Abstract
The aim of this review was to present the metabolism of vitamin D3, as well as to discuss the role of vitamin D3 in bone metabolism, temporomandibular joint osteoarthritis (TMJ OA), and autoimmune thyroid diseases (AITD) on the basis of the literature. Vitamin D3 plays a significant role in human health, as it affects the calcium-phosphate balance and regulates the bone metabolism. Calcitriol impresses the pleiotropic effect on human biology and metabolism. Its modulative function upon the immune system is based on the reduction of Th1 cell activity and increased immunotolerance. Vitamin D3 deficiency may lead to an imbalance in the relationship between Th1/Th17 and Th2, Th17/Th reg, and is considered by some authors as one of the possible backgrounds of autoimmune thyroid diseases (AITD), e.g., Hashimoto’s thyroiditis or Graves’ disease. Moreover, vitamin D3, through its direct and indirect influence on bones and joints, may also play an important role in the development and progression of degenerative joint diseases, including temporomandibular joint osteoarthritis. Further randomized, double blind studies are needed to unequivocally confirm the relationship between vitamin D3 and abovementioned diseases and to answer the question concerning whether vitamin D3 supplementation may be used in the prevention and/or treatment of either AITD or OA diseases.
1. Introduction
Calcium ions play a significant role in several vital processes in the human body, including blood clotting, muscle contraction, proper nerve activity, and bone turnover, as well as affect the cell membrane activity [1]. The physiologic concentration of calcium within serum depends on calcium absorption from the gastrointestinal tract, the intensity of calcification processes, and finally on the urine calcium excretion. Calcium homeostasis is controlled by three hormones: parathyroid hormone (PTH), calcitonin, and 1,25-dihydroxycholecalciferol, also known as 1,25-dihydroxyvitamin D3 [1].
Vitamin D3 is a fat-soluble steroid prohormone, which is well-known in medicine for over 100 years, especially for its invaluable role in rickets prevention [2], maintenance of calcium-phosphate balance, and finally due to its role in bone metabolism [3]. The disorders of calcium metabolism are most commonly related to the imbalance of osteoblasts and osteoclast activity, leading to osteopenia and osteoporosis [4,5,6]. Moreover, deficits of vitamin D3 have also been found to affect the skin issues, autoimmune disorders, cancers, cardiovascular, and metabolic diseases [7,8]. In addition, 1,25-dihydroxyvitamin D3 increases the concentration of calcium ions within the extracellular fluid by increasing the renal calcium and phosphate reabsorption, by increasing the intestinal calcium absorption, as well as by increasing bone resorption [9].
Vitamin D3 is in great interest of many different specialties, including among others endocrinology, and dentistry. Low levels of vitamin D3 have been linked with the presence of recurrent aphthous stomatitis [10] and may increase in the risk of the development of periodontal disease [11].
Vitamin D3 presents immunomodulative properties, and therefore it may affect autoimmune thyroid diseases (AITD), including Hashimoto thyroiditis (HT) and Graves’ disease (GD) [12,13]. AITDs are considered as one of the most common autoimmune diseases, affecting 5% of the population [14]. HT is a form of chronic, lymphocytic inflammation. Auto-antigens, e.g., thyroid peroxidase or thyroglobulin, are responsible for the immunization and activation auto-reactive T cells, recruitment of B cells, and auto-antibody production. This chronic inflammatory process leads to disfunction of the thyroid gland with hypothyroidism [15]. Contrary to HT, GD is a thyroid lymphocytic inflammatory process ongoing with hyperthyroidism. Activated Treg and B cells produce TSH receptor stimulating autoantibodies, resulting in uncontrolled and excessive thyroid hormone production and subsequent growth of thyroid tissue [16].
Low serum levels of vitamin D3 may increase the risk of the development of autoimmune thyroid diseases [12]. Moreover, it has also been reported that autoimmune thyroid diseases affect the bone metabolism [17,18].
There has been found a correlation between the low level of 25-hydroxycholecalciferol and both the presence and the intensity of many rheumatic diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), spondyloarthropathies (SpA), and osteoarthritis (OA) [19]. Furthermore, the polymorphisms of vitamin D receptor gene appeared to be related to the osteoarthritis susceptibility in the spine [20].
OA is one of the most common joint diseases, which encompasses: articular cartilage, synovium, subchondral bone, adjacent muscles, as well as ligaments [21]. Several different factors which may lead to OA have been listed. Those are: mechanical, metabolic, and inflammatory ones [21]. Some authors have found the presence of the correlation between the low serum levels of 25-hydroxyvitamin D3 and knee OA both in younger and older patients [22,23]. However, low serum levels of 25-hydroxyvitamin D3 did not correlate with the clinical and radiological symptoms indicating the severity of knee OA [23].
Degenerative joint disease (DJD), including OA, is also the most common disease of the temporomandibular joints (TMJs). The most common symptom of TMJ OA is pain [24]. DJD is also accompanied by below listed radiographic findings, namely: pseudocysts, erosion of the articular surface, osteophytes, generalized sclerosis, subcortical sclerosis, and articular surface flattening [25].
As far as the serum levels of vitamin D3 are correlated with the presence of knee OA, it may be speculated that vitamin D3 also affects the presence of TMJ OA. If so, especially very low levels of vitamin D3 in serum might be one of the TMJ OA risk factors. So far it has been found that polymorphisms of vitamin D3 receptor may be correlated with the development of the temporomandibular disorders (TMD) [26]. Additionally, due to the fact that vitamin D3 may affect autoimmune thyroid diseases, which consequently are able to affect bone metabolism, it may be also speculated that there exists a relationship between autoimmune thyroid diseases and the development of TMJ OA.
Therefore, the aim of this review was to present the metabolism of vitamin D3, as well as to discuss the role of vitamin D3 in bone metabolism, TMJ OA, and autoimmune thyroid diseases on the basis of the literature.
2. Vitamin D3—Biochemical Structure and Metabolism
Vitamin D is a group of different steroid forms which encompasses among others vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) [27]. Figure 1 presents the chemical structure of vitamin D2 and vitamin D3 on the basis of the literature [27].
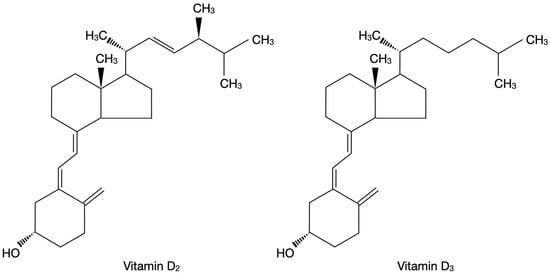
Figure 1.
Chemical structure of vitamin D2 and vitamin D3 on the basis of the literature [27].
Vitamin D3 is a pro-hormone, but it was initially categorized as a vitamin, because it can be absorbed from dietary products, mainly oily fish, fish oil, dairy products, meat, eggs and some mushrooms. Nevertheless, the main source of vitamin D3 in humans is biosynthesis from 7-dehydrocholesterol in the course of photochemical reaction within the human skin [28].
Cholecalciferol is a prohormone that having been biosynthesized within the skin must undergo further transformations to become a biologically active hormone. Cholecalciferol becomes hydroxylated in the position of C25 by the enzyme 25-hydroxylase (CYP2R1, cytochrome P450 family) in the endoplasmic reticulum of the hepatocytes, or by CYP27A1 in the hepatic mitochondria. The product of the above described reaction is 25-hydroxyvitamin D3 (25(OH)D3) [29,30]. Enzymatic activity is regulated by negative feedback, where increased blood serum concentration of 25(OH)D3 decreases hydroxylation of vitamin D3. An excessive amount of vitamin D3 is stored in liver, muscles, or adipocytes [31]. 25-hydroxyvitamin-D3 is the major form of vitamin D3 circulating in the bloodstream with a long half-life time of 2–3 weeks. Therefore, measurement of blood serum concentration of 25(OH)D3 is a golden standard in the assessment of vitamin D3 deficits in humans [27].
25(OH)D3 is subsequently bound to the vitamin-D3 binding protein (DBP) and transported to the kidneys. Human DBP, also called group-specific component (GC), is a protein composed of 458 amino acids [32]. Most of the total plasma 25(OH)D3 is transported being bound to DBP, and only less than 10% is carried by the albumins [33]. The affinity of DBP is 10–100 times greater for 25(OH)D3 than to 1,25(OH)2D3 [34].
Subsequently, mainly in renal mitochondria, 25(OH)D3 becomes hydroxylated at the C1α position by the 1α-hydroxylase (CYP27B1) to the biologically active hormone 1,25(OH)2D3 (calcitriol). Enzyme CYP27B1 has also been found in other tissues, including skin, placenta, and many cells of the immune system that are able to produce calcitriol locally on tissue demand [35]. Renal 1α-hydroxylase activity is directly controlled (stimulated) by PTH, and inhibited by calcium, phosphates, or fibroblast growth factor 23 (FGF-23). Contrary to this, local biosynthesis of 1,25(OH)2D3 in peripheral tissues (e.g., by monocytes and macrophages) is controlled among others by the activity of the immune system, implying auto- and paracrine properties of vitamin D (apart from calcium and phosphate metabolism) [36].
