Abstract
Severe obesity (SO) can accelerate atherosclerosis and the onset of acute cardiovascular events. The diagnosis of atherosclerosis in the context of a high body mass index (BMI) can be challenging, making the identification of biomarkers clinically relevant. We aimed to assess the usefulness of irisin as a biomarker for subclinical atherosclerosis in participants with SO. This prospective observational study included 61 participants undergoing bariatric surgery for SO, defined as a BMI >40 kg/m2 or >35 kg/m2 with at least one comorbidity. Atherosclerotic plaques were detected by ultrasound. Plasma samples were obtained 1 month before and at 6 and 12 months after bariatric surgery to measure irisin by ELISA. Additionally, subcutaneous samples of adipose tissue were taken and genotyped to identify irisin polymorphism rs3480. Irisin levels were positively correlated with BMI (r = 0.23, p = 0.0064), negatively correlated with atheroma-related parameters (e.g., carotid intima-media thickness), and lower in subjects with atheroma (p < 0.0002). Irisin also showed good overall accuracy for discriminating plaque presence (AUC, 0.81; 95% CI, 0.6956–0.9156). However, the rs3480 polymorphism correlated with neither the irisin levels nor the presence of atheromas. Iirisin could identify subclinical atherosclerosis in SO and might facilitate clinical diagnosis.
1. Introduction
The prevalence of overweight and obesity has increased globally over recent decades to become a major public health challenge. Currently, 13% of adults in the world have obesity and 8% of global deaths were attributed to obesity [1]. Severe obesity (SO) is a complex chronic disease that can lead to serious health conditions [2]. Some of these comorbidities can revert after bariatric surgery (BS), which is currently the most effective approach for treating SO [3]. Moreover, although SO is a well-known risk factor for atherosclerosis, the mechanisms underlying this association are not fully understood [4].
Atherosclerosis is a chronic disease that begins with the formation of atheromas and predisposes individuals to cardiovascular disease (CVD) and increased mortality [5]. An estimated 17.9 million people died from CVDs in 2019, representing 32% of all global deaths [6]. Carotid ultrasound is the recommended method to detect subclinical atheromatous plaques [7], but the fat accumulation in cases of SO can make the diagnoses based on arterial wall imaging challenging [8]. Accordingly, the identification of the biomarkers of subclinical atherosclerosis is clinically relevant in the context of SO. Our previous research in this area has revealed abnormalities in several molecules implicated in the metabolic and inflammatory mechanisms that also promote atherogenesis [9]. Adipose and muscle tissues play key roles in producing many of these molecules, among which the novel adipomyokine irisin appears to promote metabolic health and affect CVD [10,11]. Hence, it seems reasonable to suggest that irisin could be involved in both obesity and atherosclerosis.
Concerning energy metabolism, irisin induces the upregulation of uncoupling protein-1 and the conversion of white adipose tissue to brown adipose tissue, resulting in heightened thermogenesis and energy expenditure [12]. In this way, conflicting results suggest that irisin may play a direct role in obesity [13,14]. Current knowledge of the biological functions of irisin also includes regulating glucose and lipid metabolism, anti-inflammation, and anti-oxidation [15,16,17,18]. The dysregulation of these processes impairs metabolic responses, which in turn, can lead to atherosclerosis [9,19]. Nevertheless, the role of irisin in the development of atherosclerosis requires further research to understand its mechanisms of action fully [10,11]. To the best of our knowledge, no previous studies in the context of SO and BS have considered the use of irisin as a serological and genetic biomarker (FNDC5 gene) for detecting subclinical atherosclerosis. Accordingly, research to elucidate the functions of irisin might provide new opportunities for developing more effective approaches for early diagnoses of atherosclerosis and preventing CVD.
This study aimed to assess the usefulness of irisin as a biomarker for subclinical atherosclerosis in cases of SO. To accomplish our main objective, we propose the following specific aims: (i) to analyze the serum levels of irisin and its genetic variability in cases with and without atheroma undergoing BS, (ii) to evaluate the association between irisin levels and the biochemical parameters involved in atherosclerosis, and (iii) to assess the usefulness of irisin as a biomarker of subclinical atherosclerosis in patients with SO undergoing BS.
2. Results
2.1. General Characteristics
Table 1 summarizes the anthropometric and biochemical characteristics of the study groups with and without atheromatous plaques. Atheromatous plaques did not remit 12 months after BS, but the BMIs decreased by about 33% after BS, and did not differ statistically between groups. Regarding biochemical parameters, triacylglycerides (TAG), glucose, glycated hemoglobin (Hba1c), and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) results were significantly higher in subjects with plaques, but they did decrease after BS. Referring comorbidities, SO and atheroma were also associated with a higher incidence of hypertension, dyslipidemia, and type 2 diabetes mellitus (T2DM), but their prevalence also decreased after BS.

Table 1.
