Impact of Deoxynivalenol and Zearalenone as Single and Combined Treatment on DNA, Cell Cycle and Cell Proliferation in HepG2 Cells
Abstract
1. Introduction
2. Results
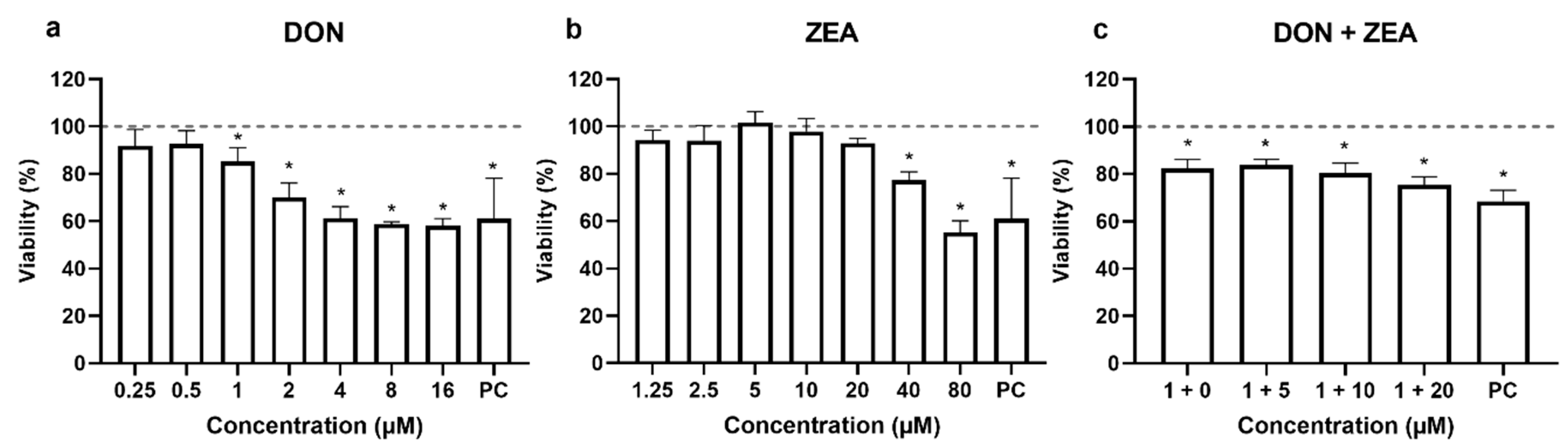
2.1. Cytotoxicity of DON, ZEA and Their Mixtures to HepG2 Cells
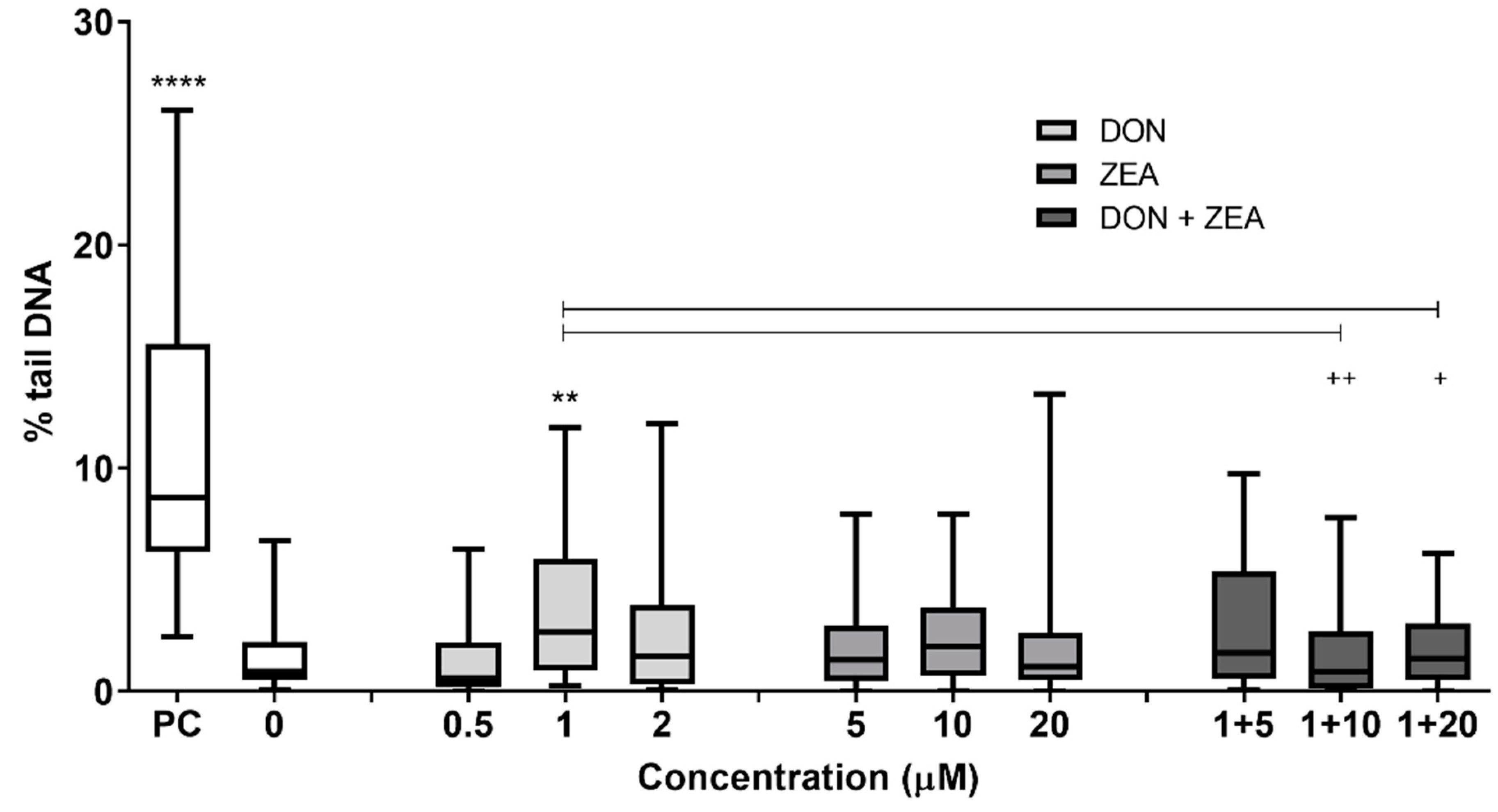
2.2. Genotoxic Activity of DON, ZEA and Their Mixtures in HepG2 Cells
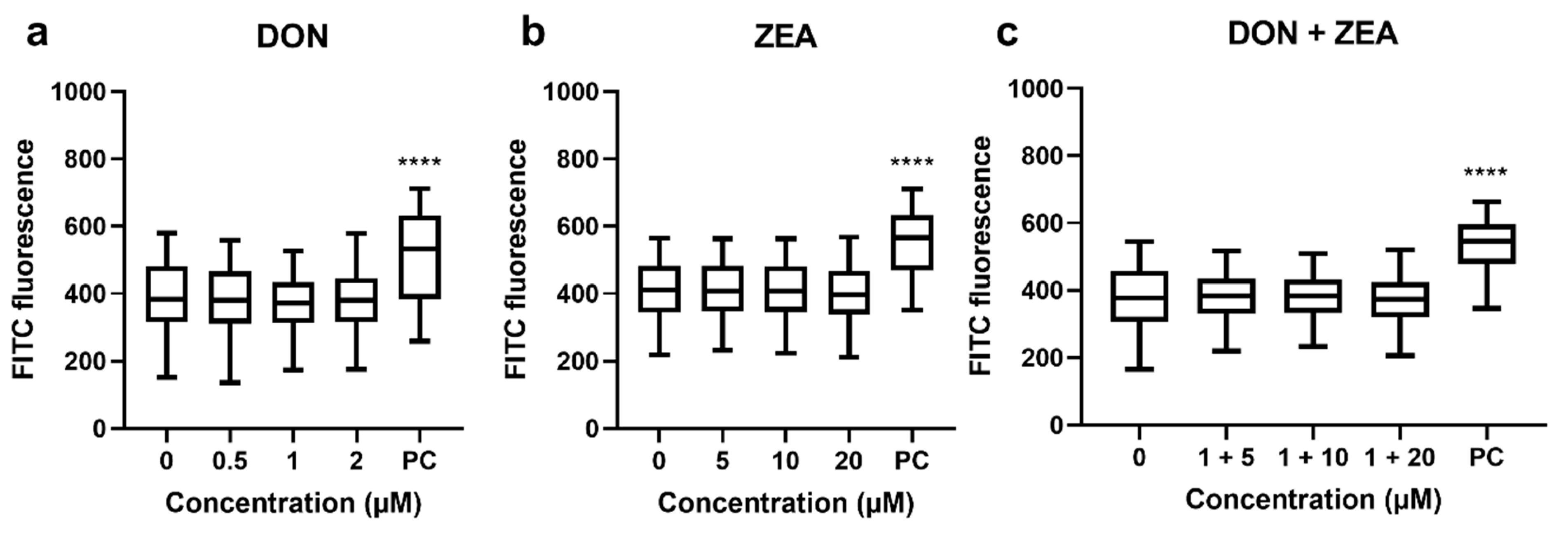
2.3. Effect of DON, ZEA and Their Mixtures on Cell Cycle Progression of HepG2 Cells
2.4. Effect of DON, ZEA and Their Mixtures on the Proliferation of HepG2 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Preparation of Mycotoxins’ Standard Solution
4.2. Cell Culture
4.3. Cytotoxicity—The MTS Assay
4.4. DNA Strand Breaks
4.5. Simultaneous Determination of the Gamma-H2AX Positive Cells, Cell Proliferation and Cell Cycle by Flow Cytometry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Sokolović, M.; Perši, N.; Zadravec, M.; Jaki, V.; Vulić, A. Contamination of maize with deoxynivalenol and zearalenone in Croatia. Food Control 2012, 28, 94–98. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Rastelli, S.; Mulazzi, A.; Donadini, G.; Pietri, A. Mycotoxin occurrence in beer produced in several European countries. Food Control 2011, 22, 2059–2064. [Google Scholar] [CrossRef]
- Peters, J.; Van Dam, R.; Van Doorn, R.; Katerere, D.; Berthiller, F.; Haasnoot, W.; Nielen, M.W.F. Mycotoxin profiling of 1000 beer samples with a special focus on craft beer. PLoS ONE 2017, 12, e0185887. [Google Scholar] [CrossRef] [PubMed]
- Heyndrickx, E.; Sioen, I.; Huybrechts, B.; Callebaut, A.; De Henauw, S.; De Saeger, S. Human biomonitoring of multiple mycotoxins in the Belgian population: Results of the BIOMYCO study. Environ. Int. 2015, 84, 82–89. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Gambacorta, L.; Visconti, A. Assessment of Multi-Mycotoxin Exposure in Southern Italy by Urinary Multi-Biomarker Determination. Toxins 2014, 6, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.C.; Rothwell, J.; White, K.L.M.; Gong, Y.Y.; Cade, J.; Wild, C.P. Urinary Deoxynivalenol Is Correlated with Cereal Intake in Individuals from the United Kingdom. Environ. Health Perspect. 2008, 116, 21–25. [Google Scholar] [CrossRef]
