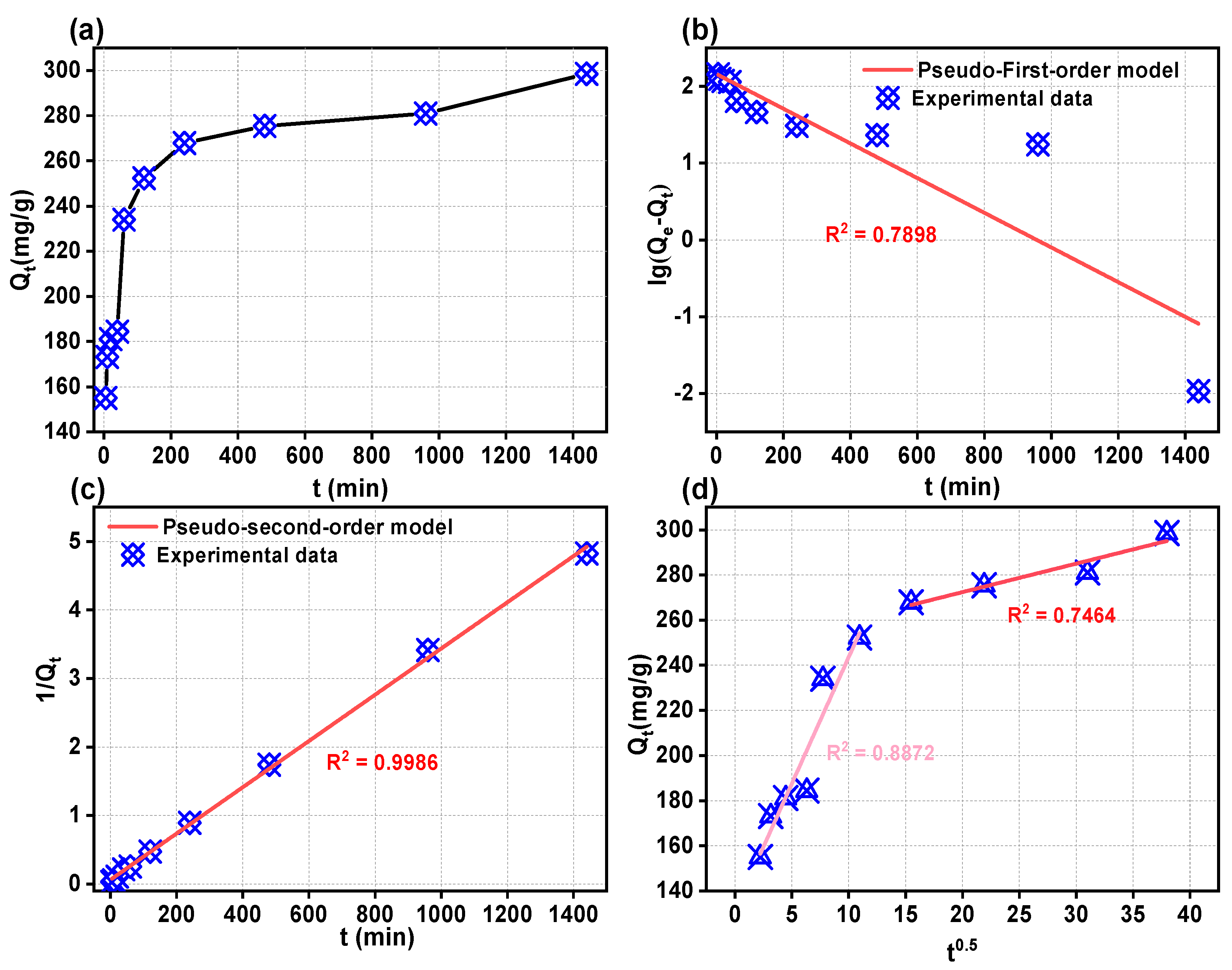
2.3.1. Adsorption Kinetics
2.3.2. Adsorption Isotherms
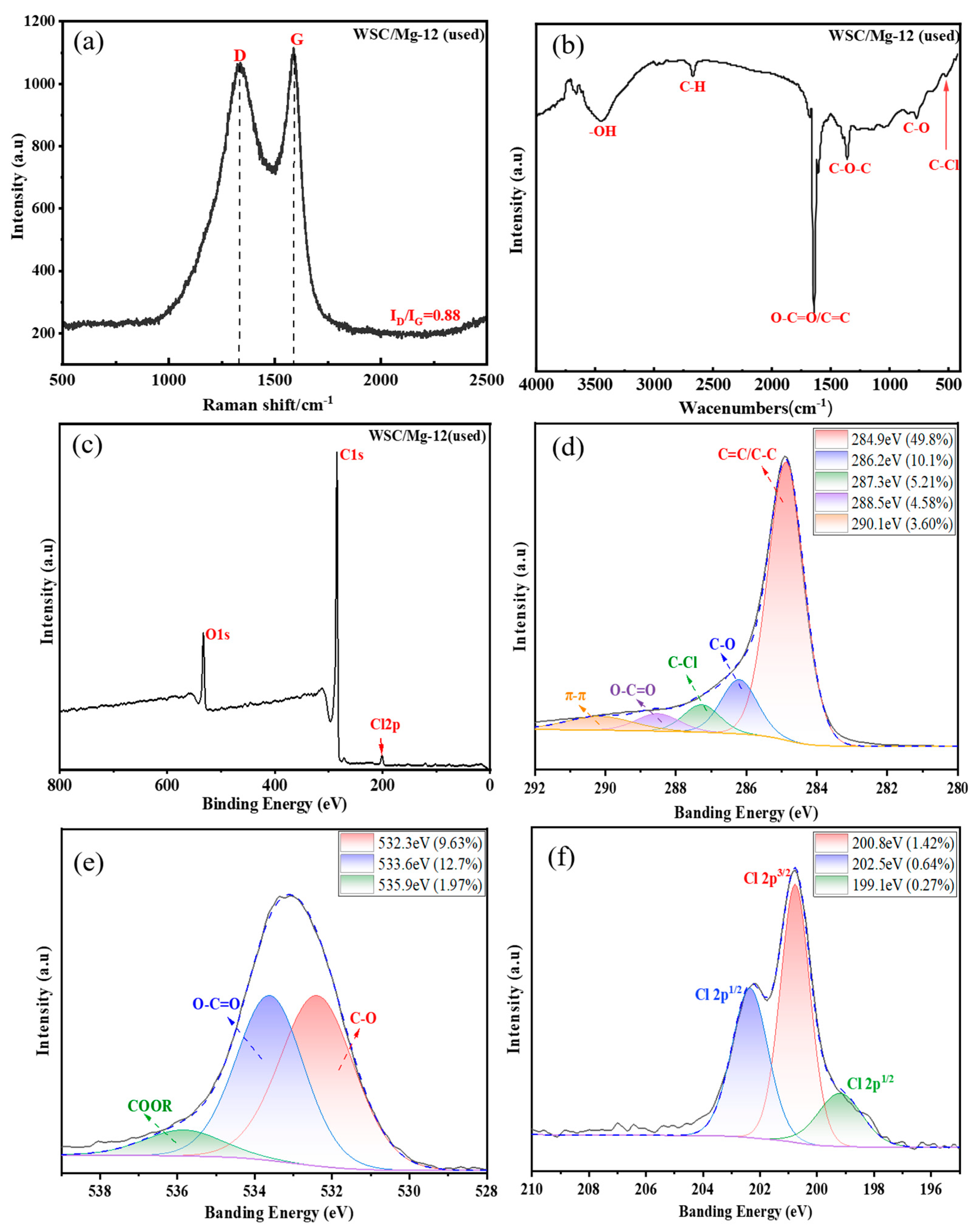
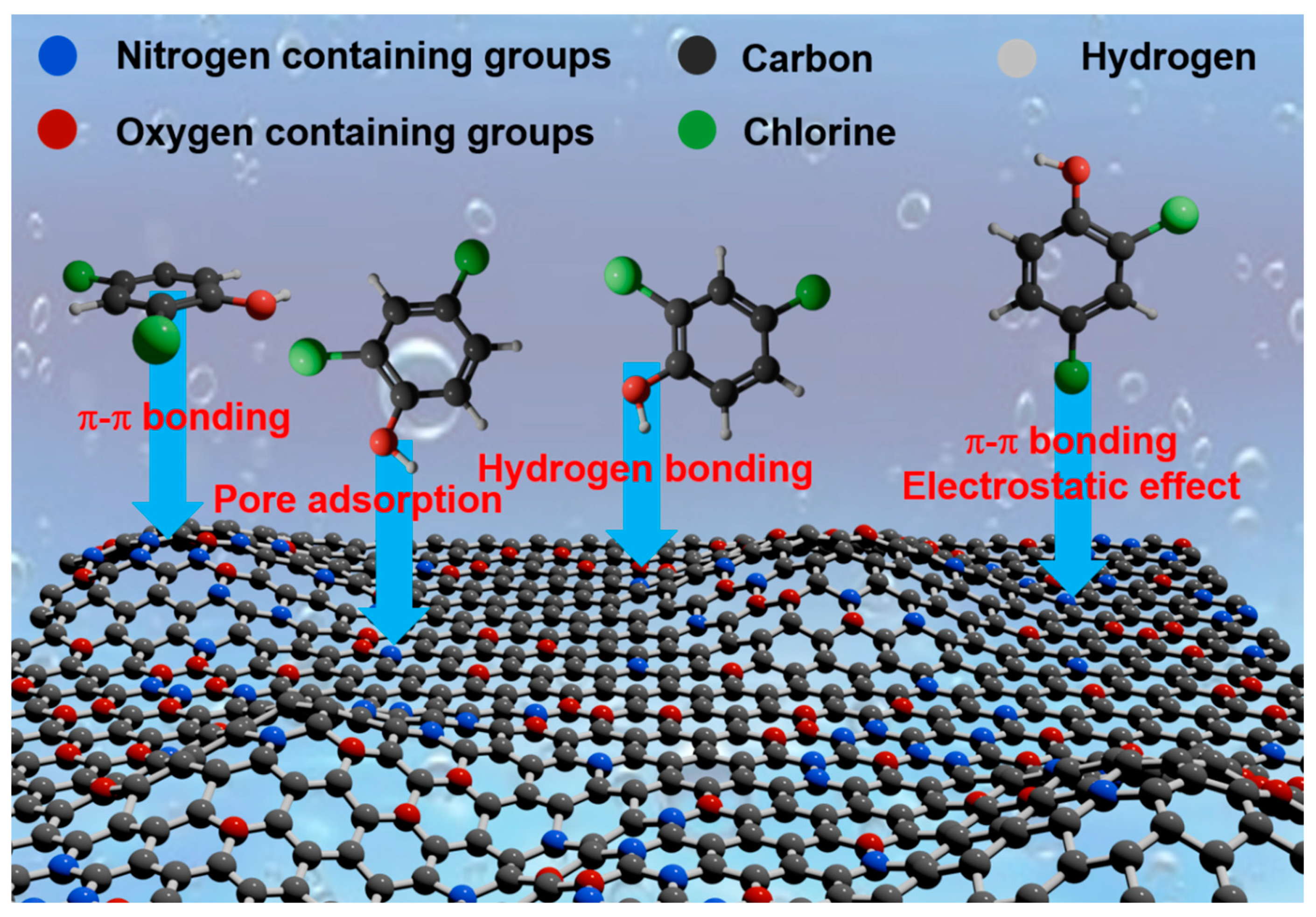
2.3.3. Adsorption Mechanism Analysis
2.4. Anti-Interference Performance
2.5. Recyclability and Applicability Study
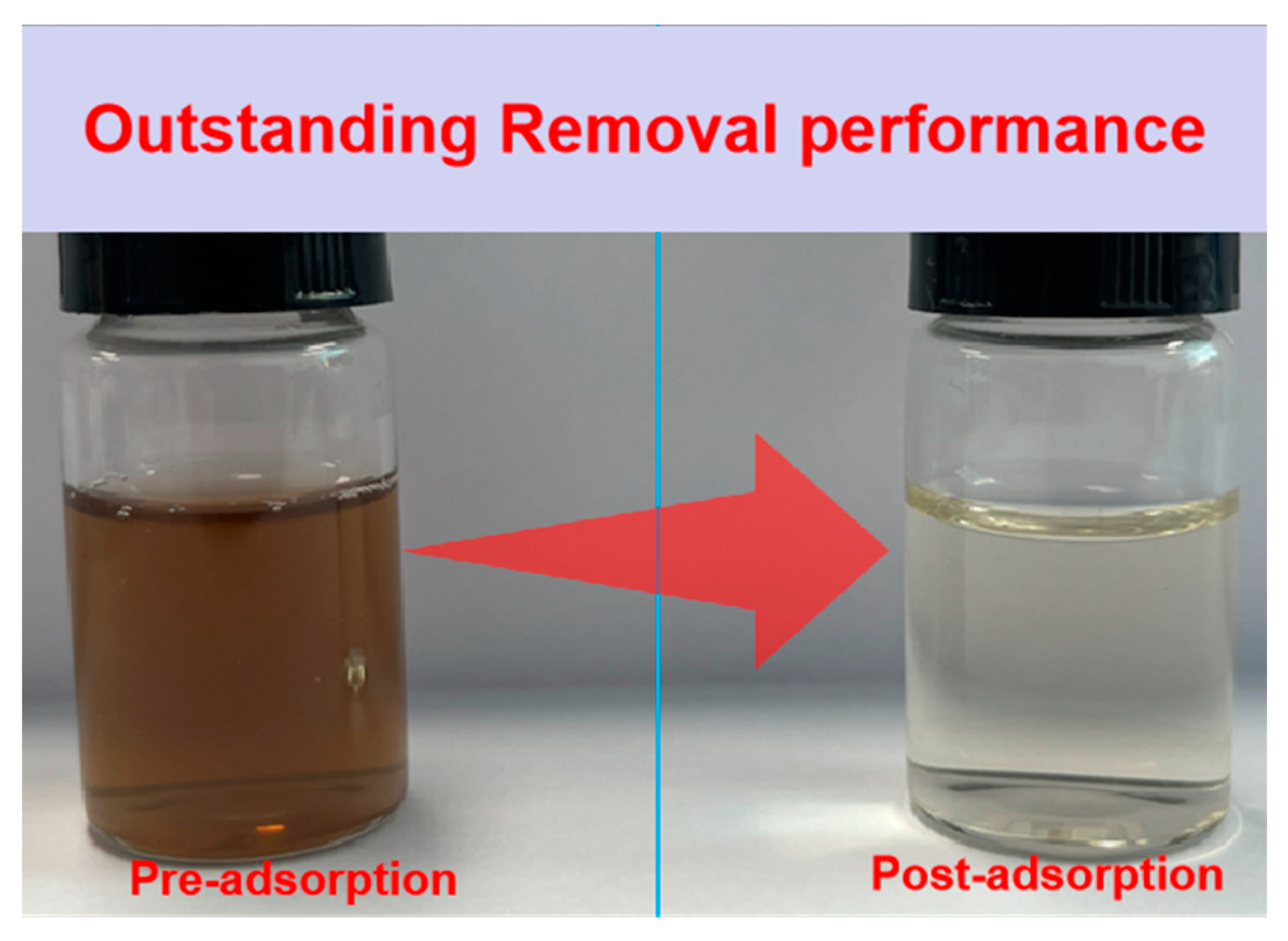
2.6. Multifunctional Performance
3. Materials and Methods
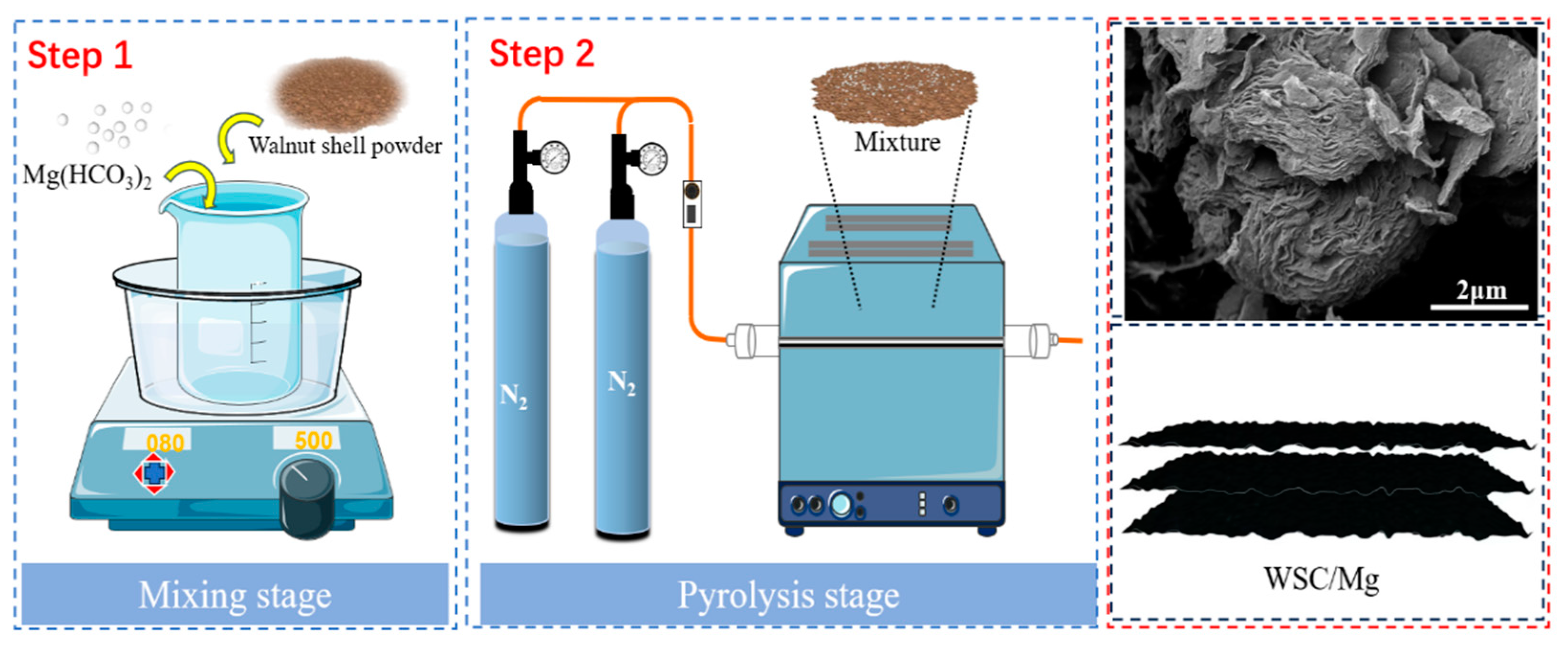
3.1. Synthesis of WSC/Mg
3.2. Adsorption Experiments
3.3. Detection of Environmental Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parveen, N.; Chowdhury, S.; Goel, S. Environmental impacts of the widespread use of chlorine-based disinfectants during the COVID-19 pandemic. Environ. Sci. Pollut. Res. 2022, 29, 85742–85760. [Google Scholar] [CrossRef] [PubMed]
- Kali, S.; Khan, M.; Ghaffar, M.S.; Rasheed, S.; Waseem, A.; Iqbal, M.M.; Khan Niazi, M.B.; Zafar, M.I. Occurrence, influencing factors, toxicity, regulations, and abatement approaches for disinfection by-products in chlorinated drinking water: A comprehensive review. Environ. Pollut. 2021, 281, 116950. [Google Scholar] [CrossRef] [PubMed]
- Gilca, A.F.; Teodosiu, C.; Fiore, S.; Musteret, C.P. Emerging disinfection byproducts: A review on their occurrence and control in drinking water treatment processes. Chemosphere 2020, 259, 127476. [Google Scholar] [CrossRef]
- Sinha, R.; Gupta, A.K.; Ghosal, P. A review on Trihalomethanes and Haloacetic acids in drinking water: Global status, health impact, insights of control and removal technologies. J. Environ. Chem. Eng. 2021, 9, 106511. [Google Scholar] [CrossRef]
- Albolafio, S.; Marín, A.; Allende, A.; García, F.; Simón-Andreu, P.J.; Soler, M.A.; Gil, M.I. Strategies for mitigating chlorinated disinfection byproducts in wastewater treatment plants. Chemosphere 2022, 288, 132583. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.L.; Zhu, J.N.; Li, Z.; Fu, C.Y.; He, G.L.; Lin, Q.F.; Li, C.; Song, S. The occurrence, formation and transformation of disinfection byproducts in the water distribution system: A review. Sci. Total Environ. 2023, 867, 161497. [Google Scholar] [CrossRef] [PubMed]
- Pandian Muthu Kumara, A.; Rajamehala, M.; Vijay Pradhap Singh, M.; Sarojini, G.; Rajamohan, N. Potential risks and approaches to reduce the toxicity of disinfection by-product–A review. Sci. Total Environ. 2022, 822, 153323. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, M.A.; Khan, N.A.; Ahmed, S.; Khan, A.H.; Hussain, A.; Changani, F.; Yousefi, M.; Ahmadi, S.; Vambol, V. Chlorination disinfection by-products in municipal drinking water–a review. J. Clean. Prod. 2020, 273, 123159. