Enhanced CO2 Capture of Poly(amidoamine)-Modified Graphene Oxide Aerogels with the Addition of Carbon Nanotubes
Abstract
1. Introduction
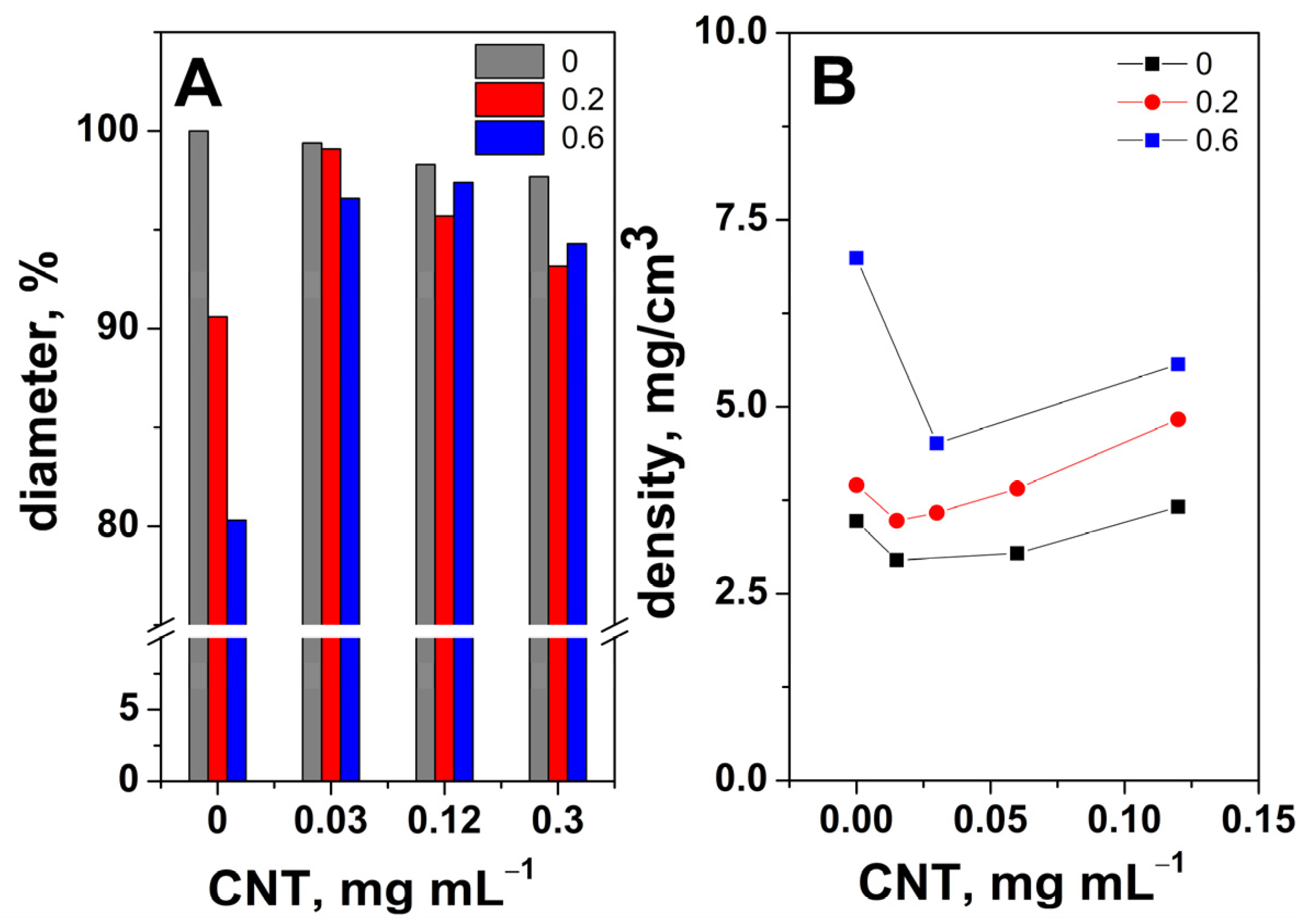
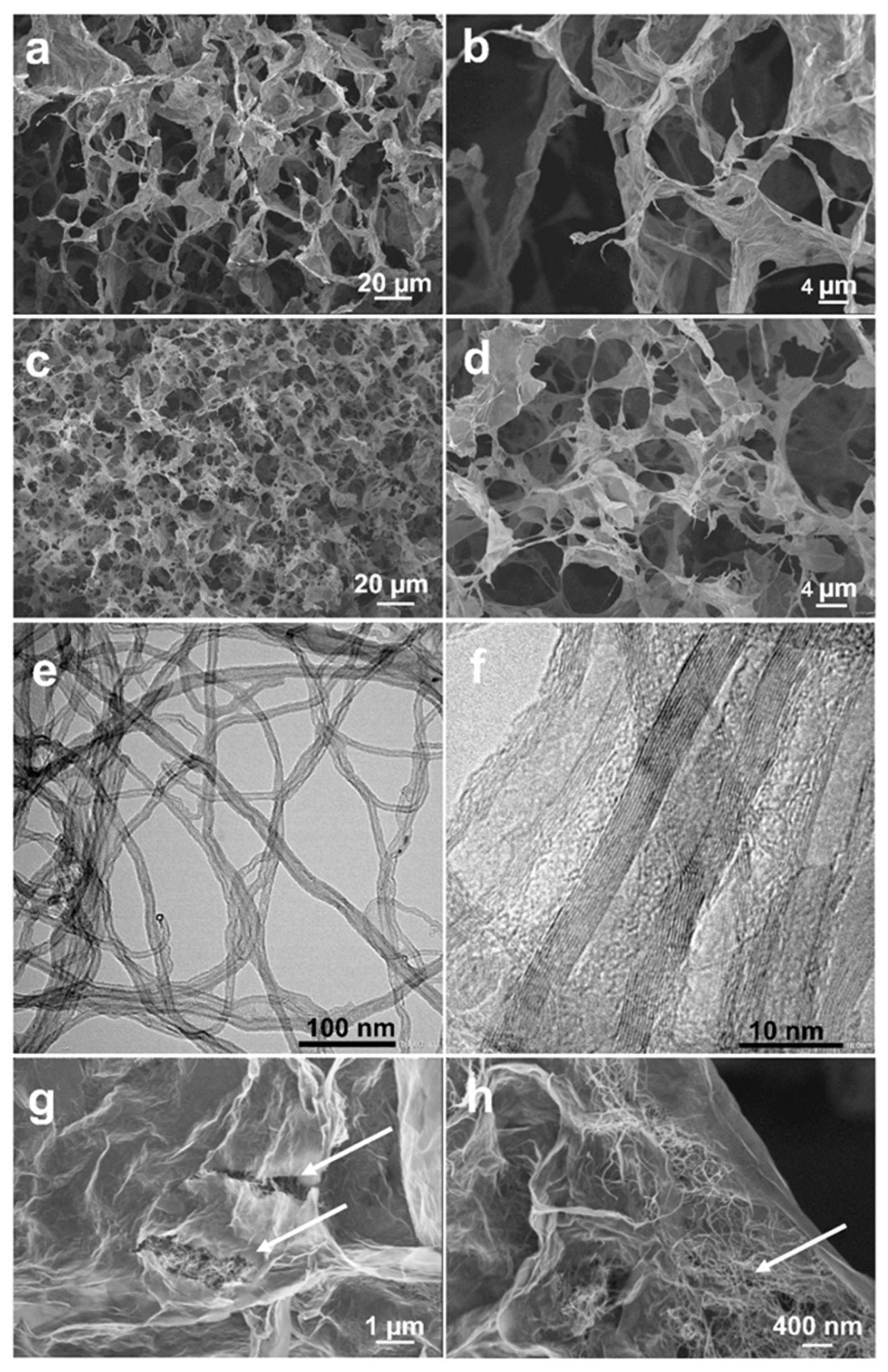
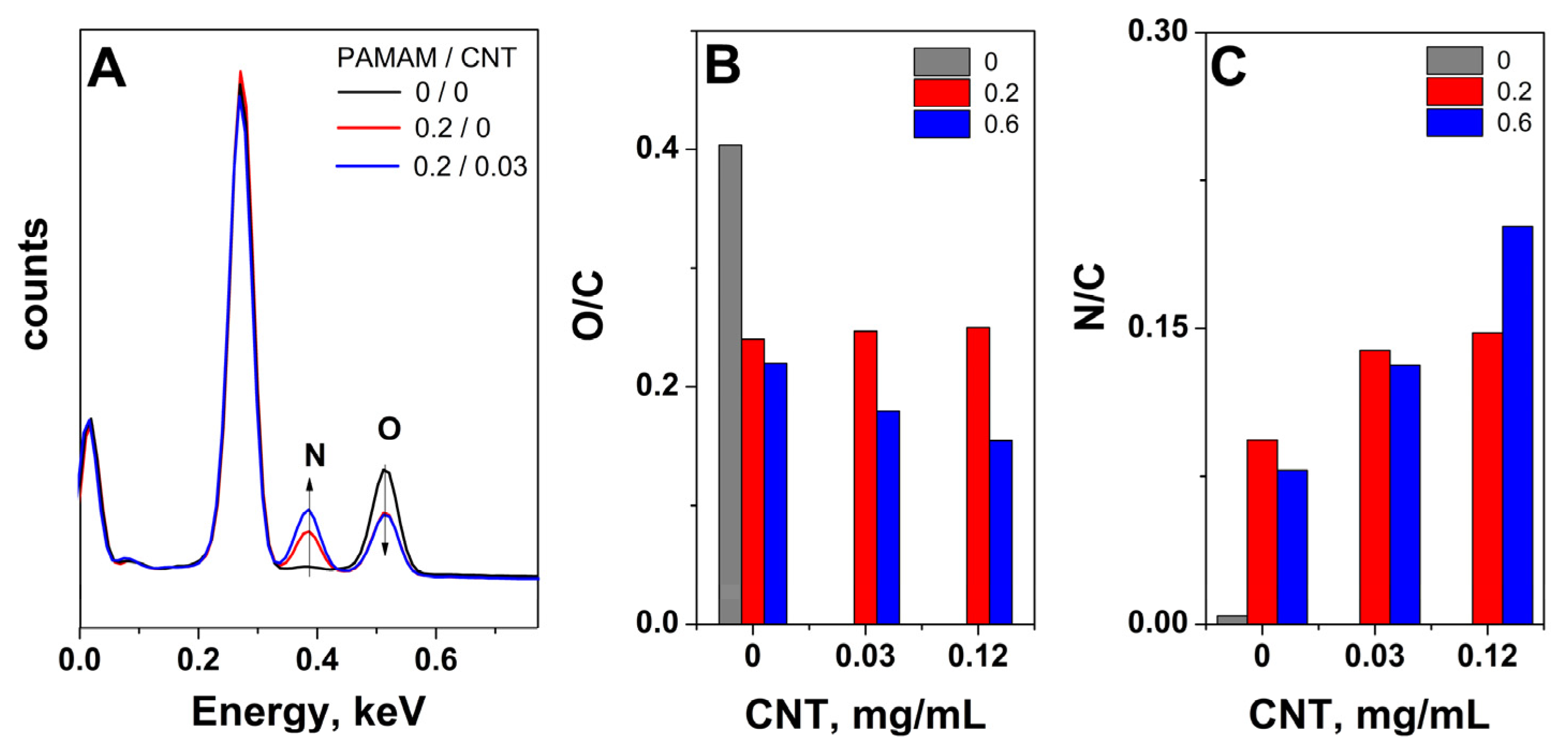
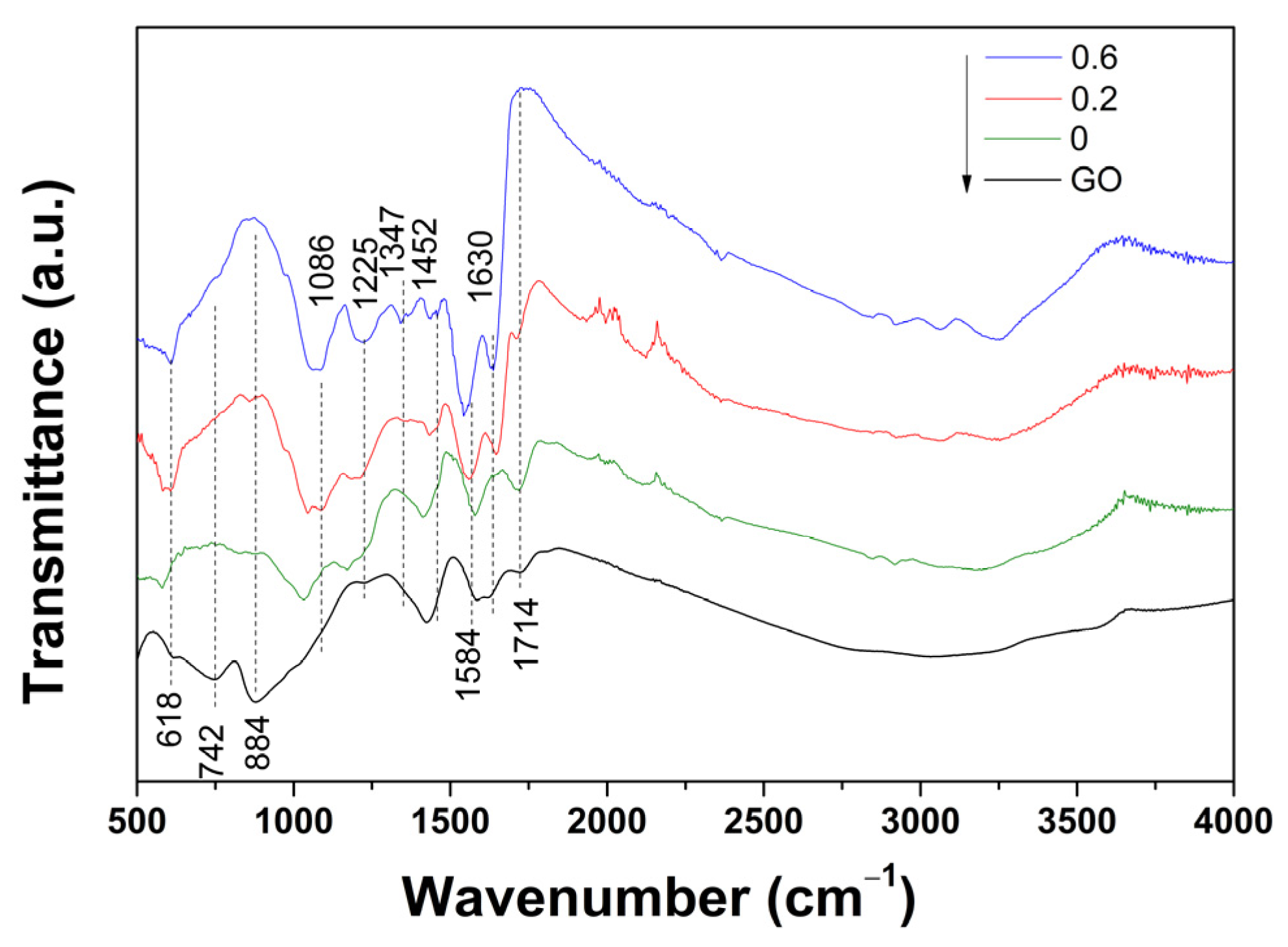
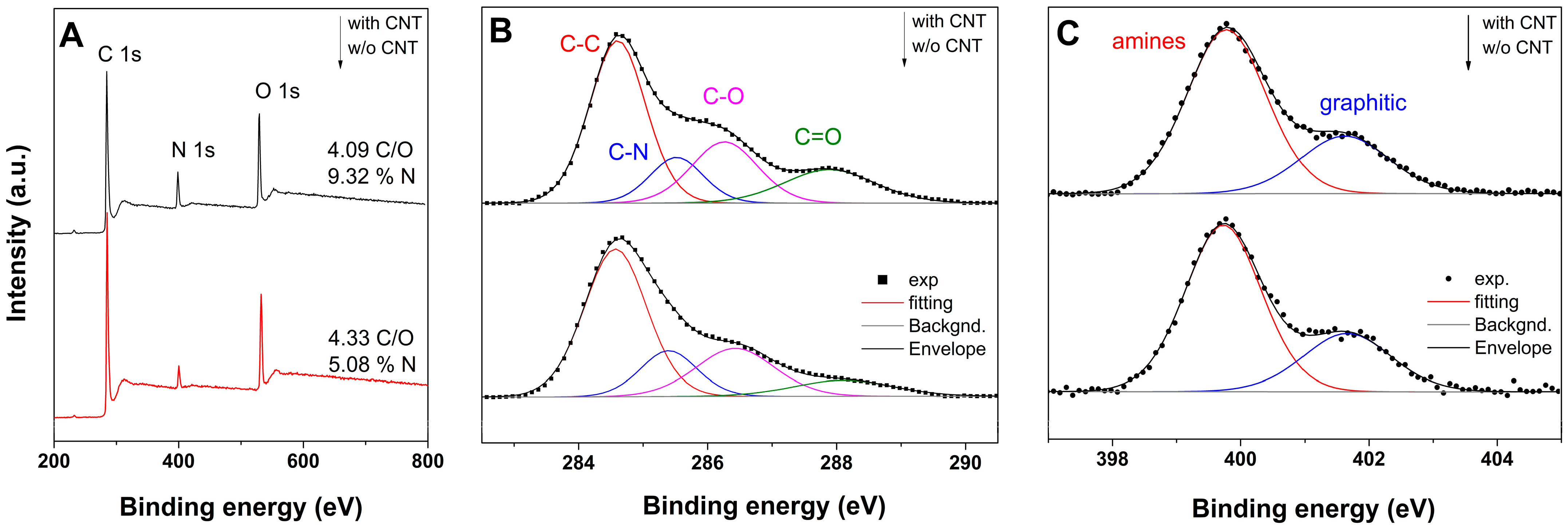
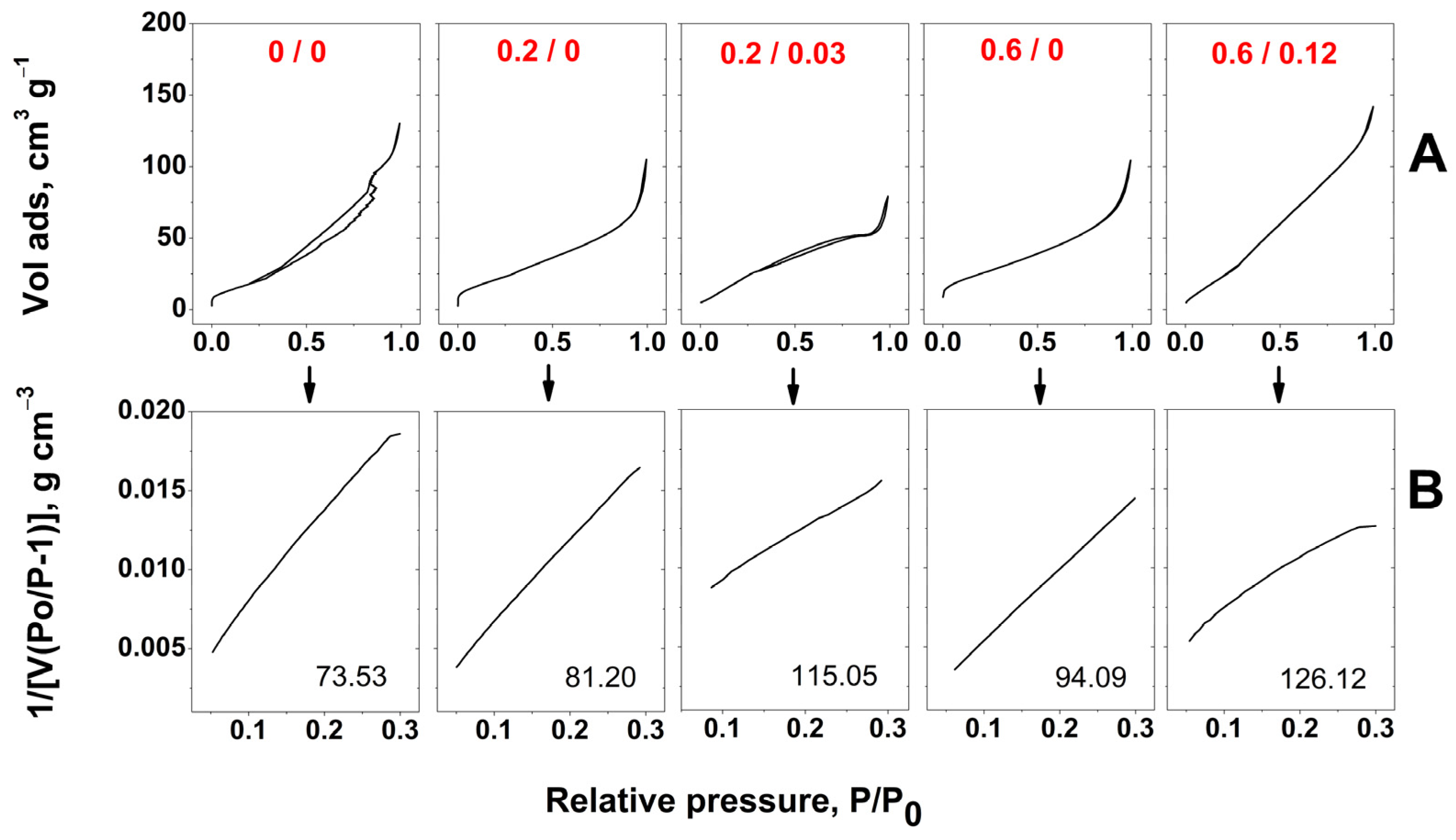
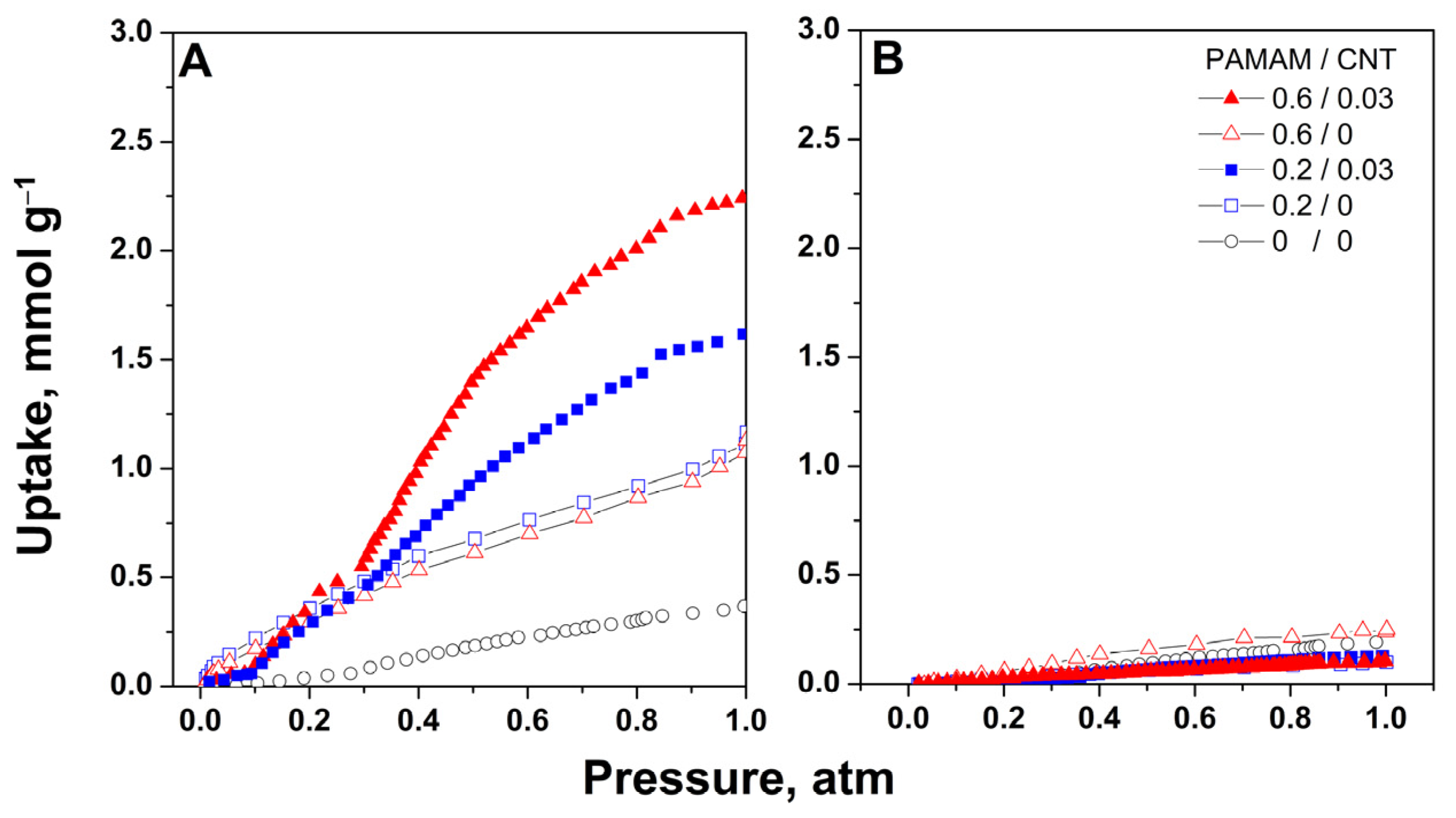
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younas, M.; Rezakazemi, M.; Daud, M.; Wazir, M.B.; Ahmad, S.; Ullah, N.; Inamuddin; Ramakrishna, S. Recent progress and remaining challenges in post-combustion CO2 capture using metal-organic frameworks (MOFs). Prog. Energy Combust. Sci. 2020, 80, 100849. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Ismail, I.; Vaithilingam, B.V.; Polychronopoulou, K.; Singaravel, G.; Morin, S.; Berthod, M.; Al Wahedi, Y. Synthesis of hierarchical porous Zeolite-Y for enhanced CO2 capture. Microporous Mesoporous Mater. 2020, 303, 110261. [Google Scholar] [CrossRef]
- Wang, H.; Chen, C.; Chen, Y.; Wan, H.; Dong, L.; Guan, G. Construction of ultramicropore-enriched N-doped carbons for CO2 capture: Self-decomposition of polyethyleneimine-based precursor to promote pore formation and surface polarity. J. Environ. Chem. Eng. 2021, 9, 105046. [Google Scholar] [CrossRef]
