Abstract
Thermophilic cyanobacteria are cosmopolitan and abundant in the thermal environment. Their light-harvesting complexes, phycobilisomes (PBS), are highly important in photosynthesis. To date, there is limited information on the PBS composition of thermophilic cyanobacteria whose habitats are challenging for survival. Herein, genome-based methods were used to investigate the molecular components of PBS in 19 well-described thermophilic cyanobacteria. These cyanobacteria are from the genera Leptolyngbya, Leptothermofonsia, Ocullathermofonsia, Thermoleptolyngbya, Trichothermofonsia, Synechococcus, Thermostichus, and Thermosynechococcus. According to the phycobiliprotein (PBP) composition of the rods, two pigment types are observed in these thermophiles. The amino acid sequence analysis of different PBP subunits suggests several highly conserved cysteine residues in these thermophiles. Certain amino acid contents in the PBP of thermophiles are significantly higher than their mesophilic counterparts, highlighting the potential roles of specific substitutions of amino acid in the adaptive thermostability of light-harvesting complexes in thermophilic cyanobacteria. Genes encoding PBS linker polypeptides vary among the thermophiles. Intriguingly, motifs in linker apcE indicate a photoacclimation of a far-red light by Leptolyngbya JSC-1, Leptothermofonsia E412, and Ocullathermofonsia A174. The composition pattern of phycobilin lyases is consistent among the thermophiles, except for Thermostichus strains that have extra homologs of cpcE, cpcF, and cpcT. In addition, phylogenetic analyses of genes coding for PBPs, linkers, and lyases suggest extensive genetic diversity among these thermophiles, which is further discussed with the domain analyses. Moreover, comparative genomic analysis suggests different genomic distributions of PBS-related genes among the thermophiles, indicating probably various regulations of expression. In summary, the comparative analysis elucidates distinct molecular components and organization of PBS in thermophilic cyanobacteria. These results provide insights into the PBS components of thermophilic cyanobacteria and fundamental knowledge for future research regarding structures, functions, and photosynthetic improvement.
1. Introduction
Thermophilic cyanobacteria are ubiquitously distributed photosynthetic prokaryotes found in diverse thermal environments around the world [1,2]. In the past few decades, thermophilic cyanobacteria have been extensively explored as promising candidates for various applications related to agriculture, pharmaceutics, nutraceutical, and biofuel [3]. However, basic research and technological innovations are needed to fully explore the industrial potential of thermophilic cyanobacteria by thorough studies on each biological block of these organisms.
Phycobilisomes (PBS) are an important fragment of light-harvesting complexes present in most cyanobacteria. PBS capture light in regions of the visible spectrum and migrate energy to the photosystems [4]. Upon nitrogen starvation, the PBS also serve as a nitrogen storage and can be degraded to recover the nitrogen reserves [5]. The PBS complex is primarily composed of diverse, colored, and highly fluorescent phycobiliproteins (PBPs), while PBPs comprise several subunits, each formed by a protein backbone and a phycobilin linked by a covalent bond [6]. Based on long-wavelength absorption maxima, PBPs are divided into four major types: phycoerythrin (PE), phycocyanin (PC), phycoerythrocyanin (PEC), and allophycocyanin (APC) [7]. Four types of bilin chromophores are identified in cyanobacteria: phycoerythrobilin (PEB), phycocyanobilin (PCB), phycourobilin (PUB), and phycoviolobilin (PVB) [8]. PBPs and phycobilins from extreme environments have shown tremendous potential as antioxidant, anti-inflammatory anticancer, antimicrobial, antiplatelet, and antiviral compounds, and thus, could be applied in different industries, including food, feed, pharmaceutical, nutraceutical, and cosmetics [9]. More importantly, the PBPs from thermal environments may be more stable than that of mesophilic cyanobacteria (e.g., Arthrospira platensis, commonly known as Spirulina) or synthetic blue dyes (e.g., Brilliant Blue FCF) in a wider range of temperatures and pH conditions [10,11]. Therefore, it would be highly desirable to explore alternative sources for stable PBPs or to genetically modify the stability properties of PBPs in mesophilic species.
To date, PBS complexes in association with PSII are typically made up of two substructures: a core consisting of APC and a suite of rods arising out of the core, which may contain PC, PC, and PEC, and/or PE, depending on the species and growth environments [12]. A distinct rod-shaped PBS complex has also been described, which contains no APC but connects the rod by a unique CpcL linker protein directly to PSI [13,14]. Moreover, different groups of linker proteins and specific lyases for covalent attachment of the bilin chromophores also diversify the PBS structures [15]. Indeed, the composition of PBS varies from species to species, and individual organisms can have environmental acclimations due to external factors [7]. Obviously, understanding the molecular components of PBS is a prerequisite and will be useful for further research on structures, functions, biosynthesis, and downstream applications of a sort of microorganism from specific niches. In addition, molecular diversity can shed light on the evolution and structural characteristics (e.g., structural rigidity and thermostability) of PBPs [16,17]. The complexity of molecular components for PBS can also provide insights into metabolic engineering in mesophilic species since the heterologous production of mature PBPs is significantly challenging [18].
Recently, genomes of thermophilic cyanobacteria have been increasingly achieved by using next-generation sequencing (NGS). This affords an opportunity to rapidly investigate the molecular component of PBS in thermophilic cyanobacteria and the structural characteristics at the genomic level prior to biochemical studies. In the present study, we investigated the molecular basis of PBS by computational identification in the genome sequences of 19 thermophilic cyanobacteria. The function, evolution, and adaptations of these thermophilic cyanobacteria were further discussed in light of genetic diversity, sequence characteristics, and genomic organization of PBS components. The insights into the PBS components lay a solid foundation for future research regarding structures, functions, and photosynthetic improvement.
2. Results and Discussion
2.1. Genes Encoding PBPs in Thermophilic Cyanobacteria
Genes encoding phycobiliproteins, APC, PC, and PE, are shown in Table 1. All the studied thermophilic cyanobacteria possess one single set of apcA and apcB genes encoding α and β of APC. PBS core APC binds only the blue-colored chromophore phycocyanobilin (PCB), the biosynthesis gene (pcyA), which is also present in all the thermophiles with one single homolog (Table 1). Genes encoding PC and PE vary tremendously among these thermophiles. Two copies of α (cpcA) and β (cpcB) subunits of PC are present in Leptolyngbya JSC-1, Leptothermofonsia E412, and the six Thermostichus strains, compared to only one homolog of the two subunits in the other thermophilic cyanobacteria. Furthermore, pebA and pebB involved in the biosynthesis of phycocyanobilin (PEB) chromophore are present only in Leptolyngbya JSC-1 and Leptothermofonsia E412, suggesting that the remaining thermophilic cyanobacteria bind only to PCB and are of the C-PC type. Remarkably, PE-I encoded by cpeA and cpeB has been identified only in Leptolyngbya JSC-1 and Leptothermofonsia E412, whereas PE-II is absent in all the thermophiles. The absence of PE in these thermophiles suggests that PC might constitute the whole rod. Given the freshwater origins of these thermophilic cyanobacteria, such a high proportion of PE absence is consistent with the empirical knowledge that many freshwater cyanobacteria, e.g., Synechocystis PCC 6803 and Synechococcus PCC 7942, show no PE rods. As for the two PE-containing strains, the distal part of the PBS rods is probably composed of one type of PE (PE-I). Moreover, previous studies indicate that PE-I binds either only PEB or both PEB and phycourobilin (PUB) [19]. Herein, PE-I binds only PEB in the two thermophilic cyanobacteria in light of the absence of PUB-related biosynthesis genes (phycoerythrocyanin lyase/isomerase, pecE/F) [20]. According to the phycobiliprotein composition of the rods [21], the pigment types of the surveyed thermophilic cyanobacteria can be partitioned into two types: type 1 (T1) have only PC, and type 2 (T2) have PC and PE-I (Table 1).

