Abstract
Diabetes mellitus (DM) poses a significant challenge to global health, with its prevalence projected to rise dramatically by 2045. This narrative review explores the bidirectional relationship between periodontitis (PD) and type 1 diabetes mellitus (T1DM), focusing on cellular and molecular mechanisms derived from the interplay between oral microbiota and the host immune response. A comprehensive search of studies published between 2008 and 2023 was conducted to elucidate the association between these two diseases. Preclinical and clinical evidence suggests a bidirectional relationship, with individuals with T1DM exhibiting heightened susceptibility to periodontitis, and vice versa. The review includes recent findings from human clinical studies, revealing variations in oral microbiota composition in T1DM patients, including increases in certain pathogenic species such as Porphyromonas gingivalis, Prevotella intermedia, and Aggregatibacter actinomycetemcomitans, along with shifts in microbial diversity and abundance. Molecular mechanisms underlying this association involve oxidative stress and dysregulated host immune responses, mediated by inflammatory cytokines such as IL-6, IL-8, and MMPs. Furthermore, disruptions in bone turnover markers, such as RANKL and OPG, contribute to periodontal complications in T1DM patients. While preventive measures to manage periodontal complications in T1DM patients may improve overall health outcomes, further research is needed to understand the intricate interactions between oral microbiota, host response, periodontal disease, and systemic health in this population.
1. Introduction
Diabetes mellitus (DM) is identified as a challenge to global health, impacting a substantial segment of the global population. According to projections by the International Diabetes Federation (IDF), the prevalence of diabetes is expected to rise significantly by 2045; it is estimated that 1 in 8 adults, approximately 783 million people, will have DM. The two primary types of diabetes mellitus are type 1 (T1DM) and type 2 (T2DM). T1DM, also called juvenile-onset or insulin-dependent diabetes, is characterized by the autoimmune destruction of pancreatic beta-cells resulting in insulin deficiency, which predominantly affects young individuals [1]. T2DM, also called adult-onset or non-insulin-dependent diabetes, is characterized by insulin resistance.
There is compelling evidence suggesting a bidirectional relationship between periodontitis (PD) and DM, both being chronic inflammatory conditions. Individuals with diabetes demonstrate a higher prevalence of periodontitis, and conversely, those with periodontitis are more prone to diabetes-related complications [2,3,4]. While the relationship between periodontitis and type 2 diabetes mellitus is more extensively studied in the literature, there is a growing interest in understanding its connection with type 1 diabetes mellitus as well [5].
This narrative review aims to elucidate the association between periodontitis and T1DM, focusing on cellular and molecular mechanisms, with particular emphasis on the role of oral microbiota and immune responses. Amidst the increasing focus on T1DM, this review provides a pioneering investigation, greatly enriching our comprehension of the intricate interplay between periodontitis and T1DM. We conducted a comprehensive review of articles pertaining to these two diseases, published in English between 2008 and 2023. Our search utilized the National Library of Medicine, PubMed search engine, employing specific search pathways such as: ((hyperglycemia) AND (diabetes mellitus type I)) AND (modifications in microbiota), type 1 diabetes and periodont* and inflammation, and type 1 diabetes and periodont* and immune response. Few older reference articles frequently cited in the literature were included. The review included all clinical studies published within this timeframe, focusing on the correlation and mechanisms linking periodontal disease and T1DM. All authors screened the articles and categorized them into two groups: studies on microbiological findings (22 studies, 6 of which were published before 2008 but frequently cited in recent articles) and studies on the host immune response (9 studies).
2. Type 1 Diabetes Mellitus
T1DM is the most common autoimmune disease in young patients. This pathology is characterized by the dysregulation of glucose metabolism, attributed to the gradual autoimmune destruction of pancreatic beta cells. Consequently, individuals with T1DM exhibit insulin deficiency coupled with hyperglycemia [1]. According to estimates provided by the American Diabetes Association, T1DM accounts for approximately 5–10% of all diabetes cases. Clinical manifestations commonly associated with T1DM include polydipsia, polyphagia, and polyuria [1].
The global incidence of T1DM has displayed a consistent upward trend, with rates escalating by 2–5% across various regions worldwide. Significantly considerable heterogeneity in diagnosis exists between different geographic locales or continents [6,7,8]. While the epidemiology of childhood-onset T1DM is extensively documented and routinely updated in the IDF Diabetes Atlas, the landscape of adult-onset T1DM remains less elucidated. This knowledge gap can be attributed to historical biases favoring childhood-onset cases, challenges associated with distinguishing adult-onset T1DM from T2DM, and the absence of comprehensive national diabetes registries encompassing T1DM incidence across all age groups [6,9]. Nonetheless, it is acknowledged that T1DM predominantly manifests in childhood but can occur at any age.
A recent systematic review conducted by Harding et al. (2022) [10] aimed to evaluate the incidence of adult-onset T1DM (occurring in individuals over 20 years of age) across 32 countries and regions. The findings of this review underscored a notable burden of adult-onset T1DM incidence, thereby emphasizing the urgent need to enhance both the quality and quantity of information pertaining to adult-onset T1DM, particularly in low- and middle-income countries.
3. Clinical Phenotype of T1DM
T1DM presents a multifaceted clinical phenotype predicated on two key assumptions. Firstly, the onset of T1DM is characterized by evidence of islet-directed autoimmunity preceding the manifestation of dysglycemia or hyperglycemia. Secondly, the pathogenesis of T1DM encompasses diverse pathways leading to beta cell destruction, influenced by variables such as age of onset, genetic predisposition, pancreatic pathology, metabolic dysregulation, insulin secretion dynamics, diabetic complications, and therapeutic responses [11,12].
The clinical diagnosis of T1DM relies on two primary features: insulin deficiency necessitating exogenous insulin therapy and the presence of islet-directed autoantibodies. These criteria serve as pivotal markers guiding the clinical assessment of the disease [13].
T1DM progresses through three discernible stages. The initial stage is characterized by the detection of autoantibodies in the absence of hyperglycemia, defining the prediabetic phase. Subsequently, the second stage ensues with the development of hyperglycemia. Finally, overt diabetes manifests in the third stage, marked by the emergence of clinical symptoms revealing the diabetic process [14].
4. Pathogenesis of Type 1 Diabetes Mellitus
Autoantibodies serve as vital biological markers for autoimmune diabetes, albeit their direct involvement in beta-cell destruction is limited. In children under 5 years old who develop diabetes, these autoantibodies are detected in nearly 100% of cases. They often serve as predictive indicators for diabetes occurrence in first-degree relatives of T1DM patients or in newborns from T1DM parents. Their detection typically precedes disease onset by months or even years, varying with the age of onset. Notably, the presence of two or more antibodies before the age of 3 correlates with a 75% risk of developing T1DM within 10 years, while the presence of all four antibodies indicates a 100% risk over a 20-year follow-up period [15].
