Abstract
Streptococcus suis, an encapsulated zoonotic pathogen, has been reported to cause a variety of infectious diseases, such as meningitis and streptococcal-toxic-shock-like syndrome. Increasing antimicrobial resistance has triggered the need for new treatments. In the present study, we found that isopropoxy benzene guanidine (IBG) significantly attenuated the effects caused by S. suis infection, in vivo and in vitro, by killing S. suis and reducing S. suis pathogenicity. Further studies showed that IBG disrupted the integrity of S. suis cell membranes and increased the permeability of S. suis cell membranes, leading to an imbalance in proton motive force and the accumulation of intracellular ATP. Meanwhile, IBG antagonized the hemolysis activity of suilysin and decreased the expression of Sly gene. In vivo, IBG improved the viability of S. suis SS3-infected mice by reducing tissue bacterial load. In conclusion, IBG is a promising compound for the treatment of S. suis infections, given its antibacterial and anti-hemolysis activity.
1. Introduction
Streptococcus suis is a common zoonotic pathogen, and its infection is associated with mortality in humans and animals [1]. S. suis can cause a variety of diseases in humans and pigs, including pneumonia, meningitis, endocarditis, and streptococcal-toxic-shock-like syndrome, resulting in morbidity and mortality [2,3]. Among the existing S. suis serotypes, type 2 is recognized as the most virulent and impactful serotype [4]. There are currently 35 capsular antigen-based serotypes of S. suis, of which serotypes 1, 2, and 14 are the most clinically relevant due to their virulence factors [5,6,7]. The major virulence determinants of S. suis identified to date are hemolysin (suilysin), muramidase-released protein, and extracellular protein factor [8]. A novel surface protein Fhb (factor H-binding protein), a potential new virulence factor, was identified in Streptococcus serotype two and serotypes one, seven, and nine, contributing to S. suis resistance to phagocytosis and virulence [9]. Multiple locus sequence typing (MLST) was used to study the population structure and genetic diversity of S. suis [10]. ST25, ST28, ST29, ST94, ST108, ST117, ST225, ST373, ST961, ST977, ST21, and ST31 were found to be associated with pathogenic pathotypes [11]. Pigs are important food animals, and S. suis can be transmitted to humans from sick pigs through human skin wounds and nasal mucosa [12]. At present, the human infection with S. suis has been widely reported worldwide [13]. With the rapid development of resistance, humans have encountered great challenges in treating infections caused by S. suis [14]. Therefore, there is an urgent need for the discovery and development of drugs against S. suis infection.
At present, the discovery of antibacterial drugs is mainly based on the following aspects: firstly, the screening and extraction of active compounds from natural products; secondly, the generation of compound libraries by chemical synthesis and the screening of effective compounds; thirdly, the exploration of unknown antibacterial targets and the performance of target screening [15,16,17]. For example, daptomycin, originally discovered in Streptomyces roserosi, is a cyclic lipopeptide antibiotic currently used to treat serious infections caused by Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, etc. [18,19,20]. At the same time, drug repurposing has also become an innovative way of drug development [21]. To date, many efforts have been made to screen FDA-approved drugs for antimicrobial and antiviral activity [22,23]. The antiplatelet drug ticlopidine was found to have anti-MASR effects by inhibiting wall teichoic acid biosynthesis in combination with β-lactam antibiotics [24].
In the history of antibiotic discovery, it is easy to see that natural products are representative of the substances used to discover antibiotics. However, natural products have some unavoidable drawbacks; firstly, their wide distribution in nature and the high cost of obtaining them; and secondly, their complex chemical structures, which are difficult to obtain via chemical synthesis. Compared to the natural product library, synthetic compound libraries have a much wider scope, both in terms of quantity and chemical structure variability, and we can modify their structures by conformational relationships to obtain more active compounds [25]. Guanidine functional groups are positively charged and can lead to the loss of biological function of phospholipids or disruption of membranes by binding to negatively charged cytoplasmic membranes or cell wall components, ultimately leading to cell death [26]. The guanidine group is one of the preferred functional groups in the design and development of antibacterial drugs. In our previous study, we screened isopropoxy benzene guanidine (IBG) from a series of guanidinium-based compounds with anti-Gram-positive bacterial activity, such as Staphylococcus aureus (0.125–4 µg/mL) and Enterococcus (2–16 μg/mL), by disrupting cell membranes [27,28]. This compound shows good drug properties and the possibility of being a leading compound in terms of antibacterial activity and safety. The aim of this study was to further investigate the antibacterial activity and antibacterial mechanism of action of IBG against S. suis through serial passages, assessment of the effect on the hemolytic activity of S. suis and a mouse model of intraperitoneal infection.
2. Result
2.1. IBG Is a Potential Antimicrobial Agent
IBG showed strong antibacterial activity against all S. suis isolates tested, with MICs ranging from 0.25–8 μg/mL. The MIC of IBG against S. suis ATCC 43765 was 8 μg/mL. The activity of IBG against S. suis was not potentially related to its multi-locus sequence type (MLST; Table 1). In addition, serum concentration has a greater effect on the bactericidal activity of IBG. In the absence of serum, the MIC of IBG was 2 µg/mL, whereas, in the presence of 10% serum, the MIC increased 8-fold to 16 µg/mL. In resistance studies, IBG did not show strongly induced resistance, and its MIC only increased only fourfold within 28 days and then recovered (Figure 1B). In contrast, the MIC of CIP increased 1024-fold within 28 days. The time-kill kinetics assay showed that IBG had a significant bactericidal effect that was concentration-dependent (Figure 1C). IBG was able to kill most cells at a concentration of 10 × MIC (80 μg/mL) for 4 h. The bactericidal activity of IBG was weaker at 1× and 4 × MIC. At 1 × MIC, there was a weak bactericidal effect within two hours of exposure, after which bacterial growth resumed. Toxicity is one of the key factors limiting the clinical use of drugs [29]. The results of the safety assessment showed that IBG had a very high cut-off range (Figure 1C). It had no apparent hemolytic effect at low concentrations. At the concentration of 10 × MIC, the hemolysis rate was less than 20%. In general, IBG is a promising antibacterial compound.