Table 1.
Species and PBP characteristics of thermophilic cyanobacteria studied.
Apart from α and β subunits of APC, all the thermophilic cyanobacteria also possess one homolog of apcD and apcF (Table S1), encoding the minor α-B and β-18 APC subunits, respectively. Both subunits have a lower abundance and replace α and β subunits in different APC trimers of membrane-contacted cylinders that form the core of the PBS [22]. Importantly, apcD and apcF, as well as the globular domain (PB domain) of the linker core–membrane (apcE), are indispensable for energy transfer to the photosystems [23].
2.2. Sequence Characteristics and Phylogenies of PBP Genes in Thermophilic Cyanobacteria
Functionally, each subunit of PBPs can have one to three phycobilin molecules attached to the polypeptide skeleton in highly preserved cysteine residues [24]. The amino acid sequences analysis of different PBP subunits in these thermophilic cyanobacteria suggests that there are several highly conserved cysteine residues, namely Cys-81 residues of APC subunits (Figure S1), Cys-85 residues of PC α subunit, Cys-83/110/154 residues of PC β subunit (Figure S2), Cys-82/139 residues of PE α subunit, and Cys-51/62/83/168 residues of PE β subunit (Figure S3). Notably, the Cys residue around the 81st site is conserved in all the PBP subunits of these thermophilic cyanobacteria, which is equivalent to the reported Cys-81 residue in other cyanobacteria that always show a bonded phycobilin [8].
Extensive comparisons of the amino acid content of each PBP polypeptide indicate that certain amino acid contents in the PBP of thermophiles are significantly higher than their mesophilic counterparts (Table 2). These results are consistent with previous reports that protein molecular adaptations to temperature are partially due to specific substitutions of amino acids, with glycine, serine, lysine, and asparagine in mesophiles, which are generally replaced in thermophiles by alanine, threonine, arginine, and glutamate, respectively [17,25], thus highlighting the potential roles of specific substitutions of amino acid in adaptive thermostability of light-harvesting complexes in thermophilic cyanobacteria. Interestingly, alanine is the most abundant amino acid category of PBP proteins in the studied thermophiles. Such alanine accumulation in PBP proteins may increase hydrophobicity and therefore decrease molecular flexibility, further enhancing protein thermostability to survive in thermal environments [26].

Table 2.
Comparisons of amino acid content of each PBP polypeptide between thermophiles (the 19 thermophilic cyanobacteria investigated in this study) and mesophiles (Synechocystis PCC 6803 and Synechococcus PCC 7942).
Phylogenetic analyses suggest extensive genetic diversity in these PBP genes, as indicated by the assignments of these thermophilic cyanobacteria into different clusters or clades (Figure 1). Nevertheless, the grouping of the thermophilic strains from specific genus is consistent with the taxonomic lineages in all the phylograms, indicating high sequence conservation as revealed by extremely short branches (Figure 1). Among the thermophilic strains, a high degree of homology is noticed in apcA (>81.3%), apcB (>82.7%), cpeA (95.1%), and cpeB (83.6%) proteins, whereas the identity of cpcA/B proteins dramatically varies at the intergenus level, ranging from 53.0 to 93.8% and from 62.2 to 90.6%, respectively. In addition, unlike Synechococcus PCC 7942 and Gloeobacter PCC 7421 that harbor two identical copies of cpcA/B in the genome, the cpcA and cpcB homologs in the thermophiles are distinct, and are particularly divergent in Thermostichus strains (Figure 1c,d). Such a significant discrepancy of Thermostichus strains in the phylograms is further revealed by the low sequence conservation (cpcA: 60.3–60.9%; cpcB: 66.2–66.8%) between the corresponding opponents. However, domains analysis by PFAM tools suggests similar structures of cpcA or cpcB in Thermostichus strains. Experimental studies are required in future to elucidate the actual functions of the two copies of cpcA/B that differ in amino acid sequences. Moreover, the phylograms of apcD and apcF are in line with that of APC subunits (Figure S4).
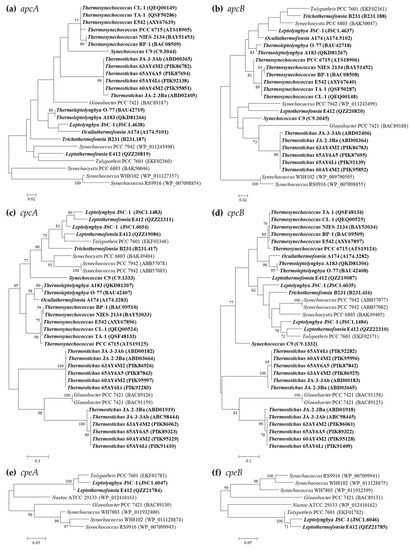
Figure 1.
Phylogenetic inference of protein sequences encoding PBP subunits. The thermophilic cyanobacteria surveyed in this study are indicated in bold. Only bootstrap values > 50% are indicated at nodes. (a) apcA, (b) apcB, (c) cpcA, (d) cpcB, (e) cpeA, (f) cpeB.
Intriguingly, most of the thermophilic strains do not group with any non-thermophilic strains (Figure 1), indicating the specificity of PBP genes in the thermophiles. Exceptions are also noticed. The homologs of cpcA/B and cpeA/B within Leptolyngbya JSC-1 and Leptothermofonsia E412 cluster with that of freshwater filamentous Tolypothrix PCC 7601 (Figure 1c–f), showing high identities (>80%) to each other. Although the clustering of thermophilic strains does not completely comply with morphology, e.g., filamentous strains cluster with unicellular strains, the apcA/B and cpeA/B of marine strains (Synechococcus WH8102 and RS9916) are clearly discrepant to that of thermophilic and freshwater strains (Figure 1a,b,e,f), suggesting that habitats might be related to the genetic diversity of PBPs in cyanobacteria. However, future phylogenetic inferences of these molecular markers on a much larger scale may be helpful for the elaboration of the evolutionary relationship with cyanobacterial habitats and environments.
2.3. Genes Encoding PBS Linker Polypeptides in Thermophilic Cyanobacteria
The higher-order structure of PBS is stabilized by linker polypeptides that contribute to the building of a protein environment around the phycobilins [8]. Two types of APC-associated linker genes, apcC (core linker, LC) and apcE (core–membrane linker, LCM), are present in all the surveyed thermophilic cyanobacteria (Table 3). A gene copy of apcE varies among genera, while intragenus variation is evident only within the genus Thermostichus. Such an intragenus discrepancy may be brought about possibly by gene loss, recently gene acquisition, or limited duplication events that only occurred in certain strains.

Table 3.
Availability of genes encoding linker polypeptides in the genomes of thermophilic cyanobacteria studied.
Sequence analysis suggests a conserved pattern of apcC among the thermophilic cyanobacteria, exhibiting > 81% identities to that of Synechocystis PCC 6803. This is in accordance with the phylogram topology (Figure 2a). Remarkably, the sequence length of the apcE gene fluctuates from 778 (Leptolyngbya JSC-1) to 1139 (the six Thermosynechococcus strains) amino acids. The phylogram of apcE distributes the thermophilic cyanobacteria into three clusters (Figure 2b), which contain none of the non-thermophilic reference strains. Only Synechococcus C9 is an outlier and is solely located in a separate branch. Further domain analyses indicate that two predicted repeat (or linker-like) domains are possessed by apcE in strains belonging to cluster III, three in Synechococcus C9, Trichothermofonsia B231, and Thermostichus strains, and four domains in the remaining thermophiles (Figure 2b). The presence of additional LCM domains in these strains suggests that their PBS core may have additional half-cylinders [27].