Contemporary understanding characterizes T1DM as a multifactorial and heterogeneous disease, with diverse trajectories among patients. While the precise mechanisms remain elusive, genetic, and environmental factors are implicated, suggesting an intricate interplay [16]. Among environmental influences, infections, probiotics, micronutrients, and microbiota have emerged as significant factors in either triggering or exacerbating the disease process [17,18].
Current consensus within the scientific community underscores the autoimmune response to beta cells as precipitated by a myriad of environmental triggers in genetically predisposed individuals. Notably, variations in T1DM incidence among countries likely reflect disparities in susceptibility genetic loci rather than environmental exposures. However, the declining occurrence of high-risk Human leukocyte antigen (HLA) alleles in T1DM cases suggests a pivotal role for gene-environment interactions [19]. The initiation of beta-cell autoimmunity involves diverse environmental factors and gene-environment interactions, mediated by the activation of autoreactive CD4+ helper T cells and CD8+ cytotoxic T cells, ultimately leading to beta-cell apoptosis [13,20].
The subsequent chapter delves into evidence discussing the association between T1DM and periodontitis, incorporating findings from both animal and human studies (Figure 1).
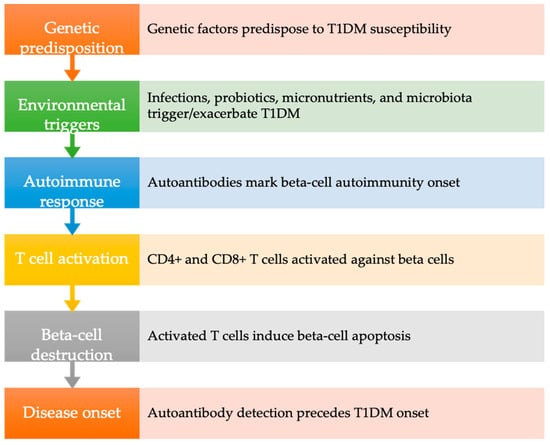
Figure 1.
Pathogenesis of type 1 diabetes mellitus. T1DM: type 1 diabetes mellitus.
5. Preclinical and Clinical Evidence
Animal studies have utilized rodent models, including chemically induced and genetically caused T1DM, to investigate the bidirectional relationship between periodontitis and T1DM. Streptozotocin and alloxan can chemically induce an immune-mediated form of T1DM in rodents, while the non-obese diabetic mouse develops an autoimmune affection in the pancreas resembling human T1DM development [21]. Studies have revealed that periodontal bone loss in T1DM rats is three times higher than in normal rats [22,23] corroborating findings from human studies on the impact of diabetes on the periodontium. However, few rodent studies have explored the consequences of periodontitis on diabetes.
In human studies the association between the two conditions was explored at the level of prevalence, clinical periodontal parameters, and metabolic control. For instance, a cross-sectional study found that periodontitis affected 15.0% of controls and 57.9% of diabetic patients, with severe periodontitis more prevalent in poorly controlled diabetic patients [24]. Another study reported that almost one in five T1DM patients also suffer from periodontal disease [25]. Clinically, diabetic subjects exhibit more plaque, gingival inflammation, and attachment loss compared to controls [26,27]. Additionally, poor glycemic control is associated with worse periodontal clinical parameters [28]. These parameters are strongly correlated with diabetes duration and HbA1c levels [29]. Moreover, T1DM patients exhibit not only increased susceptibility to periodontal diseases, but also to dental caries particularly when metabolic control is poor [26,30]. Meta-analyses have consistently shown higher prevalence and severity of periodontal disease in diabetic patients, with elevated HbA1c levels linked to worse clinical parameters [2,31,32,33]. Interestingly, even patients with relatively good metabolic control, such as those treated with continuous subcutaneous insulin infusion, show a higher prevalence of mild gingivitis compared to non-diabetic individuals [34].
The effect of periodontal treatment on glycemic control in T1DM patients with periodontitis has equally been studied. While periodontal health improved after treatment, no significant effect on glycemic control was observed [35,36]. Nevertheless, providing periodontal care to diabetic patients remains important.
It is evident that the clinical periodontal status of patients with type 1 diabetes is characterized by a heightened susceptibility to periodontal disease, manifesting as gingival inflammation, periodontal pocket formation, and alveolar bone resorption [28]. As mentioned before, longitudinal studies indicate a progression of periodontal disease in diabetic individuals, with a significant correlation between glycemic control and periodontal health [37]. Therapeutically, managing periodontal disease in patients with type 1 diabetes requires a comprehensive approach that integrates periodontal treatment modalities with meticulous glycemic control strategies. Effective management not only aims to arrest periodontal disease progression but also potentially mitigates the systemic complications associated with both diabetes and periodontitis. Effective management of periodontal health in T1DM patients necessitates regular monitoring, stringent glycemic control, interdisciplinary collaboration between healthcare providers, and personalized treatment plans. Emphasizing patient education on the critical connection between diabetes and periodontal disease can also play a pivotal role in mitigating these adverse outcomes. Preventive measures tailored to managing periodontal complications and the use of host modulatory agents in individuals with T1DM can help to improve their overall oral and systemic health outcomes [38].
6. Type 1 Diabetes Mellitus and Oral Microbiota
Environmental factors have been recognized as significant contributors to the onset and progression of T1DM, with emerging attention on the role of the gut microbiota as evidenced by “The Environmental Determinants of Diabetes in the Young Study” [39]. It is hypothesized that the gut microbiome influences T1DM risk through mechanisms such as altered intestinal permeability, modulation of the gut immune system, and molecular mimicry. Animal studies have demonstrated associations between gut flora composition and autoimmune diabetes risk, with lactobacillus and bifidobacterium linked to diabetes resistance, and bacteroides associated with susceptibility [40].
Similarly, human studies reveal disparities in gut microbiota between diabetic and healthy individuals, with T1DM patients exhibiting less diverse and potentially harmful organisms [41]. These variations are influenced by factors such as antibiotic use, dietary components, hygiene practices, and geographical location, all which impact microbiome composition.
Clinical investigations on the gut microbiome consistently report increased Bacteroides species in T1DM subjects compared to controls, alongside increases in Bacilli, Enterobacteriaceae, Streptococcus, Ruminococcus, and Prevotella, with decreases noted for Bifidobacteria, Butyrate-producing bacteria, Haemophilus, Veillonella, and occasionally Prevotella. Recently, Abudqwider et al. (2023) conducted a systematic review exploring the interplay between the gut microbiome, inflammation, and blood glucose parameters in T1DM patients, finding correlations between HbA1c levels and specific microbial abundances, with Prevotella, Faecalibacterium, and Ruminococcaceae negatively correlated, and Dorea formicigenerans, Bacteroidetes, Lacrobacillales, and Bacteroides positively correlated. Bifidobacteria showed a negative correlation with fasting blood glucose levels [42].
These studies collectively indicate that T1DM is associated with gut dysbiosis and increased intestinal permeability, suggesting modulation of the gut microbiome as a novel therapeutic avenue.