Table 1.
Activity of IBG against a wide variety of MLST S. suis. MLST, MLST, multi-locus sequence type; MIC, minimum inhibitory concentration; /, none.
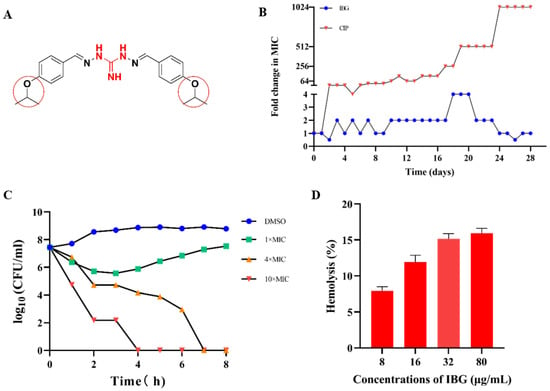
Figure 1.
IBG is a potential antimicrobial agent against S. suis. (A) The chemical structure of IBG, the guanidine group, is labeled in red, and 4-OCH(CH3)2 is marked with a red circle. (B) No resistance to IBG occurred in the 28-day serial passage. CIP, ciprofloxacin, was used as a control group. (C) Time-kill curves of IBG against Streptococcus suis with 1 × MIC (8 μg/mL), 4 × MIC (32 μg/mL), and 10 × MIC (80 μg/mL) concentrations. The 1% DMSO-treated group served as the control group. (D) Hemolytic activity of IBG on defibrillated sheep blood erythrocytes.
2.2. IBG Disrupts S. suis Cell Membrane
Membrane damage was reflected by an increase in the fluorescence signal of S. suis cells stained with PI. As shown in Figure 2E, the fluorescence signal was greatly enhanced in the presence of IBG in a dose-dependent manner compared to the IBG-untreated group. The effect of IBG on PMF was then measured. ΔΨm and ΔpH are two important factors of PMF [30]. The fluorescent probe DiSC3(5) was used to detect changes in ΔΨm. The results showed that IBG significantly (p < 0.01) decreased the fluorescence value of DiSC3(5), especially at 80 μg/mL, indicating that IBG disrupted the ΔΨm of the cell membrane (Figure 2A). ΔpH was then detected using the fluorescent probe BCECF-AM. IBG significantly dissipated ΔpH in a dose-dependent manner (Figure 2B). Since PMF disruption can affect cellular ATP [31], the levels of intracellular and extracellular ATP were measured. The results showed that, correspondingly, IBG significantly decreased the level of intracellular ATP in a dose-dependent manner (Figure 2C). In contrast, a dose-dependent increase in extracellular ATP was observed (Figure 2C). As the generation of ROS is thought to be a typical mechanism of bactericidal antibiotics [32], we next investigated the effect of IBG on ROS levels. The results showed that, as with classical antibiotics, IBG significantly (p < 0.001) increased the level of ROS in a dose-dependent manner after 60 min of treatment (Figure 2D).
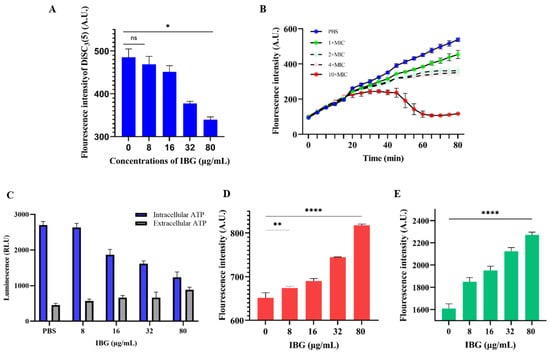
Figure 2.
The antibacterial mechanism of IBG against Streptococcus suis. (A) Fluorescent probe DiSC3(5) was used to detect the membrane potential (ΔΨm). Disruption of membrane potential in IBG-treated S. suis ATCC 43765. p values were determined using a non-parametric one-way ANOVA. (B) The ΔpH of S. suis ATCC 43765 treated with different concentrations of IBG was detected by the fluorescent probe BCECF-AM. (C) The intracellular and extracellular ATP levels of IBG-treated S. suis ATCC 43765. (D) The effect of low concentrations of IBG on ROS accumulation in S. suis ATCC 43765 was negligible. (E) The permeability of S. suis ATCC 43765 membranes was probed by PI. 1 × MIC, 8 μg/mL; 2 × MIC, 16 μg/mL; 4 × MIC, 32 μg/mL; and 10 × MIC, 80 μg/mL. *, p < 0.01, ****, p < 0.0001.
2.3. Significant Anti-Hemolysis Activity of IBG against S. suis
The effect of IBG against suilysin was assessed by hemolysis assays. As shown in Figure 3A, S. suis ATCC 43765 culture supernatant had strong hemolytic activity, and approximately 41.36% of sheep erythrocytes were lysed. However, in the presence of IBG, the hemolytic activity of the supernatant was significantly (p < 0.0001) decreased in a dose-dependent manner. When the final concentration of IBG added was 80 μg/mL, the hemolytic capacity of the supernatant was only 5.10%. We then examined the effect of IBG on S. suis ATCC 43765 Sly gene expression. The results showed that IBG could significantly (p < 0.01) reduce the expression of the Sly gene in a dose-dependent manner compared to the control group (Figure 3B). In addition, the expression of the Sly gene results in the production of the Sly protein, a cholesterol-dependent toxin that destroys cells by creating microscopic pores in the host membrane containing cholesterol [33]. Subsequently, molecular docking was performed to analyze the binding between IBG and the Sly protein. The results showed that IBG had a good affinity for the Sly protein with a LibDockScore of 82.4514. Meanwhile, molecular docking further revealed the potential interaction of IBG with the Sly protein. Potentially critical active residues were observed to be involved in the binding sites of IBG, such as VAL232, TYP264, PRO235, LEU124, LYS240, GLY100, THR125, ASP233, GLU234, PRO145, and ASP123 (Figure 3C,D).
