Unraveling the Potential Role of Tecomella undulata in Experimental NASH
Abstract
1. Introduction
2. Results
2.1. Quality Evaluation and Dose Selection of Tecomella undulata for Preclinical Studies
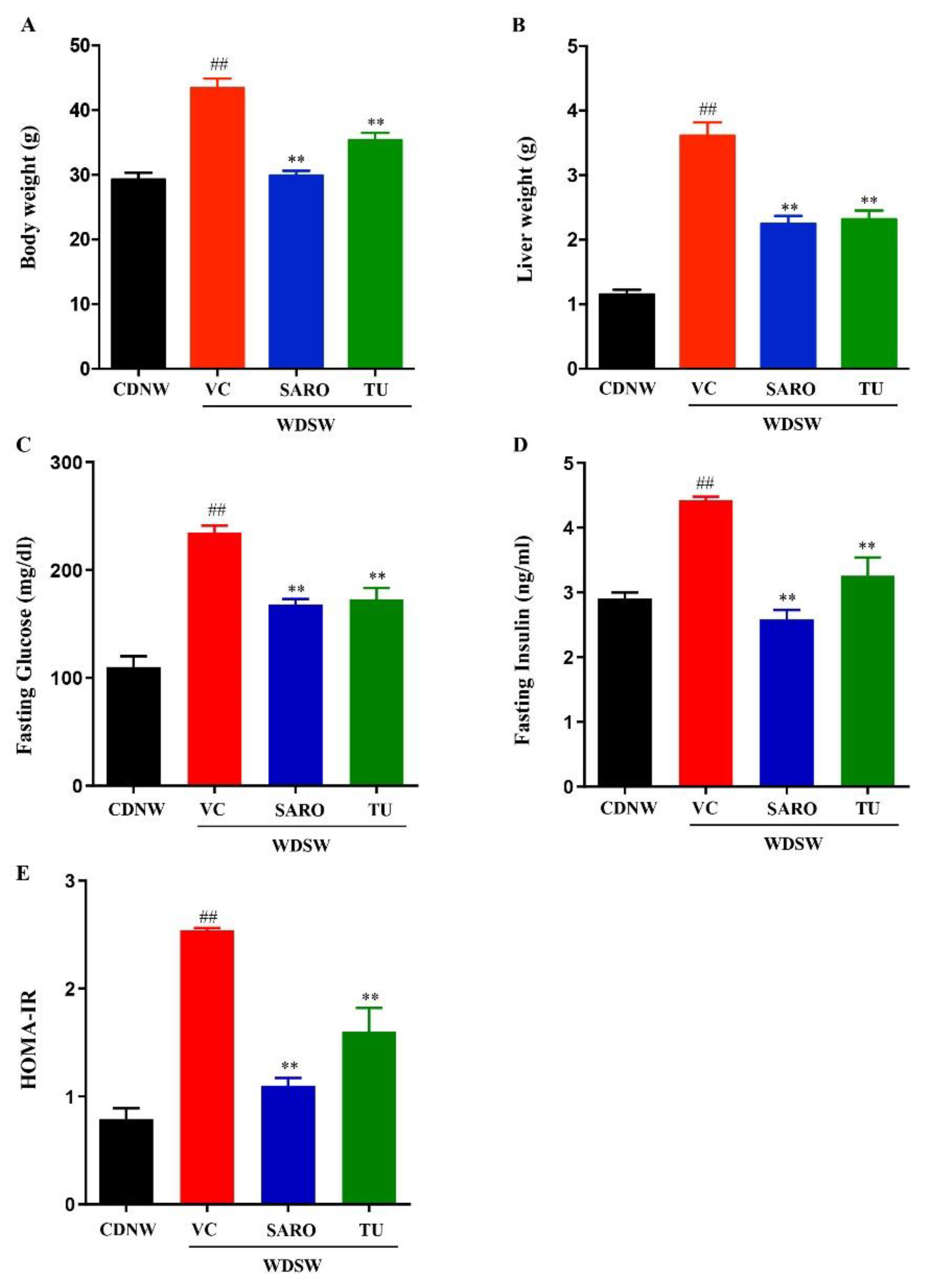
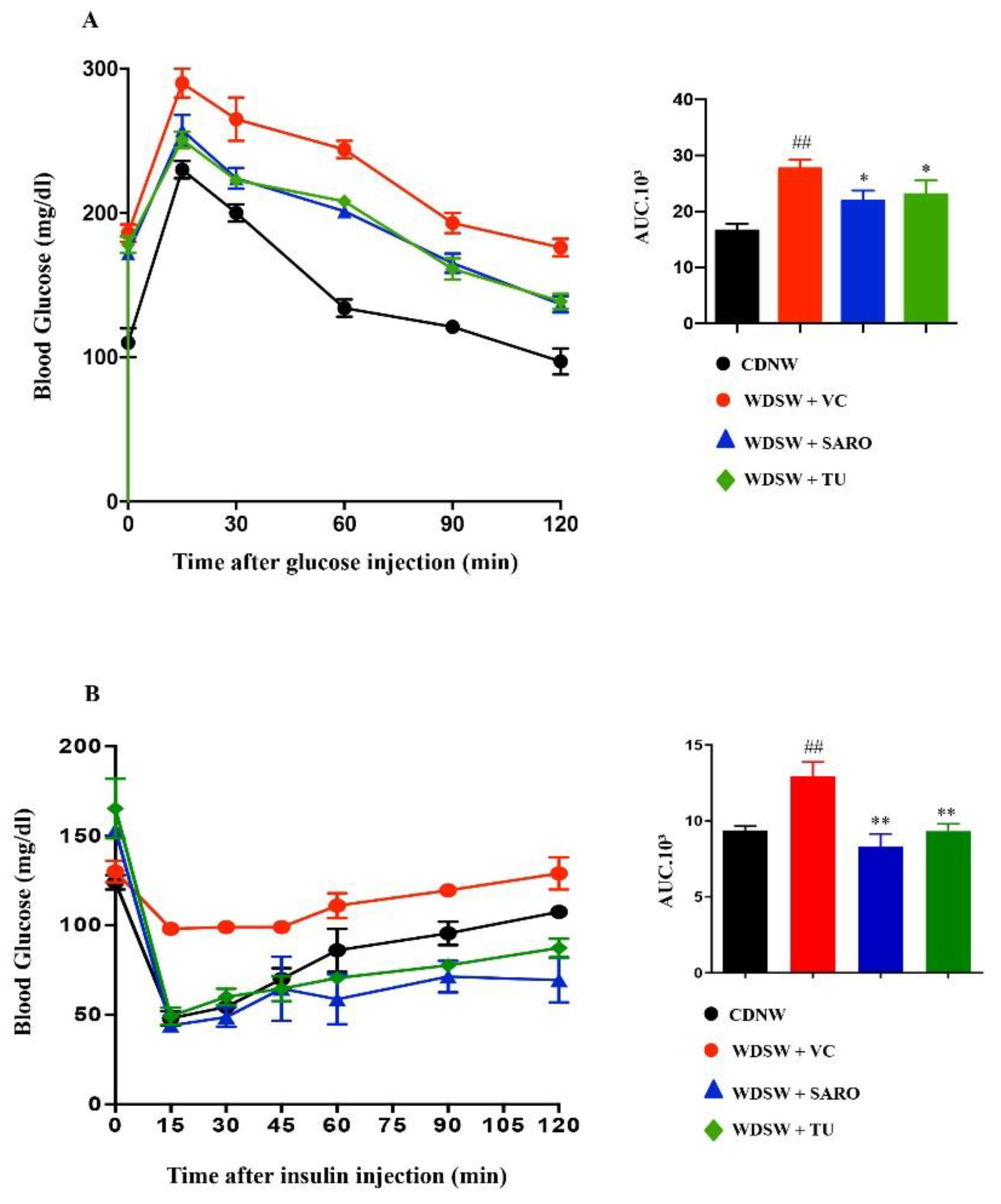
2.2. Tecomella undulata Treatment Ameliorates Western Diet Sugar Water-induced Obesity and Insulin Resistance
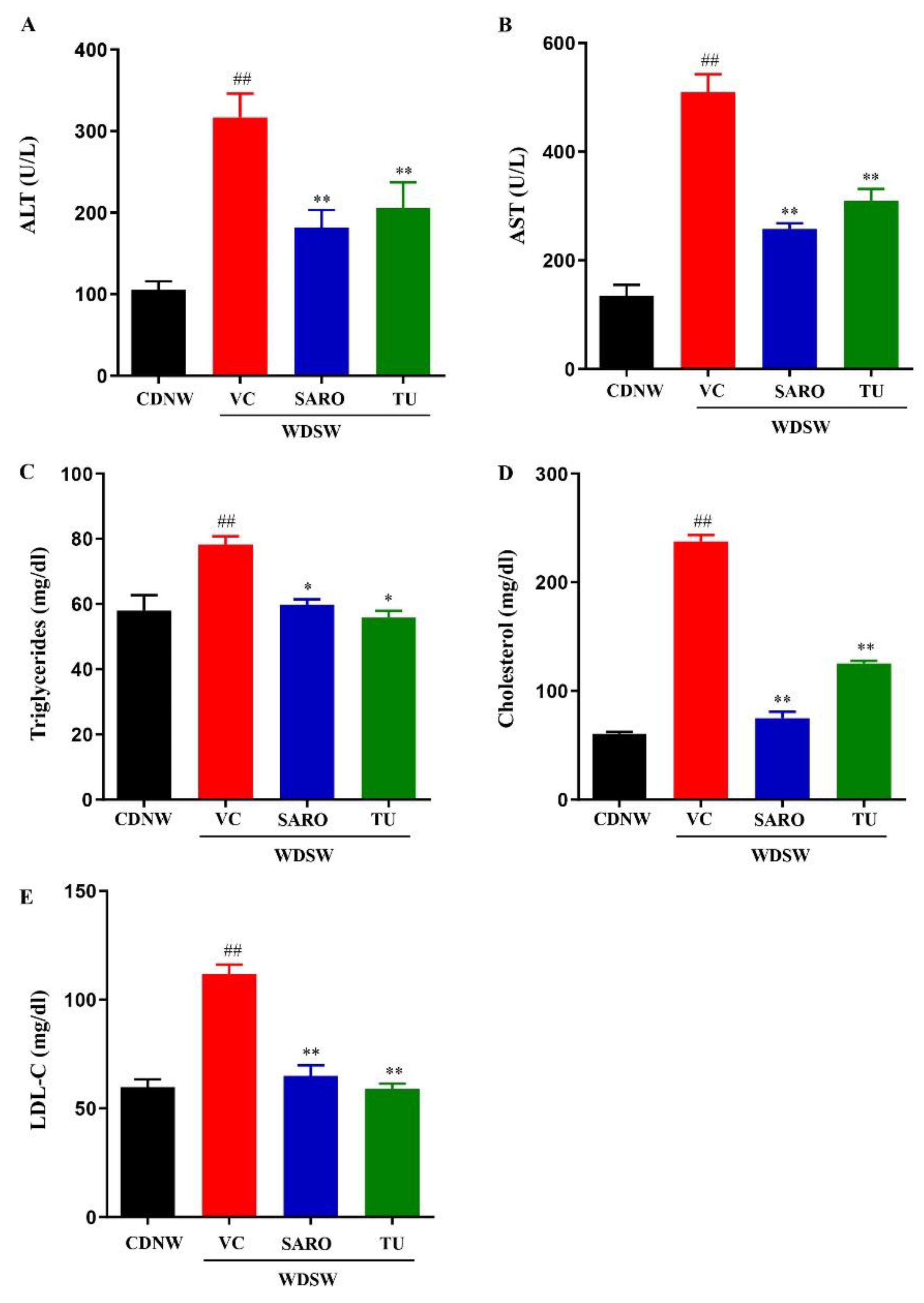
2.3. Tecomella undulata Prevented Liver Injury and Hyperlipidemia
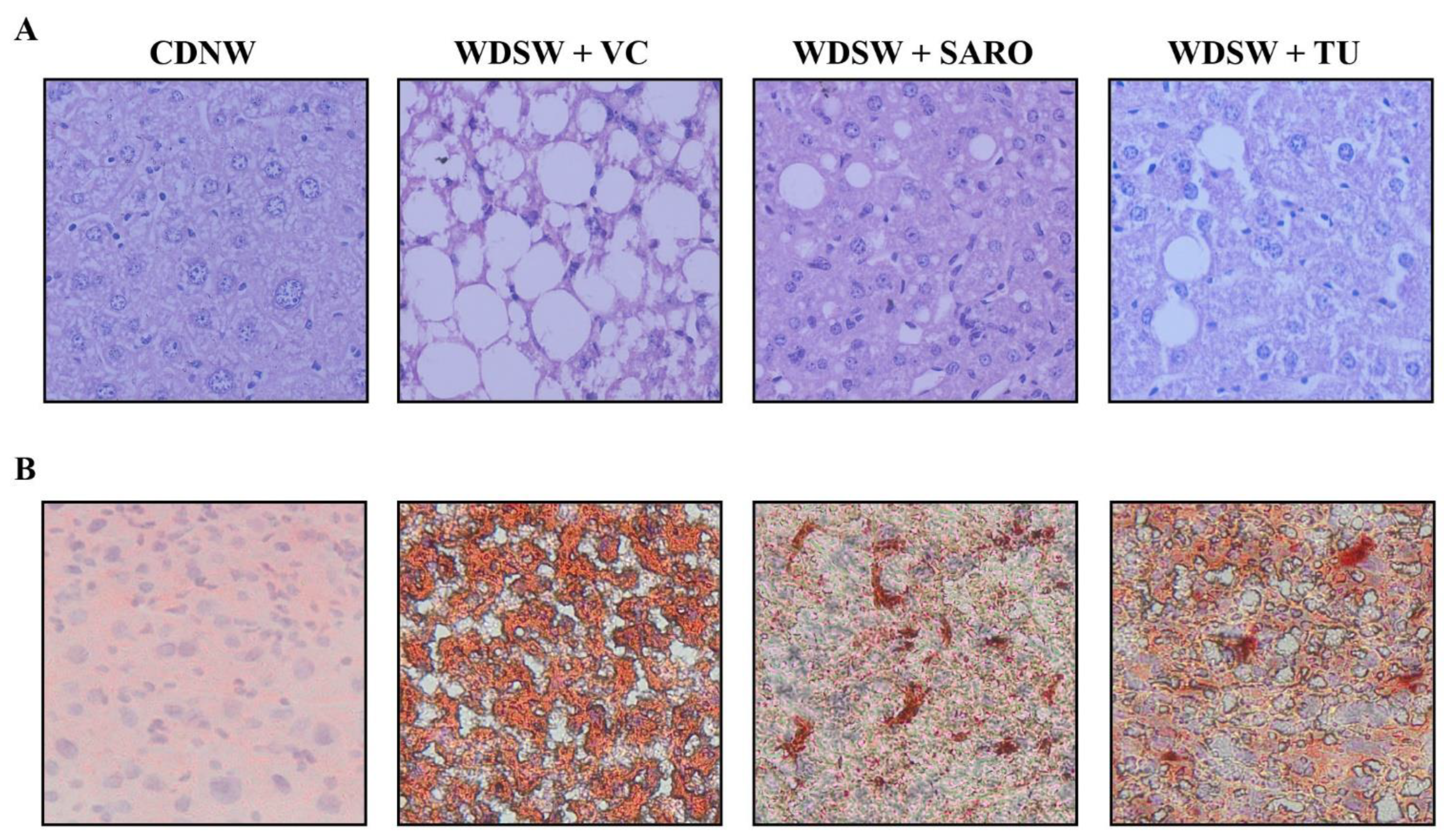
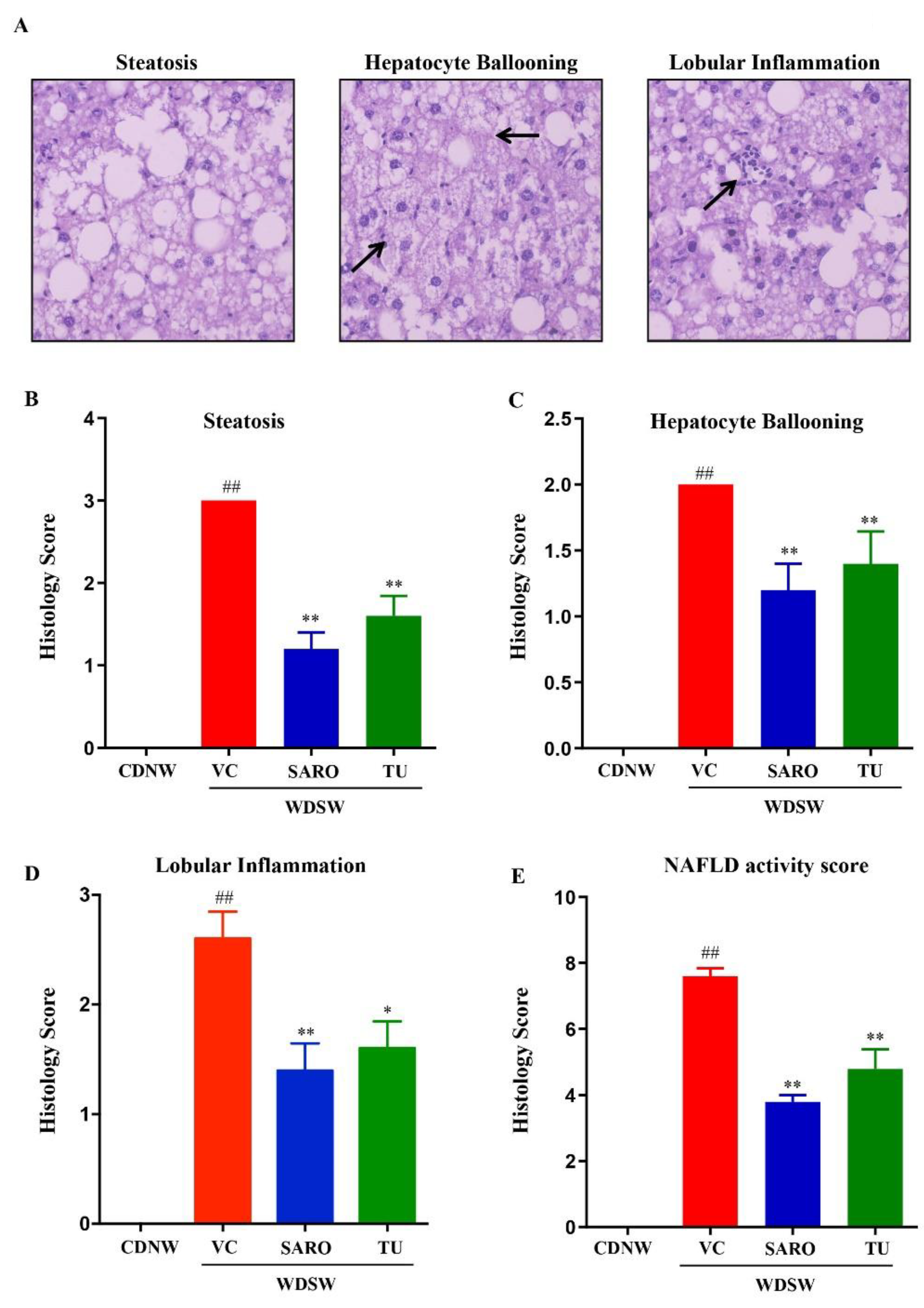
2.4. Tecomella undulata Improves Hepatic Steatosis and Steatohepatitis
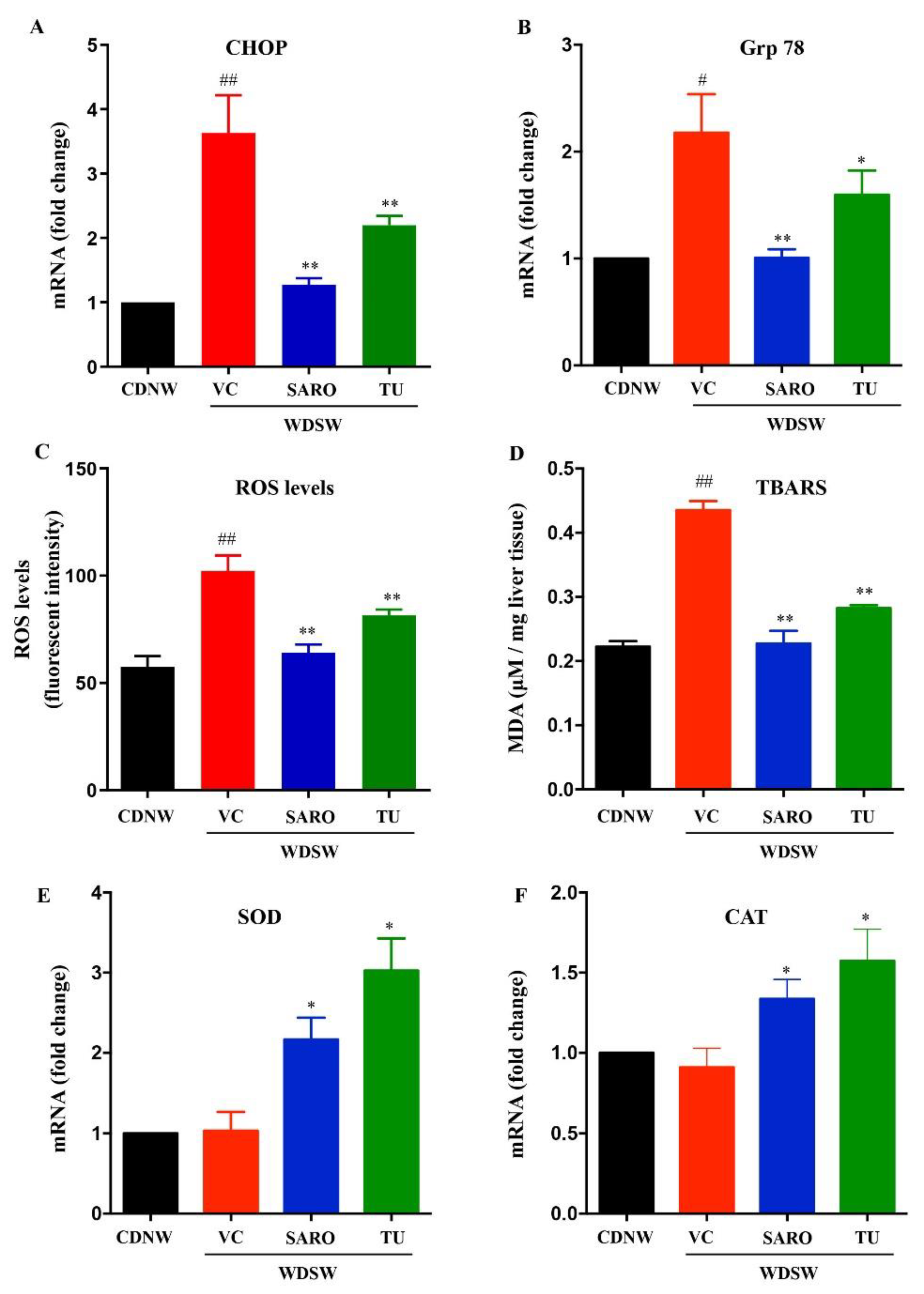
2.5. Tecomella undulata Alleviates Hepatic ER Stress and Oxidative Stress
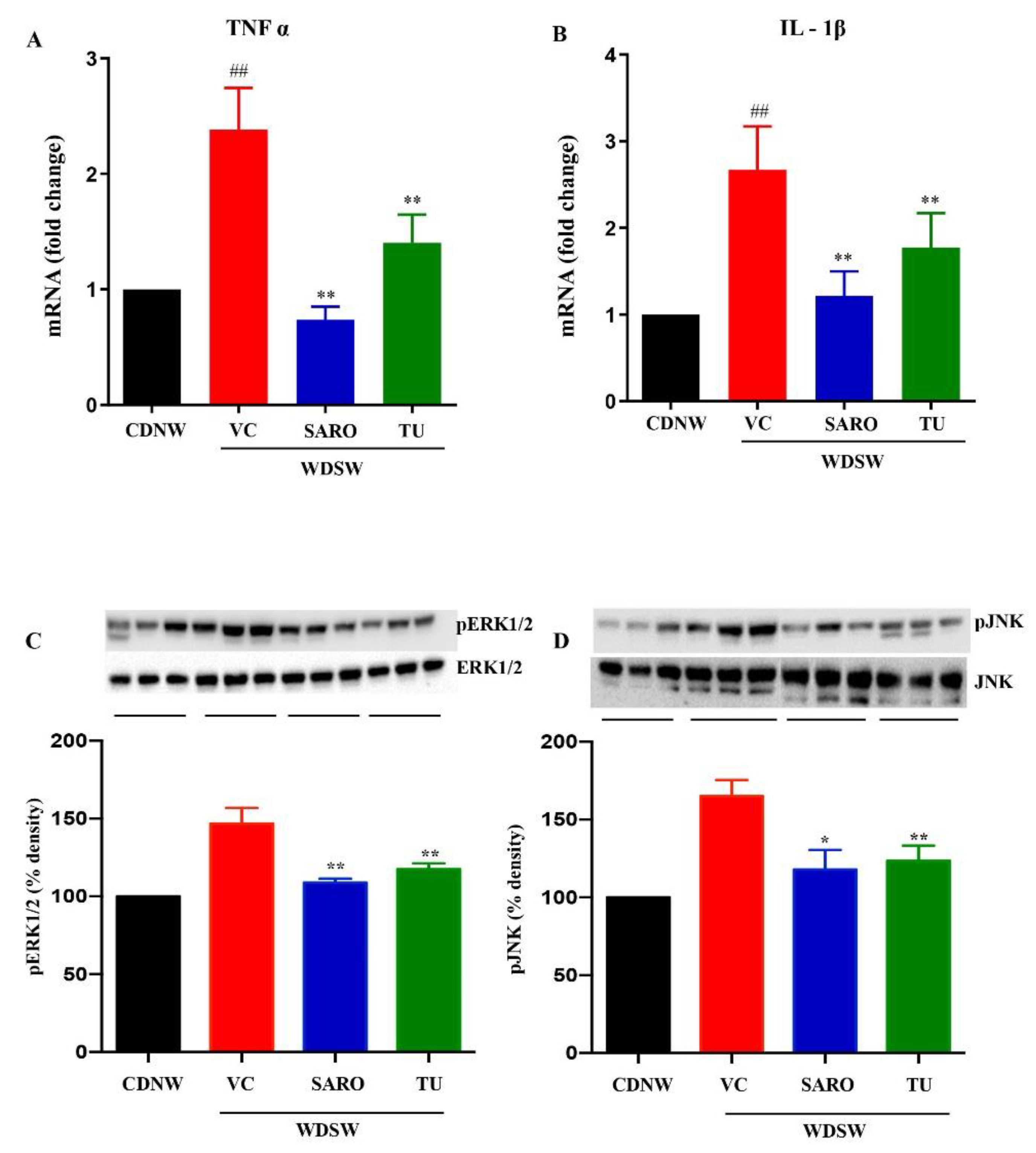
2.6. Anti-Inflammatory Effects of Tecomella undulata in NASH
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Cell Culture
4.4. In Vitro Cytotoxicity Assay
4.5. Animal Model
4.6. Study Design and Interventions
4.7. Choice of Tecomella undulata Dosage
4.8. Blood Collection and Biochemical Analysis
4.9. Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
4.10. Histological Analysis
4.11. TBARS Assay
4.12. ROS Assay
4.13. RNA Isolation and Quantitative Real Time PCR
4.14. Western Blot
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2017, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef]
