Abstract
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia. To date, a lot of research has been conducted to investigate the underlying mechanisms of this disease at both molecular and cellular levels. There is increasing evidence suggesting that autoimmunity is an important factor in the initiation and perpetuation of AF. Autoantibodies are thought to play a pivotal role in the regulation of heart rhythm and the conduction system and, therefore, are associated with AF development. In this review, we have summarized current knowledge concerning the role of autoantibodies in AF development as well as their prognostic and predictive value in this disease. The establishment of the autoantibody profile of separate AF patient groups may appear to be crucial in terms of developing novel treatment approaches for those patients; however, the exact role of various autoantibodies in AF is still a matter of ongoing debate.
1. Introduction
Autoimmune diseases, such as type 1 diabetes mellitus, rheumatoid arthritis, and psoriasis, derive from the failure of normal self-tolerance mechanisms. Although autoantibodies have been proven to be the cause of many diseases, factors that are involved in their production and their exact role in pathogenesis remain largely unresolved [1].
Autoimmune diseases affect about 5–7% of the population and they have a significant contribution to the general mortality and morbidity [2]. The number of illnesses attributed to autoimmunity is still growing as new findings are being discovered in ongoing trials.
Recently, autoimmunity has been evidenced to be a contributor, a cause, and a predictive factor of cardiovascular system diseases such as cardiomyopathies, myocarditis, heart failure, and atherosclerosis [3,4,5]. Lately, many research groups have investigated the role of autoimmunity in the development of cardiac arrhythmias including atrial fibrillation (AF) [6,7,8,9].
Atrial fibrillation is the most prevalent sustained cardiac arrhythmia in adults [10]. In the United States the lifetime risk of AF occurrence was estimated to be approximately 1 in 3 among White People, and 1 in 5 among Black People [11]. Currently, the prevalence of AF in adults ranges between 2% and 4%, and a 2.3-fold rise by 2030 is expected [12,13]. Atrial fibrillation is related to an increased risk of stroke and peripheral embolism. Moreover, it is associated with an increased mortality rate. Many independent risk factors for AF occurrence have been identified so far in population-based cohort studies including e.g., diabetes, hypertension, congestive heart failure, and valve disease [14]. Nevertheless, the pathophysiological mechanisms of AF remain unclear despite detailed research in this field [15,16]. Extensive clinical and scientific evidence demonstrates that inflammatory mechanisms play an important role in promoting AF [17,18]. Many studies have highlighted the role of electrical and structural changes in the atria in the initiation and maintenance of AF. These changes include ion channels remodeling, atrial fibrosis, and altered autonomic tone [19,20].
The search for biomarkers enabling diagnosis, prognosis, and treatment response prediction in different cardiovascular diseases has included not only various microparticles, such as micro-ribonucleic acids or extracellular vesicles, but also autoantibodies [21,22,23]. Autoantibodies have been found to target various cardiac receptors modulating the impact of the autonomic nervous system on signaling pathways [7,24]. Autoantibodies can bind to the extracellular receptor causing a direct change in the protein function, but they can also lead to cell death in a complement-mediated manner. Growing evidence suggests that autoantibodies play a pivotal role in the regulation of heart rhythm and conduction system and, therefore, are associated with AF development [6,25,26] (Figure 1). In this review, we have summarized the current knowledge concerning the role of autoantibodies in AF development as well as their prognostic and predictive value in this disease.
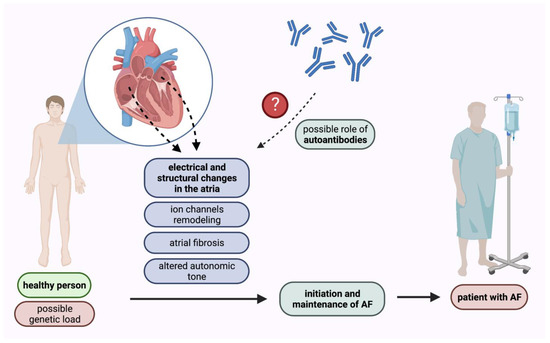
Figure 1.
Pathomechanisms of AF initiation and maintenance with the possible role of autoantibodies. AF—atrial fibrillation.
2. Autoantibodies in Patients Suffering from Atrial Fibrillation
We searched the PubMed Database and eventually included 31 original research publications (both clinical and preclinical) relevant to the discussed field, excluding only the records that were found by coincidence. We divided these studies into the following groups: (i) research investigating only antibodies against β-adrenergic receptors (anti-β antibodies), (ii) research investigating only antibodies against M2-muscarinic acetylcholine receptor (anti-M2 antibodies), (iii) research investigating both anti-β and anti-M2 antibodies, (iv) research investigating anti-heat shock protein (anti-HSP) antibodies, (v) research investigating other types of autoantibodies.