Clinical characteristics of participants with SO with/without plaques before and after BS. Continuous variables are expressed as the mean ± standard error of the mean. Statistical analysis for age and sex used Mann–Whitney U tests. Continuous variables were analyzed by two-way ANOVA (group and time of follow-up) and the Bonferroni post-hoc test. Statistical analyses for categorical variables were performed using chi-square or Fisher exact tests. Symbol (o) denotes differences vs. SO; symbol (*) denotes differences vs. with-plaque group. One, two, or three of these symbols denotes p < 0.05, <0.01, or <0.001, respectively. Abbreviations: 6M, 6 months after surgery; 12M, 12 months after surgery; BMI, body mass index; BS, bariatric surgery; cHDL, high-density lipoprotein cholesterol; cLDL, low-density lipoprotein cholesterol; DLP, dyslipidemia; HbA1c, glycated hemoglobin; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; HT, hypertension; n, number of subjects; ns, non-significant; SO, severe obesity at 1 month before bariatric surgery; T2DM, type 2 diabetes mellitus; TAG, triacylglyceride; TC, total cholesterol.
2.2. Irisin Levels and Genetic Variability in Subclinical Atherosclerosis
Figure 1 shows irisin concentrations 1 month before and at 6 and 12 months after BS for the groups with and without plaques. When comparing the differences between participants with and without plaque, irisin concentrations were significantly lower in those with atheromatous plaques before and after BS (p = 0.0002). When focusing on the effect of BS, irisin concentration decreased 6 months post BS and increased again after one year (time p = 0.0043). Overall, irisin levels were significantly different during the study and depended on the presence of plaques.
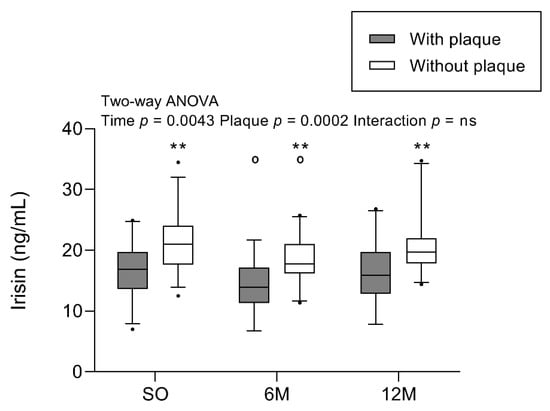
Figure 1.
Irisin concentration in participants with SO with/without plaques before and after BS. Results are represented in a boxplot showing the median and first to the third quartiles, with whiskers showing the 5th and 95th percentiles. Points below and above the whiskers are drawn as individual points. Two-way ANOVA (group and time of follow-up) was performed with the Bonferroni post-hoc test. n = 61 subjects; n = 30 with plaque, and n = 31 without plaque. Statistical differences were considered significant when p < 0.05. Symbol (o) denotes differences in the post-test vs. SO for the same group. Symbol (*) denotes differences in the post-test vs. with-plaque group for the same time. One or two of either symbols denote p < 0.05 or p < 0.01, respectively. Abbreviations: 6M, 6 months after surgery; 12M, 12 months after surgery; BS, bariatric surgery; ns, non-significant; SO, severe obesity at 1 month before bariatric surgery.
To delineate the association of irisin levels with obesity and weight loss, we studied the correlation between irisin levels and BMI at each follow-up (Figure 2). This revealed a positive correlation (r = 0.261; p < 0.01) with higher irisin levels in individuals with higher BMIs. The reported correlation can only be observed globally, not separately at each time point.
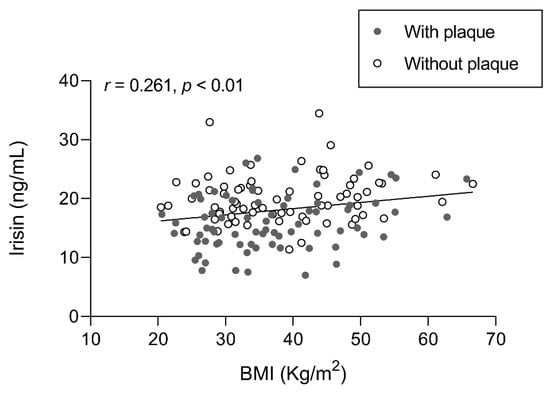
Figure 2.
Association of irisin levels with BMI in participants with SO with/without atheromatous plaques before and after BS. Results were obtained after Pearson’s test and expressed as the Pearson’s coefficient (r). n = 61 subjects. In gray, subjects with plaque; in white, subjects without plaque. Abbreviations: BMI, body mass index.
Next, we measured the distribution of FNDC5 rs3480 polymorphism frequencies by plaque presence to assess whether the reported differences in irisin levels reflected genetic variability (Table 2). Nevertheless, there was no significant difference in the allele and genotype frequencies between individuals with and without atheromas.

Table 2.
Genotype frequencies of the FNDC5 gene rs3480 (A/G) polymorphism and irisin levels in participants with SO with/without plaques. Genotype frequencies are presented as number of subjects and the percentage versus the total per group. Irisin concentrations are represented as the mean ± standard error of the mean. Abbreviations: SO, severe obesity at 1 month before bariatric surgery.
