Deletion of pbpC Enhances Bacterial Pathogenicity on Tomato by Affecting Biofilm Formation, Exopolysaccharides Production, and Exoenzyme Activities in Clavibacter michiganensis
Abstract
1. Introduction
2. Results
2.1. Expression Level of Pathogenesis-Related Genes in C. michiganensis pbpC Mutants
2.2. Endocellulase and Amylase Secreted by pbpC Derivatives
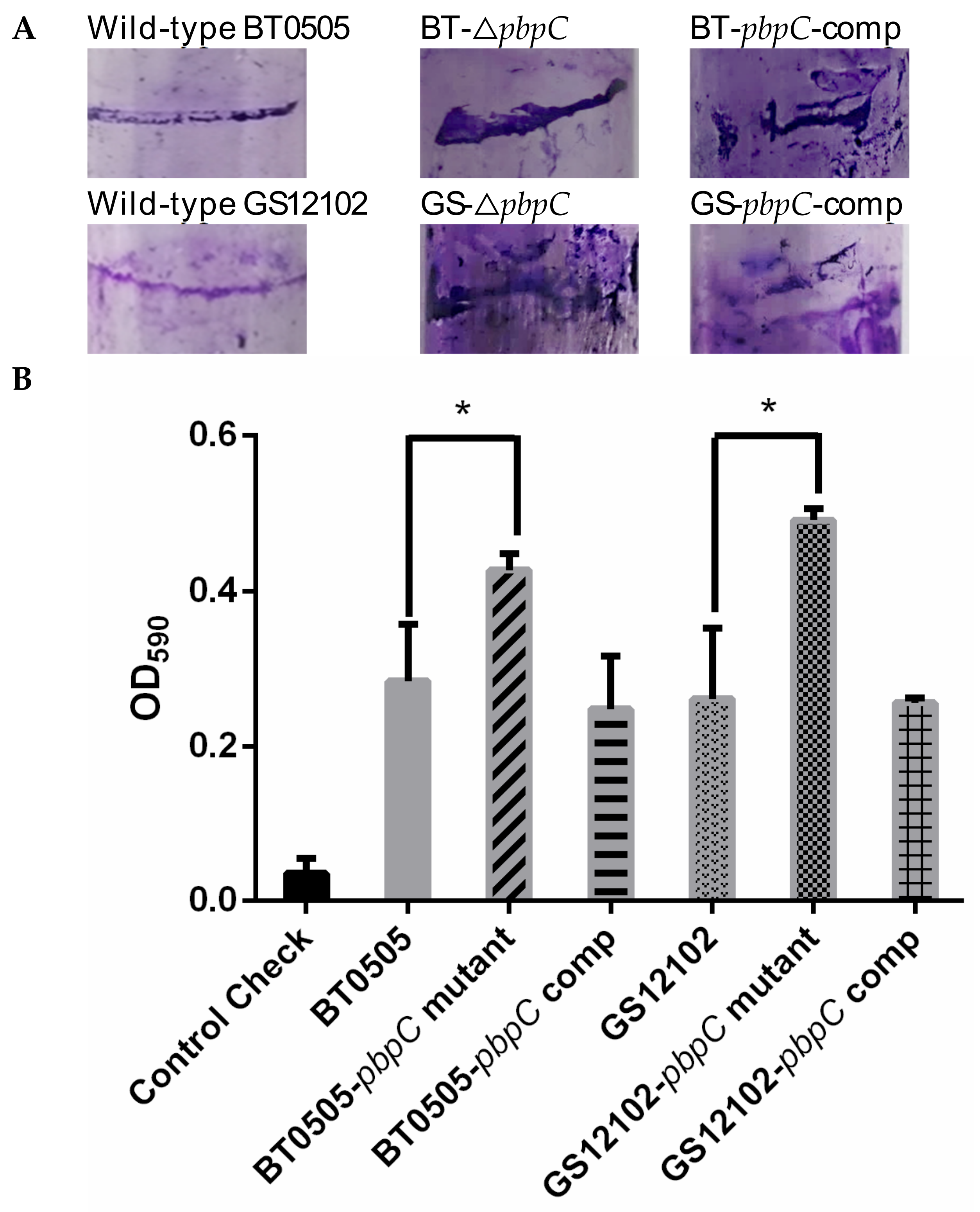
2.3. Biofilm Formation of pbpC Derivatives
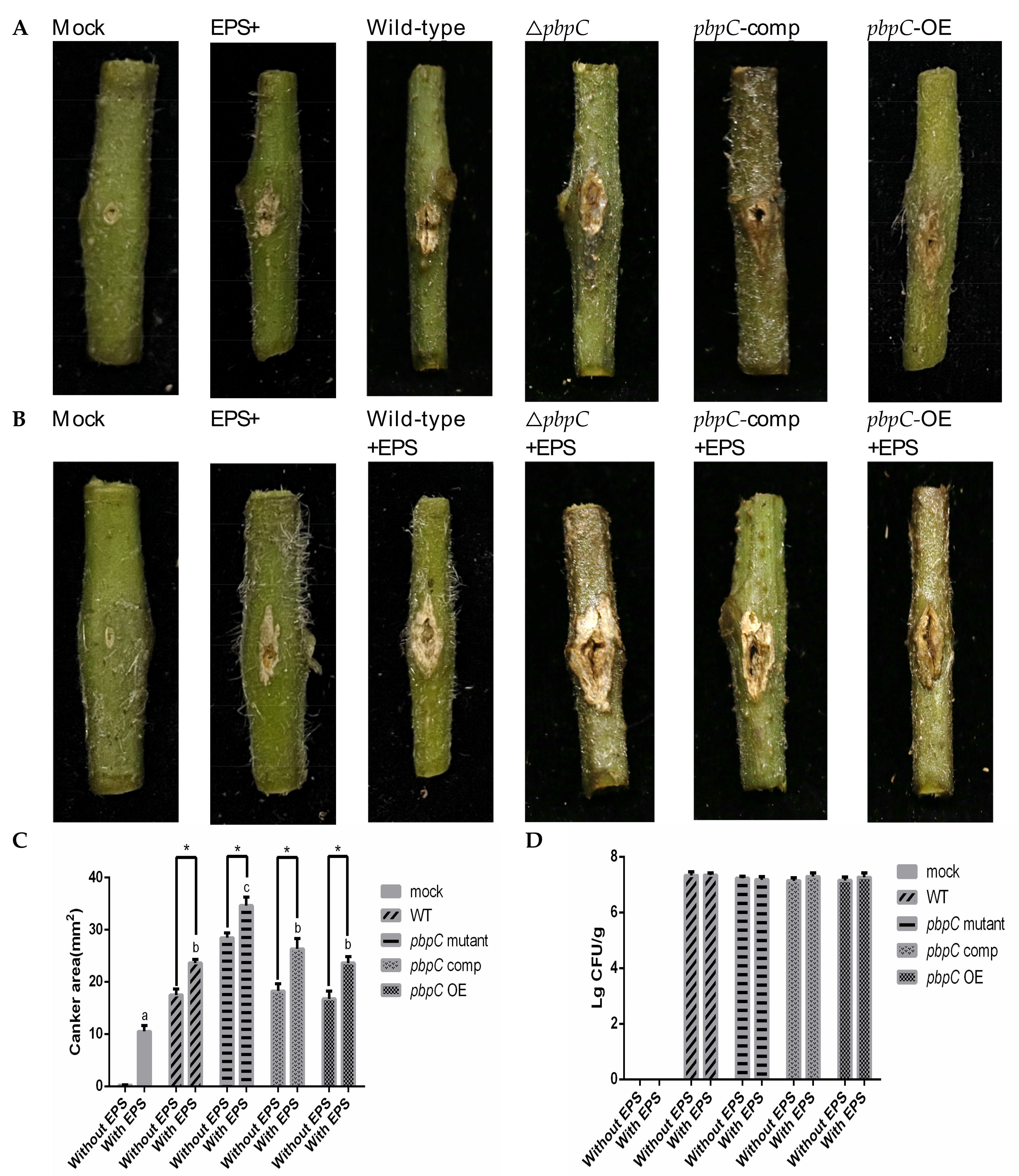
2.4. EPS Isolation of pbpC Derivatives and Virulence Testing In Vitro
2.5. Effect of EPS on Tomato and Tobacco Leaves
3. Discussion
4. Materials and Methods
4.1. Strains, Plasmids, and Growth Conditions
4.2. Construction of pbpC Over-Expression Strains
4.3. RNA Extraction and cDNA Synthesis
4.4. Gene Expression Analysis by qRT-PCR
4.5. Exoenzyme Activity Measurement Assays
4.6. Biofilm Measurement Assays
4.7. Isolation and Quantification of Bacterial EPS
4.8. Plant Material and Growth Conditions
4.9. Virulence and HR Test
4.10. Bacterial Population Measurement and Validation in Planta
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de León, L.; Siverio, F.; López, M.M.; Rodríguez, A. Clavibacter michiganesis subsp. michiganensis, a seedborne tomato pathogen: Healthy seeds are still the goal. Plant Dis. 2011, 95, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Sen, Y.; van der Wolf, J.; Visser, R.G.; van Heusden, S. Bacterial canker of tomato: Current knowledge of detection, management, resistance, and interactions. Plant Dis. 2015, 99, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Eichenlaub, R.; Gartemann, K.H. The Clavibacter michiganensis subspecies: Molecular investigation of gram-positive bacterial plant pathogens. Annu. Rev. Phytopathol. 2011, 49, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Baek, K.-H.; Moon, E. Antimicrobial effects of a hexapetide KCM21 against Pseudomonas syringae pv. tomato DC3000 and Clavibacter michiganensis subsp. michiganensis. Plant Pathol. J. 2014, 30, 245. [Google Scholar] [CrossRef]
- Xu, X.; Kumar, A.; Deblais, L.; Pina-Mimbela, R.; Nislow, C.; Fuchs, J.R.; Miller, S.A.; Rajashekara, G. Discovery of novel small molecule modulators of Clavibacter michiganensis subsp. michiganensis. Front. Microbiol. 2015, 6, 1127. [Google Scholar] [CrossRef][Green Version]
- Basim, H.; Basim, E.; Tombuloglu, H.; Unver, T. Comparative transcriptome analysis of resistant and cultivated tomato lines in response to Clavibacter michiganensis subsp. michiganensis. Genomics 2021, 113, 2455–2467. [Google Scholar] [CrossRef]
