Abstract
Inflammatory bowel disease is a chronic condition characterized by recurrent intestinal inflammation. Its etiopathogenesis is driven by a series of events that disrupt the mucosal barrier, alter the healthy balance of intestinal microbiota, and abnormally stimulate intestinal immune responses. Therefore, numerous studies suggest the use of short-chain fatty acids and their immunomodulatory effects as a therapeutic approach in this disease. The objective of this systematic review was to synthesize previous evidence on the relevance and therapeutic use of short-chain fatty acids, particularly butyrate, in the immune regulation of inflammatory bowel disease. This systematic review of articles linking inflammatory bowel disease with short-chain fatty acids was conducted according to the PRISMA-2020 guidelines. The Medline and the Web of Science databases were searched in August 2024. The risk of bias was assessed using the Joanna Briggs Institute checklists. A total of 1460 articles were reviewed, of which, 29 met the inclusion criteria. Short-chain fatty acids, particularly butyrate, play a critical role in the regulation of intestinal inflammation and can be used as a strategy to increase the levels of short-chain fatty acid-producing bacteria for use in therapeutic approaches.
1. Introduction
Inflammatory bowel disease (IBD) is a chronic disease with recurrent intestinal inflammation. It is a general term for a group of diseases that mainly includes Crohn’s disease (CD), ulcerative colitis (UC), and unclassified inflammatory bowel disease (UC-IBD). Of unknown etiopathogenesis, it involves the inappropriate and persistent activation of the intestinal mucosal immune system. Environmental factors play a critical role in the development of inflammatory bowel disease (IBD). In particular, the adoption of a Western diet high in saturated fat and low in fiber disrupts the balance of the gut microbiota. In addition, the prolonged use of antibiotics negatively affects microbial diversity, while high levels of hygiene are associated with reduced exposure to beneficial microorganisms. These factors contribute to the development of dysbiosis, creating a pro-inflammatory environment that promotes inappropriate immune activation in genetically predisposed individuals [1].
CD can affect any part of the gastrointestinal tract, including the mouth, esophagus, stomach, small intestine, rectum, and anus. It is mainly associated with abdominal pain and problems such as fistulas, rectal lesions, and rectal bleeding, which are more common in UC and occur in areas of the large intestine, including the colon and rectum [1]. The etiopathogenesis of both diseases is caused by a series of events that disrupt the mucosal barrier, alter the healthy balance of the intestinal microbiota, and inappropriately stimulate the intestinal immune response.
It is estimated that more than 1 million people in the United States and 2.5 million people in Europe have IBD, resulting in significant health care costs [2]. The disease can begin in childhood, although the incidence usually peaks in adulthood [3]. According to several studies [4,5,6], UC is slightly more common in men (60%). However, CD is slightly more common in women, suggesting some involvement of hormonal factors in the expression of the disease. The prevalence of IBD is increasing, particularly in developed and industrialized countries. It is estimated that it will affect up to 30 million people worldwide by 2025 [7].
The gut microbiome has attracted increasing interest as a factor regulating the balance in the gut of healthy individuals. Several environmental and lifestyle factors, such as hygiene, the use of antibiotics, and the adoption of a typical Western diet, have been linked to imbalances in the gut microbiota, known as dysbiosis. This alteration of the microbiome can lead to an imbalance in the production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), and a decrease in anti-inflammatory cytokines, such as interleukin-10 (IL-10). This shift creates a chronic pro-inflammatory environment in the GI tract, disrupting intestinal homeostasis and contributing to the development of inflammatory diseases such as IBD. [8]. The gut microbiome of a healthy individual consists of a balanced community of diverse microorganisms, including bacteria, bacteriophages, viruses, archaea, and fungi. Among the bacterial species of particular importance are those that feed on indigestible dietary fiber and produce metabolites such as short-chain fatty acids (SCFAs), especially acetate, propionate, and butyrate. Butyrate acts as a major energy source for colonic cells and also contributes to the maintenance of intestinal homeostasis through its anti-inflammatory activity [8,9].
