Metal-Free, PPA-Mediated Fisher Indole Synthesis via Tandem Hydroamination–Cyclization Reaction between Simple Alkynes and Arylhydrazines
Abstract
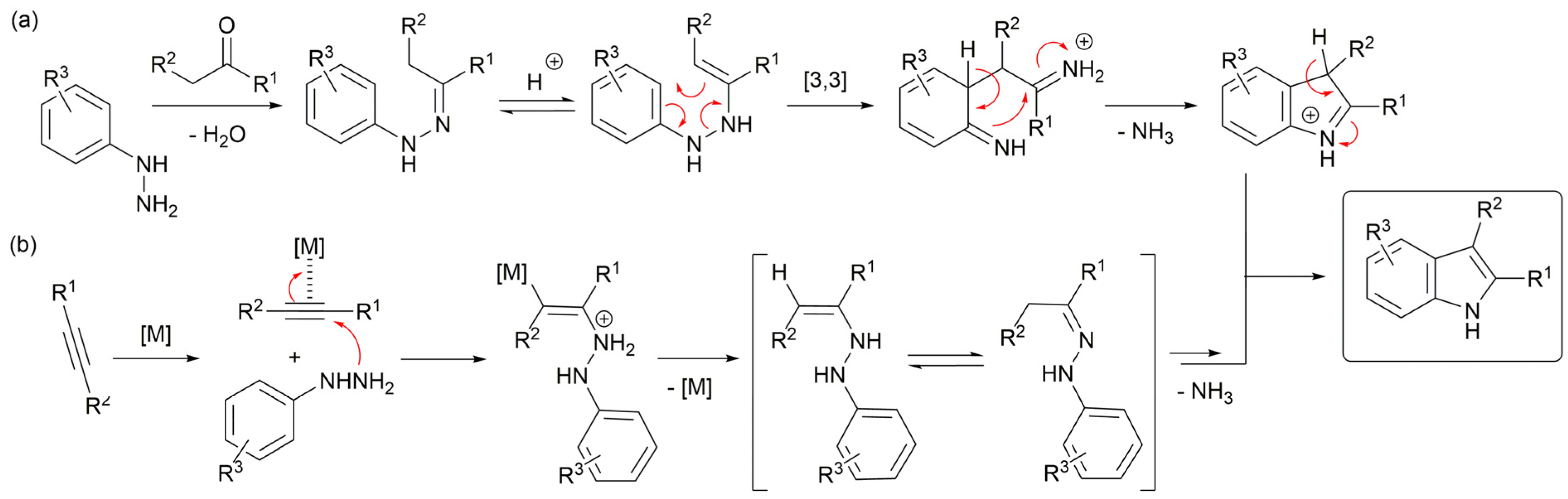
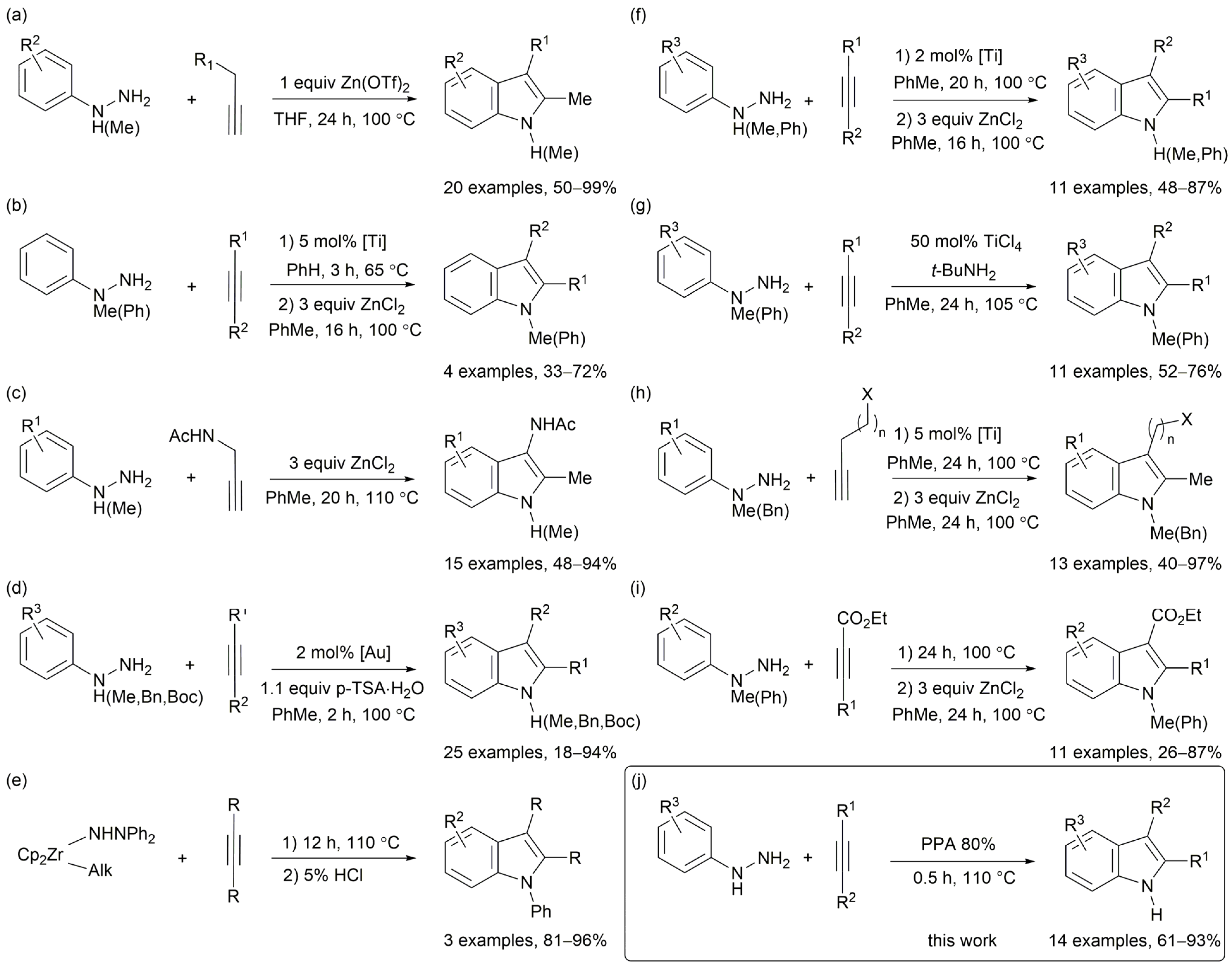
1. Introduction
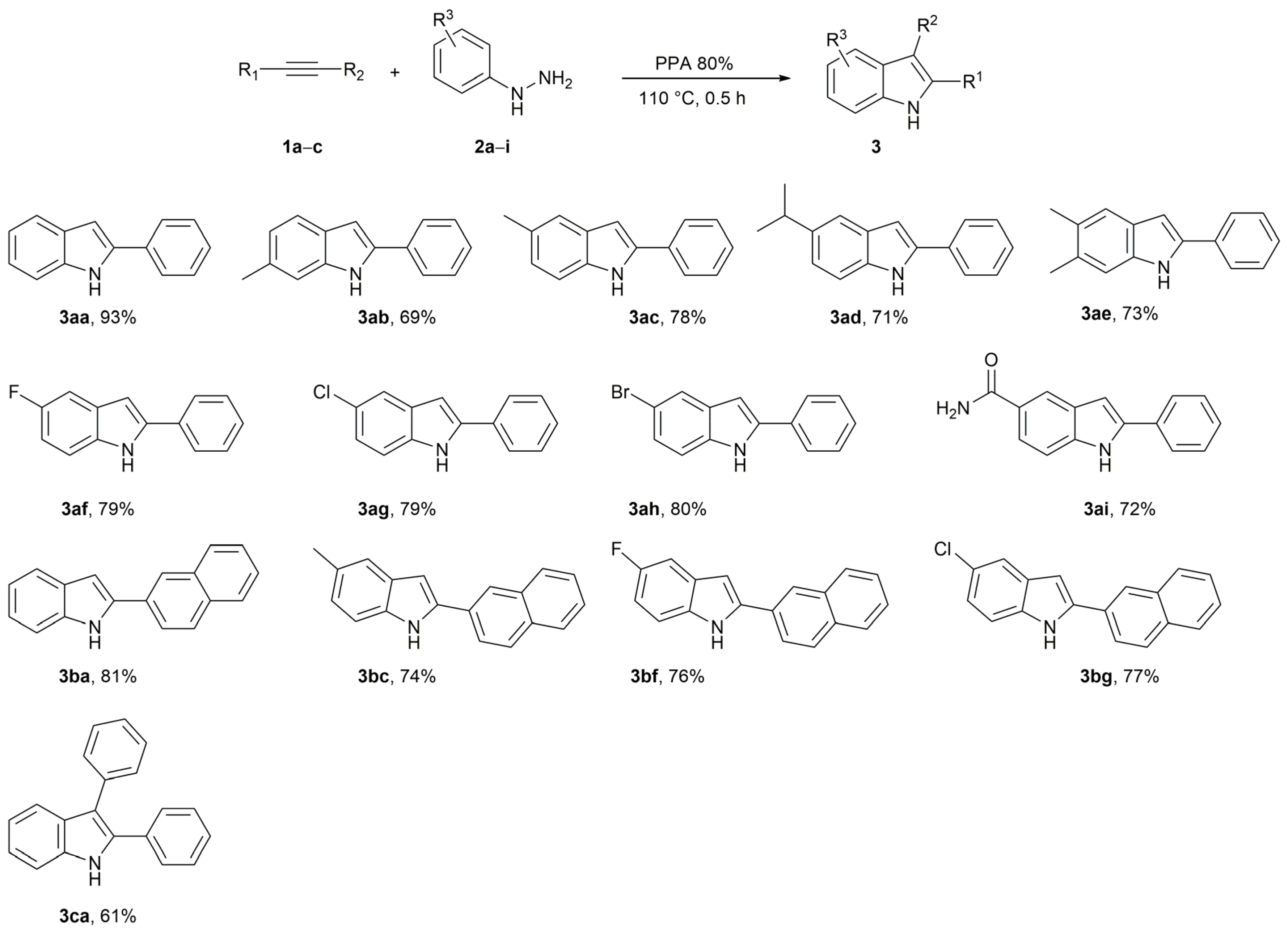
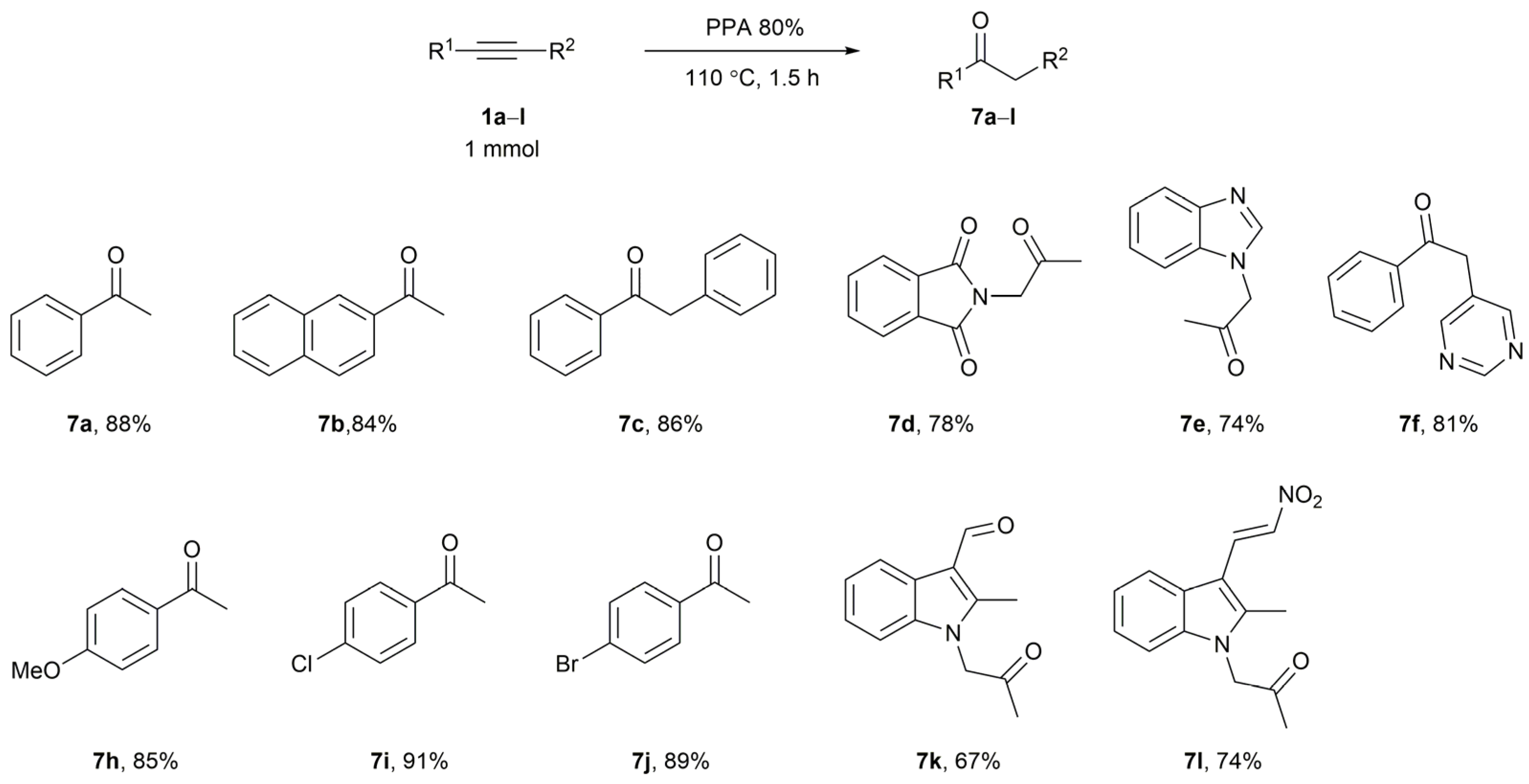
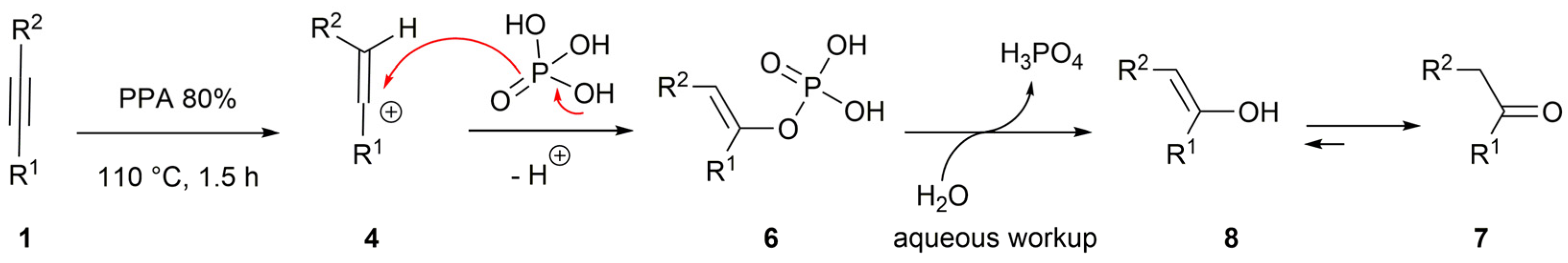
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Preparation of Indoles 3 (General Procedure)
3.3. Preparation of Acetophenones 7 (General Procedure)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaushik, N.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.; Verma, A.; Choi, E. Biomedical Importance of Indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sharma, S.; Kalra, S.; Singh, G.; Monga, V.; Kumar, B. Medicinal Perspective of Indole Derivatives: Recent Developments and Structure-Activity Relationship Studies. Curr. Drug Targets 2020, 21, 864–891. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Han, C.; Mohammed, S.; Li, S.; Song, Y.; Sun, F.; Du, Y. Indole-Containing Pharmaceuticals: Targets, Pharmacological Activities, and SAR Studies. RSC Med. Chem. 2024, 15, 788–808. [Google Scholar] [CrossRef] [PubMed]
- Gribble, G.W. Indole Ring Synthesis: From Natural Products to Drug Discovery; Wiley: Hoboken, NJ, USA, 2016; ISBN 9780470512180. [Google Scholar]
- Das, S. Indole Frameworks via Transition-Metal-Free Annulation: A Current Perspective. New J. Chem. 2023, 47, 13729–13775. [Google Scholar] [CrossRef]
- Bugaenko, D.I.; Karchava, A.V.; Yurovskaya, M.A. Synthesis of Indoles: Recent Advances. Russ. Chem. Rev. 2019, 88, 99–159. [Google Scholar] [CrossRef]
- Youn, S.W.; Ko, T.Y. Metal-Catalyzed Synthesis of Substituted Indoles. Asian J. Org. Chem. 2018, 7, 1467–1487. [Google Scholar] [CrossRef]
- Ma, J.; Feng, R.; Dong, Z. Recent Advances in Indole Synthesis and the Related Alkylation. Asian J. Org. Chem. 2023, 12, e202300092. [Google Scholar] [CrossRef]
- Mathada, B.S.; Yernale, N.G. Current Advances in Transition Metal-Free Access to Indoles. A Review. Org. Prep. Proced. Int. 2023, 55, 299–316. [Google Scholar] [CrossRef]
- De, S. Synthesis of Some Heterocyclic Compounds Using Named Reactions. In Applied Organic Chemistry; Wiley: Hoboken, NJ, USA, 2021; pp. 469–506. [Google Scholar] [CrossRef]
- Inman, M.; Moody, C.J. Indole Synthesis—Something Old, Something New. Chem. Sci. 2013, 4, 29–41. [Google Scholar] [CrossRef]
- Humphrey, G.R.; Kuethe, J.T. Practical Methodologies for the Synthesis of Indoles. Chem. Rev. 2006, 106, 2875–2911. [Google Scholar] [CrossRef]