The process of vitamin D3 inactivation, both 1,25(OH)2D3 and 25(OH)D3, starts with C24 hydroxylation by 24-hydroxylase (CYP24A1), mainly in kidneys, intestine, and bones. Further oxidation ends with calcitroic acid, water soluble molecules excreted to the bile [27].
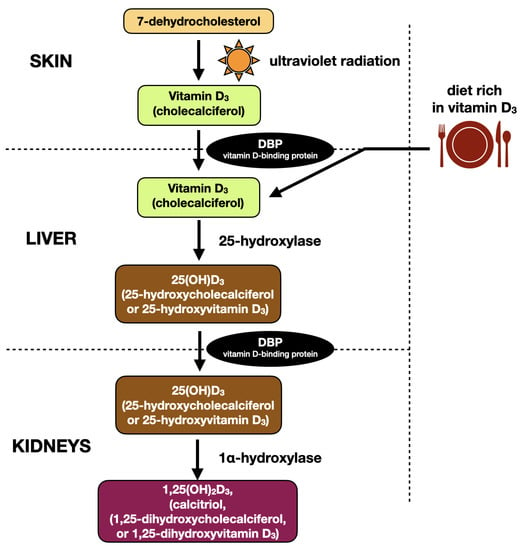
Figure 2.
Metabolic pathway of vitamin D3 on the basis of the literature [27,31].
3. Vitamin D3 Receptor and Mechanisms of Action
Two different mechanisms describing biological activity of vitamin D3 have been discovered, namely: genomic (via vitamin D receptor) and non-genomic (pleiotropic effect) [37].
Vitamin D3 enters the cytoplasm of the cell either as a free molecule or in the process of endocytosis supported by the LDL receptor-related protein 2 (LRP2)—cubilin (CUBN) complex [38]. Within the genomic pathway, vitamin D3 binds to the vitamin-D receptor (VDR) and therefore forms the vitamin D3—VDR complex. VDR belongs to the steroid hormone nuclear receptors family, which includes: glucocorticoid, mineralocorticoid, estrogen, androgen, progesterone receptors [38]. VDR is a gene transcription factor consisting of three domains: C-terminal ligand-bind domain, N-terminal DNA-binding domain with two zinc fingers to link up with accessible DNA sites, and a clip area that binds these two together [39,40]. The VDR is activated by binding to calcitriol (1,25(OH)2D3) and subsequently transforms into heterodimer with retinoid receptor X (RXR). The calcitriol-VDR-RXR complex binds to the specific gene promoter region of the DNA and therefore is able to either promote or inhibit the RNA polymerase II specific VDR-dependent genes [10]. Figure 3 presents the classic vitamin D3 pathway on the basis of the literature [38].
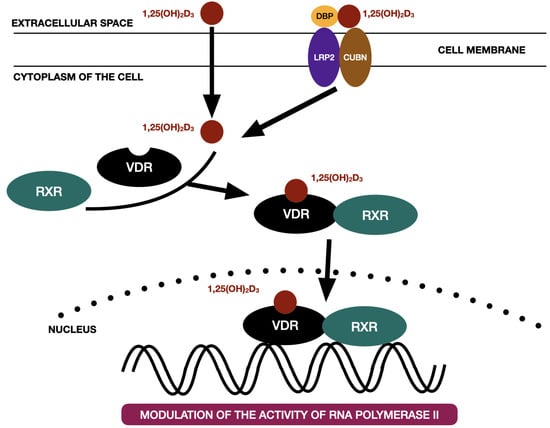
Figure 3.
The classic vitamin D3 pathway on the basis of the literature [38]. 1,25(OH)2D3—calcitriol, CUBN—cubilin, DBP—vitamin D binding protein, LRP2—LDL receptor-related protein 2, RXR—retinoid X receptor, VDR—vitamin D receptor.
1,25(OH)2D3 regulates more than 1000 genes in more than 200 tissues and cells within the human body [38,41]. In an in vitro experiment, when a cell culture model was exposed to a calcitriol concentration higher than physiologic (10–100 nM 1,25(OH)2D3), it took few hours to observe first biological effects [42].
The so-called non-genomic mechanism of action takes seconds to minutes and does not depend on VDR activation, nor gene transcription [38]. 1,25(OH)2D3 can cause calcium influx in cells. In a study from 1990 on osteogenic sarcoma cell line ROS 17/2.8, there was observed a rapid calcium inflow in cell culture treated with 1,25(OH)2D3 [43]. Another example of rapid, hormonally stimulated transport of calcium by enterocytes is called transcalathia [44]. Furthermore, calcitriol enables rapid (1–10 min) tissue uptake of calcium in myocardial chicken cells. Hormonal stimulations lead to microsomal membrane protein phosphorylation and activation of cyclic-AMP pathway [45]. According to the free hormone hypothesis, vitamin D3 is a hydrophobic molecule, which is able to naturally enter the phospholipid membrane, thus probably no membrane receptor or specific protein is needed in transmembrane transport [46]. Therefore, various pathways are known to be regulated by vitamin D3. This creates a possibility for clinical practice to individualize cell-specified therapy by vitamin D3 and its analogues.
4. Vitamin D3 and Immune System
Various immune cells express VDR, thus active vitamin D3 plays a vital role in human immune system. The VDR is present in both B and T lymphocytes, as well as in antigen presenting cells (APC), including monocytes, macrophages and dendritic cells [47]. Interestingly, leukocytes, especially APCs, are also able to activate circulating 25(OH)D3 to 1,25(OH)2D3 through 1α -hydroxylase (CYP27B1) [48]. Biological effects of calcitriol on leukocytes affect: cell proliferation, differentiation, maturation, and apoptosis (programmed cell death). Furthermore, 1,25(OH)2D3 affects the immunological balance between cell-mediated (Th1) and humoral (Th2) response [49].
The antigen presenting cells specialize in presenting hostile antigen to T lymphocytes. Dendritic cells (DCs) are the most common and powerful APCs. The presentation of antigen affects through major histocompatibility complex protein (MHC). The DCs play crucial role in immunization and immunotolerance balance, which is strongly associated with cell maturation [50]. Immature dendritic cells stimulate regulatory (suppressor) T cell proliferation, whereas mature DCs, capable of antigen presenting, promote naive T cells to differentiate to Th1 lymphocytes and enhance pro-inflammatory response.
Calcitriol, through several cytokines, e.g., anti-inflammatory IL-10, suppresses DCs maturation and MHCs type II expression and diminishes co-stimulating of antigens CD40, CD80, CD84. These processes increase the immunotolerance. The switch in interleukin production involves activation of nuclear factor kB (NF-kB) and mitogen activated protein kinase (MAPK) pathways in DCs. Subsequently, vitamin D3 inhibits pro-inflammatory interleukins (IL-12, TNF-α, INF-γ) and promotes T-cell inhibitory factors (programmed death-1, PD-1), decreasing activity and differentiation to Th1 and Th17 cells [51,52].
The T cell population differentiates into T helpers (Th, CD4+) and cytotoxic T cells (Tc, CD8+). Within the group of Th cells, there can be distinguished, among others, Th1, Th2, and Th17 cells. Maturation from naive T CD4+ to Th1 cells involves presenting the antigen by APCs in lymph nodes. VDR is present within the naive T cells, thus the vitamin D3 can directly influence T cell responses. Furthermore, calcitriol affects the differentiation of the T cell subclasses by inhibiting naive CD4+ T cells proliferation to Th1 cells and promoting maturation of Th2 cells. Moreover, vitamin D3 suppresses pro-inflammatory cytokine production (IL-2, INF-γ) by Th1 cells and simultaneously increases the production of anti-inflammatory cytokines by Th2 cells (IL-4, IL-5, IL-10) [53]. Therefore, vitamin D3 controls the Th1/Th2 immune balance and limits the Th1-induced destructive impact on tissues. Immune cells producing IL-17 (Th17) represent a quite new T cell subclass. The cytokine profile of Th17 cells (TNF-α, IL-6, IL-17, IL-21, IL-22) indicates that Th17 cells stimulate pro-inflammatory response in many diseases [54,55]. Probably, Th17 cells play a significant role in autoimmune diseases through pro-inflammatory cytokines, as there are many tissues that express receptors for IL-17 and IL-22. There have been published studies that provide the evidence of suppressive action of vitamin D3 on Th17 cells, leading to the decreased production of IL-17 and other cytokines (IL-1, IL-6, IL-12) and inhibition of CD4+ cells differentiation to Th17 [56]. Moreover, vitamin D3 stimulates IL-10 production by regulatory T cells (Treg) and proliferation of Treg, which subsequently regulate Th activation and cytokine production, affecting general immunological response [57]. 1,25(OH)2D3 is also speculated to affect B lymphocytes, but this relationship remains controversial. Vitamin D3 inhibits production of immunoglobulins (IgG and IgM), decreases the proliferation and maturation of memory B cells, and promotes the apoptosis of B cells [58]. Furthermore, B cells also express enzymes concerned in vitamin D3 metabolism (1α-hydroxylase and 24-hydroxylase), indicating a possible important role of vitamin D3 in B cell activity [53]. Unfortunately, mature B cells seem to be resistant to 1,25(OH)2D3 influence [58].