Figure 1 shows a synergistic interaction between SCFA-producing bacteria and anti-inflammatory drugs in the treatment of IBD. Anti-inflammatory drugs reduce the production of inflammatory mediators, while SCFA-producing bacteria have anti-inflammatory effects, strengthen the intestinal barrier, and promote the production of anti-inflammatory mediators. This joint interaction may improve disease symptoms and promote remission. IL-10 and NF-κB are two key molecules that regulate the inflammatory response in the gut. IL-10 acts as an inhibitor of intestinal inflammation, while NF-κB enhances it. The imbalance between these two molecules contributes to the development and progression of IBD. Therapeutic strategies that increase IL-10 or decrease NF-κB activity may be promising for the treatment of IBD. In conclusion, SCFA-producing bacteria, anti-inflammatory drugs, IL-10, and NF-κB play important roles in gut health. Synergistic interactions between SCFA-producing bacteria and anti-inflammatory drugs may be a valuable treatment option for people with IBD, while strategies that modulate IL-10 and NF-κB may also have therapeutic potential [8,9].
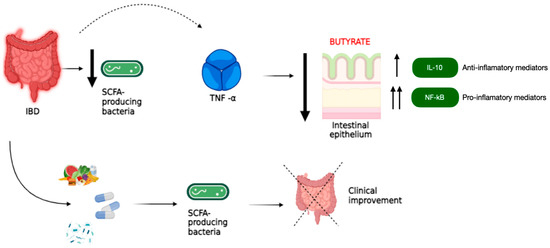
Figure 1.
Synergistic interactions between SCFA-producing bacteria and anti-inflammatory drugs in the treatment of IBD: The figure shows the interaction between SCFA-producing bacteria and anti-inflammatory drugs in the treatment of IBD. SCFA-producing bacteria are beneficial inhabitants of the gut that ferment dietary carbohydrates to produce SCFAs such as acetate, propionate, and butyrate. These SCFAs are essential for gut health because they nourish the intestinal cells: they are the main source of energy for the cells that line the intestine, maintaining their protective function. They fight inflammation by reducing the production of inflammatory substances such as TNF-α and increasing the production of other anti-inflammatory substances such as IL-10. They also help strengthen the intestinal barrier, which protects the body from pathogens and harmful substances. The anti-inflammatory drugs that are commonly used to treat inflammation in IBD work by reducing the production of pro-inflammatory mediators, including TNF-α. SCFA-producing bacteria and anti-inflammatory drugs may have a synergistic effect in reducing inflammation and strengthening the intestinal barrier, helping to improve IBD symptoms and promote remission.
Butyrate is particularly important in the therapeutic use of SCFAs due to its central role in regulating intestinal inflammation and maintaining microbiome homeostasis. As the primary energy source for colonic epithelial cells, butyrate is essential for maintaining the integrity of the intestinal barrier. In addition, butyrate exerts significant anti-inflammatory effects by inhibiting histone deacetylases (HDACs), leading to epigenetic modifications in immune cells that help regulate the immune response within the gastrointestinal tract. These properties have been shown to be particularly beneficial in conditions such as IBD, where butyrate can help reduce inflammation and restore a balanced gut microbiome, making it a key SCFA in therapeutic interventions. Therefore, the aim of this study was to synthesize previous evidence on the relevance and the therapeutic use of short-chain fatty acids, especially butyrate, in the immune regulation of inflammatory bowel disease.
2. Materials and Methods
A systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [10], which was registered in the records of the Open Science Framework (https://osf.io/9738p; accessed on 1 September 2024). The PRISMA 2020 checklist (Supplementary Data S1) was also applied.
2.1. Research Question and Eligibility Criteria
The review was designed to answer the following question: “What is the effect of restoring butyrate-producing bacteria by controlled administration of prebiotics, probiotics, and specific dietary treatments on the anti-inflammatory activity of short-chain fatty acids in patients with inflammatory bowel disease (IBD)?”. The PICO strategy was used, with P (population), I (intervention/exposure), C (comparison), and O (outcome) defined as follows.
2.1.1. Inclusion Criteria
- Population: adults diagnosed with ulcerative colitis or Crohn’s disease.
- Intervention: the administration of prebiotics, probiotics, or dietary treatments aimed at restoring butyrate-producing bacteria.
- Comparator: comparison with patients who did not receive such interventions.
- Outcome: the evidence of anti-inflammatory effects associated with short-chain fatty acids.
- Publication language: English or Spanish.
- Publication date: articles published between January 2000 and August 2024.
- Study design: prospective or retrospective observational studies (cross-sectional, case-control, or cohort studies), reviews, case studies and case series.
2.1.2. Exclusion Criteria
We excluded the following works:
- Articles that did not directly address the impact of SCFAs or their immunomodulatory role in inflammatory bowel disease.