- Cong, H.P.; Chen, J.F.; Yu, S.H. Graphene-based macroscopic assemblies and architectures: An emerging material system. Chem. Soc. Rev. 2014, 43, 7295–7325. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Wei, Z.; Xia, X.; Hong, Z.; Li, S. CO2 adsorption performance of CuBTC/graphene aerogel composites. J. Nanopart. Res. 2020, 22, 191. [Google Scholar] [CrossRef]
- Pruna, A.; Cárcel, A.C.; Benedito, A.; Giménez, E. Effect of synthesis conditions on CO2 capture of ethylenediamine-modified graphene aerogels. Appl. Surf. Sci. 2019, 487, 228–235. [Google Scholar] [CrossRef]
- Pruna, A.I.; Cárcel, A.C.; Benedito, A.; Giménez, E. The effect of solvothermal conditions on the properties of three-dimensional N-doped graphene aerogels. Nanomaterials 2019, 9, 350. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, Q.; Tang, L.A.L.; Zhong, Y.; Loh, K.P. Hydrothermal dehydration for the “green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties. Chem. Mater. 2009, 21, 2950–2956. [Google Scholar] [CrossRef]
- Garcia-Bordejé, E.; Benito, A.M.; Maser, W.K. Graphene aerogels via hydrothermal gelation of graphene oxide colloids: Fine-tuning of its porous and chemical properties and catalytic applications. Adv. Colloid Interface Sci. 2021, 292, 102420. [Google Scholar] [CrossRef]
