Melatonin Receptor Expression in Primary Uveal Melanoma
Abstract
1. Introduction
1.1. Uveal Melanoma
1.2. Melatonin
1.3. Melatonin Receptors
1.3.1. Melatonin Receptor Type 1A and Melatonin Receptor Type 1B
1.3.2. N-Ribosyldihydronicotinamide:Quinone Oxidoreductase 2
1.3.3. Retinoic Acid-Related Orphan Receptor Alpha
1.3.4. G Protein-Coupled Receptor 50
1.4. Aim of the Study
2. Results
2.1. Descriptive Statistics
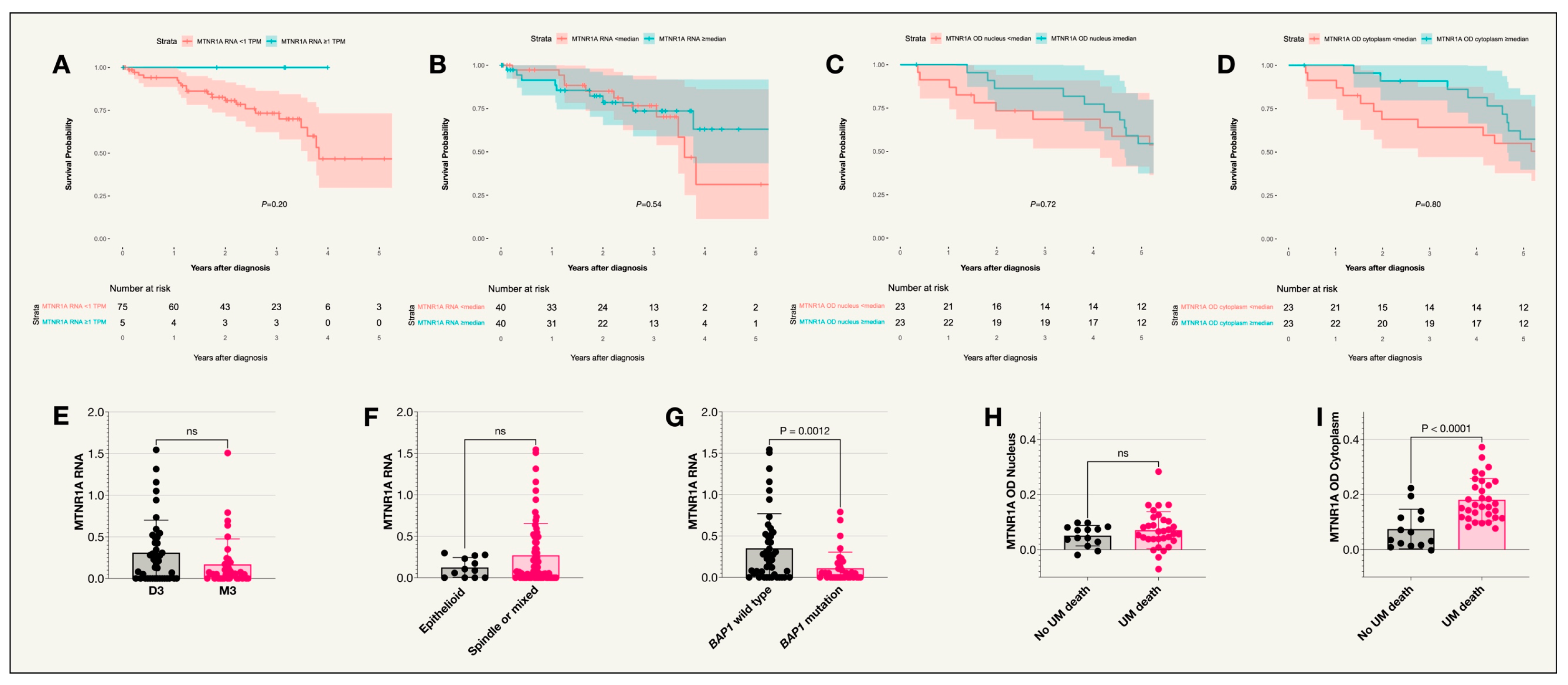
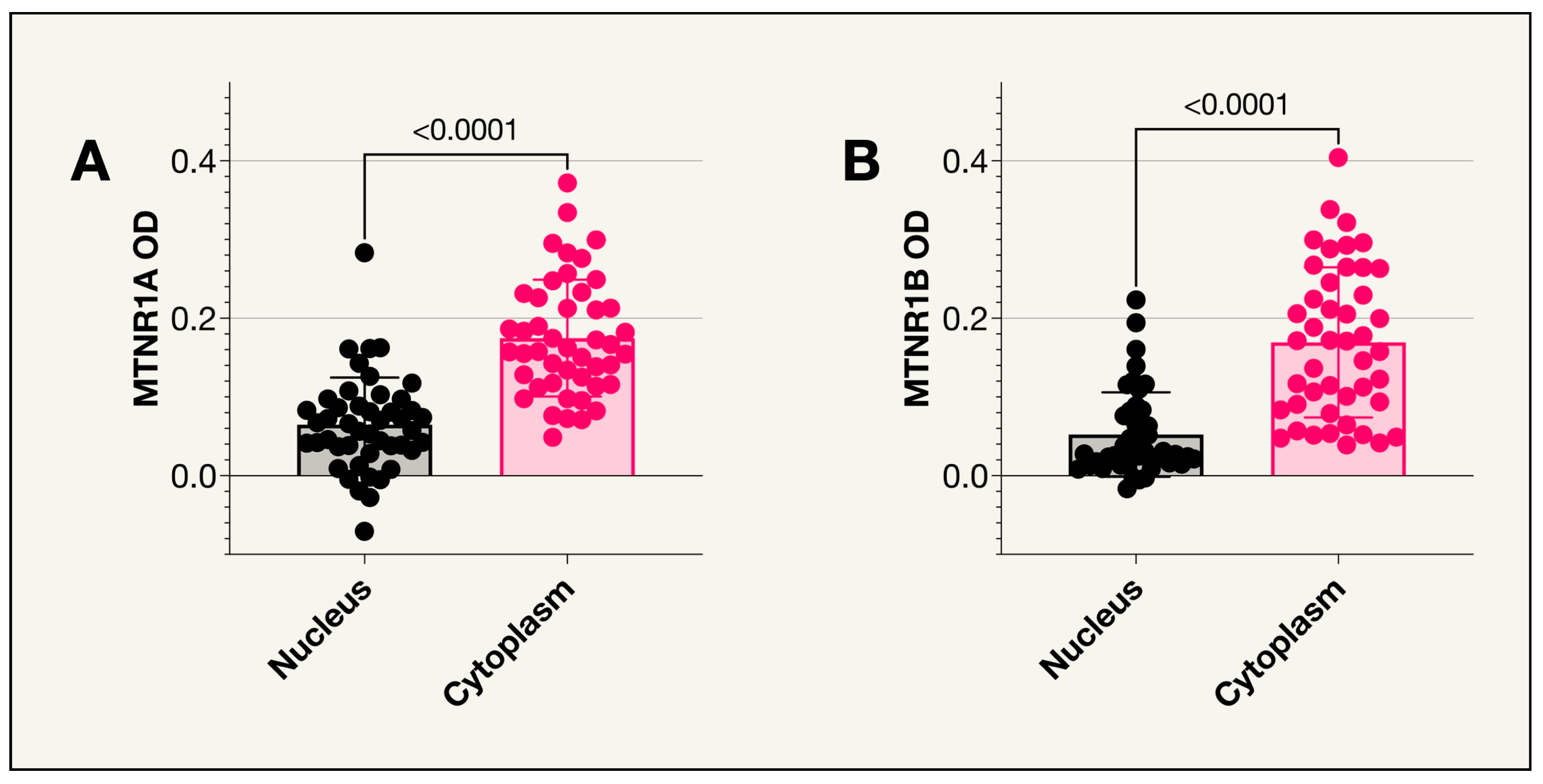
2.2. Melatonin Receptor Type 1A
2.3. Melatonin Receptor Type 1B
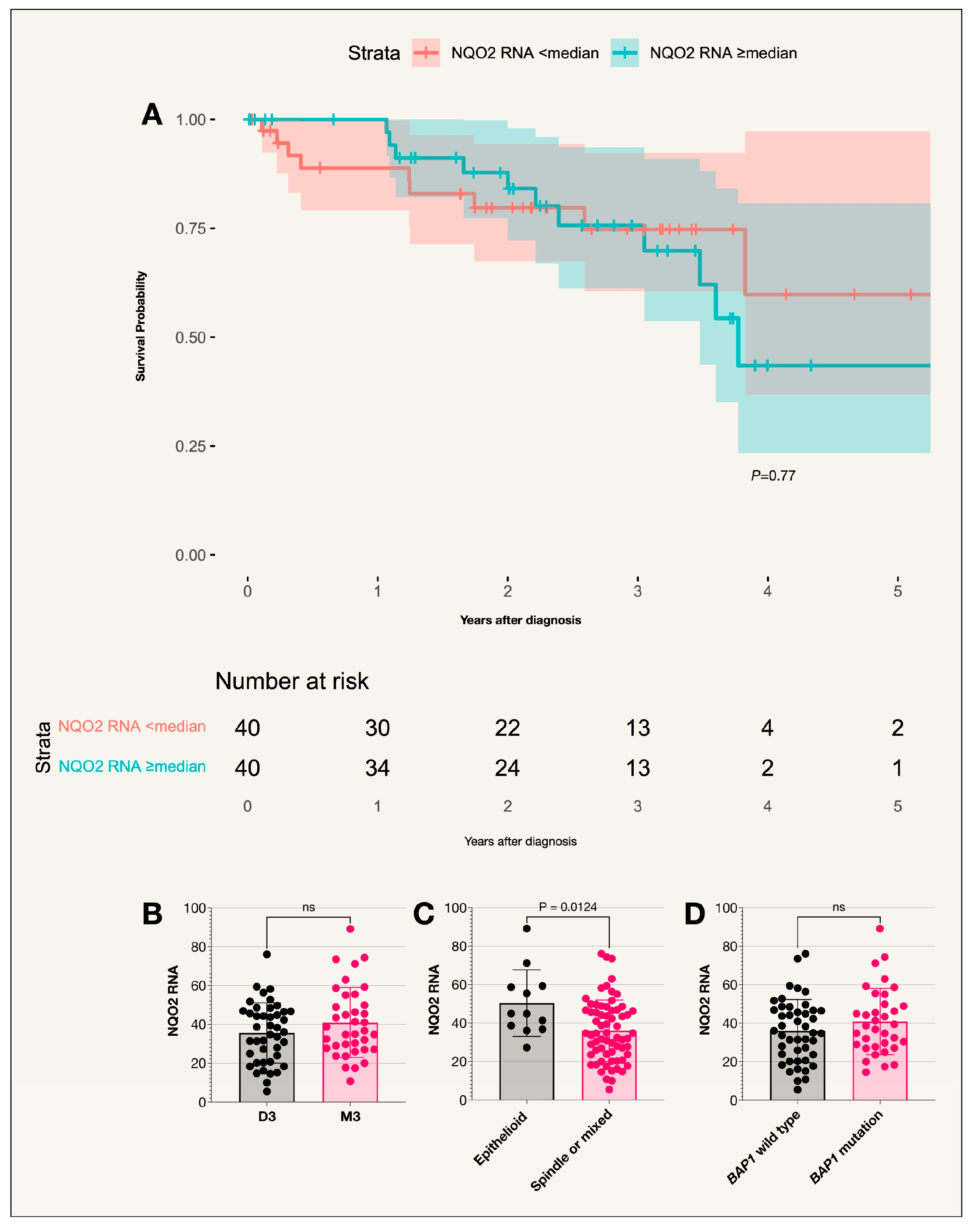
2.4. N-Ribosyldihydronicotinamide:Quinone Oxidoreductase 2
2.5. Retinoic Acid-Related Orphan Receptor Alpha
2.6. G Protein-Coupled Receptor 50
3. Materials and Methods
3.1. Patients and Samples
3.2. Statistical Methods
4. Discussion
4.1. Main Findings
4.2. Context
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal Melanoma: Trends in Incidence, Treatment, and Survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef]
- Stålhammar, G. Forty-year prognosis after plaque brachytherapy of uveal melanoma. Sci. Rep. 2020, 10, 11297. [Google Scholar] [CrossRef]
- Kujala, E.; Mäkitie, T.; Kivelä, T. Very Long-Term Prognosis of Patients with Malignant Uveal Melanoma. Investig. Opthalmol. Vis. Sci. 2003, 44, 4651. [Google Scholar] [CrossRef]
- COMS. The COMS Randomized Trial of Iodine 125 Brachytherapy for Choroidal Melanoma: V. Twelve-Year Mortality Rates and Prognostic Factors: COMS Report No. 28. Arch. Ophthalmol. 2006, 124, 1684–1693. [Google Scholar] [CrossRef]
- Eskelin, S.; Pyrhönen, S.; Summanen, P.; Hahka-Kemppinen, M.; Kivelä, T. Tumor doubling times in metastatic malignant melanoma of the uvea: Tumor progression before and after treatment. Ophthalmology 2000, 107, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, R.D.; Schwartz, G.K.; Tezel, T.; Marr, B.; Francis, J.H.; Nathan, P.D. Metastatic disease from uveal melanoma: Treatment options and future prospects. Br. J. Ophthalmol. 