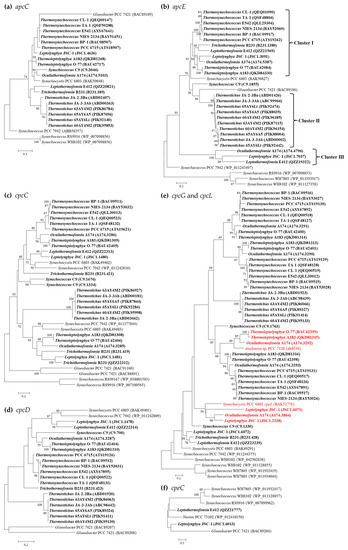
Figure 2.
Phylogenetic inference of protein sequences encoding linker polypeptides. The thermophilic cyanobacteria surveyed in this study are indicated in bold. The putative cpcL is indicated in red. Only bootstrap values > 50% are indicated at nodes. (a) apcC, (b) apcE, (c) cpcC, (d) cpcG and cpcL, (e) cpcD, (f) cpeC.
More interestingly, Leptolyngbya JSC-1, Leptothermofonsia E412, and Ocullathermofonsia A174 comprise two distinct apcE (Figure 2b). The amino acid sequences of apcE affiliated to cluster III show conserved VIPEDV-like motifs (Figure 3), which are identical to a recently identified far-red-specific area within apcE2 of cyanobacteria [28]. This result suggests that the three apcE of thermophilic cyanobacteria encode a phycobilisome linker associated with FRL photosynthesis, meanwhile highlighting their capabilities of adjusting a photosynthetic apparatus through chromatic adaptation. The other, conventional, apcE sequences show a highly conserved phytochrome-binding cysteine (Figure 3) that is missing in far-red sequences. The missing cysteine in apcE implies the non-covalent binding of phycocyanobilin and might relate to an important red-shift in the absorbance (e.g., 700 nm in the far-red Synechococcus PCC 7335, as opposed to 660 nm for covalent binding) [29]. Photoacclimation of a far-red light by Leptolyngbya JSC-1 and Leptothermofonsia E412 has been experimentally verified [30,31], and the responses to the ambient light environment might be mediated by two-component systems involved in signal transduction [32]. Moreover, the Ocullathermofonsia strain might expand the diverse array of far-red photosynthesizing cyanobacteria.
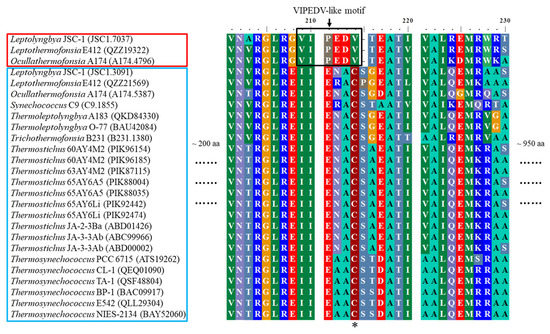
Figure 3.
Conserved far-red specific-motifs present in apcE of thermophilic cyanobacteria. Thermophiles outlined in red contain conserved far-red-specific motifs in apcE sequences, while thermophiles in the blue box encompass conventional (white light) apcE sequences. The black box highlights the far-red-specific motif and the phytochrome-binding cysteine is indicated by an asterisk.
Four types of PC-associated linker genes, cpcC/D (rod linker, LR), cpcG (rod–core linker, LRC), and cpcL (LRM, rod–membrane linker) have been identified in the surveyed thermophilic cyanobacteria (Table 3). Except for cpcD, intergenus variations are noticed in the cpcC and cpcG gene numbers. The six filamentous thermophiles and unicellular Synechococcus C9 harbor two cpcC genes, while the other unicellular thermophiles contain only one homolog. The cpcC phylogram indicates that the two homologs from the same strain are quite distinct and all the thermophilic cyanobacteria are divergent from non-thermophilic reference strains, as suggested by long branches (Figure 2c). Identity calculation reveals a low sequence conservation of cpcC homologs between thermophilic cyanobacteria and reference Synechococcus PCC 7942 (41.4–63.3%). Nevertheless, domain analysis suggests a similar structure of cpcC between all the thermophilic cyanobacteria and Synechococcus PCC 7942. In addition, the presence of cpcC/D in Leptolyngbya JSC-1 and Leptothermofonsia E412 is contrary to the previous findings in marine Synechococcus strains that PE-I-containing strains possess none of the cpcC and cpcD homologs [21,33]. Taken together, our results indicate that cyanobacteria strains belonging to a given pigment type may own different structures of PBS rods. Moreover, for the case with multiple cpcC, determining which cpcC is located in a central position in the PC rod or in a peripheral position requires future experimental study, e.g., using rod mutants [34]. Similar to cpcC, phylogenetic analysis and identity calculation suggest a relatively low sequence conservation of cpcD homologs as revealed by long branches (Figure 2d) and low identities to Synechococcus PCC 7942 (30.0–50.6%), and similar domain structures are observed.
As for cpcG, the homolog number tremendously varies among thermophilic cyanobacteria, from one to three (Table 3). The phylogram of CpcG proteins (Figure 2e) indicates that they are categorized into subgroups, which appears to fit within the structural variations of the phycobilisome core. For example, the multi-type of cpcG genes in Thermosynechococcus strains may suggest a large pentacylindrical core [35], while the Thermostichus strains, such as Synechococcus PCC 7942, may correspond to a small bicylindrical core due to the single cpcG gene [36]. These indicate that various CpcG proteins have evolved to play a specific role in the assembly of the rods with diverged core structures. In addition, six homologs from Leptolyngbya JSC-1, Ocullathermofonsia A174, Thermoleptolyngbya A183 and O-77 cluster, with the cpcL of Synechocystis PCC 6803 [37] and Anabaena PCC 7120 [14], respectively (Figure 2e). A hydrophobic segment is also characterized in the C-terminal region of these homologs (Table S2), which differs from the hydrophilic sequence of cpcG that associates with the APC core [38]. Taken together, these homologs from thermophilic cyanobacteria could be putative cpcL, which may anchor cpcL-PBS to the thylakoid membrane or PSI complex [37]. Moreover, this result suggests that these cpcL-owned cyanobacteria may utilize CpcL–PBS as an alternative form to harvest energy in association with PSI. Moreover, the phylogram reveals that the cpcL clusters are mixed with cpcG clusters in a large phylogenetic clade. This implies that the acquisition or loss of the hydrophobic C-terminal transmembrane helix may occur frequently, enabling cyanobacteria to flexibly alter light energy distribution to the photosystems via the domain reorganization of CpcG and CpcL family proteins [13]. Herein, whether the sequence discrimination of these cpcL genes results in any biological function shift needs further careful investigations. Intriguingly, Ocullathermofonsia A174 possesses two types of cpcL, and what the actual functions of the two cpcL are also requires further investigations.
Only one putative PE-associated linker gene, cpeC (LR), is present in the two PE-I-containing thermophilic strains (Table 3). Of the cpeC phylogram (Figure 2f), Leptolyngbya JSC-1 and Leptothermofonsia E412, together with another freshwater cyanobacterium, Nostoc punctiforme PCC 73102, form a distinct clade apart from the clade composed of marine Synechococcus strains, both of which are supported by robust bootstrap values. This result suggests that cpeC may be subjected to different evolution related to cyanobacterial habitats and environments.