- Shetty, S.R.; Kumar, S.; Mathur, R.; Sharma, K.H.; Jaiswal, A.D. Observational study to evaluate the safety and efficacy of saroglitazar in Indian diabetic dyslipidemia patients. Indian Heart J. 2015, 67, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Kakazu, E.; Mauer, A.S.; Yin, M.; Malhi, H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1α-dependent manner. J. Lipid Res. 2016, 57, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, N.U.; Sheikh, T.A. Endoplasmic reticulum stress and Oxidative stress in the pathogenesis of Non-alcoholic fatty liver disease. Free Radic. Res. 2015, 49, 1405–1418. [Google Scholar] [CrossRef]
- García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial Oxidative Stress and Antioxidants Balance in Fatty Liver Disease. Hepatol. Commun. 2018, 2, 1425–1439. [Google Scholar] [CrossRef]
- Schuster, S.; Cabrera, D.; Arrese, M.; Feldstein, A.E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 349–364. [Google Scholar] [CrossRef]
- Li, H.-Y.; Gan, R.-Y.; Shang, A.; Mao, Q.-Q.; Sun, Q.-C.; Wu, D.-T.; Geng, F.; He, X.-Q.; Li, H.-B. Plant-Based Foods and Their Bioactive Compounds on Fatty Liver Disease: Effects, Mechanisms, and Clinical Application. Oxidative Med. Cell. Longev. 2021, 2021, 6621644. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, R.; Sharma, S.; Kumar, S. An overview for various aspects of multifaceted, health care Tecomella undulata Seem. plant. Acta Pol. Pharm. 2012, 69, 993–996. [Google Scholar] [PubMed]