Three-factor ANOVA was then used to determine if the genotype alone and combined with plaque or time after surgery affected irisin concentrations (Table 2). This revealed no statistically significant differences in the irisin levels by the genotype alone (p = 0.0952). Furthermore, the interactions of the genotype with plaque presence or time were not statistically significant (p = 0.8805 and p = 0.9113, respectively).
2.3. Association between Irisin Levels and Biochemical Features of Atherosclerosis
The parameters implicated in atherogenesis, such as inflammation, oxidative stress, and endothelial function, were previously measured in this cohort [9,19,20]. Table 3 shows the analysis of the correlations between irisin and some of these parameters. Of note, we found a negative correlation with pro-inflammatory parameters, such as non-esterified fatty acids (NEFA) and plasminogen activator inhibitor 1 (PAI1), and a positive correlation with leptin. The levels of oxidized low-density lipoprotein cholesterol (a marker of oxidative stress) correlated negatively with irisin levels. Endothelial function, including the inflammatory adhesion molecule P-selectin and the carotid intima-media thickness (cIMT), also correlated negatively with irisin.

Table 3.
Association of irisin levels with atherosclerosis features in participants with SO with/without plaques. Calculated in the total cohort with SO with/without plaques (before bariatric surgery or throughout the study). Statistical analyses were performed using Pearson’s test and expressed as the Pearson’s coefficient (r). n = 61 subjects. Abbreviations: cIMT, carotid intima-media thickness; HbA1c, glycated hemoglobin; NEFA, non-esterified fatty acids; oxLDL, oxidized low-density lipoproteins; PAI-1, plasminogen activator inhibitor-1; P-sel, P-selectin; SO, severe obesity at 1 month before bariatric surgery; T2DM, type 2 diabetes mellitus.
2.4. Irisin as a Biomarker of Subclinical Atherosclerosis
Given the differences between the groups with and without atheromas, and the correlations of irisin with atherogenesis-related parameters, we evaluated the potential of irisin as a biomarker for subclinical atherosclerosis. The area under the receiver operating characteristic (ROC) curve (AUC) was used to calculate the accuracy of irisin as a potential diagnostic test (Figure 3). The high AUC (0.81; i.e., 81% predictive capacity) indicated that irisin levels have good overall accuracy for discriminating the presence of plaque.
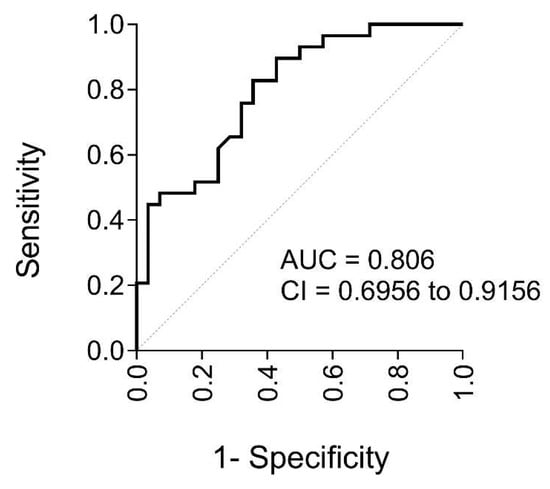
Figure 3.
Goodness of irisin level prediction in participants with SO before BS. Quantified using ROC curve analysis with the AUC and its 95% CI (n = 61 subjects). The dotted line is the reference line (AUC = 0.5). Abbreviations: AUC, area under the ROC curve; CI, confidence interval; ROC, receiver operating characteristic.
3. Discussion
Identifying subjects with subclinical atherosclerosis is crucial to enhance the diagnosis and prevention of CVD. We found that irisin may not only be a good biomarker for subclinical atherosclerosis, but could also be related to the SO itself.
Studies on the potential relationship between irisin levels and BMI or obesity have shown inconsistent findings [21]. Our results were consistent with the most frequently reported finding that plasma irisin levels correlate positively with BMI [22]. It has been suggested that adipose tissue could oversecrete irisin to tackle obesity-induced metabolic dysregulation [23], thereby explaining the observed positive association between irisin and leptin in SO [9,24]. Leptin is an adipokine that reflects dysregulated metabolism.
When we studied circulating irisin levels after surgery, a marked reduction was observed at 6 months following BS. However, at 12 months, the levels had returned to their pre-surgical baseline. These findings are consistent with previous research indicating a decrease in FNDC5 expression in muscle tissue after BS [25]. However, it remains to be confirmed whether the decrease in circulating irisin levels at 6 months were due to decreasing muscle mass, the surgery itself, or a direct effect of the resulting weight loss. Recovery to the initial values 12 months after BS has been suggested to reflect adaptation to the altered energy intake and body weight [26]. Extended investigations are required to determine whether irisin levels remain stable over the long term after BS.
The observed correlation between irisin and BMI suggests that irisin could also be a key molecule in atherosclerosis because most processes influenced by irisin are also disrupted in atherosclerosis. Indeed, low serum irisin levels have been linked to increased risk of CVD [27], and more recently, to the presence of plaques in patients with axial spondyloarthritis [28]. Despite these reports, little is known about the role of irisin in atherosclerosis and its exact mechanism of modulation. Hence, it is important to highlight that we found lower irisin levels in subjects with atheromas throughout the study, even after BS.