- Mayer, E.; Novak, B.; Springler, A.; Schwartz-Zimmermann, H.E.; Nagl, V.; Reisinger, N.; Hessenberger, S.; Schatzmayr, G. Effects of deoxynivalenol (DON) and its microbial biotransformation product deepoxy-deoxynivalenol (DOM-1) on a trout, pig, mouse, and human cell line. Mycotoxin Res. 2017, 33, 297–308. [Google Scholar] [CrossRef]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and Potential Effects on Humans. J. Toxicol. Environ. Health Part B 2005, 8, 39–69. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; He, X.; Cheng, W.-H.; Xu, W.; Luo, Y.; Liang, R.; Luo, H.; Huang, K. Analysis of Individual and Combined Effects of Ochratoxin A and Zearalenone on HepG2 and KK-1 Cells with Mathematical Models. Toxins 2014, 6, 1177–1192. [Google Scholar] [CrossRef]
- Liu, X.-L.; Wu, R.-Y.; Sun, X.-F.; Cheng, S.-F.; Zhang, R.-Q.; Zhang, T.-Y.; Zhang, X.-F.; Zhao, Y.; Shen, W.; Li, L. Mycotoxin zearalenone exposure impairs genomic stability of swine follicular granulosa cells in vitro. Int. J. Biol. Sci. 2018, 14, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.-L.; Wang, B.-J.; Wang, L.; Shan, Y.-P.; Zou, H.; Song, R.-L.; Wang, T.; Gu, J.-H.; Yuan, Y.; Liu, X.-Z.; et al. ROS-Mediated Cell Cycle Arrest and Apoptosis Induced by Zearalenone in Mouse Sertoli Cells via ER Stress and the ATP/AMPK Pathway. Toxins 2018, 10, 24. [Google Scholar] [CrossRef]
- IARC. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic aromatic Amines and Mycotoxins; World Health Organization, International Agency for Research on Cancer: Geneva, Switzerland, 1993. [Google Scholar]
- European Commission. Commission Recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, 229, 7–9. [Google Scholar]
- Payros, D.; Dobrindt, U.; Martin, P.; Secher, T.; Bracarense, A.P.F.L.; Boury, M.; Laffitte, J.; Pinton, P.; Oswald, E.; Oswald, I.P. The Food Contaminant Deoxynivalenol Exacerbates the Genotoxicity of Gut Microbiota. Mbio 2017, 8, e00007-17. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Guanghui, Z.; Xi, W.; Yingting, W.; Xialu, L.; Fangfang, Y.; Goldring, M.B.; Xiong, G.; Lammi, M.J. Cellular responses to T-2 toxin and/or deoxynivalenol that induce cartilage damage are not specific to chondrocytes. Sci. Rep. 2017, 7, 2231. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Li, Y.-H.; Lin, M.-F. Chronic Exposure to the Fusarium Mycotoxin Deoxynivalenol: Impact on Performance, Immune Organ, and Intestinal Integrity of Slow-Growing Chickens. Toxins 2017, 9, 334. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Banerjee, S.; Chattopadhyay, P.; Borthakur, S.K.; Veer, V. Deoxynivalenol induces cytotoxicity and genotoxicity in animal primary cell culture. Toxicol. Mech. Methods 2015, 25, 184–191. [Google Scholar] [CrossRef]