- Xie, X.; Zhou, Y.; Bi, H.; Yin, K.; Wan, S.; Sun, L. Large-range control of the microstructures and properties of three-dimensional porous graphene. Sci. Rep. 2013, 3, 2117. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.-M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef]
- Deville, S. Freeze-casting of porous ceramics: A review of current achievements and issues. Adv. Eng. Mater. 2008, 10, 155–169. [Google Scholar]
- Zhu, X.; Yang, C.; Wu, P.; Ma, Z.; Shang, Y.; Bai, G.; Liu, X.; Chang, G.; Li, N.; Dai, J.; et al. Precise control of versatile microstructure and properties of graphene aerogel: Via freezing manipulation. Nanoscale 2020, 12, 4882–4894. [Google Scholar] [CrossRef] [PubMed]
- Pruna, A.I.; Barjola, A.; Cárcel, A.C.; Alonso, B.; Giménez, E. Effect of varying amine functionalities on CO2 capture of carboxylated graphene oxide-based cryogels. Nanomaterials 2020, 10, 1446. [Google Scholar] [CrossRef] [PubMed]
- Fadhel, B.; Hearn, M.; Chaffee, A. CO2 adsorption by PAMAM dendrimers: Significant effect of impregnation into SBA-15. Microporous Mesoporous Mater. 2009, 123, 140–149. [Google Scholar] [CrossRef]
- Duan, S.; Kai, T.; Taniguchi, I.; Kazama, S. Development of poly(amidoamine) dendrimer/poly(ethylene glycol) hybrid membranes for CO2 capture at elevated pressures. Energy Procedia 2014, 63, 167–173. [Google Scholar]