To complement the assessment of irisin at the protein level, we also assessed whether the observed differences resulted from genetic variability. Although previous research has reported contradictory findings on this association [29], irisin polymorphisms have been associated with obesity and related comorbidities [30]. The rs3480 noncoding polymorphism of FNDC5 is of interest here, given its association with blood pressure and lipid profiles [31]. Furthermore, the G allele of rs3480 has been associated with increased risk of myocardial infarction [32]. However, our analysis of the FNDC5 rs3480 polymorphism and its possible association with the presence of plaques revealed that is not associated with plaque presence and did not modulate irisin concentrations at any assessment point. Hence, plasma irisin levels were independent of any variability in the rs3480 polymorphism.
The positive correlation of irisin with BMI and the increase in irisin levels among patients without plaques support the hypothesis that irisin has an atheroprotective role [13,33,34,35]. Different explanations support this effect of irisin against the development of atherosclerosis in SO. These include indirect crosslinks that exist between irisin and comorbidities [36,37] and/or its role in the inflammation and oxidative stress processes leading to endothelial dysfunction [28], one of the first stages of atherosclerosis [38].
These results are further supported by our finding of a negative association between irisin levels and both glucose and HbA1c, which is indicative of the adverse blood sugar levels in our population before BS. Irisin appears to improve insulin resistance and T2DM [39], with evidence that increased levels in SO may be protective against T2DM, which is a known risk factor for CVD [40].
The anti-inflammatory and anti-oxidative potentials of irisin are of great interest because these processes are often associated with the development of atherosclerosis [41,42]. We have previously demonstrated that SO is often characterized by increased inflammation [9,19]. Supporting the anti-inflammatory function of irisin [41], we also reported that plasma irisin was negatively correlated with non-esterified fatty acid levels, which can induce inflammation by modulating adipokine production [43]. It is also worth highlighting that there exists an inverse relationship between irisin and plasminogen activator inhibitor-1 levels, a recognized risk factor for thrombosis and atherosclerosis in inflammatory conditions [44].
Consistent with other reports demonstrating that irisin reduces oxidative stress [45], it correlated negatively with the levels of oxidized low-density lipoprotein cholesterol, a well-known marker of oxidative stress that contributes to the formation and progression of atherosclerotic plaques [46].
Irisin may also help to prevent endothelial dysfunction and damage [15,41,47,48], which are crucial to the development of CVD. We previously demonstrated that our population with SO and early atherosclerosis had evidence of endothelial dysfunction [9]. Supporting the potential protective role of irisin, the present study indicates a strong negative correlation with P-selectin, an adhesion molecule that indicates the presence of inflammation in the vascular bed and enhances pro-coagulant activity [49]. Importantly, circulating irisin levels also correlated negatively with the carotid intima-media thickness, a characteristic morphological change in early atherosclerosis [50]. Accordingly, several studies have reported that irisin may suppress neointima formation, which reflects endothelial injury in the atherosclerotic vasculature [51,52]. Thus, lower irisin levels might reflect a worse pathological status in patients with early atherosclerosis.
Given the important functions of irisin and its beneficial effects on homeostasis [53], together with the many correlations highlighted in this research, we suggest that irisin could serve as a potential biomarker of subclinical atherosclerosis. AUC analysis of how well irisin distinguishes between subjects with and without plaques indicated an 81% predictive capability, which suggests that irisin alone can determine the presence of plaques. This fact, combined with the difficulty of diagnosing atherosclerosis by ultrasound in patients with SO, emphasizes the usefulness of irisin as a biomarker of subclinical atherosclerosis in this population. To the best of our knowledge, this is the first study to consider this possibility.
We are aware that this exploratory research has some limitations, such as a small sample size. It would also have benefited from additional data on body composition (muscle and fat mass) to gain a better understanding of the role of irisin in obesity metabolism. Measuring plaque composition could provide valuable information about irisin as a marker of atherosclerosis degree and severity. Further studies are needed to confirm or refute our hypothesis about the usefulness of irisin as a biochemical marker of atherosclerotic plaque presence. Nevertheless, the differences in irisin levels between subjects with and without atheroma are noteworthy and open up the possibility to use irisin as a biomarker of subclinical atherosclerosis.
4. Materials and Methods
4.1. Study Design and Participants
This prospective observational study included 61 participants with SO recruited at the Arnau de Vilanova University Hospital (Lleida, Spain) after being enrolled in out BS program, with follow-up at 6 and 12 months after BS. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Hospital Universitari Arnau de Vilanova (CEIC-1369). For inclusion, participants were required to have a body mass index (BMI) of >40 kg/m2 or >35 kg/m2 with at least one of the following comorbidities: hypertension, T2DM, dyslipidemia, obstructive sleep apnea, and weight-induced rheumatic disease [54]. All presented a clinical history of SO for at least 5 years, none had received an inflammatory or infectious disease diagnosis, and none received anti-obesity or anti-inflammatory drugs during the study. We excluded cases with previous neoplastic, renal, hepatic, or active systemic diseases, as well as those with a CVD or endocrine disease other than T2DM.