2017, 101, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Steeb, T.; Schlager, J.G.; Rosumeck, S.; Dressler, C.; Ruzicka, T.; Nast, A.; Berking, C. Immune checkpoint blockade for unresectable or metastatic uveal melanoma: A systematic review. Cancer Treat Rev. 2017, 60, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Say, E.A.T.; Hasanreisoglu, M.; Saktanasate, J.; Lawson, B.M.; Landy, J.E.; Badami, A.U.; Sivalingam, M.D.; Mashayekhi, A.; Shields, J.A.; et al. Cytogenetic Abnormalities in Uveal Melanoma Based on Tumor Features and Size in 1059 Patients: The 2016 W. Richard Green Lecture. Ophthalmology 2017, 124, 609–618. [Google Scholar] [CrossRef]
- Herrspiegel, C.; See, T.R.O.; Mendoza, P.R.; Grossniklaus, H.E.; Stålhammar, G. Digital morphometry of tumor nuclei correlates to BAP-1 status, monosomy 3, gene expression class and survival in uveal melanoma. Exp. Eye Res. 2020, 193, 107987. [Google Scholar] [CrossRef]
- Stålhammar, G.; Gill, V.T. Digital morphometry and cluster analysis identifies four types of melanocyte during uveal melanoma progression. Commun. Med. 2023, 3, 60. [Google Scholar] [CrossRef]
- Scheuermann, J.C.; de Ayala Alonso, A.G.; Oktaba, K.; Ly-Hartig, N.; McGinty, R.K.; Fraterman, S.; Wilm, M.; Muir, T.W.; Müller, J. Histone H2A deubiquitinase activity of the Polycomb repressive complex. Nature 2010, 465, 243–247. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.J.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.E.; Proctor, M.; Marquis, S.T.; Gardner, H.P.; Ha, S.I.; Chodosh, L.A.; Ishov, A.M.; Tommerup, N.; Vissing, H.; Sekido, Y.; et al. BAP1: A novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene 1998, 16, 1097–1112. [Google Scholar] [CrossRef] [PubMed]
- Szalai, E.; Wells, J.R.; Ward, L.; Grossniklaus, H.E. Uveal Melanoma Nuclear BRCA1-Associated Protein-1 Immunoreactivity Is an Indicator of Metastasis. Ophthalmology 2018, 125, 203–209. [Google Scholar] [CrossRef]
- Koopmans, A.E.; Verdijk, R.M.; Brouwer, R.W.W.; van den Bosch, T.P.P.; van den Berg, M.M.P.; Vaarwater, J.; Kockx, C.E.; Paridaens, D.; Naus, N.C.; Nellist, M.; et al. Clinical significance of immunohistochemistry for detection of BAP1 mutations in uveal melanoma. Mod. Pathol. 