The Functions of Pt-DIC and Pt-Lamin B in Spermatogenesis of Portunus trituberculatus
Abstract
1. Introduction
2. Results
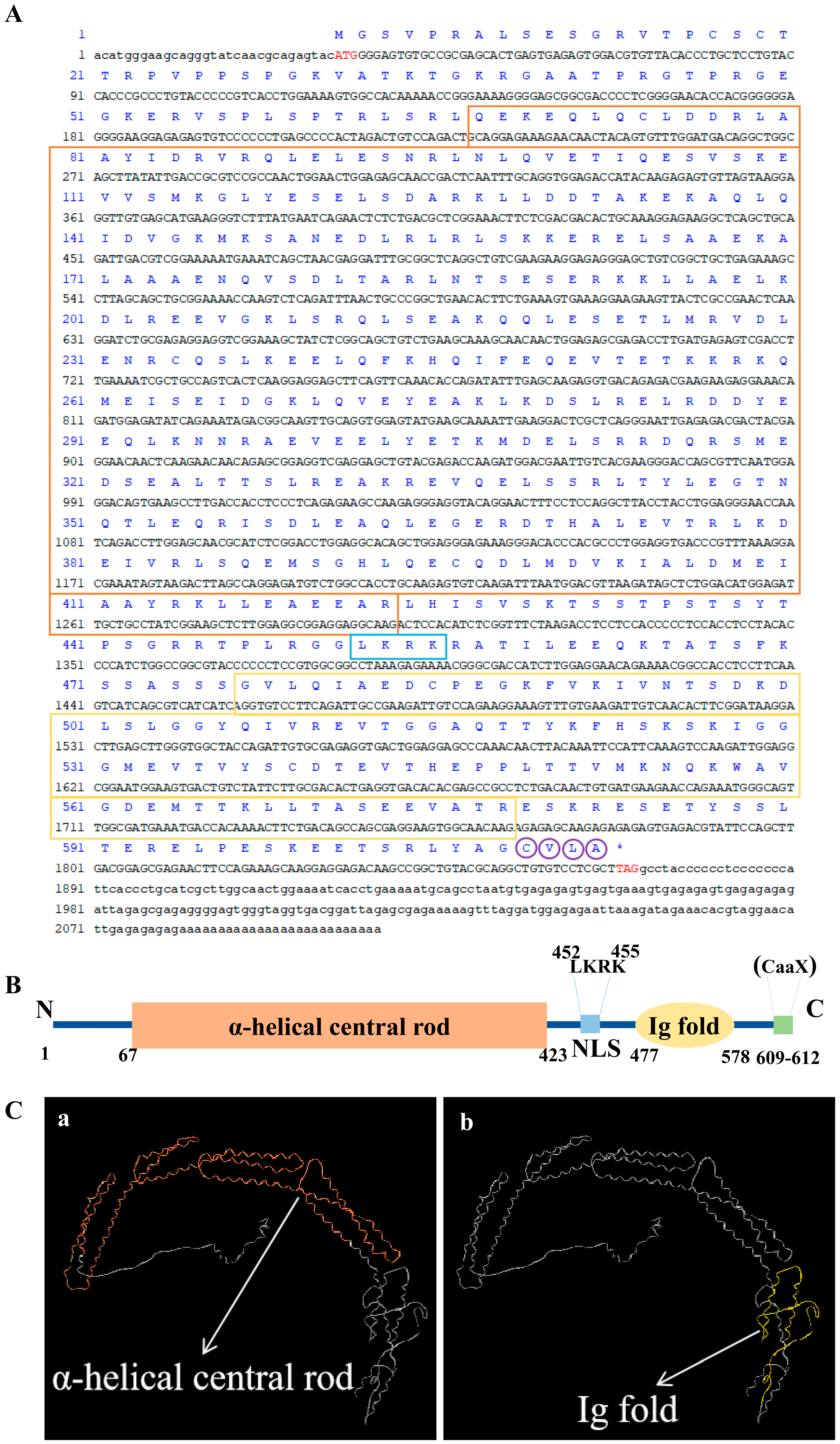
2.1. Prediction of the Full-Length Sequence of Pt-Lamin B cDNA and Its Protein Structure
2.2. Pt-Lamin B Multiple Sequence Alignment and Phylogenetic Tree Analysis
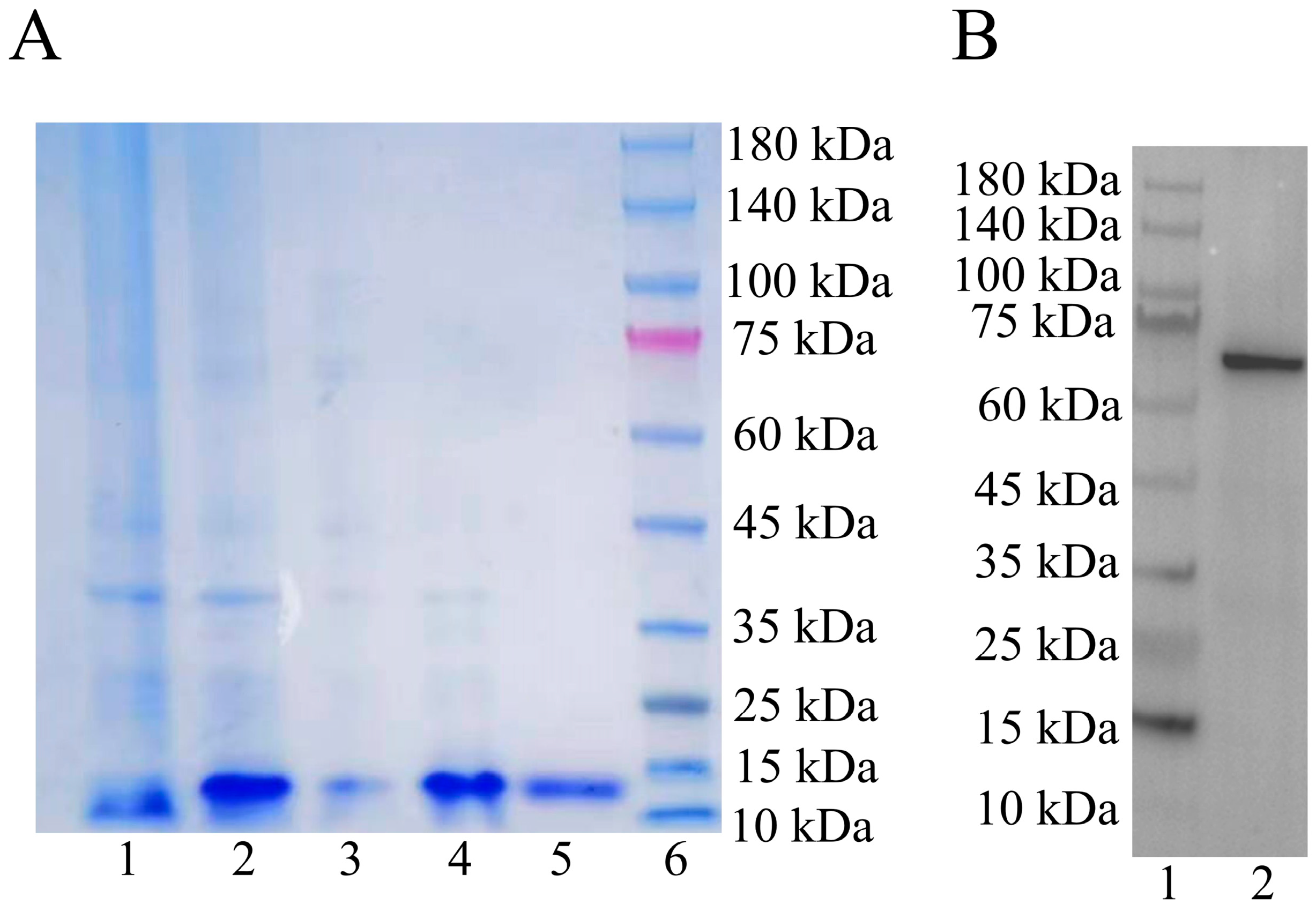
2.3. Validation of the Pt-Lamin B Antibody
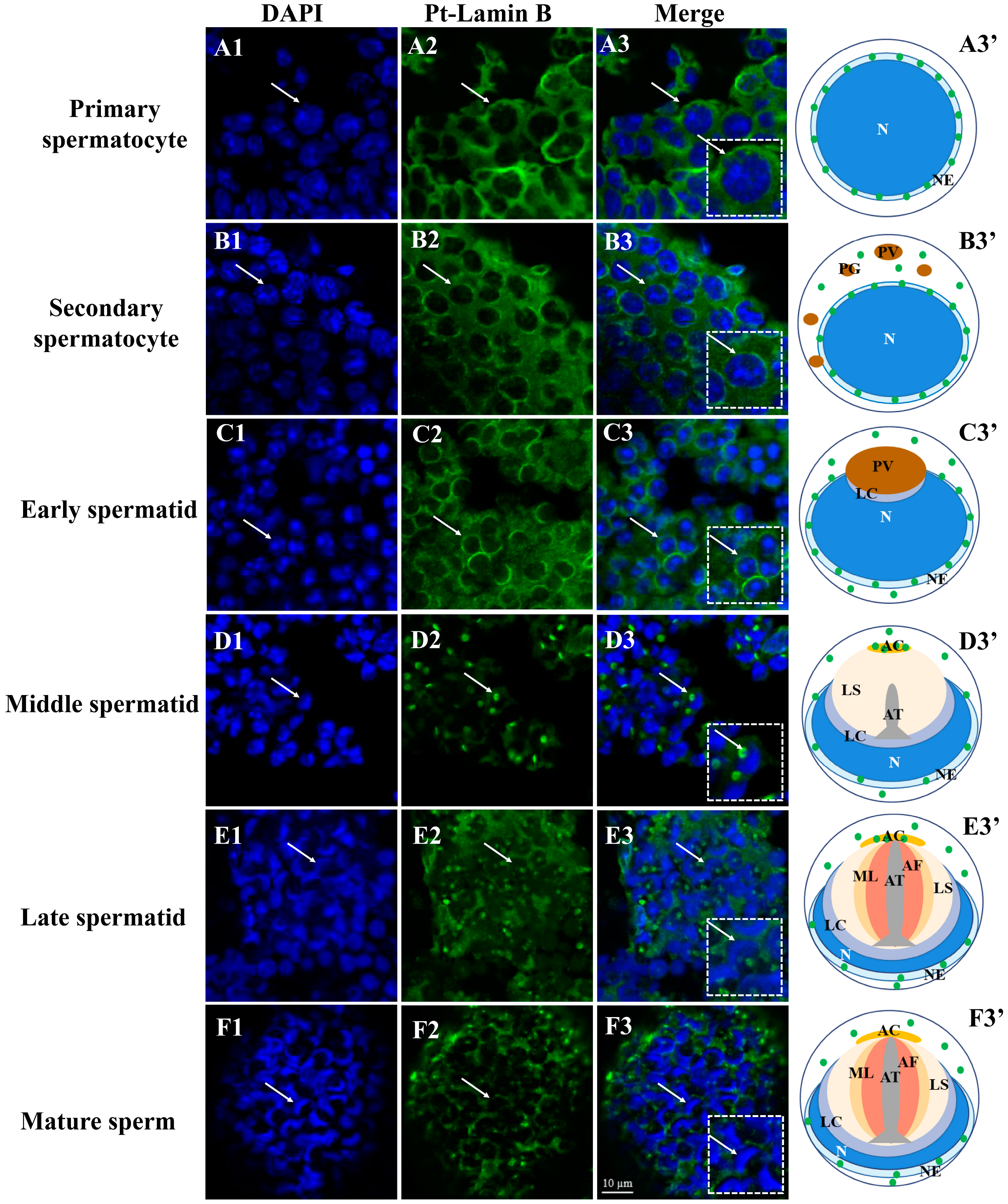
2.4. Expression and Distribution of Pt-Lamin B during Spermatogenesis in P. trituberculatus
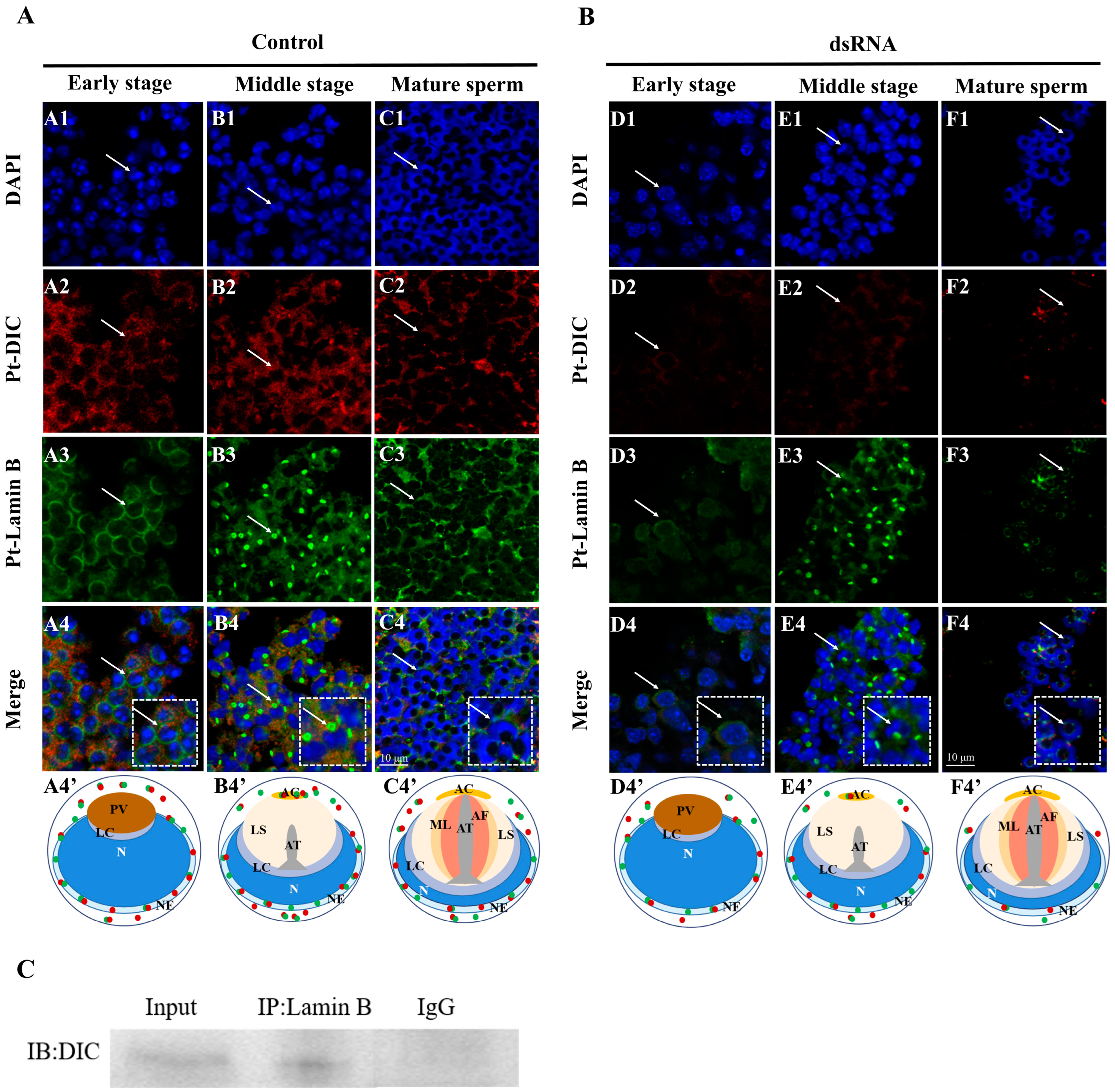
2.5. Colocalization of Pt-DIC and Pt-Lamin B during Spermiogenesis in P. trituberculatus
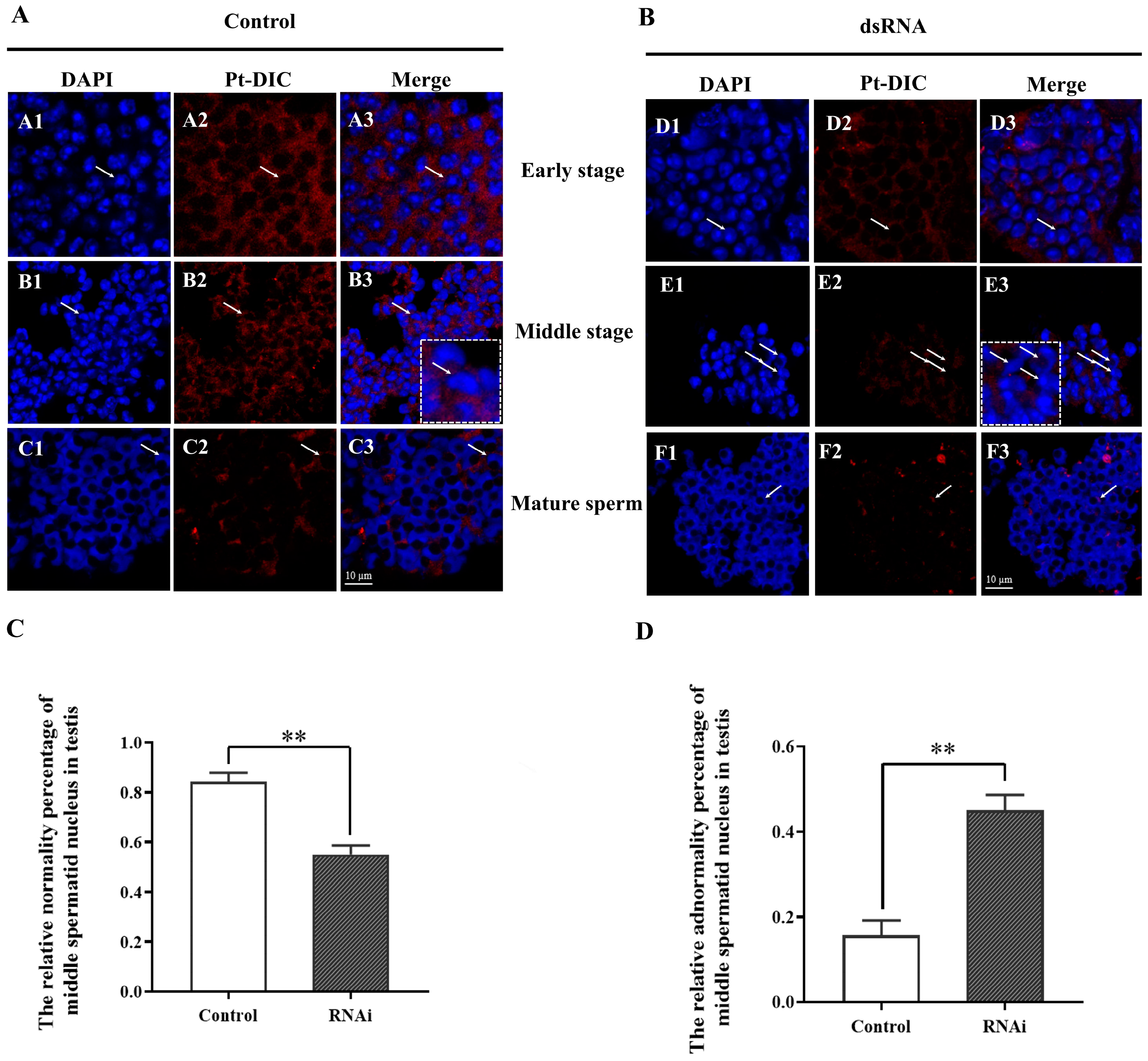
2.6. Effects of Interference with Pt-DIC at the Individual Level on Spermatid Nucleus Deformation and Spermiogenesis
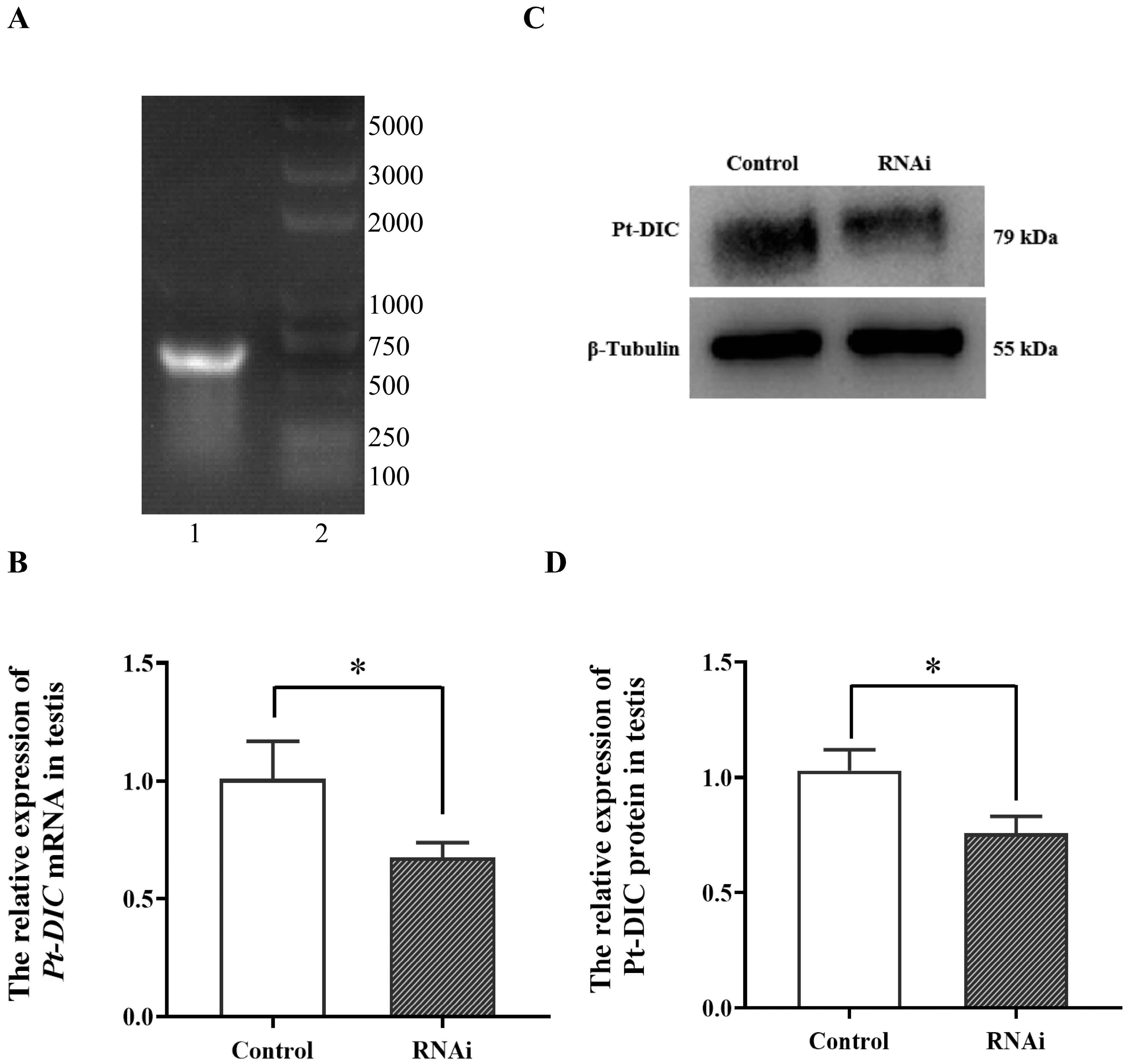
2.6.1. Preparation of Pt-DIC Gene dsRNA
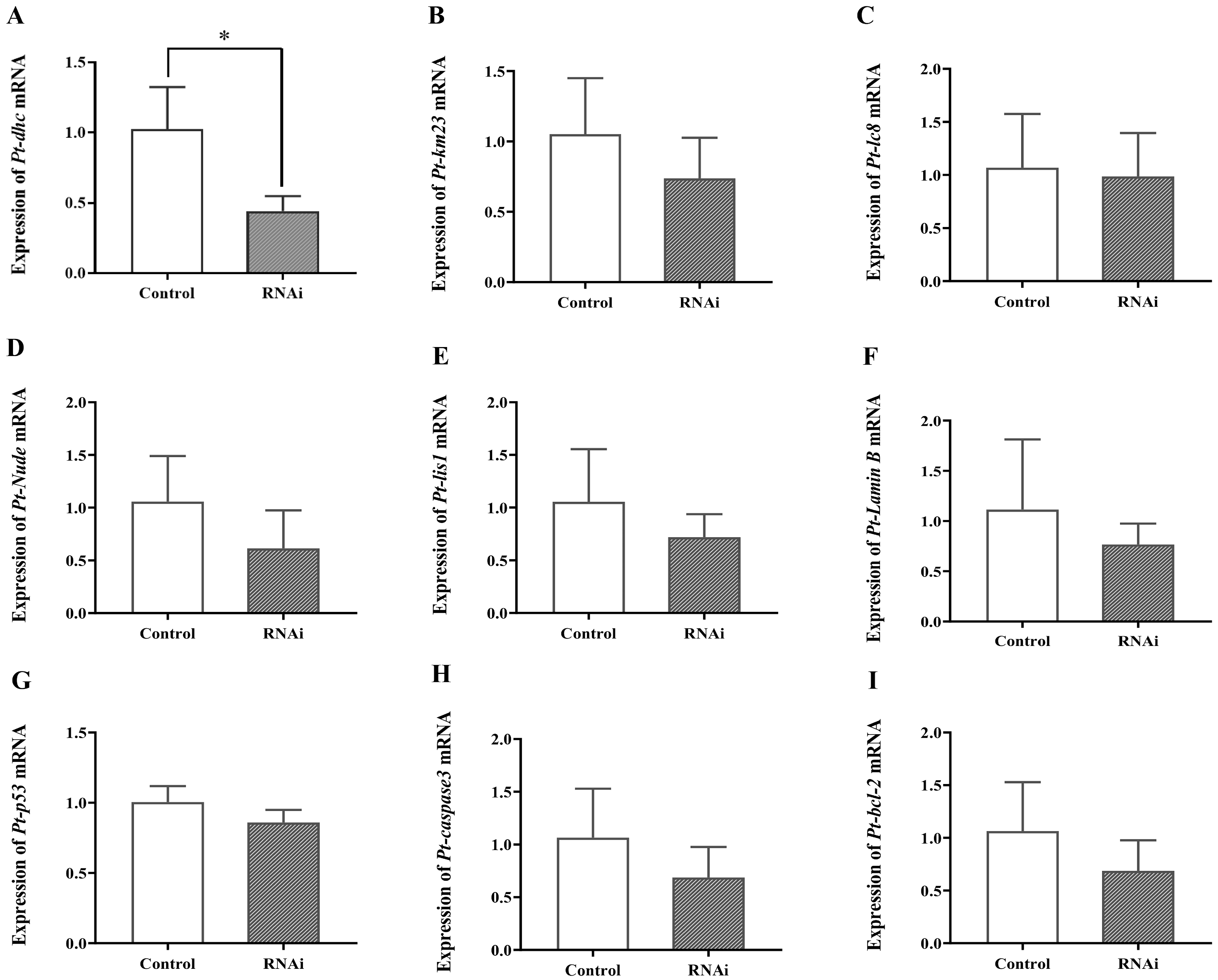
2.6.2. Detection of Related Genes after In Vivo Interference of Pt-DIC Gene dsRNA
2.6.3. The Deformation of Spermatids Was Blocked after dsRNA Interference with the Pt-DIC Gene In Vivo
2.7. Changes in the Distribution of Pt-Lamin B after In Vivo Interference with the Pt-DIC Gene dsRNA
2.8. Analysis of the Interaction between the Pt-DIC Protein and Pt-Lamin B Protein
3. Discussion
3.1. Protein Structural Characteristics of Pt-Lamin B
3.2. Lamin B Is Involved in Nuclear Deformation and Acrosome Formation
3.3. Dynein-1 Is Involved in the Nuclear Deformation Process of Spermiogenesis in P. trituberculatus
3.4. Dynein-1 May Be Involved in the Transport of Pt-Lamin B in Middle and Late Spermatids
4. Materials and Methods
4.1. Animals, Dissection, and Sample Preparation
4.2. RNA Extraction and cDNA Reverse Transcription
4.3. Full-Length Cloning of Pt-Lamin B cDNA
4.4. Sequence Alignment, Phylogenetic Tree Building and Analysis, and Protein Structure PREDICTION
4.5. RNA Interference of Pt-DIC
4.6. qPCR
4.7. Antibodies
4.7.1. Prokaryotic Expression
4.7.2. Western Blot (Verification of Pt-Lamin B Antibody)