At the study onset, 61 individuals were distributed into two groups based on the presence (n = 30) or absence (n = 31) of atherosclerotic plaques, which we detected using a Vivid I ultrasound machine (General Electric Healthcare, Waukesha, WI, USA) [55]. Arterial ultrasound was performed in the common, bifurcation, internal, and external regions of both carotid arteries [55]. Participants underwent either laparoscopic Roux-en-Y gastric bypass (n = 31) or sleeve gastrectomy (n = 30) at the surgeon’s discretion. Plasma samples were obtained 1 month before and at 6 and 12 months after BS. Subcutaneous adipose tissue samples were obtained during BS for genotyping assays.
4.2. Clinical and Biochemical Evaluations
Serum levels of total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triacylglycerides were measured using colorimetric enzymatic methods (CHOD-PAP cholesterol, HDL-C plus, and GPO-PAP triglycerides, Modular P800; Roche Diagnostics, Manheim, Germany). Glucose was determined by the hexokinase glucose method; HbA1c was measured using a high-resolution ion exchange liquid chromatography technique (Varian II Hemoglobin A1c; Bio Rad, Hercules, CA, USA) and HOMA-IR was then calculated [56]. Plasma irisin concentrations were measured by enzyme-linked immunosorbent assay ELISA (Ref: EK-067-29; Phoenix Europe GmbH, Karlsruhe, Germany).
4.3. Irisin Polymorphism Selection and Genotyping
All participants underwent genotyping for the irisin rs3480 (A/G) polymorphism, which has previously been linked to CVD. We extracted DNA from subcutaneous adipose tissue samples using the Canvax Extraction kit (Tissue Genomic DNA Purification Kit; Canvax Biotech SL, Córdoba, Spain). The concentration of the extracted DNA was measured by the fluorometer Qubit with two standards (High Sensitivity Qubit Kit; Life Technologies; ThermoFisher Scientifics, Waltham, MA, USA). The rs3480 single nucleotide polymorphism was genotyped using the TaqMan 5′ exonuclease assay (Applied Biosystems; Foster City, CA, USA). Assays were run in a 384-well plate on QuantStudio 6 and 7 Pro Real-Time PCR Systems, using standard conditions. Genotype data were analyzed using the Design and Analysis Software (version 2.5, Applied Biosystems, Foster City, CA, USA).
4.4. Statistical Analysis
Data were analyzed in GraphPad Prism (version 8.1, GraphPad Software, San Diego, CA, USA) with an alpha level of 0.05 used to determine statistical significance. The Shapiro–Wilk test was used to determine the normality of continuous variables, which were reported as means ± standard error of the mean. By contrast, categorical variables were represented as percentages (yes/no). Box plots were used to indicate the medians and first to third quartiles of the distribution.
Statistical analysis of age and sex was performed using the Mann–Whitney U test. For categorical variables, chi-squared or Fisher exact tests were performed. For continuous variables, two-way ANOVA with the Bonferroni post-hoc test was used to evaluate differences between groups. Differences in genotype frequencies were assessed by the chi-squared test, while the determination of whether the genotype affected irisin concentrations or plaque presence was determined by time three-way ANOVA. The correlation of biomolecules with irisin was assessed by Pearson’s test. Finally, ROC curve analysis was used to describe the discriminatory accuracy of irisin for subclinical atherosclerosis when used as the primary method to determine the presence of disease as a dichotomous outcome.
5. Conclusions
Plasma irisin concentrations were positively associated with BMI in patients with SO undergoing BS. Irisin overexpression might act by tackling the deregulated metabolism induced by obesity and exerting a protective role against atherosclerosis. Overall, irisin appears to have a high predictive value for the presence of atherosclerotic plaques, and either alone or in combination with other markers, it could offer a valuable tool for the diagnosis of subclinical atherosclerosis in SO. This might improve the accuracy and detection of plaques at earlier stages, thereby helping to prevent acute CVD.
Author Contributions
Conceptualization, E.P. (Eva Pardina) and J.C.-M.; methodology, J.P.-O. and J.A.B.-F.; validation, E.P. (Eva Pardina), and D.R.-J.; formal analysis, A.M.; investigation, E.P. (Elionora Peña), J.C.-M. and N.A.-G.; resources, D.R.-J., J.C.D. and A.V.; data curation, A.M.; writing—original draft preparation, J.C.-M.; writing—review and editing, E.P. (Eva Pardina) and A.R.; visualization, A.V., D.R.-J. and M.D.L.-T.; supervision, E.P. (Eva Pardina); project administration, J.P.-O. and E.P. (Eva Pardina); funding acquisition, J.P.-O. and J.A.B.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministerio de Sanidad y Consumo, Carlos III Institute of Health (ISCIII) [grant numbers PI15/00190 and PI15/00332], and the EU FEDER Programme (European Regional Development Fund: “Una manera de hacer Europa”).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Hospital Universitari Arnau de Vilanova (CEIC-1369).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are unavailable due to privacy or ethical restrictions.