2.1. Research Investigating Only Anti-β Antibodies
Novikova et al. studied patients with arrhythmias of various etiology. The group of patients was further subdivided into: (i) primary (idiopathic) electrical abnormalities patients, (ii) chronic myocarditis (CM) and dilated cardiomyopathy (DCM) patients, and (iii) ischemic heart disease (IHD) patients. They evidenced that there was an increased level of anti-β1 antibodies in ventricular tachycardia, ventricular extrasystole or AF patients (excluding patients with IHD) compared to controls [27].
Another research team evaluated the C-reactive protein (CRP) levels and the incidence of anti-β1 antibodies in patients suffering from supraventricular tachyarrhythmias. They found that patients with arrhythmias (including AF) showed a significantly higher prevalence of anti-β1 antibodies than controls. Moreover, patients with supraventricular arrhythmias (along with DCM or CM) had higher median CRP values than patients with idiopathic arrhythmias, those with supraventricular arrhythmias with coexisting arterial hypertension (AH), and healthy controls [28].
Nikitina et al. investigated the prevalence of anti-β1 antibodies and the heart rate variability (HRV) in 42 arrhythmic patients subdivided into two groups: (i) idiopathic arrhythmias patients: 13 paroxysmal AF or flutter, two paroxysmal atrial tachycardia (PAT), and 16 paroxysmal ventricular tachycardia (PVT), (ii) 11 patients suffering from PVT with coexisting DCM or CM. An increased occurrence of anti-β1 antibodies was found in arrhythmic patients as compared to healthy controls. Additionally, within the idiopathic arrhythmic patients’ group, it was demonstrated that patients with anti-β1 antibodies had decreased HRV parameters compared to anti-β1 antibodies negative patients. These findings indicate that anti-β1 antibodies might contribute to the dysfunction of chronotropic heart regulation and might lead to arrhythmias evolution [29].
Interestingly, it was proved that immunizing rabbits with β2-adrenergic receptors (β2) caused atrial arrhythmias mainly in the form of sustained atrial tachycardia [30]. Similarly, Li et al. induced sustained atrial tachycardia in rabbits by immunizing them with β2 with the following injection of thyroxine (T4). On the other hand, rabbits immunized with β1-adrenergic receptors (β1) and injected with T4 presented with sustained sinus tachycardia [31]. Noteworthily, T4 may exert the immune response (both physiological and pathological) including the activation of lymphocytes and thus further increasing autoantibody levels.
Shang et al. studied anti-β1 antibody levels relative to left atrium diameter (LAD) and circulating fibrosis biomarker levels in a group of 70 patients with paroxysmal AF. The anti-β1 antibody levels were found to be positively correlated with LAD and circulating fibrosis biomarker levels. Furthermore, they established a rabbit model overexpressing anti-β1 antibodies. It was discovered that anti-β1 antibodies increased AF inducibility by facilitating atrial fibrosis, transforming growth factor-β1 (TGF-β1) signaling activation, and collagen accumulation [32]. All studies discussed in this section have been summarized in Table 1.

Table 1.
Summary of recent research investigating only antibodies against β-adrenergic receptors.
2.2. Research Investigating Only Anti-M2 Antibodies
Baba et al. demonstrated that the presence of anti-M2 antibodies was the only independent predictor of AF in DCM patients. Moreover, they conducted an experiment in which chick embryos were injected with IgG derived from: (i) DCM patients anti-M2 positive, (ii) idiopathic AF patients anti-M2 positive, (iii) healthy controls, (iv) DCM patients anti-M2 negative, (v) idiopathic AF patients anti-M2 negative. It was shown that purified anti-M2 IgG from both AF and DCM patients exhibited a negative chronotropic effect - indicating that anti-M2 autoantibodies could affect sinus node function and induce supraventricular arrhythmias [33].
Another group evaluated the effects of immunizing rabbits with a synthetic peptide corresponding to the M2 with further assessment of atrial electrophysiology in isolated perfused rabbit hearts. Immunized rabbits produced anti-M2 antibodies and showed a significantly shorter atrial effective refractory period and a longer intra-atrial activation time compared to control rabbits. Furthermore, they showed overexpression of the M2-receptor-IK,Ach (muscarinic-gated potassium channel) pathway, and increased atrial fibrosis. Taken together, it was demonstrated that anti-M2 antibodies were able to induce atrial remodeling in terms of both structure and electrophysiology, which pointed out that these antibodies might participate in the induction and perpetuation of AF [34].
Zou et al. studied the predictive value of preprocedural anti-M2 antibodies levels for the recurrence of AF in a group of 76 AF patients (paroxysmal or persistent) with preserved left ventricular ejection fraction who were enrolled for the ablation procedure. In patients with AF, the frequency and levels of the anti-M2 antibodies were higher than those in patients with sinus rhythm. Persistent AF patients had higher frequency and titers of these antibodies when compared to paroxysmal AF patients. Furthermore, serum anti-M2 antibodies levels correlated with LAD and plasma NT-proBNP levels. The preprocedural level of anti-M2 was proved to be an independent predictor for the recurrence of AF at one-year follow-up after ablation [35].