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Li, Y.K.; Liu, R.; Chen, Q.; Chu, Q.C. Hierarchical porous zeolitic imidazolate framework-8 supported hollow-fiber liquid-phase microextraction of nine typical phenolic pollutants in water samples followed by electrophoretic analysis. J. Chromatogr. A. 2023, 1706, 464264. [Google Scholar] [CrossRef]
- Wu, M.R.; Liang, Y.M.; Zhang, Y.G.; Xu, H.X.; Liu, W. The effects of biodegradation on the characteristics and disinfection by-products formation of soluble microbial products chemical fractions. Environ. Pollut. 2019, 253, 1047–1055. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Z.C.; Hu, M.; Zhao, J.; Zhou, P.J.; Zhang, Y.J.; Zheng, X.; Zhang, Y.F.; Hu, Z.T.; Pan, Z.Y. Cometabolic biodegradation system employed subculturing photosynthetic bacteria: A new degradation pathway of 4-chlorophenol in hypersaline wastewater. Bioresour. Technol. 2022, 361, 127670. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; He, Z.L.; Cao, W.R.; Zhang, J.; Chen, W.H.; Jin, Q.; Que, W.X.; Wang, S.G. Rational construction of S-scheme BN/MXene/ZnIn2S4 heterojunction with interface engineering for efficient photocatalytic hydrogen production and chlorophenols degradation. Sep. Purif. Technol. 2023, 309, 123004. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, Q.; Ahmad, M.; Dong, S.S.; Zhang, Y.Z.; Fang, D.X.; Wang, X.J.; Peng, H.; Lei, Y.J.; Wu, G.X.; et al. Carbonate mediated hole transfer boosting the photocatalytic degradation of organic pollutants over carbon nitride nanosheets. Sep. Purif. Technol. 2023, 306, 122580. [Google Scholar] [CrossRef]
- Yu, C.L.; Wu, Z.; Liu, R.Y.; Dionysiou, D.; Yang, K.; Wang, C.Y.; Liu, H. Novel fluorinated Bi2MoO6 nanocrystals for efficient photocatalytic removal of water organic pollutants under different light source illumination. Appl. Catal. B Environ. Energy 2017, 209, 1–11. [Google Scholar] [CrossRef]
- Dai, T.Y.; Huang, J.; Han, W.; Chen, Y.W.; Meng, T.Y.; Zhou, W.L.; Xu, Z.; Chen, M.L.; Wen, L.; Cheng, Y.H.; et al. N-doped porous carbon modified by polyacrylic acid for efficient removal of disinfection products in environmental waters under extensive conditions. Sep. Purif. Technol. 2024, 341, 126897. [Google Scholar] [CrossRef]
- Wang, W.Q.; Li, H.; Ma, X.M.; Pan, J.M. Construction of ionic liquid-filled silica shell microcapsules based on emulsion template and evaluation of their adsorption properties toward 3, 4, 5-trichlorophenol after various surface functionalization. Sep. Purif. Technol. 2023, 309, 123020. [Google Scholar] [CrossRef]
