Circulating Proteins as Diagnostic Markers in Gastric Cancer
Abstract
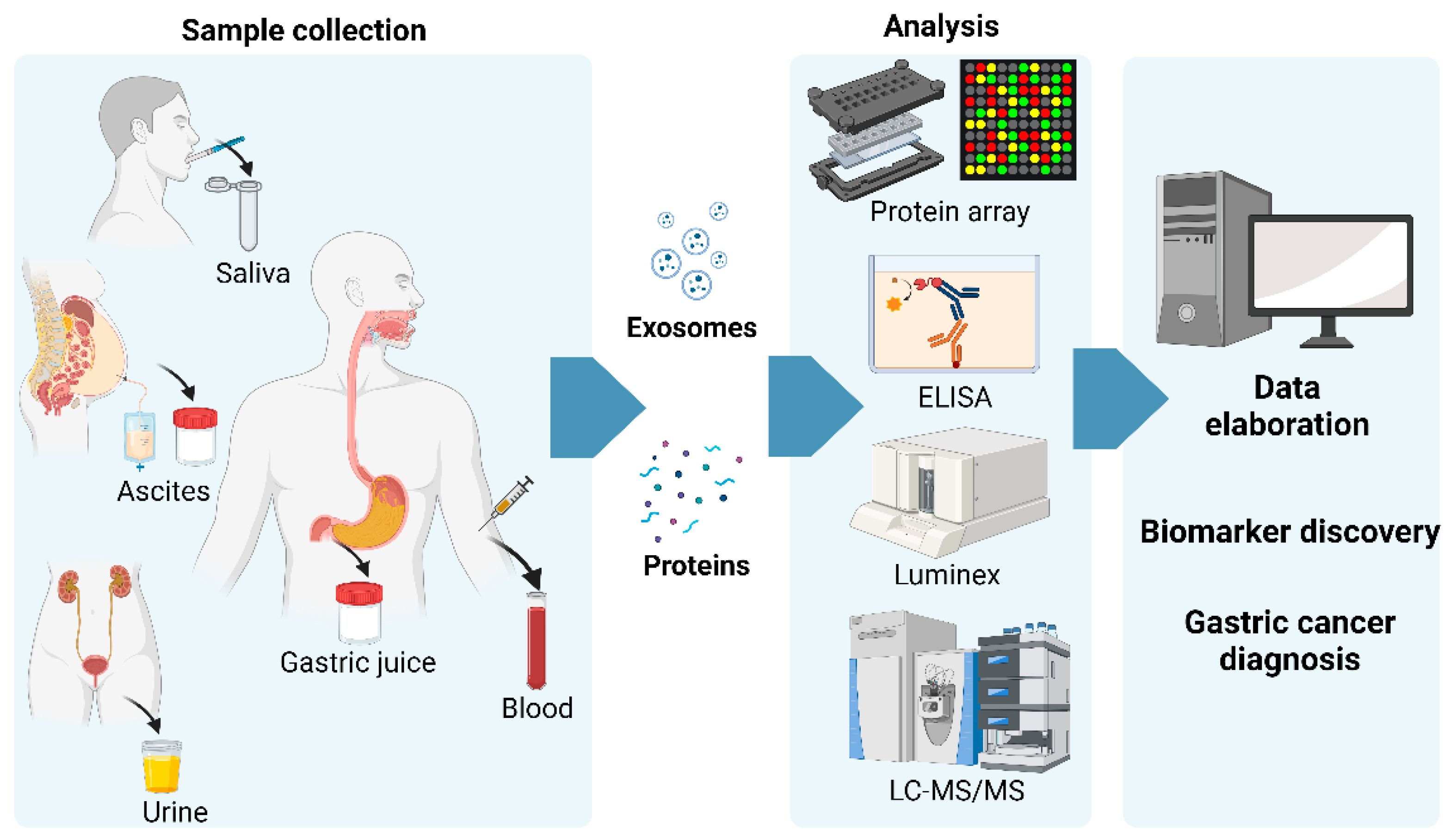
1. Gastric Cancer
2. Circulating Protein Biomarkers: An Update over the Past 10 Years
2.1. Blood-Based Circulating Biomarkers
2.2. Non-Blood-Based Circulating Biomarkers
3. Glycosylation of Circulating Proteins for GC Diagnosis
4. Serum Protein Marker Currently Used for Gastric Preneoplastic Evaluation
5. Circulating Exosomal Proteins
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Song, I.-C.; Yun, H.-J.; Jo, D.-Y.; Kim, S. CXC Chemokines and Chemokine Receptors in Gastric Cancer: From Basic Findings towards Therapeutic Targeting. World J. Gastroenterol. 2014, 20, 1681–1693. [Google Scholar] [CrossRef]
- Fock, K.M. Review Article: The Epidemiology and Prevention of Gastric Cancer. Aliment. Pharmacol. Ther. 2014, 40, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Vitelli-Storelli, F.; Rubín-García, M.; Pelucchi, C.; Benavente, Y.; Bonzi, R.; Rota, M.; Palli, D.; Ferraroni, M.; Lunet, N.; Morais, S.; et al. Family History and Gastric Cancer Risk: A Pooled Investigation in the Stomach Cancer Pooling (STOP) Project Consortium. Cancers 2021, 13, 3844. [Google Scholar] [CrossRef]
- Ilic, M.; Ilic, I. Epidemiology of Stomach Cancer. World J. Gastroenterol. 2022, 28, 1187–1203. [Google Scholar] [CrossRef]
- Verbeke, H.; Geboes, K.; Van Damme, J.; Struyf, S. The Role of CXC Chemokines in the Transition of Chronic Inflammation to Esophageal and Gastric Cancer. Biochim. Biophys. Acta 2012, 1825, 117–129. [Google Scholar] [CrossRef]
- Lauren, P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef]
- Piazuelo, M.B.; Carneiro, F.; Camargo, M.C. Considerations in Comparing Intestinal- and Diffuse-Type Gastric Adenocarcinomas. Helicobacter 2023, 28, e12975. [Google Scholar] [CrossRef]
- Gregory, S.N.; Davis, J.L. CDH1 and Hereditary Diffuse Gastric Cancer: A Narrative Review. Chin. Clin. Oncol. 2023, 12, 25. [Google Scholar] [CrossRef]
- Babu, S.D.; Jayanthi, V.; Devaraj, N.; Reis, C.A.; Devaraj, H. Expression Profile of Mucins (MUC2, MUC5AC and MUC6) in Helicobacter Pylori Infected Pre-Neoplastic and Neoplastic Human Gastric Epithelium. Mol. Cancer 2006, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Valente, P.; Garrido, M.; Gullo, I.; Baldaia, H.; Marques, M.; Baldaque-Silva, F.; Lopes, J.; Carneiro, F. Epithelial Dysplasia of the Stomach with Gastric Immunophenotype Shows Features of Biological Aggressiveness. Gastric Cancer 2015, 18, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Molinari, C.; Tedaldi, G.; Rebuzzi, F.; Morgagni, P.; Capelli, L.; Ravaioli, S.; Tumedei, M.M.; Scarpi, E.; Tomezzoli, A.; Bernasconi, R.; et al. Early Gastric Cancer: Identification of Molecular Markers Able to Distinguish Submucosa-Penetrating Lesions with Different Prognosis. Gastric Cancer 2021, 24, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive Molecular Characterization of Gastric Adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Conti, C.B.; Agnesi, S.; Scaravaglio, M.; Masseria, P.; Dinelli, M.E.; Oldani, M.; Uggeri, F. Early Gastric Cancer: Update on Prevention, Diagnosis and Treatment. Int. J. Environ. Res. Public Health 2023, 20, 2149. [Google Scholar] [CrossRef] [PubMed]