- Letters to the editor, personal opinions, books, book chapters, non-original reports, conference abstracts, editorials, commentaries, or articles that did not contribute to or complement the objectives of the study.
- Studies involving patients with additional serious medical conditions, known allergies to prebiotics or probiotics, pregnancy, or participation in other clinical trials that could confound the results.
- Articles for which the full text could not be obtained.
2.2. Sources of Information and Search Strategy
A comprehensive search of the Medline and the Web of Science databases was performed in August 2024. The search strategy used the following Medical Subject Headings (MeSHs) terms and keywords: “inflammatory bowel disease”, “short-chain fatty acids”, “butyric acid”, and “butyrate”. These terms were combined using the Boolean operators “AND” and “OR”. Search strategies are reported in Supplementary Table S1.
In addition, a hand search of the grey literature and the bibliographic references of the included studies was carried out to include papers that might have been overlooked.
2.3. Selection of Studies
After eliminating duplicate articles, the initial screening of titles and abstracts was carried out, followed by a review of the full text. The initial screening was performed by two authors, I.V. and M.C.-G, and the full-text review was performed by all of the authors.
2.4. Data Extraction
The following data were extracted from the articles that were finally included for the full review: the year of publication, the country of study, sample characteristics, and measures of anti-inflammatory activity related to short-chain fatty acids.
2.5. Quality Assessment
Joanna Briggs Institute (JBI) checklists [11], appropriate for each type of study, were used to assess the study’s design and quality. These checklists have 4 response options: yes, no, unclear, or not applicable. Items were rated by the number of positive answers to the total number of possible answers as percentages, dividing the number of possible answers by the number of “not applicable” items. A score of less than 50% was considered to be of low quality. The quality assessment of each study was reviewed independently by two authors: I.V. and M.C.-G. Any disagreements were resolved by discussion with the other reviewers.
3. Results
The literature search yielded 1460 results, as shown in Figure 2. Eighty-four articles were selected for the full-text review, of which, 29 studies met the inclusion criteria, after duplicates were eliminated and the remaining articles were screened. Table 1 summarizes the results of the search of the selected studies. Supplementary Tables S2–S6 show the quality assessment of the studies.
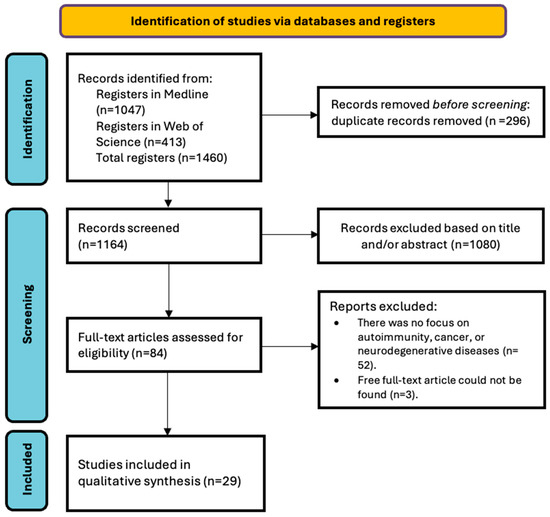
Figure 2.
PRISMA flowchart of the study selection process.

Table 1.
Summary of articles admitted for review.
The reviewed papers were mainly focused on evaluating the impact of different treatments and dietary supplements in IBD patients, especially in CD [12,16,19,21,22,23,27,35] and UC [24,30,31,32,33,36]. The objectives included the evaluation of the effect of prebiotics such as galactooligosaccharides (GOS) [13,14,18], pectin, and different SCFAs, such as butyrate, on gut microbiota, inflammation, and clinical parameters [14]. In addition, the effects of dietary interventions, such as the Mediterranean diet, and the efficacy of fecal microbiota transplantation (FMT) were investigated [13,16,20].
Regarding prebiotics and SCFAs, it was observed that GOS supplementation increased the presence of bifidobacteria, although it did not significantly reduce clinical scores or inflammation in UC patients [12]. On the other hand, the use of pectin together with FMT helped to maintain the composition and diversity of the intestinal microbiota [13]. Butyrate studies showed an increase in SCFA-producing bacteria and, in some cases, improvements in inflammation and clinical parameters. Diets such as CD-TREAT and the Mediterranean diet have shown beneficial changes in the microbiota and reductions in inflammation in IBD patients [17]. A low-fat, high-fiber diet increased acetate levels, benefiting the intestinal barrier and reducing inflammation. FMT studies revealed a complex relationship between microbial colonization and butyrate production [16]. Although an improvement in microbial diversity was observed, increasing the genetic capacity for butyrate production through FMT proved challenging [8].