4.8. Immunofluorescence
4.9. Coimmunoprecipitation (Co-IP)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hess, R.A.; Renato de Franca, L.R. Spermatogenesis and cycle of the seminiferous epithelium. Adv. Exp. Med. Biol. 2008, 636, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.Z.; Hamer, G.; Repping, S.; de Rooij, D.G.; van Pelt, A.M.M.; Vormer, T.L. Molecular control of rodent spermatogenesis. Biochim. Biophys. Acta 2012, 1822, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; L’Hernault, S.W. Spermatogenesis. Curr. Biol. 2017, 27, R988–R994. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.R.; Mu, S.M.; Wu, J.A.; Zhang, Z.H.; Ge, S.Q.; Li, G.L.; Kang, X.J. Progress of histone dynamics during spermatogenesis in Decapoda. Sci. China Press 2021, 51, 1199–1207, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Wei, C.G.; Mu, D.L.; Tang, D.J.; Zhu, J.Q.; Hou, C.C. Expression and functional analysis of cytoplasmic dynein during spermatogenesis in Portunus trituberculatus. Cell Tissue Res. 2021, 386, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.E. Importance of mitochondrial and nuclear sperm DNA in sperm quality assessment and assisted reproduction outcome. Hum. Fertil. 2002, 5, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Ghédir, H.; Braham, A.; Viville, S.; Saad, A.; Ibala-Romdhane, S. Comparison of sperm morphology and nuclear sperm quality in SPATA16- and DPY19L2-mutated globozoospermic patients. Andrologia 2019, 51, e13277. [Google Scholar] [CrossRef] [PubMed]
- Fabbretti, F.; Iannetti, I.; Guglielmi, L.; Perconti, S.; Evangelistella, C.; Proietti De Santis, L.; Bongiorni, S.; Prantera, G. Confocal analysis of nuclear lamina behavior during male meiosis and spermatogenesis in Drosophila melanogaster. PLoS ONE 2016, 11, e0151231. [Google Scholar] [CrossRef]
- Deolal, P.; Mishra, K. Regulation of diverse nuclear shapes: Pathways working independently, together. Commun. Integr. Biol. 2021, 14, 158–175. [Google Scholar] [CrossRef]