Acknowledgments
We thank Teresa Vidal, Virtudes Maria, and Angels Betriu from the Vascular and Renal Translational Research Group, IRBLleida, for the detection of the atheromatous plaques. This work was supported by IRBLleida Biobank (B.0000682) and PLATAFORMA BIOBANCOS PT17/0015/0027/.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO European Regional Obesity Report 2022; WHO Regional Office for Europe: København, Denmark, 2022. [Google Scholar]
- Nguyen, N.T.; Varela, J.E. Bariatric Surgery for Obesity and Metabolic Disorders: State of the Art. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, M.P.; Raja, R.; Jamil, A.; Csendes, D.; Gutlapalli, S.D.; Prakash, K.; Swarnakari, K.M.; Bai, M.; Desai, D.M.; Desai, A.; et al. Obesity and Coronary Artery Disease: An Updated Systematic Review 2022. Cureus 2022, 14, e29480. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of All-Cause Mortality with Overweight and Obesity Using Standard Body Mass Index Categories: A Systematic Review and Meta-Analysis. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef]
- World Health Organization. Cardiovascular Diseases (CVDs), Key Facts. Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 3 May 2022).
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Ellenberger, K.; Jeyaprakash, P.; Sivapathan, S.; Sangha, S.; Kitley, J.; Darshni, A.; Chen, D.; Negishi, K.; Pathan, F. The Effect of Obesity on Echocardiographic Image Quality. Heart Lung Circ. 2022, 31, 207–215. [Google Scholar] [CrossRef]
- Carmona-Maurici, J.; Cuello, E.; Ricart-Jané, D.; Miñarro, A.; Baena-Fustegueras, J.A.; Peinado-Onsurbe, J.; Pardina, E. Effect of Bariatric Surgery on Inflammation and Endothelial Dysfunction as Processes Underlying Subclinical Atherosclerosis in Morbid Obesity. Surg. Obes. Relat. Dis. 2020, 16, 1961–1970. [Google Scholar] [CrossRef]
- Novelle, M.G.; Contreras, C.; Romero-Picó, A.; López, M.; Diéguez, C. Irisin, Two Years Later. Int. J. Endocrinol. 2013, 2013, 746281. [Google Scholar] [CrossRef]
- Aronis, K.N.; Moreno, M.; Polyzos, S.A.; Moreno-Navarrete, J.M.; Ricart, W.; Delgado, E.; de la Hera, J.; Sahin-Efe, A.; Chamberland, J.P.; Berman, R.; et al. Circulating Irisin Levels and Coronary Heart Disease: Association with Future Acute Coronary Syndrome and Major Adverse Cardiovascular Events. Int. J. Obes. 2015, 39, 156–161. [Google Scholar] [CrossRef]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-Alpha-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Yang, M.; Sun, J.; Zhao, Y.; Tang, D. The Effect of Irisin as a Metabolic Regulator and Its Therapeutic Potential for Obesity. Int. J. Endocrinol. 2021, 2021, 6572342. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Makras, P.; Perakakis, N.; Kountouras, J.; Mantzoros, C.S. Irisin in Metabolic Diseases. Endocrine 2018, 59, 260–274. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, H.; Zhang, J.; Zhang, X.; Xin, C.; Zhang, F.; Lee, Y.; Zhang, L.; Lian, K.; Yan, W.; et al. Irisin Improves Endothelial Function in Type 2 Diabetes through Reducing Oxidative/Nitrative Stresses. J. Mol. Cell. Cardiol. 2015, 87, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I.; Pocheć, E.; Zarawski, M. Anti-Inflammatory Properties of Irisin, Mediator of Physical Activity, Are Connected with TLR4/MyD88 Signaling Pathway Activation. Int. J. Mol. Sci. 2017, 18, 701. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating Irisin in Relation to Insulin Resistance and the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Shen, J.; Liu, Q.; Zhang, Y.; Kuang, J.; Pu, S.; Cheng, S.; Zou, M.; Jiang, W.; Jiang, C.; et al. Irisin Is Regulated by CAR in Liver and Is a Mediator of Hepatic Glucose and Lipid Metabolism. Mol. Endocrinol. 2016, 30, 533–542. [Google Scholar] [CrossRef]
- Carmona-Maurici, J.; Cuello, E.; Ricart-Jané, D.; Miñarro, A.; Olsina Kissler, J.J.; Baena-Fustegueras, J.A.; Peinado-Onsurbe, J.; Pardina, E. Effect of Bariatric Surgery in the Evolution of Oxidative Stress Depending on the Presence of Atheroma in Patients with Morbid Obesity. Surg. Obes. Relat. Dis. 2020, 16, 1258–1265. [Google Scholar] [CrossRef]