The studies conclude that prebiotics and dietary supplements, such as butyrate, may have beneficial effects on the microbiota and reduce inflammation in IBD patients [13,14,15,18,20,25,26,28,29,32,37,38,39,40]. However, their efficacy is variable, and many studies highlight the need for additional controlled clinical trials to validate these findings. Dietary interventions show significant potential to improve symptoms and the quality of life in IBD patients, suggesting a comprehensive approach, combining specific diets with microbial therapies. In conclusion, research shows a growing interest in the role of microbiota and SCFAs in the management of IBD. Despite variations in results, there is an emerging consensus on the potential of special diets and prebiotic and butyrate supplementation to improve gut health in these patients. Most agree that it is essential to continue with larger, controlled studies to establish definitive clinical guidelines.
4. Discussion
The aim of this work was to investigate how the controlled manipulation of the gut microbiota, as well as the use of prebiotics, probiotics, and specific dietary treatments, can effectively modulate inflammation and, thus, improve clinical outcomes in patients with IBD. To this end, we focused on the hypothesis that the restoration of butyrate-producing bacteria appears to play a pivotal role in promoting long-term remission in UC and CD [15,19].
Studies conducted by several authors on IBD patients have shown that SCFAs, and, thus, SCFA-producing bacteria, play a beneficial role in disease control [20,21]. SCFAs have been identified as playing a critical role in this mechanism by targeting mammalian G-protein coupled receptors (GPRs), particularly GPR41 and GPR43 [37]. Accumulating evidence suggests that SCFAs may enhance T-cell regulatory (Treg) function through GPR activation and histone deacetylase (HDAC) inhibition due to epigenetic effects [37]. In summary, GPRs may protect against intestinal inflammation by maintaining the integrity of the epithelial barrier and regulating the immune response [37]. Furthermore, by analyzing the dynamics of microbial colonization and persistence in IBD patients, focusing on butyrate production gene transfer, it was concluded that butyrate production plays metabolic, regulatory, and immune functions [37]. This allows us to link the decrease in microbiota populations associated with butyrate production to IBD [16,23,26,28].
Both pectin and inulin-type fructans (ITF) are soluble fibers with prebiotic properties that are beneficial for gut health [36]. Pectin, which is fermented by the gut microbiota into SCFAs, particularly butyrate, has significant effects in the gut. Its use in UC patients undergoing fecal microbiota transplantation (FMT) not only preserves the diversity of the intestinal flora, but also enhances the effect of the transplantation [13,16,23,24,36]. On the other hand, inulin-type fructans, such as short-chain fructooligosaccharides (scFOS), are considered prebiotics and produce short-chain fatty acids when fermented in the gastrointestinal tract. The consumption of 15 g/day of inulin-type fructans during a 9-week study demonstrated significant improvements in UC markers and led to remarkable changes in the gut microbiota compared to the baseline. These results suggest that both pectin and inulin-type fructans may play an important role in the treatment and management of inflammatory bowel disease (IBD) [13,27].
Diet also emerges as an important component in the management of IBD. Three proposals have been made. The first is to study how an individualized diet based on foods rich in short-chain fatty acids can affect IBD patients [17]. This work concludes that this type of diet mimics beneficial changes in the microbiome, reduces intestinal inflammation, and is, therefore, a well-tolerated and effective diet for patients with CD. The Mediterranean diet has a higher proportion of short-chain fatty acids than other diets, such as the usual Canadian diet (CHD) [28]. This Mediterranean diet (MDP) is associated with changes in the microbiome and an improvement in intestinal inflammation and may, therefore, lead to clinical improvement in inactive UC [19]. Finally, a low-fat, high-fiber diet has been shown to reduce the markers of inflammation and dysbiosis in UC patients and improve their quality of life [24,25]. This type of diet leads to an increase in Bacteroides, which contain the main producers of acetate, thus increasing the levels of this short-chain fatty acid and having a beneficial effect on the intestinal barrier and inflammatory responses [22]. In other words, fiber intake is associated with a reduction in flares in patients with Crohn’s disease [36].