2.4. Genes Encoding Phycobilin Lyases in Thermophilic Cyanobacteria
Three families or clans of phycobilin lyases (E/F, cpcS/cpcU, and T families), enzymes involved in the chromophorylation of phycobiliproteins, have been described to date based on phylogenetic, structural, and biochemical studies (respective substrates and enzymatic activities) [39]. Four types of PC-associated phycobilin lyases have been identified in the thermophilic cyanobacteria (Table 4). One homolog of cpcE/F genes encoding a heterodimeric complex are present in all the surveyed thermophilic cyanobacteria, except for Thermostichus strains, which have an additional homolog (Table 4). Though the sequence differences of cpcE/F are evident, as suggested by the long branches (Figure 4a,b), most of the thermophilic cyanobacteria cluster with the reference strain Nostoc sp. PCC 7120, whose crystal structure of cpcE/F is primarily α-helical, consisting of crescent-shaped elongated superhelices or solenoids [40]. Only the two homologs of Thermostichus strains form two distinct clades. Further domain analysis suggests that such a discrepancy might be mainly ascribed to the existence of multiple HEAT-repeat or HEAT-like-repeat domains that are both assigned to clan CL0020. Among the cpcE gene, one HEAT-repeat and one HEAT-like-repeat domain are present in the six filamentous thermophiles and Synechococcus C9. Two types of domain patterns are present in the six Thermosynechococcus strains, namely one HEAT-repeat and one HEAT-like-repeat domain (BP-1 and NIES-2134), and two HEAT-repeat and one HEAT-like-repeat domains (PCC 6715, CL-1, TA-1, and E542). As for the two distinct homologs of cpcE in Thermostichus strains, group I (Figure 4a) contains one HEAT-repeat domain, while two HEAT-repeat and one HEAT-like-repeat domains are shown by group II, except for JA-2-3Ba, which comprises one HEAT-repeat and one HEAT-like-repeat domain.

Table 4.
Availability of genes encoding putative phycobilin lyases in the genomes of thermophilic cyanobacteria studied.
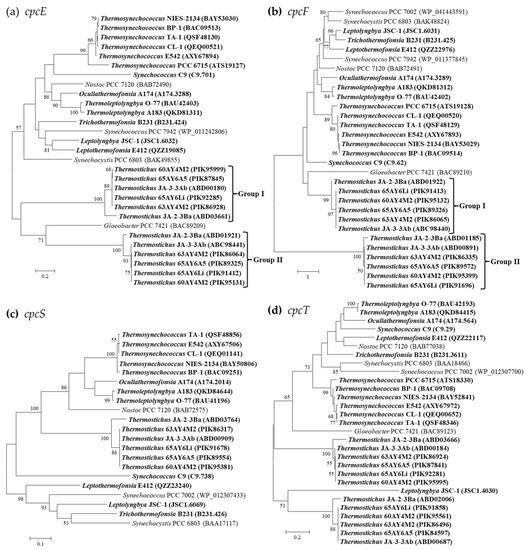
Figure 4.
Phylogenetic inference of protein sequences encoding PC-associated phycobilin lyases. The thermophilic cyanobacteria surveyed in this study are indicated in bold. Only bootstrap values > 50% are indicated at nodes. (a) cpcE, (b) cpcF, (c) cpcS, (d) cpcT.
Similarly, variations of the HEAT-repeat and HEAT-like-repeat domains exist in the cpcF gene among the thermophilic cyanobacteria. One HEAT-repeat and one HEAT-like-repeat domain are present in Ocullathermofonsia A174, while the other five filamentous thermophiles and Synechococcus C9 contain only one HEAT-repeat domain. Thermosynechococcus PCC 6715 comprises one HEAT-repeat domain, whereas the remaining Thermosynechococcus strains possess one HEAT-repeat and one HEAT-like-repeat domain. One HEAT-repeat and one HEAT-like-repeat domains are present for both the two homologs of cpcF in Thermostichus JA-2-3, while the other Thermostichus strains show a different pattern of domains, including one HEAT-repeat for group I and three HEAT-repeat domains for group II. Moreover, the number of HEAT-repeat and HEAT-like-repeat domains insignificantly matched to the Pfam database differs among the cpcE/F of thermophilic cyanobacteria, also contributing to the sequence divergences as indicated by the phylograms (Figure 4a,b). The cpcE/F genes in the thermophilic cyanobacteria might be involved in ligating chromophores to the α-subunits of phycobiliproteins and the HEAT-repeat motifs within them may facilitate protein–protein interactions [41,42].
Homolog of cpcS is present in all the thermophilic cyanobacteria except for Thermosynechococcus PCC 6715 (Table 4). Such an absence of cpcS may be caused by gene loss. Although the phylogram suggests the classification of these strains into several divergent clusters (Figure 4c), a similar domain architecture is shared by all the cyanobacteria studied. Two homologs of cpcT are shown in Thermostichus strains, while the other thermophilic cyanobacteria possess only one. In addition, the two homologs in Thermostichus strains are distinct from each other, as indicated in the phylogram (Figure 4d). However, domain analysis again confirms a consistent pattern for this lyase.
PE-associated phycobilin lyases are only in possession of Leptolyngbya JSC-1 and Leptothermofonsia E412 (Table 4), showing a consistent pattern of gene category and number. The two thermophiles exhibit a high degree of homology (71.9–78.2%) with the cpeF of filament Tolypothrix PCC 7601, an experimentally confirmed bilin lyase that is responsible for the attachment of the doubly ligated PEB to Cys-48/Cys-59 of cpeB [43]. The clade formed by the three strains is divergent from the marine Synechococcus strains, as revealed by the cpeF phylogram (Figure 5). The phylograms of cpeU and cpeZ are congruent with the cpeF phylogram, whereas the cpeS and cpeT of the two thermophilic cyanobacteria appear to be divergent from that of Tolypothrix PCC 7601 (Figure 5). Homologs of cpeZ in the two thermophiles may function as chaperones, facilitating cpeF and cpeB interaction by stabilizing cpeBs conformation [44]. Moreover, homologs of cpeS, cpeT, and cpeU in the two thermophiles may attach PEB to Cys residues of the cpeB subunit of PE [44,45].
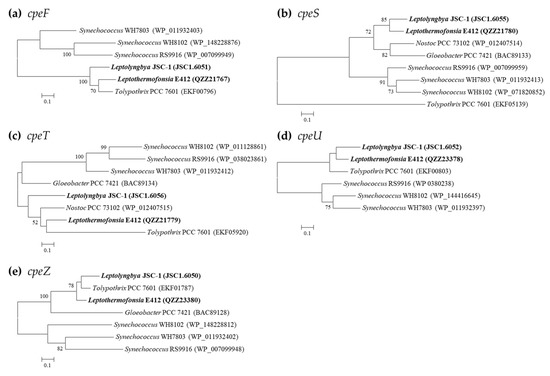
Figure 5.
Phylogenetic inference of protein sequences encoding PE-associated phycobilin lyases. The thermophilic cyanobacteria surveyed in this study are indicated in bold. Only bootstrap values > 50% are indicated at nodes. (a) cpeF, (b) cpeS, (c) cpeT, (d) cpeU, (e) cpeZ.
Overall, all the specific bilin lyases might ensure the binding of the correct bilin to the corresponding cysteine residue with the correct stereochemistry, finally contributing to the post-translational modification and assembly of PBP in vivo into mature light-harvesting complexes. Future experimental studies should be carried out to elucidate how these bilin lyases chromophorylate the PEB-binding sites on PBP in the thermophilic cyanobacteria using, e.g., biochemical assays and site-directed mutagenesis.
2.5. Genomic Distribution Pattern of PBS-Related Genes in Thermophilic Cyanobacteria
The genomic organization of PBS-related genes in the genomes of the surveyed thermophilic cyanobacteria may provide useful insights into the function and evolution of these genes. Herein, the genomic organization of PBS-related genes is graphically presented in Figure 6. Generally, the comparative genomic analysis suggests diversified molecular components and the organization of PBS-related genes among the surveyed thermophilic cyanobacteria, which is especially evident at the genus level. Moreover, the different genomic distribution of PBS-related genes among the thermophiles probably indicates various regulations of expression. Intriguingly, the distribution of PBS rod-related genes in these thermophiles is quite divergent from that of marine Synechococcus representatives with all known pigment types. The PBS rod-related genes for marine Synechococcus strains of different pigment types are primarily located in a dedicated genomic region, which is oriented from the phenylalanine tRNA (left) to the conserved low molecular weight tyrosine phosphatase ptpA (right) [33]. No such distributions are noticed in the thermophiles. It is not surprising that freshwater Synechococcus C9 shows a distinct distribution pattern to marine Synechococcus strains, since the latter are phylogenetically divergent from the former [46]; recently, a revision of its name to Parasynechococcus has been proposed.