2014, 27, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Prescher, G.; Bornfeld, N.; Hirche, H.; Horsthemke, B.; Jöckel, K.H.; Becher, R. Prognostic implications of monosomy 3 in uveal melanoma. Lancet 1996, 347, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Sanchez, A.; Ferguson, J.A.; Balmer, C.; Daniel, C.; Cohn, A.; Robinson, W.A. Melatonin therapy of advanced human malignant melanoma. Melanoma Res. 1991, 1, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- Tricoire, H.; Møller, M.; Chemineau, P.; Malpaux, B. Origin of cerebrospinal fluid melatonin and possible function in the integration of photoperiod. Reprod. Suppl. 2003, 61, 311–321. [Google Scholar] [CrossRef]
- Majidinia, M.; Sadeghpour, A.; Mehrzadi, S.; Reiter, R.J.; Khatami, N.; Yousefi, B. Melatonin: A pleiotropic molecule that modulates DNA damage response and repair pathways. J. Pineal. Res. 2017, 63, e12416. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J.; Manchester, L.C.; Yan, M.T.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and Physical Properties and Potential Mechanisms: Melatonin as a Broad Spectrum Antioxidant and Free Radical Scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef]
- Miller, S.C.; Pandi, P.S.R.; Esquifino, A.I.; Cardinali, D.P.; Maestroni, G.J.M. The role of melatonin in immuno-enhancement: Potential application in cancer. Int. J. Exp. Pathol. 2006, 87, 81–87. [Google Scholar] [CrossRef]
- Lissoni, P.; Barni, S.; Ardizzoia, A.; Tancini, G.; Conti, A.; Maestroni, G. A randomized study with the pineal hormone melatonin versus supportive care alone in patients with brain metastases due to solid neoplasms. Cancer 1994, 73, 699–701. [Google Scholar] [CrossRef]
- Lissoni, P.; Meregalli, S.; Nosetto, L.; Barni, S.; Tancini, G.; Fossati, V.; Maestroni, G. Increased Survival Time in Brain Glioblastomas by a Radioneuroendocrine Strategy with Radiotherapy plus Melatonin Compared to Radiotherapy Alone. Oncology 1996, 53, 43–46. [Google Scholar] [CrossRef]
- Lissoni, P.; Barni, S.; Mandalà, M.; Ardizzoia, A.; Paolorossi, F.; Vaghi, M.; Longarini, R.; Malugani, F.; Tancini, G. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur. J. Cancer 1999, 35, 1688–1692. [Google Scholar] [CrossRef]
- Mills, E.; Wu, P.; Seely, D.; Guyatt, G. Melatonin in the treatment of cancer: A systematic review of randomized controlled trials and meta-analysis. J. Pineal. Res. 2005, 39, 360–366. [Google Scholar] [CrossRef]