The gut microbiota plays an important role in the pathogenesis of IBD, and studying it allows us to identify the key markers of dysbiosis in these patients. Some of the signs found are decreased acetate CoA transferase, decreased absolute levels of total SCFAs, and the ratio of certain to major SCFAs [29,30,31,34]. All of these may indicate an inhibition of the functional activity and the number of anaerobic microflora and/or an alteration in the utilization of SCFAs by colonocytes, which could be used as potential targets for the development of personalized treatments for IBD patients [28,36]. One example is the use of butyrate enemas, which can prevent atrophy and inflammation in the bypassed GI mucosa of IBD patients [18]. We also found the use of sodium butyrate microcapsules as a possible potential strategy in IBD patients. The term “microcapsule” refers to a spherical body that encapsulates the active ingredient [41]. These sodium butyrate microcapsules increase the growth of SCFA-producing bacteria, resulting in benefits for these IBD patients, particularly in UC [14].
A preliminary evaluation of the butyrate and short-chain fatty acid profile was performed in patients with ulcerative colitis during a flare, who were treated with mesalamine or a combination of myrrh, chamomile flowers, and coffee charcoal. The results showed a significant reduction in the total amount of short-chain fatty acids and butyrate during a flare in the patients treated with mesalamine, whereas no significant changes were observed in the patients treated with the herbal preparation [21].
Studies by Hamilton et al. [20] show the importance of SCFA-producing bacteria in IBD. Fecal microbiota transplantation has identified the type of bacteria associated with sustained remission of UC. This procedure restores butyrate-producing bacteria and increases ButCoA levels, suggesting an important role for butyrate in long-term remission of UC. Studies of the fecal microbiota of Chinese and Japanese patients have shown that IBD is associated with a decrease in SCFA-producing bacteria [23,24]. One of the most important bacteria in this regard is Fecalibacterium prausnitzii. This bacterium is an acetate consumer that produces butyrate and bioactive anti-inflammatory molecules, such as shikimic acid and salicylic acid. It has been shown that high levels of F. prausnitzii in our microbiota lead to an improvement in the gut ecosystem and that patients with low levels of it have worse inflammatory parameters [25]. It has also been found that IBD patients have lower levels of this bacterium, resulting in reduced anti-inflammatory activities of these butyrate-producing bacteria [26,30,31,32,33,34,35,36,39,40].
Short-chain fatty acids, especially butyrate, play a key role as an energy source for the intestinal epithelium [42]. In addition, people with ulcerative colitis have significantly lower levels of total short-chain fatty acids, as well as acetate, propionate and valerate, than people without the disease [38]. This allows us to consider the possibility of using this type of bacteria as a possible treatment strategy [20]. It is important to note that not all scientific evidence supports the idea of the benefits of SCFAs, but some authors have conducted research suggesting that short-chain fatty acids have no clear benefit in IBD patients. These studies conclude that supplementation with prebiotic galactooligosaccharides (GOS) did not improve bowel motility or inflammation, although it did normalize stool [12,15]. In addition, the ratio of Bifidobacterium to Christensenellaceae was found to be increased only in patients with a less active disease, suggesting that the prebiotic effect may depend on disease status, although a controlled trial is needed to confirm these observations [12]. The trial of sodium butyrate supplementation for 12 weeks as an adjunctive therapy in newly diagnosed children and adolescents also failed to show efficacy in IBD [15].
Finally, studies of the epithelial response to butyrate have shown that the response to this SCFA is not intrinsically altered in resting IBD patients, supporting the beneficial effects of butyrate in the absence of inflammation. Nevertheless, due to the downregulation of butyrate transporters and enzymes associated with both active UC and CD patients, supplementation with butyrate extracts or SCFAs may not have beneficial effects as long as inflammation persists, highlighting the need for further studies in this area [29,31].
The studies reviewed have shown the relationship between SCFA-producing bacteria, anti-inflammatory drugs, IL-10, and NF-κB on gut health. SCFA-producing bacteria are beneficial bacteria in the gut that ferment dietary carbohydrates to produce SCFAs such as acetate, propionate, and butyrate. These SCFAs are essential for gut health because they nourish gut cells, fight inflammation, and strengthen the gut barrier. Scientific evidence supports the role of SCFA-producing bacteria in the treatment of IBD [39,40]. These studies have shown that SCFA supplements can improve the symptoms of ulcerative colitis and Crohn’s disease, and that people with IBD who have higher levels of SCFA-producing bacteria are less likely to experience flares.