- Haydari, I.; Aziz, K.; Kaya, S.; Daştan, T.; Ouazzani, N.; Mandi, L.; Aziz, F. Green synthesis of reduced graphene oxide and their use on column adsorption of phenol from olive mill wastewater. Process Saf. Environ. Prot. 2023, 170, 1079–1091. [Google Scholar] [CrossRef]
- Juela, D.M. Promising adsorptive materials derived from agricultural and industrial wastes for antibiotic removal: A comprehensive review. Sep. Purif. Technol. 2022, 284, 120286. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, R.R.; Bi, H.H.; Lu, Y.H.; Ma, L.B.; He, X.J. A review of porous carbons produced by template methods for supercapacitor applications. New Carbon Mater. 2021, 36, 69–81. [Google Scholar] [CrossRef]
- Pavlenko, V.; Żółtowska, S.; Haruna, A.B.; Zahid, M.; Mansurov, Z.; Supiyeva, Z.; Galal, A.; Ozoemena, K.I.; Abbas, Q.; Jesionowski, T. A comprehensive review of template-assisted porous carbons: Modern preparation methods and advanced applications. Mater. Sci. Eng. R. Rep. 2022, 149, 100682. [Google Scholar] [CrossRef]
- Feng, Z.Y.; Meng, L.Y. Hierarchical porous carbons derived from corncob: Study on adsorption mechanism for gas and wastewater. Carbon Lett. 2021, 31, 643–653. [Google Scholar] [CrossRef]
- Xu, G.W.; Shang, H.R.; Gong, W.T.; Zhang, X.L.; Shan, Y.L.; Ding, J.W.; Yu, W.L. One-pot fabrication of petroleum pitch derived hierarchical porous carbon via a recyclable MgO-templating strategy for p-nitrophenol removal. J. Environ. Chem. Eng. 2022, 10, 108458. [Google Scholar] [CrossRef]
- Chen, G.X.; Hu, Z.W.; Pan, Z.M.; Wang, D.W. Design of honeycomb-like hierarchically porous carbons with engineered mesoporosity for aqueous zinc-ion hybrid supercapacitors applications. J. Energy Storage 2021, 38, 102534. [Google Scholar] [CrossRef]
- Yan, D.Y.; Han, Y.; Ma, Z.H.; Wang, Q.Y.; Wang, X.; Li, Y.; Sun, G.W. Magnesium lignosulfonate-derived N, S co-doped 3D flower-like hierarchically porous carbon as an advanced metal-free electrocatalyst towards oxygen reduction reaction. Int. J. Biol. Macromol. 2022, 209, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Ji, G.Z.; Li, C.J.; Wang, X.X.; Li, A.M. Templating synthesis of hierarchical porous carbon from heavy residue of tire pyrolysis oil for methylene blue removal. Chem. Eng. J. 2020, 390, 124398. [Google Scholar] [CrossRef]
