Abstract
Parasitemia and inflammatory markers are cross-sectionally associated with chronic Chagas cardiomyopathy (CCC) among patients with Trypanosoma cruzi. However, the prospective association of the parasite load and host immune response-related characteristics with CCC (that is, progressors) among T. cruzi seropositive individuals has only been partially defined. In a cohort of T. cruzi seropositive patients in Montes Claros and São Paulo, Brazil who were followed over 10 years, we identified the association of a baseline T. cruzi parasite load and systemic markers of inflammation with a decline in cardiac function and/or the presence of cardiac congestion 10 years later. The progressors (n = 21) were individuals with a significant decline in the left ventricular ejection fraction and/or elevated markers of cardiac congestion after 10 years. The controls (n = 31) had normal markers of cardiac function and congestion at the baseline and at the follow-up. They were matched with the progressors on age, sex, and genetic ancestry. The progressors had higher mean parasite loads at the baseline than the controls (18.3 vs. 0.605 DNA parasite equivalents/20 mL, p < 0.05). Of the 384 inflammation-related proteins analyzed, 47 differed significantly at a false discovery rate- (FDR-) corrected p < 0.05 between the groups. There were 44 of these 47 proteins that were significantly higher in the controls compared to in the progressors, including the immune activation markers CCL21, CXCL12, and HCLS1 and several of the tumor necrosis factor superfamily of proteins. Among the individuals who were seropositive for T. cruzi at the baseline and who were followed over 10 years, those with incident CCC at the 10-year marker had a comparatively higher baseline of T. cruzi parasitemia and lower baseline markers of immune activation and chemotaxis. These findings generate the hypothesis that the early impairment of pathogen-killing immune responses predisposes individuals to CCC, which merits further study.
1. Introduction
Approximately 30% of Trypanosoma cruzi-infected individuals progress to chronic forms of Chagas disease, including chronic Chagas cardiomyopathy (CCC) [1], and a third of patients with CCC develop ventricular dysfunction, heart failure, arrhythmia, or sudden death. Cross-sectional investigations have revealed comparatively higher markers of inflammation and immune activation profiles among patients with CCC versus T. cruzi seropositive controls without CCC [2,3,4,5]. Moreover, detection of T. cruzi DNA in the blood has been associated with CCC, and the parasitic load has been found to inversely correlate with left ventricular dysfunction among CCC patients [6,7]. Broadly, these studies raise the hypothesis that a long-standing T. cruzi infection induces a pro-inflammatory milieu and may implicate Th1 cells and IFNgamma pathways in the pathogenesis of CCC; however, little is known regarding the prospective association of immune activation markers with cardiac decline in T. cruzi seropositive individuals.
In this analysis, we evaluated circulating markers of immune activation and inflammation associated prospectively with worsening cardiac dysfunction and/or congestion after 10 years among T. cruzi seropositive patients. We hypothesized that the parasite DNA levels and the markers of immune activation and inflammation would be higher at the baseline for individuals who had progressive cardiac dysfunction at the 10-year marker compared with controls who remained free from cardiac dysfunction after 10 years.
2. Results
The baseline demographic and clinical characteristics were largely similar for progressors and controls, except for hypertension being more common in progressors (Table 1). The mean baseline T. cruzi DNA levels were significantly higher in progressors than in controls (18.3 vs. 0.605 DNA parasite equivalents/20 mL; p = 0.018).

Table 1.
Baseline Characteristics.
Of the 384 proteins analyzed, 47 had NPX levels that differed significantly across the two groups (Table 2; mean NPX of all proteins across both groups are listed in Supplementary Table S1). The levels were lower in the progressors compared to in the controls for 44 of the 47 proteins, including CCL21, CCL25, CD4, CXCL12, TNF, TNFSF12, and TNFSF13. The levels of the other three proteins (EIF5A, IDS, and JCHAIN) were higher in the progressors compared to in the controls (Figure 1).

Table 2.
Proteins with significantly different normalized expression levels for progressors versus controls (referent).