- Heravi, M.M.; Amiri, Z.; Kafshdarzadeh, K.; Zadsirjan, V. Synthesis of Indole Derivatives as Prevalent Moieties Present in Selected Alkaloids. RSC Adv. 2021, 11, 33540–33612. [Google Scholar] [CrossRef]
- Heravi, M.M.; Rohani, S.; Zadsirjan, V.; Zahedi, N. Fischer Indole Synthesis Applied to the Total Synthesis of Natural Products. RSC Adv. 2017, 7, 52852–52887. [Google Scholar] [CrossRef]
- Huang, L.; Arndt, M.; Gooßen, K.; Heydt, H.; Gooßen, L.J. Late Transition Metal-Catalyzed Hydroamination and Hydroamidation. Chem. Rev. 2015, 115, 2596–2697. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Nájera, C.; Yus, M. Catalysis and Regioselectivity in Hydrofunctionalization Reactions of Unsaturated Carbon Bonds. Part III. Russ. Chem. Rev. 2021, 90, 70–93. [Google Scholar] [CrossRef]
- Neto, J.S.S.; Zeni, G. Recent Advances in the Synthesis of Indoles from Alkynes and Nitrogen Sources. Org. Chem. Front. 2020, 7, 155–210. [Google Scholar] [CrossRef]
- Neto, J.S.S.; Zeni, G. Recent Developments in the Cyclization of Alkynes and Nitrogen Compounds for the Synthesis of Indole Derivatives. Asian J. Org. Chem. 2021, 10, 1282–1318. [Google Scholar] [CrossRef]
- Krüger (née Alex), K.; Tillack, A.; Beller, M. Catalytic Synthesis of Indoles from Alkynes. Adv. Synth. Catal. 2008, 350, 2153–2167. [Google Scholar] [CrossRef]
- Alex, K.; Tillack, A.; Schwarz, N.; Beller, M. Zinc-Promoted Hydrohydrazination of Terminal Alkynes: An Efficient Domino Synthesis of Indoles. Angew. Chemie Int. Ed. 2008, 47, 2304–2307. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.C.H.; Bexrud, J.A.; Ayinla, R.O.; Leitch, D.C.; Schafer, L.L. Bis(Amidate)Bis(Amido) Titanium Complex: A Regioselective Intermolecular Alkyne Hydroamination Catalyst. J. Org. Chem. 2014, 79, 2015–2028. [Google Scholar] [CrossRef]
- Pews-Davtyan, A.; Tillack, A.; Schmöle, A.-C.; Ortinau, S.; Frech, M.J.; Rolfs, A.; Beller, M. A New Facile Synthesis of 3-Amidoindole Derivatives and Their Evaluation as Potential GSK-3β Inhibitors. Org. Biomol. Chem. 2010, 8, 1149. [Google Scholar] [CrossRef]
- Pews-Davtyan, A.; Beller, M. Efficient and Simple Zinc-Mediated Synthesis of 3-Amidoindoles. Org. Biomol. Chem. 2011, 9, 6331–6334. [Google Scholar] [CrossRef] [PubMed]
- Vedekhina, T.; Lukin, A.; Rogacheva, E.; Kraeva, L.; Krasavin, M. Zn(OTf)2-Catalyzed Arenehydrazination of Protected Propargylamines Leading to 3-Amidoindoles. Tetrahedron Lett. 2020, 61, 151430. [Google Scholar] [CrossRef]
- Patil, N.T.; Konala, A. Mechanistic Dichotomy with Alkynes in the Formal Hydrohydrazination/Fischer Indolization Tandem Reaction Catalyzed by a Ph3PAuNTf2/p-TSA Binary System. Eur. J. Org. Chem. 2010, 2010, 6831–6839. [Google Scholar] [CrossRef]