- Ji, Z.; Jia, Y.; Luo, T.; Kong, L.T.; Sun, B.; Shen, W.; Meng, F.L.; Liu, J.H. Efficient removal of fluoride by hierarchical MgO microspheres: Performance and mechanism study. Appl. Surf. Sci. 2015, 357, 1080–1088. [Google Scholar]
- Yan, L.; Wang, X.J.; Wu, B.; Tang, H.J.; Xie, Y.Y. Effect of temperature on decomposition of magnesium bicarbonate. Appl. Mech. Mater. 2013, 423, 488–492. [Google Scholar] [CrossRef]
- Botha, A.; Strydom, C.A. DTA and FT-IR analysis of the rehydration of basic magnesium carbonate. J. Therm. Anal. Calorim. 2003, 71, 987–996. [Google Scholar] [CrossRef]
- Mainali, K.; Mood, S.H.; Pelaez-Samaniego, M.R.; Sierra-Jimenez, V.; Garcia-Perez, M. Production and applications of N-doped carbons from bioresources: A review. Catal. Today 2023, 423, 114248. [Google Scholar] [CrossRef]
- Rafiqul Bari, G.A.K.M.; Kang, H.J.; Lee, T.G.; Hwang, H.J.; An, B.H.; Seo, H.W.; Ko, C.H.; Hong, W.H.; Jun, Y.S. Dual-templating-derived porous carbons for low-pressure CO2 capture. Carbon Lett. 2023, 33, 811–822. [Google Scholar] [CrossRef]
- Li, X.G.; Guan, B.Y.; Gao, S.Y.; David Lou, X.W. A general dual-templating approach to biomass-derived hierarchically porous heteroatom-doped carbon materials for enhanced electrocatalytic oxygen reduction. Energy Environ. Sci. 2019, 12, 648–655. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Li, F.Y.; Zheng, Y.; Cao, Y.N.; Xiao, Y.H.; Liang, S.J.; Liu, F.J.; Jiang, L.L. Dual-template approach to designing nitrogen functionalized, hierarchical porous carbons for efficiently selective capture and separation of SO2. Sep. Purif. Technol. 2022, 284, 120272. [Google Scholar] [CrossRef]
- Zou, W.X.; Gao, B.; Yong, S.O.; Dong, L. Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: A critical review. Chemosphere 2019, 218, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Fahad, S.A.K.; Nabisab, M.M.; Yie, H.T.; Rama, R.K.; Mohammad, K.; Rashmi, W.; Ezzat, C.A.; Sabzoi, N.; Shaukat, A.M. Magnetic nanocomposites for sustainable water purification—A comprehensive review. Environ. Sci. Pollut. Res. 2021, 28, 19563–19588. [Google Scholar] [CrossRef]
- Gusain, R.; Neeraj, K.; Suprakas, S. Ray. Recent advances in carbon nanomaterial-based adsorbents for water purification. Coordin. Chem. Rev. 2020, 405, 213111. [Google Scholar]
- Fordos, S.; Abid, N.; Gulzar, M.; Pasha, I.; Oz, F.; Shahid, A.; Khan, M.K.I.; Khaneghah, A.M.; Aadil, R.M. Recent development in the application of walnut processing by-products (walnut shell and walnut husk). Biomass Convers. Biorefin. 2023, 13, 14389–14411. [Google Scholar] [CrossRef]