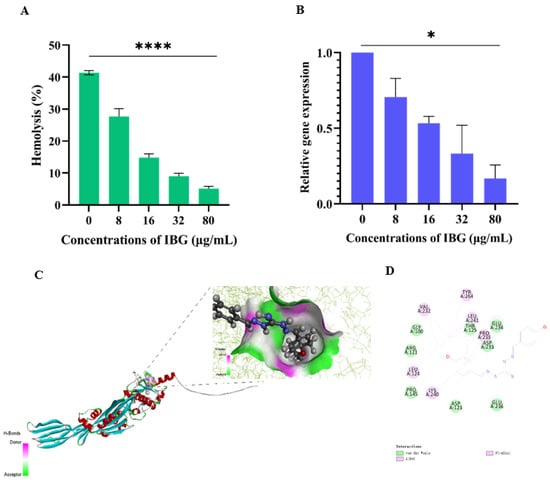
Figure 3.
IBG alleviated the hemolytic activity of S. suis ATCC 43765. (A) Hemolytic activity of supernatants of S. suis ATCC 43765 cultures treated with different concentrations of IBG. (B) Relative expression levels of the Sly gene of S. suis ATCC 43765 in the presence of IBG. (C,D) The interaction pattern of IBG and Sly proteins. *, p < 0.01, ****, p < 0.0001.
2.4. In Vivo Efficacy
The in vivo therapeutic efficacy of IBG was evaluated using a mouse model of S. suis SS3 infection. The protective effect of IBG on infected mice was assessed by survival rate. Compared to untreated infected mice, 80 mg/kg of IBG increased the survival rate of infected mice by 66%. In testing the degree of infection for different tissues of mice from S. suis SS3, SS3 caused severe pathological damage in mice, and a large number of bacterial colonies were found in lung, liver, kidney, and spleen tissues. By treating infected mice with IBG, we found that IBG significantly alleviated the pathological damage caused by SS3. The bacterial load of IBG-treated mouse tissues was significantly (p < 0.001) reduced, particularly in the lung and spleen (Figure 4). The survival rate of IBG-treated mice was higher than that of untreated mice, while the survival rate of mice increased with increasing doses of IBG (Figure 5A). In addition, the effect of IBG on blood biochemical levels (ALP, AST, ALT, CREA, UREA) was assessed. The results showed that IBG significantly (p < 0.01) reduced the blood biochemical enzyme levels in the infected mice (Figure 5). Tissue sections showed that IBG significantly alleviated pathological damage in infected mice, including liver hemorrhaging and hepatocyte necrosis, renal tubular epithelial cell degeneration and necrosis, and lung hemorrhaging (Figure 6). In conclusion, IBG showed good therapeutic effects in SS3-infected mice.
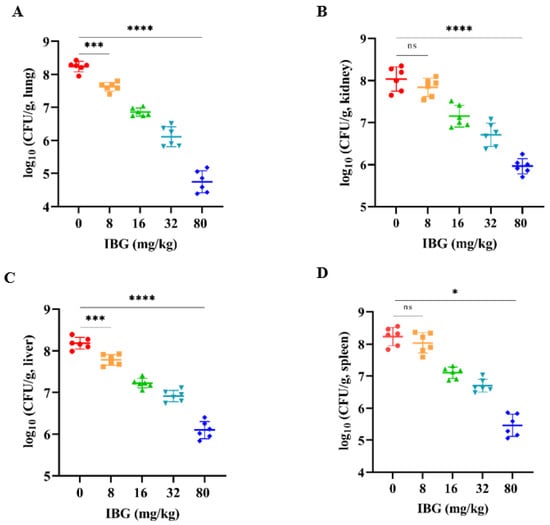
Figure 4.
Bacterial load of (A) lung, (B) kidney, (C) liver, and (D) spleen tissues of SS3-infected mice after treatment with different doses of IBG. IBG significantly reduced the bacterial load of lung, kidney, liver, and spleen tissues. An unpaired two-tailed t-test was used for statistical analysis. *, p < 0.01, ***, p < 0.001, ****, p < 0.0001.
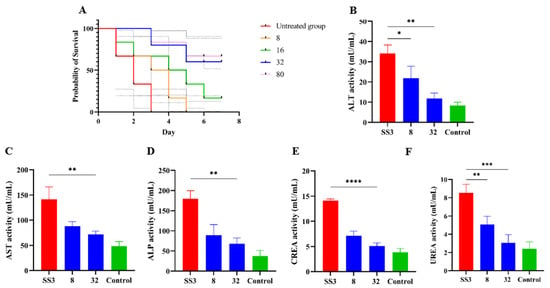
Figure 5.
The efficacy of IBG against S. suis in vivo. (A) The survival curve of various concentrations of IBG cured S. suis SS3-infected mice model. Blood levels of (B) ALT, (C) AST, (D) ALP, (E) CREA, and (F) UREA in mice. SS3 is the infected group; eight were in the treated group with 8 μg/mL IBG, and 16 were in the treated group with 16 μg/mL IBG; Control is the blank group without any treatment. *, p < 0.01, **, p < 0.01, ***, p < 0.001, ****, p < 0.0001.
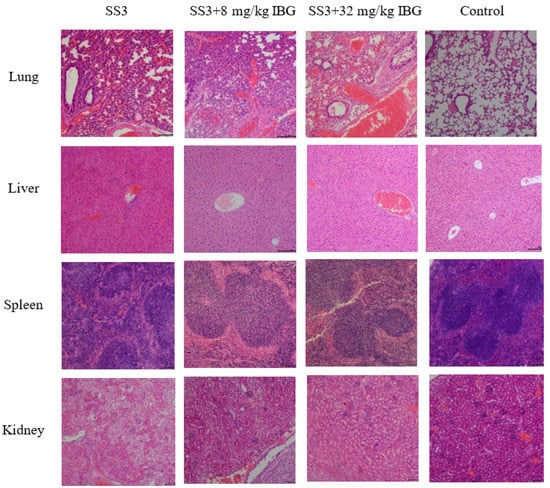
Figure 6.
Histologic analysis of different organs using hematoxylin-eosin (HE) staining. Pathological changes in lung, liver, spleen, and kidney histology after IBG treatment. IBG alleviated the pathological damage and inflammatory response in infected mice.