- Shah, K.J.; Imae, T.; Shukla, A. Selective capture of CO2 by poly(amido amine) dendrimer-loaded organoclays. RSC Adv. 2015, 5, 35985–35992. [Google Scholar] [CrossRef]
- Pruna, A.; Cárcel, A.; Benedito, A.; Giménez, E. Hydrothermal-freeze-casting of poly(amidoamine)-modified graphene aerogels towards CO2 adsorption. Int. J. Mol. Sci. 2021, 22, 9333. [Google Scholar] [CrossRef] [PubMed]
- Bahadir, E.B.; Sezgintürk, M.K. Poly(amidoamine) (PAMAM): An emerging material for electrochemical bio(sensing) applications. Talanta 2016, 148, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Wu, T.; Chen, B. One-step synthesis of graphene oxide-polyamidoamine dendrimer nanocomposite hydrogels by self-assembly. Ind. Eng. Chem. Res. 2016, 55, 6113–6121. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, L.; Liu, D. Ultralight graphene/carbon nanotubes aerogels with compressibility and oil absorption properties. Materials 2018, 11, 641. [Google Scholar] [CrossRef]
- De Marco, M.; Menzel, R.; Bawaked, S.M.; Mokhtar, M.; Obaid, A.Y.; Basahel, S.N.; Shaffer, M.S.P. Hybrid effects in graphene oxide/carbon nanotube-supported layered double hydroxides: Enhancing the CO2 sorption properties. Carbon N. Y. 2017, 123, 616–627. [Google Scholar] [CrossRef]
