Abstract
DNA methylation (leading to gene silencing) is one of the best-studied epigenetic mechanisms. It is also essential in regulating the dynamics of dopamine release in the synaptic cleft. This regulation relates to the expression of the dopamine transporter gene (DAT1). We examined 137 people addicted to nicotine, 274 addicted subjects, 105 sports subjects and 290 people from the control group. After applying the Bonferroni correction, our results show that as many as 24 out of 33 examined CpG islands had statistically significantly higher methylation in the nicotine-dependent subjects and athletes groups compared to the control group. Analysis of total DAT1 methylation revealed a statistically significant increase in the number of total methylated CpG islands in addicted subjects (40.94%), nicotine-dependent subjects (62.84%) and sports subjects (65.71%) compared to controls (42.36%). The analysis of the methylation status of individual CpG sites revealed a new direction of research on the biological aspects of regulating dopamine release in people addicted to nicotine, people practicing sports and people addicted to psychoactive substances.
1. Introduction
DNA methylation is a post-replication process in which a methyl group (-CH3) is added to the cytosine in the appropriate sequence context. DNA methylation (leading to gene silencing) is one of the best-studied epigenetic mechanisms. This is often done within the CpG islands, where cytosine conversion to 5-methyl-cytosine blocks gene expression [1]. This causes gene silencing by several mechanisms. One of these mechanisms is direct interference with the DNA binding of specific transcription factors, such as AP-2, c-Myc/Myn, cyclic AMP-dependent activator CREB, E2F and NF-κB, which recognize sequences containing CpG residues [2]. The CpG-rich regions overlap with the promoters of 50–60% of human genes and are usually more methylated than the CpG dinucleotides found outside the islands [3,4,5].
The DNA methylation pattern is stable through cell division and somatically inherited. It can be formed by de novo methylation or by demethylation. DNA methylation is a unique way to encode information in a stable but reversible way [1].
Correct control of DNA methylation is essential for proper cell function. DNA methylation dysfunction has been linked to many human pathologies [6,7,8,9].
The presented project aims to determine epigenetic factors which may significantly influence operational differences in the individual regulation of the dopaminergic system related to the different pathways of dopamine arousing. We chose three groups of people who, at first glance, are entirely unrelated: addicts to psychoactive substances, addicts to nicotine and professional athletes. In the first of them, an addictive substance is a source of dopaminergic reward, while in the second one, the natural forms of stimulation, i.e., sport or competition, result in a dopamine feast, and the postsynaptic dopaminergic receptors furiously demand a new dose to supplement the dopamine, which has not been effective enough so far.
Traditional epigenetic studies emphasize the importance of the complex interplay between the DAT1 gene and the environment [10]. The dopamine transporter DAT1 is crucial in dopamine (DA) neurotransmission. It is a protein located on nerve endings that modulates the dynamics and levels of dopamine release (by recycling extracellular dopamine back to the presynaptic terminal, thus terminating its action). Dysregulated DA activity may be due to altered release or reuptake; therefore, properly regulating DAT1 gene expression is crucial for maintaining homeostasis in dopaminergic systems.
The human DAT1 gene is extremely GC-dense and has multiple CpG islands, unlike most vertebrate genes, which only have a CpG island overlapping the pro-motor. The inactive promoters of the human cytosine and guanine genes account for an average of 57% of nucleotides [11], but GCs account for 79% of the DAT1 promoter sequence. The most notable feature of human DAT1 is its high sensitivity to epigenetic regulation: in contrast to the relative enrichment of GC nucleotides in the proximal promoter region, as is the case in most human genes, the entire DAT1 locus has a sequence composition of GC-bias (0, 55) and includes multiple CpG sites comprising 27 bona fide CGIs (CpG islands). The epigenetic sensitivity of the DAT1 gene has increased throughout evolution. The genetic drift of the GC nucleotide accumulation-oriented DAT1 sequence may reflect its amplifying epigenetic potential, which is essential in the regulatory processes resulting from the increasingly complex functions of the human brain [12].