Vitamin D3 also affects the function of phagocytes (macrophages, monocytes). Vitamin D3 deficiency may manifest by diminished antimicrobial action of monocytes. 1,25(OH)2D3 decreases the expression of toll-like receptor (TLR2 and TLR4), decreases the production of pro-inflammatory molecules like TNF-α, but also stimulates monocytes to differentiate into macrophages, which take part in phagocytosis, chemotaxis and IL-1 production [59,60]. Moreover, vitamin D3 stimulates leukocytes and some epithelial cells (e.g., oral epithelial, intestines, vagina, keratinocytes) to produce antimicrobial proteins (defensin, cathelicidin). Antimicrobial proteins have strong antibacterial, antifungal and antiviral activity. Mechanisms of action include cell membrane destruction, suppression of microbe protein biosynthesis, and inhibition of nucleic acid biosynthesis or cell division [61].
5. Role of Vitamin D3 in Autoimmune Thyroid Diseases
Autoimmune thyroid diseases (AITD), including two main clinical manifestations: Hashimoto thyroiditis (HT) and Graves’ disease (GD), represent some of the most common autoimmune diseases, concerning about 5% of the population [12]. AITD are caused by autoimmunization to native antigens, e.g., thyroid stimulating hormone (TSH), thyroid peroxidase (TPO), thyroglobulin (Tg), and TSH-receptor (TSHR). Anti-TPO and anti-Tg are mostly connected with HT, whereas the anti-TSHR (TRAb) is related to GD.
HT is characterized by the lymphocytic infiltration and imbalance between Th1/Th2 lymphocytes [62]. This chronic inflammatory process leads to the destruction of thyroid follicles and is the most common cause of hypothyroidism in the iodine-sufficient population. Within the thyroid, activated Th1 lymphocytes produce TNF-α and INF-γ, which stimulate thyrocytes to secrete CXC10 (C-X-C motif chemokine ligand 10), responsible for the chemotaxis of monocytes, macrophages, T cells, and NK cells, and enhances the autoimmune vicious circle [13].
Having considered the fact that vitamin D3 plays immune-modulative effect, it may be speculated that vitamin D3 influences autoimmune inflammation in the thyroid gland in a particular way. Calcitriol affects antigen presenting cells, such as dendritic cells, decreases lymphocyte Th1 activation, and decreases the production of proinflammatory cytokines (IL-2, IL-12, TNF-α). Furthermore, vitamin D3 suppresses naive T cells differentiation into Th17 lymphocytes, inhibits Th17-connected interleukins secretion (IL-6, IL-17, IL-21, TNF-α), and thus promotes Treg activity and influences Treg/Th17 ratio. Moreover, vitamin D3 can influence the expression of MHC class II within the thyroid by its downregulation, and consequently limit thyroid autoantigens presentation by APCs to lymphocytes. Additionally, vitamin D3 decreases maturation and proliferation of B lymphocytes. Therefore, secretion of immunoglobulins IgG and IgM is limited [10,63].
GD is one of the most frequent form of hyperthyroidism in population. Although, the exact cause of GD is still unclear, it is assumed that particular environmental triggers activate proliferation of Treg cells, stimulate maturation of B cells to DCs, and lead to production of thyroid-antibodies. Autoantibodies in GD (TRAb) constantly stimulate TSH-receptors, and subsequently lead to proliferation of thyrocytes and synthesis of thyroid hormones (triiodothyronine, thyroxine), thyroid-specific proteins, and enzymes [64]. Vitamin D3 inhibits maturation of dendritic cells and inhibits secretion of inflammatory interleukins (IL-2, IL-12, IL-23, TNF-α, INF-γ). Furthermore, vitamin D3 modulates immune response by a direct influence on Th1 and Th2 lymphocytes, namely by down-regulating Th1 cells activity and up-regulating Th2 cells, as well as Th2-derived cytokines. As mentioned, vitamin D3 can also reduce proliferation of B lymphocytes and production of immunoglobulins that are associated with GD [10].
The relationship between vitamin D3 and AITD has been investigated in recent studies. Kivity et al. [65] noticed that vitamin D3 deficiency was statistically higher in patients with AITD in comparison to healthy individuals (72% versus 30.6%; p < 0.001). What is more, patients with hypothyroidism with AITD and no-AITD were also characterized with lower vitamin D3 levels (79% versus 52%; p < 0.05). Decreased concentrations of vitamin D3 appeared to be correlated with increased titer of antithyroid antibodies (p = 0.01). Bozkurt et al. [66] analyzed in their study in total 580 patients, including: newly diagnosed AITD, ongoing AITD and healthy volunteers. The author noticed that the concentration of vitamin 25(OH)D3 in patients with HT was significantly lower compared to the control group (p < 0.001) and the severity of vitamin D3 deficiency was corelated with the duration of HT and the titer of TPO and Tg antibodies (p < 0.001). Ma et al. [67] observed lower levels of vitamin D3 in patients with AITD (HT and GD) and each 5 nmol/L increase in serum 25(OH)D3 concentrations was associated with a 1.55- and 1.62-fold reduction in GD and HT morbidity. Botelho et al. [68] compared the group of 88 patients with HT with 71 healthy, euthyroid individuals and found an association between vitamin D3 deficiency and cytokines produced by Th1, Th2 and Th17 cells like TNF-α, IL-5 and IL-17 in patients with AITD. Fang et al. [69] confirmed a positive correlation between antithyroid antibodies, vitamin D3 deficiency (odds ratio (OR): 2.428, 95% confidence interval (CI): 1.383–4.261), and 25(OH)D3 inadequacy (OR: 1.198, 95% CO: 0.828–1.733; p = 0.008). The authors also found significantly higher quantities of Th1 and Th17 cells, as well as Th1 and Th17 associated cytokines in HT patients. Chao et al. [70] revealed that the level of 25(OH)D3 in the HT group was lower than in the non-HT group. The authors noticed a significant difference in thyroid function, namely that the thyroid-stimulating hormone (TSH) levels were significantly higher in both the 25(OH)D3 insufficiency group as well as the 25(OH)D3 deficiency group comparing to the 25(OH)D3 sufficiency group. In addition, the free triiodothyronine (FT3) and thyroxine (FT4) levels were significantly lower in the 25(OH)D3 insufficiency group. Moreover, the multiple regression analysis showed that HT was significantly correlated with male sex, body mass index (BMI), waist circumference, and TSH [70].
Within the literature, there can also be found a few studies which did not confirm the relationship between AITD and the vitamin D3 deficits. Effraimidis et al. [71] examined 156 participants and did not find association between low vitamin D3 level and AITD. D’Aurizio et al. [72] analysed 100 patients affected AITD (both HT and GD) and 126 healthy subjects. The authors did not find a significant correlation between vitamin D3 levels and presence of AITD. Ke et al. [73] observed that, in patients diagnosed with AITD, serum 25(OH)D3 levels were not associated with thyroid function, presence of antithyroid antibodies, or serum cytokines IL-4, IL-17, and TNF-α. The authors found moderately lower vitamin D3 levels in HT patients, whereas in GD patients the levels of 25(OH)D3 were comparable to the values presented within the control group. Ma et al. [67] did not find any significant relationship between serum 25(OH)D3 level or any of the below listed: titer of anti-TPO, anti-TG, or TSH serum level [67].
Although, there have been mentioned a few manuscripts which did not confirm the relationship between vitamin D3 and AITD, there are still a lot of publications which state that the relationship between vitamin D3 with AITD remains indisputable. Further studies are needed to thoroughly evaluate the clinical effects of vitamin D3 on AITD [74].
Table 1 presents the relationship between vitamin D3 and AITD on the basis of the literature [65,66,67,68,69,70,71,73].

Table 1.
The relationship between vitamin D3 and AITD on the basis of the literature [65,66,67,68,69,70,71,73].
6. Vitamin D3 and Bone Metabolism
Vitamin D3 plays a prominent role in calcium-phosphate and bone metabolism. The overall feature of vitamin D3 is to increase and maintain the accurate concertation of calcium and phosphate within the extracellular fluid (ECF). Thus, it creates adequate conditions for proper growth, bone remodeling, as well as mineralization of skeleton [3]. Health bone homeostasis is controlled by the osteoblasts (bone-forming mesenchymal-derived cells) and osteoclasts (bone resorption, multi-nucleus hematopoietic stem-derived cells) [75].