- Tancos, M.A.; Chalupowicz, L.; Barash, I.; Manulis-Sasson, S.; Smart, C.D. Tomato fruit and seed colonization by Clavibacter michiganensis subsp. michiganensis through external and internal routes. Appl. Environ. Microbiol. 2013, 79, 6948–6957. [Google Scholar] [CrossRef]
- Yadeta, K.A.; BP, J.T. The xylem as battleground for plant hosts and vascular wilt pathogens. Front. Plant Sci. 2013, 4, 97. [Google Scholar] [CrossRef]
- Xu, X.; Rajashekara, G.; Paul, P.A.; Miller, S.A. Colonization of tomato seedlings by bioluminescent Clavibacter michiganensis subsp. michiganensis under different humidity regimes. Phytopathology 2012, 102, 177–184. [Google Scholar] [CrossRef]
- Nandi, M.; Macdonald, J.; Liu, P.; Weselowski, B.; Yuan, Z.C. Clavibacter michiganensis ssp. michiganensis: Bacterial canker of tomato, molecular interactions and disease management. Mol. Plant Pathol. 2018, 19, 2036–2050. [Google Scholar] [CrossRef]
- Sharabani, G.; Shtienberg, D.; Borenstein, M.; Shulhani, R.; Lofthouse, M.; Sofer, M.; Chalupowicz, L.; Barel, V.; Manulis-Sasson, S. Effects of plant age on disease development and virulence of Clavibacter michiganensis subsp. michiganensis on tomato. Plant Pathol. 2013, 62, 1114–1122. [Google Scholar] [CrossRef]
- Murray, T.; Popham, D.L.; Setlow, P. Identification and characterization of pbpC, the gene encoding Bacillus subtilis penicillin-binding protein 3. J. Bacteriol. 1996, 178, 6001–6005. [Google Scholar] [CrossRef][Green Version]
- Pazos, M.; Vollmer, W. Regulation and function of class A Penicillin-binding proteins. Curr. Opin. Microbiol. 2021, 60, 80–87. [Google Scholar] [CrossRef]
- Straume, D.; Piechowiak, K.W.; Olsen, S.; Stamsas, G.A.; Berg, K.H.; Kjos, M.; Heggenhougen, M.V.; Alcorlo, M.; Hermoso, J.A.; Havarstein, L.S. Class A PBPs have a distinct and unique role in the construction of the pneumococcal cell wall. Proc. Natl. Acad. Sci. USA 2020, 117, 6129–6138. [Google Scholar] [CrossRef]
- Cho, H.; Wivagg, C.N.; Kapoor, M.; Barry, Z.; Rohs, P.D.A.; Suh, H.; Marto, J.A.; Garner, E.C.; Bernhardt, T.G. Bacterial cell wall biogenesis is mediated by SEDS and PBP polymerase families functioning semi-autonomously. Nat. Microbiol. 2016, 1, 16172. [Google Scholar] [CrossRef]
- Ropp, P.A.; Hu, M.; Olesky, M.; Nicholas, R.A. Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2002, 46, 769–777. [Google Scholar] [CrossRef]
- Chen, X.; Bai, K.; Lyu, Q.; Jiang, N.; Li, J.; Luo, L. Role of Penicillin-Binding Proteins in the Viability, Morphology, Stress Tolerance, and Pathogenicity of Clavibacter michiganensis. Phytopathology 2021, 111, 1301–1312. [Google Scholar] [CrossRef]