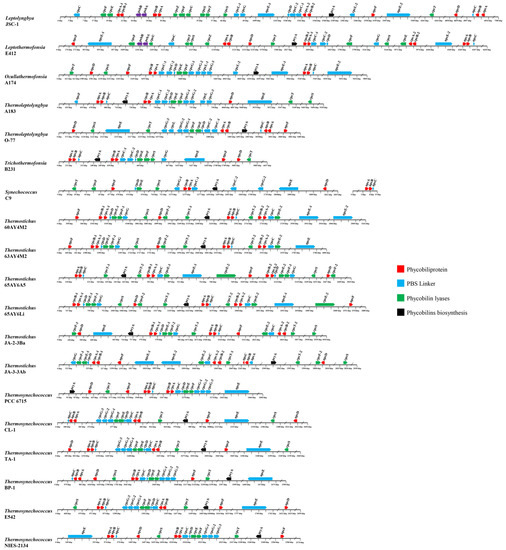
Figure 6.
Genomic organization of PBS-related genes in the 19 thermophilic cyanobacteria surveyed. Solid arrow boxes refer to genes and the direction of transcription.
A similar distribution pattern of APC-associated genes is shared by all the genomes of the thermophiles (Figure 6). Briefly, small groups cluster together with three APC core genes, in the order apcA-B-C, while three other core genes, apcD, apcE, and apcF, have no APC genes in their close vicinity. This result indicates that in these thermophiles, apcC might be co-regulated with apcA/B, while apcD, apcE, and apcF may be independently regulated. The standalone regulation of apcD and apcF might be important for adjusting energy transfer and state transitions, since the role of these proteins could be different in different strains [23]. The cluster in these thermophiles is different from the apcE-A-B-C cluster of marine Synechococcus strains [21], suggesting distinct regulations of the core–membrane linker. The expression of apcE in a locus remote from the core operon may provide the thermopiles with the flexibility of the PBS assembling, energy transfer to PSII, and photoprotection [47].
Most of the PBS rod genes are located in a much larger cluster, the size of which increases with the complexity of the rod structure (Figure 6). For the PC-associated genes, several distribution patterns are observed in the genomes of the thermophiles. First, a large cluster of PC-associated genes is found in the genomes of Ocullathermofonsia A174, Thermoleptolyngbya A183 and O-77, Trichothermofonsia B231, and the six Thermosynechococcus strains. The cluster is in the order cpcB-A-C-D-E-F-G, within which the number of cpcC and cpcG homologs varies from one to two and from one to three, respectively. Only cpcS and cpcT positionally disperse from the core cluster. The large cluster may reveal the co-regulation of these genes and the core components of the PC complex. Nevertheless, the cases in Ocullathermofonsia A174 and Thermoleptolyngbya A183 and O-77 might be more complex. A structure of cpcG1-cpcG2-cpcL-cpcG3 is observed in the three strains, which is consistent with the operon in Anabaena PCC 7120. The conventional PBS of Anabaena PCC 7120 only contains the products of CpcG1, CpcG2, and CpcG3 [14]. Therefore, regulation of cpcL for the rod-shaped PBS may be different from the regulation of the large gene cluster. The functional role of cpcL in acclimation to changes in light conditions has been reported to be widely distributed in cyanobacteria [13,48], indicating the plastic regulation of cpcL expression in response to changes in environmental conditions. Second, the genomes of Thermostichus strains comprise two clusters of PC-associated genes, namely cpcB-A-D-E-F g and cpcB-A-C-E. One homolog of cpcT is positionally close to the latter cluster but shows an opposite transcription direction. Furthermore, a homolog of cpcE, -F, g, and -T is also found to be distant from the two clusters. Third, the distribution pattern of PC-associated genes is complex in Leptolyngbya JSC-1 and Leptothermofonsia E412. Both genomes possess a cluster in the order of cpcB-A-C-C-D, whereas clusters cpcB-A-E-F and cpcB-A-E are present in JSC-1 and E412, respectively. The other PC-associated genes exhibit scattered distribution in the two genomes. Fourth, Synechococcus C9 shows a distinct distribution pattern of PC-associated genes, comprising two small clusters (cpcB-A-C and cpcD-E) and dispersal distribution of the other genes. Additional to the clusters in each genome, the isolated lyase genes, e.g., cpcS and cpcT, may flexibly modulate both the chromophore binding and detachment in these thermophiles to optimize light harvesting of the PBS to changing light environments [49].
The vast majority of PE-associated genes in the genomes of Leptolyngbya JSC-1 and Leptothermofonsia E412 are located in a large cluster ranging from 8 to 10 kbp, while only cpeC in JSC-1 and cpeT and cpeU in E412 are the outliers of this cluster (Figure 6). Although PE-associated genes are widely distributed in a large region, they may directly or indirectly regulate each other. For instance, the cpeT mutant strain of Tolypothrix PCC 7601 showed the downregulation of cpeB-A and the upregulation of the cpeCDESTR operon, suggesting potential regulatory roles of cpeT on the expression of these genes in addition to its role as a PEB lyase for chromophorylation on the β-subunit of PE [45]. The chromophorylation efficiency of CpeT can be improved with the help of the chaperone-like protein CpeZ, which can also facilitate the lyase activity of CpeS, CpeT, and CpeU [43,50]. Thus, these studies imply the underlying regulatory network of these PE-associated genes in the two thermophilic cyanobacteria. Taken together, the two thermophiles exhibit a sophisticated distribution pattern of PC and PE-associated genes and that chromatic acclimation can be regulated to adapt to different light conditions.
3. Materials and Methods
3.1. Genome Collection of Thermophilic Cyanobacteria
Cyanobacteria with available genomes and closely related to thermophilic or hot-spring strains were retrieved as a preliminary dataset from the genomic resources of the NCBI at the time of this study (1 June 2022). Further, the rigid collection of thermophilic cyanobacteria was verified and retained based on the literature searches and validation of thermophilic characteristics through contacting culture collections, leading authors of the manuscripts, and submissions. Then, quality control for all genomes was performed using the following criteria to reduce data redundancy and biased genome representation of cyanobacteria. First, genomes with average nucleotide identity (ANI, calculated using an online tool: http://enve-omics.ce.gatech.edu/ani/, accessed on 20 July 2022) values greater than 99.9% were considered as redundant genomes, and then only one of these genomes was randomly kept for the analysis. Second, the quality of the genomes was evaluated using CheckM [51] to ensure a more reliable genome dataset with near completeness (>98%) and low contamination (<2%). Finally, a dataset comprising 19 thermophilic cyanobacteria was established (Table S3). The genome, protein sequences, and genomic annotations of the thermophilic cyanobacteria studied were retrieved from the database of NCBI. The genomes with no or incomplete annotations were annotated using the RAST annotation system [52], which is provided in Table S4.
Detailed information regarding ecology, morphology, and genome characteristics of the 19 thermophilic strains was summarized in Table S3. Briefly, the 19 thermophiles were taxonomically affiliated to six families of order Pseudanabaenales, Synechococcales, and Thermostichales, including Leptolyngbyaceae: Leptolyngbya sp. JSC-1 [53] and Leptothermofonsia sichuanensis E412 [31]; Oculatellaceae: Ocullathermofonsia sinensis A174, Thermoleptolyngbya sp. O-77 [54], and T. sichuanensis A183 [55]; Synechococcaceae: Synechococcus sp. C9 [56]; Thermostichaceae: Thermostichus sp. 60AY4M2, 63AY4M2, 65AY6A5, 65AY6Li [57], JA-2-3B, and JA-3-3Ab [58]; Thermosynechococcaceae: Thermosynechococcus lividus PCC 6715 [59], Thermosynechococcus sp. CL-1 [60] and TA-1 [61], T. vestitus BP-1 [62], and E542 [63], T. vulcanus NIES-2134 [16]; and Trichocoleusaceae: Trichothermofonsia sichuanensis B231.