3. Discussion
The development of new antibiotics lags far behind the development of drug resistance. Meanwhile, the exploration of new targets and new chemical structures of antibacterial drugs is an important direction for the development of antibacterial drugs [34]. Guanidine functional groups have an important place in medicinal chemistry because of their high stability and base modulation [35]. The high pKa value of the guanidine group, protonated under physiological conditions, is the main reason for its pharmacological action, while the versatility of its side chains confers a high degree of modifiability of its physical properties to adapt to different use scenarios. Chitosan derivatives containing a guanidine functional group, synthesized by a direct reaction between chitosan and cyanamide, have broad-spectrum antibacterial activity and can effectively inhibit a variety of bacteria, including Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa [36]. Biguanide-derived nanoparticles FTP NPs showed potent anti-MRSA activity, both inside and outside biofilms, with the bactericidal mechanism involving membrane permeabilization [37].
Similar to other guanidine derivatives, IBG can disrupt the integrity of S. suis cell membranes through electrostatic interactions with negatively charged cell envelopes. In previous studies, we also found that IBG had antibacterial activity against Enterococcus and S. aureus, with the best antibacterial activity against S. aureus (0.125–4 μg/mL) and the worst against S. suis (0.25–8 μg/mL), which may be related to the differences in bacterial membrane composition [27,28]. The structural and functional integrity of cell membranes is essential for cell survival, and therefore, cell membranes are worth considering as targets for antimicrobial drugs [38]. From a safety perspective, IBG is not genotoxic, reproductive genotoxic, embryotoxic, or teratogenic and has an oral acute toxicity classification of low toxicity in rats (LD50 of 1870.83 mg/kg b.w.) [39]. The main guanidine derivatives currently in clinical use are metformin and robenidine. The biguanides, metformin, phenformin, and buformin are a class of hypoglycemic drugs developed in the 1950s for the treatment of type 2 diabetes, and only metformin is now approved for use in most countries [40]. Phenformin and buformin have been withdrawn from the market due to an increased risk of lactic acidosis [41].
More important for S. suis is its virulence [42]. Of course, IBG is not only bactericidal against S. suis, but it also reduces the virulence of S. suis in vivo and in vitro, such as suilysin (Sly). Suilysin plays an important role in the inflammatory response by destroying eukaryotic cells and inducing the release of cytokines from immune cells [43]. Given that suilysin is a key virulence factor for S. suis for the colonization of host cells, escape from host immunity, and the production of cytotoxicity, suilysin is considered a new target to explore anti-virulence compounds for the treatment of S. suis infections [44,45]. In the present study, IBG showed excellent hemolytic activity against S. suis ATCC43765 culture supernatant and significantly inhibited the expression of the Sly gene. At the same time, IBG had no significant hemolytic activity in the therapeutic range. Compared with IBG, quercetin, piceatannol, and baicalein only inhibited the hemolytic activity of Sly protein and reduced the inflammatory response to streptococcus suis infection, but had no anti-streptococcal activity [46,47,48]. In conclusion, IBG has a more comprehensive function in clinical use.
In terms of chemical structure, IBG is a two-part symmetrical diaminoguanidine derivative containing two isopropoxyphenyls, which means that it is highly lipid-soluble and poorly water-soluble, severely limiting the route to clinical use. The most chemically similar compound to IBG is robenidine, which differs only in the substituent on the benzene ring [IBG: 4-OCH(CH3)2, robenidine: 4-Cl] (Figure 1A). Robenidine is widely used in the treatment of coccidial infections in poultry and rabbits, and its antimicrobial efficacy was later evaluated when it was found to be more effective against Gram-positive bacteria, S. pseudintermedius and beta-hemolysis streptococcs, and showed good synergistic effects when used in combination with EDTA against Gram-negative bacteria [49]. In the structure-activity relationship (SAR) investigation of robenidine, it was found that the antibacterial activity of the 4-OH robenidine analog was significantly reduced, and its methylated analog (4-OCH3) had no antibacterial activity, in contrast, whereas the alkyl substituent analog was more effective, with 4-CH3 and 4-CH(CH3)2 being more prominent [50,51]. This shows that 4-OCH(CH3)2 is essential for the antibacterial activity of IBG. Obviously, the search for a more optimal chemical structure is still a work in progress.
4. Materials and Methods
4.1. Bacterial Strains and Chemicals
The S. suis strain, ATCC 43765, and 12 clinical S. suis isolates were used in this study (Table 1). All strains were grown in tryptose soya broth (TSB; Hopebio, Qingdao, China) or plated on tryptose soya agar (TSA; Hopebio, Qingdao, China) with 5% (v/v) newborn fetal bovine serum at 37 °C. Isopropoxy benzene guanidine (IBG; purity: 99.9%) was synthesized by the Guangzhou Insighter Biotechnology Co., Ltd. (Guangzhou, China).
4.2. Antimicrobial Susceptibility Testing
The minimum inhibitory concentration (MIC) of isopropoxy benzene guanidine (IBG) was determined according to the Clinical and Laboratory Standards Institute (CLSI) guidelines. The microbroth dilution method was applied to 96-well plates with Mueller–Hinton broth (MHB; 5% newborn fetal bovine serum; Hopebio, Qingdao, China). The overnight bacteria cultures were adjusted to 5 × 105 colony-forming units (CFU)/mL. After 18 h of incubation at 37 °C, the MICs were defined as the lowest antibiotic concentration without bacterial growth by visual inspection.
4.3. Kill Kinetic Assay
The time-dependent killing of S. suis ATCC 43765 with various concentrations of IBG was investigated. The overnight cultures of S. suis ATCC 43765 were diluted by 1:100 and treated with 0, 8, 16, 32, and 80 μg/mL of IBG. Colony counts were obtained by serial dilutions and plate counts at 0, 1, 2, 3, 4, 6, and 8 h. The time-kill curves were plotted as log CFU/mL versus time.
4.4. Resistance Studies
Cultures were serially passaged for 28 days while being treated with various concentrations of IBG in order to better understand how resistance to IBG develops. Overnight cultures of S. suis ATCC 43765 were inoculated into MHB containing 1×, 2×, 4×, and 10× MIC of IBG. The ciprofloxacin-treated group was used as a positive control. The MIC of each generation of cultures against IBG or ciprofloxacin was measured by the microbroth dilution method using 96-well plates. Fold changes in MICs for IBG and ciprofloxacin compared to initial values were calculated.