- Li, J.; Tang, J.; Yuan, J.; Zhang, K.; Yu, X.; Sun, Y.; Zhang, H.; Qin, L.-C. Porous carbon nanotube/graphene composites for high-performance supercapacitors. Chem. Phys. Lett. 2018, 693, 60–65. [Google Scholar] [CrossRef]
- Hu, K.; Xie, X.; Szkopek, T.; Cerruti, M. Understanding hydrothermally reduced graphene oxide hydrogels: From reaction products to hydrogel properties. Chem. Mater. 2016, 28, 1756–1768. [Google Scholar] [CrossRef]
- Acik, M.; Lee, G.; Mattevi, C.; Pirkle, A.; Wallace, R.M.; Chhowalla, M.; Cho, K.; Chabal, Y. The role of oxygen during thermal reduction of graphene oxide studied by infrared absorption spectroscopy. J. Phys. Chem. C 2011, 115, 19761–19781. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon N. Y. 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Seol, Y.G.; Trung, T.Q.; Yoon, O.-J.; Sohn, I.-Y.; Lee, N.-E. Nanocomposites of reduced graphene oxide nanosheets and conducting polymer for stretchable transparent conducting electrodes. J. Mater. Chem. 2012, 22, 23759–23766. [Google Scholar] [CrossRef]
- Ma, Y.-X.; Xing, D.; Shao, W.-J.; Du, X.-Y.; La, P.-Q. Preparation of polyamidoamine dendrimers functionalized magnetic graphene oxide for the adsorption of Hg(II) in aqueous solution. J. Colloid Interface Sci. 2017, 505, 352–363. [Google Scholar] [CrossRef]