Furthermore, it was demonstrated that the anti-M2 antibodies level was significantly increased in patients with lone paroxysmal AF when compared to healthy controls. It was also proved that, in the case of lone paroxysmal AF patients, serum anti-M2 antibodies level was a marker of left atrial fibrosis and thus an independent predictor of late AF recurrence [36].
Ma et al. compared long-standing persistent AF patients undergoing hybrid ablation and patients in sinus rhythm scheduled to undergo coronary artery bypass grafting (CABG) surgery. The study revealed that the first group had higher anti-M2 antibody levels, which were found to be associated with the severity of atrial fibrosis. In addition, serum anti-M2 antibody levels were proved to be positively correlated with TGF-β1 and connective tissue growth factor (CTGF) expression in the left atrial appendage [37].
Patients suffering from lone paroxysmal AF had a higher prevalence of increased levels of anti-M2 antibodies compared to patients with both AF and AH. However, there was no difference in the prevalence of increased levels of anti-M2 immunoglobulin M (IgM) between these groups [38].
Deng et al. used an animal model with two groups: (i) six rabbits immunized with M2 and treated with T4, and (ii) four rabbits treated only with T4. Increased inducibility of AF and sinus tachycardia was found in the first group and most likely it was gained through the disruption of electrophysiological properties mediated by anti-M2 antibodies and T4 collectively [39]. All studies discussed in this section have been summarized in Table 2.

Table 2.
Summary of recent research investigating only antibodies against M2-muscarinic acetylcholine receptor.
2.3. Research Investigating Both Anti-β and Anti-M2 Antibodies
Stavrakis et al. studied Graves’ hyperthyroidism patients with AF or in sinus rhythm. It was shown that anti-β1 and anti-M2 antibodies were the strongest predictors of AF occurrence. Moreover, IgG electrophysiologic effects were analyzed using intracellular recordings from isolated canine pulmonary veins. It was shown that these antibodies induced hyperpolarization, decreased action potential duration, enhanced early afterdepolarization formation, and facilitated triggered firing in pulmonary veins by the local autonomic nerve stimulation [40]. Another research group evidenced that anti-β1 and anti-M2 antibody levels were determinants of AF occurrence in Graves’ disease patients [41]. Moreover, Yalcin et al. showed that anti-β1 and anti-M2 antibodies were independent biomarkers of lone paroxysmal AF presence in patients without concomitant cardiovascular diseases [42].
It was proved that preprocedural anti-β1 and anti-M2 antibody levels were independent predictors of late AF recurrence following cryoballoon-based pulmonary vein isolation in paroxysmal AF patients [43]. Hu et al. measured the serum levels of anti-β1 and anti-M2 antibodies, antinuclear antibodies, interleukin-6 (IL-6), and CRP in patients with non-valvular AF. It was demonstrated that left atrium diameter and the levels of anti-β1 antibodies, anti-M2 antibodies, and IL-6 were independent risk factors for non-valvular AF [44].
Li et al. immunized rabbits with peptides from the extracellular loops of both β1-adrenergic receptors and M2-muscarinic receptors to produce both types of antibodies. Antibodies expression triggered sustained sinus, junctional and atrial tachycardias, but not AF. Sustained AF was induced by addition of excessive T4. Interestingly, AF induction was blocked by the neutralization of these antibodies despite continued hyperthyroidism [45].
It was found that when considering patients with hypertrophic cardiomyopathy (HCM), the subgroup of patients with AF had remarkably higher concentrations of anti-β1 and anti-M2 antibodies than the subgroup without this disease. Atrial fibrillation in HCM patients led to atrial systolic dysfunction and lowered cardiac output in addition to ventricular diastolic dysfunction, leading to further deterioration of heart failure and elevation of anti-β1 and anti-M2 antibodies concentration [46]. All studies discussed in this section have been summarized in Table 3.

Table 3.
Summary of recent research investigating both antibodies against β-adrenergic receptors and antibodies against M2-muscarinic acetylcholine receptor.
2.4. Research Investigating Anti-HSP Antibodies
The heat shock protein 65 (HSP65) is known to be expressed on the cell membrane only under stressful conditions. When HSP65 is attacked by circulating anti-HSP65 antibodies, it can cause a damaging autoimmune response mediated by complement-dependent cytotoxicity, leading to myocyte injury, electro-anatomical substrate formation, and in turn AF. Mandal et al. investigated the association between the preoperative levels of anti-HSP65 autoantibodies and the occurrence of postoperative AF in 329 patients undergoing elective primary CABG. It was confirmed that there was a positive association between anti-HSP65 antibody levels and postoperative AF occurrence independent of age, sex, or any other cardiovascular risk factors. Interestingly, preoperative CRP levels were similar in patients who had postoperative AF and in patients who did not develop this tachyarrhythmia [47].
Oc et al. researched the difference between preoperative and postoperative levels of anti-HSP60 antibodies in 20 patients undergoing elective primary CABG. The study revealed that increased preoperative levels of anti-HSP60 antibodies positively correlated with the development of postoperative AF [48].
The same research group in another prospective study proved that anti-HSP70 antibodies were a predictor of AF development in patients undergoing the CABG procedure. In addition, there was no significant difference in the serum CRP levels between the study and the control group [49].