- Mehta, I.S.; Elcock, L.S.; Amira, M.; Kill, I.R.; Bridger, J.M. Nuclear motors and nuclear structures containing A-type lamins and emerin: Is there a functional link? Biochem. Soc. Trans. 2008, 36, 1384–1388. [Google Scholar] [CrossRef]
- Gonzalo, S.; Kreienkamp, R.; Askjaer, P. Hutchinson-gilford progeria syndrome: A premature aging disease caused by LMNA gene mutations. Ageing Res. Rev. 2017, 33, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.D.; Gruenbaum, Y.; Moir, R.D.; Shumaker, D.K.; Spann, T.P. Nuclear lamins: Building blocks of nuclear architecture. Genes Dev. 2002, 16, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Spann, T.P.; Moir, R.D.; Goldman, A.E.; Stick, R.; Goldman, R.D. Disruption of nuclear lamin organization alters the distribution of replication factors and inhibits DNA synthesis. J. Cell Biol. 1997, 136, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, T.A.; Misteli, T. The lamin protein family. Genome Biol. 2011, 12, 222. [Google Scholar] [CrossRef]
- Burke, B.; Stewart, C.L. The nuclear lamins: Flexibility in function. Nat. Rev. Mol. Cell Biol. 2013, 14, 13–24. [Google Scholar] [CrossRef]
- Camps, J.; Erdos, M.R.; Ried, T. The role of lamin B1 for the maintenance of nuclear structure and function. Nucleus 2015, 6, 8–14. [Google Scholar] [CrossRef]
- Stuurman, N.; Heins, S.; Aebi, U. Nuclear lamins: Their structure, assembly, and interactions. J. Struct. Biol. 1998, 122, 42–66. [Google Scholar] [CrossRef]
- Ben-Harush, K.; Wiesel, N.; Frenkiel-Krispin, D.; Moeller, D.; Soreq, E.; Aebi, U.; Herrmann, H.; Gruenbaum, Y.; Medalia, O. The supramolecular organization of the C. elegans nuclear lamin filament. J. Mol. Biol. 2009, 386, 1392–1402. [Google Scholar] [CrossRef]
- de Leeuw, R.; Gruenbaum, Y.; Medalia, O. Nuclear lamins: Thin filaments with major functions. Trends Cell Biol. 2018, 28, 34–45. [Google Scholar] [CrossRef]
- Mishra, S.; Levy, D.L. Nuclear F-actin and Lamin A antagonistically modulate nuclear shape. J. Cell Sci. 2022, 135, jcs259692. [Google Scholar] [CrossRef]