- Vigouroux, A.; Cordier, B.; Aristov, A.; Alvarez, L.; Ozbaykal, G.; Chaze, T.; Oldewurtel, E.R.; Matondo, M.; Cava, F.; Bikard, D.; et al. Class-A penicillin binding proteins do not contribute to cell shape but repair cell-wall defects. Elife 2020, 9, e51998. [Google Scholar] [CrossRef]
- Levy, N.; Bruneau, J.-M.; Le Rouzic, E.; Bonnard, D.; Le Strat, F.; Caravano, A.; Chevreuil, F.; Barbion, J.; Chasset, S.; Ledoussal, B. Structural basis for E. coli penicillin binding protein (PBP) 2 inhibition, a platform for drug design. J. Med. Chem. 2019, 62, 4742–4754. [Google Scholar] [CrossRef]
- Freischem, S.; Grimm, I.; López-Pérez, A.; Willbold, D.; Klenke, B.; Vuong, C.; Dingley, A.J.; Weiergräber, O.H. Interaction mode of the novel monobactam AIC499 targeting penicillin binding protein 3 of Gram-negative bacteria. Biomolecules 2021, 11, 1057. [Google Scholar] [CrossRef]
- Flugel, M.; Becker, A.; Gartemann, K.H.; Eichenlaub, R. Analysis of the interaction of Clavibacter michiganensis subsp. michiganensis with its host plant tomato by genome-wide expression profiling. J. Biotechnol. 2012, 160, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Savidor, A.; Teper, D.; Gartemann, K.H.; Eichenlaub, R.; Chalupowicz, L.; Manulis-Sasson, S.; Barash, I.; Tews, H.; Mayer, K.; Giannone, R.J.; et al. The Clavibacter michiganensis subsp. michiganensis-tomato interactome reveals the perception of pathogen by the host and suggests mechanisms of infection. J. Proteome Res. 2012, 11, 736–750. [Google Scholar] [CrossRef] [PubMed]
- Chalupowicz, L.; Cohen-Kandli, M.; Dror, O.; Eichenlaub, R.; Gartemann, K.-H.; Sessa, G.; Barash, I.; Manulis-Sasson, S. Sequential expression of bacterial virulence and plant defense genes during infection of tomato with Clavibacter michiganensis subsp. michiganensis. Phytopathology 2010, 100, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, M.; González, M.; Velásquez, A.; Dorta, F.; Montenegro, I.; Besoain, X.; Salvà-Serra, F.; Jaén-Luchoro, D.; Moore, E.R.; Seeger, M. Analyses of virulence genes of Clavibacter michiganensis subsp. michiganensis strains reveal heterogeneity and deletions that correlate with pathogenicity. Microorganisms 2021, 9, 1530. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.K.; Teper, D. Immune recognition of the secreted serine protease ChpG restricts the host range of Clavibacter michiganensis from eggplant varieties. Mol. Plant Pathol. 2022, 23, 933–946. [Google Scholar] [CrossRef]
