Abstract
Propolis is a sticky substance produced by honeybees (Apis mellifera) through the collection of plant resins, which they mix with secretions from their palate and wax glands. Propolis can inhibit tumor invasion and metastasis, thereby reducing the proliferation of tumor cells and inducing cell apoptosis. Previous research has shown that propolis has an inhibitory effect on skin squamous cell carcinoma A431 cells. Nevertheless, its inhibitory mechanism is unclear because of many significantly different Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways between the ethanol extract of the propolis (EEP) group and the control group of cells. In this study, the main components of EEP and the antitumor mechanism at an IC50 of 29.04 μg/mL EEP were determined via untargeted metabolomics determined using ultra high-performance liquid chromatography tandem mass spectrometry (UHPLC‒MS/MS), respectively. The results revealed 43 polyphenolic components in the EEP and 1052 metabolites, with 160 significantly upregulated and 143 significantly downregulated metabolites between cells treated with EEP and solvent. The KEGG enrichment results revealed that EEP significantly inhibited A431 cell proliferation via the steroid hormone biosynthesis and linoleic acid metabolism pathways. These findings may provide valuable insights for the development of targeted therapies for the treatment of cutaneous squamous cell carcinoma.
1. Introduction
Propolis is a sticky, solid colloid formed by a mixture of plant resins collected by Western worker bees and secretions from their maxillary glands, wax glands, etc. [1]. There are many known types of propolis worldwide, such as Brazilian green propolis (Baccharis dracunculifolia as the main plant source), Brazilian red propolis (Dalbergia ecastophyllum), European propolis (Populus nigra L.), Russian propolis (Betula verrucosa Ehrh), and Cuban and Venezuelan red propolis (Clusia spp.) [2]. Their chemical compositions include more than 800 compounds, which are mainly composed of resin (70%), wax (10%), volatile substances (1%), and other organic compounds, including phenolic compounds, esters, flavonoids, terpenes, beta steroids, aromatic aldehydes, alcohols, vitamins, and minerals [3]. Propolis has a wide range of biological activities, such as antibacterial, anti-inflammatory, antioxidant, antitumor, and immune regulatory activities [3].
The antitumor effect of propolis occurs mainly through the regulation of multiple signaling pathways, which include blocking the tumor cell cycle, inducing autophagy and epigenetic regulation, inhibiting tumor invasion and metastasis, inhibiting cancer cell proliferation, and inducing cell apoptosis. Previous studies have shown that propolis has antitumor activity against a range of human cancer cell lines, including oral cancer [4], gastric cancer [5], cervical cancer [6], colon cancer [7], breast cancer [8], and prostate cancer [9] in vitro. Most of these studies have measured the effects of propolis harvested from different geographical regions on the growth, proliferation, and metastasis of cancer cells in vitro [10]. By studying the gene-suppressing potential of 93 propolis components in breast cancer, new potential bioactive compounds in the ethanol extract of propolis (EEP), 3′,4′,7-trihydroxyisoflavone, and baicalein-7-O-β-D glucopyranoside, can bind to ERα and HSP90 to exert anti-breast cancer effects [11]. The cytotoxicity of nanopropolis to human breast cancer cells is greater than that of propolis [12]. In addition, propolis enhances the antitumor effect of 5-fluorouracil in a dose-dependent manner and reduces the side effects on colorectal cancer through intracellular reactive oxygen species production [13]. Ethanol (70%) extract of Cuban red propolis exhibited antiproliferative and cytotoxic effects on MDA MB-231 cells, probably related to PI3K/Akt and ERK1/2 pathways [14]. Ethanol (95%) extract of Chinese propolis has a dose- and time-dependent cytotoxic effect on both MCF-7 (human breast cancer ER(+)) and MDA-MB-231 (human breast cancer ER(-)) cells by inducing apoptosis, regulating the levels of ANXA7, p53, and NF-κB p65, upregulating intracellular ROS, and decreasing mitochondrial membrane potential [15].
Cutaneous squamous cell carcinoma (CSCC), known as squamous cell carcinoma, is a cancer originating from keratinocytes in the epidermis and appendages. This is the leading cause of death among nonmelanoma skin cancers [16]. Squamous cell carcinoma has become a global problem that endangers the physical and mental health of citizens because of its very high incidence and impact on both physical and mental well-being [17]. In recent years, 15 to 35 people have been diagnosed with CSCC per 100,000 people, and its incidence is increasing at a rate of 2% to 4% per year [18]. Studies have shown that itraconazole inhibits the growth of cutaneous squamous cell carcinoma by targeting the 3-hydroxy-3-methylglutaryl-CoA synthase 1 (HMGCS1) and acyl-CoA synthetase long-chain family member 4 (ACSL4) (HMGCS1/ACSL4) axis, depending on the integrated analysis of transcriptomic and proteomic results [19]. Moreover, dihydroartemisinin also inhibits the proliferation, invasion, and migration and promotes apoptosis of A431 cells. Mechanistically, dihydroartemisinin promotes autophagy and modulates the activation of the melanoma II inflammasome pathway and the NF-κB/HIF-1α/VEGF pathway [20]. The proliferation and migration of the CSCC cell line A431 were inhibited by overexpression of LINC00641 at the cellular level through downregulating the expression of miR-424. The same result was also found in the tumor volume in nude mice [21]. The UV-induced apoptosis and doxorubicin-induced apoptosis of CSCC cell lines were more sensitive when E2F7 was inhibited [22]. The survival rate and metastasis of A431 cells are inhibited when MMP9 in the A431 cell line is inhibited by CRISPR/Cas9-mediated transfection of guide RNA (gRNA), which causes a decrease in viability, migration, and the mRNA expression levels of the oncogenes TGF-β, FGF, PI3K, VEGF-A, and vimentin [23]. Our previous research revealed that EEP inhibited the A431 cell line via extracellular matrix (ECM)-receptor interactions, amoebiasis, cell adhesion molecules (CAMs), nonalcoholic fatty liver disease (NAFLD), retrograde endocannabinoid signaling, and Alzheimer’s disease pathways [24]. However, the potential mechanism was also unclear because of complex protein–protein interactions between the differentially expressed proteins. New strategies are needed to explore the potential antitumor mechanism involved.
