Abstract
Human gut microbiota seems to drive the interaction with host metabolism through microbial metabolites, enzymes, and bioactive compounds. These components determine the host health–disease balance. Recent metabolomics and combined metabolome–microbiome studies have helped to elucidate how these substances could differentially affect the individual host pathophysiology according to several factors and cumulative exposures, such as obesogenic xenobiotics. The present work aims to investigate and interpret newly compiled data from metabolomics and microbiota composition studies, comparing controls with patients suffering from metabolic-related diseases (diabetes, obesity, metabolic syndrome, liver and cardiovascular diseases, etc.). The results showed, first, a differential composition of the most represented genera in healthy individuals compared to patients with metabolic diseases. Second, the analysis of the metabolite counts exhibited a differential composition of bacterial genera in disease compared to health status. Third, qualitative metabolite analysis revealed relevant information about the chemical nature of metabolites related to disease and/or health status. Key microbial genera were commonly considered overrepresented in healthy individuals together with specific metabolites, e.g., Faecalibacterium and phosphatidylethanolamine; and the opposite, Escherichia and Phosphatidic Acid, which is converted into the intermediate Cytidine Diphosphate Diacylglycerol-diacylglycerol (CDP-DAG), were overrepresented in metabolic-related disease patients. However, it was not possible to associate most specific microbiota taxa and metabolites according to their increased and decreased profiles analyzed with health or disease. Interestingly, positive association of essential amino acids with the genera Bacteroides were observed in a cluster related to health, and conversely, benzene derivatives and lipidic metabolites were related to the genera Clostridium, Roseburia, Blautia, and Oscillibacter in a disease cluster. More studies are needed to elucidate the microbiota species and their corresponding metabolites that are key in promoting health or disease status. Moreover, we propose that greater attention should be paid to biliary acids and to microbiota–liver cometabolites and its detoxification enzymes and pathways.
1. Introduction
Gut microbiota is considered a complex ecosystem with a wide array of microorganisms linked to host health. Multiple studies suggested that the structure and composition of the gut microbiota in metabolic-related diseases, such as atherosclerosis, colitis, diabetes, hyperlipidemia, hypertension, metabolic syndrome, non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), obesity, and steatosis, exhibit significant changes compared to healthy individuals and that those changes are related to host physiopathology. In this context, the analysis and description of trends in microbial populations associated with disease and health status become a key issue to elucidate possible signatures of metabolic-related diseases.
The gut microbiota of patients with metabolic-related diseases shows differences at different taxonomic levels. Many studies showed that Parabacteroides, Bifidobacterium, Oscillospira, and Bacteroides were decreased in patients with obesity [1,2,3,4,5,6,7,8,9,10,11,12,13]. Moreover, Faecalibacterium and Bifidobacterium were decreased [14,15,16,17,18,19,20,21] and species from Lactobacillaceae family [22] and Blautia were increased [7,13,19,20,21,22,23,24,25,26,27] in diabetic patients. Other metabolic diseases related to intestinal diseases seem to be related to increased Escherichia and decreased Faecalibacterium [28,29,30,31,32,33,34,35,36,37].
Recently, the combination of metagenomics and metabolomics has received extensive attention due to the growing number of studies that establish positive and negative correlations between gut microbiota taxa, metabolites, and health status. Therefore, future studies will contribute to elucidate the essential role of gut microbiota in metabolite synthesis, metabolite modifications, and metabolic pathway regulations.
In this sense, metabolites such as short-chain fatty acids (SCFA), amino acids (AA), or bile acids (BA) can play a crucial role in maintaining metabolic functions or, on the contrary, they might be involved in disease development, such as choline derivatives in the case of cardiovascular diseases [38,39,40,41]. Metabolite influences are not restricted to the intestine and distribution to other physiological locations has been described through different axes, such as the gut–liver axis, in which the gut microbiota is related to liver diseases, including NAFLD, NASH, fibrosis, or liver cancer [42]. Gut microbiota partially impacts the host BA profile as it is involved in primary bile acid transformation into secondary free bile acids, such as deoxycholic acid, lithocholic acid, and ursodeoxycholic acid, contributing to the modulation of host total bile acid production [43].
The chemical structure of many endogenous compounds, including gut microbiota metabolites, can be modified, resulting in changes in their bioactivity and half-life [44]. This kind of modifications are related to the development of complex metabolic networks between host and gut microbiota, where final substances could be potentially more toxic than the original ones [45].
Traditional probiotics, mainly consisting of species from Lactobacillaceae and Bifidobacteria and a few from other genera, have been largely applied as a useful strategy in the context of clinical intervention in metabolic-related diseases [46,47]. However, the development of new procedures using Next Generation Probiotics (NGP) opens a new world of possibilities due to the beneficial effects that have already been described in murine models and, to a lesser extent, in humans. In this context, murine models show Akkermansia muciniphila, Faecalibacterium prausnitzii, Bacteroides uniformis, Bacteroides acidifaciens, Clostridium butyricum, and Prevotella copri as interesting microorganisms with potential applications in obesity [48,49,50,51,52,53], liver diseases [52,54,55,56,57,58,59], diabetes [48,49,50,51,52,53,58,60,61], colitis [62], and hyperlipidemia [53,58].
This work will contribute to finding out microbial and metabolite patterns and their correlation with diseases that have been studied independently or not yet extensively studied. Therefore, the principal aim of this work is to identify and describe the association between human gut microbiota taxa changes in metabolic-related diseases, incorporating the correlations with metabolites, and how they can modulate host health.
2. Results
2.1. Differential Microbiota Taxa Composition and Stratification According to Their Representation in Metabolic Diseases
2.1.1. PRISMA Analysis
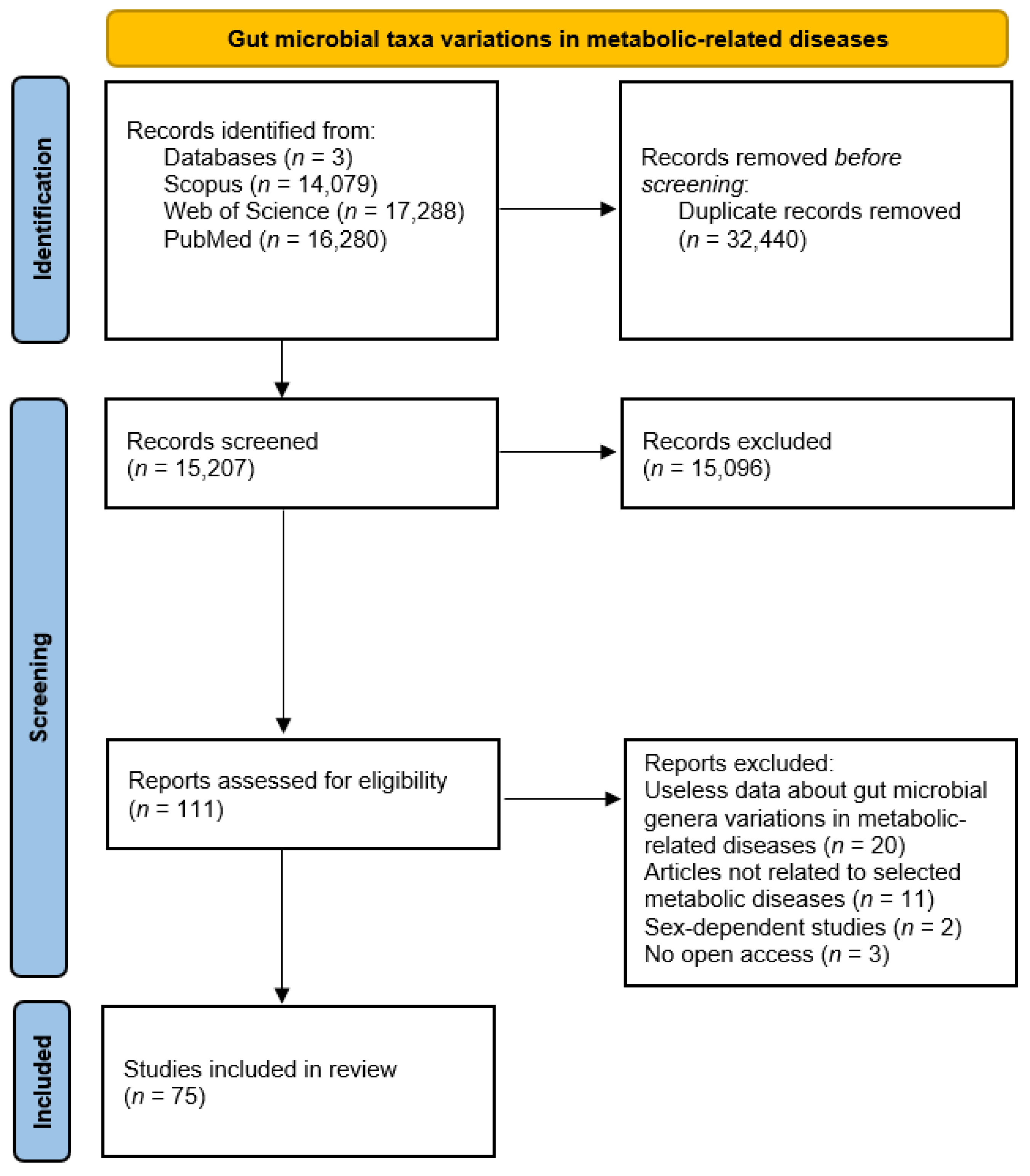
Gut microbial taxa differences in diabetes, obesity, metabolic syndrome, and liver and cardiovascular diseases, highlight links between gut microbiota and host health status. In this context, Figure 1 summarizes updated and available information about gut microbial taxa changes in these metabolic-related diseases.
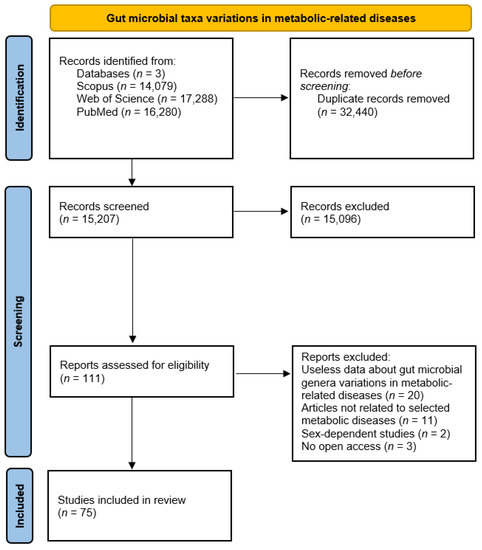
Figure 1.
PRISMA diagram for gut microbial taxa changes in metabolic diseases.
2.1.2. Microbial Taxa Decreased in Patients Suffering from Metabolic-Related Diseases
Increased and decreased trends in gut microbiota taxa were assessed through an extensive literature search including information about metabolic diseases investigated by different authors. In this context, the approach we followed offered some drivers of specific changes in gut microbiota composition that could be related to host health.