- Zlotos, D.P. Recent progress in the development of agonists and antagonists for melatonin receptors. Curr. Med. Chem. 2012, 19, 3532–3549. [Google Scholar] [CrossRef]
- Hagström, A.; Kal Omar, R.; Williams, P.A.; Stålhammar, G. The rationale for treating uveal melanoma with adjuvant melatonin: A review of the literature. BMC Cancer 2022, 22, 398. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 2010, 62, 343–380. [Google Scholar] [CrossRef]
- Roberts, J.E.; FWiechmann, A.; Hu, D.N. Melatonin receptors in human uveal melanocytes and melanoma cells. J. Pineal. Res. 2000, 28, 165–171. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, X.L.; Holtzclaw, W.D.; Talalay, P. Unexpected genetic and structural relationships of a long-forgotten flavoenzyme to NAD(P)H:quinone reductase (DT-diaphorase). Proc. Natl. Acad. Sci. USA 1997, 94, 1669–1674. [Google Scholar] [CrossRef]
- Calamini, B.; Santarsiero, B.D.; Boutin, J.A.; Mesecar, A.D. Kinetic, thermodynamic and X-ray structural insights into the interaction of melatonin and analogues with quinone reductase 2. Biochem. J. 2008, 413, 81–91. [Google Scholar] [CrossRef]
- Reybier, K.; Perio, P.; Ferry, G.; Bouajila, J.; Delagrange, P.; Boutin, J.A.; Nepveu, F. Insights into the redox cycle of human quinone reductase 2. Free Radic. Res. 2011, 45, 1184–1195. [Google Scholar] [CrossRef]
- Lozinskaya, N.A.; Bezsonova, E.N.; Dubar, M.; Melekhina, D.D.; Bazanov, D.R.; Bunev, A.S.; Grigor’eva, O.B.; Klochkov, V.G.; Sokolova, E.V.; Babkov, D.A.; et al. 3-Arylidene-2-oxindoles as Potent NRH:Quinone Oxidoreductase 2 Inhibitors. Molecules 2023, 28, 1174. [Google Scholar] [CrossRef]
- Giguère, V.; Tini, M.; Flock, G.; Ong, E.; Evans, R.M.; Otulakowski, G. Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev. 1994, 8, 538–553. [Google Scholar] [CrossRef]
- Jetten, A.M. Immunology: A helping hand against autoimmunity. Nature 2011, 472, 421–422. [Google Scholar] [CrossRef]
- Halim, T.Y.F.; MacLaren, A.; Romanish, M.T.; Gold, M.J.; McNagny, K.M.; Takei, F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity 2012, 37, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Solt, L.A.; Kojetin, D.J.; Burris, T.P. Regulation of p53 stability and apoptosis by a ROR agonist. PLoS ONE 2012, 7, e34921. [Google Scholar] [CrossRef]