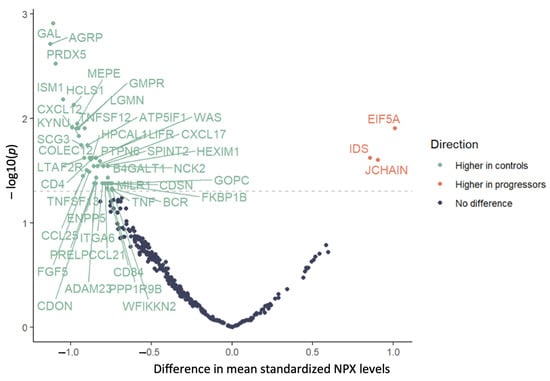
Figure 1.
Mean difference in normalized protein expression levels in progressors vs. controls; dashed line indicates p = 0.05, and statistically significant mean differences indicated above the line.
In secondary analyses restricted to participants with an EF ≥ 55 at the baseline (to exclude those without potential pre-existing CCC with ventricular dysfunction), 39 of the 384 proteins had NPX levels that differed significantly across the two groups, including several that were lower among the progressors (CCL21, CXCL12, HCLS1, and TNFSF12). The levels of only one protein (JCHAIN) were higher in the progressors compared to in the controls in this analysis (Table 2).
Taken together, 31 proteins of the 384 proteins analyzed had NPX levels that differed significantly across both clinical groups in both the primary and secondary analyses (Table 2). Of these, 30 proteins showed higher expression in the controls than in the progressors, including CCL21, CXCL12, HCLS1, and TNFSF12. Only the levels of JCHAIN were elevated in the progressors vs. the controls in both analyses (Table 2).
3. Discussion
In a nested case–control study of a cohort of T. cruzi seropositive individuals followed prospectively for 10 years, we observed that those with progressive cardiac dysfunction and/or congestion had a higher baseline T. cruzi parasite load and lower baseline markers of immune activation and inflammation.
Our findings ran counter to our central hypothesis that there would be higher indices of inflammation at the baseline among T. cruzi seropositive individuals who subsequently had progressive cardiac dysfunction, compared to those without progressive dysfunction. This hypothesis had been informed by cross-sectional studies that noted higher indices of inflammation among patients with prevalent CCC compared to controls without CCC [2,3,4,5]. Nevertheless, these prior studies were cross-sectional, leaving open the possibility that the heightened inflammation among patients who had already progressed to CCC relates to generally heightened inflammatory activation from heart failure [8]. Based on our observations of a comparatively higher parasite load and generally lower markers of immune response and inflammation among progressors versus controls, a plausible hypothesis generated from our findings is that the T. cruzi seropositive individuals with persistent parasitemia and reduced pathogen-killing immunity are at a higher prospective risk of cardiac decline. Prior investigations have shown that Chagas disease in general and CCC in particular are associated with reduced immune responsiveness to antigens during acute and chronic infections [9,10,11]. Moreover, they have shown that Chagas disease in general and CCC in particular are associated with dysfunctional/exhausted CD8+, CD4+, and CD4+CD8+ T cell responses, with increased membrane expression of inhibitory receptors, and with lower antigen-specific multifunctional capacity compared to that of asymptomatic patients [12,13]. Our group found that markers of potentially protective cytotoxic peripheral blood NK or CD8+ T cells were more highly expressed among moderate CCC patients without ventricular dysfunction, compared to severe CCC patients with ventricular dysfunction [14].