- Janissen, R.; Murillo, D.M.; Niza, B.; Sahoo, P.K.; Nobrega, M.M.; Cesar, C.L.; Temperini, M.L.; Carvalho, H.F.; de Souza, A.A.; Cotta, M.A. Spatiotemporal distribution of different extracellular polymeric substances and filamentation mediate Xylella fastidiosa adhesion and biofilm formation. Sci. Rep. 2015, 5, 9856. [Google Scholar] [CrossRef]
- Xiao, M.; Ren, X.; Cui, J.; Li, R.; Liu, Z.; Zhu, L.; Kong, Q.; Fu, X.; Mou, H. A novel glucofucobiose with potential prebiotic activity prepared from the exopolysaccharides of Clavibacter michiganensis M1. Food Chem. 2022, 377, 132001. [Google Scholar] [CrossRef]
- Yaacob, M.F.; Murata, A.; Nor, N.H.M.; Jesse, F.F.A.; Raja Yahya, M.F.Z. Biochemical composition, morphology and antimicrobial susceptibility pattern of Corynebacterium pseudotuberculosis biofilm. J. King Saud Univ. Sci. 2021, 33, 101225. [Google Scholar] [CrossRef]
- Yaacob, M.F.; Abdullah, F.F.J.; Jamil, N.M.; Yunus, N.M.; Aazmi, S.; Yahya, M.F.Z.R. The effect of dimethyl sulfoxide on Corynebacterium pseudotuberculosis biofilm: An in silico prediction and experimental validation. J. Phys. Conf. Ser. 2021, 1874, 012055. [Google Scholar] [CrossRef]
- de Sa, M.C.A.; da Silva, W.M.; Rodrigues, C.C.S.; Rezende, C.P.; Marchioro, S.B.; Rocha Filho, J.T.R.; Sousa, T.J.; de Oliveira, H.P.; da Costa, M.M.; Figueiredo, H.C.P.; et al. Comparative proteomic analyses between biofilm-forming and non-biofilm-forming strains of Corynebacterium pseudotuberculosis isolated from goats. Front. Vet. Sci. 2021, 8, 614011. [Google Scholar] [CrossRef]
- Al-Wrafy, F.; Brzozowska, E.; Gorska, S.; Gamian, A. Pathogenic factors of Pseudomonas aeruginosa—The role of biofilm in pathogenicity and as a target for phage therapy. Postępy Hig. Med. Doświadczalnej (Online) 2017, 71, 78–91. [Google Scholar] [CrossRef]
- Brindhadevi, K.; LewisOscar, F.; Mylonakis, E.; Shanmugam, S.; Verma, T.N.; Pugazhendhi, A. Biofilm and quorum sensing mediated pathogenicity in Pseudomonas aeruginosa. Process Biochem. 2020, 96, 49–57. [Google Scholar] [CrossRef]
- Whitfield, G.B.; Marmont, L.S.; Howell, P.L. Enzymatic modifications of exopolysaccharides enhance bacterial persistence. Front. Microbiol. 2015, 6, 471. [Google Scholar] [CrossRef]
- Bottimarino, M. Epiphytic Survival and Biofilm Formation of the Goss’s Wilt Pathogen Clavibacter michiganensis subsp. nebraskensis; Michigan State University: East Lansing, MI, USA, 2017. [Google Scholar]
- Rossi, E.; Paroni, M.; Landini, P. Biofilm and motility in response to environmental and host-related signals in Gram negative opportunistic pathogens. J. Appl. Microbiol. 2018, 125, 1587–1602. [Google Scholar] [CrossRef]