Kornej et al. investigated changes in the levels of HSP70 and anti-HSP70 antibodies as well as rhythm outcomes in 67 patients with AF undergoing catheter ablation. Anti-HSP70 antibody levels were proved to be associated with AF type: patients with persistent AF had higher anti-HSP70 antibody titers than those with paroxysmal AF. Moreover, the level of HSP70 and anti-HSP70 antibodies increased after AF catheter ablation. Interestingly, the HSP70 level increase was associated with total ablation time and energy. Furthermore, in univariate analysis, the growth in the level of HSP70 and anti-HSP70 antibodies was associated with a higher AF recurrence rate during 6 months follow-up after the ablation procedure [50]. All studies discussed in this section have been summarized in Table 4.

Table 4.
Summary of recent research investigating antibodies against heat shock proteins.
2.5. Research Investigating Other Types of Autoantibodies
Patients with idiopathic paroxysmal AF had an increased prevalence of anti-cardiac myosin heavy chain (anti-cMHC) IgG as compared to healthy controls with sinus rhythm. Moreover, all AF patients with anti-cMHC IgG had also specific reactivity in their sera against both ventricular and atrial cardiac MHC isoforms [51].
Baba et al. compared the levels of anti-myosin, anti-β1, and anti-Na+-K+-ATPase antibodies between patients suffering from heart failure with reduced ejection fraction and control patients with hypertension alone. They discovered that antibodies against Na+-K+-ATPase were an independent risk factor for the occurrence of paroxysmal AF in congestive heart failure patients [52]. Another research group undertook a study to find out whether anti-serum amyloid A (SAA) antibodies could be detected in a group of AF patients and whether these antibodies were associated with inflammatory states, using CRP as an inflammation marker. They discovered the association between elevated levels of anti-SAA antibodies and the occurrence of AF. Interestingly, there was no association between anti-SAA levels and CRP levels [53].
Furthermore, Xu et al. demonstrated that among patients with cardiovascular manifestations of Graves’ disease, the frequency of AF was significantly higher in patients who were anti-angiotensin II-1 receptor (anti-AT1) antibodies positive compared to those who were anti-AT1 antibodies negative [54].
Blagova et al. analyzed anti-heart antibodies in 34 coronavirus disease 2019 (COVID-19) pneumonia patients. Anti-smooth muscle antibodies (ASMA) levels correlated with the presence of AF at admission and the need for oxygen therapy [55].
Moreover, it was shown that in the group of patients with acute ischemic stroke, patients with positive initial antiphospholipid antibodies (lupus anticoagulant screening, anti-cardiolipin antibody or anti-β2glycoprotein I antibodies) had more AF episodes and higher levels of CRP than patients without these antibodies [56]. Importantly, Sveen et al. studied the incidence of AF and the levels of anti-apoB100 antibodies (meaningfully against peptides p45 and p210) in a large population-based cohort. It was shown that increased levels of IgM against apoB100 p210 were independently associated with a decreased risk of AF development but only in women. Moreover, the association between IgM and AF was significantly different between sexes even after adjusting risk factors and comorbidities [57]. All studies discussed in this section have been summarized in Table 5.

Table 5.
Summary of recent research investigating other types of autoantibodies.
3. Conclusions
The significance of autoimmune processes and particular autoantibodies in the development of AF has been the field of extensive research. Serum concentrations of various autoantibodies have been proven to be significantly increased in patients with different types of AF when compared to healthy people. Most scientific attention has been applied to activating autoantibodies against receptors of the autonomous nervous system. Moreover, a growing number of studies has shown that other autoantibodies may affect pathogenetic pathways leading to AF (Figure 2). Importantly, there are many gaps in evidence concerning mainly the cause-and-effect relationships and, therefore, observed correlations do not indicate the unambiguous role of autoantibodies in AF development and they need to be further investigated.
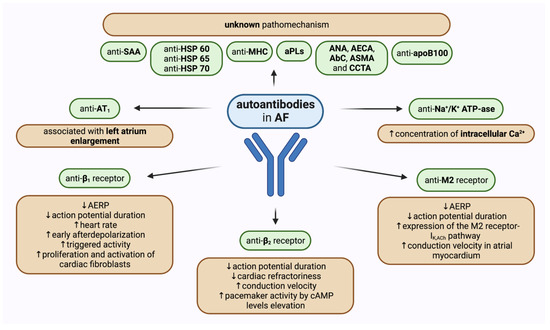
Figure 2.
Graphical summary of the role of different autoantibodies in atrial fibrillation with a probable pathomechanism of action if known. ↑—increased, ↓—decreased, AbC—anti-cardiomyocyte antibodies, AECA—anti-endothelial cell antibodies, AERP—atrial effective refractory period AF—atrial fibrillation, ANA—anti-nuclear antibodies, aPLs—antiphospholipid antibodies, ASMA—anti-smooth muscle antibodies, AT1—angiotensin II receptor type 1, β1—β1-adrenergic receptors, β2—β2-adrenergic receptors, cAMP—cyclic adenosine monophosphate, CCTA—cardiac conducting tissue antibodies, HSP—heat shock protein, IK,Ach—muscarinic-gated potassium channel, M2—M2-muscarinic acetylcholine receptor, MHC— myosin heavy chain, SAA—serum amyloid A.