Metabolomics is an increasingly powerful research tool in the natural sciences and life sciences that has been widely used to elucidate the effects of internal or external stimuli on biological disturbances. Untargeted metabolomics involves the detection of as many metabolites as possible in a single analysis, holding the potential to identify new biomarkers [25]. There are 34 biomarkers related to urine samples from 25 gastric cancer patients and 17 healthy volunteers identified via untargeted metabolomics, which can provide a basis for the early diagnosis of gastric cancer [26]. A total of 31 potential markers of lung tumor cells were also identified via untargeted metabolomics methods, which provides a new method for the discovery and early detection of lung cancer cell markers [27]. In an untargeted metabolomics study of ovarian cancer SKOV3 cells, B-norcholesterol benzimidazole compounds inhibited the intracellular metabolism and protein synthesis and decreased the energy metabolism of SKOV3 cells. These changes lead to the inhibition of proliferation and signal transduction, the elimination of invasiveness and metastasis, and the induction of cell apoptosis, thereby exerting an antitumor effect [28]. The relevance of specific antitumor mechanisms and metabolites of EEP in A431 cells remains to be elucidated.
To address this knowledge gap, ultra high-performance liquid chromatography tandem mass spectrometry (UHPLC‒MS/MS), a cell counting kit-8 (CCK-8), and untargeted metabolomics assays were employed to further reveal the potential antitumor mechanism of propolis against the proliferation of A431 cells.
2. Results
2.1. Polyphenols of EEP
The UHPLC‒MS/MS ion map of EEP components is shown in Figure 1. There are 43 polyphenolic compounds, the spectra of which are shown in Table 1.
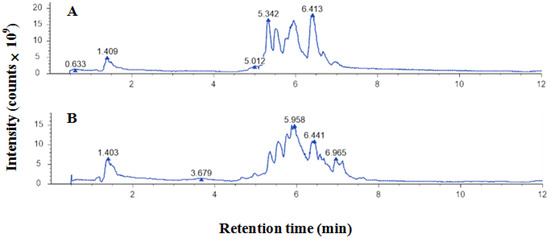
Figure 1.
UHPLC‒MS/MS ion spectrum of ethanol-extracted propolis: (A) negative ions; (B) positive ions.

Table 1.
Polyphenols (flavonoids ID 1–24 and phenols ID 25–43) of ethanol-extracted propolis determined by untargeted metabolomics.
2.2. Inhibitory Effect of EEP on the Proliferation of A431 Cells
EEP inhibited the proliferation of A431 cells in a dose-dependent manner after 48 h of treatment (Figure 2A). The IC50 of EEP in A431 cells was 29.04 μg/mL. The IC50 of 5-fluorouracil in A431 cells was 7.81 μg/mL (Figure 2B).
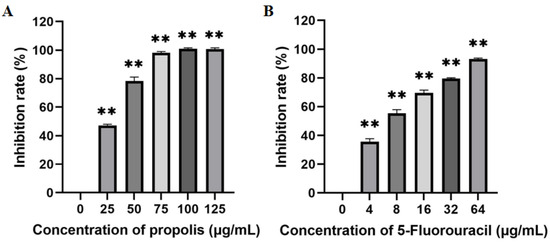
Figure 2.
Inhibitory effects of ethanol-extracted propolis (A) and 5-fluorouracil (B) on the proliferation of A431 cells (there are three biological replicates, each with 6 wells; “**” means significantly different inhibition rates between the treatment group and control group).
2.3. Differential Metabolites of A431 Cells Induced by EEP
Among the positive ion metabolites (Figure 3A,B), organic acids and their derivatives accounted for 27.15%, and lipids and lipid-like molecules accounted for 26.08%. Among the negative ion metabolites (Figure 3C,D), lipids and lipid-like molecules accounted for 40.51%, and organic acids and their derivatives accounted for 20.25%.
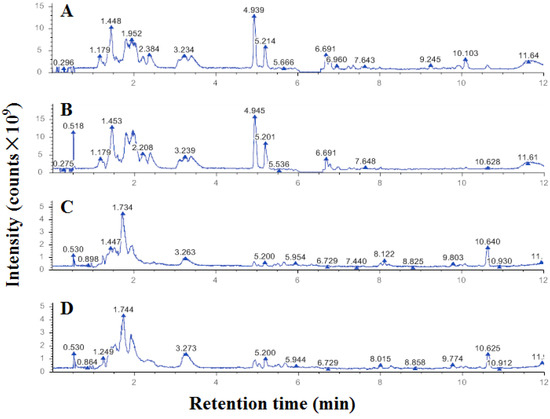
Figure 3.
UHPLC‒MS/MS ion spectra of metabolites from A431 cells treated with ethanol-extracted propolis ((B) positive and (D) negative) and solvent ((A) positive and (C) negative).
A total of 571 metabolites in positive ion mode and 481 metabolites in negative ion mode were identified in this study. There were 160 metabolites in positive ion mode and 143 metabolites in negative ion mode significantly different between A431 cells in the control group and the propolis group, which were screened according to the criteria of VIP > 1.0, FC > 1.5 or FC < 0.667, and p < 0.05 were used (Table 2).

Table 2.
Significantly differentially abundant metabolites between A431 cells in the control group and the ethanol-extracted propolis group (p < 0.05).
The proportions of the level 1 classification of differentially abundant metabolites of A431 cells treated with EEP in positive ion mode were as follows: metabolism, 68.57%; organismal systems, 13.33%; human diseases, 8.57%; environmental information processing, 4.76%; cellular processes, 2.86%; and genetic information processing, 1.90% (Figure 4A). The proportions of the level 1 classification of differentially abundant metabolites in negative ion mode were as follows: metabolism, 63.23%; organismal systems, 14.19%; human diseases, 9.68%; environmental information processing, 7.10%; cellular processes, 3.87%; and genetic information processing, 1.94% (Figure 4B).
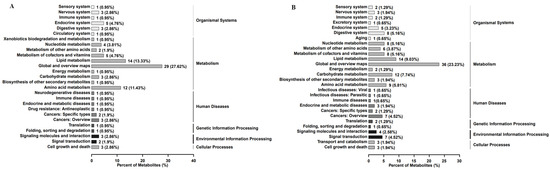
Figure 4.
The proportions of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways enriched with differentially abundant metabolites in A431 cells treated with ethanol-extracted propolis account for the level 1 classification in positive ion mode (A) and negative ion mode (B).
The significantly different pathways (p < 0.05) enriched with differentially abundant metabolites were steroid hormone biosynthesis (both positive and negative ion modes) and linoleic acid metabolism (positive ion mode), the differentially abundant metabolites of which are shown in Table 3.

Table 3.
The significantly different pathways (p < 0.05) enriched the differentially abundant metabolites.