Limitations
The weaknesses of this work include the heterogeneity of the studies reviewed, which makes the direct comparison of results and the generalization of conclusions difficult. In addition, most of the available studies are observational, which limits the ability to establish firm causal relationships between short-chain fatty acids and the immune regulation of IBD. It also faces the limitation of publication biases and variability in the study designs, methodologies, and populations studied. Finally, although the systematic review covers an extensive database, there may be relevant studies not included due to language or access restrictions, which could influence the completeness of the conclusions.
5. Conclusions
This study has shown that SCFAs, especially butyrate, play an important role in immune regulation in IBD. A systematic review of the literature has demonstrated that SCFAs may have a beneficial effect on reducing intestinal inflammation and modulating the immune response, suggesting their therapeutic potential in the treatment of IBD.
However, the heterogeneity of the reviewed studies poses certain limitations in generalizing the results. The variability in the study designs, populations studied, and methodologies used makes it difficult to make direct comparisons and draw definitive conclusions. It is essential that future studies address these limitations by using rigorous and standardized methodological designs and including diverse populations to validate and extend these findings.
Although the preliminary results are promising, further research is needed to confirm the therapeutic role of CCFAs in IBD and to establish clinically applicable treatment protocols. Future research should focus on conducting randomized clinical trials with representative samples and developing strategies for the clinical implementation of SCFAs to improve the outcomes in patients with IBD.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms252010879/s1.
Author Contributions
Conceptualization, I.V. and M.C.-G.; methodology, M.P.-B., M.E.L.-G. and M.T.M.-L.; validation, I.V. and M.P.-B.; investigation, M.C.-G., F.T.-A. and A.P.-F.; writing—original draft preparation, I.V., M.C.-G., M.P.-B., M.E.L.-G., F.T.-A., A.P.-F. and M.T.M.-L.; writing—review and editing, M.P.-B., M.E.L.-G., F.T.-A. and M.T.M.-L.; supervision, I.V. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the Catholic University of Valencia San Vicente Mártir for its contribution and help with the payment of the Open Access publication fee.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.M.; Bornemann, P.H. Ulcerative colitis. Am. Fam. Physician 2013, 87, 699–705. [Google Scholar]
- Bernstein, C.N.; Wajda, A.; Svenson, L.W.; MacKenzie, A.; Koehoorn, M.; Jackson, M.; Fedorak, R.; Israel, D.; Blanchard, J.F. The epidemiology of inflammatory bowel disease in Canada: A population-based study. Am. J. Gastroenterol. 2006, 101, 1559–1568. [Google Scholar] [CrossRef]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef]
- Loftus, E.V., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Juliao-Baños, F.; Grillo-Ardila, C.F.; Alfaro, I.; Andara-Ramírez, M.T.; Avelar-Escobar, O.; Barahona-Garrido, J.; Bautista-Martínez, S.; Bosques-Padilla, F.J.; De Paula, J.A.; Ernest Suárez, K.; et al. Update of the PANCCO clinical practice guidelines for the treatment of ulcerative colitis in the adult population. Rev. Gastroenterol. Mex. 2022, 87, 342–361. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)- Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Joanna Briggs Institute. Critical Appraisal Tools. Available online: https://jbi.global/critical-appraisal-tools (accessed on 28 February 2024).