- Geier, G.; Geider, K. Characterization and influence on virulence of the levansucrase gene from the fireblight pathogen Erwinia amylovora. Physiol. Mol. Plant Pathol. 1993, 42, 387–404. [Google Scholar] [CrossRef]
- Nimtz, M.; Mort, A.; Domke, T.; Wray, V.; Zhang, Y.; Qiu, F.; Coplin, D.; Geider, K. Structure of amylovoran, the capsular exopolysaccharide from the fire blight pathogen Erwinia amylovora. Carbohydr. Res. 1996, 287, 59–76. [Google Scholar] [CrossRef]
- Koczan, J.M.; McGrath, M.J.; Zhao, Y.; Sundin, G.W. Contribution of Erwinia amylovora exopolysaccharides amylovoran and levan to biofilm formation: Implications in pathogenicity. Phytopathology 2009, 99, 1237–1244. [Google Scholar] [CrossRef]
- Vrancken, K.; Holtappels, M.; Schoofs, H.; Deckers, T.; Valcke, R. Pathogenicity and infection strategies of the fire blight pathogen Erwinia amylovora in Rosaceae: State of the art. Microbiology 2013, 159, 823–832. [Google Scholar] [CrossRef]
- Yuan, X.; Eldred, L.I.; Sundin, G.W. Exopolysaccharides amylovoran and levan contribute to sliding motility in the fire blight pathogen Erwinia amylovora. Environ. Microbiol. 2022, 24, 4738–4754. [Google Scholar] [CrossRef]
- Thapa, S.P.; Pattathil, S.; Hahn, M.G.; Jacques, M.-A.; Gilbertson, R.L.; Coaker, G. Genomic analysis of Clavibacter michiganensis reveals insight into virulence strategies and genetic diversity of a Gram-positive bacterial pathogen. Mol. Plant Microbe Interact. 2017, 30, 786–802. [Google Scholar] [CrossRef]
- Hwang, I.S.; Oh, E.J.; Lee, H.B.; Oh, C.S. Functional Characterization of Two Cellulase Genes in the Gram-Positive Pathogenic Bacterium Clavibacter michiganensis for Wilting in Tomato. Mol. Plant Microbe Interact. 2019, 32, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Jahr, H.; Dreier, J.; Meletzus, D.; Bahro, R.; Eichenlaub, R. The endo-β-1, 4-glucanase CelA of Clavibacter michiganensis subsp. michiganensis is a pathogenicity determinant required for induction of bacterial wilt of tomato. Mol. Plant Microbe Interact. 2000, 13, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.; Grafen, I.; Engemann, J.; Niermann, E.; Pieper, M.; Kirchner, O.; Gartemann, K.H.; Eichenlaub, R. Identification of homologues to the pathogenicity factor Pat-1, a putative serine protease of Clavibacter michiganensis subsp. michiganensis. Microbiol. Res. 2005, 160, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Chalupowicz, L.; Barash, I.; Reuven, M.; Dror, O.; Sharabani, G.; Gartemann, K.H.; Eichenlaub, R.; Sessa, G.; Manulis-Sasson, S. Differential contribution of Clavibacter michiganensis ssp. michiganensis virulence factors to systemic and local infection in tomato. Mol. Plant Pathol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- Stork, I.; Gartemann, K.H.; Burger, A.; Eichenlaub, R. A family of serine proteases of Clavibacter michiganensis subsp. michiganensis: chpC plays a role in colonization of the host plant tomato. Mol. Plant Pathol. 2008, 9, 599–608. [Google Scholar] [CrossRef]