- Douda, L.; Cyrany, J.; Tachecí, I. Early Gastric Cancer. Vnitr. Lek. 2022, 68, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Borggreve, A.S.; Goense, L.; Brenkman, H.J.F.; Mook, S.; Meijer, G.J.; Wessels, F.J.; Verheij, M.; Jansen, E.P.M.; van Hillegersberg, R.; van Rossum, P.S.N.; et al. Imaging Strategies in the Management of Gastric Cancer: Current Role and Future Potential of MRI. Br. J. Radiol. 2019, 92, 20181044. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.-L.; Zhao, S.-Y.; Xu, Y.-H.; Zhang, W.-H.; Liu, K.; Chen, X.-Z.; Yang, K.; Zhang, B.; Chen, Z.-X.; et al. Prognostic Significance of Preoperative Serum CA125, CA19-9 and CEA in Gastric Carcinoma. Oncotarget 2016, 7, 35423–35436. [Google Scholar] [CrossRef]
- Yang, A.-P.; Liu, J.; Lei, H.-Y.; Zhang, Q.-W.; Zhao, L.; Yang, G.-H. CA72-4 Combined with CEA, CA125 and CAl9-9 Improves the Sensitivity for the Early Diagnosis of Gastric Cancer. Clin. Chim. Acta 2014, 437, 183–186. [Google Scholar] [CrossRef]
- Oh, J.-H.; Rhyu, M.-G.; Jung, S.-H.; Choi, S.-W.; Kim, S.-I.; Hong, S.-J. Slow Overmethylation of Housekeeping Genes in the Body Mucosa Is Associated with the Risk for Gastric Cancer. Cancer Prev. Res. 2014, 7, 585–595. [Google Scholar] [CrossRef]
- Cheng, R.; Peng, Y.; Sun, X.; Zhang, S.; Li, P. Circulating Tumor Cells as Diagnostic Markers of Early Gastric Cancer and Gastric Precancerous Lesions. Oncology 2023, 101, 512–519. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Yang, L.; Zeng, Q.; Wang, L.; Chen, M.-L.; Zhao, Z.-H.; Ye, G.-D.; Luo, Q.-C.; Lv, P.-Y.; Guo, Q.-W.; et al. Tumor-Originated Exosomal lncUEGC1 as a Circulating Biomarker for Early-Stage Gastric Cancer. Mol. Cancer 2018, 17, 84. [Google Scholar] [CrossRef]
- Qian, C.; Ju, S.; Qi, J.; Zhao, J.; Shen, X.; Jing, R.; Yu, J.; Li, L.; Shi, Y.; Zhang, L.; et al. Alu-Based Cell-Free DNA: A Novel Biomarker for Screening of Gastric Cancer. Oncotarget 2017, 8, 54037–54045. [Google Scholar] [CrossRef]
- Zhu, X.-L.; Ren, L.-F.; Wang, H.-P.; Bai, Z.-T.; Zhang, L.; Meng, W.-B.; Zhu, K.-X.; Ding, F.-H.; Miao, L.; Yan, J.; et al. Plasma microRNAs as Potential New Biomarkers for Early Detection of Early Gastric Cancer. World J. Gastroenterol. 2019, 25, 1580–1591. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, N.; Ji, N.; Chen, Z.-S. Proteomics Technologies for Cancer Liquid Biopsies. Mol. Cancer 2022, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, A.; Corrales, F.; Čolović, M.; Krstić, D.; Oliver-Martos, B.; Martínez-Cáceres, E.; Jakasa, I.; Gajski, G.; Brun, V.; Kyriacou, K.; et al. Analytical Techniques for Multiplex Analysis of Protein Biomarkers. Expert Rev. Proteom. 