Noteworthily, not only have concentrations of various antibodies and their correlation with specific types of AF been investigated, but also their predictive values, their correlations with other concomitant heart conditions, and possible pathophysiological mechanisms that determine their impact on atrial structural and electrophysiological changes. Nevertheless, the exact role of various autoantibodies in AF is still a matter of ongoing debate.
4. Future Perspectives
We propose that inflammatory cytokines and immune cells should be evaluated in future investigations concerning autoantibodies in AF. The exact characteristics of immune response could contribute to a better understanding of autoantibodies’ roles in the autoimmune process and therefore cause-and-effect relationships could be demonstrated. The identification of immunological autoantibody-mediated mechanisms would reveal new perspectives in the treatment and prevention of AF, including the use of immunosuppressive agents or neutralizing antibodies. Moreover, autoantibodies could potentially be used as a screening tool to determine the risk of developing AF in the future, especially in patients with underlying cardiovascular system diseases.
Further investigations in larger prospective studies are needed to determine specific autoantibodies level profiles as a pre-ablation screening tool to spot patients likely to have arrhythmia recurrence, and thus to contribute to the improvement of the efficacy of this treatment method. It would lead to a more individualized therapy with an estimated better risk-benefit ratio.
Additionally, there remains a substantial need to establish cut-off values to use the discussed circulating antibodies as a screening tool for patients with different structural heart diseases to prevent AF evolution.
Finally, as recent evidence suggests that neutralization of anti-β1 and anti-M2 antibodies blocks induction of AF, it is warranted to undertake studies, both in vivo and in vitro, investigating the use of decoy peptide therapy. Observations made in these studies could then be translated into clinical medicine in the form of new treatment regimens.
Author Contributions
Conceptualization, J.Z. and G.P.; writing—original draft preparation, J.Z.; writing—review and editing, G.P.; visualization, J.Z. and G.P.; supervision, P.B., P.L., M.G. and A.G.; funding acquisition, J.Z. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The figures were created with BioRender.com, a licensed version by A.G.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sinha, A.A.; Lopez, M.T.; McDevitt, H.O. Autoimmune diseases: The failure of self tolerance. Science 1990, 248, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, F.S.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.; Mahon, N.J.; Tona, F.; McKenna, W.J. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: Pathogenetic and clinical significance. Eur. J. Heart Fail. 2002, 4, 411–417. [Google Scholar] [CrossRef]
- Liang, K.P.; Kremers, H.M.; Crowson, C.S.; Snyder, M.R.; Therneau, T.M.; Roger, V.L.; Gabriel, S.E. Autoantibodies and the risk of cardiovascular events. J. Rheumatol. 2009, 36, 2462–2469. [Google Scholar] [CrossRef] [PubMed]
- Nussinovitch, U.; Shoenfeld, Y. Autoimmunity and heart diseases: Pathogenesis and diagnostic criteria. Arch. Immunol. Ther. Exp. 2009, 57, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Capecchi, P.L.; Guideri, F.; Acampa, M.; Selvi, E.; Bisogno, S.; Galeazzi, M.; Laghi-Pasini, F. Autoantibody-mediated cardiac arrhythmias: Mechanisms and clinical implications. Basic Res. Cardiol. 2008, 103, 1–11. [Google Scholar] [CrossRef]
- Lee, H.C.; Huang, K.T.; Wang, X.L.; Shen, W.K. Autoantibodies and cardiac arrhythmias. Heart Rhythm. 2011, 8, 1788–1795. [Google Scholar] [CrossRef]
- Li, J. The Role of Autoantibodies in Arrhythmogenesis. Curr. Cardiol. Rep. 2020, 23, 3. [Google Scholar] [CrossRef]
- Amaya-Amaya, J.; Montoya-Sánchez, L.; Rojas-Villarraga, A. Cardiovascular involvement in autoimmune diseases. Biomed. Res. Int. 2014, 2014, 367359. [Google Scholar] [CrossRef]