In addition, a T. cruzi infection is exacerbated in settings of impaired innate immune response [15,16,17]. Moreover, it has been found that chronic Chagas disease patients with positive blood T. cruzi DNA PCR displayed higher plasma levels of IL-10, a regulatory/immunosuppressive cytokine, than those with negative T. cruzi DNA PCR [18]. Our findings of an increased parasite load and decreased levels of several proteins associated with innate immune response and chemotaxis in the progressors vs. the controls, including CCL21, CXCL12, TNFSF12, and HCLS1, are potentially consistent with this body of literature. CCL21-CCR7 signaling is essential for IFN-gamma-mediated Toxoplasma gondii parasite clearance [19,20], and CCL21 mRNA is increased in end-stage CCC heart tissue [21]. CXCL12 also plays a role in protozoan parasite clearance [22,23] and shows increased levels in established CCC [24]. TNFSF12/TWEAK signaling activates NF-kB-mediated production of proinflammatory mediators [25], while HCLS1 is an established mediator more broadly of leukocyte recruitment and chemotaxis [26] and is an upregulated hub gene in hearts from established end-stage CCC patients and T. cruzi-infected mice [23]. Other markers that were comparatively less expressed at the baseline in the progressors included HCLS1, COLEC12, and MILR1, which is suggestive of impaired pathogen-induced leukocyte activation and antigen presentation [27,28,29,30]. Given our concomitant finding that the progressors displayed a higher amount of T. cruzi DNA in their peripheral blood than the controls, this may suggest that progressors are less capable of controlling the parasitism than the controls. The strengths of this study included a long-term follow-up of the participants for Chagas cardiomyopathy in a cohort with extensive clinical and sociodemographic profiling [31]. The key limitations included a limited sample size and a limited number of progressors, prompting the inclusion of individuals with some degree of LV dysfunction at the baseline as progressors, if their function became significantly more impaired at the follow-up. Additionally, some of the 31 proteins that differed in both analyses were minimally documented in the existing literature and did not have a clear putative relationship with the progression of Chagas disease. Notably, JCHAIN levels were found to be higher in the progressors than in the controls, and this protein has previously been noted to be expressed by mucosal and glandular plasma cells [32]. In addition, proteins were only measured at one point in time (the baseline) and not after 10 years at the follow-up. Moreover, we had a limited ability to account for any residual confounding in the regression analyses due to this sample size.
Nevertheless, our findings suggest that, although immune activation and inflammatory markers may be higher in individuals with prevalent CCC, this may not be the case prior to the development of CCC. Indeed, the results inform a new hypothesis that impairment in immune responses related to pathogen clearance early on—manifested here as decreased markers of immune response and inflammation—may predispose individuals to inferior parasite control and progression to CCC. Specifically, a markedly diminished inflammatory/immune response biomarker profile may develop early in the T. cruzi infection, prior to the onset of CCC/dilated cardiomyopathy, and a considerably more inflammatory profile may emerge once CCC/dilated cardiomyopathy develops.
4. Materials and Methods
4.1. Study Design
We performed a nested case–control study of baseline-circulating protein biomarkers associated with decline in left ventricular function and/or cardiac congestion 10 years later. The participants in this study were recruited between 2008 and 2010 from cohorts of T. cruzi seropositive individuals at two sites in Brazil: São Paulo, São Paulo and Montes Claros, Minas Gerais [31]. The participants underwent standardized health questionnaires and medical evaluations at the baseline and at the 10-year follow-up, including their age, their sex, their hypertension and diabetes statuses, an electrocardiogram (ECG), an echocardiogram (Echo), and a phlebotomy with a cryopreservation of samples for subsequent blinded analyses of T. cruzi PCR and protein biomarkers. The blood samples were collected in EDTA and serum tubes and processed for parasite detection (described below) or spun and aliquoted. All specimens were frozen and maintained at −70 °C.
The progressors were defined as individuals with a substantial decline in left ventricular function on the echocardiography between the baseline and 2018 and/or with evidence of cardiac congestion by 2018 as marked by elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP > 500 pg/mL). This group of progressors is described in Supplementary Figure S1 (n = 21). The controls (n = 31) had normal echocardiographic, ECG, and NT-proBNP findings at the baseline and in 2018 and were propensity matched 2:3 with the progressors on age, sex, and ancestry, based on GWAS data [33]. One progressor and two controls from the original twenty-two and thirty-three, respectively, were excluded due to incomplete data. For the two groups, we compared the baseline T. cruzi parasitemia and the normalized protein expression levels of a panel of 384 proteins relevant to immune response and inflammation.
4.2. Blood Bank Screening Procedures
The Fundação Pro-Sangue blood center performed T. cruzi antibody screening during the initial recruitment using three serological methods, including ELISA, hemagglutination, and immunofluorescence, described previously [34,35]. The participants were included if they were positive in all three assays at the time of the donation. In Montes Claros, the Hemominas blood center screened blood donations with ELISA and hemagglutination. Donors who were positive in both assays at the time of the donation were considered to be eligible for this study.