4.5. Membrane Integrity Test
To assess the effect of IBG on the integrity of bacterial membranes in S. suis ATCC 43765, the membrane permeability of S. suis ATCC 43765 induced by IBG was tested using the fluorescent dye propidium iodide (PI; Aladdin, Shanghai, China) at a final concentration of 10 nM. The S. suis ATCC 43765 cells were washed three times with PBS (pH = 7.4) and adjusted to a final concentration of OD600 nm of 0.5. After incubation with PI at 37 °C for 20 min, S. suis ATCC 43765 was treated with different final concentrations of IBG (8, 16, 32, and 80 μg/mL) for 1 h, and the fluorescence was measured at excitation wavelength 535 and emission wavelength 615.
4.6. Membrane Potential Assay
The fluorescent probe DiSC3(5) (Thermo Scientific, Massachusetts, United States) was used to determine the effect of IBG on the cell membrane potential (ΔΨm) of S. suis. In total, overnight cultures of S. suis ATCC 43765 were washed three times with HEPES containing 20 mM glucose, and the bacterial suspensions were adjusted to an OD600 nm of 0.5. DiSC3(5) was then added to a final concentration of 5 μM and incubated at 37 °C for 20 min to plateau. Cultures were treated with IBG at final concentrations of 8, 16, 32, and 80 μg/mL, and changes in DiSC3(5) fluorescence values were detected by spectrofluorometer at Ex.622 nm/Em.670 nm over 60 min.
4.7. ΔpH Assay
ΔpH, another component of the proton motive force (PMF), was measured by pH-sensitive fluorescent probes BCECF-AM. S. suis ATCC 43765 were washed and resuspended to obtain an OD600 nm of approximately 0.5 with 5 mM HEPES containing 20 mM glucose. After treatment with BCECF-AM for 20 min, various concentrations of IBG were added, and then ΔpH of S. suis ATCC 43765 was measured using the excitation wavelength of 488 nm and emission wavelength of 535 nm for 60 min.
4.8. ATP Determination
Intracellular and extracellular ATP levels of S. suis ATCC 43765 were detected by an Enhanced ATP Assay Kit (Beyotime, Shanghai, China). The overnight cultured S. suis ATCC 43765 cells were washed three times with PBS (pH = 7.4) and resuspended to an OD600 nm of 0.5. Different concentrations of IBG were added to the resuspension and incubated at 37 °C for 1 h. The bacterial cultures were then centrifuged at 12,000 rpm for 5 min at 4 °C. The supernatants were collected for the determination of extracellular ATP levels. Meanwhile, the precipitates were lysed with lysozyme, and the intracellular ATP was detected after centrifugation. The assay working solution was added to a 96-well plate and incubated for 5 min at room temperature. The supernatant was then added to a 96-well plate, rapidly mixed, and then ATP levels were measured using an EnSight® Multimode Plate Reader.
4.9. ROS Detection
Reactive oxygen species (ROS) levels of IBG-treated S. suis ATCC 43765 were measured by ROS Assay Kit (Beyotime, Shanghai, China). Briefly, the overnight cultured S. suis ATCC 43765 cells were washed with PBS (pH = 7.4) and resuspended to an OD600 nm of 0.5. DCFH-DA (10 µM) was added to the culture and incubated at 37 °C for 20 min. Different concentrations of IBG were then added to treat the cultures for 60 min, and the fluorescence values were measured with 488 nm excitation and 525 nm emission filters.
4.10. Safety Assessment
The hemolytic activity of IBG was determined by defibrillating sheep blood erythrocytes. Briefly, 2% defibrillated sheep blood erythrocytes were treated with various concentrations of IBG (0–80 μg/mL) for 1 h. A PBS (pH = 7.4) treatment, with or without 2.5% Triton X-100, was used as positive and negative controls, respectively. The fluorescence value at OD543 nm was measured to evaluate the rate of hemolysis, and the following formula was used to evaluate the rate of hemolysis, Equation (1):
4.11. Evaluation of the Effect of IBG on the Hemolysis Activity of the Culture Supernatant of S. suis ATCC 43765
The overnight cultured S. suis ATCC 43765 was centrifuged at 12,000 rpm for 10 min at 4 °C. The supernatants were collected and incubated with different concentrations of IBG at 37 °C for 30 min. After adding 2% defibrillated sheep blood cells to the culture for 1 h, the culture was centrifuged at 1000 rpm for 5 min at 4 °C, and the supernatant was collected to measure its absorbance at OD543 nm. Samples treated with 2.5% Triton X-100 were set as positive controls. The PBS (pH = 7.4) treatment served as a negative control.
4.12. RT-qPCR
RT-qPCR was used to determine the effect of IBG on Sly gene expression. The overnight cultured S. suis ATCC 43765 cells were transferred 1:100 to fresh TSB medium and grown at 37 °C with shaking at 200 rpm to the logarithmic growth phase. After the cultures were washed with PBS (pH = 7.4) and incubated with different final concentrations of IBG for 4 h, total RNA was extracted, and cDNA was synthesized using the PrimeScript™ 1st Strand cDNA Synthesis Kit (Takara, Japan) according to the manufacturer’s instructions. A real-time PCR assay was performed with the SYBR Premix Ex Taq (Takara, China) following the manufacturer’s instructions. The primers used are listed in Table 2 [52]. The 16S rRNA served as the endogenous control. Relative expression levels of the Sly gene were calculated by the 2−ΔΔCT method.

Table 2.
The primers used for RT-qPCR in this study.
4.13. Molecular Docking
The model structure of the Sly protein was found in the UniProt Knowledgebase (https://www.uniprot.org/uniprotkb accessed on 25 October 2022). The protein sequence was A4FS11. The 2D structure of IBG was displayed using ChemDraw 20.0. The LibDock protocol of Discovery Studio 2019 was used to perform the molecular docking of the Sly protein and IBG.