- Xie, W.; Burke, B. Lamins. Curr. Biol. 2016, 26, R348–R350. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, D.; Gray, H.L.; Sammak, P.J.; Schatten, G.P.; Csoka, A.B. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells 2006, 24, 177–185. [Google Scholar] [CrossRef]
- Elkhatib, R.; Longepied, G.; Paci, M.; Achard, V.; Grillo, J.M.; Levy, N.; Mitchell, M.J.; Metzler-Guillemain, C. Nuclear envelope remodelling during human spermiogenesis involves somatic B-type lamins and a spermatid-specific B3 lamin isoform. Mol. Hum. Reprod. 2015, 21, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Koncicka, M.; Cervenka, J.; Jahn, D.; Sucha, R.; Vodicka, P.; Gad, A.; Alsheimer, M.; Susor, A. Expression of lamin C2 in mammalian oocytes. PLoS ONE 2020, 15, e0229781. [Google Scholar] [CrossRef] [PubMed]
- Broers, J.L.; Machiels, B.M.; Kuijpers, H.J.; Smedts, F.; van den Kieboom, R.; Raymond, Y.; Ramaekers, F.C. A- and B-type lamins are differentially expressed in normal human tissues. Histochem. Cell Biol. 1997, 107, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Schütz, W.; Alsheimer, M.; Ollinger, R.; Benavente, R. Nuclear envelope remodeling during mouse spermiogenesis: Postmeiotic expression and redistribution of germline lamin B3. Exp. Cell Res. 2005, 307, 285–291. [Google Scholar] [CrossRef]
- Melcer, S.; Gruenbaum, Y.; Krohne, G. Invertebrate lamins. Exp. Cell Res. 2007, 313, 2157–2166. [Google Scholar] [CrossRef]
- Schulze, S.R.; Curio-Penny, B.; Speese, S.; Dialynas, G.; Cryderman, D.E.; McDonough, C.W.; Nalbant, D.; Petersen, M.; Budnik, V.; Geyer, P.K.; et al. A comparative study of Drosophila and human A-type lamins. PLoS ONE 2009, 4, e7564. [Google Scholar] [CrossRef]
- Liu, J.; Rolef Ben-Shahar, T.; Riemer, D.; Treinin, M.; Spann, P.; Weber, K.; Fire, A.; Gruenbaum, Y. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol. Biol. Cell 2000, 11, 3937–3947. [Google Scholar] [CrossRef]
- Furukawa, K.; Hotta, Y. cDNA cloning of a germ cell specific lamin B3 from mouse spermatocytes and analysis of its function by ectopic expression in somatic cells. EMBO J. 1993, 12, 97–106. [Google Scholar] [CrossRef]