- Park, S.; Dikin, D.A.; Nguyen, S.T.; Ruoff, R.S. Graphene oxide sheets chemically cross-linked by polyallylamine. J. Phys. Chem. C 2009, 113, 15801–15804. [Google Scholar] [CrossRef]
- Nonahal, M.; Rastin, H.; Saeb, M.R.; Sari, M.G.; Moghadam, M.H.; Zarrintaj, P.; Ramezanzadeh, B. Epoxy/PAMAM dendrimer-modified graphene oxide nanocomposite coatings: Nonisothermal cure kinetics study. Prog. Org. Coat. 2018, 114, 233–243. [Google Scholar] [CrossRef]
- Rafi, M.; Samiey, B.; Cheng, C.-H. GO/PAMAM as a high capacity adsorbent for removal of alizarin red S: Selective separation of dyes. Acta Chim. Slov. 2020, 2020, 1124–1138. [Google Scholar] [CrossRef]
- Ma, H.-L.; Zhang, H.-B.; Hu, Q.-H.; Li, W.-J.; Jiang, Z.-G.; Yu, Z.-Z.; Dasari, A. Functionalization and reduction of graphene oxide with p-phenylene diamine for electrically conductive and thermally stable polystyrene composites. ACS Appl. Mater. Interfaces 2012, 4, 1948–1953. [Google Scholar] [CrossRef]
- Bera, M.; Gupta, P.; Maji, P.K. Efficacy of ultra-low loading of amine functionalized graphene oxide into glycidol-terminated polyurethane for high-performance composite material. React. Funct. Polym. 2019, 139, 60–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, K.; Liu, X.; Ma, W.; Li, S.; Wang, J.; Fan, S. Functionalization of partially reduced graphene oxide hydrogels with 2-Aminopyridine for high-performance symmetric supercapacitors. J. Mater. Sci. Mater. Electron. 