4.3. Parasite DNA Detection
At the time of the baseline interviews and examinations, 20 mL of EDTA-anti-coagulated blood was collected from each enrolled subject and immediately mixed with an equal volume of 6 M of guanidine HCl–0.2 M EDTA solution, boiled for 15 min, and ultimately vortexed and aliquoted into (1.0 mL) aliquots for the qPCR reactions, as described previously [8].
4.4. Serum Biomarkers
We analyzed the normalized protein expression (NPX) levels from a multiplex immunoassay of 384 inflammatory biomarkers (384 analytes, plus internal controls per panel, Olink Explore 384, Olink Bioscience, Uppsala, Sweden) [36]. The NPX levels were expressed on a log2 scale whereby a one unit increase in the NPX level indicated a doubling of the protein concentration. Each NPX variable was centered to have a mean of 0 and scaled to have a standard deviation of 1 before analysis.
4.5. Statistical Analysis
We compared protein expression in the progressors versus the controls using two-sided t-tests to identify the proteins with significantly different expressions across the groups. To account for multiple comparisons, a false discovery rate- (FDR-) adjusted p-value of <0.05 was required for the protein expression levels to be considered significantly different between the groups. The primary analyses were performed in the complete case group (21 progressors and 31 controls). To investigate whether the associations remained when considering only individuals with a normal LVEF at the baseline, we repeated this comparison in the subgroup of patients with an LVEF > 55% at the baseline (n = 43, n = 12 progressors, and n = 31 controls). The differences in the mean NPX levels and the corresponding FDR-adjusted p-values for each analysis are reported below.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25010044/s1.
Author Contributions
Conceptualization, M.J.F. and E.C.-N.; methodology, M.J.F., E.C.-N., A.S., L.M.C., M.A. and S.A.; software, M.J.F., E.C.-N., A.S., L.M.C., M.A. and S.A.; validation, M.J.F. and A.S.; formal analysis, M.J.F., A.S. and S.A.; investigation, M.J.F., E.C.-N., A.M.F., L.C.d.O.-d.S., A.L.P.R., M.d.C.P.N., E.C.S., A.L., J.K. and C.C.; resources, M.J.F., E.C.-N., A.M.F., L.C.d.O.-d.S., A.L.P.R., M.d.C.P.N., E.C.S., A.L., J.K. and C.C.; data curation, E.C.-N. and M.J.F.; writing—original draft preparation, M.J.F., E.C.-N., A.S., L.M.C., M.A. and S.A.; writing—review and editing, M.J.F., E.C.-N., A.S., L.M.C., M.A., S.A., A.L. and C.C.; visualization, A.S.; supervision, M.J.F. and E.C.-N.; project administration, M.J.F. and E.C.-N.; funding acquisition, M.J.F., E.C.-N., A.M.F., L.C.d.O.-d.S., A.L.P.R., M.d.C.P.N., E.C.S., A.L., J.K. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Northwestern University Havey Institute for Global Health, global health catalyst grant project #1022, which was acquired through collaboration by the authors of this manuscript. Christophe Chevillard was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM); the Aix-Marseille University (grant number: AMIDEX “Internation-al_2018” MITOMUTCHAGAS); the French Agency for Research (Agence Nationale de la Recherche—ANR (grant numbers: “Br-Fr-Chagas” and “landscardio”); and the Inserm Cross-Cutting Project GOLD. This project has received funding from the Excellence Initiative of Aix-Marseille University—A*Midex, a French “Investissements d’Avenir programme”—Institute MarMaRa AMX-19-IET-007. This work was supported by the CAPES-COFECUB program (Me987/23). This work was funded by an NIH grant #1U01AI168383.