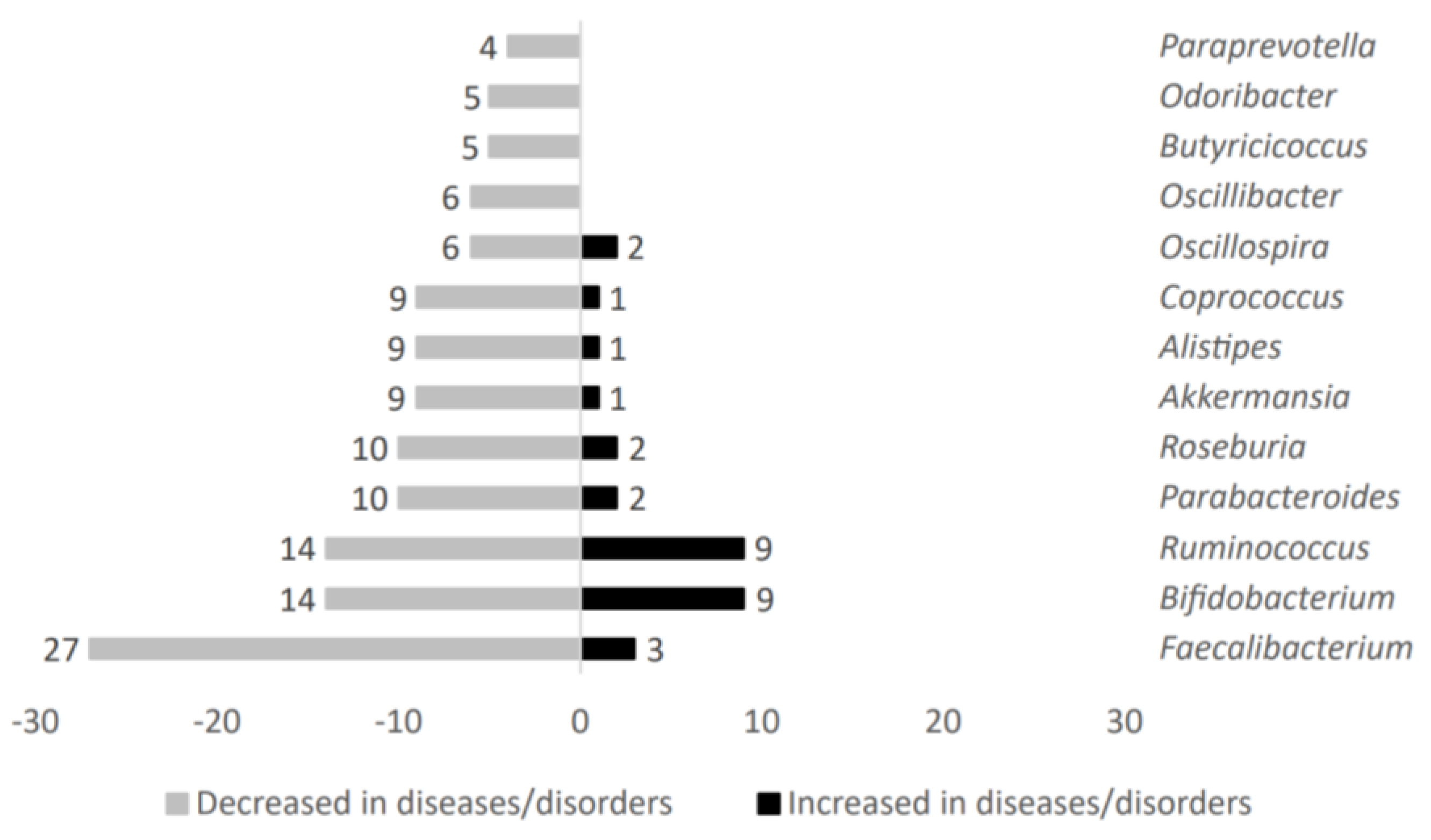
The analysis of 75 studies involving changes of the main taxa altered in patients suffering metabolic-related diseases disclosed 121 differentially abundant microbial genera (complete data are available in Supplementary Material S1). Figure 2 shows representative genera count value comparison obtained in metabolic diseases after microbial taxa variation analysis.
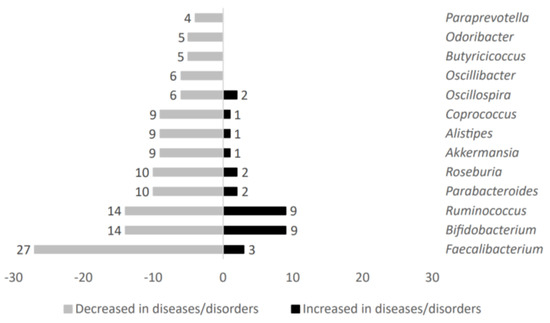
Figure 2.
Analysis of main taxa stratified according to high representativeness in patients without metabolic-related diseases.
Gut microbiota genera such as Oscillibacter, Butyricicoccus, Odoribacter, and Paraprevotella were exclusively decreased in individuals affected by metabolic diseases. On the other hand, Faecalibacterium, Bifidobacterium, Ruminococcus, Parabacteroides, Roseburia, Akkermansia, Alistipes, Coprococcus, and Oscillospira were both decreased and increased in metabolic-related diseases. However, overall, these microbial genera showed a negative association with the metabolic diseases studied here.
2.1.3. Microbial Taxa Increased in Patients Suffering Metabolic-Related Diseases
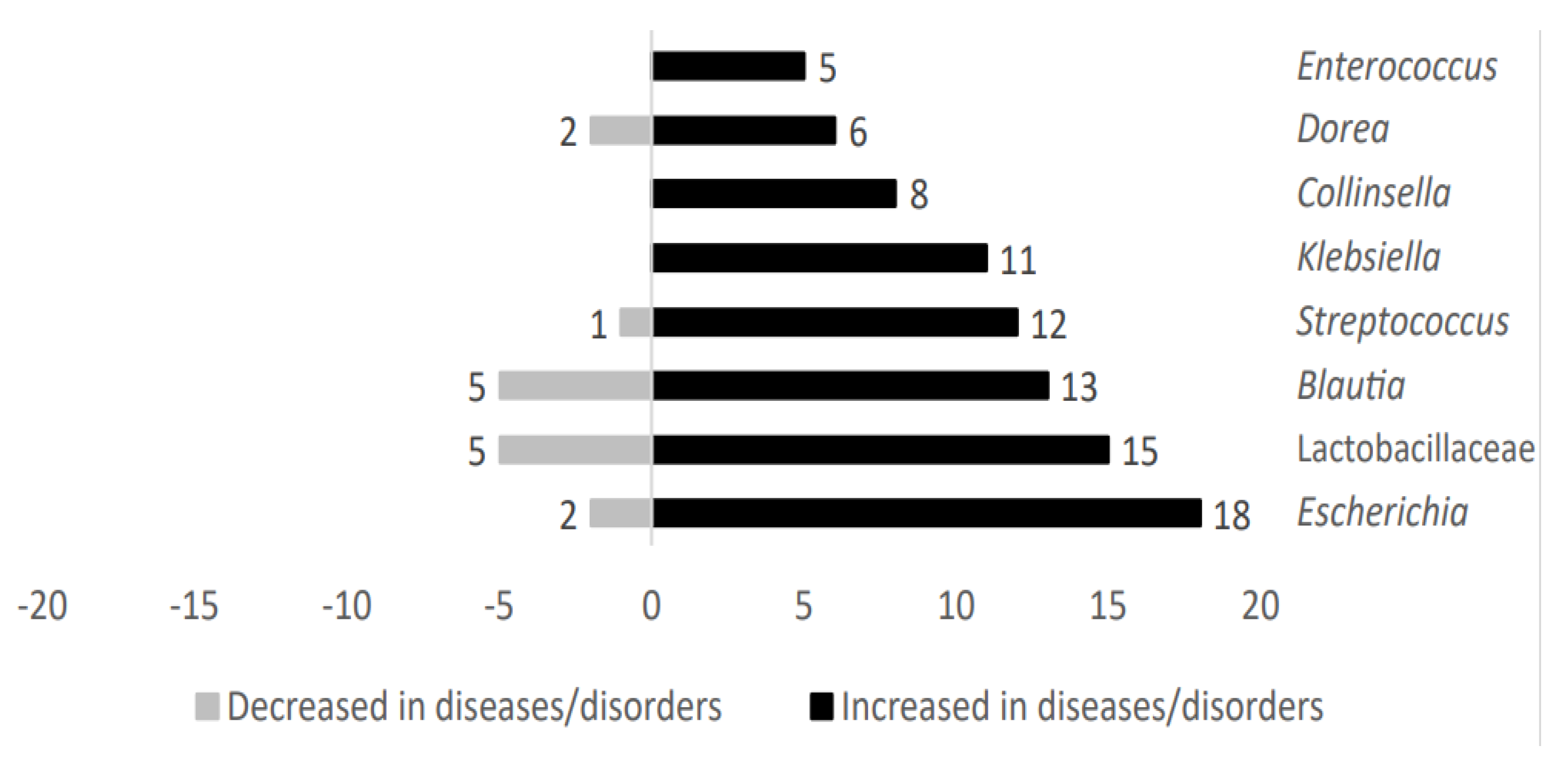
Microbial genera such as Klebsiella, Collinsella, and Enterococcus were exclusively present in those cases in which individuals were affected by metabolic diseases. However, taxa belonging to Escherichia, Lactobacillaceae, Blautia, Streptococcus, and Dorea were also identified in patients without metabolic-related diseases. These microbial genera showed an upward trend in metabolic-related diseases studied here. Figure 3 shows the distribution of representative microbial taxa linked to metabolic-related diseases.
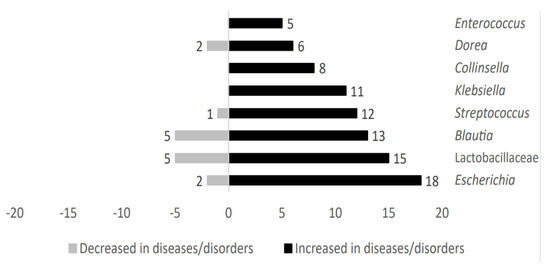
Figure 3.
Analysis of main taxa stratified according to high representativeness in metabolic−related diseases patients.
In a previous study exploring next generation probiotics for metabolic and microbiota dysbiosis linked to xenobiotic exposure [63], we tried the first approach to describe changes in gut microbial taxa associated to metabolic-related disease. As a result, potential associations between bacterial genera and metabolic diseases were described despite the lesser number of analyzed studies. In this case, Table 1 shows an expansion of the current knowledge available in this field, including the relevant information identified in the previous study.

Table 1.
Changes in the main microbiota taxa found in patients suffering metabolic-related diseases.