- Gerace, L.; Blobel, G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell 1980, 19, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Moir, R.D.; Yoon, M.; Khuon, S.; Goldman, R.D. Nuclear lamins A and B1: Different pathways of assembly during nuclear envelope formation in living cells. J. Cell Biol. 2000, 151, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Laberge, R.M.; Demaria, M.; Campisi, J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell 2012, 23, 2066–2075. [Google Scholar] [CrossRef] [PubMed]
- Shimi, T.; Butin-Israeli, V.; Adam, S.A.; Hamanaka, R.B.; Goldman, A.E.; Lucas, C.A.; Shumaker, D.K.; Kosak, S.T.; Chandel, N.S.; Goldman, R.D. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 2011, 25, 2579–2593. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.X.; Xu, C.Y.; Donahue, G.; Shimi, T.; Pan, J.A.; Zhu, J.J.; Ivanov, A.; Capell, B.C.; Drake, A.M.; Shah, P.P.; et al. Autophagy mediates degradation of nuclear lamina. Nature 2015, 527, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Supp, D.M.; Potter, S.S.; Brueckner, M. Molecular motors: The driving force behind mammalian left-right development. Trends Cell Biol. 2000, 10, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Pfister, K.K.; Shah, P.R.; Hummerich, H.; Russ, A.; Cotton, J.; Annuar, A.A.; King, S.M.; Fisher, E.M. Genetic analysis of the cytoplasmic dynein subunit families. PLoS Genet. 2006, 2, e1. [Google Scholar] [CrossRef]
- Koonce, M.P. Identification of a microtubule-binding domain in a cytoplasmic dynein heavy chain. J. Biol. Chem. 1997, 272, 19714–19718. [Google Scholar] [CrossRef]
- Pfister, K.K. Distinct functional roles of cytoplasmic dynein defined by the intermediate chain isoforms. Exp. Cell Res. 2015, 334, 54–60. [Google Scholar] [CrossRef]
- Wen, Q.; Tang, E.I.; Lui, W.Y.; Lee, W.M.; Wong, C.K.C.; Silvestrini, B.; Cheng, C.Y. Dynein 1 supports spermatid transport and spermiation during spermatogenesis in the rat testis. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E924–E948. [Google Scholar] [CrossRef]