- Lorenz, N.; Dänicke, S.; Edler, L.; Gottschalk, C.; Lassek, E.; Marko, D.; Rychlik, M.; Mally, A. A critical evaluation of health risk assessment of modified mycotoxins with a special focus on zearalenone. Mycotoxin Res. 2019, 35, 27–46. [Google Scholar] [CrossRef]
- Ehrlich, V.; Darroudi, F.; Uhl, M.; Steinkellner, H.; Zsivkovits, M.; Knasmueller, S. Fumonisin B1 is genotoxic in human derived hepatoma (HepG2) cells. Mutagenesis 2002, 17, 257–260. [Google Scholar] [CrossRef]
- Knasmüller, S.; Mersch-Sundermann, V.; Kevekordes, S.; Darroudi, F.; Huber, W.W.; Hoelzl, C.; Bichler, J.; Majer, B.J. Use of human-derived liver cell lines for the detection of environmental and dietary genotoxicants; current state of knowledge. Toxicology 2004, 198, 315–328. [Google Scholar] [CrossRef]
- Waldherr, M.; Mišík, M.; Ferk, F.; Tomc, J.; Žegura, B.; Filipič, M.; Mikulits, W.; Mai, S.; Haas, O.; Huber, W.W.; et al. Use of HuH6 and other human-derived hepatoma lines for the detection of genotoxins: A new hope for laboratory animals? Arch. Toxicol. 2018, 92, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Azqueta, A.; Ladeira, C.; Giovannelli, L.; Boutet-Robinet, E.; Bonassi, S.; Neri, M.; Gajski, G.; Duthie, S.; Del Bo’, C.; Riso, P.; et al. Application of the comet assay in human biomonitoring: An hCOMET perspective. Mutat. Res. Rev. Mutat. Res. 2020, 783, 108288. [Google Scholar] [CrossRef] [PubMed]
- Gerić, M.; Gajski, G.; Garaj-Vrhovac, V. γ-H2AX as a biomarker for DNA double-strand breaks in ecotoxicology. Ecotoxicol. Environ. Saf. 2014, 105, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Žegura, B.; Filipič, M. Application of In Vitro Comet Assay for Genotoxicity Testing. In Optimization in Drug Discovery, Methods in Pharmacology and Toxicology; Yan, Z., Caldwell, G., Eds.; Hu-Mana Press: Totowa, NJ, USA, 2004; pp. 301–313. [Google Scholar]
- Khanna, K.K.; Jackson, S.P. DNA double-strand breaks: Signaling, repair and the cancer connection. Nat. Genet. 2001, 27, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Kopp, B.; Khoury, L.; Audebert, M. Validation of the γH2AX biomarker for genotoxicity assessment: A review. Arch. Toxicol. 2019, 93, 2103–2114. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA Double-stranded Breaks Induce Histone H2AX Phosphorylation on Serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef]