2020, 17, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.; Reed, D.A.; Pereira, B.A.; Timpson, P. The Cancer Cell Secretome Drives Cooperative Manipulation of the Tumour Microenvironment to Accelerate Tumourigenesis. Fac. Rev. 2021, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Padgaonkar, M.; Shendre, S.; Chatterjee, P.; Banerjee, S. Cancer Secretome: Finding out Hidden Messages in Extracellular Secretions. Clin. Transl. Oncol. 2023, 25, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Papaleo, E.; Gromova, I.; Gromov, P. Gaining Insights into Cancer Biology through Exploration of the Cancer Secretome Using Proteomic and Bioinformatic Tools. Expert Rev. Proteom. 2017, 14, 1021–1035. [Google Scholar] [CrossRef]
- Patel, S.; Ngounou Wetie, A.G.; Darie, C.C.; Clarkson, B.D. Cancer Secretomes and Their Place in Supplementing Other Hallmarks of Cancer. Adv. Exp. Med. Biol. 2014, 806, 409–442. [Google Scholar] [CrossRef]
- Núñez, C. Blood-Based Protein Biomarkers in Breast Cancer. Clin. Chim. Acta 2019, 490, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Issaq, H.J.; Xiao, Z.; Veenstra, T.D. Serum and Plasma Proteomics. Chem. Rev. 2007, 107, 3601–3620. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.-R.; Ding, W.; Xu, S.-H.; Xu, Z.; Xiao, C.-L.; Yin, X.-F.; He, Q.-Y. Characterization of Phosphoproteins in Gastric Cancer Secretome. OMICS 2011, 15, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, A.; Subbannayya, Y.; Sahasrabuddhe, N.A.; Balakrishnan, L.; Syed, N.; Sekhar, N.R.; Katte, T.V.; Pinto, S.M.; Srikanth, S.M.; Kumar, P.; et al. SILAC-Based Quantitative Proteomic Analysis of Gastric Cancer Secretome. Prot. Clin. Appl. 2013, 7, 355–366. [Google Scholar] [CrossRef]
- Cui, M.; Cheng, C.; Zhang, L. High-Throughput Proteomics: A Methodological Mini-Review. Lab. Invest. 2022, 102, 1170–1181. [Google Scholar] [CrossRef]
- Dayon, L.; Cominetti, O.; Affolter, M. Proteomics of Human Biological Fluids for Biomarker Discoveries: Technical Advances and Recent Applications. Expert. Rev. Proteom. 2022, 19, 131–151. [Google Scholar] [CrossRef]
- Castagnola, M.; Scarano, E.; Passali, G.C.; Messana, I.; Cabras, T.; Iavarone, F.; Di Cintio, G.; Fiorita, A.; De Corso, E.; Paludetti, G. Salivary Biomarkers and Proteomics: Future Diagnostic and Clinical Utilities. Acta Otorhinolaryngol. Ital. 2017, 37, 94–101. [Google Scholar] [CrossRef]
- Felípez, N.; Montori, S.; Mendizuri, N.; Llach, J.; Delgado, P.G.; Moreira, L.; Santamaría, E.; Fernández-Irigoyen, J.; Albéniz, E. The Human Gastric Juice: A Promising Source for Gastric Cancer Biomarkers. Int. J. Mol. Sci. 2023, 24, 9131. [Google Scholar] [CrossRef]