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Northwestern University (protocol code STU 00212524 approved 5 November 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are openly available in https://biolincc.nhlbi.nih.gov/studies/chagas/ (accessed on 23 November 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Frade, A.F.; Pissetti, C.W.; Ianni, B.M.; Saba, B.; Lin-Wang, H.T.; Nogueira, L.G.; Borges, A.d.M.; Buck, P.; Dias, F.; Baron, M.; et al. Genetic susceptibility to Chagas disease cardiomyopathy: Involvement of several genes of the innate immunity and chemokine-dependent migration pathways. BMC Infect. Dis. 2013, 13, 587. [Google Scholar] [CrossRef] [PubMed]
- Abel, L.C.; Rizzo, L.V.; Ianni, B.; Albuquerque, F.; Bacal, F.; Carrara, D.; Edimar, A.B.; Henrique, C.T.; Charles, M.; Jorge, K.; et al. Chronic Chagas’ disease cardiomyopathy patients display an increased IFN-gamma response to Trypanosoma cruzi infection. J. Autoimmun. 2001, 17, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Bahia-Oliveira, L.; Rocha, M.; Martins-Filho, O.; Gazzinelli, G.; Correa-Oliveira, R. Evidence that development of severe cardiomyopathy in human Chagas’ disease is due to a Th1-specific immune response. Infect. Immun. 2003, 71, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Vitelli-Avelar, D.M.; Sathler-Avelar, R.; Teixeira-Carvalho, A.; Dias, J.C.P.; Gontijo, E.D.; Faria, A.M.; Elói-Santos, S.M.; Martins-Filho, O.A. Strategy to Assess the Overall Cytokine Profile of Circulating Leukocytes and its Association with Distinct Clinical Forms of Human Chagas Disease. Scand. J. Immunol. 2008, 68, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Pinho, R.T.; Waghabi, M.C.; Cardillo, F.; Mengel, J.; Antas, P.R.Z. Scrutinizing the Biomarkers for the Neglected Chagas Disease: How Remarkable! Front. Immunol. 2016, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Sabino, E.C.; Ribeiro, A.L.; Lee, T.H.; Oliveira, C.L.; Carneiro-Proietti, A.B.; Antunes, A.P.; Menezes, M.M.; Ianni, B.M.; Salemi, V.M.; Nastari, L.; et al. Detection of Trypanosoma cruzi DNA in blood by PCR is associated with Chagas cardiomyopathy and disease severity. Eur. J. Heart Fail. 2015, 17, 416–423. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.T.; Schmidt, A.; da Silva, M.C.; Donadi, E.A.; da Silva, J.S.; Marin-Neto, J.A. Parasitic Load Correlates With Left Ventricular Dysfunction in Patients With Chronic Chagas Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 741347. [Google Scholar] [CrossRef]
- Keating, S.M.; Deng, X.; Fernandes, F.; Cunha-Neto, E.; Ribeiro, A.L.; Adesina, B.; Beyer, A.; Contestable, P.; Custer, B.; Busch, M.; et al. Inflammatory and cardiac biomarkers are differentially expressed in clinical stages of Chagas disease. Int. J. Cardiol. 2015, 199, 451–459. [Google Scholar] [CrossRef]
- Kierszenbaum, F. Immunologic deficiency during experimental Chagas’ disease (Trypanosoma cruzi infection): Role of adherent, nonspecific esterase-positive splenic cells. J. Immunol. 1982, 129, 2202–2205. [Google Scholar] [CrossRef]
- Cetron, M.S.; Basilio, F.P.; Paes, J.N.; Sousa, A.Q.; Van Voorhis, W.C.; Kahn, S.J.; Wener, M.H.; Moraes, A.P. Humoral and cellular immune response of adults from northeastern Brazil with chronic Trypanosoma cruzi infection: Depressed cellular immune response to T. cruzi antigen among Chagas’ disease patients with symptomatic versus indeterminate infection. Am. J. Trop. Med. Hyg. 1993, 49, 370–382. [Google Scholar] [CrossRef]