4.14. Establishment of a Mouse Model of Intraperitoneal Infection
Male BALB/C mice, 6 weeks old, were obtained from the Guangdong Medical Laboratory Animal Center. The mice were randomly divided into five groups of six mice each. All procedures used in this experiment were performed according to the guidelines developed by the Laboratory Animal Center of South China Agricultural University. After 5 days of acclimatization, mice were injected intraperitoneally with 100 μL of a 1.5 × 108 CFU culture of S. suis SS3. One hour later, infected mice were treated with PBS and IBG (8, 16, 32, 80 mg/kg) by intraperitoneal injection. After 7 days, the survival curve of the mice was constructed based on the detection data.
In addition, the effect of IBG on blood biochemical enzymes in S. suis SS3-infected mice was evaluated, and the mice were treated as described above. Mice (six per group) were injected intraperitoneally with 100 µL of 1.5 × 108 CFU of S. suis SS3 culture, and 1 h later with PBS and different doses of IBG (8, 32 mg/kg) were injected as described above. After 12 h of treatment, cardiac blood was collected from anesthetized mice and assayed for the levels of alanine transaminase (ALT), aspartate transaminase (AST), creatinine (CREA), UREA, and alkaline phosphatase (ALP). Meanwhile, the lung, kidney, spleen, and liver tissues were collected, ground, diluted, and counted on a drop plate to determine the amounts of infected bacteria. Finally, the lung, kidney, spleen, and brain tissues of the mice were fixed with 4% paraformaldehyde, and their pathological changes were analyzed.
4.15. Statistical Analysis
GraphPad Prism 9.0 software was used for statistical analysis. All data were expressed as mean ± standard deviation. ns, not significant. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
5. Conclusions
IBG shows surprising anti-streptococcal activity in vivo and in vitro. IBG acts by disrupting the physical structure and function of bacterial cell membranes. It is a concentration-dependent, fast-acting bactericidal agent and is not susceptible to induced resistance. In addition, suilysin (sly) is one of the major virulence factors of S. suis and is closely associated with pathogenicity. IBG shows an exceptional ability to reduce the hemolysis activity of S. suis by reducing the expression of the sly gene and binding to suilysin. Taken together, it is clear that IBG is an excellent potential antimicrobial agent for the treatment of S. suis infections.
Author Contributions
Conceptualization, N.H.; methodology, N.H. and J.L.; software, N.H.; validation, N.H., J.L., F.Z., Y.L. and J.W.; formal analysis, N.H.; resources, X.D.; data curation, N.H.; writing—original draft preparation, N.H.; writing—review and editing, Z.Z., D.Z. and W.X.; visualization, N.H.; supervision, Z.Z.; project administration, Z.Z.; funding acquisition, Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 32273057).
Institutional Review Board Statement
All specific pathogen-free male BALB/C mice (Guangdong Medical Laboratory Animal Center) were 5–7 weeks old, weighing 20 ± 2 g. All procedures were approved by the Laboratory Animal Center of South China Agricultural University and performed in accordance with the guidelines of the Guangdong Laboratory Animal Welfare and Ethics Committee and the Laboratory Animal Center of South China Agricultural University (2022b165).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article.
Acknowledgments
Ning Han designed and drafted the article, analyzed the data, and plotted the images. Jie Li performed the membrane integrity test, membrane Potential Assay, ΔpH assay, and ATP determination. Feifei Zhao, Yangyang Li, and Jun Wang performed a mouse model of intraperitoneal infection assay. Dongping Zeng and Wenguang Xiong revised the manuscript. Zhenling Zeng revised and supervised the manuscript. All authors have been read and agreed with the version to be submitted.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Lun, Z.-R.; Wang, Q.-P.; Chen, X.-G.; Li, A.-X.; Zhu, X.-Q. Streptococcus suis: An emerging zoonotic pathogen. Lancet Infect. Dis. 2007, 7, 201–209. [Google Scholar] [CrossRef]
- Wangsomboonsiri, W.; Luksananun, T.; Saksornchai, S.; Ketwong, K.; Sungkanuparph, S. Streptococcus suis infection and risk factors for mortality. J. Infect. 2008, 57, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, B.; Zhang, Q.; Liu, L.; Zhang, A.; Yang, Y.; Huang, K.; Yan, S.; Yu, J.; Sun, X.; et al. Streptococcus suis 2 Transcriptional Regulator TstS Stimulates Cytokine Production and Bacteremia to Promote Streptococcal Toxic Shock-Like Syndrome. Front. Microbiol. 2018, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Goyette-Desjardins, G.; Auger, J.-P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 2014, 3, e45. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.J.; Hunt, B.W. Streptococcus suis serotypes 3 to 28 associated with disease in pigs. Vet. Rec. 2001, 148, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.; Gottschalk, M.; Boudreau, M.; Lebrun, A.; Henrichsen, J. Description of six new capsular types (29–34) of Streptococcus suis. J. Vet. Diagn. Investig. 1995, 7, 405–406. [Google Scholar] [CrossRef]