- Carmona-Maurici, J.; Cuello, E.; Sánchez, E.; Miñarro, A.; Rius, F.; Bueno, M.; de la Fuente, M.C.; Olsina Kissler, J.J.; Vidal, T.; Maria, V.; et al. Impact of Bariatric Surgery on Subclinical Atherosclerosis in Patients with Morbid Obesity. Surg. Obes. Relat. Dis. 2020, 16, 1419–1428. [Google Scholar] [CrossRef]
- Jia, J.; Yu, F.; Wei, W.-P.; Yang, P.; Zhang, R.; Sheng, Y.; Shi, Y.-Q. Relationship between Circulating Irisin Levels and Overweight/Obesity: A Meta-Analysis. World J. Clin. Cases 2019, 7, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Belviranli, M.; Okudan, N.; Çelik, F. Association of Circulating Irisin with Insulin Resistance and Oxidative Stress in Obese Women. Horm. Metab. Res. 2016, 48, 653–657. [Google Scholar] [CrossRef]
- Pérez-Sotelo, D.; Roca-Rivada, A.; Baamonde, I.; Baltar, J.; Castro, A.I.; Domínguez, E.; Collado, M.; Casanueva, F.F.; Pardo, M. Lack of Adipocyte-Fndc5/Irisin Expression and Secretion Reduces Thermogenesis and Enhances Adipogenesis. Sci. Rep. 2017, 7, 16289. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Gusdon, A.M.; Qu, S. Irisin: A New Molecular Marker and Target in Metabolic Disorder. Lipids Health Dis. 2015, 14, 2. [Google Scholar] [CrossRef]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and Irisin in Humans: I. Predictors of Circulating Concentrations in Serum and Plasma and II. MRNA Expression and Circulating Concentrations in Response to Weight Loss and Exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef]
- Demirpence, M.; Yilmaz, H.; Colak, A.; Yalcin, H.; Toprak, B.; Turkon, H.; Ugurlu, L.; Aydin, C. The Effect of Sleeve Gastrectomy on Serum Irisin Levels in Patients with Morbid Obesity. Endokrynol. Pol. 2016, 67, 481–486. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Miura, K.; Arima, H.; Fujiyoshi, A.; Kadota, A.; Kadowaki, S.; Zaid, M.; Miyagawa, N.; Satoh, A.; Kunimura, A.; et al. Relationship of Serum Irisin Levels to Prevalence and Progression of Coronary Artery Calcification: A Prospective, Population-Based Study. Int. J. Cardiol. 2018, 267, 177–182. [Google Scholar] [CrossRef]
- Remuzgo-Martínez, S.; Rueda-Gotor, J.; Pulito-Cueto, V.; López-Mejías, R.; Corrales, A.; Lera-Gómez, L.; Pérez-Fernández, R.; Portilla, V.; González-Mazón, Í.; Blanco, R.; et al. Irisin as a Novel Biomarker of Subclinical Atherosclerosis, Cardiovascular Risk and Severe Disease in Axial Spondyloarthritis. Front. Immunol. 2022, 13, 894171. [Google Scholar] [CrossRef] [PubMed]
- Onalan Etem, E.; Dis, O.; Tektemur, A.; Korkmaz, H.; Buran Kavuran, I. Common Single Nucleotide Polymorphisms in the FNDC5 Gene and Serum Irisin Levels in Acute Myocardial Infarction. Anatol. J. Cardiol. 2021, 25, 528–535. [Google Scholar] [CrossRef]
- Cao, R.Y.; Zheng, H.; Redfearn, D.; Yang, J. FNDC5: A Novel Player in Metabolism and Metabolic Syndrome. Biochimie 2019, 158, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Brondani, L.A.; Boelter, G.; Assmann, T.S.; Leitão, C.B.; Canani, L.H.; Crispim, D. Irisin-Encoding Gene (FNDC5) Variant Is Associated with Changes in Blood Pressure and Lipid Profile in Type 2 Diabetic Women but Not in Men. Metabolism 2015, 64, 952–957. [Google Scholar] [CrossRef]
- Badr, E.A.; Mostafa, R.G.; Awad, S.M.; Marwan, H.; Abd El-Bary, H.M.; Shehab, H.E.; Ghanem, S.E. A Pilot Study on the Relation between Irisin Single-Nucleotide Polymorphism and Risk of Myocardial Infarction. Biochem. Biophys. Rep. 2020, 22, 100742. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, F.; Tang, Y.; Cai, L.; Zeng, C.; Yang, Y.; Yang, J. The Emerging Role of Irisin in Cardiovascular Diseases. J. Am. Heart Assoc. 2021, 10, e022453. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Li, H.; Zhang, T.; Yang, L.; Yao, S.; Chen, S.; Zheng, M.; Zhao, Q.; Tian, H. Irisin Protects Macrophages from Oxidized Low Density Lipoprotein-Induced Apoptosis by Inhibiting the Endoplasmic Reticulum Stress Pathway. Saudi J. Biol. Sci. 2018, 25, 849–857. [Google Scholar] [CrossRef]
- Pan, J.-A.; Zhang, H.; Yu, Q.; Zhang, J.-F.; Wang, C.-Q.; Gu, J.; Chen, K. Association of Circulating Irisin Levels and the Characteristics and Prognosis of Coronary Artery Disease. Am. J. Med. Sci. 2021, 362, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Anandakumar, P.; Debela, T. A Review on the Role of Irisin in Insulin Resistance and Type 2 Diabetes Mellitus. J. Pharmacopunct. 2017, 20, 235–242. [Google Scholar] [CrossRef]