- Kottorou, A.E.; Antonacopoulou, A.G.; Dimitrakopoulos, F.I.; Tsamandas, A.C.; Scopa, C.D.; Petsas, T.; Kalofonos, H.P. Altered expression of NFY-C and RORA in colorectal adenocarcinomas. Acta Histochem. 2012, 114, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.M.; Marelli, M.M.; Motta, M.; Polizzi, D.; Monestiroli, S.; Pratesi, G.; Limonta, P. Activation of the orphan nuclear receptor RORalpha induces growth arrest in androgen-independent DU 145 prostate cancer cells. Prostate 2001, 46, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xu, R. RORα, a potential tumor suppressor and therapeutic target of breast cancer. Int. J. Mol. Sci. 2012, 13, 15755–15766. [Google Scholar] [CrossRef]
- Brożyna, A.; Jóźwicki, W.; Skobowiat, C.; Jetten, A.; Slominski, A. RORα and RORγ expression inversely correlates with human melanoma progression. Oncotarget 2016, 7, 63261–63282. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Kim, T.-K.; Janjetovic, Z.; Brożyna, A.A.; Podgorska, E.; Dixon, K.M.; Mason, R.S.; Tuckey, R.C.; Sharma, R.; Crossman, D.K.; et al. Malignant Melanoma: An Overview, New Perspectives, and Vitamin D Signaling. Cancers 2024, 16, 2262. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Kang, J.; Fan, W.; He, H.; Huang, F. ROR: Nuclear Receptor for Melatonin or Not? Molecules 2021, 26, 2693. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Levoye, A.; Dam, J.; Ayoub, M.A.; Guillaume, J.L.; Couturier, C.; Delagrange, P.; Jockers, R. The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J. 2006, 25, 3012–3023. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.N.; Roberts, J.E. Melatonin inhibits growth of cultured human uveal melanoma cells. Melanoma Res. 1997, 7, 27–31. [Google Scholar] [CrossRef]
- Hu, D.N.; McCormick, S.A.; Roberts, J.E. Effects of melatonin, its precursors and derivatives on the growth of cultured human uveal melanoma cells. Melanoma Res. 1998, 8, 205–210. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Robertson, A.G.; Shih, J.; Yau, C.; Gibb, E.A.; Oba, J.; Mungall, K.L.; Hess, J.M.; Uzunangelov, V.; Walter, V.; Danilova, L.; et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell 2017, 32, 204–220. [Google Scholar] [CrossRef]