- Rashid, S.; Grzmil, P.; Drenckhahn, J.D.; Meinhardt, A.; Adham, I.; Engel, W.; Neesen, J. Disruption of the murine dynein light chain gene Tcte3-3 results in asthenozoospermia. Reproduction 2010, 139, 99–111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fatima, R. Drosophila Dynein intermediate chain gene, Dic61B, is required for spermatogenesis. PLoS ONE 2011, 6, e27822. [Google Scholar] [CrossRef] [PubMed]
- Wong, X.R.; Melendez-Perez, A.J.; Reddy, K.L. The Nuclear Lamina. Cold Spring Harb. Perspect. Biol. 2022, 14, a040113. [Google Scholar] [CrossRef] [PubMed]
- Uchino, R.; Sugiyama, S.; Katagiri, M.; Chuman, Y.; Furukawa, K. Non-farnesylated B-type lamin can tether chromatin inside the nucleus and its chromatin interaction requires the Ig-fold region. Chromosoma 2017, 126, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, S.M.; Sharakhov, I.V. Reorganization of the nuclear architecture in the Drosophila melanogaster Lamin B mutant lacking the CaaX box. Nucleus 2020, 11, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.L.; Ni, F.D.; Yang, W.X. The dynamics and regulation of chromatin remodeling during spermiogenesis. Gene 2019, 706, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Salina, D.; Bodoor, K.; Eckley, D.M.; Schroer, T.A.; Rattner, J.B.; Burke, B. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell 2002, 108, 97–107. [Google Scholar] [CrossRef]
- Helfand, B.T.; Mikami, A.; Vallee, R.B.; Goldman, R.D. A requirement for cytoplasmic dynein and dynactin in intermediate filament network assembly and organization. J. Cell Biol. 2002, 157, 795–806. [Google Scholar] [CrossRef]
- Xiang, Q.M.; Wei, C.G.; Gao, X.M.; Chen, Y.E.; Tang, D.J.; Zhu, J.Q.; Hou, C.C. Molecular cloning of Dynein Heavy Chain and the effect of Dynein inhibition on the testicular function of Portunus trituberculatus. Animals 2021, 11, 3582. [Google Scholar] [CrossRef]
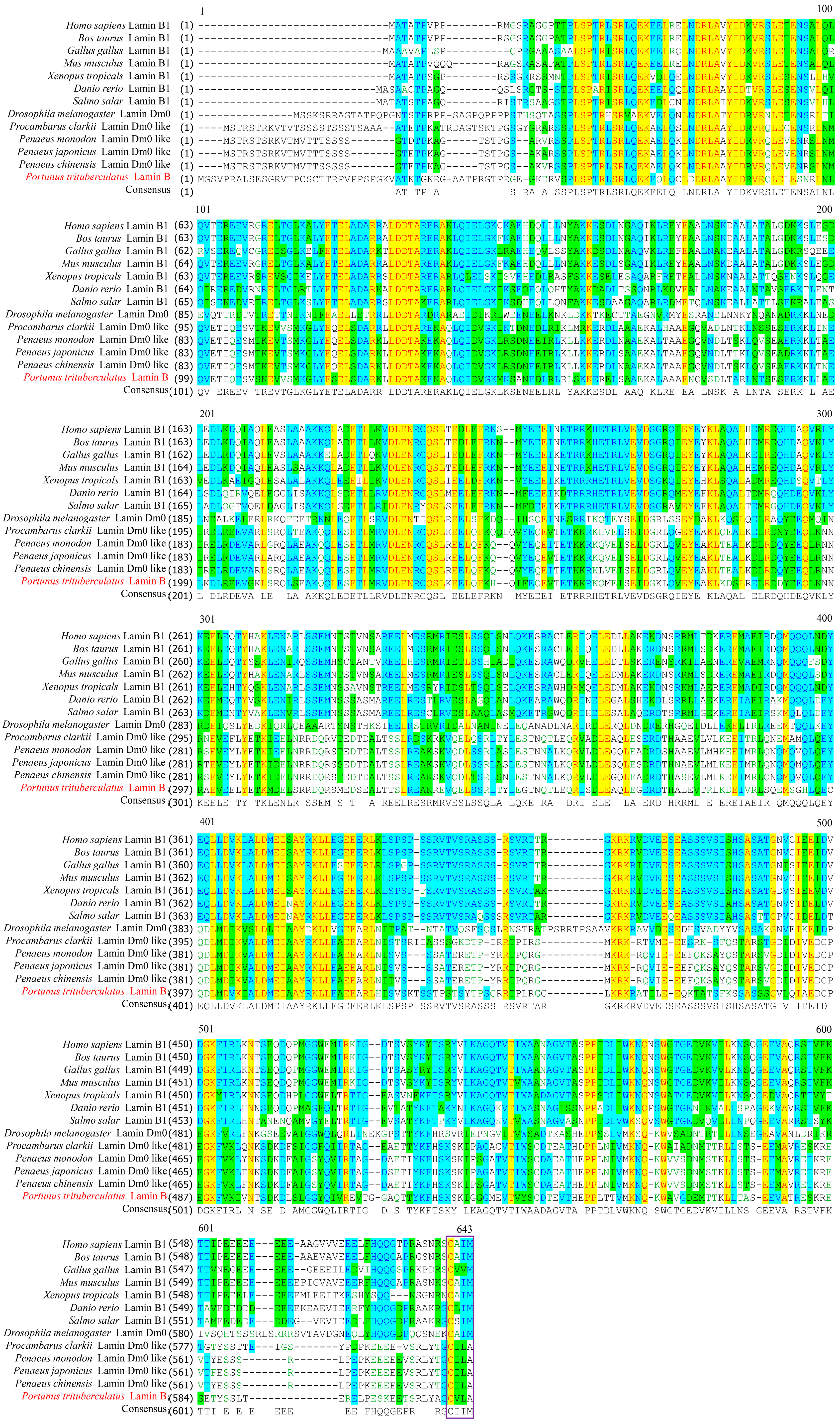
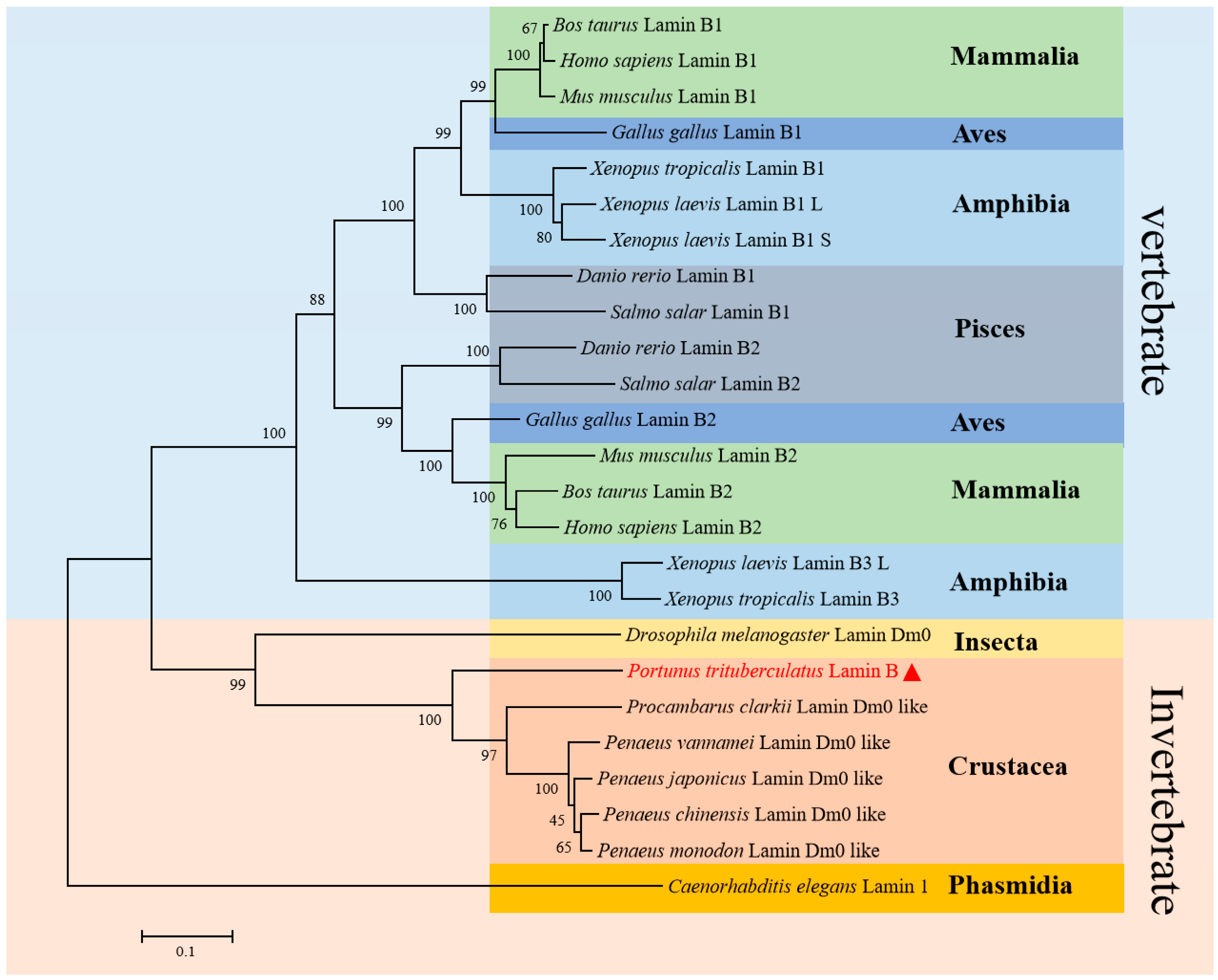
| Species | Identity |
|---|---|