3. Discussion
The polyphenolic components in propolis determined in this study are different from previous reports [24,29]. The difference may be influenced by the extraction procedure, storage, and detection database. Genistein is a flavonoid compound in propolis that can inhibit the growth of prostate cancer cells [30]. Quercetin is a flavonoid in propolis that also induces cell apoptosis [31]. Chrysin, caffeic acid, p-coumaric acid, and ferulic acid are phenolic compounds in propolis that can also induce cell-dependent apoptosis [10,32,33]. These compounds affect the proliferation process of tumor cells.
Propolis has antitumor activity against different cell lines. Brazilian red propolis inhibits the growth of cancer cells. After 24 h of treatment, the IC50 values in Hep-2 cells and HeLa cells were 63.48 ± 3.30 µg/mL and 81.40 ± 6.40 µg/mL, respectively [34]. Iranian Ardabil propolis has a dose-dependent toxic effect on both KB and A431 cells. After 48 h incubation, the IC50 values of the EEP for the KB and A431 cell lines were 40 ± 8.9 µg/mL and 98 µg/mL, respectively [35]. After 24 h of Polish propolis treatment, the IC50 values for tongue cancer cells were approximately 88 µg/mL, 110 µg/mL, and 150 µg/mL [36]. The IC50 values of serial samples of Serbian propolis for the human colon cancer cell line HCT-16 were 26.33~143.09 µg/mL [37]. The IC50 values of propolis from Thailand for A549 cells were 106 ± 0.004 µg/mL, 199 ± 0.009 µg/mL, and 87 ± 0.012 µg/mL, respectively, and their IC50 values for HeLa cells were 81 ± 0.006 µg/mL, 116 ± 0.023 µg/mL, and 54 ± 0.005 µg/mL, respectively [38]. In this study, the IC50 of propolis for A431 cells incubated for 48 h was 29.04 μg/mL, which was similar to the previous result of 39.17 μg/mL [24]. This difference may be caused by the representativeness of the raw propolis samples. These different IC50 values for different tumor cell lines may be related to the cancer cell type, cancer cell concentration, incubation time, plant source of propolis, propolis extraction process, and storage of propolis.
Propolis has an antitumor effect on A431 cells via extracellular matrix (ECM)-receptor interactions, amoebiasis, cell adhesion molecules (CAMs), nonalcoholic fatty liver disease (NAFLD), retrograde endocannabinoid signaling, and Alzheimer’s disease pathways, as determined by label-free proteomics [24]. However, the interactions among the differentially expressed proteins affected the determination of the potential antitumor mechanism against A431 cells. Untargeted metabolomics was employed to estimate the potential mechanism involved in lung cancer cells and ovarian cancer SKOV3 cells after drug treatment [26,27]. In this study, untargeted metabolomics revealed 1052 small-molecule metabolites, of which 160 metabolites were significantly upregulated and 143 metabolites were significantly downregulated. KEGG enrichment analysis revealed that the two pathways of steroid hormone biosynthesis and linoleic acid metabolism were significantly different.
Among the metabolites enriched in positive ion mode, the significantly enriched pathway was steroid hormone biosynthesis, whose key differentially abundant metabolites were cholesterol, androsterone, estrone, dehydroepiandrosterone (DHEA), and corticosterone. Skin cells contain the entire biochemical apparatus required to produce glucocorticoids, androgens, and estrogens, either from systemic precursors or through the conversion of cholesterol to pregnenolone, which is subsequently converted into biologically active steroids. The differentially abundant metabolite production of this series of steroids by A431 skin cell carcinoma in response to propolis is consistent with this result [39]. Cholesterol is a metabolite associated with multiple biological functions that play complex roles in supporting cancer progression and suppressing immune responses. Preclinical and clinical studies have shown that controlling cholesterol metabolism can inhibit tumor growth, reshape the immune landscape, and enhance antitumor immunity [40]. In our previous study [24], propolis inhibited A431 proliferation by downregulating proteins related to the nonalcoholic fatty liver disease (NAFLD) pathway, which requires activation of the transcription factor sterol regulatory binding protein-1c (SREBP-1c). This NAFLD pathway is induced by dietary cholesterol through activation of the ligand-activated nuclear receptor liver X receptor [41], which is consistent with the downregulation of the metabolite cholesterol in this study. Skin is an androgen target tissue in which 3β-hydroxysteroid dehydrogenase (3β-HSD), 17β-hydroxysteroid dehydrogenase (17β-HSD), and 5α-reductase convert dehydroepiandrosterone, androstenedione, and testosterone into the most effective natural androgen dihydrotestosterone (DHT). This androgen is mainly converted into two phase I metabolites, androstane (ADT) and androstane-3α, 17 β-diol (3α-DIOL) in situ [42]. 3α-DIOL and ADT are then converted into two inactive and easily excreted 3α-DIOL-17 glucuronide (3α-DIOL-17G) and ADT-3 glucuronide (ADT-3G). This metabolic process is also present in prostate cancer [43]. In this study, both dehydroepiandrosterone and androsterone were upregulated, which may be related to this metabolic process after propolis acts on A431 cells.
The biosynthesis of steroid hormones is also a significantly different enrichment pathway in the negative ion mode metabolites, which are tetrahydrocorticosterone, cortodoxone, hydrocortisone, rosterone glucuronide, and adrenosterone. Among them, androsterone is also significantly upregulated, similar to positive ions. Androsterone glucuronide (AoG), a metabolite of circulating androgens under the influence of 5α-reductase activity, is associated with inflammatory lesions [44]. The immune system recognizes and eliminates pathogens and tumor cells, thereby inhibiting tumor growth [45]. Here, the metabolite AoG was downregulated and associated with the inhibition effect on A431 tumor cell proliferation.
The biosynthesis of steroid hormones is also closely related to changes in lipid metabolism and is also a therapeutic intervention point for the treatment of prostate cancer [46,47]. The steroid hormone biosynthesis pathway was the main mechanism of the antitumor effect of Hericium erinaceus petroleum ether extract on H22 tumor-bearing mice, according to untargeted metabolomics mechanism analysis [48]. Moreover, apolipoprotein A-1 containing steroid hormones reduces the number of 4,3-keto groups and accelerates the biosynthesis rate of DNA and protein in liver cancer HA-1 mice [49]. Owing to the association between steroidogenesis and breast cancer progression, steroid determination is an important tool for diagnosing breast cancer through the measurement of several steroid hormones and metabolites [50]. Steroid hormones can also regulate the expression of transforming growth factor and epidermal growth factor receptors in endometrial cancer cells to inhibit their proliferation [51].