- Xiong, X.-Q.; Chen, D.; Sun, H.-J.; Ding, L.; Wang, J.-J.; Chen, Q.; Li, Y.-H.; Zhou, Y.-B.; Han, Y.; Zhang, F.; et al. FNDC5 Overexpression and Irisin Ameliorate Glucose/Lipid Metabolic Derangements and Enhance Lipolysis in Obesity. Biochim. Biophys. Acta 2015, 1852, 1867–1875. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Tang, E.H.C.; Feletou, M. Endothelial Dysfunction and Vascular Disease. Acta Physiol. 2009, 196, 193–222. [Google Scholar] [CrossRef]
- Martinez Muñoz, I.Y.; Camarillo Romero, E.D.S.; Garduño Garcia, J.d.J. Irisin a Novel Metabolic Biomarker: Present Knowledge and Future Directions. Int. J. Endocrinol. 2018, 2018, 7816806. [Google Scholar] [CrossRef]
- Dal Canto, E.; Ceriello, A.; Rydén, L.; Ferrini, M.; Hansen, T.B.; Schnell, O.; Standl, E.; Beulens, J.W. Diabetes as a Cardiovascular Risk Factor: An Overview of Global Trends of Macro and Micro Vascular Complications. Eur. J. Prev. Cardiol. 2019, 26, 25–32. [Google Scholar] [CrossRef]
- Askari, H.; Fatima, S.; Poorebrahim, M.; Haghi-Aminjan, H.; Raeis-Abdollahi, E.; Abdollahi, M. A Glance at the Therapeutic Potential of Irisin against Diseases Involving Inflammation, Oxidative Stress, and Apoptosis: An Introductory Review. Pharmacol. Res. 2018, 129, 44–55. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Bilski, J.; Pochec, E.; Brzozowski, T. New Insight into the Direct Anti-Inflammatory Activity of a Myokine Irisin against Proinflammatory Activation of Adipocytes. Implication for Exercise in Obesity. J. Physiol. Pharmacol. 2017, 68, 243–251. [Google Scholar]
- De Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, Inflammation and the Immune System. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef]
- Sillen, M.; Declerck, P.J. Targeting PAI-1 in Cardiovascular Disease: Structural Insights Into PAI-1 Functionality and Inhibition. Front. Cardiovasc. Med. 2020, 7, 622473. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Kozlowska, K.; Pochec, E.; Bilski, J.; Brzozowski, T. Myokine Irisin-Induced Protection against Oxidative Stress in Vitro. Involvement of Heme Oxygenase-1 and Antioxidazing Enzymes Superoxide Dismutase-2 and Glutathione Peroxidase. J. Physiol. Pharmacol. 2018, 69, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Han, F.; Sun, X. The Relationship between Circulating Irisin Levels and Endothelial Function in Lean and Obese Subjects. Clin. Endocrinol. 2015, 83, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Askin, L.; Uzel, K.E.; Tanriverdi, O.; Turkmen, S. Serum Irisin: Pathogenesis and Clinical Research in Cardiovascular Diseases. Cardiovasc. Innov. Appl. 2020, 4, 195–200. [Google Scholar] [CrossRef]
- Woollard, K.J.; Chin-Dusting, J. Therapeutic Targeting of P-Selectin in Atherosclerosis. Inflamm. Allergy Drug Targets 2007, 6, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Bots, M.L. Carotid Intima-Media Thickness as a Surrogate Marker for Cardiovascular Disease in Intervention Studies. Curr. Med. Res. Opin. 2006, 22, 2181–2190. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, Q.; Zhou, Z.; Song, H.; Zhang, Y.; Wu, F.; Jiang, M.; Wang, F.; Zhang, W.; Li, L.; et al. Protective Effect of Irisin on Atherosclerosis via Suppressing Oxidized Low Density Lipoprotein Induced Vascular Inflammation and Endothelial Dysfunction. PLoS ONE 2016, 11, e0158038. [Google Scholar] [CrossRef]
- Lu, J.; Xiang, G.; Liu, M.; Mei, W.; Xiang, L.; Dong, J. Irisin Protects against Endothelial Injury and Ameliorates Atherosclerosis in Apolipoprotein E-Null Diabetic Mice. Atherosclerosis 2015, 243, 438–448. [Google Scholar] [CrossRef]
- Korta, P.; Pocheć, E.; Mazur-Biały, A. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Medicina 2019, 55, 485. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Sabetai, M.M.; Tegos, T.J.; Nicolaides, A.N.; Dhanjil, S.; Pare, G.J.; Stevens, J.M. Reproducibility of Computer-Quantified Carotid Plaque Echogenicity: Can We Overcome the Subjectivity? Stroke 2000, 31, 2189–2196. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).