2.2. Differential Microbial Metabolites and Stratification According to Their Representation in Metabolic Diseases
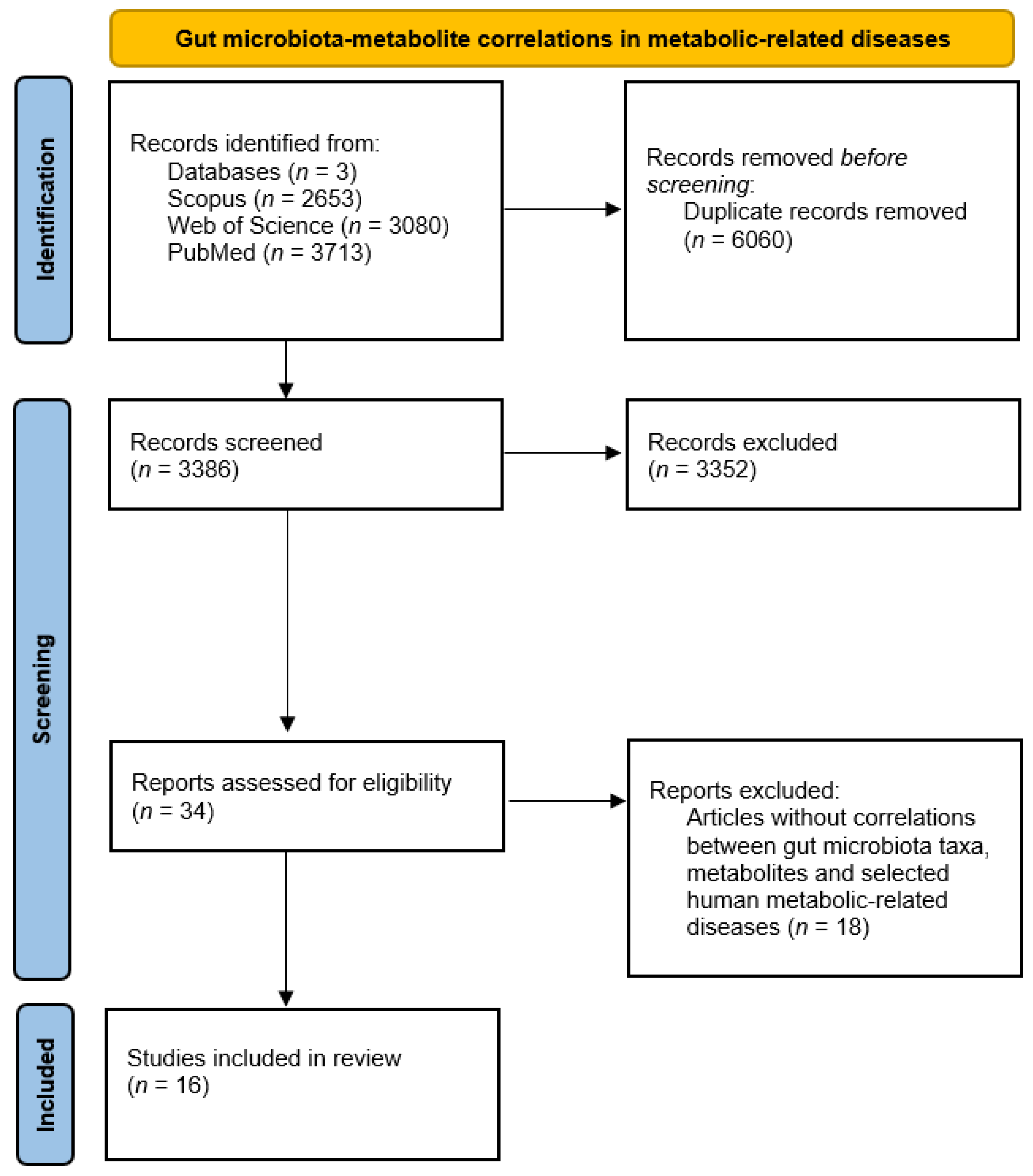
The analysis of the 16 selected studies involving correlations between gut microbiota taxa altered in patients suffering from metabolic diseases, metabolites, and host health status allowed us to shed light on potential critical pathways to modulate homeostatic processes (complete data are available in Supplementary Material S2 [103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118] Figure 4 summarizes available information about gut microbiota–metabolite correlations and host health status.
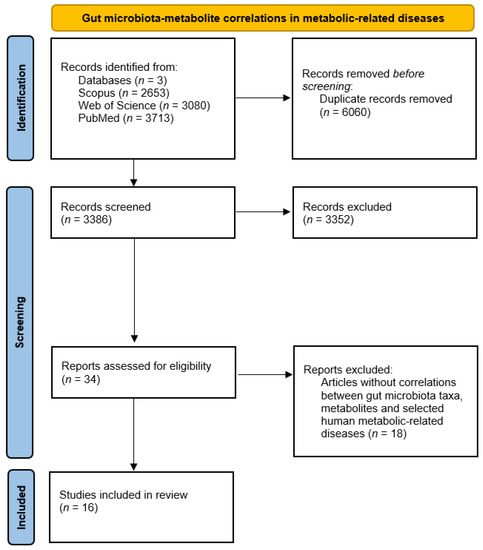
Figure 4.
PRISMA diagram for gut microbiota–metabolite correlations and host status.
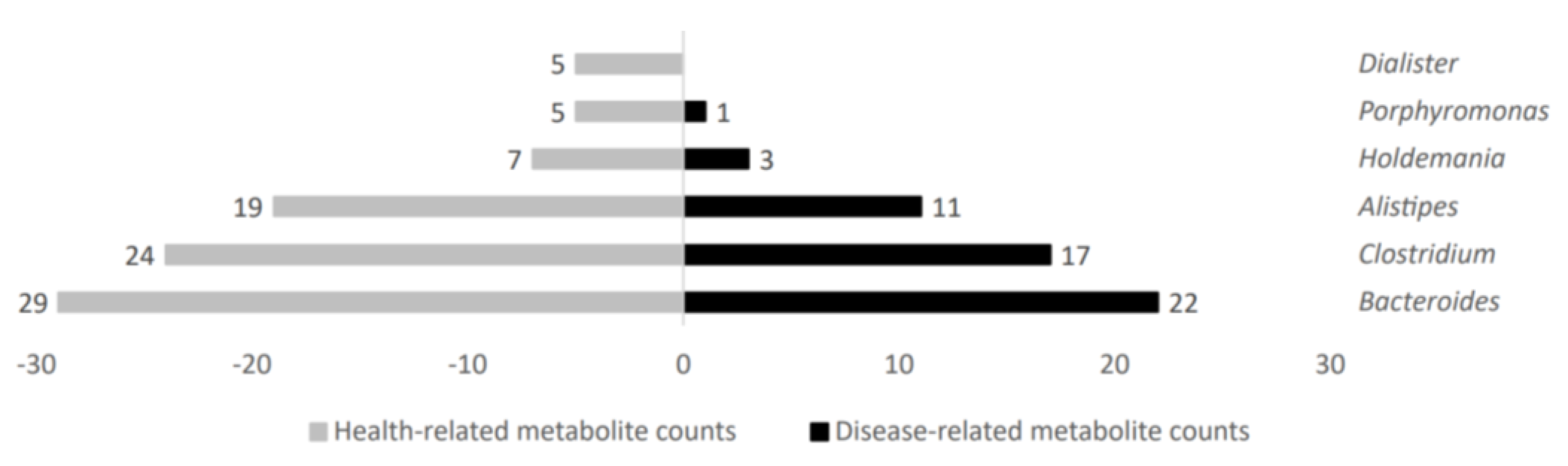
Several gut microbiota taxa showed a high metabolite count linked to disease or health status. In that regard, increased microbial metabolite counts in health status were obtained in gut microbiota genera such as Holdemania, Porphyromonas, and Dialister; further, they were also higher for Bacteroides, Clostridium, and Alistipes, but with more similar counts in both groups. Figure 5 shows representative genera differential values associated to health-related metabolite count analysis.
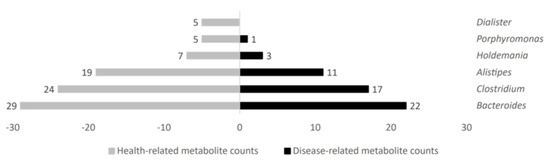
Figure 5.
Health−related metabolite counts stratified according to gut microbiota taxa producers.
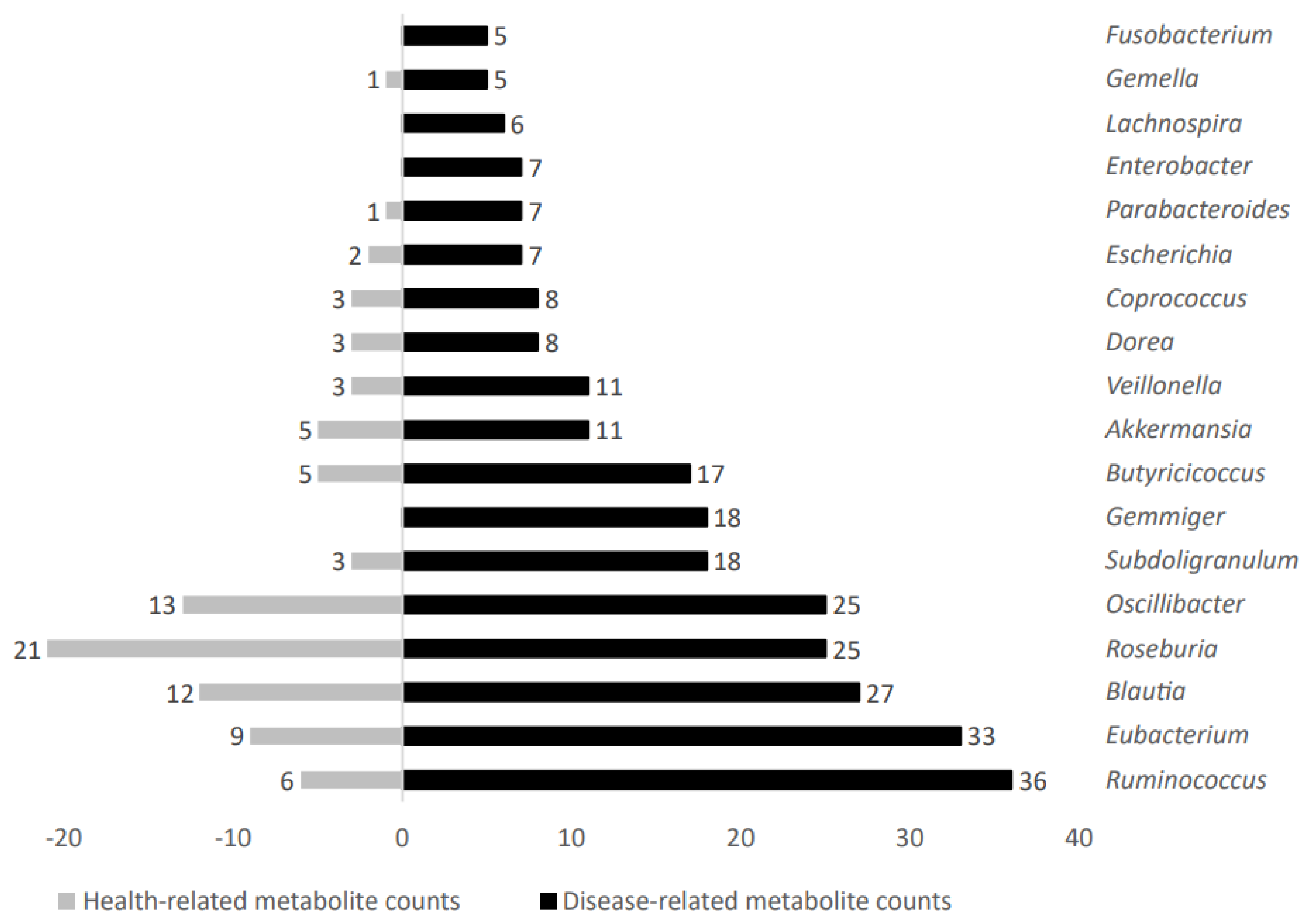
Increased metabolite counts related to disease status were linked to gut microbiota taxa such as Ruminococcus, Eubacterium, Blautia, Roseburia, Oscillibacter, Subdoligranulum, Gemmiger, Butyricicoccus, Akkermansia, Veillonella, Dorea, Coprococcus, Escherichia, Parabacteroides, Enterobacter, Lachnospira, Gemella, and Fusobacterium. Figure 6 shows representative genera differential values associated to disease-related metabolite count analysis.