- Walsh, P.J.; Carney, M.J.; Bergman, R.G. Generation, Dative Ligand Trapping, and N-N Bond Cleavage Reactions of the First Monomeric H1-Hydrazido Zirconocene Complex, Cp2Zr=NNPh2. A Zirconium-Mediated Synthesis of Indoles. J. Am. Chem. Soc. 1991, 113, 6343–6345. [Google Scholar] [CrossRef]
- Banerjee, S.; Barnea, E.; Odom, A.L. Titanium-Catalyzed Hydrohydrazination with Monosubstituted Hydrazines: Catalyst Design, Synthesis, and Reactivity. Organometallics 2008, 27, 1005–1014. [Google Scholar] [CrossRef]
- Cao, C.; Shi, Y.; Odom, A.L. Intermolecular Alkyne Hydroaminations Involving 1,1-Disubstituted Hydrazines. Org. Lett. 2002, 4, 2853–2856. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Born, R. TiCl4/t-BuNH2 as the Sole Catalyst for a Hydroamination-Based Fischer Indole Synthesis. Tetrahedron Lett. 2004, 45, 9541–9544. [Google Scholar] [CrossRef]
- Khedkar, V.; Tillack, A.; Michalik, M.; Beller, M. Convenient Synthesis of Tryptophols and Tryptophol Homologues by Hydroamination of Alkynes. Tetrahedron 2005, 61, 7622–7631. [Google Scholar] [CrossRef]
- Khedkar, V.; Tillack, A.; Michalik, M.; Beller, M. Efficient One-Pot Synthesis of Tryptamines and Tryptamine Homologues by Amination of Chloroalkynes. Tetrahedron Lett. 2004, 45, 3123–3126. [Google Scholar] [CrossRef]
- Schwarz, N.; Alex, K.; Sayyed, I.; Khedkar, V.; Tillack, A.; Beller, M. Titanium-Catalyzed Hydroamination of Propargyl Alcohol Derivatives: Synthesis of 3-Silyloxy-2-Methylindoles via Hydrohydrazination. Synlett 2007, 2007, 1091–1095. [Google Scholar] [CrossRef]
- Sayyed, I.A.; Alex, K.; Tillack, A.; Schwarz, N.; Michalik, D.; Beller, M. A Convenient and General Method for the Synthesis of Indole-2,3-dicarboxylates and 2-Arylindole-3-carboxylates. Eur. J. Org. Chem. 2007, 2007, 4525–4528. [Google Scholar] [CrossRef]
- Gehrmann, T.; Lloret Fillol, J.; Scholl, S.A.; Wadepohl, H.; Gade, L.H. Zirconium-Catalyzed Multistep Reaction of Hydrazines with Alkynes: A Non-Fischer-Type Pathway to Indoles. Angew. Chemie Int. Ed. 2011, 50, 5757–5761. [Google Scholar] [CrossRef]
- Zhao, D.; Shi, Z.; Glorius, F. Indole Synthesis by Rhodium(III)-Catalyzed Hydrazine-Directed C-H Activation: Redox-Neutral and Traceless by N-N Bond Cleavage. Angew. Chemie Int. Ed. 2013, 52, 12426–12429. [Google Scholar] [CrossRef]
- Li, D.Y.; Chen, H.J.; Liu, P.N. Rhodium-Catalyzed Oxidative Annulation of Hydrazines with Alkynes Using a Nitrobenzene Oxidant. Org. Lett. 2014, 16, 6176–6179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, J.; Wang, L.; Chen, K.; Song, C.; Zhu, J. Co(III)-Catalyzed, Internal and Terminal Alkyne-Compatible Synthesis of Indoles. Org. Lett. 2016, 18, 3806–3809. [Google Scholar] [CrossRef]