- Desmarais, S.M.; De Pedro, M.A.; Cava, F.; Huang, K.C. Peptidoglycan at its peaks: How chromatographic analyses can reveal bacterial cell wall structure and assembly. Mol. Microbiol. 2013, 89, 1–13. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Bai, K.; Gu, M.; Xu, X.; Jiang, N.; Chen, Y.; Li, J.; Luo, L. Class A Penicillin-Binding Protein C Is Responsible for Stress Response by Regulation of Peptidoglycan Assembly in Clavibacter michiganensis. Microbiol. Spectr. 2022, 10, e01816–e01822. [Google Scholar] [CrossRef]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef]
- Roca, C.; Alves, V.D.; Freitas, F.; Reis, M.A. Exopolysaccharides enriched in rare sugars: Bacterial sources, production, and applications. Front. Microbiol. 2015, 6, 288. [Google Scholar] [CrossRef]
- Barber, C.; Tang, J.; Feng, J.; Pan, M.; Wilson, T.; Slater, H.; Dow, J.; Williams, P.; Daniels, M. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol. Microbiol. 1997, 24, 555–566. [Google Scholar] [CrossRef]
- Bahar, O.; Goffer, T.; Burdman, S. Type IV pili are required for virulence, twitching motility, and biofilm formation of Acidovorax avenae subsp. citrulli. Mol. Plant Microbe Interact. 2009, 22, 909–920. [Google Scholar] [CrossRef]
- Westra, A.; Slack, S. Isolation and characterization of extracellular polysaccharide of Clavibacter michiganensis subsp. sepedonicus. Phytopathology 1992, 82, 1193–1199. [Google Scholar] [CrossRef]
- Van den Bulk, R.; Zevenhuizen, L.; Cordewener, J.; Dons, J. Characterization of the extracellular polysaccharide produced by Clavibacter michiganensis subsp. michiganensis. Phytopathology 1991, 81, 619–623. [Google Scholar] [CrossRef]
- Beimen, A.; Bermpohl, A.; Meletzus, D.; Eichenlaub, R.; Barz, W. Accumulation of phenolic compounds in leaves of tomato plants after infection with Clavibacter michiganense subsp. michiganense strains differing in virulence. Z. Nat. C 1992, 47, 898–909. [Google Scholar]
- Bermpohl, A.; Dreier, J.; Bahro, R.; Eichenlaub, R. Exopolysaccharides in the pathogenic interaction of Clavibacter michiganensis subsp. michiganensis with tomato plants. Microbiol. Res. 1996, 151, 391–399. [Google Scholar] [CrossRef]
- Denny, T.P. Involvement of bacterial polysaccharides in plant pathogenesis. Annu. Rev. Phytopathol. 1995, 33, 173–197. [Google Scholar] [CrossRef]