Several efforts have been made to identify if similar modifications appear in the oral microbiome based on the hypothesis that various factors, including oral and systemic diseases like diabetes, may contribute to changes in the composition of the oral microbiome [43,44]. Understanding the microbial variations is particularly complex in T1DM due to the prolonged period between initial β-pancreatic cell damage and clinical disease manifestation [45].
Studies on the oral microbial composition in individuals with T1DM (insulin-dependent diabetes mellitus), have revealed variations in the quantity and quality of bacterial species compared to healthy controls. For instance, Mashimo et al. (1983) observed a microflora predominantly composed of Capnocytophaga species and anaerobic vibrios in individuals with insulin-dependent diabetes mellitus (IDDM) [46]. In contrast, Sastrowijoto et al. (1989) reported low levels of Capnocytophaga species and elevated levels of Porphyromonas gingivalis, Prevotella intermedia, and Aggregatibacter actinomycetemcomitans in IDDM patients [47].
Some studies demonstrated a significant increase in Gram-negative rods and fusiforms in the subgingival microbial composition of T1DM patients, influenced by age, duration of diabetes, or metabolic control, as indicated by HbA1c scores [48], while others found a significantly higher percentage of Prevotella intermedia in periodontal sites with deep pockets among poorly controlled T1DM patients [49]. Examining poorly controlled T1DM patients, they noted elevated levels of pathogens at diseased sites, including Prevotella intermedia, Prevotella melaninogenica, Campylobacter gracilis, Eikenella corrodens, Fusobacterium nucleatum, and Campylobacter rectus, with a significantly higher percentage of Prevotella intermedia noted at sites with deep pockets and attachment loss. Contrary, another study also compared the periodontal condition and subgingival microbial composition of insulin-dependent juvenile diabetic patients with their non-diabetic siblings, finding no statistically significant differences for the tested microorganisms [50].
Recent research has further delved into the relationship between glycemic control, oral microbiota composition, and periodontal health in children with T1DM. Sjodin et al. (2012) compared the microbiota of young adults with T1DM since childhood to an age- and sex-matched non-diabetic control group, observing differences in the prevalence of specific bacteria, though not significant [51]. Diabetics with poor metabolic control exhibited a lower frequency of certain bacteria, emphasizing potential distinctions in microbial composition [51].
Others expanded this investigation by evaluating the subgingival microflora in both insulin-dependent and non-insulin-dependent diabetic patients with periodontitis. Although predominant organisms were identified in the insulin-dependent diabetic group, the study failed to establish statistically significant differences in subgingival microflora between diabetic and healthy individuals [52].
Other studies found no significant difference in the prevalence of putative periodontopathic bacteria between T1DM and healthy children, challenging the notion of an increased risk of periodontitis associated with specific bacterial species in T1DM subjects [53]. Similarly, Lalla et al. (2006), suggested similar subgingival infection patterns between individuals with T1DM and healthy controls, particularly under controlled periodontal disease severity conditions [54]. Accordingly, Singh-Hüsgen et al. (2016) demonstrated that the oral microflora of diabetic children did not differ significantly from that of healthy subjects, challenging previous notions of altered host responses in diabetics leading to increased tissue destruction [55].
On the contrary, some investigations on the interplay between diabetes, periodontal parameters, and microbiota, revealed variations in certain microorganisms between periodontitis patients with and without diabetes [56,57,58] with specific bacteria, such as the F. nucleatum and the Capnocytophaga spp. showing strong associations with diabetes [59].
Recently Mahalakshimi et al. (2019) evaluated the risk of periodontitis associated with specific bacteria in T1DM children, revealing differences in gingival health but no statistically significant association with bacterial prevalence [60]. Other studies continued to explore the correlation between HbA1c levels, oral microbiota, and specific bacterial species, uncovering associations with microbial diversity and periodontal disease in T1DM subjects [61,62].
Many characterized the oral microbiota in children and adults with T1DM, identifying distinctions in bacterial abundance, microbial diversity, and specific genera, providing a more comprehensive understanding of the oral microbiome in the context of T1DM [34,43,63].
In a recent clinical trial, Carelli et al. (2023) explored the association between oral microbiota, dental and periodontal diseases, and glycemic control in T1DM children and adolescents, observing consistent presence of specific bacterial species and associations with poor glycemic control, adverse metabolic outcomes, and oral hygiene practices [64].
The cross-sectional study by Selway et al. (2023) further underlined the complexity of the relationship between oral microbiota, periodontal health, and systemic factors in children with T1D, with microbial diversity influenced by periodontal risk markers and familial history of hyperlipidemia. This emphasizes the multifaceted nature of the oral microbiota in this population, suggesting a potential role of non-Porphyromonas species, such as Prevotella, in contributing to periodontal disease in children with a family history of hyperlipidemia [65]. This emphasizes the multifaceted nature of the oral microbiota in this population, suggesting a potential role of non-Porphyromonas species, such as Prevotella, in contributing to periodontal disease in children with a family history of hyperlipidemia. The disruption of microbial ecosystems in these children may involve putative pathogens that contribute to periodontitis and cardiovascular risk factors in subjects.
The current understanding of bacterial-host interactions in periodontal disease related to T1DM is still limited, emphasizing the necessity for additional research to comprehend the intricate connections between systemic health and periodontitis (Table 1). Furthermore, exploring potential connections between oral microbiota and diabetes during the latent phase offers opportunities for early intervention and potentially delaying disease onset. The main microbiological findings are summarized in Table 2.

Table 1.
Clinical studies on microbiological findings in diabetes mellitus type I and periodontal disease.

Table 2.
Main microbiological findings in diabetes mellitus type I.
7. Type I Diabetes Mellitus and Host Immune Response
The contribution of a dysregulated exaggerated host immune/inflammatory response in periodontitis is clear. Although a large number of studies describing the bi-directional relationship between periodontitis and T2DM is available in the literature [66] the potential mechanisms underlying the possible association between T1DM and periodontal diseases remain unclear. While exploring the intricate relationship between T1DM and PD, it becomes evident that the host inflammatory and immune response serve as a key player driving the progression of both diseases. However, it is increasingly recognized that periodontal pathologies may represent complications of T1DM, sharing common pathogenic mechanisms with other macro- and micro-vascular complications of diabetes, such as retinopathy and nephropathy [67].
The host immune response involves inflammation of gingiva and can be modulated by several host-related factors including diabetes mellitus, smoking, genetics and stress [68]. The immune response and inflammation emerge as central links between autoimmune disorders like T1DM and PD. Notably, hyperglycemia stands out as a crucial risk factor, triggering oxidative stress and inflammation that accelerate tissue dysfunction in the periodontium. Several human clinical studies have been undertaken to explore potential immunological pathways connecting these two diseases (Table 3).

Table 3.
Clinical studies on host immune response in diabetes mellitus type I and periodontal disease.