- Jain, M.; Kapadia, R.; Jadeja, R.N.; Thounaojam, M.C.; Devkar, R.V.; Mishra, S. Hepatoprotective potential of Tecomella undulata stem bark is partially due to the presence of betulinic acid. J. Ethnopharmacol. 2012, 143, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Thanawala, P.R.; Jolly, C.I. Pharmacognostical, phytochemical and antimicrobial studies on stem bark of tecomella undulata seem. Anc. Sci. Life 1993, 12, 414–419. [Google Scholar]
- Alvala, R.; Alvala, M.; Sama, V.; Dharmarajan, S.; Ullas, J.V.; Reddy, M.B. Scientific evidence for traditional claim of anti-obesity activity of Tecomella undulata bark. J. Ethnopharmacol. 2013, 148, 441–448. [Google Scholar] [CrossRef]
- Saggoo, M.; Kaur, N.; Gill, A. Economically valuable Tecomella undulata-endangered tree of Arid Zone. Int. J. Sci. 2017, 2, 8–13. [Google Scholar]
- Dhir, R.; Shekhawat, G.S. Critical review on Tecomella Undulata: A medicinally potent endangered plant species of Indian Thar desert. Int. J. Curr. Res. 2012, 4, 036–044. [Google Scholar]
- Saxena, P.K.; Nanda, D.; Gupta, R. Hepatoprotective Potential of Tecomella undulata Bark on Paracetamol and CCL4 Induced Hepatotoxicity in Rats: Invitro Analysis. J. Pharm. Res. Int. 2021, 33, 307–322. [Google Scholar] [CrossRef]
- Charlton, M.; Krishnan, A.; Viker, K.; Sanderson, S.; Cazanave, S.; McConico, A.; Masuoko, H.; Gores, G. Fast food diet mouse: Novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G825–G834. [Google Scholar] [CrossRef]
- Asgharpour, A.; Cazanave, S.C.; Pacana, T.; Seneshaw, M.; Vincent, R.; Banini, B.A.; Kumar, D.P.; Daita, K.; Min, H.-K.; Mirshahi, F.; et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J. Hepatol. 2016, 65, 579–588. [Google Scholar] [CrossRef]