- Arutiunov, N.A.; Aksenov, A.V.; Aksenov, D.A.; Kurenkov, I.A.; Aksenova, I.V.; Zatsepilina, A.M.; Aksenov, N.A.; Kornienko, A. Convenient Synthesis of (Z)-3-(1-Aryl-2-Nitrovinyl)-Indoles. Tetrahedron Lett. 2023, 129, 154722. [Google Scholar] [CrossRef]
- Aksenov, N.A.; Arutiunov, N.A.; Aksenov, A.V.; Kirilov, N.K.; Aksenova, I.V.; Aksenov, D.A.; Aleksandrova, E.V.; Rubin, M.; Kornienko, A. Synthesis of β-Carbolines with Electrocyclic Cyclization of 3-Nitrovinylindoles. Int. J. Mol. Sci. 2023, 24, 13107. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Yin, D. An Eco-Friendly Industrial Fischer Indole Cyclization Process. Org. Process Res. Dev. 2018, 22, 1115–1118. [Google Scholar] [CrossRef]
- Pohlki, F.; Doye, S. The Catalytic Hydroamination of Alkynes. Chem. Soc. Rev. 2003, 32, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Severin, R.; Doye, S. The Catalytic Hydroamination of Alkynes. Chem. Soc. Rev. 2007, 36, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.C.-H.; Schafer, L.L. Efficient Anti-Markovnikov-Selective Catalysts for Intermolecular Alkyne Hydroamination: Recent Advances and Synthetic Applications. Eur. J. Org. Chem. 2014, 2014, 6825–6840. [Google Scholar] [CrossRef]
- Patil, N.T.; Singh, V. Alkyne Hydroamination Triggered Cyclizations: A Powerful Tool for the Construction of Biologically Important Structural Motifs. J. Organomet. Chem. 2011, 696, 419–432. [Google Scholar] [CrossRef]
- Escorihuela, J.; Lledós, A.; Ujaque, G. Anti-Markovnikov Intermolecular Hydroamination of Alkenes and Alkynes: A Mechanistic View. Chem. Rev. 2023, 123, 9139–9203. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Saunthwal, R.K.; Verma, A.K. Base-Mediated Hydroamination of Alkynes. Acc. Chem. Res. 2017, 50, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.S.S.; Zeni, G. Synthesis of Indoles from Alkynes and a Nitrogen Source under Metal-Free Conditions. Org. Biomol. Chem. 2020, 18, 4906–4915. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.E.; Hultzsch, K.C.; Yus, M.; Foubelo, F.; Tada, M. Hydroamination: Direct Addition of Amines to Alkenes and Alkynes. Chem. Rev. 2008, 108, 3795–3892. [Google Scholar] [CrossRef] [PubMed]
- Coman, S.M.; Parvulescu, V.I. Nonprecious Metals Catalyzing Hydroamination and C–N Coupling Reactions. Org. Process Res. Dev. 2015, 19, 1327–1355. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Naájera, C.; Yus, M. Catalysis and Regioselectivity in Hydrofunctionalization Reactions of Unsaturated Carbon Bonds. Part II. Hydroamination. Russ. Chem. Rev. 2020, 89, 1074–1114. [Google Scholar] [CrossRef]