The dopaminergic system plays a significant role in amplifying the effects of drug abuse [13,14]. Three factors contribute to the development of addiction: genetic, environmental diversity and the effect of drugs on gene expression or mRNA levels [15]. Recent studies have shown that transcription factors such as non-coding RNAs, histone modifications and chromatin structures can alter the transcription potential of genes. Transcription factors also contribute significantly to many neural adaptations resulting from chronic drug exposure [16]. Growing evidence supports the hypothesis that drug abuse directly influences the mechanism of epigenetic regulation and that these adaptations are among the main processes by which drugs induce highly stable changes in the brain that mediate the addiction phenotype [17]. Important epigenetic mechanisms include DNA methylation, histone modifications and small non-coding RNAs [18,19].
Cigarette smoke is one of DNA methylation’s most potent environmental modifiers [20]. Cigarette smoke can modulate it through DNA damage followed by DNMT recruitment. DNA repair sites recruit DNMT1 [21], which methylates the CpG adjacent to the repaired nucleotides [22]. Cigarette smoke may also modulate DNA methylation through nicotine’s effect on gene expression [23].
DNA methylation is stable through cell division dynamic throughout life due to environmental stimuli, such as exercise and nutrition. Exercise epigenetics is a new and exciting field of research, and we currently have limited knowledge of whether and how epigenetic signals, such as DNA methylation, mediate responses to exercise. A study from 2012 showed lower DNA methylation in specific genes 20 min after high-intensity endurance exercise and demonstrated rapid dynamics of DNA methylation. A meta-analysis of 16 studies identified 478 loci (307 in skeletal muscle) that undergo methylation changes following either acute (one bout) or regular exercise (walking, cycling, and tai-chi). Additional studies have found that long-term activity is associated with changes in DNA methylation in human skeletal muscles [24]. However, there is little research on epigenetics, particularly methylation in athletes. Researchers analyze various genes and their methylation levels in the promoter regions. We focus on that area of research based on the reports regarding the effects of dopamine on motivation and a series of thought processes. This is not the only study into the genetics and epigenetics of the gene regions responsible for physical exercise focusing on the endurance or functional phase.
Therefore, we want to examine how various factors, such as addiction to psychoactive substances, nicotine addiction or practicing sports, affect the methylation of the DAT1 gene promoter.
2. Results
Analysis of the methylation status of individual CpG sites revealed differences in methylation levels at individual sites (islands) of the DAT1 promoter (Table 1, Figure 1). At twenty-four of the 33 CpG sites, a significant methylation level was found in the addicted, nicotine-dependent and sports subjects (sites 1, 2,3, 4, 5, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 20, 21, 23, 24, 26, 27, 32 and 33).

Table 1.
Methylation status of 33 CpG DAT1 sites in the addicted, nicotine-dependent and sports subjects. 274 addicted, 137 nicotine-dependent, 105 sports and 290 control subjects were tested to compare the methylation status at the indicated CpG sites.
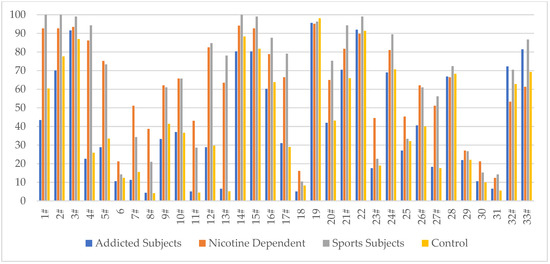
Figure 1.
The methylation level (%) of 33 CpG DAT1 sites in a group of addicted subjects, nicotine-dependent subjects, sports subjects and controls. # statistically significant differences in the methylation level—Bonferroni correction was applied, and p-value was lowered to 0.0015 (p = 0.05/33 (number of statistical tests performed)).