3.2. Identification of Orthologous Proteins
Amino acid sequences of proteins involved in PBS of Synechocystis sp. PCC 6803, and/or Synechococcus sp. PCC 7942, and/or Synechococcus sp. WH8102 were downloaded from the CyanoBase (http://genome.kazusa.or.jp/cyanobase, accessed on 20 August 2022) or BioCyc (https://biocyc.org/, accessed on 22 August 2022) as a reference protein set. Based on the bidirectional best hit (BBH) criterion [64], orthologous proteins involved in PBS of the surveyed thermophilic species were identified using BLASTP with the following thresholds: E-value cut-off of 1E-6, ≥30% identity, and 70% coverage. The identified proteins were homologous to these proteins in the three aforementioned species, namely PBP (apcA, apcB, apcD, apcF, cpcA, cpcB, cpeA, cpeB, mpeA, mpeB, rpcA, and rpcB,), PBS linkers (apcC, apcE, cpcC, cpcD, cpcG, cpeC, cpeF, mpeC, mepD, mpeE, mpeF, and mpeG), phycobilin lyases (cpcE, cpcF, cpcS, cpcT, cpeF, cpeS, cpeT, cpeU, cpeY, cpeZ, rpcE, rpcF, rpcG, rpcT, mpeU, mpeV, mpeY, and mpeZ), and phycobilin biosynthesis (pcyA, pebA, pebB, pebS, pecE, pecF, rpcG, pecE, and pecF). The accession numbers of orthologous proteins identified in each genome of the thermophilic cyanobacteria studied were summarized in Table S1.
3.3. Protein Sequence Analysis
Amino acid sequences were collected for the surveyed thermophilic species and reference cyanobacteria to reconstruct phylogenies. Multiple sequence alignments were performed by muscle complemented in Mega7 [65]. Maximum-likelihood (ML) phylogenetic analyses were carried out using PhyML v3.0 [66], and the substitution models were automatically selected by the model selection function implemented in PhyML [67] under Bayesian information criterion (BIC). Parameter settings in PhyML and bootstrap analysis of phylogenies were followed as described [68].
Domains within protein sequences were identified by the PFAM tool of European Bioinformatics Institute (EMBL-EBI) (http://pfam-legacy.xfam.org/, accessed on 20 July 2022), and domain architectures were further viewed using the HMMER webserver [69].
4. Conclusions
In the present study, the molecular components of PBS in 19 well-described thermophilic cyanobacteria are elucidated using genome-based methods. According to the PBP composition of the rods, two pigment types are observed in the surveyed thermophiles. The amino acid sequence analysis of different PBP subunits suggests several highly conserved cysteine residues in these thermophiles. Certain amino acid contents in the PBP of thermophiles are significantly higher than their mesophilic counterparts, highlighting the potential roles of the specific substitutions of amino acids in the adaptive thermostability of light-harvesting complexes in thermophilic cyanobacteria. The genes encoding PBS linker polypeptides vary among the thermophiles. Intriguingly, motifs in linker apcE indicate the photoacclimation of a far-red light by Leptolyngbya JSC-1, Leptothermofonsia E412, and Ocullathermofonsia A174. The composition pattern of phycobilin lyases is consistent among the thermophiles, except for Thermostichus strains, which have an extra homolog of cpcE, cpcF and cpcT. In addition, phylogenetic analyses of genes coding for PBPs, linkers, and lyases suggest extensive genetic diversity among these thermophiles. Moreover, the different genomic distribution of PBS-related genes among the thermophiles probably indicates various regulations of expression. In summary, the comparative analysis elucidates the distinct molecular components and organization of PBS in thermophilic cyanobacteria. These results provide insights into the PBS components of thermophilic cyanobacteria and fundamental knowledge for future research regarding structures, functions, and photosynthetic improvement.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24065632/s1.
Author Contributions
Conceptualization, J.T. and M.D.; methodology, J.T. and M.D.; software, H.Z., D.Y. and L.D.; validation, J.T.; formal analysis, H.Z., D.Y. and L.D.; investigation, H.Z. and D.Y.; resources, J.T. and M.D.; data curation, H.Z., D.Y. and L.D.; writing—original draft preparation, J.T.; writing—review and editing, J.T. and M.D.; visualization, D.Y. and H.Z.; supervision, J.T. and M.D.; project administration, J.T. and M.D.; funding acquisition, J.T. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31970092, 32071480, and 3221101094), the Shenzhen Fundamental Research Program (GXWD20201231165807007-20200806170221001), and the Tenure-Track Fund to M.D.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/genome/, accessed on 1 June 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tang, J.; Jiang, D.; Luo, Y.; Liang, Y.; Li, L.; Shah, M.; Da Roch, M. Potential new genera of cyanobacterial strains isolated from thermal springs of western Sichuan, China. Algal Res. 2018, 31, 14–20. [Google Scholar] [CrossRef]
- Mehetre, G.T.; Puia, Z.; Deka, P.; Carrie, W.; Lalrokimi; Singh, B.P. Chapter 6—Thermophilic and thermotolerant cyanobacteria: Environmental and biotechnological perspectives. In Cyanobacterial Lifestyle and Its Applications in Biotechnology; Singh, P., Fillat, M., Kumar, A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 159–178. [Google Scholar]
- Patel, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. A perspective on biotechnological applications of thermophilic microalgae and cyanobacteria. Bioresour. Technol. 2019, 278, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Su, H.-N.; Pu, Y.; Chen, J.; Liu, L.-N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular structure, production, applications, and prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef]
- Ruan, Z.; Prášil, O.; Giordano, M. The phycobilisomes of Synechococcus sp. are constructed to minimize nitrogen use in nitrogen-limited cells and to maximize energy capture in energy-limited cells. Environ. Exp. Bot. 2018, 150, 152–160. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Kumar, D.; Pathak, J.; Sinha, R.P. Phycobiliproteins and their commercial significance. In Cyanobacteria; Elsevier: Amsterdam, The Netherlands, 2019; pp. 207–216. [Google Scholar]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef] [PubMed]
- Dagnino-Leone, J.; Figueroa, C.P.; Castañeda, M.L.; Youlton, A.D.; Vallejos-Almirall, A.; Agurto-Muñoz, A.; Pérez, J.P.; Agurto-Muñoz, C. Phycobiliproteins: Structural aspects, functional characteristics, and biotechnological perspectives. Comput. Struct. Biotechnol. J. 2022, 20, 1506–1572. [Google Scholar] [CrossRef]