- Morin, D.P.; Bernard, M.L.; Madias, C.; Rogers, P.A.; Thihalolipavan, S.; Estes, N.A., 3rd. The State of the Art: Atrial Fibrillation Epidemiology, Prevention, and Treatment. Mayo Clin. Proc. 2016, 91, 1778–1810. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Colilla, S.; Crow, A.; Petkun, W.; Singer, D.E.; Simon, T.; Liu, X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am. J. Cardiol. 2013, 112, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Abbott, R.D.; Savage, D.D.; McNamara, P.M. Epidemiologic features of chronic atrial fibrillation: The Framingham study. N. Engl. J. Med. 1982, 306, 1018–1022. [Google Scholar] [CrossRef]
- Everett, T.H., IV; Olgin, J.E. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007, 4, S24–S27. [Google Scholar] [CrossRef]
- Kourliouros, A.; Savelieva, I.; Kiotsekoglou, A.; Jahangiri, M.; Camm, J. Current concepts in the pathogenesis of atrial fibrillation. Am. Heart J. 2009, 157, 243–252. [Google Scholar] [CrossRef]
- Aviles, R.J.; Martin, D.O.; Apperson-Hansen, C.; Houghtaling, P.L.; Rautaharju, P.; Kronmal, R.A.; Tracy, R.P.; Van Wagoner, D.R.; Psaty, B.M.; Lauer, M.S.; et al. Inflammation as a risk factor for atrial fibrillation. Circulation 2003, 108, 3006–3010. [Google Scholar] [CrossRef]
- Chung, M.K.; Martin, D.O.; Sprecher, D.; Wazni, O.; Kanderian, A.; Carnes, C.A.; Bauer, J.A.; Tchou, P.J.; Niebauer, M.J.; Natale, A.; et al. C-reactive protein elevation in patients with atrial arrhythmias: Inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001, 104, 2886–2891. [Google Scholar] [CrossRef]
- Schotten, U.; Verheule, S.; Kirchhof, P.; Goette, A. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiol. Rev. 2011, 91, 265–325. [Google Scholar] [CrossRef]
- Lu, Z.; Scherlag, B.J.; Lin, J.; Niu, G.; Fung, K.M.; Zhao, L.; Ghias, M.; Jackman, W.M.; Lazzara, R.; Jiang, H.; et al. Atrial fibrillation begets atrial fibrillation: Autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ. Arrhythm. Electrophysiol. 2008, 1, 184–192. [Google Scholar] [CrossRef]
- Procyk, G.; Bilicki, D.; Balsam, P.; Lodziński, P.; Grabowski, M.; Gąsecka, A. Extracellular Vesicles in Atrial Fibrillation-State of the Art. Int. J. Mol. Sci. 2022, 23, 7591. [Google Scholar] [CrossRef]
- Oikonomou, E.; Zografos, T.; Papamikroulis, G.A.; Siasos, G.; Vogiatzi, G.; Theofilis, P.; Briasoulis, A.; Papaioannou, S.; Vavuranakis, M.; Gennimata, V.; et al. Biomarkers in Atrial Fibrillation and Heart Failure. Curr. Med. Chem. 2019, 26, 873–887. [Google Scholar] [CrossRef]
- Procyk, G.; Klimczak-Tomaniak, D.; Sygitowicz, G.; Tomaniak, M. Circulating and Platelet MicroRNAs in Cardiovascular Risk Assessment and Antiplatelet Therapy Monitoring. J. Clin. Med. 2022, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Z.; Leib, C.; Katus, H.A. Autoantibodies in heart failure and cardiac dysfunction. Circ. Res. 2012, 110, 145–158. [Google Scholar] [CrossRef]
- Escobar, A.L.; Fernández-Gómez, R.; Peter, J.C.; Mobini, R.; Hoebeke, J.; Mijares, A. IgGs and Mabs against the beta2-adrenoreceptor block A-V conduction in mouse hearts: A possible role in the pathogenesis of ventricular arrhythmias. J. Mol. Cell Cardiol. 2006, 40, 829–837. [Google Scholar] [CrossRef]
- Chiale, P.A.; Garro, H.A.; Schmidberg, J.; Sánchez, R.A.; Acunzo, R.S.; Lago, M.; Levy, G.; Levin, M. Inappropriate sinus tachycardia may be related to an immunologic disorder involving cardiac beta andrenergic receptors. Heart Rhythm. 2006, 3, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Novikova, D.S.; Bekbosynova, M.S.; Antidze, T.; Loladze, N.V.; Domogatskiĭ, S.P.; Golitsyn, S.P.; Nasonov, E.L.; Denisova, I.A.; Tonevitskiĭ, S.P. Autoantibodies Against beta(1)-Adrenoreceptors in Patients With Cardiac Rhythm Disorders. Prevalence and Possible Role in Development of Arrhythmia. Kardiologiia 2004, 44, 17–22. [Google Scholar] [PubMed]
- Bekbosynova, M.S.; Nikitina, T.; Golitsyn, S.P.; Novikova, D.S.; Loladze, N.V.; Masenko, V.P.; Tonevitskiĭ, A.G. C-reactive protein level and rate of detection of autoantibodies to beta(1)-adrenoreceptors in patients with supraventricular tachyarrhythmias. Kardiologiia 2006, 46, 55–61. [Google Scholar] [PubMed]