- Cockrum, C.; Kaneshiro, K.R.; Rechtsteiner, A.; Tabuchi, T.M.; Strome, S. A primer for generating and using transcriptome data and gene sets. Development 2020, 147, dev193854. [Google Scholar] [CrossRef]
- Wang, X.T.; Chen, C.W.; Zheng, X.M.; Wang, B.; Zhang, S.X.; Yao, M.H.; Chen, H.; Huang, H.F. Expression and prognostic significance of melatonin receptor MT1 in patients with gastric adenocarcinoma. Neoplasma 2020, 67, 415–420. [Google Scholar] [CrossRef]
- Park, H.K.; Ryu, M.H.; Hwang, D.S.; Kim, G.C.; Jang, M.A.; Kim, U.K. Effects of melatonin receptor expression on prognosis and survival in oral squamous cell carcinoma patients. Int. J. Oral. Maxillofac. Surg. 2022, 51, 713–723. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Li, N.; Liu, W.T.; Liang, J.Z.; Sun, Y.; Zhang, W.X.; Fang, R.D.; Huang, S.L.; Sun, Z.H.; et al. Curcumol Overcomes TRAIL Resistance of Non-Small Cell Lung Cancer by Targeting NRH:Quinone Oxidoreductase 2 (NQO2). Adv. Sci. 2020, 7, 2002306. [Google Scholar] [CrossRef]
- Janda, E.; Boutin, J.A.; De Lorenzo, C.; Arbitrio, M. Polymorphisms and Pharmacogenomics of NQO2: The Past and the Future. Genes 2024, 15, 87. [Google Scholar] [CrossRef]
- Boutin, J.A. Quinone reductase 2 as a promising target of melatonin therapeutic actions. Expert. Opin. Ther. Targets 2016, 20, 303–317. [Google Scholar] [CrossRef]
- Harbour, J.W.; Brantley, M.A.; Hollingsworth, H.; Gordon, M. Association between choroidal pigmentation and posterior uveal melanoma in a white population. Br. J. Ophthalmol. 2004, 88, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.-K.; Slominski, R.M.; Song, Y.; Qayyum, S.; Placha, W.; Janjetovic, Z.; Kleszczyński, K.; Atigadda, V.; Song, Y.; et al. Melatonin and Its Metabolites Can Serve as Agonists on the Aryl Hydrocarbon Receptor and Peroxisome Proliferator-Activated Receptor Gamma. Int. J. Mol. Sci. 2023, 24, 15496. [Google Scholar] [CrossRef]
- Slominski, A.T.; Semak, I.; Fischer, T.W.; Kim, T.K.; Kleszczyński, K.; Hardeland, R.; Reiter, R.J. Metabolism of melatonin in the skin: Why is it important? Exp. Dermatol. 2017, 26, 563–568. [Google Scholar] [CrossRef]