- Shi, Q.Y.; Wang, W.B.; Zhang, H.M.; Bai, H.L.; Liu, K.Q.; Zhang, J.F.; Li, Z.H.; Zhu, W.H. Porous biochar derived from walnut shell as an efficient adsorbent for tetracycline removal. Bioresour. Technol. 2023, 383, 129213. [Google Scholar] [CrossRef]
- Intachai, S.; Sumanatrakul, P.; Pankam, P.; Suppaso, C.; Khaorapapong, N. Efficient removal of both anionic and cationic dyes by activated carbon/NiFe-layered double oxide. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1999–2008. [Google Scholar] [CrossRef]
- Machado, L.M.; Lütke, S.F.; Perondi, D.; Godinho, M.; Oliveira, M.L.S.; Collazzo, G.C.; Dott, G.L. Treatment of effluents containing 2-chlorophenol by adsorption onto chemically and physically activated biochars. J. Environ. Chem. Eng. 2020, 8, 104473. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, L.; Chi, T.; Fan, X.; Fang, Y.; Zou, H.B.; Bao, X.L.; Zeng, Y.L. Effect of pyrolytic temperatures on the 2,4-dichlorophenol adsorption performance of biochar derived from Populus nigra. Environ. Sci. Pollut. Res. 2024, 31, 1–11. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; Han, R. Adsorption of chlorophenols on activated pine sawdust-activated carbon from solution in batch mode. Environ. Sci. Pollut. Res. 2023, 30, 31294–31308. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Duan, Z.; You, Q.; Liao, G.; Wang, D. Chitosan modified nitrogen-doped porous carbon composite as a highly-efficient adsorbent for phenolic pollutants removal. Colloids Surf. A Physicochem. Eng. Aspects 2021, 610, 125728. [Google Scholar] [CrossRef]
- Ravi, K.; Singh, M.; Neogi, S.; Grafouté, M.; Biradar, A.V. Hierarchical porous nitrogen-doped carbon supported MgO as an excellent composite for CO2 capture at atmospheric pressure and conversion to value-added products. J. CO2 Util. 2022, 65, 102222. [Google Scholar]
- Chen, Z.; Ma, W.; Lu, G.; Hu, J.L.; Zhang, Z.; Wang, B.D.; Cheng, Z.H.; Pan, Y.Z. Parallel-slipped π−π electron-donor-acceptor in adsorption process: Molecular dynamics simulation. J. Mol. Graph. Model. 2022, 111, 108100. [Google Scholar] [CrossRef] [PubMed]
- Casco, M.E.; Martínez-Escandell, M.; Ilvestre-Albero, J.; Rodríguez-Reinoso, S.F. Effect of the porous structure in carbon materials for CO2 capture at atmospheric and high-pressure. Carbon 2014, 67, 230–235. [Google Scholar] [CrossRef]
- Wang, Y.F.; Li, J.N.; Xu, L.; Wu, D.; Li, Q.N.; Ai, Y.H.; Liu, W.; Li, D.N.; Zhou, Y.T.; Zhang, B.Y.; et al. EDTA functionalized Mg/Al hydroxides modified biochar for Pb (II) and Cd (II) removal: Adsorption performance and mechanism. Sep. Purif. Technol. 2024, 335, 126199. [Google Scholar] [CrossRef]