- Wilson, B.; Eyice, Ö.; Koumoutsos, I.; Lomer, M.C.; Irving, P.M.; Lindsay, J.O.; Whelan, K. Prebiotic Galactooligosaccharide Supplementation in Adults with Ulcerative Colitis: Exploring the Impact on Peripheral Blood Gene Expression, Gut Microbiota, and Clinical Symptoms. Nutrients 2021, 13, 3598. [Google Scholar] [CrossRef]
- Wei, Y.; Gong, J.; Zhu, W.; Tian, H.; Ding, C.; Gu, L.; Li, N.; Li, J. Pectin enhances the effect of fecal microbiota transplantation in ulcerative colitis by delaying the loss of diversity of gut flora. BMC Microbiol. 2016, 16, 255. [Google Scholar] [CrossRef]
- Facchin, S.; Vitulo, N.; Calgaro, M.; Buda, A.; Romualdi, C.; Pohl, D.; Perini, B.; Lorenzon, G.; Marinelli, C.; D’Incà, R.; et al. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol. Motil. 2020, 32, e13914. [Google Scholar] [CrossRef]
- Pietrzak, A.; Banasiuk, M.; Szczepanik, M.; Borys-Iwanicka, A.; Pytrus, T.; Walkowiak, J.; Banaszkiewicz, A. Sodium Butyrate Effectiveness in Children and Adolescents with Newly Diagnosed Inflammatory Bowel Diseases-Randomized Placebo-Controlled Multicenter Trial. Nutrients 2022, 14, 3283. [Google Scholar] [CrossRef]
- Chu, N.D.; Crothers, J.W.; Nguyen, L.T.T.; Kearney, S.M.; Smith, M.B.; Kassam, Z.; Collins, C.; Xavier, R.; Moses, P.L.; Alm, E.J. Dynamic Colonization of Microbes and Their Functions after Fecal Microbiota Transplantation for Inflammatory Bowel Disease. mBio 2021, 12, e0097521. [Google Scholar] [CrossRef]
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A.; et al. Treatment of Active Crohn’s Disease with an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology 2019, 156, 1354–1367.e6. [Google Scholar] [CrossRef]
- Luceri, C.; Femia, A.P.; Fazi, M.; Di Martino, C.; Zolfanelli, F.; Dolara, P.; Tonelli, F. Effect of butyrate enemas on gene expression profiles and endoscopic/histopathological scores of diverted colorectal mucosa: A randomized trial. Dig. Liver Dis. 2016, 48, 27–33. [Google Scholar] [CrossRef]
- Haskey, N.; Estaki, M.; Ye, J.; Shim, R.K.; Singh, S.; Dieleman, L.A.; Jacobson, K.; Gibson, D.L. A Mediterranean Diet Pattern Improves Intestinal Inflammation Concomitant with Reshaping of the Bacteriome in Ulcerative Colitis: A Randomised Controlled Trial. J. Crohns. Colitis. 2023, 17, 1569–1578. [Google Scholar] [CrossRef]
- Hamilton, A.L.; Kamm, M.A.; De Cruz, P.; Wright, E.K.; Feng, H.; Wagner, J.; Sung, J.J.Y.; Kirkwood, C.D.; Inouye, M.; Teo, S.M. Luminal microbiota related to Crohn’s disease recurrence after surgery. Gut. Microbes. 2020, 11, 1713–1728. [Google Scholar] [CrossRef]
- Langhorst, J.; Koch, A.K.; Voiss, P.; Dobos, G.J.; Rueffer, A. Distinct patterns of shortchain fatty acids during flare in patients with ulcerative colitis under treatment with mesalamine or a herbal combination of myrrh, chamomile flowers, and coffee charcoal: Secondary analysis of a randomized controlled trial. Eur. J. Gastroenterol. Hepatol. 2020, 32, 175–180. [Google Scholar] [CrossRef]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30. [Google Scholar] [CrossRef]
- Ma, H.Q.; Yu, T.T.; Zhao, X.J.; Zhang, Y.; Zhang, H.J. Fecal microbial dysbiosis in Chinese patients with inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 1464–1477. [Google Scholar] [CrossRef]
- Andoh, A.; Kuzuoka, H.; Tsujikawa, T.; Nakamura, S.; Hirai, F.; Suzuki, Y.; Matsui, T.; Fujiyama, Y.; Matsumoto, T. Multicenter analysis of fecal microbiota profiles in Japanese patients with Crohn’s disease. J. Gastroenterol. 2012, 47, 1298–1307. [Google Scholar] [CrossRef]
- O’Brien, C.L.; Allison, G.E.; Pavli, P. The more the merrier: Faecalibacterium prausnitzii in Crohn’s disease. J. Gastroenterol. Hepatol. 2013, 28, 757–759. [Google Scholar] [CrossRef]
- Fujimoto, T.; Imaeda, H.; Takahashi, K.; Kasumi, E.; Bamba, S.; Fujiyama, Y.; Andoh, A. Decreased abundance of Faecalibacterium prausnitzii in the gut microbiota of Crohn’s disease. J. Gastroenterol. Hepatol. 2013, 28, 613–619. [Google Scholar] [CrossRef]