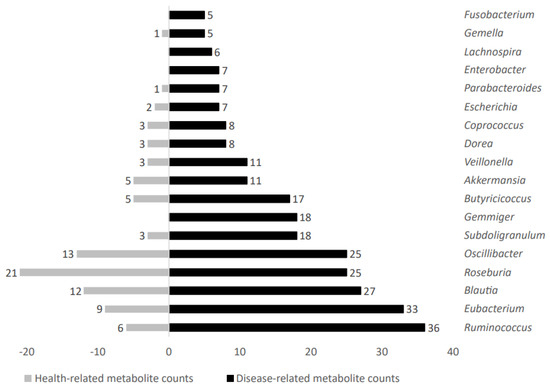
Figure 6.
Disease−related metabolite counts stratified according to gut microbiota taxa producers.
According to the total metabolites linked to disease and health status, 171 metabolites were associated with metabolic-related diseases; among these, 143 were exclusively associated with this group and 28 were shared with health status. Moreover, 63 metabolites were related to health status, and 35 were exclusively associated with this group. A qualitative metabolite analysis was performed considering total disease/health-related metabolites. Table 2 shows disease/health-related metabolites classified according to three main chemical groups: fatty acids and conjugates, amino acids and derivatives, and bile acids and derivatives.

Table 2.
Disease/health-related metabolites and chemical classification.
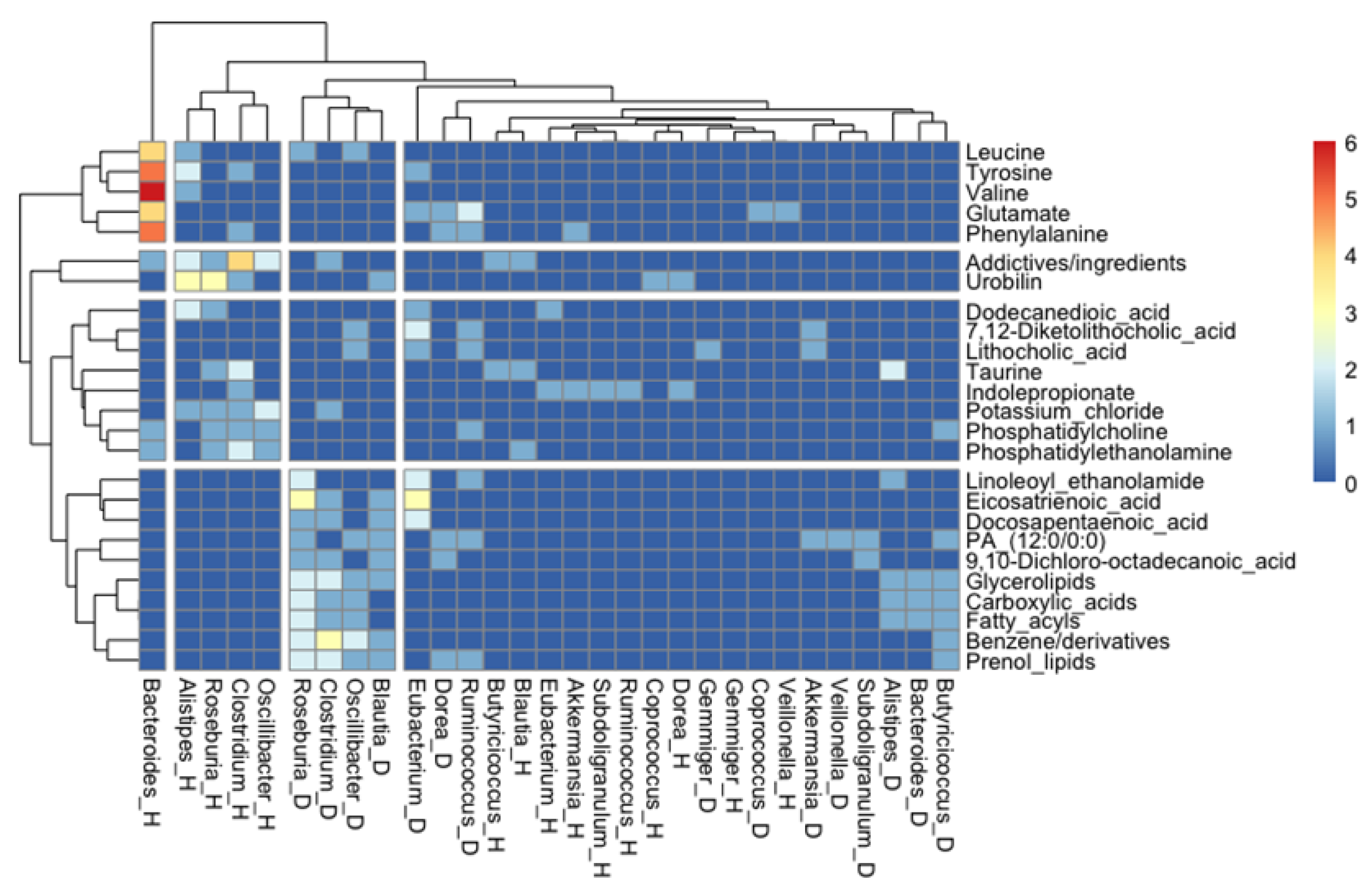
A further association analysis of the number of studies where a specific association between a metabolite and a bacterial genus was found showed very interesting clustering patterns. For instance, butyrate-producer genera when present in a healthy status associated with bile acid metabolites and, to a lesser extent, with essential amino acids; however, when they are overrepresented in metabolic diseases, they are associated with lipid metabolism, clustering in two distinct groups. We also observed that essential amino acids clustered together, and they might have an important role for the metabolism of Bacteroides in health status, according to Figure 7.
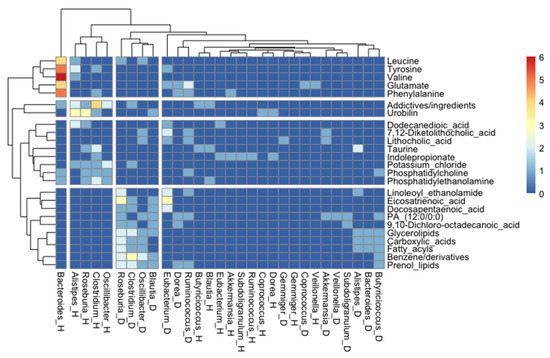
Figure 7.
Heatmap showing the analysis where specific associations between a metabolite and a bacterial genus was found in a health and/or a disease stage (as indicated by “_H” or “_D”, respectively). For simplicity, only the representative genera and the most found metabolites were included.
3. Materials and Methods
We performed a comprehensive literature search covering the period from 1995 to November 2022 using Scopus, Web of Science, and PubMed databases, using the search strategies showed in systematic review and dividing this review into two main study issues: gut microbial taxa variations in metabolic-related diseases and gut microbiota–metabolite correlations in metabolic-related diseases.
Studies involving changes in gut microbial taxa in atherosclerosis, colitis, diabetes, hyperlipidemia, hypertension, metabolic syndrome, NAFLD, NASH, obesity, and steatosis and studies involving microbiota–metabolite correlations in metabolic-related diseases were assessed, screened, and selected according to PRISMA 2020 flow diagrams (Figure 1 and Figure 4) [111].
In the microbial taxa variation analysis, gut microbial taxa identified in selected studies were divided into two groups: decreased in metabolic-related diseases and increased in metabolic-related diseases, based on research findings. Metabolite counts were calculated for each microbial genus. To determine representative gut microbiota taxa, an arbitrary criterion was applied. Microbial genera were considered representative if the absolute frequency difference between decreased–increased counts was greater than three.
In the gut microbiota–metabolite correlation analysis, gut microbiota, microbial metabolites, and host status correlations were assessed. First, gut microbial genera were classified into increased in health status or increased in diseases, according to metabolite absolute frequencies displayed for each genus. Second, considering metabolites related to representative genera in health or disease status, a qualitative metabolite analysis was performed. Metabolites correlated with health or disease status were classified into three main groups: fatty acids and conjugates (FA), amino acids and derivatives (AA), and bile acids and derivatives (BA), according to PubChem and related chemical database classification. Furthermore, a bioinformatics analysis was performed to establish potential biomarkers, which revealed the association between specific disease/health balances. Heatmap shows the analysis where a specific association between a metabolite and bacterial genera was found in a health and/or a disease stage (as indicated by “_H” or “_D”, respectively). For simplicity, only the representative genera and the most found metabolites (metabolites that appeared least five times either associated with health or disease in the studies analyzed here) were included. First, we selected only the genera with more than 10 metabolites associated and then we kept only the metabolites that appeared at least five times, either associated with health or disease, in the studies analyzed here. Figure 7 shows the performance of R (version 4.1.1.) using the package “pheatmap” [112].