In CpG islands 1, 2, 4, 12, 14, 15, 16, 20, and 24, the highest methylation was shown in sports, nicotine-dependent, control and addicted subjects, respectively. In CpG islands 3, 10, 13, 17, 21, and 27, the highest degree of methylation was shown in sports, nicotine-dependent, addicted and control subjects, respectively. In CpG islands 5, 7, 9, and 23, the highest degree of methylation was shown in nicotine-dependent, sports, control and addicted subjects, respectively. In CpG islands 8, 11, and 26, the highest degree of methylation was shown in the nicotine-dependent, sports, addicted and control subjects, respectively. In CpG island 32, the highest degree of methylation was demonstrated in the addicted, sports, control and nicotine-dependent subjects, respectively. In CpG island 33, the highest degree of methylation was shown in sports, addicted, control and nicotine-dependent subjects, respectively.
Analysis of total DAT1 methylation revealed a statistically significant increase in the number of total methylated CpG islands in addicted (40.94%), nicotine-dependent (62.84%) and sports subjects (65.71%) compared to controls (42.36%) (F3,802 = 95.185, p < 0.000001, η2 = 0.262, Table 2, Figure 2). Table 3 shows the results of the post hoc test.

Table 2.
The mean total methylation sites in a group of addicted subjects, nicotine-dependent subjects, sports subjects and controls.
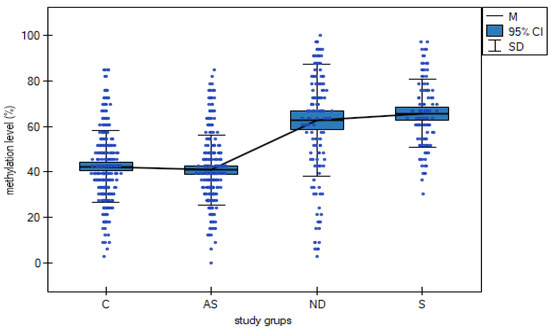
Figure 2.
The relationship between total methylation sites (%) in a group of AS—addicted subjects, ND—nicotine-dependent subjects, S—sports subjects and C—controls. Mt—mean total methylation sites. SD—standard deviation.

Table 3.
Post hoc (Fisher LSD) analysis of dependencies between a group of addicted subjects, nicotine-dependent subjects, sports subjects and controls.
3. Discussion
The study of epigenetic factors, especially methylation in the promoter region of the DAT1 gene, has been the subject of our interest for many years. The presented results culminate in analyzing groups commonly referred to as “dopamine seekers”, i.e., people using nicotine, people addicted to psychoactive substances and professional athletes. In our previous reports, we analyzed 33 CpG sites located in the promoter region of the DAT1 gene in cannabis-dependent subjects and controls. The results of our analyses showed significant differences between both groups—methylation changes are not similar across all sites. Compared with the control group, some sites in the dependent subjects were hypermethylated, while some were hypomethylated. Further, the assessment of the ability of transcription factors to bind to indicated sites revealed a substantial number of those regulators of gene expression [25].
In another study, we analyzed 33 CpG sites of the promoter region of the DAT1 gene among a sample group of athletes and a control group. The obtained data showed significant differences between the two groups as methylation changes across all sites differed. When comparing the control group and the group of athletes, the latter were hypermethylated, and some were hypomethylated. Moreover, transcription factors’ ability to bind to chosen sites revealed a great number of those regulators of gene expression [26].
In the present study, we went one step further by analyzing people addicted to various substances (not only marijuana), people using nicotine and athletes with championship successes. In our study of individual DAT1 methylation CpG islands (1,2,4,9,10,11,12,13,14,15,16,17,21,24,26,27), a significantly higher percentage of methylation was shown in nicotine-dependent and sports subjects compared to addicted subjects and controls (Table 1, Figure 1). The total percentage of methylation also showed a statistically significantly higher percentage of methylation in nicotine-dependent and sports subjects compared to addicted subjects and control (Table 2 and Table 3, Figure 2) (62.84% and 65.71% vs. 40.94% and 42.36%, respectively)
The epigenetic regulation of gene expression can be mediated by both hypermethylation and hypomethylation of DNA.