8. Oxidative Stress
Oxidative stress stands as a pivotal factor in the pathogenesis of diabetes mellitus and periodontal diseases, often serving as a reliable marker for screening diabetes-related periodontal dysregulation [69]. The cascade of hyperglycemia prompts an upsurge in Reactive Oxygen Species (ROS), culminating in structural alterations within proteins, nucleic acids, and lipids, thereby disrupting cellular functionality [78]. Notably, heightened levels of oxidative stress markers have been detected in the Gingival Crevicular Fluid (GCF), saliva, and serum of T1DM patients, showing positive correlations with glycated hemoglobin [69]. Furthermore, oral hygiene education coupled with professional scaling has demonstrated a notable reduction in oxidative stress markers among T1DM patients three months post-periodontal treatment [69]. However, a recent study among Thai adolescents and young adults with T1DM showed no disparity in salivary oxidative stress biomarkers when compared to their healthy counterparts [70]. Nevertheless, salivary total oxidative status levels were linked to both diabetes status and the extent of gingival inflammation, warranting further exploration through clinical studies encompassing varying degrees of periodontal disease [70]. Additionally, Lipski et al. (2021) revealed that reinforcing proper oral hygiene with antibacterial dentifrices notably reduced specific salivary oxidative stress biomarkers in young T1DM patients with gingivitis [79]. Moreover, analyses of GCF microbiology and metabolomics in adults with T1DM undergoing continuous subcutaneous insulin infusion suggested early alterations in the GCF microbiome and metabolite concentrations, potentially attributed to increased oxidative stress markers, thus urging further investigation [34].
9. Host Immune Markers
The interplay between high blood glucose levels and sustained chronic inflammatory mediator secretion significantly contributes to an exaggerated periodontal response in individuals with T1DM. Studies investigating the increase in host immune markers in patients with T1DM and periodontal diseases are expected to shed further light into the processes linking T1DM and periodontitis and provide significant value in understanding better the mechanisms behind diabetic complications and introducing novel therapeutic targets. Elevated plasma levels of IL-8 were found in patients with T1DM and periodontitis [71,80] but also in patients with T1DM independently on their periodontal status possibly associated with high glucose-induced oxidative stress. Furthermore, serum IL-6 levels have shown positive correlations with the extent of periodontal inflammation in T1DM patients, while serum high-density lipoprotein (HDL) cholesterol levels exhibited negative correlations [72,81]. Notably, heightened serum IL-6 levels post-periodontal therapy in T1DM patients were linked to poorer periodontal healing responses, suggesting a potential modulation of the host immune response by IL-6 in T1DM patients [81]. Additionally, increased GCF levels of IL-1β and MMP-9 have been reported in T1DM patients during experimental gingivitis, unrelated to microbial differences [73]. Conversely, Sereti et al. (2021) found no differences in GCF levels of IL-8, MMP-8, and advanced glycation end products (AGEs) between T1DM and non-diabetic individuals [82]. Despite the small sample size, gingival biopsies from adult T1DM patients with aggressive periodontitis exhibited increased expression of MMP-7, -8, -9, and -13 compared to patients without diabetes, underscoring the importance of early periodontal therapy in T1DM [83].
Controversial results have been reported regarding IL-18 levels in children with T1DM due to limitations in collecting samples from an adequate number of patients in order to draw safe conclusions. GCF IL-18 levels were found higher in children with T1DM (n = 30) and gingivitis compared to healthy children with gingivitis (n = 13) [84], while in a larger study GCF IL-18 levels were similar between diabetic (n = 44) and healthy children (n = 44) with gingivitis [85]. While most of the studies compared levels of inflammatory cytokines between diabetic and systemically healthy patients, one very interesting study compared immune markers between T1DM and T2DM with periodontitis [74]. Unexpectedly, GCF IL-1β and TNF-α levels were higher in T1DM periodontitis patients compared to T2DM periodontitis ones. The authors of this study also showed that GCF IL-1β and TNF-α levels were negatively correlated with diabetes duration are higher in cases of recent onset of the disease highlighting the need for periodontal therapy at the early stages of T1DM development. Good metabolic control affects periodontal inflammation in diabetic patients. Indeed, T1DM patients post simultaneous pancreas and kidney transplantation displayed lower GCF levels of inflammatory markers and reduced intensity of periodontitis compared to insulin-treated kidney recipients [75].
Salivary IgA levels were lower in T1DM patients with diabetic neuropathy compared to healthy individuals, offering a non-invasive method for assessing the risk of developing diabetic neuropathy [86]. Furthermore, a case–control study revealed increased salivary IL-17 levels in diabetic children, highlighting potential immune dysregulation in T1DM [87]. Additionally, Yilmaz et al. (2023) demonstrated altered salivary concentrations of macrophage activation-related chemokines and MAPKK-degrading proteolytic activity in T1DM patients showing higher levels of monokine induced by interferon gamma (MIG) and macrophage inflammatory protein-1 alpha (MIP-1α) in saliva of T1DM patients, underscoring the impact of T1DM on the host immune response [88].
Studies exploring bone markers in T1DM patients with periodontitis have revealed intriguing findings. While T1DM patients with periodontitis exhibited lower plasma RANKL:OPG ratios compared to non-diabetic counterparts, they displayed higher serum OPG levels, suggesting impaired bone turnover in T1DM patients during periods of acute periodontitis [76]. Similarly, Antonoglou et al. (2013) reported increased serum OPG levels in T1DM patients, positively correlating with the severity of periodontitis, emphasizing the need for further investigation into the role of OPG in T1DM-associated periodontal diseases [89]. Furthermore, Chairatnathrongporn et al. (2022) observed increased RANKL and RANKL:OPG ratio alongside decreased OPG gene expression levels in saliva of T1DM patients compared to healthy individuals, advocating for more extensive analysis of bone markers in oral fluids to elucidate their role in T1DM and periodontal diseases [77].
While current evidence underscores the intricate interplay between oxidative stress, host immune markers, and periodontal diseases in T1DM patients, further large-scale clinical studies are warranted to validate differences in inflammatory mediators and bone markers between diabetic and non-diabetic individuals across various stages of periodontal diseases.
10. General Conclusions and Future Suggestions
The dynamic interplay between PD and T1DM highlights the significance of comprehensive dental care in managing diabetes mellitus. It is evident from current research that both diseases share common pathogenic mechanisms. A key feature and limitation of this review is its narrative approach, distinct from the systematic methodology commonly used in systematic reviews. Consequently, the narrative format employed here may introduce subjectivity into the selection of included studies and their interpretation. Unlike systematic reviews, our aim is to provide valuable insights into the interplay between the two diseases, rather than incorporate quantitative synthesis techniques like meta-analysis. Recognizing these differences in approach is crucial, as they can shape the interpretation and implications of the findings.