Another significantly different pathway in positive ion mode is the linoleic acid metabolism pathway, which is enriched with differentially abundant metabolites, such as phosphatidylcholine (PC32:1), 12, 13-dihydroxy-9Z-octadecenoic acid ((+/−)12(13)-DiHOME), and 13-hydroperoxylinoleic acid (13-HpODE). It was reported that the increased choline consumption and phosphatidylcholine secretion of cancer cells were positively correlated with the cell proliferation rate. Phosphatidylcholine levels increased during the malignant transformation of human breast and prostate epithelial cells [52]. Lipid peroxidation processes involve linoleic acid in the production of 13-HpODE [53], while the source of choline is the degradation of choline-containing lipids [51]. Linoleic acid, which has important functions in health and disease, helps stimulate skin growth, maintain bone health, regulate metabolism, maintain reproductive system function, and enhance the metabolic adaptability and antitumor immunity of CD8+ T cells [54]. Linoleic acid can significantly inhibit the proliferation of hepatocellular carcinoma through metabolomics assessment and can also exhibit anti-proliferative and anti-invasive activities in endometrial cancer cell lines [55,56]. Metabolomic analysis of patients with precursor B-cell acute lymphoblastic leukemia revealed that the linoleic acid metabolic disorder pathway is closely related to B-cell acute lymphoblastic leukemia [57]. The linoleic acid metabolic pathway was also one of the five metabolic pathways affected in the orthotopic lung tumor model [58]. The dihydroxyflavone pinothiocyanate from plants and propolis inhibited human ileocecal colorectal adenocarcinoma OC43 cells via the linoleic acid and arachidonic acid metabolic axis through the AHR/CYP1A1 pathway [59]. In this study, untargeted metabolomics revealed the ability of propolis to inhibit the proliferation of A431 cells via the steroid hormone biosynthesis and linoleic acid metabolic pathways.
Some differentially abundant metabolites are components of EEP, which has a direct inhibitory effect on cancer cells. Chrysin, one of the significantly upregulated differentially abundant metabolites, has anticancer effects by regulating the apoptosis of breast cancer, gastrointestinal cancer, liver cancer, hepatocellular carcinoma, and bladder cancer [33,60]. Glycine sojae is an isoflavone and another upregulated metabolite in A431 cells treated with propolis. It also has significant cytotoxic effects on human gastric cancer cells by regulating cycle-related proteins [61]. Upregulated metabolite naringenin is a bioactive polyphenol that can inhibit the development of cancer in various parts of the body. Its anticancer activity is pleiotropic, including regulating different cell signaling pathways, inhibiting the production of cytokines and growth factors, and arresting the cell cycle [62]. Chrysin, glycine sojae, and naringenin in propolis are absorbed by cancer cells, thereby causing the apoptosis of A431 cells.
There are several other metabolites associated with anticancer activity. Sphingomyelin metabolism produces anticancer signals such as ceramide and sphingosine, which may inhibit cell proliferation and induce differentiation and apoptosis in colon cancer [63]. The metabolite sphingomyelin was also significantly upregulated in this study. The formation and development of adenoma is caused by excessive autonomous secretion of aldosterone [64]. The main metabolite of aldosterone is tetrahydroaldosterone [65], whose downregulation can reflect a decrease in aldosterone content. This downregulation of tetrahydroaldosterone was also observed in this study. L-palmitoylcarnitine was also downregulated in this study, which is consistent with the detection of metabolites of breast cancer cells inhibited by berberine in the hypoxic microenvironment [66].
4. Materials and Methods
4.1. Propolis Extraction and Polyphenols Determination
The raw poplar propolis sample was the same as that used in our previous report [29]. The extraction procedure for EEP was modified [29]. Briefly, 133.3 g of crushed propolis powder was dissolved in 1000 mL of a 70% ethanol solution. The mixture was stirred for 2 h and then placed in an ultrasonic cleaner (40 kHz, 60 min, 30 °C water bath). The mixture was filtered under vacuum after incubation at room temperature for 48 h, and then it was centrifuged at 8000× g for 10 min at 4 °C. These above operations were repeated three times. The ethanol in the supernatant was evaporated in a fume hood for 48 h and dried in a constant-temperature drying oven at 60 °C. The propolis extract was collected and stored in a −30 °C refrigerator for further experiments.
The chemical components of EEP were determined via an untargeted metabolomics method by a UHPLC–MS/MS system [Vanquish UHPLC system (Thermo Fisher Scientific Inc., Germering, Germany) coupled with a Q ExactiveTM HF/Q ExactiveTM HF-X mass spectrometer (ThermoFisher, Germering, Germany)], which is performed by Novogene Co., Ltd. (Beijing, China). In brief, 0.1 g of EEP was dissolved in 500 μL of 80% methanol aqueous solution in an EP tube. The mixture was vortexed and then placed in an ice bath for 5 min. The mixture was subsequently centrifuged at 15,000× g at 4 °C for 20 min (D3024R, Scilogex, Rocky Hill, CT, USA). The supernatant was diluted with a 53% methanol aqueous solution and then centrifuged again at 15,000× g and 4 °C for 20 min. The supernatant was injected into the UHPLC–MS/MS system for component determination. A Hypersil Gold column (C18, 100 × 2.1 mm, 1.9 μm, Thermo Scientific, Waltham, MA, USA) with mobile phases A (0.1% formic acid) and B (methanol) was employed. The gradient gradually changed from 98% A to 15%, 0, and 98% at the 3rd, 10th, and 10.1th minutes to 12 min at 98% A with a flow rate of 200 μL/min. The Q ExactiveTM HF/Q ExactiveTM HF-X mass spectrometer was operated in positive/negative polarity mode with a spray voltage of 3.5 kV, capillary temperature of 320 °C, sheath gas flow rate of 35 psi, aux gas flow rate of 10 L/min, S-lens RF level of 60, and aux gas heater temperature of 350 °C. MS/MS secondary scans are data-dependent scans.
The raw data files obtained from UHPLC–MS/MS were processed via Compound Discoverer 3.3 (CD3.3, Thermo Fisher) software to perform peak alignment, peak picking, and quantitation for each metabolite, whose main parameters were set as follows: retention time tolerance of 0.2 min; actual mass tolerance of 5 ppm; signal intensity tolerance of 30%; signal/noise ratio of 3; and minimum intensity. The peak area was quantified, and the target ions were integrated. The molecular formula was predicted through the molecular ion peak and fragment ions and comparison with mzCloud. The background ions were removed from the blank samples. The original quantitative results were standardized according to the following formula: sample original quantitative value/(sum of sample metabolite quantitative values/sum of quality control (QC)1 sample metabolite quantitative values) to obtain the relative peak area. The compounds with a coefficient of variation (CV) of relative peak area greater than 30% in the QC sample were deleted. The identification and relative quantitative results of the metabolites were obtained. Polyphenols were selected according to the secondary classification of the components.