Our research showed that in the group of addicts, the average total methylation level of the DAT1 gene promoter was lower than in the control group (40.94% vs. 42.36%; Table 3), which indicates the occurrence of DNA hypomethylation.
Imaging studies in heroin addicts using SPECT have shown less DAT availability in the bilateral caudate nucleus, putamen and striatum. Unfortunately, the exact mechanism of changes in the density of the dopamine transporter is still unknown [27,28].
The lower availability of DAT indicates lower gene expression in the studied regions, which our current finding of reduced levels of DNA methylation, or hypomethylation, could explain.
However, in the group of people addicted to nicotine and the group of athletes, the dominant epigenetic phenomenon turned out to be the process of DNA hypermethylation. The average total methylation level of the DAT1 gene in the group of people addicted to nicotine was significantly higher than in the control group (62.84% vs. 42.36%; Table 3).
Cigarette smoke may also modulate DNA methylation through nicotine’s effect on gene expression [23]. Cigarette smoke may indirectly affect changes in DNA methylation by modulating DNA expression and binding activity factors. For example, cigarette smoke condensate increased the expression of Sp1 and DNA binding in lung epithelial cells [29,30]. Sp1 is a general transcription factor that binds to GC-rich motifs in the promoters of genes [31]. Transcription factor Sp1 was shown to contribute to regulating DAT mRNA expression [32]
Abdelkhalek et al. measured methylation in the promoter IV of the BDNF gene in smokers compared with healthy controls within 14 days of smoking cessation. Mean methylation was significantly higher in smokers than in healthy controls at all time points [33].
Halit et al. assessed the relationship between methylation in the promoters of the APC, NR3C1, and DRD2 genes and its effect on nicotine use. They found a significant difference in APC2 methylation, comparing the study and control groups. Smokers’ methylation rate was about eight times higher than healthy controls. NR3C1 methylation was slightly higher in patients with nicotine addiction compared to controls, but the difference was not significant between the two groups (% 95.33 vs. 91.08, p = 0.269). The DRD2 methylation ratio was insignificant between nicotine-addicted patients and healthy controls (p = 0.894) [34].
These previously documented studies allow us to conclude that the increased expression of Sp1 due to tobacco smoke may also increase the intensity of the dopamine transporter gene transcription process, which was revealed in our study in the form of DAT promoter hypermethylation.
Moreover, cigarette smoke can change DNA methylation through hypoxia—cigarette smoke contains carbon monoxide, which binds to haemoglobin (competitively with oxygen), thus reducing the oxygenation of tissues [35]. Hypoxia leads to HIF-1α-dependent up-regulation of methionine adenosyltransferase 2A, an enzyme synthesizing S-adenosylmethionine, the leading biological methyl donor critical to DNA methylation processes [36].
The present study provides further evidence that changes in DNA methylation in the regulatory (promoter) regions of addiction-related genes are associated with nicotine addiction, possibly because they can result in altered gene transcription. The rationale is that promoter DNA methylation can directly interfere with binding transcription factors (TFs) to regulatory regions. We also proved hypermethylation of the DAT promoter in a group of athletes. The average total methylation level of the DAT1 gene in the group of people practicing sports was significantly higher than in the control group (65.71% vs. 42.36%; Table 3). It is visible that sport is an essential environmental factor positively influencing the expression of the DAT1 gene.
We must remember that DNA methylation patterns are susceptible to change throughout life. Environmental factors acting through DNA methylation can modify our health, both positively and negatively. Moreover, these changes can be passed on to the next generations, participating in the evolution process [37]. In our work, we proved the extraordinary effect of epigenetics on the dopaminergic system in three different groups of happiness seekers.
4. Materials and Methods
4.1. Participants
The study group consisted of three homogeneous subgroups: patients addicted to drugs (274 patients), people addicted to nicotine (137 people) and athletes (105 people). The control group consisted of 290 people who were not addicted to drugs or nicotine and were not athletes. The average age of the individual groups and the percentages of females and males are shown in Table 4. To reduce the possibility of genetic admixture and overcome potential problems from population stratification, all participants were European.