- Santhekadur, P.K.; Kumar, D.P.; Sanyal, A.J. Preclinical models of non-alcoholic fatty liver disease. J. Hepatol. 2017, 68, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.K.; Joshi, A.; Dhiman, K.S. The Ayurvedic Pharmacopoeia of India, development and perspectives. J. Ethnopharmacol. 2017, 197, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, G.; Revelo, X.; Malhi, H. Pathogenesis of Nonalcoholic Steatohepatitis: An Overview. Hepatol. Commun. 2020, 4, 478–492. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Urano, F.; Wang, X.; Bertolotti, A.; Zhang, Y.; Chung, P.; Harding, H.P.; Ron, D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000, 287, 664–666. [Google Scholar] [CrossRef]
- Khan, A.S.; Subramaniam, S.; Dramane, G.; Khelifi, D.; Khan, N.A. ERK1 and ERK2 activation modulates diet-induced obesity in mice. Biochimie 2017, 137, 78–87. [Google Scholar] [CrossRef]
- Hardy, T.; Oakley, F.; Anstee, Q.M.; Day, C.P. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 451–496. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Loomba, R.; Rinella, M.E.; Bugianesi, E.; Marchesini, G.; Neuschwander-Tetri, B.A.; Serfaty, L.; Negro, F.; Caldwell, S.H.; Ratziu, V.; et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2018, 68, 361–371. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A. Therapeutic Landscape for NAFLD in 2020. Gastroenterology 2020, 158, 1984–1998. [Google Scholar] [CrossRef]