4. Discussion
There is a growing interest in the analysis of the gut microbiome and its metabolome [113,114]. However, integrating data from both fields to understand how gut microbiota, microbial metabolites, and host status are correlated not always provide concise information. Thus, it can hinder researchers in establishing clear links between the presence of a particular gut bacterial taxa and/or metabolites and disease or health status. This task is especially challenging in the context of searching gut microbial biomarkers that allow predicting future phenotypes or classifying individuals into disease and non-disease status. This is mainly due to the fact that contradictory results about microbial taxa abundance and metabolites related to disease or non-disease status can be found in the literature. In this case, this approach showed that Faecalibacterium, Bifidobacterium, Ruminococcus, Parabacteroides, Roseburia, Akkermansia, Alistipes, Coprococcus, Oscillospira, Oscillibacter, Butyricicoccus, Odoribacter, and Paraprevotella could represent a downregulated microbial cluster in metabolic-related disease patients and, on the contrary, Escherichia, species from Lactobacillaceae family, Blautia, Streptococcus, Klebsiella, Collinsella, Dorea, and Enterococcus cluster upregulation could be involved in metabolic-related disease status. Due to relevant information underlined by many authors and results obtained in this review, Ruminococcus and Bifidobacterium, as well as taxa belonging to Lactobacillaceae family, Blautia, and Dorea should be identified at the species level to establish similarities with the results already available in the microbiological databases.
According to metabolite absolute frequencies in disease and health status and representative gut microbiota taxa, we tried to search for possible trends between those elements and host physiopathology. When we compared representative metabolites and microbial taxa results, only Alistipes, from the down-regulated proposed cluster, showed high counts in both gut microbial taxa variation analysis and metabolite count analysis related to health. In the same way, Escherichia, Blautia, Streptococcus, Collinsella, Dorea, and Enterococcus, from the proposed upregulated cluster, showed high counts in both gut microbial taxa analysis and metabolite count analysis in disease/disorder group.
Following this approach, Faecalibacterium and Akkermansia genera [115,116], frequently described as key microorganisms related to health status, were decreased in metabolic-related diseases, indicating a possible relationship with health status. However, a link with disease status could be identified according to metabolite absolute frequencies described for both genera Faecalibacterium and Akkermansia. A similar result can be observed in other microorganisms frequently associated with metabolic diseases [117], where microbial taxa analysis showed links with obesity-related diseases. However, metabolite absolute counts showed links with health status.
Interestingly, preliminary data results derived from the biomarker search have demonstrated the positive association of essential amino acids with health in the genera Bacteroides, and conversely, benzene derivatives have been related to disease and the genera Clostridium. We also observed that lipid metabolites grouped several taxa overrepresented in diseases, but it will be necessary to determine the results to the species level.
These results showed which bacterial taxa of the gut microbiota and their derived metabolites could be related to host status manifestations. However, study limitations and lack of available data in some fields make it impossible to establish final and solid conclusions in this way.
Human health is not only affected by gut microbiota composition and its derived metabolites but also many exogenous and endogenous factors, which can also impact in genotypic and phenotypic manifestations. Recently, the holistic concept of the One Health approach and the exposome include multidisciplinary analysis of a complex reality that affect different but linked items [118]. Nowadays, solid evidence about specific microbial and metabolite signatures in cases of metabolic-related disease is still limited and more concrete information on the correlations between gut microbiota, gut metabolites, and host health status is needed. This synergic approach will lead to a better management of well-known microbiota–metabolic related diseases.
To increase the availability of scientific data on the interaction between gut microbiota taxa in different health contexts, metabolite synthesis, and metabolite modification and impact on the host health, integrated metagenome and metabolome analysis should be continually reviewed, since it seems to be a possible cornerstone involved in the determination of potential microbial and metabolite signatures related to physiological alterations.
5. Conclusions
Despite the existence of microbial taxa–metabolite-health correlations, there is no evidence of a clear gut microbiota and derived metabolite patterns into healthy or metabolic-related disease status that is able to predict or classify patients into one or the other.
Most of the taxa and metabolites did not show representative oscillations between disease and health groups, so bacterial genera with potential interest should continue to be monitored as new information on their abundance in metabolic-related disease appearance.
Implementation of the One Health holistic approach combined with exposome principles can provide new perspectives and evidence about how endogenous and exogenous substances interact with gut microbiota and microbial-derived substances and how the pull of interactions finally affects human homeostasis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24054519/s1.
Author Contributions
Conceptualization, A.T.-S. and M.A.; Methodology, A.T.-S.; Writing—Original Draft Preparation, A.T.-S.; Writing—Review and Editing, P.O., A.R.-R. and M.A.; Supervision, A.R.-R. and M.A.; Project Administration, M.A.; Funding Acquisition, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out within the framework of the project and in the framework of several projects: FEDER Project Infrastructure: IE_2019-198; Junta de Andalucía Proyectos de Excelencia: Consejería de Universidad, Investigación e Innovación P21-00341 and the project Instituto de Salud Carlos III-PI20/01278. A.T.-S. holds a contract from FIBAO. A.R.-R. holds a Maria Zambrano Talent Grant (Next Generation EU-University of Granada).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Part of the results are from the doctoral thesis of Alfonso Torres-Sánchez in the Nutrition and Food Technology Doctorate Programme of the University of Granada.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Duan, M.; Wang, Y.; Zhang, Q.; Zou, R.; Guo, M.; Zheng, H. Characteristics of Gut Microbiota in People with Obesity. PLoS ONE 2021, 16, e0255446. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.C.; Monteil, M.A.; Davis, E.M. Overweight and Obesity in Children Are Associated with an Abundance of Firmicutes and Reduction of Bifidobacterium in Their Gastrointestinal Microbiota. Child Obes 2020, 16, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, H.; Jiang, F.; Shen, Y.; Li, X.; Hu, X.; Shen, X.; Wei, P. Alteration of the Gut Microbiota Associated with Childhood Obesity by 16S RRNA Gene Sequencing. PeerJ 2020, 8, e8317. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Zhu, C.; Li, H.; Yin, M.; Pan, C.; Huang, L.; Kong, C.; Wang, X.; Zhang, Y.; Qu, S.; et al. Dysbiosis Signatures of Gut Microbiota Along the Sequence from Healthy, Young Patients to Those with Overweight and Obesity. Obesity 2018, 26, 351–361. [Google Scholar] [CrossRef]
- Hou, Y.-P.; He, Q.-Q.; Ouyang, H.-M.; Peng, H.-S.; Wang, Q.; Li, J.; Lv, X.-F.; Zheng, Y.-N.; Li, S.-C.; Liu, H.-L.; et al. Human Gut Microbiota Associated with Obesity in Chinese Children and Adolescents. BioMed Res. Int. 2017, 2017, 7585989. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Borgo, F.; Lassandro, C.; Verduci, E.; Morace, G.; Borghi, E.; Berry, D. Pediatric Obesity Is Associated with an Altered Gut Microbiota and Discordant Shifts in Firmicutes Populations. Environ. Microbiol. 2017, 19, 95–105. [Google Scholar] [CrossRef]
- Kashtanova, D.A.; Tkacheva, O.N.; Doudinskaya, E.N.; Strazhesko, I.D.; Kotovskaya, Y.V.; Popenko, A.S.; Tyakht, A.V.; Alexeev, D.G. Gut Microbiota in Patients with Different Metabolic Statuses: Moscow Study. Microorganisms 2018, 6, E98. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, J.; Liu, J.; Wang, Z.; Chen, M.; Zhou, S. Metagenome of Gut Microbiota of Children with Nonalcoholic Fatty Liver Disease. Front. Pediatr. 2019, 7, 518. [Google Scholar] [CrossRef]
- Nistal, E.; Sáenz de Miera, L.E.; Ballesteros Pomar, M.; Sánchez-Campos, S.; García-Mediavilla, M.V.; Álvarez-Cuenllas, B.; Linares, P.; Olcoz, J.L.; Arias-Loste, M.T.; García-Lobo, J.M.; et al. An Altered Fecal Microbiota Profile in Patients with Non-Alcoholic Fatty Liver Disease (NAFLD) Associated with Obesity. Rev. Esp. Enferm. Dig. 2019, 111, 275–282. [Google Scholar] [CrossRef]