- Nikitina, T.; Bekbosynova, M.S.; Golitsyn, S.P.; Novikova, D.S.; Loladze, N.V.; Tonevitskiĭ, A.G. Spectral parameters of heart rate variability and frequency of detection of autoantibodies to beta(1)-adrenoreceptors in patients with tachyarrhythmias: Idiopathic and at the background of primary myocardial diseases. Kardiologiia 2006, 46, 13–19. [Google Scholar] [PubMed]
- Li, H.; Scherlag, B.J.; Kem, D.C.; Zillner, C.; Male, S.; Thirunavukkarasu, S.; Shen, X.; Pitha, J.V.; Cunningham, M.W.; Lazzara, R.; et al. Atrial tachycardia provoked in the presence of activating autoantibodies to β2-adrenergic receptor in the rabbit. Heart Rhythm. 2013, 10, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Scherlag, B.J.; Kem, D.C.; Benbrook, A.; Zhang, L.; Huang, B.; Cunningham, M.W.; Lazzara, R.; Yu, X. Atrial tachyarrhythmias induced by the combined effects of β1/2-adrenergic autoantibodies and thyroid hormone in the rabbit. J. Cardiovasc. Transl. Res. 2014, 7, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Zhang, L.; Shao, M.; Feng, M.; Shi, J.; Dong, Z.; Guo, Q.; Xiaokereti, J.; Xiang, R.; Sun, H.; et al. Elevated β1-Adrenergic Receptor Autoantibody Levels Increase Atrial Fibrillation Susceptibility by Promoting Atrial Fibrosis. Front. Physiol. 2020, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.; Yoshikawa, T.; Fukuda, Y.; Sugiyama, T.; Shimada, M.; Akaishi, M.; Tsuchimoto, K.; Ogawa, S.; Fu, M. Autoantibodies against M2-muscarinic acetylcholine receptors: New upstream targets in atrial fibrillation in patients with dilated cardiomyopathy. Eur. Heart J. 2004, 25, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.M.; Zheng, Q.S.; Liu, X.T.; Shang, F.J.; Wang, H.T.; Jiang, W.R. Effects of autoantibodies against M2 muscarinic acetylcholine receptors on rabbit atria in vivo. Cardiology 2009, 112, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Zhang, Z.; Zhao, W.; Li, G.; Ma, G.; Yang, X.; Zhang, J.; Zhang, L. Predictive value of pre-procedural autoantibodies against M2-muscarinic acetylcholine receptor for recurrence of atrial fibrillation one year after radiofrequency catheter ablation. J. Transl. Med. 2013, 11, 7. [Google Scholar] [CrossRef]
- Gurses, K.M.; Yalcin, M.U.; Kocyigit, D.; Kesikli, S.A.; Canpolat, U.; Yorgun, H.; Sahiner, M.L.; Kaya, E.B.; Hazirolan, T.; Ozer, N.; et al. M2-muscarinic acetylcholine receptor autoantibody levels predict left atrial fibrosis severity in paroxysmal lone atrial fibrillation patients undergoing cryoablation. Europace 2015, 17, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wu, X.; Zeng, L.; Jin, J.; Liu, X.; Zhang, J.; Zhang, L. Association of Autoantibodies against M2-Muscarinic Acetylcholine Receptor with Atrial Fibrosis in Atrial Fibrillation Patients. Cardiol. Res. Pract. 2019, 2019, 8271871. [Google Scholar] [CrossRef] [PubMed]
- Mironova, E.S.; Mironova, N.A.; Sharf, T.V.; Efremov, E.E.; Azmuko, A.A.; Molokoedov, A.S.; Zykov, K.A.; Golitsyn, S.P. Autoantibodies to M2-cholinoreceptors as a potential development factor of arrhythmia in patients with paroxysmal atrial fibrillation. Ter. Arkh. 2019, 91, 101–107. [Google Scholar] [CrossRef]
- Deng, J.; Guo, Y.; Zhang, G.; Zhang, L.; Kem, D.; Yu, X.; Jiang, H.; Li, H. M(2) muscarinic autoantibodies and thyroid hormone promote susceptibility to atrial fibrillation and sinus tachycardia in an autoimmune rabbit model. Exp. Physiol. 2021, 106, 882–890. [Google Scholar] [CrossRef]
- Stavrakis, S.; Yu, X.; Patterson, E.; Huang, S.; Hamlett, S.R.; Chalmers, L.; Pappy, R.; Cunningham, M.W.; Morshed, S.A.; Davies, T.F.; et al. Activating autoantibodies to the beta-1 adrenergic and m2 muscarinic receptors facilitate atrial fibrillation in patients with Graves’ hyperthyroidism. J. Am. Coll. Cardiol. 2009, 54, 1309–1316. [Google Scholar] [CrossRef]