- Gottschalk, M.; Higgins, R.; Jacques, M.; Beaudoin, M.; Henrichsen, J. Characterization of six new capsular types (23 through 28) of Streptococcus suis. J. Clin. Microbiol. 1991, 29, 2590–2594. [Google Scholar] [CrossRef]
- Vecht, U.; Wisselink, H.J.; Jellema, M.L.; Smith, H.E. Identification of two proteins associated with virulence of Streptococcus suis type 2. Infect. Immun. 1991, 59, 3156–3162. [Google Scholar] [CrossRef]
- Pian, Y.; Gan, S.; Wang, S.; Guo, J.; Wang, P.; Zheng, Y.; Cai, X.; Jiang, Y.; Yuan, Y. Fhb, a novel factor H-binding surface protein, contributes to the antiphagocytic ability and virulence of Streptococcus suis. Infect. Immun. 2012, 80, 2402–2413. [Google Scholar] [CrossRef]
- Hatrongjit, R.; Fittipaldi, N.; Gottschalk, M.; Kerdsin, A. Tools for Molecular Epidemiology of Streptococcus suis. Pathogens 2020, 9, 81. [Google Scholar] [CrossRef]
- Estrada, A.A.; Gottschalk, M.; Rossow, S.; Rendahl, A.; Gebhart, C.; Marthaler, D.G. Serotype and Genotype (Multilocus Sequence Type) of Streptococcus suis Isolates from the United States Serve as Predictors of Pathotype. J. Clin. Microbiol. 2019, 57, e00377-19. [Google Scholar] [CrossRef] [PubMed]
- Yongkiettrakul, S.; Wongsurawat, T.; Jenjaroenpun, P.; Acheampong, D.A.; Srimanote, P.; Maneerat, K.; Visessanguan, W.; Nookaew, I. Genome sequences of antibiotic-resistant Streptococcus suis strains isolated from human patients and diseased and asymptomatic pigs in Thailand. Infect. Genet. Evol. 2021, 87, 104674. [Google Scholar] [CrossRef]
- Weinert, L.A.; Chaudhuri, R.R.; Wang, J.; Peters, S.E.; Corander, J.; Jombart, T.; Baig, A.; Howell, K.J.; Vehkala, M.; Välimäki, N.; et al. Genomic signatures of human and animal disease in the zoonotic pathogen Streptococcus suis. Nat. Commun. 2015, 6, 6740. [Google Scholar] [CrossRef]
- Haenni, M.; Lupo, A.; Madec, J.-Y. Antimicrobial Resistance in Acinetobacter spp. and Pseudomonas spp. Microbiol. Spectr. 2018, 6, 6-3. [Google Scholar] [CrossRef] [PubMed]
- Wohlleben, W.; Mast, Y.; Stegmann, E.; Ziemert, N. Antibiotic drug discovery. Microb. Biotechnol. 2016, 9, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef]
- Chang, M.; Mahasenan, K.V.; Hermoso, J.A.; Mobashery, S. Unconventional Antibacterials and Adjuvants. Acc. Chem. Res. 2021, 54, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Debono, M.; Barnhart, M.; Carrell, C.B.; Hoffmann, J.A.; Occolowitz, J.L.; Abbott, B.J.; Fukuda, D.S.; Hamill, R.L.; Biemann, K.; Herlihy, W.C. A21978C, a complex of new acidic peptide antibiotics: Isolation, chemistry, and mass spectral structure elucidation. J. Antibiot. (Tokyo) 1987, 40, 761–777. [Google Scholar] [CrossRef]
- Pujol, M.; Miró, J.-M.; Shaw, E.; Aguado, J.-M.; San-Juan, R.; Puig-Asensio, M.; Pigrau, C.; Calbo, E.; Montejo, M.; Rodriguez-Álvarez, R.; et al. Daptomycin Plus Fosfomycin Versus Daptomycin Alone for Methicillin-resistant Staphylococcus aureus Bacteremia and Endocarditis: A Randomized Clinical Trial. Clin. Infect. Dis. 2021, 72, 1517–1525. [Google Scholar] [CrossRef]
- Stefani, S.; Campanile, F.; Santagati, M.; Mezzatesta, M.L.; Cafiso, V.; Pacini, G. Insights and clinical perspectives of daptomycin resistance in Staphylococcus aureus: A review of the available evidence. Int. J. Antimicrob. Agents 2015, 46, 278–289. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, Z.; Shi, J.; Li, R.; Upton, M.; Wang, Z. Drug repurposing for next-generation combination therapies against multidrug-resistant bacteria. Theranostics 2021, 11, 4910–4928. [Google Scholar] [CrossRef]
- Dittmar, M.; Lee, J.S.; Whig, K.; Segrist, E.; Li, M.; Kamalia, B.; Castellana, L.; Ayyanathan, K.; Cardenas-Diaz, F.L.; Morrisey, E.E.; et al. Drug repurposing screens reveal cell-type-specific entry pathways and FDA-approved drugs active against SARS-Cov-2. Cell. Rep. 2021, 35, 108959. [Google Scholar] [CrossRef] [PubMed]
- Miró-Canturri, A.; Ayerbe-Algaba, R.; Smani, Y. Drug Repurposing for the Treatment of Bacterial and Fungal Infections. Front. Microbiol. 2019, 10, 41. [Google Scholar] [CrossRef]
- Farha, M.A.; Leung, A.; Sewell, E.W.; D’Elia, M.A.; Allison, S.E.; Ejim, L.; Pereira, P.M.; Pinho, M.G.; Wright, G.D.; Brown, E.D. Inhibition of WTA synthesis blocks the cooperative action of PBPs and sensitizes MRSA to β-lactams. ACS Chem. Biol. 2013, 8, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Quartararo, A.J.; Gates, Z.P.; Somsen, B.A.; Hartrampf, N.; Ye, X.; Shimada, A.; Kajihara, Y.; Ottmann, C.; Pentelute, B.L. Ultra-large chemical libraries for the discovery of high-affinity peptide binders. Nat. Commun. 2020, 11, 3183. [Google Scholar] [CrossRef]
- Song, X.; Yuan, G.; Li, P.; Cao, S. Guanidine-Containing Polyhydroxyl Macrolides: Chemistry, Biology, and Structure-Activity Relationship. Molecules 2019, 24, 3913. [Google Scholar] [CrossRef]
- Zhang, X.; Han, D.; Pei, P.; Hao, J.; Lu, Y.; Wan, P.; Peng, X.; Lv, W.; Xiong, W.; Zeng, Z. In vitro Antibacterial Activity of Isopropoxy Benzene Guanidine Against Multidrug-Resistant. Infect. Drug. Resist. 2019, 12, 3943–3953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiong, W.; Peng, X.; Lu, Y.; Hao, J.; Qin, Z.; Zeng, Z. Isopropoxy Benzene Guanidine Kills Without Detectable Resistance. Front. Microbiol. 2021, 12, 633467. [Google Scholar] [CrossRef]