- Wang, M.; Yan, J.L.; Diao, Y.S.; Zhou, X.Q.; Luo, T.; Wang, H.; Quan, G.X.; Sun, X.Y.; Wang, J. Ball milled Mg/Al hydroxides modified nitrogen-rich biochar for arsenic removal: Performance and governing mechanism. Carbon Res. 2023, 2, 30. [Google Scholar] [CrossRef]
- Ren, X.L.; Yang, L.; Liu, M. Kinetic and thermodynamic studies of acid scarlet 3R adsorption onto low-cost adsorbent developed from sludge and straw. Chin. J. Chem. Eng. 2014, 22, 208–213. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Cheng, Y.Z.; Li, A.W.; Shi, W.; Zhao, L.S. Magnetic chitosan-functionalized waste carton biochar composites for efficient adsorption of anionic and cationic dyes. Chem. Eng. J. 2024, 481, 148535. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Wilson, L.D. Sequestration of agrochemicals from aqueous media using cross-linked chitosan-based sorbents. Adsorption 2016, 22, 1025–1034. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Lowry, G.V.; Cao, Z.; Zhao, H.; Zhou, J.L.; Xu, X.H. Dechlorination mechanism of 2,4-dichlorophenol by magnetic MWCNTs supported Pd/Fe nanohybrids: Rapid adsorption, gradual dechlorination and desorption of phenol. ACS Appl. Mater. Interfaces 2016, 8, 7333–7342. [Google Scholar] [CrossRef]
- Abiko, Y.; Yamada, Y.; Hayasaki, T.; Kimura, Y.; Almarasy, A.A.; Fujimori, A. Adsorption immobilization of biomolecules from subphase on Langmuir monolayers of organo-modified single-walled carbon nanotube. Colloids Surf. A Physicochem. Eng. Aspects 2021, 621, 126559. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, Y.H.; Dai, L.M.; Liu, D.H.; Du, W. Pyrolysis regulation of ZIF-8 to construct a robust multifunctional N-doped macroporous carbon for lipase immobilization. Chem. Eng. J. 2023, 473, 145218. [Google Scholar] [CrossRef]
- Aijaz, A.; Masa, J.; Rosler, C.; Xia, W.; Weide, P.; Botz, A.J.R.; Fischer, R.A.; Schuhmann, W.; Muhler, M. Co@Co3O4 encapsulated in carbon nanotube grafted nitrogen-doped carbon polyhedra as an advanced bifunctional oxygen electrode. Angew. Chem. Int. Ed. 2016, 55, 4087–4091. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.J.; Yu, H.Y.; Wu, J.; Wang, Z.B.; Zhang, S.Q.; Zhang, X.Q.; Gong, X.Y. The study on adsorption behavior of 2,4-DCP in solution by biomass carbon modified with CTAB-KOH. Water Sci. Technol. 2020, 82, 1535–1546. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, W.W.; Sun, D.J.; Li, Y.J.; Wu, T. Hollow nanoshell of layered double oxides for removal of 2,4-dichlorophenol from aqueous solution: Synthesis, characterization, and adsorption performance study. Colloid Surface A 2019, 561, 244–253. [Google Scholar] [CrossRef]