2021, 32, 18728–18740. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Kirilenko, D.A.; Ulin, N.V.; Baidakova, M.V.; Shnitov, V.V.; Pavlov, S.I.; Chumakov, R.G.; Stolyarova, D.Y.; Besedina, N.A.; et al. From graphene oxide towards aminated graphene: Facile synthesis, its structure and electronic properties. Sci. Rep. 2020, 10, 6902. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Albero, J.; Wahby, A.; Sepúlveda-Escribano, A.; Martínez-Escandell, M.; Kaneko, K.; Rodríguez-Reinoso, F. Ultrahigh CO2 adsorption capacity on carbon molecular sieves at room temperature. Chem. Commun. 2011, 47, 6840–6842. [Google Scholar] [CrossRef]
- Chowdhury, S.; Balasubramanian, R. Highly efficient, rapid and selective CO2 capture by thermally treated graphene nanosheets. J. CO2 Util. 2016, 13, 50–60. [Google Scholar] [CrossRef]
- López-Aranguren, P.; Builes, S.; Fraile, J.; López-Periago, A.; Vega, L.F.; Domingo, C. Hybrid aminopolymer-silica materials for efficient CO2 adsorption. RSC Adv. 2015, 5, 104943–104953. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Alshahrani, A.F.; Khdary, N.H.; Alharthi, F.A.; Alattas, H.A.; Adil, S.F. Enhanced CO2 adsorption by nitrogen-doped graphene oxide sheets (N-GOs) prepared by employing polymeric precursors. Materials 2018, 11, 578. [Google Scholar] [CrossRef]