| Analysis | UM-Related Death, Mean DAB OD (SD) | Non UM-Related Death, Mean DAB OD (SD) | p (Holm–Bonferroni Corrected p Value) |
|---|---|---|---|
| MTNR1A OD in Nucleus and UM death | 0.07 (0.07) | 0.05 (0.04) | ns (ns) |
| MTNR1A OD in Cytoplasm and UM death | 0.18 (0.08) | 0.16 (0.07) | *** (**) |
| MTNR1B OD in Nucleus and UM death | 0.04 (0.04) | 0.07 (0.07) | ns (ns) |
| MTNR1B OD in Cytoplasm and UM death | 0.16 (0.09) | 0.20 (0.11) | ns (ns) |
| (A) | |||
|---|---|---|---|
| Analysis | Epithelioid, Mean TPM (SD) | Non-Epithelioid, Mean TPM (SD) | p (Holm-Bonferroni Corrected p Value) |
| NQO2 vs. epithelioid or non-epithelioid | 50 (17) | 35 (16) | * (ns) |
| RORA vs. epithelioid or non-epithelioid | 1 (0.8) | 0.9 (1) | ns (ns) |
| GPR50 vs. epithelioid or non-epithelioid | <0.05 (<0.05) | <0.05 (<0.05) | ns (ns) |
| MTNR1B vs. epithelioid or non-epithelioid | 0 (0) | <0.05 (<0.05) | ns (ns) |
| MTNR1A vs. epithelioid or non-epithelioid | 0.1 (0.1) | 0.3 (0.4) | ns (ns) |
| (B) | |||
| Analysis | Monosomy 3, Mean TPM (SD) | Disomy 3, Mean TPM (SD) | p (Holm–Bonferroni Corrected p Value) |
| NQO2 vs. M3 or D3 | 41 (18) | 36 (15) | ns (ns) |
| RORA vs. M3 or D3 | 1 (1) | 0.9 (1) | ns (ns) |
| GPR50 vs. M3 or D3 | <0.05 (<0.05) | <0.05 (<0.05) | ns (ns) |
| MTNR1B vs. M3 or D3 | <0.05 (<0.05) | 0 (<0.05) | ns (ns) |
| MTNR1A vs. M3 or D3 | 0.4 (0.3) | 0.3 (0.2) | ns (ns) |
| (C) | |||
| Analysis | BAP1 Mutation, Mean TPM (SD) | BAP1 Wildtype, Mean TPM (SD) | p (Holm–Bonferroni Corrected p Value) |
| NQO2 vs. BAP1 mutation or wildtype | 41 (17) | 36 (16) | ns (ns) |
| RORA vs. BAP1 mutation or wildtype | 1.1 (1.1) | 0.9 (0.9) | ns (ns) |
| GPR50 vs. BAP1 mutation or wildtype | <0.05 (<0.05) | <0.05 (<0.05) | ns (ns) |
| MTNR1B vs. BAP1 mutation or wildtype | <0.05 (<0.05) | <0.05 (<0.05) | ns (ns) |
| MTNR1A vs. BAP1 mutation or wildtype | 0.1 (0.2) | 0.4 (0.4) | *** (***) |
| St. Erik Eye Hospital Cohort | TCGA Cohort | |
|---|---|---|
| n | 47 | 80 |
| Sex, n (%) | ||
| Female | 19 (40) | 35 (44) |
| Male | 28 (60) | 45 (56) |
| Ciliary body involvement, n (%) | 8 (16) | 10 (13) |
| Extraocular extension, n (%) | 7 (14) | 6 (8) |
| Tumor thickness at diagnosis, mean mm (SD) | 7.8 (3.8) | 10.8 (2.6) |
| Tumor diameter at diagnosis, mean mm (SD) | 14.7 (4.9) | 16.6 (3.8) |