Table 4.
Primary statistics include 274 addicted subjects, 137 nicotine-dependent subjects, 105 sports subjects and 290 control subjects.
The study was approved by the bioethics committee of the Pomeranian Medical University in Szczecin and was conducted following the guidelines of the Declaration of Helsinki. All participants were informed about the rules of the study, familiarized with its course and told about the possibility of withdrawing from the study at any time.
None of the study participants was financially rewarded for participating in the project, and the study was wholly anonymized following the principles of personal data protection. The addicted subjects were recruited in addiction treatment facilities in the province of Lubuskie after at least 3 months of abstinence. Nicotine dependence was tested with the Fagerstrom test. Athletes have been defined as people who practice sports professionally. The control group was selected in terms of age and sex; people who had not smoked more than a pack of cigarettes in their lifetime and were not addicted to other psychoactive substances at the time of the examination were not athletes. All procedures for comfort and concentration were performed.
4.2. Assessment of the Methylation Status of the Dopamine Gene Transporter (DAT1) Promoter
As previously described, DNA was isolated from peripheral blood using a DNA isolation kit (A&A Biotechnology, Gdynia, Poland) [37]. Bisulfite modifications of 250 ng DNA were performed following the manufacturer’s instructions using the EZ DNA Methylation Kit (Zymo Research, Orange, CA, USA). The methylation-specific PCR assay was performed on a Mastercycler epgradient S (Eppendorf, Germany).
Oligonucleotide primers designed using metprimer (http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi, accessed 29 April 2022) were obtained from Genomed.pl (Warsaw, Poland). The status of the DAT1 promoter (ENSG00000142319) was assessed by PCR using primers specific for the gene fragment, i.e., DATF: 5’-GGTTTTTGTTTTTTTTATGTTGAG-3’; DATR: 5’-AAATCCCCTAAACCTAATCCC-3’. Table 5 shows the PCR conditions used to amplify the 447 bp fragment spanning the 33 CpG sites in the DAT1 gene promoter.

Table 5.
PCR reaction conditions for the amplification of a 447 bp fragment encompassing 33 CpG sites in the promoter of the DAT1 gene.
The magnesium chloride ion concentration was 2.5 mM. After the amplification, the PCR products were sequenced as previously described [38]. Briefly, samples were verified by sequencing with the BigDye v3.1 kit (Applied Biosystems, Darmstadt, Germany) and separation by ethanol extraction with ABI Prism 3130XL (Applied Biosystems, Darmstadt, Germany) in a 36 cm POP7 Polymer capillary, using a reverse-primer.
Sequencing chromatograms were analyzed by the 4peaks software (v. 1.8., Mek & Tosj, Amsterdam, The Netherlands, https://nucleobytes.com/4peaks/index.html, accessed 29 April 2022). Cytosine methylation was considered positive when the G/A+G ratio was at least 20% of the total signal. The mathematical equation to calculate the percentage of methylation in each participant was: (G/(G + A) × 100)
4.3. Statistical Analysis
Analysis and comparison of the total methylation level (%) of 33 CpG DAT1 sites in the four analyzed groups of test subjects were performed using the One-Way Analysis of Variance (addicted subjects; nicotine-dependent subjects; sports subjects; control)).
The chi-squared test was used to show differences in the percentage of methylation in individual CpG islands in the analyzed four groups of subjects, with p < 0.05 regarded as statistically significant. The Bonferroni, multiple comparisons correction was applied for these variables, and the accepted significance level was 0.0015 (0.05/33). All computations were performed using STATISTICA 13 (TIBCO Software, Inc., Palo Alto, CA, USA) and PQStat Software (v. 1.8.2., Poznań, Poland).
5. Conclusions
Our analysis of the methylation status of individual CpG sites revealed a new direction of research on the biological aspects of regulating dopamine release in people addicted to nicotine, people practicing sports and people addicted to psychoactive substances.