4.2. Antitumor Effects of Propolis on the Proliferation of A431 Cells
The human skin squamous cell carcinoma A431 cell line (purchased from Wuhan Purosai Life Sciences Co., Ltd., Wuhan, China) was cultured with a special complete culture or serum-free cell freezing medium (Wuhan Purosai Life Sciences Co., Ltd.) in a 5% CO2 humidified incubator at 37 °C (C150, Binder, Tuttlingen, Germany).
After the cells were washed 2–3 times with phosphate-buffered saline (PBS, pH 7.2–7.4, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), they were digested with 1 mL of 0.25% trypsin (HyClone, Thermo Scientific, Waltham, MA, USA). The digestion was terminated with the complete culture medium. The digested cells were subsequently centrifuged at 137× g for 5 min and resuspended using the complete culture medium in a centrifuge tube. The cells were diluted with the complete culture medium to a concentration of 5 × 104 cells/mL by staining count with 0.4% trypan blue (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). A cell suspension (0.1 mL) was added to each well of a 96-well plate, which was placed in a cell culture incubator at 37 °C and 5% CO2 for 24 h.
EEP (0.1 g) was dissolved in a mixture of 0.5 mL of dimethyl sulfoxide (DMSO, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and 9.5 mL of complete cell culture medium. This solution was subpackaged and placed in a −20 °C refrigerator for further use. During the experiment, the propolis solution was diluted with the complete culture medium to the required concentration for further experiments.
The EEP solutions were diluted to 25, 50, 75, 100, and 125 μg/mL in a complete culture medium. The concentrations of 5-fluorouracil (HPLC grade; purchased from Sigma-Aldrich Co., St. Louis, MO, USA) solution used were 4, 8, 16, 32, and 64 μg/mL complete culture medium. The complete culture medium containing 0.0625% DMSO (equal to the quantity of DMSO in 125 μg/mL EEP solution) was used as the control group. There were triplicate and six duplicate wells for each treatment. The treatments and control group solutions (100 μL) were added and then cultured in the cell culture incubator for 48 h. The cells were washed with PBS, and then 110 μL of complete culture medium containing 10 μL of CCK-8 solution (DOJINDO, Kumamoto, Japan) was added in the dark. The absorbances at 450 nm were determined via a microplate reader (1510, Thermo Fisher Waltham, MA, USA) after 2 h of incubation in a cell culture incubator. The proliferation inhibition rate (%) was calculated as [(OD of control wells having cells and culture medium containing CCK-8 − OD of experimental wells having different concentrations of propolis solutions)/(OD of control wells having cells and culture medium containing CCK-8 − OD of blank wells containing CCK-8 and cells and culture medium free)] × 100%.
4.3. Untargeted Metabolomic Effects of Propolis on the Proliferation of A431 Cells
The cells were added to a 6-well plate after cell counting. They were treated with the complete culture medium containing 0.0625% DMSO (equal to DMSO in 125 μg/mL EEP solution) and the IC50 concentration of EEP (29.04 μg/mL) after 24 h of incubation. These cells were washed with 4 °C PBS (pH 7.2–7.4) 3 times after 48 h of incubation. After digestion with 0.25% trypsin, the cells were washed with 4 °C PBS (pH 7.2–7.4) 3 times again. These cells were transferred in a 1.5 mL centrifuge tube and quickly frozen with liquid nitrogen for 15 min. They were stored at −80 °C.
Aqueous methanol (80%, 300 μL) was added to the cells. Then, the samples were put into liquid nitrogen for quick freezing for 5 min. The frozen cells were thawed on ice and vortexed for 30 s. The samples were treated with the assistance of an ultrasonic cleaner for 6 min and centrifuged at 2560× g at 4 °C for 1 min. The supernatant was transferred into a new centrifuge tube and freeze-dried. A 10% methanol solution was used to dissolve the powder. The solution was injected into the UHPLC‒MS/MS system for analysis.
Each experiment had six replicates, and equal volumes of samples were taken from each experimental sample and mixed as QC samples. A 53% methanol aqueous mixture was used as a blank sample. The pretreatment procedure for the blank sample was the same as that for the experimental samples.
The conditions of the UHPLC‒MS/MS system are the same as those used for the determination of propolis components described above.
4.4. Statistical Analysis
All the experiments were performed in triplicate except for untargeted metabolomic effects of propolis on the proliferation of A431 cells, and the results are expressed as the means ± standard errors. The cell death (%) was transformed to arcsin (degree) values (according to the formula: arc sin√p) before ANOVA, which was performed via GraphPad Prism 9.5.1 for Windows (GraphPad Software, Inc. San Diego, CA, USA) to analyze the significance of differences (p < 0.01: extremely statistically significant differences between the different treatment groups; p < 0.05: statistically significant differences).
The identified metabolites were annotated via the KEGG database. For multivariate statistical analysis, the metabolomics data processing software metaX was used to transform the data, and then principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were performed to obtain the VIP value of each metabolite. In the univariate analysis, the statistical significance (p-value) of each metabolite between the two groups was calculated via a t-test, and the fold change (FC value) of the metabolite between the two groups was calculated. The default criteria for differentially abundant metabolite screening are VIP > 1, p < 0.05, and FC ≥ 1.5 or FC ≤ 0.667.
5. Conclusions
There was a total of 43 polyphenolic components in the ethanol extraction of propolis (EEP), of which the IC50 in A431 cells was 29.04 μg/mL. Untargeted metabolomics revealed 1052 small-molecule metabolites, of which 160 were significantly upregulated and 143 were significantly downregulated. The KEGG enrichment results revealed that EEP significantly inhibited A431 cell proliferation via the steroid hormone biosynthesis and linoleic acid metabolism pathways. These results provide evidence for the development of targeted drugs for the treatment of cutaneous squamous cell carcinoma.