Calcitriol increases the concentrations of calcium and phosphates in ECF through intensified intestinal absorption from nutrients and renal reabsorption from primary urine in proximal tubule. Moreover, it increases the expression of transmembrane glycoprotein Receptor Activator for Nuclear Factor κβ Ligand (RANKL) on osteoblasts. RANKL binds to Receptor Activator for Nuclear Factor κβ (RANK) on preosteoclasts and stimulates their differentiation to mature osteoclasts and accelerates bone resorption [76]. Secondly, 1,25(OH)2D3 stimulates osteoblasts to produce proteins essential in the processes of bone remodeling and mineralization of bone matrix, namely collagen, osteopontin, osteocalcin (dependent on vitamin K bone protein). Moreover, 1,25(OH)2D3 stimulates the activity of alkaline phosphatase, which is essential in the process of bone mineralization [77]. Furthermore, vitamin D3 promotes bone forming by accelerating the differentiation of monocytes to macrophages, as well as through binding them to the osteoclasts, which enhances bone resorption and calcium release from the bones [78]. Moreover, 1,25(OH)2D3 also plays a significant role in the upregulation of osteoprotegerin (OPG), a soluble glycoprotein, which is a competitive RANKL inhibitor. OPG after being bound to RANKL, inhibits the activation of RANK by its ligand, and subsequently inhibits the processes of osteoclasts activity, maturation and differentiation [79]. Thus, the vitamin D3 apparently affects bone development, mineralization, and remodeling through its resorption.
Parathormone (PTH) is the one of the most important hormones in bone metabolism. PTH is produced in parathyroid glands. Parathyroid cells, along with renal tubules, brain, heart, skin, stomach, and C cells, express calcium sensing receptors (CaSR), a Class III or Family C G-protein coupled receptor [80]. Serum ionized calcium binds to CaSR and transduces signals through phospholipase C, which hydrolyses phosphatidylinositol 4,5-bisphosphonate to diacyl glycerol (DAG) and inositol 1,4,5-triphosphate (IP3). IP3/DAG pathway leads to the degranulation of calcium in endoplasmic reticulum, increased intracellular calcium concentration, and finally blocks degranulation of the vesicles with PTH to the cell membrane. Thus, the secretion of PTH through parathyroids is inhibited [81].
Parathormone activity is strictly corelated with 1,25(OH)2D3 by negative feedback. Increased concentration of calcitriol inhibits CaSR and decreases PTH serum level. Parathormone plays dual role: both anabolic (bone remodeling), and catabolic (bone resorption) ones. On the one hand, pulsating secretion of PTH, through parathyroid receptor type 1 (which belongs to G protein-coupled receptor family), stimulates osteoblasts to produce important compounds for bone matrix composition, namely insulin-like growth factor 1 (IGF-1), fibroblast growth factor (FGF), matrix metalloproteinase (MM-13), or Wnt/β-catenin [82]. Moreover, PTH decreases osteoblasts’ apoptosis and intensifies bone matrix formation [83]. On the other hand, PTH indirectly leads to bone resorption through the activation of osteoclasts. PTH downregulates the production of osteoprotegerin, promotes binding RANKL to RANK, and stimulates the differentiation of osteoclasts. Having been activated, osteoclasts produce hydrogen ions, via carbonic anhydrase, to dissolve mineralized matrix into water and ions: calcium, magnesium, phosphates, and other organic substances. Simultaneously, particular hydrolytic enzymes, including cathepsin K and MM-13, are secreted to degrade proteins from bone matrix [84]. During the process of bone resorption, calcium, magnesium and phosphate ions are released to ECF and subsequently to blood circulation. Parathormone also affects kidneys. As mentioned before, it increases the activity of 1α-hydroxylase, and therefore 1,25(OH)2D3 is produced. Furthermore, PTH increases the reabsorption of calcium in renal proximal tubule [85]. To sum up, PTH controls the calcium homeostasis within three stages, namely: bone resorption, vitamin D3 activation, and both intestine and renal calcium absorption.
7. Vitamin D3 and Osteoarthritis
Osteoarthritis (OA) is a chronic, degenerative disease involving joint cartilage, synovium, periarticular ligaments, and subchondral bone, affecting up to one in eight adults. It is the most common chronic articular disease. Only a few effective methods of OA treatment have been discussed, but none of them is able to stop or effectively delay the development of the disease [86].
The constantly increasing number of patients diagnosed with OA is associated with increasing life expectancy of population. The cause of the disease is multifactorial, including modifiable and non-modifiable, local and systemic factors. Among the risk factors, there have been mentioned: female sex, race, genetics, old age, type of diet, overweight and obesity, joint injury and mechanical factors, repetitive use of joints, bone density, muscle weakness and joint laxity, low-grade-inflammatory processes, and hormonal system [87,88]. OA can be characterized pathologically, radiographically, and clinically. Diagnosis of OA is not always evident, because some patients with radiological symptoms do not present any clinical manifestation and, at the same time, not everyone with joint symptoms present radiological changes. Therefore, OA should be diagnosed with a diversity of methods, including pathological, clinical, and radiological [89].
The articular cartilage is made of water (>70%) and organic matrix, mostly type II collagen, aggrecan and other proteoglycans [90]. The pathophysiological background of OA involves the whole group of pro-inflammatory cytokines (interleukins IL-1β, IL-6, IL-8), as well as pro-catabolic signalization with nuclear factor kB (NF-kB), mitogen activated protein kinase (MAPK) pathways, and the activation of synovial macrophages and fibroblasts [91]. The inflammatory stimulation of chondrocytes results in upregulation of proteinases, especially aggrecanase and collagenase. The main enzymes responsible for degradation of cartilage matrix are A Disintegrin and Metalloproteinase with Thrombospondin motifs (ADAMTS) and zinc-dependent metalloproteinases (MMPs) belonging to the MMP families. The MMPs group includes collagenases MMP-1, MMP-13 (type II collagen proteinase), and MMP-3 (effective aggrecanase) [92]. The additive effect of pro-inflammatory mediators, mechanical injuries, and oxidative stress affect the function and vitality of chondrocytes, resulting in further degeneration of cartilage and bone underneath.
Recent studies suggest that vitamin D3 may play a significant role in osteoarthritis. Several studies revealed that chondrocytes express VDR. Orfanidou et al. [93] showed increased expression of VDR in the areas of cartilage erosion in OA. In a prospective study with 418 participants with already diagnosed OA Zhang et al. [94] found that participants with both decreased concentration vitamin D3 and high concentration of PTH had a more than three-fold increased risk of OA progression. Heidari et al. [95] presented similar conclusions, however the significant difference was observed in the younger group of patients (<55 years, p = 0.01), whereas in patients aged more than 60 years old the association between serum 25(OH)D3 deficiency and OA was not statistically significant. In another, two-year prospective study with 413 enrolled participants with knee OA and low 25(OH)D3 serum level, Jin X et al. [96] revealed that treatment with 50 000 IU of vitamin D3 monthly did not result in significant changes in MRI-measured tibial cartilage volume or WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) knee pain score. Contrary to this, Gao XR et al. [97] found that daily supplementation of more than 2000 IU of vitamin D3 significantly decreased pain and improved function of the joint on the basis of the WOMAC scale. However, the authors did not find any beneficial effect of vitamin D3 supplementation on the prevention of tibial cartilage loss. What is more, Divjak et al. [98] administrated 4000 IU of 25(OH)D3 daily to patients with primary knee OA and compared the cytokine profile before and after intervention. The authors observed that as the concentration of IL-1β (p < 0.01), IL-23 (p < 0.01), and IL-33 (p < 0.01) significantly increased, the concentration of TNF-α (p < 0.01), IL-13 (p < 0.01), and IL-17 (p < 0.01) significantly decreased, whereas the concentration of IL-4 did not change significantly. The prescribed treatment with vitamin D3 appeared to reduce joint pain, joint stiffness, and to improve physical function. The authors suggested that vitamin D3 supplementation may be recommended as a new co-therapeutic treatment in the course of knee OA.
Vitamin D3 is also known to affect bone regeneration, bone malformation, as well as osseointegration of implants. There have been published several metanalyses and systematic reviews regarding the relationship between vitamin D3 and abovementioned processes [99,100,101]. Salomó-Coll et al. [102] performed an animal study and noticed reduced crestal bone loss as well as increased osteointegration by 10% around implants supplemented with vitamin D. Dvorak et al. [103] described that vitamin D deficiency negatively affected implants osseointegration in rats. Werny et al. [100] stated that 75% of the analyzed studies had confirmed the positive effect of vitamin D supplementation on bone regeneration. Unfortunately, most of the abovementioned studies were performed on animals. So far, several studies have been performed on human patients, but the results are often contradictory. Kwiatek et al. [104] showed, in a prospective, randomized clinical trial on a group of 122 patients, that vitamin D supplementation and treatment of vitamin D deficiency led to an increased bone level surrounding the implant 12 weeks after surgery. Contrary to this study, Grønborg et al. [105] performed a randomized double-blinded placebo-controlled trial with 143 women diagnosed with vitamin D deficiency who received special diet reach in vitamin D. After 12 weeks the author noticed a statistically significant increase in vitamin D serum concentration (p < 0.05). However, they did not notice significant changes in bone turnover biomarkers, nor improvement in muscle strength. Having considered all of the above mentioned, further randomized human studies are necessary to establish the role of vitamin D3 in the process of implant osseointegration.