- Bernoud, E.; Lepori, C.; Mellah, M.; Schulz, E.; Hannedouche, J. Recent Advances in Metal Free- and Late Transition Metal-Catalysed Hydroamination of Unactivated Alkenes. Catal. Sci. Technol. 2015, 5, 2017–2037. [Google Scholar] [CrossRef]
- Kang, H.-J.; Lee, J.-H.; Kim, D.-H.; Cho, C.-G. Imidazole-Selective Alkyne Hydroamination under Physiological Conditions. Org. Lett. 2020, 22, 7588–7593. [Google Scholar] [CrossRef]
- Aksenov, A.V.; Grishin, I.Y.; Aksenov, N.A.; Malyuga, V.V.; Aksenov, D.A.; Nobi, M.A.; Rubin, M. Electrophilically Activated Nitroalkanes in Synthesis of 3,4-Dihydroquinozalines. Molecules 2021, 26, 4274. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, A.V.; Smirnov, A.N.; Aksenov, N.A.; Bijieva, A.S.; Aksenova, I.V.; Rubin, M. Benzimidazoles and Benzoxazoles via the Nucleophilic Addition of Anilines to Nitroalkanes. Org. Biomol. Chem. 2015, 13, 4289–4295. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, N.A.; Malyuga, V.V.; Abakarov, G.M.; Aksenov, D.A.; Voskressensky, L.G.; Aksenov, A.V. Synthesis of 3,4-Dihydroisoquinolines Using Nitroalkanes in Polyphosphoric Acid. Russ. Chem. Bull. 2019, 68, 1047–1051. [Google Scholar] [CrossRef]
- Aksenov, A.V.; Khamraev, V.; Aksenov, N.A.; Kirilov, N.K.; Domenyuk, D.A.; Zelensky, V.A.; Rubin, M. Electrophilic Activation of Nitroalkanes in Efficient Synthesis of 1,3,4-Oxadiazoles. RSC Adv. 2019, 9, 6636–6642. [Google Scholar] [CrossRef] [PubMed]
- Popp, F.D.; McEwen, W.E. Polyphosphoric Acids as a Reagent in Organic Chemistry. Chem. Rev. 1958, 58, 321–401. [Google Scholar] [CrossRef]
- Dodd, J.H. Polyphosphoric Acid. In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd.: Chichester, UK, 2001; pp. 183–202. ISBN 0471936235. [Google Scholar]
- Pinchukova, N.A.; Chebanov, V.A.; Gorobets, N.Y.; Gudzenko, L.V.; Ostras, K.S.; Shishkin, O.V.; Hulshof, L.A.; Voloshko, A.Y. Beneficial Energy-Efficiencies in the Microwave-Assisted Vacuum Preparation of Polyphosphoric Acid. Chem. Eng. Process. Process Intensif. 2011, 50, 1193–1197. [Google Scholar] [CrossRef]
- Guy, A.; Guetté, J.-P.; Lang, G. Utilization of Polyphosphoric Acid in the Presence of a Co-Solvent. Synthesis 1980, 1980, 222–223. [Google Scholar] [CrossRef]
- Vekariya, R.H.; Prajapati, N.P.; Patel, H.D. Silica-Supported Polyphosphoric Acid (PPA-SiO2): An Efficient and Reusable Heterogeneous Catalyst for Ecofriendly Organic Synthesis. Synth. Commun. 2016, 46, 197–219. [Google Scholar] [CrossRef]