- Rai, P.V.; Strobel, G.A. Phytotoxic glycopeptides produced by Corynebacterium michiganense. II. Biological properties. Phytopathology 1969, 59, 53–57. [Google Scholar]
- Henningson, P.J.; Gudmestad, N.C. Comparison of exopolysaccharides from mucoid and nonmucoid strains of Clavibacter michiganensis subspecies sepedonicus. Can. J. Microbiol. 1993, 39, 291–296. [Google Scholar] [CrossRef]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef]
- Lolle, S.; Stevens, D.; Coaker, G. Plant NLR-triggered immunity: From receptor activation to downstream signaling. Curr. Opin. Immunol. 2020, 62, 99–105. [Google Scholar] [CrossRef]
- Boulanger, M.; Delvaux, C.; Quinton, L.; Joris, B.; De Pauw, E.; Far, J. Bacillus licheniformis peptidoglycan characterization by CZE–MS: Assessment with the benchmark RP-HPLC-MS method. Electrophoresis 2019, 40, 2672–2682. [Google Scholar] [CrossRef] [PubMed]
- Humann, J.; Bjordahl, R.; Andreasen, K.; Lenz, L.L. Expression of the p60 autolysin enhances NK cell activation and is required for Listeria monocytogenes expansion in IFN-γ-responsive mice. J. Immunol. 2007, 178, 2407–2414. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Grabherr, H.M.; Willmann, R.; Kolb, D.; Brunner, F.; Bertsche, U.; Kuhner, D.; Franz-Wachtel, M.; Amin, B.; Felix, G.; et al. Host-induced bacterial cell wall decomposition mediates pattern-triggered immunity in Arabidopsis. Elife 2014, 3, e01990. [Google Scholar] [CrossRef] [PubMed]
- Ruhmann, B.; Schmid, J.; Sieber, V. Methods to identify the unexplored diversity of microbial exopolysaccharides. Front. Microbiol. 2015, 6, 565. [Google Scholar] [CrossRef] [PubMed]
- Frère, J.-M.; Page, M.G. Penicillin-binding proteins: Evergreen drug targets. Curr. Opin. Pharmacol. 2014, 18, 112–119. [Google Scholar] [CrossRef]
- Lyu, Q.; Bai, K.; Kan, Y.; Jiang, N.; Thapa, S.P.; Coaker, G.; Li, J.; Luo, L. Variation in Streptomycin Resistance Mechanisms in Clavibacter michiganensis. Phytopathology 2019, 109, 1849–1858. [Google Scholar] [CrossRef]
- Laine, M.J.; Nakhei, H.; Dreier, J.; Lehtilä, K.; Meletzus, D.; Eichenlaub, R.; Metzler, M.C. Stable transformation of the gram-positive phytopathogenic bacterium Clavibacter michiganensis subsp. sepedonicus with several cloning vectors. Appl. Environ. Microbiol. 1996, 62, 1500–1506. [Google Scholar] [CrossRef]
- Kirchner, O.; Gartemann, K.-H.; Zellermann, E.-M.; Eichenlaub, R.; Burger, A. A highly efficient transposon mutagenesis system for the tomato pathogen Clavibacter michiganensis subsp. michiganensis. Mol. Plant Microbe Interact. 2001, 14, 1312–1318. [Google Scholar] [CrossRef]