- Miller, I.; Min, M.; Yang, C.; Tian, C.; Gookin, S.; Carter, D.; Spencer, S.L. Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep. 2018, 24, 1105–1112.e5. [Google Scholar] [CrossRef]
- Wan, L.Y.M.; Turner, P.C.; El-Nezami, H. Individual and combined cytotoxic effects of Fusarium toxins (deoxynivalenol, nivalenol, zearalenone and fumonisins B1) on swine jejunal epithelial cells. Food Chem. Toxicol. 2013, 57, 276–283. [Google Scholar] [CrossRef]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Kachlek, M.; Szabó-Fodor, J.; Bodnár, Z.B.; Horvatovich, K.; Kovács, M. Preliminary results on the interactive effects of deoxynivalenol, zearalenone and fumonisin B1 on porcine lymphocytes. Acta Vet. Hung. 2017, 65, 340–353. [Google Scholar] [CrossRef]
- Zhou, H.; George, S.; Hay, C.; Lee, J.; Qian, H.; Sun, X. Individual and combined effects of Aflatoxin B 1, Deoxynivalenol and Zearalenone on HepG2 and RAW 264.7 cell lines. Food Chem. Toxicol. 2017, 103, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Juan-García, A.; Taroncher, M.; Font, G.; Ruiz, M.-J. Micronucleus induction and cell cycle alterations produced by deoxynivalenol and its acetylated derivatives in individual and combined exposure on HepG2 cells. Food Chem. Toxicol. 2018, 118, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Kouadio, J.H.; Dano, S.D.; Moukha, S.; Mobio, T.A.; Creppy, E.E. Effects of combinations of Fusarium mycotoxins on the inhibition of macromolecular synthesis, malondialdehyde levels, DNA methylation and fragmentation, and viability in Caco-2 cells. Toxicon 2007, 49, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chung, D.H.; Kim, Y.B.; Choi, Y.H.; Moon, Y. Ribotoxic mycotoxin deoxynivalenol induces G2/M cell cycle arrest via p21Cip/WAF1 mRNA stabilization in human epithelial cells. Toxicology 2008, 243, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current Situation of Mycotoxin Contamination and Co-occurrence in Animal Feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, L.; Geng, C.; Cao, J.; Zhong, L. The role of oxidative stress in deoxynivalenol-induced DNA damage in HepG2 cells. Toxicon 2009, 54, 513–518. [Google Scholar] [CrossRef]