- Aksenov, N.A.; Aksenov, A.V.; Kornienko, A.; De Carvalho, A.; Mathieu, V.; Aksenov, D.A.; Ovcharov, S.N.; Griaznov, G.D.; Rubin, M. A Nitroalkane-Based Approach to One-Pot Three-Component Synthesis of Isocryptolepine and Its Analogs with Potent Anti-Cancer Activities. RSC Adv. 2018, 8, 36980–36986. [Google Scholar] [CrossRef]
- Sil, S.; Krishnapriya, A.U.; Mandal, P.; Kuniyil, R.; Mandal, S.K. Cross-Coupling Between Aryl Halides and Aryl Alkynes Catalyzed by an Odd Alternant Hydrocarbon. Chem. Eur. J. 2024, 30, e202400895. [Google Scholar] [CrossRef]
- Liang, S.; Hammond, G.B.; Xu, B. Efficient Hydration of Alkynes through Acid-Assisted Brønsted Acid Catalysis. Chem. Commun. 2015, 51, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Hagemeyer, H.J.; Hull, D.C. Reactions of Isopropenyl Acetate. Ind. Eng. Chem. 1949, 41, 2920–2924. [Google Scholar] [CrossRef]
- Hintermann, L.; Labonne, A. Catalytic Hydration of Alkynes and Its Application in Synthesis. Synthesis 2007, 2007, 1121–1150. [Google Scholar] [CrossRef]
- Salvio, R.; Bassetti, M. Sustainable Hydration of Alkynes Promoted by First Row Transition Metal Complexes. Background, Highlights, and Perspectives. Inorganica Chim. Acta 2021, 522, 120288. [Google Scholar] [CrossRef]
- Sedikides, A.T.; Lennox, A.J.J. Silver-Catalyzed (Z)-β-Fluoro-Vinyl Iodonium Salts from Alkynes: Efficient and Selective Syntheses of Z-Monofluoroalkenes. J. Am. Chem. Soc. 2024, 146, 15672–15680. [Google Scholar] [CrossRef] [PubMed]
- Didaskalou, C.; Kupai, J.; Cseri, L.; Barabas, J.; Vass, E.; Holtzl, T.; Szekely, G. Membrane-Grafted Asymmetric Organocatalyst for an Integrated Synthesis–Separation Platform. ACS Catal. 2018, 8, 7430–7438. [Google Scholar] [CrossRef]
- Gholinejad, M.; Esmailoghli, H.; Khosravi, F.; Sansano, J.M. Ionic Liquid Modified Carbon Nanotube Supported Palladium Nanoparticles for Efficient Sonogashira-Hagihara Reaction. J. Organomet. Chem. 2022, 963, 122295. [Google Scholar] [CrossRef]
- Hering, T.; Hari, D.P.; König, B. Visible-Light-Mediated α-Arylation of Enol Acetates Using Aryl Diazonium Salts. J. Org. Chem. 2012, 77, 10347–10352. [Google Scholar] [CrossRef]
- Yu, X.; Park, E.-J.; Kondratyuk, T.P.; Pezzuto, J.M.; Sun, D. Synthesis of 2-Arylindole Derivatives and Evaluation as Nitric Oxide Synthase and NFκB Inhibitors. Org. Biomol. Chem. 2012, 10, 8835. [Google Scholar] [CrossRef]
- Lai, R.-Y.; Surekha, K.; Hayashi, A.; Ozawa, F.; Liu, Y.-H.; Peng, S.-M.; Liu, S.-T. Intra- and Intermolecular Hydroamination of Alkynes Catalyzed by Ortho-Metalated Iridium Complexes. Organometallics 2007, 26, 1062–1068. [Google Scholar] [CrossRef]