- Valcheva, R.; Koleva, P.; Martínez, I.; Walter, J.; Gänzle, M.G.; Dieleman, L.A. Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels. Gut. Microbes. 2019, 10, 334–357. [Google Scholar] [CrossRef]
- Danilova, N.A.; Abdulkhakov, S.R.; Grigoryeva, T.V.; Markelova, M.I.; Vasilyev, I.Y.; Boulygina, E.A.; Ardatskaya, M.D.; Pavlenko, A.V.; Tyakht, A.V.; Odintsova, A.K.; et al. Markers of dysbiosis in patients with ulcerative colitis and Crohn’s disease. Ter. Arkhiv 2019, 91, 17–24. [Google Scholar] [CrossRef]
- Ferrer-Picón, E.; Dotti, I.; Corraliza, A.M.; Mayorgas, A.; Esteller, M.; Perales, J.C.; Ricart, E.; Masamunt, M.C.; Carrasco, A.; Tristán, E.; et al. Intestinal Inflammation Modulates the Epithelial Response to Butyrate in Patients with Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2020, 26, 43–55. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Zhou, R.; Wang, X.; Song, L.; Huang, S.; Wang, G.; Xia, B. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrateproducing bacteria in inflammatory bowel disease. J. Clin. Microbiol. 2014, 52, 398–406. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Enrich-Capó, N.; Aldeguer, X.; Sabat-Mir, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Alterations in the Abundance and Co-occurrence of Akkermansia muciniphila and Faecalibacterium prausnitzii in the Colonic Mucosa of Inflammatory Bowel Disease Subjects. Front. Cell Infect. Microbiol. 2018, 8, 281. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Martinez-Medina, M.; Abellà, C.; Busquets, D.; Sabat-Mir, M.; Duncan, S.H.; Aldeguer, X.; Flint, H.J.; Garcia-Gil, L.J. Mucosa-associated Faecalibacterium prausnitzii phylotype richness is reduced in patients with inflammatory bowel disease. Appl. Environ. Microbiol. 2015, 81, 7582–7592. [Google Scholar] [CrossRef]
- Brotherton, C.S.; Martin, C.A.; Long, M.D.; Kappelman, M.D.; Sandler, R.S. Avoidance of Fiber Is Associated with Greater Risk of Crohn’s Disease Flare in a 6-Month Period. Clin. Gastroenterol. Hepatol. 2016, 14, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Ahuja, V.; Paul, J. Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World J. Gastroenterol. 2013, 19, 3404–3414. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 2016, 93, 59–65, Erratum in Digestion 2016, 93, 174. [Google Scholar] [CrossRef]
- Zhuang, X.; Li, T.; Li, M.; Huang, S.; Qiu, Y.; Feng, R.; Zhang, S.; Chen, M.; Xiong, L.; Zeng, Z. Systematic Review and Meta-analysis: Short-Chain Fatty Acid Characterization in Patients with Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2019, 25, 1751–1763. [Google Scholar] [CrossRef]
- Xu, H.M.; Zhao, H.L.; Guo, G.J.; Xu, J.; Zhou, Y.L.; Huang, H.L.; Nie, Y.Q. Characterization of short-chain fatty acids in patients with ulcerative colitis: A meta-analysis. BMC Gastroenterol. 2022, 22, 117. [Google Scholar] [CrossRef]
- Cao, Y.; Shen, J.; Ran, Z.H. Association between Faecalibacterium prausnitzii Reduction and Inflammatory Bowel Disease: A Meta-Analysis and Systematic Review of the Literature. Gastroenterol. Res. Pract. 2014, 2014, 872725. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, H.; Chen, S.; He, J.; Zhou, Y.; Nie, Y. Systematic review and meta-analysis of the role of Faecalibacterium prausnitzii alteration in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2021, 36, 320–328. [Google Scholar] [CrossRef]
- del Toro Añel, A.Y.; Pérez Tabío, Y.; Gorguet Pí, M.M.; Díaz del Toro, C.E. Estrés académico en estudiantes de Medicina durante la pandemia de covid-19. MEDISAN 2023, 27, e4398. [Google Scholar]
- Burisch, J.; Pedersen, N.; Čuković-Čavka, S.; Brinar, M.; Kaimakliotis, I.; Duricova, D.; Shonová, O.; Vind, I.; Avnstrøm, S.; Thorsgaard, N.; et al. East-West gradient in the incidence of inflammatory bowel disease in Europe: The ECCO-EpiCom inception cohort. Gut 2014, 63, 588–597. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).