Author Contributions
Conceptualization, A.G. and K.C.; methodology, A.G. and K.C.; formal analysis, K.C.; investigation, J.C. and J.M.; resources, K.C.; data curation, A.S.-P.; writing—original draft preparation, K.C., A.G., J.C, A.S.-P., J.M., A.B., M.L. and P.C.; writing—review and editing, K.C., A.G., J.C., A.S.-P., J.M., A.B., D.L. and M.D.-Z.; visualization, K.C.; supervision, A.G.; project administration, K.C.; funding acquisition, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Approval was obtained from the Bioethical Committee of the Pomeranian Medical University in Szczecin (KB-0012/106/16) and approved by KOMISJA BIOETYCZNA przy Okręgowej Izbie Lekarskiej w Szczecinie, ul. Marii Skłodowskiej-Curie 11, 71-332 (protocol nr 13/KB/VI/2016, 8 December 2016). Consent from study participants was informed and written. The study was conducted in the Independent Health Promotion Laboratory.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rogalska, S.; Achrem, M.; Wojciechowski, A. Chromatyna. Molekularne Mechanizmy Epigenetyczne; Wydawnictwo Uniwersytetu Przyrodniczego w Poznaniu: Poznań, Poland, 2010. [Google Scholar]
- Das, P.M.; Singal, R. DNA Methylation and Cancer. J. Clin. Oncol. 2004, 22, 4632–4642. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, F.C.C. An Evaluation of New Criteria for CpG Islands in the Human Genome as Gene Markers. Bioinformatics 2004, 20, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Chmielowiec, J.; Chmielowiec, K.; Strońska-Pluta, A.; Suchanecka, A.; Humińska-Lisowska, K.; Lachowicz, M.; Niewczas, M.; Białecka, M.; Śmiarowska, M.; Grzywacz, A. Methylation in the Promoter Region of the Dopamine Transporter DAT1 Gene in People Addicted to Nicotine. Int. J. Environ. Res. Public Health 2022, 19, 8602. [Google Scholar] [CrossRef] [PubMed]
- Kordi-Tamandani, D.M.; Tajoddini, S.; Salimi, F. Promoter Methylation and BDNF and DAT1 Gene Expression Profiles in Patients with Drug Addiction. Pathobiology 2015, 82, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Hatchwell, E.; Greally, J.M. The Potential Role of Epigenomic Dysregulation in Complex Human Disease. Trends Genet. 2007, 23, 588–595. [Google Scholar] [CrossRef]
- Mill, J.; Petronis, A. Pre- and Peri-Natal Environmental Risks for Attention-Deficit Hyperactivity Disorder (ADHD): The Potential Role of Epigenetic Processes in Mediating Susceptibility. J. Child Psychol. Psychiatry 2008, 49, 1020–1030. [Google Scholar] [CrossRef]
- Ikegame, T.; Bundo, M.; Murata, Y.; Kasai, K.; Kato, T.; Iwamoto, K. DNA Methylation of the BDNF Gene and Its Relevance to Psychiatric Disorders. J. Hum. Genet. 2013, 58, 434–438. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Ehrlich, S.; Walton, E.; White, T.; Perrone-Bizzozero, N.; Bustillo, J.; Turner, J.A.; Calhoun, V.D. Methylation Patterns in Whole Blood Correlate With Symptoms in Schizophrenia Patients. Schizophr. Bull. 2014, 40, 769–776. [Google Scholar] [CrossRef]
- Wiers, C.E.; Lohoff, F.W.; Lee, J.; Muench, C.; Freeman, C.; Zehra, A.; Marenco, S.; Lipska, B.K.; Auluck, P.K.; Feng, N.; et al. Methylation of the Dopamine Transporter Gene in Blood Is Associated with Striatal Dopamine Transporter Availability in ADHD: A Preliminary Study. Eur. J. Neurosci. 2018, 48, 1884. [Google Scholar] [CrossRef]