- Bhunia, S.K.; Polley, A.; Natarajan, R.; Jana, R. Through-Space 1,4-Palladium Migration and 1,2-Aryl Shift: Direct Access to Dibenzo[a,c]Carbazoles through a Triple C-H Functionalization Cascade. Chem. Eur. J. 2015, 21, 16786–16791. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Annaka, K.; Fujita, A.; Sato, A.; Konakahara, T. InBr3-Promoted Divergent Approach to Polysubstituted Indoles and Quinolines from 2-Ethynylanilines: Switch from an Intramolecular Cyclization to an Intermolecular Dimerization by a Type of Terminal Substituent Group. J. Org. Chem. 2008, 73, 4160–4165. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Lou, Y.; Wang, C.; Qi, L.; Fang, T.; Zhang, F.; Xu, H.; Zhou, L.; Li, W.; Zhang, G.; et al. Chiral Brønsted Acid from Chiral Phosphoric Acid Boron Complex and Water: Asymmetric Reduction of Indoles. Angew. Chem. Int. Ed. 2020, 59, 3294–3299. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Leslie, B.E.; Driver, T.G. Dirhodium(II)-Catalyzed Intramolecular C-H Amination of Aryl Azides. Angew. Chem. Int. Ed. 2008, 47, 5056–5059. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xia, Y.; Jin, Y.; Lu, M. Green and Reusable Homogeneous Oxidative System with Ceric Ammonium Nitrate/[Imim-PEG 1000-TEMPO] for Efficient Aerobic Oxidation of Alcohols and One-pot Synthesis of Benzimidazoles from Alcohols under Ambient Conditions. Appl. Organomet. Chem. 2015, 29, 109–112. [Google Scholar] [CrossRef]
- Jung, J.; Kim, J.; Park, G.; You, Y.; Cho, E.J. Selective Debromination and A-Hydroxylation of α-Bromo Ketones Using Hantzsch Esters as Photoreductants. Adv. Synth. Catal. 2016, 358, 74–80. [Google Scholar] [CrossRef]
- Chai, H.; Wang, L.; Liu, T.; Yu, Z. A Versatile Ru(II)-NNP Complex Catalyst for the Synthesis of Multisubstituted Pyrroles and Pyridines. Organometallics 2017, 36, 4936–4942. [Google Scholar] [CrossRef]
- Li, X.; Che, X.; Chen, G.-H.; Zhang, J.; Yan, J.-L.; Zhang, Y.-F.; Zhang, L.-S.; Hsu, C.-P.; Gao, Y.Q.; Shi, Z.-J. Direct Oxidation of Aliphatic C–H Bonds in Amino-Containing Molecules under Transition-Metal-Free Conditions. Org. Lett. 2016, 18, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, B.; El-Apasery, M.A.; Elnagdi, M.H. Synthesis of New Azolyl Azoles and Azinyl Azoles. J. Heterocycl. Chem. 2005, 42, 483–486. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Pan, X.; Wang, F.; Huang, S. Oxidation of Tertiary Aromatic Alcohols to Ketones in Water. Adv. Synth. Catal. 2018, 360, 3607–3612. [Google Scholar] [CrossRef]