Undoubtedly, vitamin D3 affects the metabolism of bone and chondrocytes by the modulation of pro- and anti-inflammatory responses. However, there are no specific guidelines regarding the use of vitamin D3 in the treatment of OA. In light of the latest research, the use of vitamin D3 in patients diagnosed with OA seems to be promising.
Table 2 presents the relationship between vitamin D3 and OA on the basis of the literature [93,94,95,96,98].

Table 2.
The relationship between vitamin D3 and OA on the basis of the literature [93,94,95,96,98].
8. Vitamin D3 and Temporomandibular Joint Osteoarthritis
Temporomandibular disorders (TMD) is an umbrella term describing the pathology within the temporomandibular joints and/or adjacent muscles [106]. The most typical symptoms for TMD include: pain in the area of TMJs, limited mouth opening, and noises within the TMJs [106]. The etiology of TMD is multifactorial and encompasses several different items, which may be allocated into one of the subgroups, namely: host-adaptive capacity factors (genetics, age, estrogens, systemic diseases, abnormal remodeling of subchondral bone) and mechanical factors (excessive mechanical stress, parafunctions, functional overloading, microtrauma) [107,108]. In the past, it was believed that occlusion played a major role in the development of TMD [109,110]. However, according to the most recent research, there is not enough evidence to confirm that occlusion may lead to TMD [111,112,113]. TMD is a complex condition, modified by many factors, and which often binds with many diseases [114,115].
Because of the fact that TMD is not a dental, but an interdisciplinary issue, the treatment of TMD, including temporomandibular joint osteoarthritis (TMJ OA), requires the cooperation of different specialists, including physiotherapists, dentists, rheumatologists, endocrinologists, laryngologists, maxillofacial surgeons, psychiatrists, psychologists, and speech therapists.
TMJ OA is a degenerative joint disease (DJD). According to the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD), to diagnose DJD, it is necessary to meet the following criteria: noises within the TMJ that occur during the movement of the mandible within the last 30 days or noise within the TMJ which is reported by the patient during the examination [116]. Moreover, it is necessary to detect the crepitus with palpation during mandibular movement [116]. Nonetheless, the diagnosis based only on anamnesis and clinical examination, without imaging, is characterized by sensitivity of 0.55 and specificity of 0.61 [116]. Radiological symptoms typical for DJD are: erosion, osteophytes, generalized sclerosis, and subchondral cysts. According to the DC/TMD, articular surface flattening and cortical sclerosis are regarded as indeterminant findings for DJD [116].
As previously stated, vitamin D3 plays a significant role in calcium-phosphate and bone metabolism [3,75]. It was also reported that vitamin D3 deficits may be correlated with the development and progression of osteoarthritis [93,94]. Although it may be speculated that low serum concentration of vitamin D3 is correlated with the development and/or progression of TMJ OA, so far there have been published only few studies regarding this topic and the results remain inconsistent.
Shen et al. [117] examined 25(OH)D3 1α-hydroxylase knockout mice which had been fed a rescue diet. The authors found that 1,25(OH)2D3 deficient mice presented reduced bone mineral density and reduced subchondral bone volume within the mandibular condyles. Shen et al. [117] also reported that 1,25(OH)2D3 deficiency was associated with the changes in the shape of articular surfaces, as well as in the thickness of articular cartilage. The authors observed articular surface erosion in 1,25(OH)2D3 deficient mice. Shen et al. [117] concluded that 1,25(OH)2D3 deficiency was correlated with erosive TMJ OA phenotype, increased DNA damage, cellular senescence, as well as with the production of inflammatory cytokines associated with senescence.
Hong et al. [118] noticed that 1,25(OH)2D3 was significantly correlated with TMJ OA, both development and progression, in young and postmenopausal women. The authors indicated that vitamin D3 could be considered a therapeutic agent for TMJ OA. Jagur et al. [119] indicated that decreasing bone mineral density as well as low serum concentration of 25(OH)D3 may be considered predictors of bone destruction in the area of TMJs. Gupta et al. [120] found that in patients diagnosed with TMD, who were also vitamin D3 deficient, supplementation of vitamin D3 in addition to stabilization splint therapy led to quicker alleviation of pain in the area of TMJs. Demir et al. [121] compared healthy individuals and patients with TMD. The authors did not find statistically significant differences between the examined groups regarding the serum concentration of: calcium, magnesium, phosphorus, calcitonin, and 25(OH)D3. Patients diagnosed with TMD presented significantly increased concentration of parathyroid hormone. According to Demir et al. [121], vitamin D3 deficiency in patients diagnosed with TMD requires assessment and correction. Madani et al. [122] performed a case-control study and found that there were no statistically significant differences in the serum concentrations of: alkaline phosphatase, phosphate, calcium, PTH, and vitamin D3 between patients diagnosed with TMD and healthy individuals.
To sum up, it may be speculated that vitamin D3 concentrations may be different in various TMDs, which may consequently be the cause of inconsistency in the obtained results from the above presented research. Studies in which only cases with TMJ OA were included clearly indicated that 1,25(OH)2D3 was significantly correlated with TMJ OA [110,111]. Kui et al. [123] concluded that further studies regarding the relationship between vitamin D3 concentration and TMDs are absolutely needed and that supplementation of vitamin D3 is recommended for vitamin D3 deficient patients who suffer from TMD.
9. Conclusions
Calcitriol impresses a pleiotropic effect on the human biology and metabolism. Its modulative function upon the immune system is based on the reduction of Th1 cell activity and increased immunotolerance. Severe vitamin D3 deficiency may lead to an imbalance in relationship between Th1/Th17 and Th2, Th17/Th reg, and is considered by some authors as one of the possible backgrounds of autoimmune thyroid diseases (AITD), e.g., Hashimoto’s thyroiditis or Graves’ disease. Moreover, vitamin D3, through a direct and indirect influence on bones and joints, may also play an important role in the development and progression of degenerative joint diseases, including temporomandibular joint osteoarthritis. Further randomized, double blind studies are needed to unequivocally confirm the relationship between vitamin D3 and abovementioned diseases and to answer the question concerning whether vitamin D3 supplementation may be used in the prevention and/or treatment of either AITD or OA diseases.