| Homo sapiens | 28.0% |
| Bos taurus | 27.8% |
| Gallus gallus | 26.4% |
| Mus musculus | 27.5% |
| Xenopus tropicals | 22.3% |
| Danio rerio | 28.6% |
| Salmo salar | 28.2% |
| Drosophila melanogaster | 39.1% |
| Procambarus clarkii | 66.1% |
| Penaeus vannamei | 43.8% |
| Penaeus monodon | 63.7% |
| Penaeus japonicus | 64.2% |
| Penaeus chinensis | 63.7% |
| Caenorhabditis elegans | 26.6% |
| Primer | Sequence (5′→3′) | Purpose |
|---|---|---|
| Lamin B-F | GTGTTTGAATGACAGGCTGGC | PCR |
| Lamin B-R | GAGCCGCAAATCCTCGTTAG | PCR |
| 3′LaminB-F1 | CAACTGGAACTGGAGAGCAACCGACT | 3′RACE |
| 3′Lamin B-F2 | TTAGTAAGGAGGTTGTGAGCATGAAGGG | 3′RACE |
| 5′Lamin B-R1 | CGAGAAGTTTCCGAGCGTCAGAGAGTT | 5′RACE |
| 5′Lamin B-R2 | TTGAGTCGGTTGCTCTCCAGTTCCA | 5′RACE |
| DIC-mbF | CGACGGGCACGGTTACTAT | RNAi |
| DIC-mbR | AGGCTTCGTTTCCTTGACA | RNAi |
| T7dsDICF | TAATACGACTCACTATAGGGGGATGGAGAGGCTAACGA | Positive template |
| dsDICR | ACATCTTGCCATCAGTGCT | RNAi |
| dsDICF | GGATGGAGAGGCTAACGA | RNAi |
| T7dsDICR | TAATACGACTCACTATAGGGACATCTTGCCATCAGTGCT | Reverse template |
| LaminYH-F | CGCGGATCCCAACTGGAACTGGAGAGCAA | Prokaryotic expression |
| LaminYH-R | CGCGGATCCCAACTGGAACTGGAGAGCAA | Prokaryotic expression |
| DIC-F | TATGAGGTAGCCTGGTCCCC | qPCR |
| DIC-R | GCTGGCCAGAGTTAGTCCAA | qPCR |
| P53-F | GGGTAACGCCATGAACGAGA | qPCR |
| P53-R | GCTGCATCTCCGTGTGTTTC | qPCR |
| Caspase3-F | TCACAGATTGACAAAGAGCGG | qPCR |
| Caspase3-R | TCCTCAGGTCAGTAGTGGAAATG | qPCR |
| km23-F | TGTCAGCAGAGGTTGAAGAAA | qPCR |
| km23-R | AGGAATGTGAGGTCGTTGGT | qPCR |
| Lamin B-qF | TTACTCGCCGAACTCAAGGA | qPCR |
| Lamin B-qR | TGAAGTCCTCGTAGTAGCAGCA | qPCR |
| BCL2-F | AGCTTACAACTGGATGCGCT | qPCR |
| BCL2-R | TCGAGAGTGATTTAGGCGGC | qPCR |
| LC8-F | ACATCGCCGCCTACATCAA | qPCR |
| LC8-R | GGGGCACACTTAGCCACTCT | qPCR |
| Lis1-F | TGCCACAGATAACCGAAAGC | qPCR |
| Lis1-R | TTGTCGTGACCCACCAGAGA | qPCR |
| Nude-F | AGGAGTTTCAGAGTGGCAGCA | qPCR |
| Nude-R | TTTTGTAGCCTTTCCAGACGC | qPCR |
| GAPDHF | TGAGGTGAAGGTAGAGGAT | qPCR |
| GAPDHR | CCAGTGAAGTGAGCAGAG | qPCR |