- Batool, S.; Idrees, M.; Ahmad, M.; Ahmad, M.; Hussain, Q.; Iqbal, A.; Kong, J. Design and characterization of a biomass template/SnO2 nanocomposite for enhanced adsorption of 2,4-dichlorophenol. Environ. Res. 2020, 181, 108955. [Google Scholar] [CrossRef]
- Garmia, D.; Zaghouane-Boudiaf, H.; Ibbora, C.V. Preparation and characterization of new low cost adsorbent beads based on activated bentonite encapsulated with calcium alginate for removal of 2,4-dichlorophenol from aqueous medium. Int. J. Biol. Macromol. 2018, 115, 257–265. [Google Scholar] [CrossRef]
- Khedri, D.; Hassani, A.H.; Moniri, E.; Panahi, H.A.; Khaleghian, M. Temperature-responsive graphene oxide/N-isopropylacrylamide/2-allylphenol nanocomposite for the removal of phenol and 2,4-dichlorophenol from aqueous solution. Environ. Sci. Pollut. Res. 2023, 30, 2494–2508. [Google Scholar] [CrossRef]
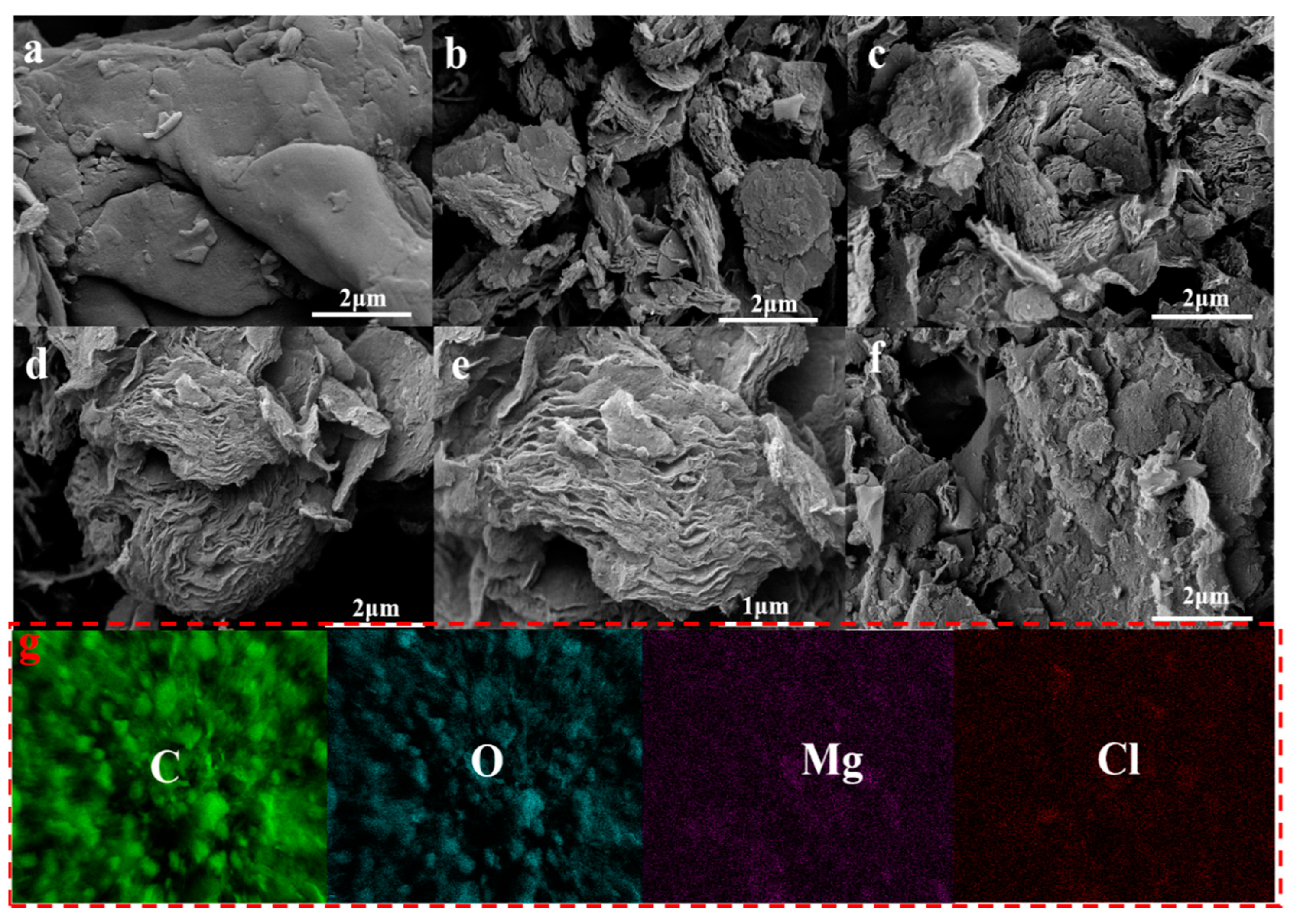
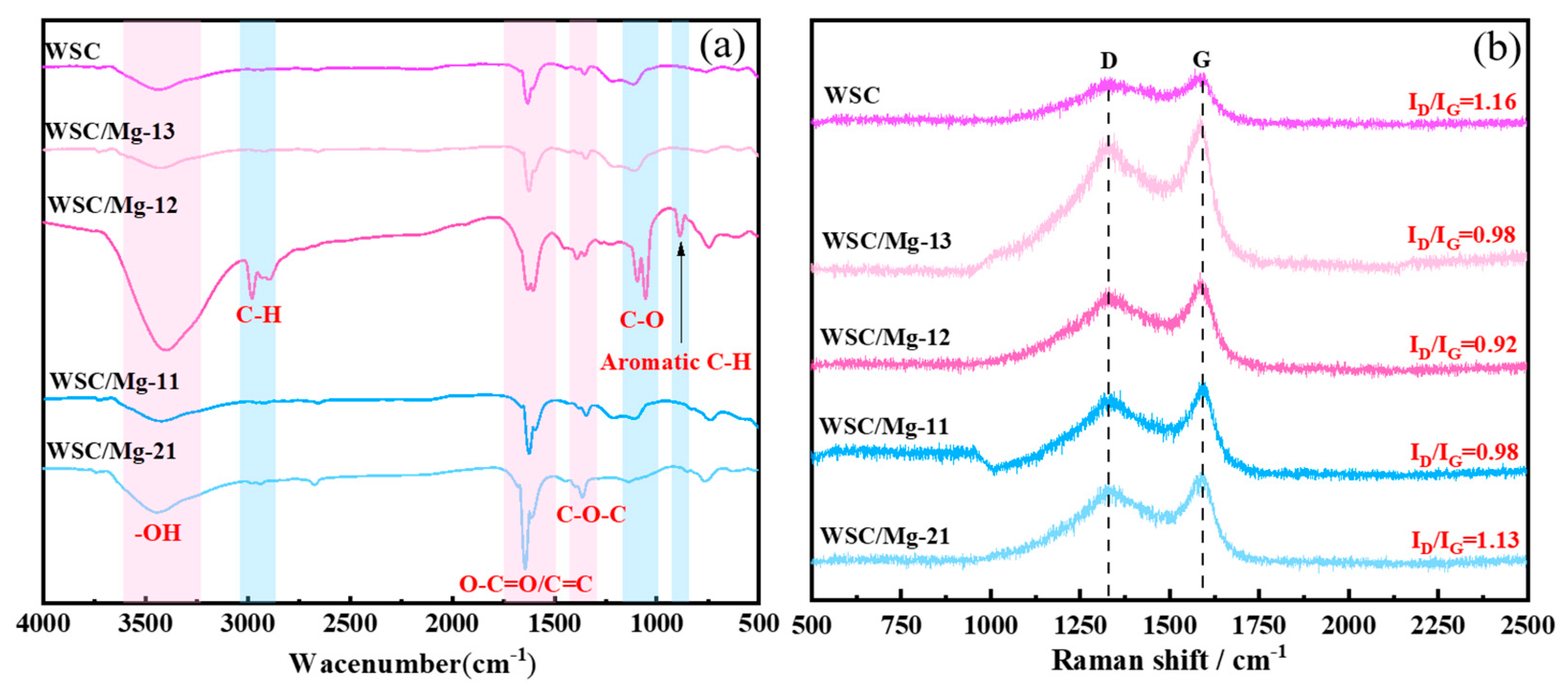
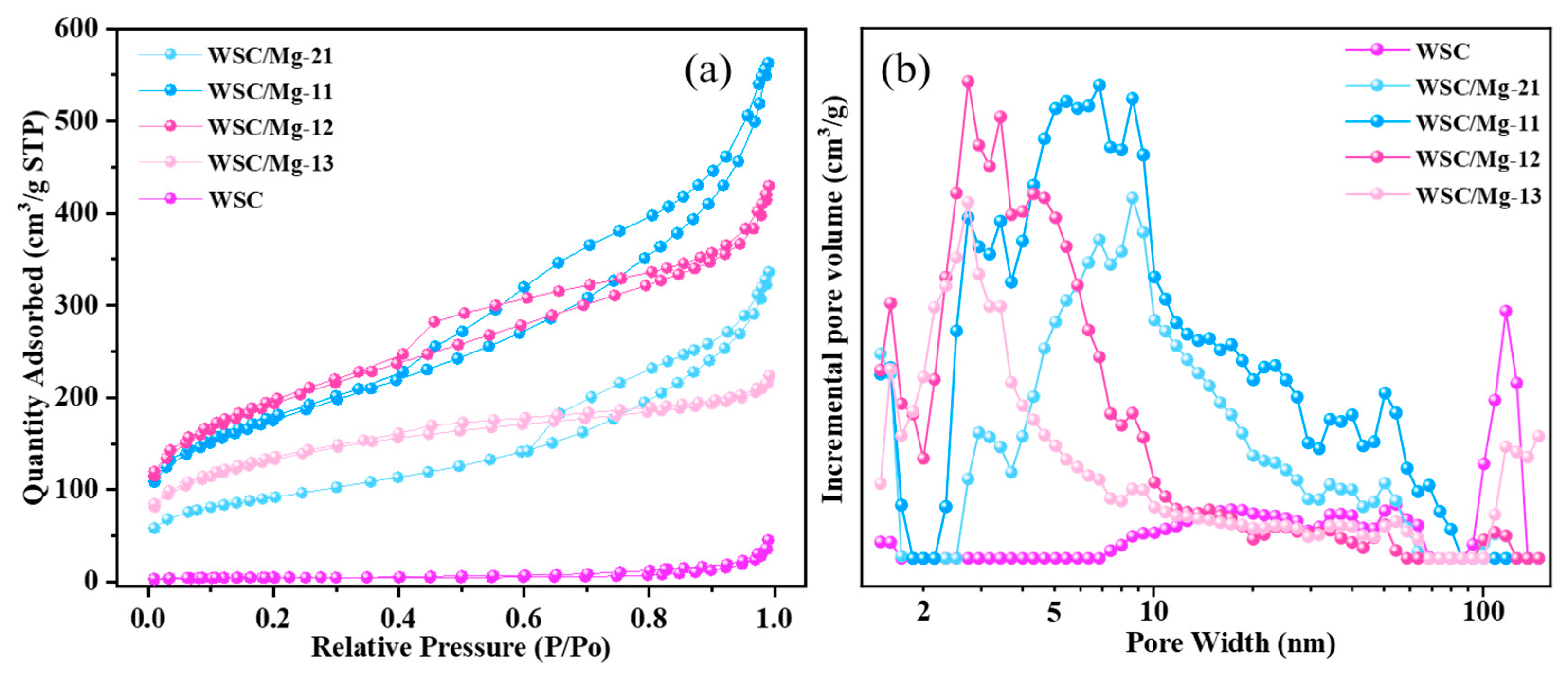
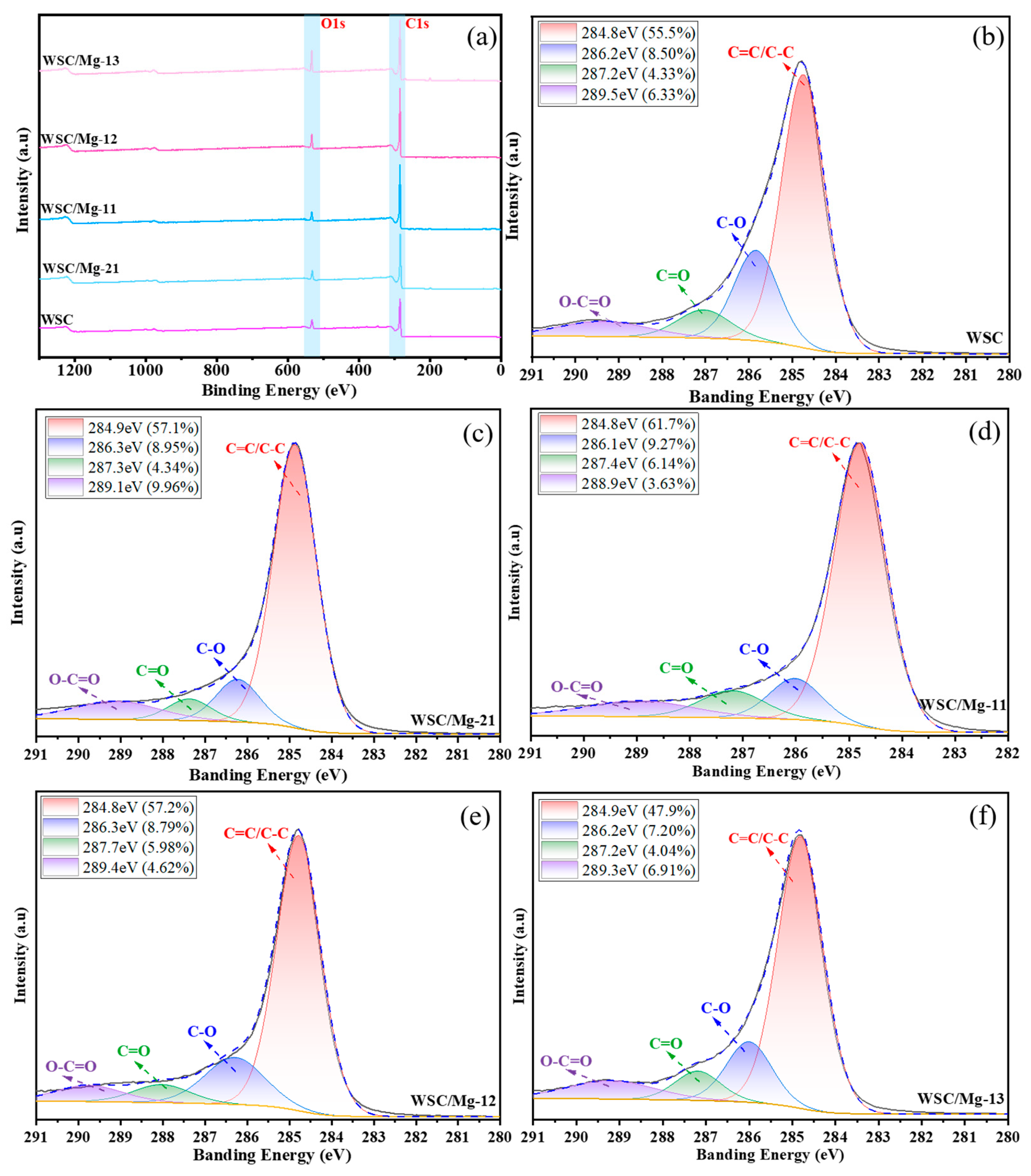



| Adsorbent. | SBET (m2/g) | Smicro (m2/g) | Vtotal (cm3/g) | Vmicro (cm3/g) | BJH Average Pore Diameter (nm) |
|---|---|---|---|---|---|
| WSC | 16.07 | 9.241 | 0.06605 | 0.004161 | 9.335 |
| WSC/Mg-21 | 324.7 | 78.59 | 0.2834 | 0.03477 | 5.538 |
| WSC/Mg-11 | 630.2 | 83.97 | 0.5150 | 0.03295 | 4.901 |
| WSC/Mg-12 | 696.1 | 82.64 | 0.3834 | 0.03054 | 3.408 |
| WSC/Mg-13 | 475.9 | 81.55 | 0.2858 | 0.03250 | 2.671 |
| Attribution | Relative Content (%) | ||||
|---|---|---|---|---|---|
| WSC | WSC/Mg-21 | WSC/Mg-11 | WSC/Mg-12 | WSC/Mg-13 | |
| C−C/C=C | 55.5 | 57.1 | 61.7 | 57.2 | 47.9 |
| C−O | 8.5 | 8.95 | 9.27 | 8.79 | 7.20 |
| C=O | 4.33 | 4.34 | 6.14 | 5.98 | 4.04 |
| O=C−O | 6.33 | 9.96 | 3.63 | 4.62 | 6.91 |
| Attribution | Relative Content (%) | ||||
|---|---|---|---|---|---|
| WSC | WSC/Mg-21 | WSC/Mg-11 | WSC/Mg-12 | WSC/Mg-13 | |
| C=O | 10.2 | - | - | - | - |
| C−O | 7.8 | 8.9 | 8.01 | 5.05 | 16.4 |
| O−C=O | 4.36 | 10.7 | 10.3 | 17.6 | 13.8 |
| Kinetic Model | Parameter | 2,4-DCP |
|---|---|---|
| Pseudo-first order | Qe(exp) (mg/g) | 298.5 |
| k1 (min−1) | −0.0023 | |
| Qe(cal) (mg/g) | 268.1 | |
| R2 | 0.7898 | |
| Pseudo-second order | k2 (g·mg−1·min−1) | 0.0034 |
| Qe(cal) (mg/g) | 296.7 | |
| R2 | 0.9986 | |
| Intra-particle diffusion model | ki1 (g·mg−1·min−1/2) | 8.877 |
| C1 | 142.9 | |