- Jadeja, R.; Devkar, R.V.; Nammi, S. Herbal Medicines for the Treatment of Nonalcoholic Steatohepatitis: Current Scenario and Future Prospects. Evid. Based Complement. Altern. Med. 2014, 2014, 648308. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Kapadia, R.; Jadeja, R.N.; Thounaojam, M.C.; Devkar, R.V.; Mishra, S.H. Traditional uses, phytochemistry and pharmacology of Tecomella undulata—A review. Asian Pac. J. Trop. Biomed. 2012, 2, S1918–S1923. [Google Scholar] [CrossRef]
- Goyal, R.; Ravishanka, B.; Shukla, V.; Singh, M. Hepatoprotective Activity of Rohitaka ghrita against Paracetamol Induced Liver Injury in Rat. Pharmacologia 2012, 3, 227–232. [Google Scholar] [CrossRef]
- Ahmad, F.; Khan, R.A.; Rasheed, S. Preliminary screening of methanolic extracts of Celastrus peniculatus and Tecomella undulata for analgesic and anti inflammatory activities. J. Ethnopharmacol. 1994, 42, 193–198. [Google Scholar] [CrossRef]
- Choudhary, G.P. Immunomodulatory activity of alcoholic extract of Tecomella undulata Linn. in mice. Asian J. Pharm. Biol. Res. 2011, 1, 67–70. [Google Scholar]
- Goyal, O.; Nohria, S.; Goyal, P.; Kaur, J.; Sharma, S.; Sood, A.; Chhina, R.S. Saroglitazar in patients with non-alcoholic fatty liver disease and diabetic dyslipidemia: A prospective, observational, real world study. Sci. Rep. 2020, 10, 21117. [Google Scholar] [CrossRef]
- Kumar, D.P.; Caffrey, R.; Marioneaux, J.; Santhekadur, P.K.; Bhat, M.; Alonso, C.; Koduru, S.V.; Philip, B.; Jain, M.R.; Giri, S.R.; et al. The PPAR α/γ Agonist Saroglitazar Improves Insulin Resistance and Steatohepatitis in a Diet Induced Animal Model of Nonalcoholic Fatty Liver Disease. Sci. Rep. 2020, 10, 9330. [Google Scholar] [CrossRef]