- Del Chierico, F.; Nobili, V.; Vernocchi, P.; Russo, A.; De Stefanis, C.; Gnani, D.; Furlanello, C.; Zandonà, A.; Paci, P.; Capuani, G.; et al. Gut Microbiota Profiling of Pediatric Nonalcoholic Fatty Liver Disease and Obese Patients Unveiled by an Integrated Meta-Omics-Based Approach. Hepatology 2017, 65, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.M.B.; Stefano, J.T.; Miele, L.; Ponziani, F.R.; Souza-Basqueira, M.; Okada, L.S.R.R.; de Barros Costa, F.G.; Toda, K.; Mazo, D.F.C.; Sabino, E.C.; et al. Gut Microbiome Composition in Lean Patients with NASH Is Associated with Liver Damage Independent of Caloric Intake: A Prospective Pilot Study. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martín, A.; Iglesias-Aguirre, C.E.; Meoro, A.; Selma, M.V.; Espín, J.C. There Is No Distinctive Gut Microbiota Signature in the Metabolic Syndrome: Contribution of Cardiovascular Disease Risk Factors and Associated Medication. Microorganisms 2020, 8, 416. [Google Scholar] [CrossRef]
- Lippert, K.; Kedenko, L.; Antonielli, L.; Kedenko, I.; Gemeier, C.; Leitner, M.; Kautzky-Willer, A.; Paulweber, B.; Hackl, E. Gut Microbiota Dysbiosis Associated with Glucose Metabolism Disorders and the Metabolic Syndrome in Older Adults. Benef. Microbes 2017, 8, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Thingholm, L.B.; Rühlemann, M.C.; Koch, M.; Fuqua, B.; Laucke, G.; Boehm, R.; Bang, C.; Franzosa, E.A.; Hübenthal, M.; Rahnavard, A.; et al. Obese Individuals with and without Type 2 Diabetes Show Different Gut Microbial Functional Capacity and Composition. Cell Host Microbe 2019, 26, 252–264.e10. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, Z.; Xiong, X.; Chen, X.; Peng, J.; Yao, H.; Pu, J.; Chen, Q.; Zheng, M. Gut Microbiota Composition and Fecal Metabolic Profiling in Patients with Diabetic Retinopathy. Front. Cell Dev. Biol. 2021, 9, 2684. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Yang, Y.; Wang, Z.; Wang, B.; Zhang, B.; Yu, J.; Lu, W.; Pan, M.; Zhao, J.; et al. The Diversity of Gut Microbiota in Type 2 Diabetes with or without Cognitive Impairment. Aging Clin. Exp. Res. 2021, 33, 589–601. [Google Scholar] [CrossRef]
- Li, Q.; Chang, Y.; Zhang, K.; Chen, H.; Tao, S.; Zhang, Z. Implication of the Gut Microbiome Composition of Type 2 Diabetic Patients from Northern China. Sci. Rep. 2020, 10, 5450. [Google Scholar] [CrossRef]
- Navab-Moghadam, F.; Sedighi, M.; Khamseh, M.E.; Alaei-Shahmiri, F.; Talebi, M.; Razavi, S.; Amirmozafari, N. The Association of Type II Diabetes with Gut Microbiota Composition. Microb. Pathog. 2017, 110, 630–636. [Google Scholar] [CrossRef]
- Sedighi, M.; Razavi, S.; Navab-Moghadam, F.; Khamseh, M.E.; Alaei-Shahmiri, F.; Mehrtash, A.; Amirmozafari, N. Comparison of Gut Microbiota in Adult Patients with Type 2 Diabetes and Healthy Individuals. Microb. Pathog. 2017, 111, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.; Hoseini-Tavassol, Z.; Khatami, S.; Zangeneh, M.; Behrouzi, A.; Badi, S.A.; Moshiri, A.; Hasani-Ranjbar, S.; Soroush, A.; Vaziri, F.; et al. Main Gut Bacterial Composition Differs between Patients with Type 1 and Type 2 Diabetes and Non-Diabetic Adults. J. Diabetes Metab. Disord. 2020, 19, 265–271. [Google Scholar] [CrossRef]
- Leiva-Gea, I.; Sánchez-Alcoholado, L.; Martín-Tejedor, B.; Castellano-Castillo, D.; Moreno-Indias, I.; Urda-Cardona, A.; Tinahones, F.J.; Fernández-García, J.C.; Queipo-Ortuño, M.I. Gut Microbiota Differs in Composition and Functionality between Children with Type 1 Diabetes and MODY2 and Healthy Control Subjects: A Case-Control Study. Diabetes Care 2018, 41, 2385–2395. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Wittouck, S.; Mattarelli, P.; Zheng, J.; Lebeer, S.; Felis, G.E.; Gänzle, M.G. After the Storm—Perspectives on the Taxonomy of Lactobacillaceae. JDS Commun. 2022, 3, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Sroka-Oleksiak, A.; Młodzińska, A.; Bulanda, M.; Salamon, D.; Major, P.; Stanek, M.; Gosiewski, T. Metagenomic Analysis of Duodenal Microbiota Reveals a Potential Biomarker of Dysbiosis in the Course of Obesity and Type 2 Diabetes: A Pilot Study. J. Clin. Med. 2020, 9, 369. [Google Scholar] [CrossRef] [PubMed]
- Gaike, A.H.; Paul, D.; Bhute, S.; Dhotre, D.P.; Pande, P.; Upadhyaya, S.; Reddy, Y.; Sampath, R.; Ghosh, D.; Chandraprabha, D.; et al. The Gut Microbial Diversity of Newly Diagnosed Diabetics but Not of Prediabetics Is Significantly Different from That of Healthy Nondiabetics. mSystems 2020, 5, e00578-19. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lou, H.; Peng, Y.; Chen, S.; Zhang, Y.; Li, X. Comprehensive Relationships between Gut Microbiome and Faecal Metabolome in Individuals with Type 2 Diabetes and Its Complications. Endocrine 2019, 66, 526–537. [Google Scholar] [CrossRef]
- Chen, P.-C.; Chien, Y.-W.; Yang, S.-C. The Alteration of Gut Microbiota in Newly Diagnosed Type 2 Diabetic Patients. Nutrition 2019, 63–64, 51–56. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.-W.; Shao, L.; Sun, S.-H.; Wu, J.; Song, Q.-H.; Zou, H.-S.; Ling, Z.-X. Gut Microbiota Dysbiosis in Chinese Children with Type 1 Diabetes Mellitus: An Observational Study. World J. Gastroenterol. 2021, 27, 2394–2414. [Google Scholar] [CrossRef]
- Ahmed, E.A.; Ahmed, S.M.; Zakaria, N.H.; Baddour, N.M.; Header, D.A. Estudio del microbioma intestinal en pacientes egipcios con colitis ulcerosa crónica idiopática. Rev. Gastroenterol. México 2022, 843, 1–10. [Google Scholar] [CrossRef]
- Zakerska-Banaszak, O.; Tomczak, H.; Gabryel, M.; Baturo, A.; Wolko, L.; Michalak, M.; Malinska, N.; Mankowska-Wierzbicka, D.; Eder, P.; Dobrowolska, A.; et al. Dysbiosis of Gut Microbiota in Polish Patients with Ulcerative Colitis: A Pilot Study. Sci. Rep. 2021, 11, 2166. [Google Scholar] [CrossRef]
- Dai, L.; Tang, Y.; Zhou, W.; Dang, Y.; Sun, Q.; Tang, Z.; Zhu, M.; Ji, G. Gut Microbiota and Related Metabolites Were Disturbed in Ulcerative Colitis and Partly Restored after Mesalamine Treatment. Front. Pharmacol. 2021, 11, 620724. [Google Scholar] [CrossRef]
- Knoll, R.L.; Forslund, K.; Kultima, J.R.; Meyer, C.U.; Kullmer, U.; Sunagawa, S.; Bork, P.; Gehring, S. Gut Microbiota Differs between Children with Inflammatory Bowel Disease and Healthy Siblings in Taxonomic and Functional Composition: A Metagenomic Analysis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 312, G327–G339. [Google Scholar] [CrossRef] [PubMed]
- Heidarian, F.; Alebouyeh, M.; Shahrokh, S.; Balaii, H.; Zali, M.R. Altered Fecal Bacterial Composition Correlates with Disease Activity in Inflammatory Bowel Disease and the Extent of IL8 Induction. Curr. Res. Transl. Med. 2019, 67, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-Q.; Yu, T.-T.; Zhao, X.-J.; Zhang, Y.; Zhang, H.-J. Fecal Microbial Dysbiosis in Chinese Patients with Inflammatory Bowel Disease. World J. Gastroenterol. 2018, 24, 1464–1477. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut Microbiome Structure and Metabolic Activity in Inflammatory Bowel Disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Chang, T.-E.; Luo, J.-C.; Yang, U.-C.; Huang, Y.-H.; Hou, M.-C.; Lee, F.-Y. Fecal Microbiota Profile in Patients with Inflammatory Bowel Disease in Taiwan. J. Chin. Med. Assoc. 2021, 84, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Petito, V.; Graziani, C.; Schiavoni, E.; Paroni Sterbini, F.; Poscia, A.; Gaetani, E.; Franceschi, F.; Cammarota, G.; Sanguinetti, M.; et al. Gut Microbiota in Health, Diverticular Disease, Irritable Bowel Syndrome, and Inflammatory Bowel Diseases: Time for Microbial Marker of Gastrointestinal Disorders. Dig. Dis. 2018, 36, 56–65. [Google Scholar] [CrossRef]
- Budinska, E.; Gojda, J.; Heczkova, M.; Bratova, M.; Dankova, H.; Wohl, P.; Bastova, H.; Lanska, V.; Kostovcik, M.; Dastych, M.; et al. Microbiome and Metabolome Profiles Associated with Different Types of Short Bowel Syndrome: Implications for Treatment. J. Parenter. Enter. Nutr. 2020, 44, 105–118. [Google Scholar] [CrossRef]
- Ikeda, T.; Nishida, A.; Yamano, M.; Kimura, I. Short-Chain Fatty Acid Receptors and Gut Microbiota as Therapeutic Targets in Metabolic, Immune, and Neurological Diseases. Pharmacol. Ther. 2022, 239, 108273. [Google Scholar] [CrossRef]
- Su, X.; Gao, Y.; Yang, R. Gut Microbiota-Derived Tryptophan Metabolites Maintain Gut and Systemic Homeostasis. Cells 2022, 11, 2296. [Google Scholar] [CrossRef]
- Qian, B.; Zhang, K.; Li, Y.; Sun, K. Update on Gut Microbiota in Cardiovascular Diseases. Front. Cell. Infect. Microbiol. 2022, 12, 1694. [Google Scholar] [CrossRef]
- Cai, J.; Rimal, B.; Jiang, C.; Chiang, J.Y.L.; Patterson, A.D. Bile Acid Metabolism and Signaling, the Microbiota, and Metabolic Disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef]
- Ohtani, N.; Kawada, N. Role of the Gut–Liver Axis in Liver Inflammation, Fibrosis, and Cancer: A Special Focus on the Gut Microbiota Relationship. Hepatol. Commun. 2019, 3, 456–470. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Li, Z.-J.; Gou, H.-Z.; Song, X.-J.; Zhang, L. The Gut Microbiota–Bile Acid Axis: A Potential Therapeutic Target for Liver Fibrosis. Front. Cell. Infect. Microbiol. 2022, 12, 1416. [Google Scholar] [CrossRef] [PubMed]
- Koppel, N.; Maini Rekdal, V.; Balskus, E.P. Chemical Transformation of Xenobiotics by the Human Gut Microbiota. Science 2017, 356, eaag2770. [Google Scholar] [CrossRef]
- Abdelsalam, N.A.; Ramadan, A.T.; ElRakaiby, M.T.; Aziz, R.K. Toxicomicrobiomics: The Human Microbiome vs. Pharmaceutical, Dietary, and Environmental Xenobiotics. Front. Pharmacol. 2020, 11, 390. [Google Scholar] [CrossRef]