Author Contributions
Conceptualization, methodology, funding acquisition, software, and writing—review and editing, W.Y.; formal analysis, writing—original draft preparation, J.W.; data curation, L.C., J.L., Y.W., S.C. and Z.W.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded for the Undergraduate Innovation Program by Fujian Agriculture and Forestry University, grant number FAFUXMPC20230718001-00201.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Bobiş, O. Plants: Sources of diversity in propolis properties. Plants 2022, 11, 2298. [Google Scholar] [CrossRef] [PubMed]
- Moise, A.R.; Bobiş, O. Baccharis dracunculifolia and Dalbergia ecastophyllum, main plant sources for bioactive properties in green and red Brazilian propolis. Plants 2020, 9, 1619. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Sharma, A.; Chopra, H.K.; Nanda, V. Impact of biodiversification on propolis composition, functionality, and application in foods as natural preservative: A review. Food Control 2024, 155, 110097. [Google Scholar] [CrossRef]
- Vagish Kumar, L.S. Propolis in dentistry and oral cancer management. N. Am. J. Med. Sci. 2014, 6, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Yañez, N.; Ruiz-Hurtado, P.A.; Rivera-Yañez, C.R.; Arciniega-Martínez, I.M.; Yepez-Ortega, M.; Mendoza-Arroyo, B.; Rebollar-Ruíz, X.A.; Méndez-Cruz, A.R.; Reséndiz-Albor, A.A.; Nieto-Yañez, O. The role of propolis as a natural product with potential gastric cancer treatment properties: A systematic review. Foods 2023, 12, 415. [Google Scholar] [CrossRef]
- Souza, R.P.; Bonfim-Mendonça, P.S.; Damke, G.M.Z.F.; de-Assis Carvalho, A.R.B.; Ratti, B.A.; Dembogurski, D.S.O.; da-Silva, V.R.S.; Silva, S.O.; Da-Silva, D.B.; Bruschi, M.L.; et al. Artepillin C induces selective oxidative stress and inhibits migration and invasion in a comprehensive panel of human cervical cancer cell lines. Anti-Cancer Agents Med. Chem. 2018, 18, 1750–1760. [Google Scholar] [CrossRef]
- Frión-Herrera, Y.; Gabbia, D.; Scaffidi, M.; Zagni, L.; Cuesta-Rubio, O.; De Martin, S.; Carrara, M. Cuban brown propolis interferes in the crosstalk between colorectal cancer cells and M2 macrophages. Nutrients 2020, 12, 2040. [Google Scholar] [CrossRef]
- Meghalatha, T.S.; Muninathan, N. Antitumor activity of withaferin-A and propolis in benz (a) pyrene-induced breast cancer. Bioinformation 2022, 18, 841–844. [Google Scholar] [CrossRef]
- Goto, T.; Kimura, H.; Yoshino, T.; Sawada, A.; Akamatsu, S.; Kobayashi, T.; Yamasaki, T.; Tazawa, S.; Fujimoto, M.; Hidaka, Y.; et al. Efficacy and safety of Brazilian green propolis in biochemically recurrent prostate cancer after radical prostatectomy: A single-arm phase II study. Int. J. Transl. Med. 2022, 2, 618–632. [Google Scholar] [CrossRef]
- Chiu, H.; Han, Y.; Shen, Y.; Golovinskaia, O.; Venkatakrishnan, K.; Wang, C. Chemopreventive and chemotherapeutic effect of propolis and its constituents: A mini-review. J. Cancer Prev. 2020, 25, 70–78. [Google Scholar] [CrossRef]
- Simanjuntak, M.V.; Jauhar, M.M.; Syaifie, P.H.; Arda, A.G.; Mardliyati, E.; Shalannanda, W.; Hermanto, B.R.; Anshori, I. Revealing propolis potential activity on inhibiting estrogen receptor and heat shock protein 90 overexpressed in breast cancer by bioinformatics approaches. Bioinf. Biol. Insights 2024, 18, 11779322231224187. [Google Scholar] [CrossRef] [PubMed]
- Ay, E.N.; Caner, A.; Özsoy Hepokur, C.; Danışman Kalındemirtaş, F.; ÖZEN EROĞLU, G.; Kariper, I.A. Propolis nanoparticles synthesis and characterization with cytotoxic and apoptotic effects on breast cancer cells. J. Taibah Univ. Med. Sci. 2023, 17, 2249628. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M.; Durmus, E.; Yenigun, V.B.; Kanimdan, E.; Ozman, Z.; Yasar, O.; Goren, A.C.; Hekimoglu, E.R.; Oruc, H.H.; et al. Propolis enhances 5-fluorouracil mediated antitumor efficacy and reduces side effects in colorectal cancer: An in vitro and in vivo study. Chem. Biodivers. 2023, 20, e202300591. [Google Scholar] [CrossRef] [PubMed]
- Frión-Herrera, Y.; Díaz-García, A.; Ruiz-Fuentes, J.; Rodríguez-Sánchez, H.; Sforcin, J.M. The cytotoxic effects of propolis on breast cancer cells involve PI3K/Akt and ERK1/2 pathways, mitochondrial membrane potential, and reactive oxygen species generation. Inflammopharmacology 2019, 27, 1081–1089. [Google Scholar] [CrossRef]
- Xuan, H.; Li, Z.; Yan, H.; Sang, Q.; Wang, K.; He, Q.; Wang, Y.; Hu, F. Antitumor activity of chinese propolis in human breast cancer MCF-7 and MDA-MB-231 cells. Evid. Based. Complement. Alternat. Med. 2014, 2014, 280120. [Google Scholar] [CrossRef]
- Corchado-Cobos, R.; García-Sancha, N.; González-Sarmiento, R.; Pérez-Losada, J.; Cañueto, J. Cutaneous squamous cell carcinoma: From biology to therapy. Int. J. Mol. Sci. 2020, 21, 2956. [Google Scholar] [CrossRef]
- Cleavenger, J.; Johnson, S.M. Non melanoma skin cancer review. J. Ark. Med. Soc. 2014, 110, 230–234. [Google Scholar] [PubMed]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef]
- Xu, C.; Zhuo, Y.; Liu, Y.; Chen, H. Itraconazole inhibits the growth of cutaneous squamous cell carcinoma by targeting HMGCS1/ACSL4 Axis. Front. Pharmacol. 2022, 13, 828983. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Teng, M.; Liu, J. Dihydroartemisinin inhibits activation of the AIM2 inflammasome pathway and NF-κB/HIF-1α/VEGF pathway by inducing autophagy in A431 human cutaneous squamous cell carcinoma cells. Int. J. Med. Sci. 2021, 18, 2705–2715. [Google Scholar] [CrossRef]