Author Contributions
Conceptualization, M.S. and M.D.; methodology, M.S. and M.D.; validation, M.S., M.D., R.Ś.-S. and E.P.; formal analysis, M.S. and M.D.; investigation, M.S.; resources, M.S. and M.D.; writing—original draft preparation, M.S. and M.D.; writing—review and editing, M.S., M.D., R.Ś.-S. and E.P.; visualization, M.S. and M.D.; supervision, M.D., R-Ś.-S. and E.P.; project administration, M.S. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data underlying this article are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lumachi, F.; Motta, R.; Cecchin, D.; Ave, S.; Camozzi, V.; Basso, S.M.; Luisetto, G. Calcium metabolism & hypercalcemia in adults. Curr. Med. Chem. 2011, 18, 3529–3536. [Google Scholar] [CrossRef] [PubMed]
- Creo, A.L.; Thacher, T.; Pettifor, J.; Strand, M.A.; Fischer, P.R. Nutritional rickets around the world: An update. Ann. Trop. Paediatr. 2016, 37, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Bhattoa, H.P.; Konstantynowicz, J.; Laszcz, N.; Wojcik, M.; Pludowski, P. Vitamin D: Musculoskeletal health. Rev. Endocr. Metab. Disord. 2017, 18, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.B.; Camacho, P.M.; Lewiecki, E.M.; Petak, S.M. AACE/ACE Postmenopausal Osteoporosis Guidelines Task Force. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis-2020 Update. Endocr. Pract. 2021, 27, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wu, W.; Gu, J.; Zhang, X.; Dang, J.; Wang, J.; Zheng, Y.; Huang, F.; Yuan, J.; Xue, Y.; et al. Human gingival tissue-derived MSC suppress osteoclastogenesis and bone erosion via CD39-adenosine signal pathway in autoimmune arthritis. Ebiomedicine 2019, 43, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Lan, Q.; Chen, M.; Zheng, T.; Su, W.; Wang, J.; Yang, Z.; Park, R.; Dagliyan, G.; Conti, P.S.; et al. Induced T regulatory cells suppress osteoclastogenesis and bone erosion in collagen-induced arthritis better than natural T regulatory cells. Ann. Rheum. Dis. 2012, 71, 1567–1572. [Google Scholar] [CrossRef]
- Bellan, M.; Andreoli, L.; Mele, C.; Sainaghi, P.P.; Rigamonti, C.; Piantoni, S.; De Benedittis, C.; Aimaretti, G.; Pirisi, M.; Marzullo, P. Pathophysiological Role and Therapeutic Implications of Vitamin D in Autoimmunity: Focus on Chronic Autoimmune Diseases. Nutrients 2020, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Bouchemal, M.; Hakem, D.; Azzouz, M.; Touil-Boukoffa, C.; Mezioug, D. Vitamin D Levels Correlate with Metabolic Syndrome Criteria in Algerian Patients: The Ex-vivo Immunomodulatory Effect of α, 25 Dihydroxyvitamin D3. Endocr. Metab. Immune Disord Drug Targets 2020, 20, 1282–1294. [Google Scholar] [CrossRef]
- Song, L. Calcium and Bone Metabolism Indices. Adv. Clin. Chem. 2017, 82, 1–46. [Google Scholar] [CrossRef]
- Zakeri, M.; Parsian, H.; Bijani, A.; Shirzad, A.; Neamati, N. Serum levels of vitamin D in patients with recurrent aphthous stomatitis. Dent. Med. Probl. 2021, 58, 27–30. [Google Scholar] [CrossRef]
- Krawiec, M.; Dominiak, M. Prospective evaluation of vitamin D levels in dental treated patients: A screening study. Dent. Med. Probl. 2021, 58, 321–326. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, W.; Ma, C.; Zhao, Y.; Xiong, R.; Wang, H.; Chen, W.; Zheng, S.G. Immunomodulatory Function of Vitamin D and Its Role in Autoimmune Thyroid Disease. Front. Immunol. 2021, 12, 574967. [Google Scholar] [CrossRef] [PubMed]
- Kim, D. The Role of Vitamin D in Thyroid Diseases. Int. J. Mol. Sci. 2017, 18, 1949. [Google Scholar] [CrossRef]
- Vieira, I.H.; Rodrigues, D.; Paiva, I. Vitamin D and Autoimmune Thyroid Disease-Cause, Consequence, or a Vicious Cycle? Nutrients 2020, 12, 2791. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Corrado, A.; Di Domenicantonio, A.; Fallahi, P. Autoimmune thyroid disorders. Autoimmun. Rev. 2015, 14, 174–180. [Google Scholar] [CrossRef]
- Saravanan, P.; Dayan, C.M. Thyroid autoantibodies. Endocrinol. Metab. Clin. N. Am. 2001, 30, 315–337. [Google Scholar] [CrossRef]
- Takedani, K.; Notsu, M.; Yamauchi, M.; Nawata, K.; Sugimoto, T.; Kanasaki, K. Graves’ disease and vertebral fracture: Possible pathogenic link in postmenopausal women. Clin. Endocrinol. 2020, 93, 204–211. [Google Scholar] [CrossRef]
- Wu, J.; Huang, H.; Yu, X. How does Hashimoto’s thyroiditis affect bone metabolism? Rev. Endocr. Metab. Disord. 2022. Epub ahead of print. [Google Scholar]
- Charoenngam, N. Vitamin D and Rheumatic Diseases: A Review of Clinical Evidence. Int. J. Mol. Sci. 2021, 22, 10659. [Google Scholar] [CrossRef]
- Li, H.-M.; Liu, Y.; Zhang, R.-J.; Ding, J.-Y.; Shen, C.-L. Vitamin D receptor gene polymorphisms and osteoarthritis: A meta-analysis. Rheumatology 2021, 60, 538–548. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Bergink, A.P.; Zillikens, M.C.; Van Leeuwen, J.P.; Hofman, A.; Uitterlinden, A.G.; van Meurs, J.B. 25-Hydroxyvitamin D and osteoarthritis: A meta-analysis including new data. Semin. Arthritis Rheum. 2016, 45, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.K.; Gantaguru, A.; Nanda, S.N.; Velagada, S.; Srinivasan, A.; Mangaraj, M. Association of vitamin D and knee osteoarthritis in younger individuals. World J. Orthop. 2020, 11, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Interdisciplinary Approach to the Temporomandibular Joint Osteoarthritis—Review of the Literature. Medicina 2020, 56, 225. [Google Scholar] [CrossRef] [PubMed]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Morphology of the Temporomandibular Joints Regarding the Presence of Osteoarthritic Changes. Int. J. Environ. Res. Public Health 2020, 17, 2923. [Google Scholar] [CrossRef]
- Ferrillo, M.; Lippi, L.; Giudice, A.; Calafiore, D.; Paolucci, T.; Renò, F.; Migliario, M.; Fortunato, L.; Invernizzi, M.; de Sire, A. Temporomandibular Disorders and Vitamin D Deficiency: What Is the Linkage between These Conditions? A Systematic Review. J. Clin. Med. 2022, 11, 6231. [Google Scholar] [CrossRef]
- Tuckey, R.C.; Cheng, C.; Slominski, A.T. The serum vitamin D metabolome: What we know and what is still to discover. J. Steroid Biochem. Mol. Biol. 2019, 186, 4–21. [Google Scholar] [CrossRef]
- Żmijewski, M.A. Nongenomic Activities of Vitamin, D. Nutrients 2022, 14, 5104. [Google Scholar] [CrossRef]
- Zhu, J.; DeLuca, H.F. Vitamin D 25-hydroxylase-Four decades of searching, are we there yet? Arch Biochem. Biophys. 2012, 523, 30–36. [Google Scholar] [CrossRef]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.; Wallace, J. Vitamin D and Bone Health; Potential Mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Farrell, C.-J.L.; Pusceddu, I.; Fabregat-Cabello, N.; Cavalier, E. Assessment of vitamin D status–a changing landscape. Clin. Chem. Lab. Med. 2017, 55, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.G.; Hillman, L.; Rojanasathit, S. Human Serum Binding Capacity and Affinity for 25-Hydroxyergocalciferol and 25-Hydroxycholecalciferol. J. Clin. Endocrinol. Metab. 1976, 43, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch. Biochem. Biophys. 2012, 523, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef]
- Bourguet, W.; Germain, P.; Gronemeyer, H. Nuclear receptor ligand-binding domains: Three-dimensional structures, molecular interactions and pharmacological implications. Trends Pharmacol. Sci. 2000, 21, 381–388. [Google Scholar] [CrossRef]
- Rochel, N.; Wurtz, J.; Mitschler, A.; Klaholz, B.; Moras, D. The Crystal Structure of the Nuclear Receptor for Vitamin D Bound to Its Natural Ligand. Mol. Cell 2000, 5, 173–179. [Google Scholar] [CrossRef]
- Van de Peppel, J.; van Leeuwen, J.P. Vitamin D and gene networks in human osteoblasts. Front. Physiol. 2014, 5, 137. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D and Its Target Genes. Nutrients 2022, 14, 1354. [Google Scholar] [CrossRef] [PubMed]
- Civitelli, R.; Kim, Y.S.; Gunsten, S.L.; Fujimori, A.; Huskey, M.; Avioli, L.V.; Hruska, K.A. Nongenomic Activation of the Calcium Message System by Vitamin D Metabolites in Osteoblast-like Cells. Endocrinology 1990, 127, 2253–2262. [Google Scholar] [CrossRef]