- Galloway, A.; Li, H.; Vanderlinde-Wood, M.; Khan, M.; Benbrook, A.; Liles, C.; Zillner, C.; Rao, V.; Cunningham, M.W.; Yu, X.; et al. Activating autoantibodies to the β1/2-adrenergic and M2 muscarinic receptors associate with atrial tachyarrhythmias in patients with hyperthyroidism. Endocrine 2015, 49, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, M.U.; Gurses, K.M.; Kocyigit, D.; Kesikli, S.A.; Ates, A.H.; Evranos, B.; Yorgun, H.; Sahiner, M.L.; Kaya, E.B.; Oto, M.A.; et al. Elevated M2-muscarinic and β1-adrenergic receptor autoantibody levels are associated with paroxysmal atrial fibrillation. Clin. Res. Cardiol. 2015, 104, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, M.U.; Gurses, K.M.; Kocyigit, D.; Kesikli, S.A.; Dural, M.; Evranos, B.; Yorgun, H.; Sahiner, L.; Kaya, E.B.; Oto, M.A.; et al. Cardiac Autoantibody Levels Predict Recurrence Following Cryoballoon-Based Pulmonary Vein Isolation in Paroxysmal Atrial Fibrillation Patients. J. Cardiovasc. Electrophysiol. 2015, 26, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Sun, Y.; Li, S.; Sun, J.; Liu, T.; Wu, Z.; Feng, L.I. Association of β1-Adrenergic, M2-Muscarinic Receptor Autoantibody with Occurrence and Development of Nonvalvular Atrial Fibrillation. Pacing Clin. Electrophysiol. 2016, 39, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Murphy, T.; Zhang, L.; Huang, B.; Veitla, V.; Scherlag, B.J.; Kem, D.C.; Yu, X. β1-Adrenergic and M2 Muscarinic Autoantibodies and Thyroid Hormone Facilitate Induction of Atrial Fibrillation in Male Rabbits. Endocrinology 2016, 157, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Liu, R.; Luo, X.L.; Gao, X.J.; Hu, F.H.; Guo, C.; Wang, J.; Hu, X.Y.; Chun, Y.S.; Yuan, J.S.; et al. The relationship between β(1) -adrenergic and M(2) -muscarinic receptor autoantibodies and hypertrophic cardiomyopathy. Exp. Physiol. 2020, 105, 522–530. [Google Scholar] [CrossRef]
- Mandal, K.; Jahangiri, M.; Mukhin, M.; Poloniecki, J.; Camm, A.J.; Xu, Q. Association of anti-heat shock protein 65 antibodies with development of postoperative atrial fibrillation. Circulation 2004, 110, 2588–2590. [Google Scholar] [CrossRef]
- Oc, M.; Ucar, H.I.; Pinar, A.; Akbulut, B.; Oc, B.; Akinci, S.B.; Akyon, Y.; Kanbak, M.; Boke, E.; Dogan, R. Heat shock protein 60 antibody. A new marker for subsequent atrial fibrillation development. Saudi Med. J. 2007, 28, 844–847. [Google Scholar]
- Oc, M.; Ucar, H.I.; Pinar, A.; Akbulut, B.; Oc, B.; Akyon, Y.; Kanbak, M.; Dogan, R. Heat shock protein70: A new marker for subsequent atrial fibrillation development? Artif. Organs 2008, 32, 846–850. [Google Scholar] [CrossRef]
- Kornej, J.; Reinhardt, C.; Kosiuk, J.; Arya, A.; Hindricks, G.; Adams, V.; Husser, D.; Bollmann, A. Response of circulating heat shock protein 70 and anti-heat shock protein 70 antibodies to catheter ablation of atrial fibrillation. J. Transl. Med. 2013, 11, 49. [Google Scholar] [CrossRef]
- Maixent, J.M.; Paganelli, F.; Scaglione, J.; Lévy, S. Antibodies against myosin in sera of patients with idiopathic paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 1998, 9, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.; Yoshikawa, T.; Chino, M.; Murayama, A.; Mitani, K.; Nakagawa, S.; Fujii, I.; Shimada, M.; Koyama, T.; Akaishi, M.; et al. Autoantibodies: New upstream targets of paroxysmal atrial fibrillation in patients with congestive heart failure. J. Cardiol. 2002, 40, 217–223. [Google Scholar] [PubMed]
- Rosenau, B.J.; Schur, P.H. Antibody to serum amyloid A. J. Autoimmun. 2004, 23, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, L.; Xiang, G.; Xu, C. Relationship between autoantibody to the angiotensin II-1 receptor and cardiovascular manifestations of Graves’ disease. Exp. Clin. Endocrinol. Diabetes 2014, 122, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Blagova, O.; Varionchik, N.; Zaidenov, V.; Savina, P.; Sarkisova, N. Anti-heart antibodies levels and their correlation with clinical symptoms and outcomes in patients with confirmed or suspected diagnosis COVID-19. Eur. J. Immunol. 2021, 51, 893–902. [Google Scholar] [CrossRef]
- Yoo, J.S.; Kim, Y.S.; Kim, H.Y.; Kwon, H.S.; Koh, S.H.; Heo, S.H.; Kim, B.J.; Bushnell, C.D.; Chang, D.I. Comparison of patients with transient and sustained increments of antiphospholipid antibodies after acute ischemic stroke. J. Neurol. 2021, 268, 2541–2549. [Google Scholar] [CrossRef]
- Sveen, K.A.; Smith, G.; Björkbacka, H.; Orho-Melander, M.; Engström, G.; Gonçalves, I.; Melander, O.; Nilsson, J.; Bengtsson, E. High levels of autoantibodies against apoB100 p210 are associated with lower incidence of atrial fibrillation in women. J. Intern. Med. 2022, 291, 207–217. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).