- Liu, W.; Liu, X. LINC00641 inhibits the development of cutaneous squamous cell carcinoma by downregulating miR-424 in A431 Cells. Cancer Biother. Radiopharm. 2021, 39, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Endo-Munoz, L.; Dahler, A.; Teakle, N.; Rickwood, D.; Hazar-Rethinam, M.; Abdul-Jabbar, I.; Sommerville, S.; Dickinson, I.; Kaur, P.; Paquet-Fifield, S.; et al. E2F7 can regulate proliferation, differentiation, and apoptotic responses in human keratinocytes: Implications for cutaneous squamous cell carcinoma formation. Cancer Res. 2009, 69, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.W.; Elderdery, A.; Rampal, S.; Subbiah, S.K.; Mok, P.L. Clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 transfection of guide RNA targeting on MMP9 as anti-cancer therapy in human cutaneous squamous cell carcinoma cell line A431. Wspolczesna Onkol. 2023, 27, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tian, Y.; Yang, A.; Tan, W.; Liu, X.; Yang, W. Antitumor effect of poplar propolis on human cutaneous squamous cell carcinoma A431 cells. Int. J. Mol. Sci. 2023, 24, 16753. [Google Scholar] [CrossRef]
- Ribbenstedt, A.; Ziarrusta, H.; Benskin, J.P. Development, characterization and comparisons of targeted and non-targeted metabolomics methods. PLoS ONE 2018, 13, e0207082. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, H.; Jiang, Y.; Gao, Y.; Liu, S. Urinary metabonomics of stomach cancer assessed by rapid resolution liquid chromatography/time-of-fight mass spectrometry. Chin. Med. J. (Engl.) 2013, 126, 1930–1933. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Q.; Lu, W.; Wang, Y.; Ma, X.; Chen, Z.; Yan, C. Metabonomics study of lung cancer cells based on liquid l chromatography-mass spectrometry. SePu 2013, 31, 691–696. [Google Scholar] [CrossRef]
- Gan, C.; Huang, X.; Wu, Y.; Zhan, J.; Zhang, X.; Liu, Q.; Huang, Y. Untargeted metabolomics study and pro-apoptotic properties of B-norcholesteryl benzimidazole compounds in ovarian cancer SKOV3 cells. J. Steroid Biochem. Mol. Biol. 2020, 202, 105709. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Y.; Yang, A.; Zhang, C.; Miao, X.; Yang, W. Antitumor effects of poplar propolis on DLBCL SU-DHL-2 cells. Foods 2023, 12, 283. [Google Scholar] [CrossRef]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J.A. Biological properties of propolis extracts: Something new from an ancient product. Chem. Phys. Lipids 2017, 207, 214–222. [Google Scholar] [CrossRef]
- Zheng, Y.; Deng, G.; Liang, Q.; Chen, D.; Guo, R.; Lai, R. Antioxidant activity of quercetin and its glucosides from propolis: A theoretical study. Sci. Rep. 2017, 7, 7543. [Google Scholar] [CrossRef] [PubMed]
- Zulhendri, F.; Felitti, R.; Fearnley, J.; Ravalia, M. The use of propolis in dentistry, oral health, and medicine: A review. J. Oral Biosci. 2021, 63, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Celińska-Janowicz, K.; Zaręba, I.; Lazarek, U.; Teul, J.; Tomczyk, M.; Pałka, J.; Miltyk, W. Constituents of propolis: Chrysin, caffeic acid, p-coumaric acid, and ferulic acid induce PRODH/POX-dependent apoptosis in human tongue squamous cell carcinoma cell (CAL-27). Front. Pharmacol. 2018, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Frozza, C.O.; Garcia, C.S.; Gambato, G.; de Souza, M.D.; Salvador, M.; Moura, S.; Padilha, F.F.; Seixas, F.K.; Collares, T.; Borsuk, S.; et al. Chemical characterization, antioxidant and cytotoxic activities of Brazilian red propolis. Food Chem. Toxicol. 2013, 52, 137–142. [Google Scholar] [CrossRef]
- Asgharpour, F.; Moghadamnia, A.A.; Zabihi, E.; Kazemi, S.; Ebrahimzadeh Namvar, A.; Gholinia, H.; Motallebnejad, M.; Nouri, H.R. Iranian propolis efficiently inhibits growth of oral streptococci and cancer cell lines. BMC Complement. Altern. Med. 2019, 19, 266. [Google Scholar] [CrossRef]
- Wezgowiec, J.; Wieczynska, A.; Wieckiewicz, W.; Kulbacka, J.; Saczko, J.; Pachura, N.; Wieckiewicz, M.; Gancarz, R.; Wilk, K.A. Polish propolis-chemical composition and biological effects in tongue cancer cells and macrophages. Molecules 2020, 25, 2426. [Google Scholar] [CrossRef]
- Žižić, J.B.; Vuković, N.L.; Jadranin, M.B.; Anđelković, B.D.; Tešević, V.V.; Kacaniova, M.M.; Sukdolak, S.B.; Marković, S.D. Chemical composition, cytotoxic and antioxidative activities of ethanolic extracts of propolis on HCT-116 cell line. J. Sci. Food Agric. 2013, 93, 3001–3009. [Google Scholar] [CrossRef]
- Khacha-Ananda, S.; Tragoolpua, K.; Chantawannakul, P.; Tragoolpua, Y. Propolis extracts from the northern region of Thailand suppress cancer cell growth through induction of apoptosis pathways. Invest. New Drugs 2016, 34, 707–722. [Google Scholar] [CrossRef]
- Slominski, A.; Zbytek, B.; Nikolakis, G.; Manna, P.R.; Skobowiat, C.; Zmijewski, M.; Li, W.; Janjetovic, Z.; Postlethwaite, A.; Zouboulis, C.C.; et al. Steroidogenesis in the skin: Implications for local immune functions. J. Steroid Biochem. Mol. Biol. 2013, 137, 107–123. [Google Scholar] [CrossRef]
- Huang, B.; Song, B.; Xu, C. Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2020, 2, 132–141. [Google Scholar] [CrossRef]
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell. Mol. Life Sci. 2019, 76, 99–128. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, A.; Pelletier, G.; Labrie, F.; Barbier, O.; Chouinard, S. Inactivation of androgens by UDP-glucuronosyltransferase enzymes in humans. Trends Endocrinol. Metab. 2003, 14, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Gauthier-Landry, L.; Bélanger, A.; Barbier, O. Multiple roles for UDP-glucuronosyltransferase (UGT)2B15 and UGT2B17 enzymes in androgen metabolism and prostate cancer evolution. J. Steroid Biochem. Mol. Biol. 2015, 145, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Godwin, A.J.; Stanczyk, F.Z.; Lippman, J.S.; Lobo, R.A. The association of serum androsterone glucuronide with inflammatory lesions in women with adult acne. J. Endocrinol. Investig. 2002, 25, 765–768. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Targeted Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Barfeld, S.J.; Itkonen, H.M.; Urbanucci, A.; Mills, I.G. Androgen-regulated metabolism and biosynthesis in prostate cancer. Endocr.-Relat. Cancer. 2014, 21, T57–T66. [Google Scholar] [CrossRef]