Conducting longitudinal assessments to delineate close interactions between oral microbiota, host response, periodontal disease, and systemic health in T1DM patients could help to develop targeted interventions aimed to mitigate the impact of periodontitis on glycemic control and overall health in individuals with T1DM.
Author Contributions
C.G., E.P. and A.Z. conceived of the present idea. All authors analyzed and interpreted the articles and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Supported in part by USPHS grant K08DE027119 to Dr. E. Papathanasiou from the National Institute of Dental and Craniofacial Research (NIH/NIDCR).
Data Availability Statement
All data generated or analyzed for this review are included in this published article. They are also available on request from the corresponding author (A.Z.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Atkinson, M.A. The Pathogenesis and Natural History of Type 1 Diabetes. Cold Spring Harb. Perspect. Med. 2012, 2, a007641. [Google Scholar] [CrossRef]
- Ziukaite, L.; Slot, D.E.; Van der Weijden, F.A. Prevalence of diabetes mellitus in people clinically diagnosed with periodontitis: A systematic review and meta-analysis of epidemiologic studies. J. Clin. Periodontol. 2018, 45, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Löe, H. Periodontal Disease: The sixth complication of diabetes mellitus. Diabetes Care 1993, 16, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.W. Bidirectional Interrelationships Between Diabetes and Periodontal Diseases: An Epidemiologic Perspective. Ann. Periodontol. 2001, 6, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Păunică, I.; Giurgiu, M.; Dumitriu, A.S.; Păunică, S.; Stoian, A.M.P.; Martu, M.-A.; Serafinceanu, C. The Bidirectional Relationship between Periodontal Disease and Diabetes Mellitus—A Review. Diagnostics 2023, 13, 681. [Google Scholar] [CrossRef]
- Maahs, D.M.; West, N.A.; Lawrence, J.M.; Mayer-Davis, E.J. Epidemiology of Type 1 Diabetes. Endocrinol. Metab. Clin. North Am. 2010, 39, 481–497. [Google Scholar] [CrossRef]
- Mobasseri, M.; Shirmohammadi, M.; Amiri, T.; Vahed, N.; Fard, H.H.; Ghojazadeh, M. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot. Perspect. 2020, 10, 98–115. [Google Scholar] [CrossRef]
- Gregory, G.A.; Robinson, T.I.G.; Linklater, S.E. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: A modelling study (vol 10, pg 741, 2022). Lancet Diabetes Endo. 2022, 10, E11. [Google Scholar]
- A DiMeglio, L.; Evans-Molina, C.; A Oram, R. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Harding, J.L.; Wander, P.L.; Zhang, X.; Li, X.; Karuranga, S.; Chen, H.; Sun, H.; Xie, Y.; Oram, R.A.; Magliano, D.J.; et al. The Incidence of Adult-Onset Type 1 Diabetes: A Systematic Review From 32 Countries and Regions. Diabetes Care 2022, 45, 994–1006. [Google Scholar] [CrossRef]
- Ikegami, H. Which comes first in type 1 diabetes: Autoimmunity or dysglycemia? J. Diabetes Investig. 2023, 14, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Zajec, A.; Podkrajšek, K.T.; Tesovnik, T.; Šket, R.; Kern, B.; Bizjan, B.J.; Schweiger, D.; Battelino, T.; Kovač, J. Pathogenesis of Type 1 Diabetes: Established Facts and New Insights. Genes 2022, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.C. Type 1 diabetes mellitus: Much progress, many opportunities. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Insel, R.A.; Dunne, J.L.; Ziegler, A.-G. General population screening for type 1 diabetes. Curr. Opin. Endocrinol. Diabetes 2015, 22, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Pietropaolo, M.; Towns, R.; Eisenbarth, G.S. Humoral Autoimmunity in Type 1 Diabetes: Prediction, Significance, and Detection of Distinct Disease Subtypes. Cold Spring Harb. Perspect. Med. 2012, 2, a012831. [Google Scholar] [CrossRef]
- Alkanani, A.K.; Hara, N.; Gottlieb, P.A.; Ir, D.; Robertson, C.E.; Wagner, B.D.; Frank, D.N.; Zipris, D. Alterations in Intestinal Microbiota Correlate with Susceptibility to Type 1 Diabetes. Diabetes 2015, 64, 3510–3520. [Google Scholar] [CrossRef] [PubMed]
- Houeiss, P.; Luce, S.; Boitard, C. Environmental Triggering of Type 1 Diabetes Autoimmunity. Front. Endocrinol. 2022, 13, 933965. [Google Scholar] [CrossRef]
- Segerstad, E.M.H.A.; Liu, X.; Uusitalo, U.; Agardh, D.; Aronsson, C.A. Sources of dietary gluten in the first 2 years of life and associations with celiac disease autoimmunity and celiac disease in Swedish genetically predisposed children: The Environmental Determinants of Diabetes in the Young (TEDDY) study. Am. J. Clin. Nutr. 2022, 116, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Redondo, M.J.; Steck, A.K.; Pugliese, A. Genetics of type 1 diabetes. Pediatr. Diabetes 2017, 19, 346–353. [Google Scholar] [CrossRef]
- Oliveira, S.M.R.; Rebocho, A.; Ahmadpour, E.; Nissapatorn, V.; Pereira, M.d.L. Type 1 Diabetes Mellitus: A Review on Advances and Challenges in Creating Insulin Producing Devices. Micromachines 2023, 14, 151. [Google Scholar] [CrossRef]
- Andersen, C.C.P.; Flyvbjerg, A.; Buschard, K.; Holmstrup, P. Relationship Between Periodontitis and Diabetes: Lessons from Rodent Studies. J. Periodontol. 2007, 78, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, D.; Choi, S.; Cha, J.; Bak, E.; Yoo, Y. Diabetic characteristics and alveolar bone loss in streptozotocin- and streptozotocin-nicotinamide-treated rats with periodontitis. J. Periodontal Res. 2014, 49, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Lee, D.-E.; Gunawardhana, K.S.N.D.; Choi, S.-H.; Woo, G.-H.; Cha, J.-H.; Bak, E.-J.; Yoo, Y.-J. Effect of the interaction between periodontitis and type 1 diabetes mellitus on alveolar bone, mandibular condyle and tibia. Acta Odontol. Scand. 2013, 72, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Popławska-Kita, A.; Siewko, K.; Szpak, P.; Król, B.; Telejko, B.; Klimiuk, P.A.; Stokowska, W.; Górska, M.; Szelachowska, M. Association between type 1 diabetes and periodontal health. Adv. Med Sci. 2014, 59, 126–131. [Google Scholar] [CrossRef]