- Rodrigues, V., Jr.; Agrelli, G.S.; Leon, S.C.; Silva Teixeira, D.N.; Tostes, S., Jr.; Rocha-Rodrigues, D.B. Fas/Fas-L expression, apoptosis and low proliferative response are associated with heart failure in patients with chronic Chagas’ disease. Microbes Infect. 2008, 10, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Antón, E.; Egui, A.; Thomas, M.C.; Carrilero, B.; Simón, M.; López-Ruz, M.; Segovia, M.; López, M.C. A proportion of CD4+ T cells from patients with chronic Chagas disease undergo a dysfunctional process, which is partially reversed by benznidazole treatment. PLoS Negl. Trop. Dis. 2021, 15, e0009059. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Antón, E.; Egui, A.; Thomas, M.C.; Simón, M.; Segovia, M.; López, M.C. Immunological exhaustion and functional profile of CD8(+) T lymphocytes as cellular biomarkers of therapeutic efficacy in chronic Chagas disease patients. Acta Trop. 2020, 202, 105242. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.R.P.; Ferreira, F.M.; Nakaya, H.I.; Deng, X.; Cândido, D.D.S.; De Oliveira, L.C.; Billaud, J.-N.; Lanteri, M.C.; Rigaud, V.O.-C.; Seielstad, M.; et al. Blood Gene Signatures of Chagas Cardiomyopathy with or without Ventricular Dysfunction. J. Infect. Dis. 2017, 215, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Torrico, F.; Heremans, H.; Rivera, M.T.; Van Marck, E.; Billiau, A.; Carlier, Y. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J. Immunol. 1991, 146, 3626–3632. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, C.; Köhler, G.; Müller, U.; Mossmann, H.; Schaub, G.N.A.; Brombacher, F. Defective nitric oxide effector functions lead to extreme susceptibility of Trypanosoma cruzi-infected mice deficient in gamma interferon receptor or inducible nitric oxide synthase. Infect. Immun. 1998, 66, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Graefe, S.E.; Jacobs, T.; Gaworski, I.; Klauenberg, U.; Steeg, C.; Fleischer, B. Interleukin-12 but not interleukin-18 is required for immunity to Trypanosoma cruzi in mice. Microbes Infect. 2003, 5, 833–839. [Google Scholar] [CrossRef]
- Salvador, F.; Sánchez-Montalvá, A.; Martínez-Gallo, M.; Sulleiro, E.; Franco-Jarava, C.; Avilés, A.S.; Bosch-Nicolau, P.; Moure, Z.; Silgado, A.; Molina, I. Serum IL-10 Levels and Its Relationship with Parasitemia in Chronic Chagas Disease Patients. Am. J. Trop. Med. Hyg. 2020, 102, 159–163. [Google Scholar] [CrossRef]
- Noor, S.; Habashy, A.S.; Nance, J.P.; Clark, R.T.; Nemati, K.; Carson, M.J.; Wilson, E.H. CCR7-dependent immunity during acute Toxoplasma gondii infection. Infect. Immun. 2010, 78, 2257–2263. [Google Scholar] [CrossRef]
- Ploix, C.C.; Noor, S.; Crane, J.; Masek, K.; Carter, W.; Lo, D.D.; Wilson, E.H.; Carson, M.J. CNS-derived CCL21 is both sufficient to drive homeostatic CD4+ T cell proliferation and necessary for efficient CD4+ T cell migration into the CNS parenchyma following Toxoplasma gondii infection. Brain Behav. Immun. 2011, 25, 883–896. [Google Scholar] [CrossRef]
- Nogueira, L.G.; Santos, R.H.B.; Ianni, B.M.; Fiorelli, A.I.; Mairena, E.C.; Benvenuti, L.A.; Frade, A.; Donadi, E.; Dias, F.; Saba, B.; et al. Myocardial chemokine expression and intensity of myocarditis in Chagas cardiomyopathy are controlled by polymorphisms in CXCL9 and CXCL10. PLoS Negl. Trop. Dis. 2012, 6, e1867. [Google Scholar] [CrossRef] [PubMed]