- Shin, G.-J.; Rhee, K.; Park, S.-J. Improvement of CO2 capture by graphite oxide in presence of polyethylenimine. Int. J. Hydrogen Energy 2016, 41, 14351–14359. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruna, A.I.; Cárcel, A.; Benedito, A.; Giménez, E. Enhanced CO2 Capture of Poly(amidoamine)-Modified Graphene Oxide Aerogels with the Addition of Carbon Nanotubes. Int. J. Mol. Sci. 2023, 24, 3865. https://doi.org/10.3390/ijms24043865
Pruna AI, Cárcel A, Benedito A, Giménez E. Enhanced CO2 Capture of Poly(amidoamine)-Modified Graphene Oxide Aerogels with the Addition of Carbon Nanotubes. International Journal of Molecular Sciences. 2023; 24(4):3865. https://doi.org/10.3390/ijms24043865
Chicago/Turabian StylePruna, Alina Iuliana, Alfonso Cárcel, Adolfo Benedito, and Enrique Giménez. 2023. "Enhanced CO2 Capture of Poly(amidoamine)-Modified Graphene Oxide Aerogels with the Addition of Carbon Nanotubes" International Journal of Molecular Sciences 24, no. 4: 3865. https://doi.org/10.3390/ijms24043865
APA StylePruna, A. I., Cárcel, A., Benedito, A., & Giménez, E. (2023). Enhanced CO2 Capture of Poly(amidoamine)-Modified Graphene Oxide Aerogels with the Addition of Carbon Nanotubes. International Journal of Molecular Sciences, 24(4), 3865. https://doi.org/10.3390/ijms24043865