- Dicembrini, I.; Serni, L.; Monami, M.; Caliri, M.; Barbato, L.; Cairo, F.; Mannucci, E. Type 1 diabetes and periodontitis: Prevalence and periodontal destruction—A systematic review. Acta Diabetol. 2020, 57, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Gastaldi, G.; Courvoisier, D.S.; Mombelli, A.; Giannopoulou, C. Periodontal health in a cohort of subjects with type 1 diabetes mellitus. Clin. Exp. Dent. Res. 2019, 5, 243–249. [Google Scholar] [CrossRef]
- Meenawat, A.; Punn, K.; Srivastava, V.; Meenawat, A.S.; Dolas, R.; Govila, V. Periodontal disease and type I diabetes mellitus: Associations with glycemic control and complications. J. Indian Soc. Periodontol. 2013, 17, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.; Parihar, A.S.; Sood, M.; Singh, P.; Singh, N. Relationship between Severity of Periodontal Disease and Control of Diabetes (Glycated Hemoglobin) in Patients with Type 1 Diabetes Mellitus. J. Int. Oral Health 2015, 7, 17–20. [Google Scholar]
- Dakovic, D.; Pavlovic, M.D. Periodontal Disease in Children and Adolescents with Type 1 Diabetes in Serbia. J. Periodontol. 2008, 79, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Ferizi, L.; Bimbashi, V.; Kelmendi, J. Association between metabolic control and oral health in children with type 1 diabetes mellitus. BMC Oral Health 2022, 22, 502. [Google Scholar] [CrossRef]
- Costa, R.; Ríos-Carrasco, B.; Monteiro, L.; López-Jarana, P.; Carneiro, F.; Relvas, M. Association between Type 1 Diabetes Mellitus and Periodontal Diseases. J. Clin. Med. 2023, 12, 1147. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Ding, Z.; Yang, Y. The impact of diabetes on periodontal diseases. Periodontology 2000 2019, 82, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Gopalkrishna, P. Type 1 diabetes and periodontal disease: A literature review. Can. J. Dent. Hyg. 2022, 56, 22–30. [Google Scholar] [PubMed]
- Gregorczyk-Maga, I.; Fiema, M.; Kania, M.; Jachowicz-Matczak, E.; Romaniszyn, D.; Gerreth, K.; Klupa, T.; Wójkowska-Mach, J. Oral Microbiota—One Habitat or Diverse Niches? A Pilot Study of Sampling and Identification of Oral Bacterial and Fungal Biota in Patients with Type I Diabetes Mellitus Treated with Insulin Pump. Int. J. Environ. Res. Public Health 2023, 20, 2252. [Google Scholar] [CrossRef]
- Vergnes, J.; Canceill, T.; Vinel, A.; Laurencin-Dalicieux, S.; Maupas-Schwalm, F.; Blasco-Baqué, V.; Hanaire, H.; Arrivé, E.; Rigalleau, V.; Nabet, C.; et al. The effects of periodontal treatment on diabetic patients: The DIAPERIO randomized controlled trial. J. Clin. Periodontol. 2018, 45, 1150–1163. [Google Scholar] [CrossRef]
- Simpson, T.C.; E Clarkson, J.; Worthington, H.V.; MacDonald, L.; Weldon, J.C.; Needleman, I.; Iheozor-Ejiofor, Z.; Wild, S.H.; Qureshi, A.; Walker, A.; et al. Treatment of periodontitis for glycaemic control in people with diabetes mellitus. Cochrane Database Syst. Rev. 2022, 2022, CD004714. [Google Scholar] [CrossRef]
- Seppälä, B.; Seppälä, M.; Ainamo, J. A longitudinal study on insulin-dependent diabetes mellitus and periodontal disease. J. Clin. Periodontol. 1993, 20, 161–165. [Google Scholar] [CrossRef]
- Balta, M.; Papathanasiou, E.; Blix, I.; Van Dyke, T. Host Modulation and Treatment of Periodontal Disease. J. Dent. Res. 2021, 100, 798–809. [Google Scholar] [CrossRef] [PubMed]
- TEDDY Study Group; Toppari, J.; Akolkar, B.; Salami, F.; Lee, H.-S.; Freyhult, E.; Larsson, H.E.; Törn, C.; Couper, J.J.; Haller, M.J.; et al. The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Ann. New York Acad. Sci. 2008, 1150, 1–13. [Google Scholar] [CrossRef]
- Davis-Richardson, A.G.; Triplett, E.W. A model for the role of gut bacteria in the development of autoimmunity for type 1 diabetes. Diabetologia 2015, 58, 1386–1393. [Google Scholar] [CrossRef]
- Abdellatif, A.M.; Sarvetnick, N.E. Current understanding of the role of gut dysbiosis in type 1 diabetes. J. Diabetes 2019, 11, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Abuqwider, J.; Corrado, A.; Scidà, G.; Lupoli, R.; Costabile, G.; Mauriello, G.; Bozzetto, L. Gut microbiome and blood glucose control in type 1 diabetes: A systematic review. Front. Endocrinol. 2023, 14, 1265696. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, M.; Nassar, M.; Moriel, N.; Cher, A.; Faibis, S.; Ram, D.; Zangen, D.; Yassour, M.; Steinberg, D. Characterization of the Oral Microbiome Among Children With Type 1 Diabetes Compared With Healthy Children. Front. Microbiol. 2021, 12, 756808. [Google Scholar] [CrossRef]
- Pisano, M.; Giordano, F.; Sangiovanni, G.; Capuano, N.; Acerra, A.; D’ambrosio, F. The Interaction between the Oral Microbiome and Systemic Diseases: A Narrative Review. Microbiol. Res. 2023, 14, 1862–1878. [Google Scholar] [CrossRef]
- Størling, J.; Pociot, F. Type 1 Diabetes Candidate Genes Linked to Pancreatic Islet Cell Inflammation and Beta-Cell Apoptosis. Genes 2017, 8, 72. [Google Scholar] [CrossRef]
- Mashimo, P.A.; Yamamoto, Y.; Slots, J.; Park, B.H.; Genco, R.J. The Periodontal Microflora of Juvenile Diabetics: Culture, Immunofluorescence, and Serum Antibody Studies. J. Periodontol. 1983, 54, 420–430. [Google Scholar] [CrossRef]
- Sastrowijoto, S.H.; Hillemans, P.; van Steenbergen, T.J.M.; Abraham-Inpijn, L.; de Graaff, J. Periodontal condition and microbiology of healthy and diseased periodontal pockets in type 1 diabetes mellitus patients. J. Clin. Periodontol. 1989, 16, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Sandholm, L.; Swanljung, O.; Rytömaa, I.; Kaprio, E.A.; Mäenpää, J. Morphotypes of the Subgingival Microflora in Diabetic Adolescents in Finland. J. Periodontol. 1989, 60, 526–528. [Google Scholar] [CrossRef]