- Khatri, A.; Garg, A.; Agrawal, S.S. Evaluation of hepatoprotective activity of aerial parts of Tephrosia purpurea L. and stem bark of Tecomella undulata. J. Ethnopharmacol. 2009, 122, 1–5. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, S.; Vasudeva, N.; Ranga, V. In vivo anti-hyperglycemic and antioxidant potentials of ethanolic extract from Tecomella undulata. Diabetol. Metab. Syndr. 2012, 4, 33–37. [Google Scholar] [CrossRef]
- Kamata, H.; Honda, S.-I.; Maeda, S.; Chang, L.; Hirata, H.; Karin, M. Reactive Oxygen Species Promote TNFα-Induced Death and Sustained JNK Activation by Inhibiting MAP Kinase Phosphatases. Cell 2005, 120, 649–661. [Google Scholar] [CrossRef]
- Lake, A.D.; Novak, P.; Hardwick, R.N.; Flores-Keown, B.; Zhao, F.; Klimecki, W.T.; Cherrington, N.J. The Adaptive Endoplasmic Reticulum Stress Response to Lipotoxicity in Progressive Human Nonalcoholic Fatty Liver Disease. Toxicol. Sci. 2014, 137, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Song, M.J.; Malhi, H. The unfolded protein response and hepatic lipid metabolism in non alcoholic fatty liver disease. Pharmacol. Ther. 2019, 203, 107401. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Pan, X.; Luo, J.; Xiao, X.; Li, J.; Bestman, P.L.; Luo, M. Association of Inflammatory Cytokines With Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2022, 13, 880298. [Google Scholar] [CrossRef]