- Nemere, I.; Norman, A.W. The rapid, hormonally stimulated transport of calcium (transcaltachia). J. Bone Miner. Res. 1987, 2, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Selles, J.; Boland, R. Evidence on the participation of the 3’,5’-cyclic AMP pathway in the non-genomic action of 1,25-dihydroxy-vitamin D3 in cardiac muscle. Mol. Cell. Endocrinol. 1991, 82, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Mendel, C.M. The Free Hormone Hypothesis: A Physiologically Based Mathematical Model. Endocr. Rev. 1989, 10, 232–274. [Google Scholar] [CrossRef]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983, 221, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Medrano, M.; Carrillo-Cruz, E.; Montero, I.; Perez-Simon, J.A. Vitamin D: Effect on Haematopoiesis and Immune System and Clinical Applications. Int. J. Mol. Sci. 2018, 19, 2663. [Google Scholar] [CrossRef]
- Zmijewski, M.A. Vitamin D and Human Health. Int. J. Mol. Sci. 2019, 20, 145. [Google Scholar] [CrossRef]
- Ramalingam, R.; Larmonier, C.B.; Thurston, R.D.; Midura-Kiela, M.T.; Zheng, S.G.; Ghishan, F.K.; Kiela, P.R. Dendritic Cell-Specific Disruption of TGF-β Receptor II Leads to Altered Regulatory T Cell Phenotype and Spontaneous Multiorgan Autoimmunity. J. Immunol. 2012, 189, 3878–3893. [Google Scholar] [CrossRef]
- Kundu, R.; Theodoraki, A.; Haas, C.T.; Zhang, Y.; Chain, B.; Kriston-Vizi, J.; Noursadeghi, M.; Khoo, B. Cell-type-specific modulation of innate immune signalling by vitamin D in human mononuclear phagocytes. Immunology 2016, 150, 55–63. [Google Scholar] [CrossRef]
- Lan, Q.; Zhou, X.; Fan, H.; Chen, M.; Wang, J.; Ryffel, B.; Brand, D.; Ramalingam, R.; Kiela, P.R.; Horwitz, D.A.; et al. Polyclonal CD4+Foxp3+ Treg cells induce TGFβ-dependent tolerogenic dendritic cells that suppress the murine lupus-like syndrome. J. Mol. Cell Biol. 2012, 4, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Vanherwegen, A.-S.; Gysemans, C.; Mathieu, C. Vitamin D endocrinology on the cross-road between immunity and metabolism. Mol. Cell. Endocrinol. 2017, 453, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.T.; Harrington, L.E.; Mangan, P.R.; Gavrieli, M.; Murphy, K.M. Th17: An Effector CD4 T Cell Lineage with Regulatory T Cell Ties. Immunity 2006, 24, 677–688. [Google Scholar] [CrossRef]
- Schnell, A.; Littman, D.R.; Kuchroo, V.K. TH17 cell heterogeneity and its role in tissue inflammation. Nat. Immunol. 2023, 24, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhou, R.; Luger, D.; Zhu, W.; Silver, P.B.; Grajewski, R.S.; Su, S.-B.; Chan, C.-C.; Adorini, L.; Caspi, R.R. Calcitriol Suppresses Antiretinal Autoimmunity through Inhibitory Effects on the Th17 Effector Response. J. Immunol. 2009, 182, 4624–4632. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.A.; Rahimzadeh, M.; Brierley, C.; Gration, B.; Doree, C.; Kimber, C.E.; Cajide, A.P.; Lamikanra, A.A.; Roberts, D.J. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PLoS ONE 2019, 14, e0222313. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory Effects of 1,25-Dihydroxyvitamin D3 on Human B Cell Differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Okabe, T.; Ozawa, K.; Urabe, A.; Takaku, F. 1 alpha,25-Dihydroxyvitamin D3 (calcitriol) stimulates proliferation of human circulating monocytes in vitro. FEBS Lett. 1985, 185, 9–13. [Google Scholar] [CrossRef]
- Skrobot, A.; Demkow, U.; Wachowska, M. Immunomodulatory Role of Vitamin D: A Review. Adv. Exp. Med. Biol. 2018, 1108, 13–23. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The Effect of Vitamin D on Thyroid Autoimmunity in Levothyroxine-Treated Women with Hashimoto’s Thyroiditis and Normal Vitamin D Status. Exp. Clin. Endocrinol. Diabetes 2017, 125, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Tamer, G.; Arik, S.; Tamer, I.; Coksert, D. Relative vitamin D insufficiency in Hashimoto’s thyroiditis. Thyroid 2011, 21, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Klecha, A.J.; Arcos, M.L.B.; Frick, L.; Genaro, A.M.; Cremaschi, G. Immune-Endocrine Interactions in Autoimmune Thyroid Diseases. Neuroimmunomodulation 2008, 15, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Kivity, S.; Agmon-Levin, N.; Zisappl, M.; Shapira, Y.; Nagy, E.V.; Dankó, K.; Szekanecz, Z.; Langevitz, P.; Shoenfeld, Y. Vitamin D and autoimmune thyroid diseases. Cell. Mol. Immunol. 2011, 8, 243–247. [Google Scholar] [CrossRef]
- Bozkurt, N.C.; Karbek, B.; Ucan, B.; Sahin, M.; Cakal, E.; Ozbek, M.; Delibasi, T. The association between severity of vitamin D deficiency and Hashimoto’s thyroiditis. Endocr Pract. 2013, 19, 479–484. [Google Scholar] [CrossRef]
- Ma, J.; Wu, D.; Li, C.; Fan, C.; Chao, N.; Liu, J.; Li, Y.; Wang, R.; Miao, W.; Guan, H.; et al. Lower Serum 25-Hydroxyvitamin D Level is Associated With 3 Types of Autoimmune Thyroid Diseases. Medicine 2015, 94, e1639. [Google Scholar] [CrossRef]
- Botelho, I.M.B.; Neto, A.M.; Silva, C.A.; Tambascia, M.A.; Alegre, S.M.; Zantut-Wittmann, D.E. Vitamin D in Hashimoto’s thyroiditis and its relationship with thyroid function and inflammatory status. Endocr. J. 2018, 65, 1029–1037. [Google Scholar] [CrossRef]
- Fang, F.; Chai, Y.; Wei, H.; Wang, K.; Tan, L.; Zhang, W.; Fan, Y.; Li, F.; Shan, Z.; Zhu, M. Vitamin D deficiency is associated with thyroid autoimmunity: Results from an epidemiological survey in Tianjin, China. Endocrine 2021, 73, 447–454. [Google Scholar] [CrossRef]
- Chao, G.; Zhu, Y.; Fang, L. Correlation Between Hashimoto’s Thyroiditis-Related Thyroid Hormone Levels and 25-Hydroxyvitamin, D. Front. Endocrinol. 2020, 11, 4. [Google Scholar] [CrossRef]
- Effraimidis, G.; Badenhoop, K.; Tijssen, J.G.P.; Wiersinga, W.M. Vitamin D deficiency is not associated with early stages of thyroid autoimmunity. Eur. J. Endocrinol. 2012, 167, 43–48. [Google Scholar] [CrossRef]
- D’Aurizio, F.; Villalta, D.; Metus, P.; Doretto, P.; Tozzoli, R. Is vitamin D a player or not in the pathophysiology of autoimmune thyroid diseases? Autoimmun Rev. 2015, 14, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Sun, T.; Zhang, Y.; He, L.; Wu, Q.; Liu, J.; Zha, B. 25-Hydroxyvitamin D serum level in Hashimoto’s thyroiditis, but not Graves’ disease is relatively deficient. Endocr. J. 2017, 64, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H.; Alexandraki, K.I. On the Centennial of Vitamin D—Vitamin D, Inflammation, and Autoimmune Thyroiditis: A Web of Links and Implications. Nutrients 2022, 14, 5032. [Google Scholar] [CrossRef]
- Zhong, Z.; Ethen, N.J.; Williams, B.O. WNT signaling in bone development and homeostasis. Wiley Interdiscip. Rev. Dev. Biol. 2014, 3, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Irie, N. Osteoclast-osteoblast communication. Arch. Biochem. Biophys. 2008, 473, 201–209. [Google Scholar] [CrossRef] [PubMed]
- van Driel, M.; van Leeuwen, J.P. Vitamin D endocrine system and osteoblasts. Bonekey Rep. 2014, 3, 493. [Google Scholar] [CrossRef]
- van Driel, M.; Koedam, M.; Buurman, C.; Roelse, M.; Weyts, F.; Chiba, H.; Uitterlinden, A.; Pols, H.; van Leeuwen, J. Evidence that both 1α,25-dihydroxyvitamin D3 and 24-hydroxylated D3 enhance human osteoblast differentiation and mineralization. J. Cell. Biochem. 2006, 99, 922–935. [Google Scholar] [CrossRef]
- Xu, D.; Gao, H.-J.; Lu, C.-Y.; Tian, H.-M.; Yu, X.-J. Vitamin D inhibits bone loss in mice with thyrotoxicosis by activating the OPG/RANKL and Wnt/β-catenin signaling pathways. Front. Endocrinol. 2022, 13, 1066089. [Google Scholar] [CrossRef]
- Hannan, F.M.; Kallay, E.; Chang, W.; Brandi, M.L.; Thakker, R.V. The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat. Rev. Endocrinol. 2018, 15, 33–51. [Google Scholar] [CrossRef]
- Chu, H.; Qin, Z.; Ma, J.; Xie, Y.; Shi, H.; Gu, J.; Shi, B. Calcium-Sensing Receptor (CaSR)-Mediated Intracellular Communication in Cardiovascular Diseases. Cells 2022, 11, 3075. [Google Scholar] [CrossRef]
- Rendina-Ruedy, E.; Rosen, C.J. Parathyroid hormone (PTH) regulation of metabolic homeostasis: An old dog teaches us new tricks. Mol. Metab. 2022, 60, 101480. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Southgate, R.D.; Farhat, Y.M.; Loiselle, A.E.; Hammert, W.C.; Awad, H.A.; O’Keefe, R.J. Parathyroid hormone 1-34 enhances extracellular matrix deposition and organization during flexor tendon repair. J. Orthop. Res. 2014, 33, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kryśkiewicz, E.; Lorenc, R.S. Mechanizmy działania leków antykatabolicznych stosowanych w osteoporozie [Mechanisms of action of anticatabolic drugs used in osteoporosis therapy]. Endokrynol. Pol. 2009, 60, 134–144. [Google Scholar] [PubMed]