- Cooper, S.J.; Trinklein, N.D.; Anton, E.D.; Nguyen, L.; Myers, R.M. Comprehensive Analysis of Transcriptional Promoter Structure and Function in 1% of the Human Genome. Genome Res 2006, 16, 1. [Google Scholar] [CrossRef]
- Shumay, E.; Fowler, J.S.; Volkow, N.D. Genomic Features of the Human Dopamine Transporter Gene and Its Potential Epigenetic States: Implications for Phenotypic Diversity. PLoS ONE 2010, 5, 11067. [Google Scholar] [CrossRef]
- Justinova, Z.; Panlilio, L.V.; Goldberg, S.R. Drug Addiction. Curr. Top Behav. Neurosci. 2009, 1, 309–346. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Fowler, J.S.; Wang, G.J.; Swanson, J.M. Dopamine in Drug Abuse and Addiction: Results from Imaging Studies and Treatment Implications. Mol. Psychiatry 2004, 9, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Kreek, M.J.; Nielsen, D.A.; LaForge, K.S. Genes Associated with Addiction: Alcoholism, Opiate, and Cocaine Addiction. Neuromol. Med. 2004, 5, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Molecular Basis of Long-Term Plasticity Underlying Addiction. Nat. Rev. Neurosci. 2001, 2, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Robison, A.J.; Nestler, E.J. Transcriptional and Epigenetic Mechanisms of Addiction. Nat. Rev. Neurosci. 2011, 12, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Waddington, C.H. The Epigenotype. 1942. Int. J. Epidemiol. 2012, 41, 10–13. [Google Scholar] [CrossRef]
- Vassoler, F.M.; Sadri-Vakili, G. Mechanisms of Transgenerational Inheritance of Addictive-like Behaviors. Neuroscience 2014, 264, 198–206. [Google Scholar] [CrossRef]
- Breitling, L.P.; Yang, R.; Korn, B.; Burwinkel, B.; Brenner, H. Tobacco-Smoking-Related Differential DNA Methylation: 27K Discovery and Replication. Am. J. Hum. Genet. 2011, 88, 450. [Google Scholar] [CrossRef]
- Mortusewicz, O.; Schermelleh, L.; Walter, J.; Cardoso, M.C.; Leonhardt, H. Recruitment of DNA Methyltransferase I to DNA Repair Sites. Proc. Natl. Acad. Sci. USA 2005, 102, 8905. [Google Scholar] [CrossRef]
- Cuozzo, C.; Porcellini, A.; Angrisano, T.; Morano, A.; Lee, B.; di Pardo, A.; Messina, S.; Iuliano, R.; Fusco, A.; Santillo, M.R.; et al. DNA Damage, Homology-Directed Repair, and DNA Methylation. PLoS Genet. 2007, 3, 1144–1162. [Google Scholar] [CrossRef]
- Lee, E.W.; D’alonzo, G.E. Cigarette Smoking, Nicotine Addiction, and Its Pharmacologic Treatment. Arch. Intern. Med. 1993, 153, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Landen, S.; Voisin, S.; Craig, J.M.; McGee, S.L.; Lamon, S.; Eynon, N. Genetic and Epigenetic Sex-Specific Adaptations to Endurance Exercise. Epigenetics 2019, 14, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Grzywacz, A.; Barczak, W.; Chmielowiec, J.; Chmielowiec, K.; Suchanecka, A.; Trybek, G.; Masiak, J.; Jagielski, P.; Grocholewicz, K.; Rubiś, B. Contribution of Dopamine Transporter Gene Methylation Status to Cannabis Dependency. Brain Sci. 2020, 10, 400. [Google Scholar] [CrossRef]