- Farina, A. Proximal Fluid Proteomics for the Discovery of Digestive Cancer Biomarkers. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2014, 1844, 988–1002. [Google Scholar] [CrossRef]
- Ding, H.; Fazelinia, H.; Spruce, L.A.; Weiss, D.A.; Zderic, S.A.; Seeholzer, S.H. Urine Proteomics: Evaluation of Different Sample Preparation Workflows for Quantitative, Reproducible, and Improved Depth of Analysis. J. Proteome Res. 2020, 19, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Birhanu, A.G. Mass Spectrometry-Based Proteomics as an Emerging Tool in Clinical Laboratories. Clin. Proteom. 2023, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Guarnizo, L.V.; Monroy-Camacho, P.S.; Rincón-Rodríguez, D.E.; Rincón-Riveros, A.; Martinez-Vargas, D.A.; Huertas-Caro, C.A.; Oliveros-Wilches, R.; Sanchez-Pedraza, R.; Nuñez-Lemus, M.; Cristancho-Lievano, C.F.; et al. Soluble HLA-G (sHLA-G) Measurement Might Be Useful as an Early Diagnostic Biomarker and Screening Test for Gastric Cancer. Sci. Rep. 2023, 13, 13119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhou, Z.; Chen, Y.; Deng, H.; Cai, Y.; Rao, X.; Yin, Y.; Rong, L. Plasma Proteomics-Based Identification of Novel Biomarkers in Early Gastric Cancer. Clin. Biochem. 2020, 76, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, O.V.; Podlesnaya, P.A.; Chang, V.L.; Ognerubov, N.A.; Gratchev, A.N.; Kozlov, N.A.; Stilidi, I.S.; Kushlinskii, N.E. Comprehensive Analysis of Stromal and Serum Markers in Gastric Cancer. Acta Naturae 2022, 14, 75–83. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, Y.; Zhu, X.; Zhang, J.; Yuwen, D.; Wei, X.; Tang, C.; Zhang, W. Plasma Thioredoxin Reductase: A Potential Diagnostic Biomarker for Gastric Cancer. Carcinogenesis 2022, 43, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.C.; Song, M.; Shimazu, T.; Charvat, H.; Yamaji, T.; Sawada, N.; Kemp, T.J.; Pfeiffer, R.M.; Hildesheim, A.; Pinto, L.A.; et al. Circulating Inflammation Markers and Risk of Gastric and Esophageal Cancers: A Case-Cohort Study Within the Japan Public Health Center-Based Prospective Study. Cancer Epidemiol. Biomark. Prev. 2019, 28, 829–832. [Google Scholar] [CrossRef]
- Lee, K.-F.; Tsai, M.-M.; Tsai, C.-Y.; Huang, C.-G.; Ou, Y.-H.; Hsieh, C.-C.; Hsieh, H.-L.; Wang, C.-S.; Lin, K.-H. DEK Is a Potential Biomarker Associated with Malignant Phenotype in Gastric Cancer Tissues and Plasma. Int. J. Mol. Sci. 2019, 20, 5689. [Google Scholar] [CrossRef]
- Cheng, C.-W.; Chang, C.-C.; Patria, Y.N.; Chang, R.-T.; Liu, Y.-R.; Li, F.-A.; Shih, H.-M.; Lin, C.-Y. Sex Hormone-Binding Globulin (SHBG) Is a Potential Early Diagnostic Biomarker for Gastric Cancer. Cancer Med. 2018, 7, 64–74. [Google Scholar] [CrossRef]
- Pan, Y.-Q.; Ruan, Y.-Y.; Peng, J.-B.; Han, Q.-Y.; Zhang, X.; Lin, A.; Yan, W.-H. Diagnostic Significance of Soluble Human Leukocyte Antigen-G for Gastric Cancer. Hum. Immunol. 2016, 77, 317–324. [Google Scholar] [CrossRef]