- Bony, S.; Carcelen, M.; Olivier, L.; Devaux, A. Genotoxicity assessment of deoxynivalenol in the Caco-2 cell line model using the Comet assay. Toxicol. Lett. 2006, 166, 67–76. [Google Scholar] [CrossRef]
- Mishra, S.; Tewari, P.; Chaudhari, B.P.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Deoxynivalenol induced mouse skin tumor initiation: Elucidation of molecular mechanisms in human HaCaT keratinocytes. Int. J. Cancer 2016, 139, 2033–2046. [Google Scholar] [CrossRef]
- Takakura, N.; Nesslany, F.; Fessard, V.; Le Hegarat, L. Absence of in vitro genotoxicity potential of the mycotoxin deoxynivalenol in bacteria and in human TK6 and HepaRG cell lines. Food Chem. Toxicol. 2014, 66, 113–121. [Google Scholar] [CrossRef]
- Gao, F.; Jiang, L.-P.; Chen, M.; Geng, C.-Y.; Yang, G.; Ji, F.; Zhong, L.-F.; Liu, X.-F. Genotoxic effects induced by zearalenone in a human embryonic kidney cell line. Mutat. Res. Toxicol. Environ. Mutagen. 2013, 755, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Szabó-Fodor, J.; Szabó, A.; Kócsó, D.; Marosi, K.; Bóta, B.; Kachlek, M.; Mézes, M.; Balogh, K.; Kövér, G.; Nagy, I.; et al. Interaction between the three frequently co-occurring Fusarium mycotoxins in rats. J. Anim. Physiol. Anim. Nutr. 2019, 103, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Mu, P.; Huang, B.; Li, H.; Mu, H.; Deng, Y. EGR1 is essential for deoxynivalenol-induced G2/M cell cycle arrest in HepG2 cells via the ATF3ΔZip2a/2b-EGR1-p21 pathway. Toxicol. Lett. 2018, 299, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mu, P.; Wen, J.; Deng, Y. Carrier-Mediated and Energy-Dependent Uptake and Efflux of Deoxynivalenol in Mammalian Cells. Sci. Rep. 2017, 7, 5889. [Google Scholar] [CrossRef]
- Hercog, K.; Maisanaba, S.; Filipič, M.; Sollner-Dolenc, M.; Kač, L.; Žegura, B. Genotoxic activity of bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF and their mixtures in human hepatocellular carcinoma (HepG2) cells. Sci. Total. Environ. 2019, 687, 267–276. [Google Scholar] [CrossRef]
- Collins, A.; Møller, P.; Gajski, G.; Vodenková, S.; Abdulwahed, A.; Anderson, D.; Bankoglu, E.E.; Bonassi, S.; Boutet-Robinet, E.; Brunborg, G.; et al. Measuring DNA modifications with the comet assay: A compendium of protocols. Nat. Protoc. 2023. [Google Scholar] [CrossRef]
- Møller, P.; Azqueta, A.; Boutet-Robinet, E.; Koppen, G.; Bonassi, S.; Milić, M.; Gajski, G.; Costa, S.; Teixeira, J.P.; Pereira, C.C.; et al. Minimum Information for Reporting on the Comet Assay (MIRCA): Recommendations for describing comet assay procedures and results. Nat. Protoc. 2020, 15, 3817–3826. [Google Scholar] [CrossRef]