- Blaine, J.; Chonchol, M.; Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Lee, K.; Ju, J.H. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef]
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef]
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef]
- Hannan, M.T.; Felson, D.T.; Pincus, T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J. Rheumatol. 2000, 27, 1513–1517. [Google Scholar]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers. 2016, 2, 16072. [Google Scholar] [CrossRef]
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 35–44. [Google Scholar] [CrossRef]
- Troeberg, L.; Nagase, H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim. Biophys. Acta. 2012, 1824, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Orfanidou, T.; Malizos, K.N.; Varitimidis, S.; Tsezou, A. 1,25-Dihydroxyvitamin D3 and extracellular inorganic phosphate activate mitogen-activated protein kinase pathway through fibroblast growth factor 23 contributing to hypertrophy and mineralization in osteoarthritic chondrocytes. Exp. Biol. Med. 2012, 237, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Driban, J.B.; Lo, G.H.; Price, L.L.; Booth, S.; Eaton, C.B.; Lu, B.; Nevitt, M.; Jackson, B.; Garganta, C.; et al. Vitamin D Deficiency Is Associated with Progression of Knee Osteoarthritis. J. Nutr. 2014, 144, 2002–2008. [Google Scholar] [CrossRef]
- Heidari, B.; Heidari, P.; Hajian-Tilaki, K. Association between serum vitamin D deficiency and knee osteoarthritis. Int. Orthop. 2011, 35, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jones, G.; Cicuttini, F.; Wluka, A.; Zhu, Z.; Han, W.; Antony, B.; Wang, X.; Winzenberg, T.; Blizzard, L.; et al. Effect of Vitamin D Supplementation on Tibial Cartilage Volume and Knee Pain Among Patients With Symptomatic Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 2016, 315, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-R.; Chen, Y.-S.; Deng, W. The effect of vitamin D supplementation on knee osteoarthritis: A meta-analysis of randomized controlled trials. Int. J. Surg. 2017, 46, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Divjak, A.; Jovanovic, I.; Matic, A.; Lucic, A.T.; Gajovic, N.; Jurisevic, M.; Skevin, A.J.; Veselinovic, M. The influence of vitamin D supplementation on the expression of mediators of inflammation in knee osteoarthritis. Immunol. Res. 2022. Epub ahead of print. [Google Scholar]
- Muresan, G.C.; Hedesiu, M.; Lucaciu, O.; Boca, S.; Petrescu, N. Effect of Vitamin D on BoneRegeneration: A Review. Medicina 2022, 58, 1337. [Google Scholar] [CrossRef]
- Werny, J.G.; Sagheb, K.; Diaz, L.; Kämmerer, P.W.; Al-Nawas, B.; Schiegnitz, E. Does vitamin D have an effect on osseointegration of dental implants? A systematic review. Int. J. Implant. Dent. 2022, 8, 16. [Google Scholar] [CrossRef]
- Makke, A. Vitamin D Supplementation for Prevention of Dental Implant Failure: A Systematic Review. Int. J. Dent. 2022, 2022, 2845902. [Google Scholar] [CrossRef]
- Salomó-Coll, O.; Maté-Sánchez de Val, J.E.; Ramírez-Fernandez, M.P.; Hernández-Alfaro, F.; Gargallo-Albiol, J.; Calvo-Guirado, J.L. Topical applications of vitamin D on implant surface for bone-to-implant contact enhance: A pilot study in dogs part II. Clin. Oral. Implant. Res. 2016, 27, 896–903. [Google Scholar] [CrossRef]
- Dvorak, G.; Fügl, A.; Watzek, G.; Tangl, S.; Pokorny, P.; Gruber, R. Impact of dietary vitamin D on osseointegration in the ovariec-tomized rat. Clin. Oral. Implant. Res. 2012, 23, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek, J.; Jaroń, A.; Trybek, G. Impact of the 25-Hydroxycholecalciferol Concentration and Vitamin D Deficiency Treatment on Changes in the Bone Level at the Implant Site during the Process of Osseointegration: A Prospective, Randomized, Controlled Clinical Trial. J. Clin. Med. 2021, 10, 526. [Google Scholar] [CrossRef] [PubMed]
- Grønborg, I.M.; Tetens, I.; Andersen, E.W.; Kristensen, M.; Larsen, R.E.K.; Tran, T.L.L.; Andersen, R. Effect of vitamin D fortified foods on bone markers and muscle strength in women of Pakistani and Danish origin living in Denmark: A randomised controlled trial. Nutr. J. 2019, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Ohrbach, R.; Bair, E.; Fillingim, R.B.; Gonzalez, Y.; Gordon, S.M.; Lim, P.-F.; Ribeiro-Dasilva, M.; Diatchenko, L.; Dubner, R.; Greenspan, J.D.; et al. Clinical Orofacial Characteristics Associated With Risk of First-Onset TMD: The OPPERA Prospective Cohort Study. J. Pain 2013, 14 (Suppl. 12), T33–T50. [Google Scholar] [CrossRef]
- Wang, X.D.; Zhang, J.N.; Gan, Y.H.; Zhou, Y.H. Current Understanding of Pathogenesis and Treatment of TMJ Osteoarthritis. J. Dent. Res. 2015, 94, 666–673. [Google Scholar] [CrossRef]
- Tanaka, E.; Detamore, M.; Mercuri, L. Degenerative Disorders of the Temporomandibular Joint: Etiology, Diagnosis, and Treatment. J. Dent. Res. 2008, 87, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.H. Temporomandibular pain-dysfunction and occlusal relationships. Angle Orthod. 1973, 43, 136–153. [Google Scholar] [CrossRef]
- Cordray, F.E. Three-dimensional analysis of models articulated in the seated condylar position from a deprogrammed asymptomatic population: A prospective study. Part 1. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 619–630. [Google Scholar] [CrossRef]
- Manfredini, D.; Lombardo, L.; Siciliani, G. Temporomandibular disorders and dental occlusion. A systematic review of association studies: End of an era? J. Oral. Rehabil. 2017, 44, 908–923. [Google Scholar] [CrossRef]
- Michelotti, A.; Iodice, G. The role of orthodontics in temporomandibular disorders. J. Oral Rehabil. 2010, 37, 411–429. [Google Scholar] [CrossRef]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Is the Temporomandibular Joints’ Reciprocal Clicking Related to the Morphology and Position of the Mandible, as Well as to the Sagittal Position of Lower Incisors?-A Case-Control Study. Int. J. Environ. Res. Public Health 2021, 18, 4994. [Google Scholar] [CrossRef] [PubMed]
- Wiȩckiewicz, M.; Paradowska, A.; Kawala, B.; Wiȩckiewicz, W. SAPHO syndrome as a possible cause of masticatory system anomalies-A review of the literature. Adv. Clin. Exp. Med. 2011, 20, 521–525. [Google Scholar]
- Smardz, J.; Martynowicz, H.; Michalek-Zrabkowska, M.; Wojakowska, A.; Mazur, G.; Winocur, E.; Wieckiewicz, M. Sleep Bruxism and Occurrence of Temporomandibular Disorders-Related Pain: A Polysomnographic Study. Front. Neurol. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. International RDC/TMD Consortium Network, International association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [PubMed]
- Shen, M.; Luo, Y.; Niu, Y.; Chen, L.; Yuan, X.; Goltzman, D.; Chen, N.; Miao, D. 1,25(OH)2D deficiency induces temporomandibular joint osteoarthritis via secretion of senescence-associated inflammatory cytokines. Bone 2013, 55, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Kang, J.-H. Bone mineral density, bone microstructure, and bone turnover markers in females with temporomandibular joint osteoarthritis. Clin. Oral Investig. 2021, 25, 6435–6448. [Google Scholar] [CrossRef] [PubMed]
- Jagur, O.; Kull, M.; Leibur, E.; Kallikorm, R.; Loorits, D.; Lember, M.; Voog-Oras, U. Relationship between radiographic changes in the temporomandibular joint and bone mineral density: A population based study. Stomatologija 2011, 13, 42–48. [Google Scholar]
- Gupta, A.K.; Gupta, R.; Gill, S. Effectiveness of Vitamin D along with Splint therapy in the Vit D deficient patients with Temporomandibular disorder-A Randomized, double-blind, placebo-controlled clinical trial. J. Indian Prosthodont. Soc. 2022, 22, 65–73. [Google Scholar] [CrossRef]
- Demir, C.Y.; Ersoz, M.E. Biochemical changes associated with temporomandibular disorders. J. Int. Med. Res. 2018, 47, 765–771. [Google Scholar] [CrossRef]
- Madani, A.; Shamsian, S.A.; Layegh, P.; Abrisham, S.M.; Ravaghi, A.; Tayarani Najjaran, N. Are certain factors involved in calcium metabolism associated with temporomandibular disorders? Cranio 2021, 39, 202–208. [Google Scholar] [CrossRef]
- Kui, A.; Buduru, S.; Labunet, A.; Balhuc, S.; Negucioiu, M. Vitamin D and Temporomandibular Disorders: What Do We Know So Far? Nutrients 2021, 13, 1286. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).