- Puzorjov, A.; McCormick, A.J. Phycobiliproteins from extreme environments and their potential applications. J. Exp. Bot. 2020, 71, 3827–3842. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Q.; Zhao, J.; Jiang, P. Biosynthesis, spectral properties and thermostability of cyanobacterial allophycocyanin holo-α subunits. Int. J. Biol. Macromol. 2016, 88, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.-Y.; Soulier, N.T.; Canniffe, D.P.; Shen, G.; Bryant, D.A. Light regulation of pigment and photosystem biosynthesis in cyanobacteria. Curr. Opin. Plant Biol. 2017, 37, 24–33. [Google Scholar] [CrossRef]
- Hirose, Y.; Chihong, S.; Watanabe, M.; Yonekawa, C.; Murata, K.; Ikeuchi, M.; Eki, T. Diverse chromatic acclimation processes regulating phycoerythrocyanin and rod-shaped phycobilisome in cyanobacteria. Mol. Plant 2019, 12, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Semchonok, D.A.; Webber-Birungi, M.T.; Ehira, S.; Kondo, K.; Narikawa, R.; Ohmori, M.; Boekema, E.J.; Ikeuchi, M. Attachment of phycobilisomes in an antenna-photosystem I supercomplex of cyanobacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.A.; Canniffe, D.P. How nature designs light-harvesting antenna systems: Design principles and functional realization in chlorophototrophic prokaryotes. J. Phys. B 2018, 51, 033001. [Google Scholar] [CrossRef]
- Liang, Y.; Kaczmarek, M.B.; Kasprzak, A.K.; Tang, J.; Shah, M.M.R.; Jin, P.; Klepacz-Smółka, A.; Cheng, J.J.; Ledakowicz, S.; Daroch, M. Thermosynechococcaceae as a source of thermostable C-phycocyanins: Properties and molecular insights. Algal Res. 2018, 35, 223–235. [Google Scholar] [CrossRef]
- Pittera, J.; Partensky, F.; Six, C. Adaptive thermostability of light-harvesting complexes in marine picocyanobacteria. ISME J. 2017, 11, 1–13. [Google Scholar] [CrossRef]
- Puzorjov, A.; Dunn, K.E.; McCormick, A.J. Production of thermostable phycocyanin in a mesophilic cyanobacterium. Metab. Eng. Commun. 2021, 13, e00175. [Google Scholar] [CrossRef]
- Everroad, R.C.; Wood, A.M. Comparative molecular evolution of newly discovered picocyanobacterial strains reveals a phylogenetically informative variable region of beta-phycoerythrin. J. Phycol. 2010, 42, 1300–1311. [Google Scholar] [CrossRef]
- Aaron, J.T.; Alexander, N.G. Biosynthesis of the cyanobacterial light-harvesting polypeptide phycoerythrocyanin holo-alpha subunit in a heterologous host. J. Bacteriol. 2002, 184, 4666–4671. [Google Scholar]
- Six, C.; Thomas, J.C.; Garczarek, L.; Waski, M.O.; Dufresne, A.; Blot, N. Diversity and evolution of phycobilisomes in marine Synechococcus spp.: A comparative genomics study. Genome Biol. 2007, 259, 1–22. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, Z.; Li, X.; Wang, G.; Zhang, K.; Wei, P.; Zhao, J.; Gao, N. Structural insight into the mechanism of energy transfer in cyanobacterial phycobilisomes. Nat. Commun. 2021, 12, 5497. [Google Scholar] [CrossRef]
- Calzadilla, P.I.; Muzzopappa, F.; Sétif, P.; Kirilovsky, D. Different roles for ApcD and ApcF in Synechococcus elongatus and Synechocystis sp. PCC 6803 phycobilisomes. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Scheer, H.; Zhao, K.H. Biliprotein maturation: The chromophore attachment. Mol. Mircrobiol. 2008, 68, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Fields, P.A. Review: Protein function at thermal extremes: Balancing stability and flexibility. Comp. Biochem. Phys. 2001, 129, 417–431. [Google Scholar] [CrossRef]
- Kumwenda, B.; Litthauer, D.; Bishop, O.T.; Reva, O. Analysis of protein thermostability enhancing factors in industrially important thermus bacteria species. Evol. Bioinform. Online 2013, 9, 327–342. [Google Scholar] [CrossRef]
- Zhao, K.-H.; Su, P.; Böhm, S.; Song, B.; Zhou, M.; Bubenzer, C.; Scheer, H. Reconstitution of phycobilisome core–membrane linker, LCM, by autocatalytic chromophore binding to ApcE. Biochim. Biophys. Acta Bioenerg. 2005, 1706, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Antonaru, L.A.; Cardona, T.; Larkum, A.W.D.; Nürnberg, D.J. Global distribution of a chlorophyll f cyanobacterial marker. ISME J. 2020, 14, 2275–2287. [Google Scholar] [CrossRef]
- Ho, M.-Y.; Gan, F.; Shen, G.; Zhao, C.; Bryant, D.A. Far-red light photoacclimation (FaRLiP) in Synechococcus sp. PCC 7335: I. Regulation of FaRLiP gene expression. Photosynth. Res. 2017, 131, 173–186. [Google Scholar] [CrossRef]
- Gan, F.; Zhang, S.; Rockwell, N.C.; Martin, S.S.; Lagarias, J.C.; Bryant, D.A. Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light. Science 2014, 345, 1312–1317. [Google Scholar] [CrossRef]
- Tang, J.; Shah, M.R.; Yao, D.; Du, L.; Zhao, K.; Li, L.; Li, M.; Waleron, M.; Waleron, M.; Waleron, K.F.; et al. Polyphasic identification and genomic insights of Leptothermofonsia sichuanensis gen. sp. nov., a novel thermophilic cyanobacteria within Leptolyngbyaceae. Front. Microbiol. 2022, 13, 765105. [Google Scholar] [CrossRef]
- Tang, J.; Yao, D.; Zhou, H.; Wang, M.; Daroch, M. Distinct molecular patterns of two-component signal transduction systems in thermophilic cyanobacteria as revealed by genomic identification. Biology 2023, 12, 271. [Google Scholar] [CrossRef]
- Grébert, T.; Garczarek, L.; Daubin, V.; Humily, F.; Marie, D.; Ratin, M.; Devailly, A.; Farrant, G.K.; Mary, I.; Mella-Flores, D.; et al. Diversity and evolution of pigment types in marine Synechococcus cyanobacteria. Genome Biol. Evol. 2022, 14, evac035. [Google Scholar] [CrossRef] [PubMed]
- Ughy, B.; Ajlani, G. Phycobilisome rod mutants in Synechocystis sp. strain PCC6803. Microbiology 2004, 150, 4147–4156. [Google Scholar] [CrossRef]
- Chang, L.; Liu, X.; Li, Y.; Liu, C.-C.; Yang, F.; Zhao, J.; Sui, S.-F. Structural organization of an intact phycobilisome and its association with photosystem II. Cell Res. 2015, 25, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Geng, X.X.; Katayama, M.; Ikeuchi, M. Distinct roles of CpcG1 and CpcG2 in phycobilisome assembly in the cyanobacterium Synechocystis sp. PCC 6803. Photosynth. Res. 2005, 84, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Ochiai, Y.; Katayama, M.; Ikeuchi, M. The membrane-associated CpcG2-phycobilisome in Synechocystis: A new photosystem I antenna. Plant Physiol. 2007, 144, 1200–1210. [Google Scholar] [CrossRef]
- Watanabe, M.; Ikeuchi, M. Phycobilisome: Architecture of a light-harvesting supercomplex. Photosynth. Res. 2013, 116, 265–276. [Google Scholar] [CrossRef]
- Bretaudeau, A.; Coste, F.; Humily, F.; Garczarek, L.; Le Corguille, G.; Six, C.; Ratin, M.; Collin, O.; Schluchter, W.M.; Partensky, F. CyanoLyase: A database of phycobilin lyase sequences, motifs and functions. Nucleic Acids Res. 2012, 41, D396–D401. [Google Scholar] [CrossRef]
- Zhao, C.; Höppner, A.; Xu, Q.Z.; Gärtner, W.; Scheer, H.; Zhou, M.; Zhao, K.H. Structures and enzymatic mechanisms of phycobiliprotein lyases CpcE/F and PecE/F. Proc. Natl. Acad. Sci. USA 2017, 114, 13170–13175. [Google Scholar] [CrossRef]
- Fairchild, C.D.; Zhao, J.; Zhou, J.; Colson, S.E.; Bryant, D.A.; Glazer, A.N. Phycocyanin alpha-subunit phycocyanobilin lyase. Proc. Natl. Acad. Sci. USA 1992, 89, 7017–7021. [Google Scholar] [CrossRef]