- Mandell, R.L.; Dirienzo, J.; Kent, R.; Joshipura, K.; Haber, J. Microbiology of Healthy and Diseased Periodontal Sites in Poorly Controlled Insulin Dependent Diabetics. J. Periodontol. 1992, 63, 274–279. [Google Scholar] [CrossRef]
- Sbordone, L.; Ramaglia, L.; Barone, A.; Ciaglia, R.N.; Tenore, A.; Iacono, V.J. Periodontal Status and Selected Cultivable Anaerobic Microflora of Insulin-Dependent Juvenile Diabetics. J. Periodontol. 1995, 66, 452–461. [Google Scholar] [CrossRef]
- Sjödin, B.; Edblad, E.; Sondell, K.; Dahlén, G. Minor manifestations of periodontal diseases in young adults with type 1 diabetes mellitus. Periodontal and microbiological findings. Acta Odontol. Scand. 2011, 70, 589–596. [Google Scholar] [CrossRef]
- Kumar, V.H.; Kumar, K.M.; Gafoor, A.; Santhosh, V. Evaluation of Subgingival Microflora in Diabetic and Nondiabetic Patients. J. Contemp. Dent. Pr. 2012, 13, 157–162. [Google Scholar] [CrossRef]
- Krishnan, M.; Chamarthi, V.; Arangannal, P.; Santoshkumari; Krishnan, P.; Nichani, M. Detection of putative periodontopathic bacteria in type 1 diabetic and healthy children: A comparative study. Indian J. Dent. Res. 2013, 24, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Lalla, E.; Kaplan, S.; Chang, S.J.; Roth, G.A.; Celenti, R.; Hinckley, K.; Greenberg, E.; Papapanou, P.N. Periodontal infection profiles in type 1 diabetes. J. Clin. Periodontol. 2006, 33, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Singh-Hüsgen, P.; Meissner, T.; Bizhang, M.; Henrich, B.; Raab, W.H.-M. Investigation of the oral status and microorganisms in children with phenylketonuria and type 1 diabetes. Clin. Oral Investig. 2015, 20, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Olczak-Kowalczyk, D.; Pyrżak, B.; Dąbkowska, M.; Pańczyk-Tomaszewska, M.; Miszkurka, G.; Rogozińska, I.; Swoboda-Kopeć, E.; Gozdowski, D.; Kalińska, A.; Piróg, A.; et al. Candida spp. and gingivitis in children with nephrotic syndrome or type 1 diabetes. BMC Oral Health 2015, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Duque, C.; João, M.F.D.; Camargo, G.A.d.C.G.; Teixeira, G.S.; Machado, T.S.; Azevedo, R.d.S.; Mariano, F.S.; Colombo, N.H.; Vizoto, N.L.; Mattos-Graner, R.d.O. Microbiological, lipid and immunological profiles in children with gingivitis and type 1 diabetes mellitus. J. Appl. Oral Sci. 2017, 25, 217–226. [Google Scholar] [CrossRef]
- Castrillon, C.A.; Hincapie, J.P.; Yepes, F.L.; Roldan, N.; Moreno, S.M.; Contreras, A.; Botero, J.E. Occurrence of red complex microorganisms and Aggregatibacter actinomycetemcomitans in patients with diabetes. J. Investig. Clin. Dent. 2013, 6, 25–31. [Google Scholar] [CrossRef]
- Šaferis, V.; Sakalauskiene, J.; Kubilius, R.; Gleiznys, A.; Vitkauskiene, A.; Ivanauskiene, E. Relationship of Clinical and Microbiological Variables in Patients with Type 1 Diabetes Mellitus and Periodontitis. Med Sci. Monit. 2014, 20, 1871–1877. [Google Scholar] [CrossRef]
- Mahalakshmi, K.; Arangannal, P. Santoshkumari Frequency of putative periodontal pathogens among type 1 diabetes mellitus: A case–control study. BMC Res. Notes 2019, 12, 328. [Google Scholar] [CrossRef]
- Jensen, E.D.; Selway, C.A.; Allen, G.; Bednarz, J.; Weyrich, L.S.; Gue, S.; Peña, A.S.; Couper, J. Early markers of periodontal disease and altered oral microbiota are associated with glycemic control in children with type 1 diabetes. Pediatr. Diabetes 2020, 22, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Chowdhury, R.; Bhakta, A.; Mukhopahyay, P.; Ghosh, S. Microbiology of periodontal disease in adolescents with Type 1 diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102333. [Google Scholar] [CrossRef] [PubMed]
- Pachoński, M.; Koczor-Rozmus, A.; Mocny-Pachońska, K.; Łanowy, P.; Mertas, A.; Jarosz-Chobot, P. Oral microbiota in children with type 1 diabetes mellitus. Pediatr. Endocrinol. Diabetes Metab. 2021, 27, 100–108. [Google Scholar] [CrossRef]
- Carelli, M.; Maguolo, A.; Zusi, C.; Olivieri, F.; Emiliani, F.; De Grandi, G.; Unali, I.; Zerman, N.; Signoretto, C.; Maffeis, C. Oral Microbiota in Children and Adolescents with Type 1 Diabetes Mellitus: Novel Insights into the Pathogenesis of Dental and Periodontal Disease. Microorganisms 2023, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Selway, C.A.; Jensen, E.D.; Pena, A.S.; Smart, G.; Weyrich, L.S. Type 1 diabetes, periodontal health, and a familial history of hyperlipidaemia is associated with oral microbiota in children: A cross-sectional study. BMC Oral Health 2023, 23, 15. [Google Scholar] [CrossRef]
- Wu, C.-Z.; Yuan, Y.-H.; Liu, H.-H.; Li, S.-S.; Zhang, B.-W.; Chen, W.; An, Z.-J.; Chen, S.-Y.; Wu, Y.-Z.; Han, B.; et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health 2020, 20, 204. [Google Scholar] [CrossRef]
- King, G.L. The Role of Inflammatory Cytokines in Diabetes and Its Complications. J. Periodontol. 2008, 79, 1527–1534. [Google Scholar] [CrossRef]
- Papathanasiou, E.; Palaska, I.; Theoharides, T.C. Stress hormones regulate periodontal inflammation. J. Biol. Regul. Homeost. Agents 2013, 27, 621–626. [Google Scholar]
- Aral, C.A.; Nalbantoğlu; Nur, B.G.; Altunsoy, M.; Aral, K. Metabolic control and periodontal treatment decreases elevated oxidative stress in the early phases of type 1 diabetes onset. Arch. Oral Biol. 2017, 82, 115–120. [Google Scholar] [CrossRef]
- Aroonrangsee, T.; Chairatnathrongporn, R.; Surarit, R.; Tansriratanawong, K.; Santiprabhob, J.; Boriboonhirunsarn, C.; Promsudthi, A. Salivary oxidative stress biomarkers in thai adolescents and young adults with type 1 diabetes mellitus: A cross-sectional study. J. Int. Soc. Prev. Community Dent. 2023, 13, 333–341. [Google Scholar] [CrossRef]