- Tan, H.; Zhai, Q.; Chen, W. Investigations of Bacteroides Spp. towards next-Generation Probiotics. Food Res. Int. 2019, 116, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations; World Health Organization (Eds.) Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; FAO food and nutrition paper; Food and Agriculture Organization of the United Nations; World Health Organization: Rome, Italy, 2006; ISBN 978-92-5-105513-7. [Google Scholar]
- Zhao, S.; Liu, W.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Liu, R.; Hong, J. Akkermansia muciniphila Improves Metabolic Profiles by Reducing Inflammation in Chow Diet-Fed Mice. J. Mol. Endocrinol. 2017, 58, 1–14. [Google Scholar] [CrossRef]
- López-Almela, I.; Romaní-Pérez, M.; Bullich-Vilarrubias, C.; Benítez-Páez, A.; Gómez Del Pulgar, E.M.; Francés, R.; Liebisch, G.; Sanz, Y. Bacteroides uniformis Combined with Fiber Amplifies Metabolic and Immune Benefits in Obese Mice. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Lee, Y.-S.; Kim, Y.; Lee, S.-H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.-S.; Lim, H.S.; Kim, M.-S.; et al. Gut Commensal Bacteroides Acidifaciens Prevents Obesity and Improves Insulin Sensitivity in Mice. Mucosal Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A Purified Membrane Protein from Akkermansia muciniphila or the Pasteurized Bacterium Improves Metabolism in Obese and Diabetic Mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Guo, X.; Zhang, M.; Ou, Z.; Wu, D.; Deng, L.; Lu, Z.; Zhang, J.; Deng, G.; Chen, S.; et al. An Akkermansia muciniphila Subtype Alleviates High-Fat Diet-Induced Metabolic Disorders and Inhibits the Neurodegenerative Process in Mice. Anaerobe 2020, 61, 102138. [Google Scholar] [CrossRef] [PubMed]
- Fabersani, E.; Portune, K.; Campillo, I.; López-Almela, I.; la Paz, S.M.; Romaní-Pérez, M.; Benítez-Páez, A.; Sanz, Y. Bacteroides Uniformis CECT 7771 Alleviates Inflammation within the Gut-Adipose Tissue Axis Involving TLR5 Signaling in Obese Mice. Sci. Rep. 2021, 11, 11788. [Google Scholar] [CrossRef]
- Munukka, E.; Rintala, A.; Toivonen, R.; Nylund, M.; Yang, B.; Takanen, A.; Hänninen, A.; Vuopio, J.; Huovinen, P.; Jalkanen, S.; et al. Faecalibacterium prausnitzii Treatment Improves Hepatic Health and Reduces Adipose Tissue Inflammation in High-Fat Fed Mice. ISME J. 2017, 11, 1667–1679. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Kim, Y.; Seo, Y.; Lee, H.; Ha, J.; Lee, J.; Choi, Y.; Oh, H.; Yoon, Y. Akkermansia muciniphila Prevents Fatty Liver Disease, Decreases Serum Triglycerides, and Maintains Gut Homeostasis. Appl. Environ. Microbiol. 2020, 86, e03004-19. [Google Scholar] [CrossRef] [PubMed]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of Ethanol-Induced Akkermansia muciniphila Depletion Ameliorates Alcoholic Liver Disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Q.; Liu, M.; Zhang, X.; He, F.; Wang, G. Akkermansia muciniphila Can Reduce the Damage of Gluco/Lipotoxicity, Oxidative Stress and Inflammation, and Normalize Intestine Microbiota in Streptozotocin-induced Diabetic Rats. Pathog. Dis. 2018, 76, fty028. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Deng, L.; Lu, Z.; Wu, F.; Liu, W.; Huang, D.; Peng, Y. Protective Effects of Akkermansia muciniphila on Cognitive Deficits and Amyloid Pathology in a Mouse Model of Alzheimer’s Disease. Nutr. Diabetes 2020, 10, 12. [Google Scholar] [CrossRef]
- Gómez del Pulgar, E.M.; Benítez-Páez, A.; Sanz, Y. Safety Assessment of Bacteroides uniformis CECT 7771, a Symbiont of the Gut Microbiota in Infants. Nutrients 2020, 12, 551. [Google Scholar] [CrossRef]
- Jia, L.; Shan, K.; Pan, L.-L.; Feng, N.; Lv, Z.; Sun, Y.; Li, J.; Wu, C.; Zhang, H.; Chen, W.; et al. Clostridium butyricum CGMCC0313.1 Protects against Autoimmune Diabetes by Modulating Intestinal Immune Homeostasis and Inducing Pancreatic Regulatory T Cells. Front. Immunol. 2017, 8, 1345. [Google Scholar] [CrossRef]
- Péan, N.; Le Lay, A.; Brial, F.; Wasserscheid, J.; Rouch, C.; Vincent, M.; Myridakis, A.; Hedjazi, L.; Dumas, M.-E.; Grundberg, E.; et al. Dominant Gut Prevotella copri in Gastrectomised Non-Obese Diabetic Goto–Kakizaki Rats Improves Glucose Homeostasis through Enhanced FXR Signalling. Diabetologia 2020, 63, 1223–1235. [Google Scholar] [CrossRef]
- Zhai, R.; Xue, X.; Zhang, L.; Yang, X.; Zhao, L.; Zhang, C. Strain-Specific Anti-Inflammatory Properties of Two Akkermansia muciniphila Strains on Chronic Colitis in Mice. Front. Cell. Infect. Microbiol. 2019, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sánchez, A.; Ruiz-Rodríguez, A.; Ortiz, P.; Moreno, M.A.; Ampatzoglou, A.; Gruszecka-Kosowska, A.; Monteoliva-Sánchez, M.; Aguilera, M. Exploring Next Generation Probiotics for Metabolic and Microbiota Dysbiosis Linked to Xenobiotic Exposure: Holistic Approach. Int. J. Mol. Sci. 2022, 23, 12917. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Manco, M.; Gardini, S.; Guarrasi, V.; Russo, A.; Bianchi, M.; Tortosa, V.; Quagliariello, A.; Shashaj, B.; Fintini, D.; et al. Fecal Microbiota Signatures of Insulin Resistance, Inflammation, and Metabolic Syndrome in Youth with Obesity: A Pilot Study. Acta Diabetol. 2021, 58, 1009–1022. [Google Scholar] [CrossRef]
- Ahmad, A.; Yang, W.; Chen, G.; Shafiq, M.; Javed, S.; Zaidi, S.S.A.; Shahid, R.; Liu, C.; Bokhari, H. Analysis of Gut Microbiota of Obese Individuals with Type 2 Diabetes and Healthy Individuals. PLoS ONE 2019, 14, e0226372. [Google Scholar] [CrossRef]
- Doumatey, A.P.; Adeyemo, A.; Zhou, J.; Lei, L.; Adebamowo, S.N.; Adebamowo, C.; Rotimi, C.N. Gut Microbiome Profiles Are Associated with Type 2 Diabetes in Urban Africans. Front. Cell. Infect. Microbiol. 2020, 10, 63. [Google Scholar] [CrossRef]
- Adachi, K.; Sugiyama, T.; Yamaguchi, Y.; Tamura, Y.; Izawa, S.; Hijikata, Y.; Ebi, M.; Funaki, Y.; Ogasawara, N.; Goto, C.; et al. Gut Microbiota Disorders Cause Type 2 Diabetes Mellitus and Homeostatic Disturbances in Gut-Related Metabolism in Japanese Subjects. J. Clin. Biochem. Nutr. 2019, 64, 231–238. [Google Scholar] [CrossRef]
- Takagi, T.; Naito, Y.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Kamada, K.; Ishikawa, T.; Inoue, R.; Okuda, K.; Tsujimoto, Y.; et al. Changes in the Gut Microbiota Are Associated with Hypertension, Hyperlipidemia, and Type 2 Diabetes Mellitus in Japanese Subjects. Nutrients 2020, 12, 2996. [Google Scholar] [CrossRef] [PubMed]
- Mejía-León, M.E.; Petrosino, J.F.; Ajami, N.J.; Domínguez-Bello, M.G.; de la Barca, A.M.C. Fecal Microbiota Imbalance in Mexican Children with Type 1 Diabetes. Sci. Rep. 2014, 4, 3814. [Google Scholar] [CrossRef]
- Radwan, S.; Gilfillan, D.; Eklund, B.; Radwan, H.M.; Menofy, N.G.E.; Lee, J.; Kapuscinski, M.; Abdo, Z. A Comparative Study of the Gut Microbiome in Egyptian Patients with Type I and Type II Diabetes. PLoS ONE 2020, 15, e0238764. [Google Scholar] [CrossRef]
- Gradisteanu Pircalabioru, G.; Ilie, I.; Oprea, L.; Picu, A.; Petcu, L.M.; Burlibasa, L.; Chifiriuc, M.-C.; Musat, M. Microbiome, Mycobiome and Related Metabolites Alterations in Patients with Metabolic Syndrome—A Pilot Study. Metabolites 2022, 12, 218. [Google Scholar] [CrossRef]
- Lim, M.Y.; You, H.J.; Yoon, H.S.; Kwon, B.; Lee, J.Y.; Lee, S.; Song, Y.-M.; Lee, K.; Sung, J.; Ko, G. The Effect of Heritability and Host Genetics on the Gut Microbiota and Metabolic Syndrome. Gut 2017, 66, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Cortez, R.V.; Moreira, L.N.; Padilha, M.; Bibas, M.D.; Toma, R.K.; Porta, G.; Taddei, C.R. Gut Microbiome of Children and Adolescents with Primary Sclerosing Cholangitis in Association with Ulcerative Colitis. Front. Immunol. 2021, 11, 598152. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.; Jiang, H.; Xiang, S.; Zhao, Y.; Xiao, M.; Du, F.; Ji, H.; Kaboli, P.J.; Wu, X.; et al. Metagenome Analysis of Intestinal Bacteria in Healthy People, Patients with Inflammatory Bowel Disease and Colorectal Cancer. Front. Cell. Infect. Microbiol. 2021, 11, 599734. [Google Scholar] [CrossRef] [PubMed]
- Jee, J.J.; Lim, J.; Park, S.; Koh, H.; Lee, H.W. Gut Microbial Community Differentially Characterizes Patients with Nonalcoholic Fatty Liver Disease. J. Gastroenterol. Hepatol. 2022, 37, 1822–1832. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, H.; Chen, L.; Ruan, Y.; Chen, Y.; Liu, Q. Disease-Associated Gut Microbiota Reduces the Profile of Secondary Bile Acids in Pediatric Nonalcoholic Fatty Liver Disease. Front. Cell. Infect. Microbiol. 2021, 11, 698852. [Google Scholar] [CrossRef] [PubMed]
- Iino, C.; Endo, T.; Mikami, K.; Hasegawa, T.; Kimura, M.; Sawada, N.; Nakaji, S.; Fukuda, S. Significant Decrease in Faecalibacterium among Gut Microbiota in Nonalcoholic Fatty Liver Disease: A Large BMI- and Sex-Matched Population Study. Hepatol. Int. 2019, 13, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-N.; Joo, E.-J.; Cheong, H.S.; Kim, Y.; Kim, H.-L.; Shin, H.; Chang, Y.; Ryu, S. Gut Microbiota and Risk of Persistent Nonalcoholic Fatty Liver Diseases. J. Clin. Med. 2019, 8, 1089. [Google Scholar] [CrossRef]
- Li, F.; Sun, G.; Wang, Z.; Wu, W.; Guo, H.; Peng, L.; Wu, L.; Guo, X.; Yang, Y. Characteristics of Fecal Microbiota in Non-Alcoholic Fatty Liver Disease Patients. Sci. China Life Sci. 2018, 61, 770–778. [Google Scholar] [CrossRef]