- Swanson, R.V.; Zhou, J.; Leary, J.A.; Williams, T.; de Lorimier, R.; Bryant, D.A.; Glazer, A.N. Characterization of phycocyanin produced by cpcE and cpcF mutants and identification of an intergenic suppressor of the defect in bilin attachment. J. Biol. Chem. 1992, 267, 16146–16154. [Google Scholar] [CrossRef]
- Kronfel, C.M.; Hernandez, C.V.; Frick, J.P.; Hernandez, L.S.; Gutu, A.; Karty, J.A.; Boutaghou, M.N.; Kehoe, D.M.; Cole, R.B.; Schluchter, W.M. CpeF is the bilin lyase that ligates the doubly linked phycoerythrobilin on β-phycoerythrin in the cyanobacterium Fremyella diplosiphon. J. Biol. Chem. 2019, 294, 3987–3999. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Boutaghou, M.N.; Alvey, R.M.; Kronfel, C.M.; Cole, R.B.; Bryant, D.A.; Schluchter, W.M. Characterization of the activities of the CpeY, CpeZ, and CpeS bilin lyases in phycoerythrin biosynthesis in Fremyella diplosiphon strain UTEX 481. J. Biol. Chem. 2011, 286, 35509–35521. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Joseph, K.L.; Bussell, A.; Pokhrel, S.; Karty, J.; Kronfel, C.; Kehoe, D.; Schluchter, W. CpeT is the phycoerythrobilin lyase for Cys-165 on β-phycoerythrin from Fremyella diplosiphon and the chaperone-like protein CpeZ greatly improves its activity. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148284. [Google Scholar] [CrossRef]
- Tang, J.; Yao, D.; Zhou, H.; Du, L.; Daroch, M. Reevaluation of Parasynechococcus-like strains and genomic analysis of their microsatellites and compound microsatellites. Plants 2022, 11, 1060. [Google Scholar] [CrossRef] [PubMed]
- Zlenko, D.V.; Elanskaya, I.V.; Lukashev, E.P.; Bolychevtseva, Y.V.; Suzina, N.E.; Pojidaeva, E.S.; Kononova, I.A.; Loktyushkin, A.V.; Stadnichuk, I.N. Role of the PB-loop in ApcE and phycobilisome core function in cyanobacterium Synechocystis sp. PCC 6803. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Luimstra, V.M.; Schuurmans, J.M.; Hellingwerf, K.J.; Matthijs, H.C.P.; Huisman, J. Blue light induces major changes in the gene expression profile of the cyanobacterium Synechocystis sp. PCC 6803. Physiol. Plantarum 2020, 170, 10–26. [Google Scholar] [CrossRef]
- Hu, P.-P.; Hou, J.-Y.; Xu, Y.-L.; Niu, N.-N.; Zhao, C.; Lu, L.; Zhou, M.; Scheer, H.; Zhao, K.-H. The role of lyases, NblA and NblB proteins and bilin chromophore transfer in restructuring the cyanobacterial light-harvesting complex. Plant J. 2020, 102, 529–540. [Google Scholar] [CrossRef]
- Kronfel, C.M.; Biswas, A.; Frick, J.P.; Gutu, A.; Blensdorf, T.; Karty, J.A.; Kehoe, D.M.; Schluchter, W.M. The roles of the chaperone-like protein CpeZ and the phycoerythrobilin lyase CpeY in phycoerythrin biogenesis. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 549–561. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Brown, I.I.; Bryant, D.A.; Casamatta, D.; Thomas-Keprta, K.L.; Sarkisova, S.A.; Shen, G.; Graham, J.E.; Boyd, E.S.; Peters, J.W.; Garrison, D.H. Polyphasic characterization of a thermotolerant siderophilic filamentous cyanobacterium that produces intracellular iron deposits. Appl. Environ. Microbiol. 2010, 76, 6664–6672. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.S.; Nguyen, N.T.; Tran, K.T.; Tsuji, K.; Ogo, S. Nitrogen fixation genes and nitrogenase activity of the non-heterocystous cyanobacterium Thermoleptolyngbya sp. O-77. Microbes Environ. 2017, 32, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, L.; Li, M.; Du, L.; Shah, M.R.; Waleron, M.; Waleron, M.; Waleron, K.F.; Daroch, M. Description, taxonomy, and comparative genomics of a novel species, Thermoleptolyngbya sichuanensis sp. nov., isolated from Hot Springs of Ganzi, Sichuan, China. Front. Microbiol. 2021, 12, 696102. [Google Scholar] [CrossRef]
- Kono, M.; Martinez, J.N.; Sato, T.; Haruta, S. Draft genome sequence of the thermophilic unicellular cyanobacterium Synechococcus sp. strain C9. Microbiol. Resour. Announc. 2022, 11, e00294-22. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.T.; Nowack, S.; Wood, J.M.; Becraft, E.D.; LaButti, K.; Lipzen, A.; Martin, J.; Schackwitz, W.S.; Rusch, D.B.; Cohan, F.M.; et al. The molecular dimension of microbial species: 3. Comparative genomics of Synechococcus strains with different light responses and in situ diel transcription patterns of associated putative ecotypes in the Mushroom Spring microbial mat. Front. Microbiol. 2015, 6, 604. [Google Scholar] [CrossRef]
- Bhaya, D.; Grossman, A.R.; Steunou, A.-S.; Khuri, N.; Cohan, F.M.; Hamamura, N.; Melendrez, M.C.; Bateson, M.M.; Ward, D.M.; Heidelberg, J.F. Population level functional diversity in a microbial community revealed by comparative genomic and metagenomic analyses. ISME J. 2007, 1, 703–713. [Google Scholar] [CrossRef]
- Tang, J.; Zhou, H.; Yao, D.; Riaz, S.; You, D.; Klepacz-Smółka, A.; Daroch, M. Comparative genomic analysis revealed distinct molecular components and organization of CO2-concentrating mechanism in thermophilic cyanobacteria. Front. Microbiol. 2022, 12, 876272. [Google Scholar] [CrossRef]
- Cheng, Y.-I.; Chou, L.; Chiu, Y.-F.; Hsueh, H.-T.; Kuo, C.-H.; Chu, H.-A. Comparative genomic analysis of a novel strain of Taiwan hot-spring cyanobacterium Thermosynechococcus sp. CL-1. Front. Microbiol. 2020, 11, 82. [Google Scholar] [CrossRef]
- Leu, J.-Y.; Lin, T.-H.; Selvamani, M.J.P.; Chen, H.-C.; Liang, J.-Z.; Pan, K.-M. Characterization of a novel thermophilic cyanobacterial strain from Taian hot springs in Taiwan for high CO2 mitigation and C-phycocyanin extraction. Process Biochem. 2013, 48, 41–48. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kaneko, T.; Sato, S.; Ikeuchi, M.; Katoh, H.; Sasamoto, S.; Watanabe, A.; Iriguchi, M.; Kawashima, K.; Kimura, T.; et al. Complete genome structure of the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1. DNA Res. 2002, 9, 123–130. [Google Scholar] [CrossRef]
- Liang, Y.; Tang, J.; Luo, Y.; Kaczmarek, M.B.; Li, X.; Daroch, M. Thermosynechococcus as a thermophilic photosynthetic microbial cell factory for CO2 utilisation. Bioresour. Technol. 2019, 278, 255–265. [Google Scholar] [CrossRef]
- Brilli, M.; Fondi, M.; Fani, R.; Mengoni, A.; Ferri, L.; Bazzicalupo, M.; Biondi, E.G. The diversity and evolution of cell cycle regulation in alpha-proteobacteria: A comparative genomic analysis. BMC Syst. Biol. 2010, 4, 52. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate Maximum-Likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Vincent, L.; Jean-Emmanuel, L.; Olivier, G. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar]
- Yao, D.; Cheng, L.; Du, L.; Li, M.; Daroch, M.; Tang, J. Genome-wide investigation and analysis of microsatellites and compound microsatellites in Leptolyngbya-like species, Cyanobacteria. Life 2021, 11, 1258. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).