- Michałowska-Sawczyn, M.; Grzywacz, A.; Masiak, J.; Chmielowiec, K.; Chmielowiec, J.; Chycki, J.; MacUlewicz, E.; Ciȩszczyk, P. Associations Between Physical Effort and DNA Methylation in the Promotor Region of the Dopamine Transporter Gene (DAT1). J. Hum. Kinet. 2021, 77, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, Y.; Li, Y.; Deng, Y.; Yuan, J.; Lv, R.; Wang, Y.; Zhang, G.; Guo, Z.; Fu, D.; et al. Availability of Dopamine Transporters in Heroin-Dependent Subjects: A 18F-FECNT PET Imaging Study. Psychiatry Res. Neuroimaging 2017, 263, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Grover, T.; Gupta, R.; Arora, G.; Bal, C.S.; Ambekar, A.; Basu Ray, S.; Vaswani, M.; Sharma, A. Dopamine Transporter Availability in Alcohol and Opioid Dependent Subjects—A 99mTc-TRODAT-1SPECT Imaging and Genetic Association Study. Psychiatry Res. Neuroimaging 2020, 305, 111187. [Google Scholar] [CrossRef]
- Mercer, B.A.; Wallace, A.M.; Brinckerhoff, C.E.; D’Armiento, J.M. Identification of a Cigarette Smoke–Responsive Region in the Distal MMP-1 Promoter. Am. J. Respir. Cell Mol. Biol. 2009, 40, 4. [Google Scholar] [CrossRef]
- Di, Y.P.; Zhao, J.; Harper, R. Cigarette Smoke Induces MUC5AC Protein Expression through the Activation of Sp1. J. Biol. Chem. 2012, 287, 27948. [Google Scholar] [CrossRef]
- Kadonaga, J.T.; Carner, K.R.; Masiarz, F.R.; Tjian, R. Isolation of CDNA Encoding Transcription Factor Sp1 and Functional Analysis of the DNA Binding Domain. Cell 1987, 51, 1079–1090. [Google Scholar] [CrossRef]
- Green, A.L.; Hossain, M.M.; Tee, S.C.; Zarbl, H.; Guo, G.L.; Richardson, J.R. Epigenetic Regulation of Dopamine Transporter MRNA Expression in Human Neuroblastoma Cells. Neurochem. Res. 2015, 40, 1372–1378. [Google Scholar] [CrossRef]
- Abdelkhalek, K.; Rhein, M.; Deest, M.; Buchholz, V.; Bleich, S.; Lichtinghagen, R.; Vyssoki, B.; Frieling, H.; Muschler, M.; Proskynitopoulos, P.J.; et al. Dysregulated Methylation Patterns in Exon IV of the Brain-Derived Neurotrophic Factor (BDNF) Gene in Nicotine Dependence and Changes in BDNF Plasma Levels During Smoking Cessation. Front. Psychiatry 2022, 13, 897801. [Google Scholar] [CrossRef] [PubMed]
- Halit, C.; Elif, Y.N.; Hasan, M.; Sacide, P.; Yasemin, O.; Deniz, D.; Mehmet, A. Methylation of APC2, NR3C1, and DRD2 Gene Promoters in Turkish Patients with Tobacco Use Disorder. Niger. J. Clin. Pract. 2022, 25, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. Carbon Monoxide Poisoning: Mechanisms, Presentation, and Controversies in Management. J. Emerg. Med. 1984, 1, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, L.; Zhao, Y.; Zhang, J.; Wang, D.; Chen, J.; He, Y.; Wu, J.; Zhang, Z.; Liu, Z. Hypoxia Induces Genomic DNA Demethylation through the Activation of HIF-1α and Transcriptional Upregulation of MAT2A in Hepatoma Cells. Mol. Cancer Ther. 2011, 10, 1113–1123. [Google Scholar] [CrossRef]
- Boron, A. Epigenetic Impact of the Parents’ Physical Activity on the Health of Their Children. Balt. J. Health Phys. Act. 2022, 13, 11. [Google Scholar] [CrossRef]
- Jasiewicz, A.; Rubiś, B.; Samochowiec, J.; Małecka, I.; Suchanecka, A.; Jabłoński, M.; Grzywacz, A. DAT1 Methylation Changes in Alcohol-Dependent Individuals vs. Controls. J. Psychiatr. Res. 2015, 64, 130–133. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).