- Snaterse, G.; Visser, J.A.; Arlt, W.; Hofland, J. Circulating steroid hormone variations throughout different stages of prostate cancer. Endocr.-Relat. Cancer. 2017, 24, R403–R420. [Google Scholar] [CrossRef]
- Li, Z.; Bao, H. Anti-tumor effect of Inonotus hispidus petroleum ether extract in H22 tumor-bearing mice and analysis its mechanism by untargeted metabonomic. J. Ethnopharmacol. 2022, 285, 114898. [Google Scholar] [CrossRef]
- Panin, L.E.; Khoshchenko, O.M.; Poliakov, L.M. Effect of apolipoprotein A-1 containing steroid hormones on DNA and protein biosynthesis in cells of ascitic hepatoma HA-1. Vopr. Onkol. 2007, 53, 562–565. [Google Scholar]
- Valko-Rokytovská, M.; Očenáš, P.; Salayová, A.; Kostecká, Z. Breast cancer: Targeting of steroid hormones in cancerogenesis and diagnostics. Int. J. Mol. Sci. 2021, 22, 5878. [Google Scholar] [CrossRef]
- Murphy, L.J. Growth factors and steroid hormone action in endometrial cancer. J. Steroid Biochem. Mol. Biol. 1994, 48, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.F.; Andrade, L.N.S.; Bustos, S.O.; Chammas, R. Phosphatidylcholine-derived lipid mediators: The crosstalk between cancer cells and immune cells. Front. Immunol. 2022, 13, 768606. [Google Scholar] [CrossRef] [PubMed]
- Spiteller, P.; Kern, W.; Reiner, J.; Spiteller, G. Aldehydic lipid peroxidation products derived from linoleic acid. Biochim. Biophys. Acta. 2001, 1531, 188–208. [Google Scholar] [CrossRef] [PubMed]
- Nava Lauson, C.B.; Tiberti, S.; Corsetto, P.A.; Conte, F.; Tyagi, P.; Machwirth, M.; Ebert, S.; Loffreda, A.; Scheller, L.; Sheta, D.; et al. Linoleic acid potentiates CD8+ T cell metabolic fitness and antitumor immunity. Cell Metab. 2023, 35, 633–650. [Google Scholar] [CrossRef]
- Liu, J.; Geng, W.; Sun, H.; Liu, C.; Huang, F.; Cao, J.; Xia, L.; Zhao, H.; Zhai, J.; Li, Q.; et al. Integrative metabolomic characterisation identifies altered portal vein serum metabolome contributing to human hepatocellular carcinoma. Gut 2022, 71, 1203–1213. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Z.; Suo, H.; Paraghamian, S.E.; Hawkins, G.M.; Sun, W.; Zhang, X.; Hao, T.; Deng, B.; Shen, X.; et al. Linoleic acid exhibits anti-proliferative and anti-invasive activities in endometrial cancer cells and a transgenic model of endometrial cancer. Cancer Biol. Ther. 2024, 25, 2325130. [Google Scholar] [CrossRef]
- Wang, W.; Yu, L.; Li, Z.; Xiao, Y.; Jiang, H.; Tang, Y.L.; Chen, Y.; Xue, H. Dysregulated arginine metabolism in precursor B-cell acute lymphoblastic leukemia in children: A metabolomic study. BMC Pediatr. 2024, 24, 540. [Google Scholar] [CrossRef]
- Zhu, S.; Han, X.; Yang, R.; Tian, Y.; Zhang, Q.; Wu, Y.; Dong, S.; Zhang, B. Metabolomics study of ribavirin in the treatment of orthotopic lung cancer based on UPLC-Q-TOF/MS. Chem.-Biol. Interact. 2023, 370, 110305. [Google Scholar] [CrossRef]
- Zhao, L.; Yao, L.; Chen, R.; He, J.; Lin, T.; Qiu, S.; Chen, G.; Chen, H.; Qiu, S. Pinostrobin from plants and propolis against human coronavirus HCoV-OC43 by modulating host AHR/CYP1A1 pathway and lipid metabolism. Antivir. Res. 2023, 212, 105570. [Google Scholar] [CrossRef]
- Tsuji, P.A.; Winn, R.N.; Walle, T. Accumulation and metabolism of the anticancer flavonoid 5,7-dimethoxyflavone compared to its unmethylated analog chrysin in the Atlantic killifish. Chem. Biol. Interact. 2006, 164, 85–92. [Google Scholar] [CrossRef]
- Kumazawa, S.; Shimoi, K.; Hayashi, K.; Ishii, T.; Hamasaka, T.; Nakayama, T. Identification of metabolites in plasma and urine of Uruguayan propolis-treated rats. J. Agric. Food Chem. 2004, 52, 3083–3088. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and naringenin: Their mechanisms of action and the potential anticancer activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Liu, C.; Wang, A.; Ma, C.; Xu, Y.; Ye, T.; Su, W.; Zhou, P.; Gao, W.Q.; Li, L.; et al. SETD2 deficiency accelerates sphingomyelin accumulation and promotes the development of renal cancer. Nat. Commun. 2023, 14, 7572. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.C.; Negro, G.; Pinggera, A.; Tizen Laim, N.M.S.; Mohamed Rose, I.; Ceral, J.; Ryska, A.; Chin, L.K.; Kamaruddin, N.A.; Mohd Mokhtar, N.; et al. Aldosterone-producing adenomas: Histopathology-genotype correlation and identification of a novel CACNA1D mutation. Hypertension 2017, 70, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Makowski, N.; Burckhardt, B.B. Enabling insights into the maturation of the renin-angiotensin-aldosterone system in children-Development of a low-volume LC-MS assay for the simultaneous determination of aldosterone, its precursor, and main metabolite. Steroids 2019, 148, 73–81. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Chen, F.; Gao, X.; Yang, L.; Jin, X.; Wink, M.; Sharopov, F.S.; Sethi, G. Berberine inhibits breast carcinoma proliferation and metastasis under hypoxic microenvironment involving gut microbiota and endogenous metabolites. Pharmacol. Res. 2023, 193, 106817. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).