- Barinov, A.; Luo, L.; Gasse, P.; Meas-Yedid, V.; Donnadieu, E.; Arenzana-Seisdedos, F.; Vieira, P. Essential role of immobilized chemokine CXCL12 in the regulation of the humoral immune response. Proc. Natl. Acad. Sci. USA 2017, 114, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Garnica, M.R.; De Moraes, L.V.; Rizzo, L.V.; De Andrade, H.F., Jr. Supplementation of CXCL12 (CXCL12) induces homing of CD11c+ dendritic cells to the spleen and enhances control of Plasmodium berghei malaria in BALB/c mice. Immunology 2005, 115, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Berbert, L.R.; González, F.B.; Villar, S.R.; Vigliano, C.; Lioi, S.; Beloscar, J.; Bottasso, O.A.; Silva-Barbosa, S.D.; Savino, W.; Pérez, A.R.; et al. Enhanced Migratory Capacity of T Lymphocytes in Severe Chagasic Patients Is Correlated With VLA-4 and TNF-α Expression. Front. Cell Infect. Microbiol. 2021, 11, 713150. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, M.H.; Ríos, L.D.; Corral, R.S. Chronic Trypanosoma cruzi infection activates the TWEAK/Fn14 axis in cardiac myocytes and fibroblasts driving structural and functional changes that affect the heart. Exp. Parasitol. 2023, 248, 108491. [Google Scholar] [CrossRef] [PubMed]
- Castro-Ochoa, K.F.; Guerrero-Fonseca, I.M.; Schnoor, M. Hematopoietic cell-specific lyn substrate (HCLS1 or HS1): A versatile actin-binding protein in leukocytes. J. Leukoc. Biol. 2019, 105, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cao, J.; Fan, Y.; Li, C.; Hu, X. Comprehensive analysis of miRNA-mRNA regulatory network and potential drugs in chronic chagasic cardiomyopathy across human and mouse. BMC Med. Genomics 2021, 14, 283. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Clinton, J.; Yao, C.; Chang, S.H. Interleukin-17D Promotes Pathogenicity during Infection by Suppressing CD8 T Cell Activity. Front. Immunol. 2019, 10, 1172. [Google Scholar] [CrossRef]
- Nanatsue, K.; Ninomiya, T.; Tsuchiya, M.; Tahara-Hanaoka, S.; Shibuya, A.; Masuko, H.; Sakamoto, T.; Hizawa, N.; Arinami, T.; Noguchi, E. Influence of MILR1 promoter polymorphism on expression levels and the phenotype of atopy. J. Human. Genet. 2014, 59, 480–483. [Google Scholar] [CrossRef]
- Nagasawa, T. CXC chemokine ligand 12 (CXCL12) and its receptor CXCR4. J. Mol. Med. 2014, 92, 433–439. [Google Scholar] [CrossRef]
- Sabino, E.C.; Ribeiro, A.L.; Salemi, V.M.; Oliveira, C.D.L.; Antunes, A.P.; Menezes, M.M.; Ianni, B.M.; Nastari, L.; Fernandes, F.; Patavino, G.M.; et al. Ten-year incidence of Chagas cardiomyopathy among asymptomatic Trypanosoma cruzi-seropositive former blood donors. Circulation 2013, 127, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Johansen, F.E.; Braathen, R.; Brandtzaeg, P. Role of J chain in secretory immunoglobulin formation. Scand. J. Immunol. 2000, 52, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Sabino, E.C.; Cunha-Neto, E.; Ribeiro, A.L.; Ianni, B.; Mady, C.; Busch, M.P.; Seielstad, M.; International Component; The REDSII Chagas Study Group from the NHLBI Retrovirus Epidemiology Donor Study-II (REDS-II). Genome Wide Association Study (GWAS) of Chagas Cardiomyopathy in Trypanosoma cruzi Seropositive Subjects. PLoS ONE 2013, 8, e79629. [Google Scholar] [CrossRef] [PubMed]
- Salles, N.; Sabino, E.; Cliquet, M.; Eluf-Neto, J.; Mayer, A.; Almeida-Neto, C.; Mendonça, M.C.; Dorliach-Llacer, P.; Chamone, D.F.; MSc, A.S.-A.; et al. Risk of exposure to Chagas’ disease among seroreactive Brazilian blood donors. Transfusion 1996, 36, 969–973. [Google Scholar] [CrossRef]
- Saéz-Alquézar, A.; Otani, M.M.; Sabino, E.C.; Ribeiro-dos-Santos, G.; Salles, N.; Chamone, D.F. Evaluation of the performance of Brazilian blood banks in testing for Chagas’ disease. Vox Sang. 1998, 74, 228–231. [Google Scholar] [CrossRef]
- Sanders-van Wijk, S.; Tromp, J.; Beussink-Nelson, L.; Hage, C.; Svedlund, S.; Saraste, A.; Swat, S.A.; Sanchez, C.; Njoroge, J.; Tan, R.-S.; et al. Proteomic Evaluation of the Comorbidity-Inflammation Paradigm in Heart Failure With Preserved Ejection Fraction: Results From the PROMIS-HFpEF Study. Circulation 2020, 142, 2029–2044. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).