- Lappin, D.F.; Robertson, D.; Hodge, P.; Treagus, D.; Awang, R.A.; Ramage, G.; Nile, C.J. The Influence of Glycated Hemoglobin on the Cross Susceptibility Between Type 1 Diabetes Mellitus and Periodontal Disease. J. Periodontol. 2015, 86, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Passoja, A.; Knuuttila, M.; Hiltunen, L.; Karttunen, R.; Niemelä, O.; Raunio, T.; Vainio, O.; Hedberg, P.; Tervonen, T. Serum interleukin-6 may modulate periodontal inflammation in type 1 diabetic subjects. J. Clin. Periodontol. 2011, 38, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Franco, L.M.; Braun, T.M.; Lee, A.; Persson, G.R.; Lang, N.P.; Giannobile, W.V. Pro-inflammatory biomarkers during experimental gingivitis in patients with type 1 diabetes mellitus: A proof-of-concept study. J. Clin. Periodontol. 2009, 37, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Aspriello, S.D.; Zizzi, A.; Tirabassi, G.; Buldreghini, E.; Biscotti, T.; Faloia, E.; Stramazzotti, D.; Boscaro, M.; Piemontese, M. Diabetes mellitus-associated periodontitis: Differences between type 1 and type 2 diabetes mellitus. J. Periodontal Res. 2010, 46, 164–169. [Google Scholar] [CrossRef]
- Musiał, M.; Wiench, R.; Kolonko, A.; Choręza, P.; Świętochowska, E.; Niedzielski, D.; Machorowska-Pieniążęk, A.; Skaba, D.; Więcek, A.; Owczarek, A.J.; et al. Type 1 Diabetic Patients After Simultaneous Pancreas and Kidney Transplantation Have Less Intense Periodontal Inflammation Compared to Kidney Recipients Treated with Insulin. Ann. Transplant. 2021, 26, e932426-1–e932426-12. [Google Scholar] [CrossRef]
- Lappin, D.F.; Eapen, B.; Robertson, D.; Young, J.; Hodge, P.J. Markers of bone destruction and formation and periodontitis in type 1 diabetes mellitus. J. Clin. Periodontol. 2009, 36, 634–641. [Google Scholar] [CrossRef]
- Promsudthi, A.; Chairatnathrongporn, R.; Tansriratanawong, K.; Santiprabhob, J.; Boriboonhirunsarn, C. Salivary gene expression of RANK, RANKL, and OPG in type 1 diabetes mellitus and periodontal disease patients. J. Int. Soc. Prev. Community Dent. 2022, 12, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Guerra, J.F.d.C.; Magalhães, C.L.d.B.; Costa, D.C.; Silva, M.E.; Pedrosa, M.L. Dietary açai modulates ROS production by neutrophils and gene expression of liver antioxidant enzymes in rats. J. Clin. Biochem. Nutr. 2011, 49, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Lipski, J.; Duda-Sobczak, A.; Napierala, M.; Florek, E.; Zozulinska-Ziolkiewicz, D.; Wyganowska-Swiatkowska, M. Influence of Chlorhexidine and Cetylpyridine on Periodontal Status and Indicators of Oxidative Stress in Patients with Type 1 Diabetes. Antioxidants 2021, 10, 1732. [Google Scholar] [CrossRef]
- Linhartova, P.B.; Kavrikova, D.; Tomandlova, M.; Poskerova, H.; Rehka, V.; Dušek, L.; Holla, L.I. Differences in Interleukin-8 Plasma Levels between Diabetic Patients and Healthy Individuals Independently on Their Periodontal Status. Int. J. Mol. Sci. 2018, 19, 3214. [Google Scholar] [CrossRef]
- Passoja, A.; Knuuttila, M.; Hiltunen, L.; Karttunen, R.; Niemelä, O.; Raunio, T.; Vainio, O.; Hedberg, P.; Tervonen, T. Serum high-density lipoprotein cholesterol level associated with the extent of periodontal inflammation in type 1 diabetic subjects. J. Clin. Periodontol. 2011, 38, 1071–1077. [Google Scholar] [CrossRef]
- Sereti, M.; Roy, M.; Zekeridou, A.; Gastaldi, G.; Giannopoulou, C. Gingival crevicular fluid biomarkers in type 1 diabetes mellitus: A case–control study. Clin. Exp. Dent. Res. 2020, 7, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Şurlin, P.; Oprea, B.; Solomon, S.M.; Popa, S.G.; Moţa, M.; Mateescu, G.O.; Rauten, A.M.; Popescu, D.M.; Dragomir, L.P.; Puiu, I.; et al. Matrix metalloproteinase -7, -8, -9 and -13 in gingival tissue of patients with type 1 diabetes and periodontitis. Rom. J. Morphol. Embryol. 2014, 55, 1137–1141. [Google Scholar]
- Maksymenko, A.I.; Sheshukova, O.V.; Kuz, I.O.; Lyakhova, N.A.; Tkachenko, I.M. THE LEVEL OF INTERLEUKIN-18 IN THE ORAL FLUID IN PRIMARY SCHOOL CHILDREN WITH CHRONIC CATARRHAL GINGIVITIS AND TYPE I DIABETES MELLITUS. Wiadomosci Lek. (Warsaw, Pol. 1960) 2021, 74, 1336–1340. [Google Scholar] [CrossRef]
- Keles, S.; Anik, A.; Cevik, O.; Abas, B.I.; Anik, A. Gingival crevicular fluid levels of interleukin-18 and tumor necrosis factor-alpha in type 1 diabetic children with gingivitis. Clin. Oral Investig. 2020, 24, 3623–3631. [Google Scholar] [CrossRef] [PubMed]
- Steigmann, L.; Maekawa, S.; Kauffmann, F.; Reiss, J.; Cornett, A.; Sugai, J.; Venegas, J.; Fan, X.; Xie, Y.; Giannobile, W.V.; et al. Changes in salivary biomarkers associated with periodontitis and diabetic neuropathy in individuals with type 1 diabetes. Sci. Rep. 2022, 12, 11284. [Google Scholar] [CrossRef]
- Del Valle, L.M.L.; Ocasio-López, C.; Steffen, M. Comparison of Levels of Salivary Cytokines in Diabetic and Nondiabetic Puerto Rican Children: A Case-control Pilot Study. Pediatr. Dent. 2015, 37, 30–34. [Google Scholar]
- Yilmaz, N.; Polat, R.; Gürsoy, M.; Kaman, W.; Aydin, E.G.; Fteita, D.; Yilmaz, D.; Bikker, F.; Gürsoy, U.K. Salivary macrophage activation-related chemokines and mitogen-activated kinase kinase-degrading proteolytic activity in type 1 diabetes mellitus. J. Periodontol. 2023, 94, 896–904. [Google Scholar] [CrossRef]
- Antonoglou, G.; Knuuttila, M.; Nieminen, P.; Vainio, O.; Hiltunen, L.; Raunio, T.; Niemelä, O.; Hedberg, P.; Karttunen, R.; Tervonen, T. Serum osteoprotegerin and periodontal destruction in subjects with type 1 diabetes mellitus. J. Clin. Periodontol. 2013, 40, 765–770. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).