| Specie | Protein | GenBank |
|---|---|---|
| Homo sapiens | Lamin B1/B2 | NP_005564.1/NP_116126.3 |
| Bos taurus | Lamin B1/B2 | NP_001096765.1/NP_001263282.1 |
| Gallus gallus | Lamin B1/B2 | NP_990617.2/NP_990616.2 |
| Mus musculus | Lamin B1/B2 | NP_034851.2/NP_034852.3 |
| Danio rerio | Lamin B1/B2 | NP_694504.2/NP_571077.2 |
| Salmo salar | Lamin B1/B2 | XP_013999495.2/XP_013979108.1 |
| Xenopus tropicals | Lamin B1/B1S | NP_989198.1/NP_001081547.1 |
| Xenopus tropicals | Lamin B1L/B3 | NP_001080053.1/NP_001076823.1 |
| Xenopus tropicals | Lamin B3L | NP_001081545.1 |
| Drosophila melanogaster | Lamin Dm0 | NP_476616.1 |
| Procambarus clarkii | Lamin Dm0 like | NP_001289961.1 |
| Penaeus vannamei | Lamin Dm0 like | XP_027220822.1 |
| Penaeus monodon | Lamin Dm0 like | XP_037794519.1 |
| Penaeus japonicus | Lamin Dm0 like | XP_042885236.1 |
| Penaeus chinensis | Lamin Dm0 like | XP_047494326.1 |
| Caenorhabditis elegans | Lamin 1 | NP_492371.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-Y.; Xiang, Q.-M.; Zhu, J.-Q.; Mu, C.-K.; Wang, C.-L.; Hou, C.-C. The Functions of Pt-DIC and Pt-Lamin B in Spermatogenesis of Portunus trituberculatus. Int. J. Mol. Sci. 2024, 25, 112. https://doi.org/10.3390/ijms25010112
Wang S-Y, Xiang Q-M, Zhu J-Q, Mu C-K, Wang C-L, Hou C-C. The Functions of Pt-DIC and Pt-Lamin B in Spermatogenesis of Portunus trituberculatus. International Journal of Molecular Sciences. 2024; 25(1):112. https://doi.org/10.3390/ijms25010112
Chicago/Turabian StyleWang, Shuo-Yue, Qiu-Meng Xiang, Jun-Quan Zhu, Chang-Kao Mu, Chun-Lin Wang, and Cong-Cong Hou. 2024. "The Functions of Pt-DIC and Pt-Lamin B in Spermatogenesis of Portunus trituberculatus" International Journal of Molecular Sciences 25, no. 1: 112. https://doi.org/10.3390/ijms25010112
APA StyleWang, S.-Y., Xiang, Q.-M., Zhu, J.-Q., Mu, C.-K., Wang, C.-L., & Hou, C.-C. (2024). The Functions of Pt-DIC and Pt-Lamin B in Spermatogenesis of Portunus trituberculatus. International Journal of Molecular Sciences, 25(1), 112. https://doi.org/10.3390/ijms25010112