- Alikhani, M.; Esmaeili, M.; Tashakoripour, M.; Mohagheghi, M.A.; Eshagh Hosseini, M.; Touati, E.; Vosough, M.; Mohammadi, M. Alteration in Serum Levels of Tumor Necrosis Factor Alpha Is Associated with Histopathologic Progression of Gastric Cancer. Iran. Biomed. J. 2023, 27, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.; Rasheed, F.; Zahra, R.; König, S. Gastric Cancer Pre-Stage Detection and Early Diagnosis of Gastritis Using Serum Protein Signatures. Molecules 2022, 27, 2857. [Google Scholar] [CrossRef] [PubMed]
- Dondov, G.; Amarbayasgalan, D.; Batsaikhan, B.; Badamjav, T.; Batbaatar, B.; Tuvdenjamts, B.; Tumurbat, N.; Davaa, B.; Purevdorj, E.; Nyamaa, B.; et al. Diagnostic Performances of Pepsinogens and Gastrin-17 for Atrophic Gastritis and Gastric Cancer in Mongolian Subjects. PLoS ONE 2022, 17, e0274938. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, F.; Alizadeh, A.M.; Agah, S.; Irani, S.; Mokhtare, M. Insulin-like Growth Factor 1 Serum Levels in Different Stages of Gastric Cancer and Their Association with Helicobacter Pylori Status. Peptides 2022, 158, 170892. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Shou, M.; Ma, J.; Shu, Y.; Yu, Y. Correlation Analysis of Serum Pepsinogen, Interleukin, and TNF-α with Hp Infection in Patients with Gastric Cancer: A Randomized Parallel Controlled Clinical Study. Comput. Math. Methods Med. 2022, 2022, 9277847. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhao, W.; Yang, Z.; Zhang, B. Serum Amyloid A (SAA) and Interleukin-6 (IL-6) as the Potential Biomarkers for Gastric Cancer. Medicine 2022, 101, e31514. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cao, C. The Correlation of Serum Sirt6 with Clinical Outcome and Prognosis in Patients with Gastric Cancer. Medicine 2022, 101, e31568. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Zhang, Z.; Huang, D. Association of Multiple Tumor Markers with Newly Diagnosed Gastric Cancer Patients: A Retrospective Study. PeerJ 2022, 10, e13488. [Google Scholar] [CrossRef]
- Pan, Y.; Zheng, Y.; Yang, J.; Wei, Y.; Wu, H.; Liu, S.; Yin, A.; Hu, J.; Zeng, Y. A New Biomarker for the Early Diagnosis of Gastric Cancer: Gastric Juice- and Serum-Derived SNCG. Future Oncol. 2022, 18, 3179–3190. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, D.; Li, D.; Li, D.; Wang, H.; Wu, Y. Clinical Value on Combined Detection of Serum CA724, DKK1, and TK1 in Diagnosis of Gastric Cancer. J. Oncol. 2022, 2022, 6941748. [Google Scholar] [CrossRef]
- Wei, H.; Wu, F.; Mao, Y.; Zhang, Y.; Leng, G.; Wang, J.; Zhang, W.; Wang, T. Measurement of Soluble PD-1 and Soluble PD-L1 as Well as PD-L1 and PD-1 from Perioperative Patients with Gastric Carcinoma. Jpn. J. Clin. Oncol. 2022, 52, 331–345. [Google Scholar] [CrossRef]