- Osto, E.; Matter, C.M.; Kouroedov, A.; Malinski, T.; Bachschmid, M.; Camici, G.G.; Kilic, U.; Stallmach, T.; Boren, J.; Iliceto, S.; et al. c-Jun N-Terminal Kinase 2 Deficiency Protects Against Hypercholesterolemia-Induced Endothelial Dysfunction and Oxidative Stress. Circulation 2008, 118, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Bronk, S.F.; Werneburg, N.W.; Gores, G.J. Free Fatty Acids Induce JNK-dependent Hepatocyte Lipoapoptosis. J. Biol. Chem. 2006, 281, 12093–12101. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Abra, H.H.; Sultana, S.; Mir, S.R. Phytochemical Investigation of the Stem Bark of Tecomella Undulata (Sm.) Seem. Mod. Org. Chem. Res. 2017, 2, 159–171. [Google Scholar] [CrossRef]
- Tsuchida, T.; Lee, Y.A.; Fujiwara, N.; Ybanez, M.; Allen, B.; Martins, S.; Fiel, M.I.; Goossens, N.; Chou, H.-I.; Hoshida, Y.; et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J. Hepatol. 2018, 69, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Guidance for Industry, Center for Drug Evaluation and Research (CDER). Pharmacology and Toxicology; Food and Drug Administration: Rockville, MD, USA, 2005. Available online: https://www.fda.gov/media/72309/download (accessed on 1 September 2021).
- Poynard, T.; Munteanu, M.; Charlotte, F.; Perazzo, H.; Ngo, Y.; Deckmyn, O.; Pais, R.; Mathurin, P.; Ratziu, V. Impact of steatosis and inflammation definitions on the performance of NASH tests. Eur. J. Gastroenterol. Hepatol. 