- Štampar, M.; Breznik, B.; Filipič, M.; Žegura, B. Characterization of In Vitro 3D Cell Model Developed from Human Hepatocellular Carcinoma (HepG2) Cell Line. Cells 2020, 9, 2557. [Google Scholar] [CrossRef]
- Ujvárosi, A.Z.; Hercog, K.; Riba, M.; Gonda, S.; Filipič, M.; Vasas, G.; Žegura, B. The cyanobacterial oligopeptides microginins induce DNA damage in the human hepatocellular carcinoma (HepG2) cell line. Chemosphere 2020, 240, 124880. [Google Scholar] [CrossRef]
- Ramaiahgari, S.C.; den Braver, M.W.; Herpers, B.; Terpstra, V.; Commandeur, J.N.M.; Van De Water, B.; Price, L.S. A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies. Arch. Toxicol. 2014, 88, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Štampar, M.; Žabkar, S.; Filipič, M.; Žegura, B. HepG2 spheroids as a biosensor-like cell-based system for (geno)toxicity assessment. Chemosphere 2022, 291, 132805. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domijan, A.-M.; Hercog, K.; Štampar, M.; Gajski, G.; Gerić, M.; Sokolović, M.; Žegura, B. Impact of Deoxynivalenol and Zearalenone as Single and Combined Treatment on DNA, Cell Cycle and Cell Proliferation in HepG2 Cells. Int. J. Mol. Sci. 2023, 24, 4082. https://doi.org/10.3390/ijms24044082
Domijan A-M, Hercog K, Štampar M, Gajski G, Gerić M, Sokolović M, Žegura B. Impact of Deoxynivalenol and Zearalenone as Single and Combined Treatment on DNA, Cell Cycle and Cell Proliferation in HepG2 Cells. International Journal of Molecular Sciences. 2023; 24(4):4082. https://doi.org/10.3390/ijms24044082
Chicago/Turabian StyleDomijan, Ana-Marija, Klara Hercog, Martina Štampar, Goran Gajski, Marko Gerić, Marijana Sokolović, and Bojana Žegura. 2023. "Impact of Deoxynivalenol and Zearalenone as Single and Combined Treatment on DNA, Cell Cycle and Cell Proliferation in HepG2 Cells" International Journal of Molecular Sciences 24, no. 4: 4082. https://doi.org/10.3390/ijms24044082
APA StyleDomijan, A.-M., Hercog, K., Štampar, M., Gajski, G., Gerić, M., Sokolović, M., & Žegura, B. (2023). Impact of Deoxynivalenol and Zearalenone as Single and Combined Treatment on DNA, Cell Cycle and Cell Proliferation in HepG2 Cells. International Journal of Molecular Sciences, 24(4), 4082. https://doi.org/10.3390/ijms24044082