- Shen, F.; Zheng, R.-D.; Sun, X.-Q.; Ding, W.-J.; Wang, X.-Y.; Fan, J.-G. Gut Microbiota Dysbiosis in Patients with Non-Alcoholic Fatty Liver Disease. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; You, H.J.; Bajaj, J.S.; Joo, S.K.; Yu, J.; Park, S.; Kang, H.; Park, J.H.; Kim, J.H.; Lee, D.H.; et al. Distinct Signatures of Gut Microbiome and Metabolites Associated with Significant Fibrosis in Non-Obese NAFLD. Nat. Commun. 2020, 11, 4982. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, X.; Cao, M.; Ge, J.; Bao, Q.; Tang, L.; Chen, Y.; Li, L. Altered Fecal Microbiota Correlates with Liver Biochemistry in Nonobese Patients with Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2016, 6, 32002. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Liu, Y.-Y.; Lin, C.-C.; Wang, C.-C.; Wu, Y.-J.; Yong, C.-C.; Chen, K.-D.; Chuah, S.-K.; Yao, C.-C.; Huang, P.-Y.; et al. Gut Microbiota Dysbiosis in Patients with Biopsy-Proven Nonalcoholic Fatty Liver Disease: A Cross-Sectional Study in Taiwan. Nutrients 2020, 12, 820. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-Invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017, 25, 1054–1062.e5. [Google Scholar] [CrossRef] [PubMed]
- Sobhonslidsuk, A.; Chanprasertyothin, S.; Pongrujikorn, T.; Kaewduang, P.; Promson, K.; Petraksa, S.; Ongphiphadhanakul, B. The Association of Gut Microbiota with Nonalcoholic Steatohepatitis in Thais. BioMed Res. Int. 2018, 2018, e9340316. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, H.E.; Teterina, A.; Comelli, E.M.; Taibi, A.; Arendt, B.M.; Fischer, S.E.; Lou, W.; Allard, J.P. Nonalcoholic Fatty Liver Disease Is Associated with Dysbiosis Independent of Body Mass Index and Insulin Resistance. Sci. Rep. 2018, 8, 1466. [Google Scholar] [CrossRef]
- Mouzaki, M.; Comelli, E.M.; Arendt, B.M.; Bonengel, J.; Fung, S.K.; Fischer, S.E.; McGilvray, I.D.; Allard, J.P. Intestinal Microbiota in Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2013, 58, 120–127. [Google Scholar] [CrossRef]
- Bastian, W.P.; Hasan, I.; Lesmana, C.R.A.; Rinaldi, I.; Gani, R.A. Gut Microbiota Profiles in Nonalcoholic Fatty Liver Disease and Its Possible Impact on Disease Progression Evaluated with Transient Elastography: Lesson Learnt from 60 Cases. Case Rep. Gastroenterol. 2019, 13, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Dong, Y.; Zuo, K.; Han, C.; Jiao, J.; Yang, X.; Li, J. Characteristics and Variation of Fecal Bacterial Communities and Functions in Isolated Systolic and Diastolic Hypertensive Patients. BMC Microbiol. 2021, 21, 128. [Google Scholar] [CrossRef]
- Nakai, M.; Ribeiro, R.V.; Stevens, B.R.; Gill, P.; Muralitharan, R.R.; Yiallourou, S.; Muir, J.; Carrington, M.; Head, G.A.; Kaye, D.M.; et al. Essential Hypertension Is Associated with Changes in Gut Microbial Metabolic Pathways: A Multisite Analysis of Ambulatory Blood Pressure. Hypertension 2021, 78, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Silveira-Nunes, G.; Durso, D.F.; de Oliveira, L.R.A., Jr.; Cunha, E.H.M.; Maioli, T.U.; Vieira, A.T.; Speziali, E.; Corrêa-Oliveira, R.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; et al. Hypertension Is Associated With Intestinal Microbiota Dysbiosis and Inflammation in a Brazilian Population. Front. Pharmacol. 2020, 11, 258. [Google Scholar] [CrossRef]
- Yan, Q.; Gu, Y.; Li, X.; Yang, W.; Jia, L.; Chen, C.; Han, X.; Huang, Y.; Zhao, L.; Li, P.; et al. Alterations of the Gut Microbiome in Hypertension. Front. Cell. Infect. Microbiol. 2017, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut Microbiota Dysbiosis Contributes to the Development of Hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Wan, C.; Zhu, C.; Jin, G.; Zhu, M.; Hua, J.; He, Y. Analysis of Gut Microbiota in Patients with Coronary Artery Disease and Hypertension. Evid.-Based Complement. Altern. Med. 2021, 2021, 7195082. [Google Scholar] [CrossRef]
- Li, H.; Liu, B.; Song, J.; An, Z.; Zeng, X.; Li, J.; Jiang, J.; Xie, L.; Wu, W. Characteristics of Gut Microbiota in Patients with Hypertension and/or Hyperlipidemia: A Cross-Sectional Study on Rural Residents in Xinxiang County, Henan Province. Microorganisms 2019, 7, 399. [Google Scholar] [CrossRef]
- Gao, J.; Wang, J.; Zhao, L.-L.; Yao, T.-T.; Chen, Y.; Ma, J.; Zhang, X.; Wang, J.-X.; Wang, Y.; Cui, Z.; et al. Gut Lactobacillus Level Is a Predictive Marker for Coronary Atherosclerotic Lesions Progress and Prognosis in Patients with Acute Coronary Syndrome. Front. Cell. Infect. Microbiol. 2021, 11, 687827. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Chen, S.; Gu, G.; Zhou, J.; Wang, W.; Ren, J.; Wu, J.; Yang, D.; Zheng, Y. Exploration of Crucial Mediators for Carotid Atherosclerosis Pathogenesis through Integration of Microbiome, Metabolome, and Transcriptome. Front. Physiol. 2021, 12, 645212. [Google Scholar] [CrossRef] [PubMed]
- Baragetti, A.; Severgnini, M.; Olmastroni, E.; Dioguardi, C.C.; Mattavelli, E.; Angius, A.; Rotta, L.; Cibella, J.; Caredda, G.; Consolandi, C.; et al. Gut Microbiota Functional Dysbiosis Relates to Individual Diet in Subclinical Carotid Atherosclerosis. Nutrients 2021, 13, 304. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, W.; Liu, X.; Cheng, L. Metagenomic Analysis of the Gut Microbiome in Atherosclerosis Patients Identify Cross-Cohort Microbial Signatures and Potential Therapeutic Target. FASEB J. 2020, 34, 14166–14181. [Google Scholar] [CrossRef]
- Toya, T.; Corban, M.T.; Marrietta, E.; Horwath, I.E.; Lerman, L.O.; Murray, J.A.; Lerman, A. Coronary Artery Disease Is Associated with an Altered Gut Microbiome Composition. PLoS ONE 2020, 15, e0227147. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Hu, X.; Niu, H.; Tian, R.; Wang, H.; Pang, H.; Jiang, L.; Qiu, B.; Chen, X.; et al. Alterations in the Gut Microbiome and Metabolism with Coronary Artery Disease Severity. Microbiome 2019, 7, 68. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The Gut Microbiome in Atherosclerotic Cardiovascular Disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Loftfield, E.; Herzig, K.-H.; Caporaso, J.G.; Derkach, A.; Wan, Y.; Byrd, D.A.; Vogtmann, E.; Männikkö, M.; Karhunen, V.; Knight, R.; et al. Association of Body Mass Index with Fecal Microbial Diversity and Metabolites in the Northern Finland Birth Cohort. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ni, Z.; Yu, J.; Cheng, W.; Cai, Z.; Yu, C. Correlation Between Fecal Metabolomics and Gut Microbiota in Obesity and Polycystic Ovary Syndrome. Front. Endocrinol. 2020, 11, 628. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut Microbiome and Serum Metabolome Alterations in Obesity and after Weight-Loss Intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Nogacka, A.M.; de los Reyes-Gavilán, C.G.; Martínez-Faedo, C.; Ruas-Madiedo, P.; Suarez, A.; Mancabelli, L.; Ventura, M.; Cifuentes, A.; León, C.; Gueimonde, M.; et al. Impact of Extreme Obesity and Diet-Induced Weight Loss on the Fecal Metabolome and Gut Microbiota. Mol. Nutr. Food Res. 2021, 65, 2000030. [Google Scholar] [CrossRef] [PubMed]
- Nuli, R.; Azhati, J.; Cai, J.; Kadeer, A.; Zhang, B.; Mohemaiti, P. Metagenomics and Faecal Metabolomics Integrative Analysis towards the Impaired Glucose Regulation and Type 2 Diabetes in Uyghur-Related Omics. J. Diabetes Res. 2019, 2019, e2893041. [Google Scholar] [CrossRef]
- Qi, Q.; Li, J.; Yu, B.; Moon, J.-Y.; Chai, J.C.; Merino, J.; Hu, J.; Ruiz-Canela, M.; Rebholz, C.; Wang, Z.; et al. Host and Gut Microbial Tryptophan Metabolism and Type 2 Diabetes: An Integrative Analysis of Host Genetics, Diet, Gut Microbiome and Circulating Metabolites in Cohort Studies. Gut 2021, 71, 1095–1105. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, S.; Li, H.; Zhang, Z.; Zhang, Q.; Chen, L.; Zhao, Y.; Chen, Y.; Gu, J.; Min, L.; et al. Identification of Gut Microbiota and Metabolites Signature in Patients With Irritable Bowel Syndrome. Front. Cell. Infect. Microbiol. 2019, 9, 346. [Google Scholar] [CrossRef]
- Alferink, L.J.M.; Radjabzadeh, D.; Erler, N.S.; Vojinovic, D.; Medina-Gomez, C.; Uitterlinden, A.G.; de Knegt, R.J.; Amin, N.; Ikram, M.A.; Janssen, H.L.A.; et al. Microbiomics, Metabolomics, Predicted Metagenomics, and Hepatic Steatosis in a Population-Based Study of 1,355 Adults. Hepatology 2021, 73, 968–982. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kolde, R. Pheatmap: Pretty Heatmaps 2019. Pheatmap: Pretty Heatmaps. R Package Version 1.0.12. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 28 January 2023).
- Chen, M.X.; Wang, S.-Y.; Kuo, C.-H.; Tsai, I.-L. Metabolome Analysis for Investigating Host-Gut Microbiota Interactions. J. Formos. Med. Assoc. 2019, 118 (Suppl. S1), S10–S22. [Google Scholar] [CrossRef]
- Visconti, A.; Le Roy, C.I.; Rosa, F.; Rossi, N.; Martin, T.C.; Mohney, R.P.; Li, W.; de Rinaldis, E.; Bell, J.T.; Venter, J.C.; et al. Interplay between the Human Gut Microbiome and Host Metabolism. Nat. Commun. 2019, 10, 4505. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, N.; Tan, H.-Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia muciniphila in Obesity: Interactions with Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Maioli, T.U.; Borras-Nogues, E.; Torres, L.; Barbosa, S.C.; Martins, V.D.; Langella, P.; Azevedo, V.A.; Chatel, J.-M. Possible Benefits of Faecalibacterium prausnitzii for Obesity-Associated Gut Disorders. Front. Pharmacol. 2021, 12, 740636. [Google Scholar] [CrossRef]
- López-Moreno, A.; Suárez, A.; Avanzi, C.; Monteoliva-Sánchez, M.; Aguilera, M. Probiotic Strains and Intervention Total Doses for Modulating Obesity-Related Microbiota Dysbiosis: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1921. [Google Scholar] [CrossRef] [PubMed]
- Gao, P. The Exposome in the Era of One Health. Environ. Sci. Technol. 2021, 55, 2790–2799. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).