2018, 30, 384–391. [Google Scholar] [CrossRef]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Sanyal, A.J.; Neuschwander-Tetri, B.A.; Nonalcoholic Steatohepatitis Clinical Research Network. Improvements in Histologic Features and Diagnosis Associated With Improvement in Fibrosis in Nonalcoholic Steatohepatitis: Results From the Nonalcoholic Steatohepatitis Clinical Research Network Treatment Trials. Hepatology 2018, 70, 522–531. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinivas, A.N.; Suresh, D.; Suvarna, D.; Pathak, P.; Giri, S.; Suman; Satish, S.; Chidambaram, S.B.; Kumar, D.P. Unraveling the Potential Role of Tecomella undulata in Experimental NASH. Int. J. Mol. Sci. 2023, 24, 3244. https://doi.org/10.3390/ijms24043244
Srinivas AN, Suresh D, Suvarna D, Pathak P, Giri S, Suman, Satish S, Chidambaram SB, Kumar DP. Unraveling the Potential Role of Tecomella undulata in Experimental NASH. International Journal of Molecular Sciences. 2023; 24(4):3244. https://doi.org/10.3390/ijms24043244
Chicago/Turabian StyleSrinivas, Akshatha N., Diwakar Suresh, Deepak Suvarna, Pankaj Pathak, Suresh Giri, Suman, Suchitha Satish, Saravana Babu Chidambaram, and Divya P. Kumar. 2023. "Unraveling the Potential Role of Tecomella undulata in Experimental NASH" International Journal of Molecular Sciences 24, no. 4: 3244. https://doi.org/10.3390/ijms24043244
APA StyleSrinivas, A. N., Suresh, D., Suvarna, D., Pathak, P., Giri, S., Suman, Satish, S., Chidambaram, S. B., & Kumar, D. P. (2023). Unraveling the Potential Role of Tecomella undulata in Experimental NASH. International Journal of Molecular Sciences, 24(4), 3244. https://doi.org/10.3390/ijms24043244