- Jiang, N.; Lyu, Q.; Han, S.; Xu, X.; Walcott, R.R.; Li, J.; Luo, L. Evaluation of suitable reference genes for normalization of quantitative reverse transcription PCR analyses in Clavibacter michiganensis. Microbiologyopen 2019, 8, e928. [Google Scholar] [CrossRef]
- Looijesteijn, P.J.; Van Casteren, W.H.; Tuinier, R.; Doeswijk-Voragen, C.; Hugenholtz, J. Influence of different substrate limitations on the yield, composition and molecular mass of exopolysaccharides produced by Lactococcus lactis subsp. cremoris in continuous cultures. J. Appl. Microbiol. 2000, 89, 116–122. [Google Scholar] [CrossRef]
- Balaji, V.; Sessa, G.; Smart, C.D. Silencing of host basal defense response-related gene expression increases susceptibility of Nicotiana benthamiana to Clavibacter michiganensis subsp. michiganensis. Phytopathology 2011, 101, 349–357. [Google Scholar] [CrossRef]
- Kaup, O.; Gräfen, I.; Zellermann, E.-M.; Eichenlaub, R.; Gartemann, K.-H. Identification of a tomatinase in the tomato-pathogenic actinomycete Clavibacter michiganensis subsp. michiganensis NCPPB382. Mol. Plant-Microbe Interact. 2005, 18, 1090–1098. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, X.; Xu, X.; Yu, C.; Liu, Y.; Jiang, N.; Li, J.; Luo, L. Deletion of pbpC Enhances Bacterial Pathogenicity on Tomato by Affecting Biofilm Formation, Exopolysaccharides Production, and Exoenzyme Activities in Clavibacter michiganensis. Int. J. Mol. Sci. 2023, 24, 5324. https://doi.org/10.3390/ijms24065324
Li Y, Chen X, Xu X, Yu C, Liu Y, Jiang N, Li J, Luo L. Deletion of pbpC Enhances Bacterial Pathogenicity on Tomato by Affecting Biofilm Formation, Exopolysaccharides Production, and Exoenzyme Activities in Clavibacter michiganensis. International Journal of Molecular Sciences. 2023; 24(6):5324. https://doi.org/10.3390/ijms24065324
Chicago/Turabian StyleLi, Yao, Xing Chen, Xiaoli Xu, Chengxuan Yu, Yan Liu, Na Jiang, Jianqiang Li, and Laixin Luo. 2023. "Deletion of pbpC Enhances Bacterial Pathogenicity on Tomato by Affecting Biofilm Formation, Exopolysaccharides Production, and Exoenzyme Activities in Clavibacter michiganensis" International Journal of Molecular Sciences 24, no. 6: 5324. https://doi.org/10.3390/ijms24065324
APA StyleLi, Y., Chen, X., Xu, X., Yu, C., Liu, Y., Jiang, N., Li, J., & Luo, L. (2023). Deletion of pbpC Enhances Bacterial Pathogenicity on Tomato by Affecting Biofilm Formation, Exopolysaccharides Production, and Exoenzyme Activities in Clavibacter michiganensis. International Journal of Molecular Sciences, 24(6), 5324. https://doi.org/10.3390/ijms24065324