- Pathoor, R.; Bahulayan, D. MCR-Click Synthesis, Molecular Docking and Cytotoxicity Evaluation of a New Series of Indole–Triazole–Coumarin Hybrid Peptidomimetics. New J. Chem. 2018, 42, 6810–6816. [Google Scholar] [CrossRef]

 | |||||
|---|---|---|---|---|---|
| # | Acid, g | Temperature, °C | Solvent | Time, h | Yield, a % |
| 1 | PPA 80%, 2 | 90 | 0.5 | 88 | |
| 2 | PPA 80%, 2 | 100 | 0.5 | 90 | |
| 3 | PPA 80%, 2 | 110 | 0.5 | 93 | |
| 4 | PPA 80%, 1 | 110 | 1 | 85 | |
| 5 | PPA 80%, 4 | 110 | 0.5 | 92 | |
| 6 | PPA 87%, 2 | 100 | 1 | 50 | |
| 7 | H3PO4, 1 mL | 100 | 3 | 65 | |
| 8 | H3PO4, 0.5 mL | reflux | EtOH (1 mL) | 3 | 33 |
| 9 | PPA 80%, 2 | reflux | Toluene (1 mL) | 1.5 | 91 |
 | ||||
|---|---|---|---|---|
| # | Acid, g | Temperature, °C | Solvent | Yield a, % |
| 1 | PPA 80%, 2 | 90 | 88 | |
| 2 | PPA 80%, 2 | 100 | 90 | |
| 3 | PPA 80%, 2 | 110 | 93 | |
| 4 | PPA 80%, 1 | 110 | 85 | |
| 5 | PPA 80%, 4 | 110 | 92 | |
| 6 | PPA 87%, 2 | 100 | 50 | |
| 7 | H3PO4, 2 | 100 | 65 | |
| 8 | H3PO4, 0.5 | reflux | EtOH (1 mL) | 33 |
| 9 | PPA 80%, 2 | reflux | Toluene (1 mL) | 91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aksenov, A.V.; Makieva, D.C.; Arestov, R.A.; Arutiunov, N.A.; Aksenov, D.A.; Aksenov, N.A.; Leontiev, A.V.; Aksenova, I.V. Metal-Free, PPA-Mediated Fisher Indole Synthesis via Tandem Hydroamination–Cyclization Reaction between Simple Alkynes and Arylhydrazines. Int. J. Mol. Sci. 2024, 25, 8750. https://doi.org/10.3390/ijms25168750
Aksenov AV, Makieva DC, Arestov RA, Arutiunov NA, Aksenov DA, Aksenov NA, Leontiev AV, Aksenova IV. Metal-Free, PPA-Mediated Fisher Indole Synthesis via Tandem Hydroamination–Cyclization Reaction between Simple Alkynes and Arylhydrazines. International Journal of Molecular Sciences. 2024; 25(16):8750. https://doi.org/10.3390/ijms25168750
Chicago/Turabian StyleAksenov, Alexander V., Dinara C. Makieva, Rodion A. Arestov, Nikolai A. Arutiunov, Dmitrii A. Aksenov, Nicolai A. Aksenov, Alexander V. Leontiev, and Inna V. Aksenova. 2024. "Metal-Free, PPA-Mediated Fisher Indole Synthesis via Tandem Hydroamination–Cyclization Reaction between Simple Alkynes and Arylhydrazines" International Journal of Molecular Sciences 25, no. 16: 8750. https://doi.org/10.3390/ijms25168750
APA StyleAksenov, A. V., Makieva, D. C., Arestov, R. A., Arutiunov, N. A., Aksenov, D. A., Aksenov, N. A., Leontiev, A. V., & Aksenova, I. V. (2024). Metal-Free, PPA-Mediated Fisher Indole Synthesis via Tandem Hydroamination–Cyclization Reaction between Simple Alkynes and Arylhydrazines. International Journal of Molecular Sciences, 25(16), 8750. https://doi.org/10.3390/ijms25168750