- Tamma, P.D.; Avdic, E.; Li, D.X.; Dzintars, K.; Cosgrove, S.E. Association of Adverse Events With Antibiotic Use in Hospitalized Patients. JAMA Intern. Med. 2017, 177, 1308–1315. [Google Scholar] [CrossRef]
- Chen, M.-T.; Lo, C.-J. Using Biophysics to Monitor the Essential Protonmotive Force in Bacteria. Adv. Exp. Med. Biol. 2016, 915, 69–79. [Google Scholar]
- Vahidi, S.; Bi, Y.; Dunn, S.D.; Konermann, L. Load-dependent destabilization of the γ-rotor shaft in FOF1 ATP synthase revealed by hydrogen/deuterium-exchange mass spectrometry. Proc. Natl. Acad. Sci. USA 2016, 113, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.; Coenye, T. The Role of Reactive Oxygen Species in Antibiotic-Mediated Killing of Bacteria. Trends Microbiol. 2017, 25, 456–466. [Google Scholar] [CrossRef]
- Giddings, K.S.; Zhao, J.; Sims, P.J.; Tweten, R.K. Human CD59 is a receptor for the cholesterol-dependent cytolysin intermedilysin. Nat. Struct. Mol. Biol. 2004, 11, 1173–1178. [Google Scholar] [CrossRef]
- Laws, M.; Shaaban, A.; Rahman, K.M. Antibiotic resistance breakers: Current approaches and future directions. FEMS Microbiol. Rev. 2019, 43, 490–516. [Google Scholar] [CrossRef] [PubMed]
- Sączewski, F.; Balewski, Ł. Biological activities of guanidine compounds, 2008–2012 update. Expert. Opin. Ther. Pat. 2013, 23, 965–995. [Google Scholar] [CrossRef]
- Salama, A.; Hasanin, M.; Hesemann, P. Synthesis and antimicrobial properties of new chitosan derivatives containing guanidinium groups. Carbohydr. Polym. 2020, 241, 116363. [Google Scholar] [CrossRef]
- Li, J.; Zhong, W.; Zhang, K.; Wang, D.; Hu, J.; Chan-Park, M.B. Biguanide-Derived Polymeric Nanoparticles Kill MRSA Biofilm and Suppress Infection. ACS Appl. Mater. Interfaces 2020, 12, 21231–21241. [Google Scholar] [CrossRef]
- Viljoen, A.; Foster, S.J.; Fantner, G.E.; Hobbs, J.K.; Dufrêne, Y.F. Scratching the Surface: Bacterial Cell Envelopes at the Nanoscale. MBio 2020, 11, e03020-19. [Google Scholar] [CrossRef] [PubMed]
- Peng, X. Application of Diaminoguanidine Derivatives and Their Feed Compositions to the Manufacture of Veterinary Medicaments. CN201980005962.7, 22 July 2019. [Google Scholar]
- DeFronzo, R.; Fleming, G.A.; Chen, K.; Bicsak, T.A. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism 2016, 65, 20–29. [Google Scholar] [CrossRef]
- Bailey, C.J.; Turner, R.C. Metformin. N. Engl. J. Med. 1996, 334, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.; Grenier, D. Understanding the virulence of Streptococcus suis: A veterinary, medical, and economic challenge. Med. Mal. Infect. 2018, 48, 159–166. [Google Scholar] [CrossRef]
- Roodsant, T.J.; Van Der Putten, B.C.L.; Tamminga, S.M.; Schultsz, C.; Van Der Ark, K.C.H. Identification of putative zoonotic virulence factors: A systematic review and genomic meta-analysis. Virulence 2021, 12, 2787–2797. [Google Scholar] [CrossRef]
- Zhang, Y.; Zong, B.; Wang, X.; Zhu, Y.; Hu, L.; Li, P.; Zhang, A.; Chen, H.; Liu, M.; Tan, C. Fisetin Lowers serotype 2 Pathogenicity in Mice by Inhibiting the Heamolysis Activity of Suilysin. Front. Microbiol. 2018, 9, 1723. [Google Scholar] [CrossRef]
- Lecours, M.-P.; Gottschalk, M.; Houde, M.; Lemire, P.; Fittipaldi, N.; Segura, M. Critical role for Streptococcus suis cell wall modifications and suilysin in resistance to complement-dependent killing by dendritic cells. J. Infect. Dis. 2011, 204, 919–929. [Google Scholar] [CrossRef]
- Lu, H.; Li, X.; Wang, G.; Wang, C.; Feng, J.; Lu, W.; Wang, X.; Chen, H.; Liu, M.; Tan, C. Baicalein Ameliorates Streptococcus suis-Induced Infection In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 5829. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Gao, Y.; Wu, X.; Gao, X.; Zhang, M.; Liu, H.; Fang, T. Inhibitory Effect of Piceatannol on Infection Both and. Front. Microbiol. 2020, 11, 593588. [Google Scholar] [CrossRef]
- Li, G.; Shen, X.; Wei, Y.; Si, X.; Deng, X.; Wang, J. Quercetin reduces Streptococcus suis virulence by inhibiting suilysin activity and inflammation. Int. Immunopharmacol. 2019, 69, 71–78. [Google Scholar] [CrossRef]
- Khazandi, M.; Pi, H.; Chan, W.Y.; Ogunniyi, A.D.; Sim, J.X.F.; Venter, H.; Garg, S.; Page, S.W.; Hill, P.B.; McCluskey, A.; et al. Antimicrobial Activity of Robenidine, Ethylenediaminetetraacetic Acid and Polymyxin B Nonapeptide Against Important Human and Veterinary Pathogens. Front. Microbiol. 2019, 10, 837. [Google Scholar] [CrossRef]
- Abraham, R.J.; Stevens, A.J.; Young, K.A.; Russell, C.; Qvist, A.; Khazandi, M.; Wong, H.S.; Abraham, S.; Ogunniyi, A.D.; Page, S.W.; et al. Robenidine Analogues as Gram-Positive Antibacterial Agents. J. Med. Chem. 2016, 59, 2126–2138. [Google Scholar] [CrossRef] [PubMed]
- Krollenbrock, A.; Li, Y.; Kelly, J.X.; Riscoe, M.K. Robenidine Analogues Are Potent Antimalarials in Drug-Resistant. ACS Infect. Dis. 2021, 7, 1956–1968. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gong, S.; Dong, X.; Li, J.; Grenier, D.; Yi, L. Mixed Biofilm of and Impacts Antibiotic Susceptibility and Modulates Virulence Factor Gene Expression. Front. Microbiol. 2020, 11, 507. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).