| Cell type, n (%) | ||
| Spindle | 18 (38) | 28 (35) |
| Epithelioid | 11 (24) | 12 (15) |
| Mixed Spindle and Epithelioid | 18 (38) | 39 (49) |
| Not available | 0 (0) | 1 (1) |
| AJCC T-category, n (%) | ||
| T1a | 5 (11) | 0 (0) |
| T1b | 0 (0) | 0 (0) |
| T1c | 2 (4) | 0 (0) |
| T2a | 9 (19) | 12 (15) |
| T2b | 1 (2) | 2 (3) |
| T2c | 1 (2) | 0 (0) |
| T3a | 11 (24) | 26 (32) |
| T3b | 1 (2) | 5 (6) |
| T3c | 3 (6) | 1 (1) |
| T4a | 3 (6) | 20 (25) |
| T4b | 4 (9) | 9 (11) |
| T4c | 7 (15) | 2 (3) |
| T4d | 0 (0) | 2 (3) |
| T4e | 0 (0) | 1 (1) |
| AJCC stage at diagnosis, n (%) | ||
| I | 5 (11) | 0 (0) |
| IIA | 11 (23) | 4 (5) |
| IIB | 12 (26) | 32 (40) |
| IIIA | 9 (19) | 27 (34) |
| IIIB | 10 (21) | 10 (12) |
| IIIC | 0 (0) | 3 (4) |
| IV | 0 (0) | 4 (5) |
| Primary treatment, n (%) | ||
| Plaque brachytherapy | 17 (36) | 40 (50) |
| Enucleation | 30 (64) | 40 (50) |
| Median follow-up, years (IQR) | ||
| Death from metastatic uveal melanoma | 4.6 (3.4) | 1.7 (1.6) |
| Death from other cause | 9.7 (4.8) | 1.6 (1.8) |
| Alive | - | 2.3 (1.6) |
| AJCC, American Joint Committee on Cancer. IQR, Interquartile range. SD, Standard deviation. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagström, A.; Kal Omar, R.; Witzenhausen, H.; Lardner, E.; Abdiu, O.; Stålhammar, G. Melatonin Receptor Expression in Primary Uveal Melanoma. Int. J. Mol. Sci. 2024, 25, 8711. https://doi.org/10.3390/ijms25168711
Hagström A, Kal Omar R, Witzenhausen H, Lardner E, Abdiu O, Stålhammar G. Melatonin Receptor Expression in Primary Uveal Melanoma. International Journal of Molecular Sciences. 2024; 25(16):8711. https://doi.org/10.3390/ijms25168711
Chicago/Turabian StyleHagström, Anna, Ruba Kal Omar, Hans Witzenhausen, Emma Lardner, Oran Abdiu, and Gustav Stålhammar. 2024. "Melatonin Receptor Expression in Primary Uveal Melanoma" International Journal of Molecular Sciences 25, no. 16: 8711. https://doi.org/10.3390/ijms25168711
APA StyleHagström, A., Kal Omar, R., Witzenhausen, H., Lardner, E., Abdiu, O., & Stålhammar, G. (2024). Melatonin Receptor Expression in Primary Uveal Melanoma. International Journal of Molecular Sciences, 25(16), 8711. https://doi.org/10.3390/ijms25168711