| R12 | 0.8872 | |
| ki2 (g·mg−1·min−1/2) | 1.401 | |
| C2 | 242.6 | |
| R22 | 0.7464 |
| Isotherm Model | Parameter | 2,4-DCP |
|---|---|---|
| Langmuir | Qmax (mg/g) | 610.6 |
| KL (L/mg) | 1.225 | |
| R2 | 0.9941 | |
| RL | 0.0021–0.0161 | |
| Freundlich | KF (mg(1−1/n) L1/ng−1) | 10.29 |
| 1/n | 0.6835 | |
| R2 | 0.9957 | |
| Temkin | B (kJ/mol) | 103.1 |
| KT (mg/L) | 0.1140 | |
| R2 | 0.9570 | |
| Sips | Qms (mg/g) | 1006 |
| Ks (L/mg)m | 0.0019 | |
| ms (g/L) | 0.7721 | |
| R2 | 0.9957 |
| Adsorbent | Adsorption Capacity (mg/g) | pH Range | BET (m2/g) | Ref. |
|---|---|---|---|---|
| NC-1000@PAA | 240 | 3–9 | 1097 | [15] |
| Biomass template/SnO2 nanocomposite | 46.0 | 6 | 19.887 | [58] |
| ODTMA Bent/A4/1 | 392 | 2–6 | - | [59] |
| GO-COOH@ | 117 | 4–7 | 4.383 | [60] |
| LDOs | 566.08 | 6–8 | 176 | [56] |
| MBC | 85.13 | 5–6 | 221.352 | [57] |
| WSC/Mg-12 | 610.6 | 3–9 | 696.1 | This work |
| Performance Indicators | Before Adsorption (mg/g) | After Adsorption (mg/g) | Removal Rate (%) |
|---|---|---|---|
| COD | 2260 | 1819 | 19.5 |
| NH3-N | 447.5 | 378.2 | 15.5 |
| Color intensity | - | - | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Bai, X.; Leng, J.; Lou, Y.; Chen, D.; Jiang, L.; Wang, J. Magnesium Bicarbonate–Walnut Shell Dual-Template Synthesis of Multifunctional Layered Porous Carbon for Enhanced Adsorption of Aqueous Chlorinated Organic Compounds. Int. J. Mol. Sci. 2024, 25, 11761. https://doi.org/10.3390/ijms252111761
Kang J, Bai X, Leng J, Lou Y, Chen D, Jiang L, Wang J. Magnesium Bicarbonate–Walnut Shell Dual-Template Synthesis of Multifunctional Layered Porous Carbon for Enhanced Adsorption of Aqueous Chlorinated Organic Compounds. International Journal of Molecular Sciences. 2024; 25(21):11761. https://doi.org/10.3390/ijms252111761
Chicago/Turabian StyleKang, Juanxue, Xiaoli Bai, Junyang Leng, Yaxuan Lou, Daomei Chen, Liang Jiang, and Jiaqiang Wang. 2024. "Magnesium Bicarbonate–Walnut Shell Dual-Template Synthesis of Multifunctional Layered Porous Carbon for Enhanced Adsorption of Aqueous Chlorinated Organic Compounds" International Journal of Molecular Sciences 25, no. 21: 11761. https://doi.org/10.3390/ijms252111761
APA StyleKang, J., Bai, X., Leng, J., Lou, Y., Chen, D., Jiang, L., & Wang, J. (2024). Magnesium Bicarbonate–Walnut Shell Dual-Template Synthesis of Multifunctional Layered Porous Carbon for Enhanced Adsorption of Aqueous Chlorinated Organic Compounds. International Journal of Molecular Sciences, 25(21), 11761. https://doi.org/10.3390/ijms252111761