- Pawluczuk, E.; Łukaszewicz-Zając, M.; Gryko, M.; Kulczyńska-Przybik, A.; Mroczko, B. Serum CXCL8 and Its Specific Receptor (CXCR2) in Gastric Cancer. Cancers 2021, 13, 5186. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, J.; Jing, H.; Lu, Y.; Zhu, Q.; Shu, C.; Zhang, Q.; Jing, D. ITIH4 Is a Novel Serum Biomarker for Early Gastric Cancer Diagnosis. Clin. Chim. Acta 2021, 523, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhai, J.; Wu, X.; Xie, G.; Shen, L. Serum Proteome Profiling Reveals SOX3 as a Candidate Prognostic Marker for Gastric Cancer. J. Cell. Mol. Med. 2020, 24, 6750–6761. [Google Scholar] [CrossRef]
- Shen, Q.; Polom, K.; Williams, C.; de Oliveira, F.M.S.; Guergova-Kuras, M.; Lisacek, F.; Karlsson, N.G.; Roviello, F.; Kamali-Moghaddam, M. A Targeted Proteomics Approach Reveals a Serum Protein Signature as Diagnostic Biomarker for Resectable Gastric Cancer. eBioMedicine 2019, 44, 322–333. [Google Scholar] [CrossRef]
- Yoon, J.H.; Park, Y.G.; Nam, S.W.; Park, W.S. The Diagnostic Value of Serum Gastrokine 1 (GKN1) Protein in Gastric Cancer. Cancer Med. 2019, 8, 5507–5514. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, P.; Ma, J.; Xu, J.; Yang, L.; Xu, W.; Que, H.; Chen, M.; Xu, H. Serum Biomarker Panels for the Diagnosis of Gastric Cancer. Cancer Med. 2019, 8, 1576–1583. [Google Scholar] [CrossRef]
- Gunaldi, M.; Isiksacan, N.; Kocoglu, H.; Okuturlar, Y.; Gunaldi, O.; Topcu, T.O.; Karabulut, M. The Value of Serum Survivin Level in Early Diagnosis of Cancer. J. Cancer Res. Ther. 2018, 14, 570–573. [Google Scholar] [CrossRef]
- Huang, A.; Zhang, M.; Li, T.; Qin, X. Serum Proteomic Analysis by Tandem Mass Tags (TMT) Based Quantitative Proteomics in Gastric Cancer Patients. Clin. Lab. 2018, 64, 855–866. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Run, Z.-C.; Feng, W.; Liu, W.; Zhang, P.-J.; Li, Z. Multiple Cytokine Profiling in Serum for Early Detection of Gastric Cancer. World J. Gastroenterol. 2018, 24, 2269–2278. [Google Scholar] [CrossRef]
- Ning, S.; Wei, W.; Li, J.; Hou, B.; Zhong, J.; Xie, Y.; Liu, H.; Mo, X.; Chen, J.; Zhang, L. Clinical Significance and Diagnostic Capacity of Serum TK1, CEA, CA 19-9 and CA 72-4 Levels in Gastric and Colorectal Cancer Patients. J. Cancer 2018, 9, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Wu, H.; Qu, K.; Sun, Q.; Li, F.; Shi, C.; Li, Y.; Xiong, X.; Qin, Q.; Yu, T.; et al. Identification of Serum Proteins AHSG, FGA and APOA-I as Diagnostic Biomarkers for Gastric Cancer. Clin. Proteom. 2018, 15, 18. [Google Scholar] [CrossRef]
- Tas, F.; Karabulut, S.; Erturk, K.; Duranyildiz, D. Clinical Significance of Serum Leptin Level in Patients with Gastric Cancer. Eur. Cytokine Netw. 2018, 29, 52–58. [Google Scholar] [CrossRef]
- Feng, F.; Tian, Y.; Xu, G.; Liu, Z.; Liu, S.; Zheng, G.; Guo, M.; Lian, X.; Fan, D.; Zhang, H. Diagnostic and Prognostic Value of CEA, CA19-9, AFP and CA125 for Early Gastric Cancer. BMC Cancer 2017, 17, 737. [Google Scholar] [CrossRef]
- Li, J.; Xie, N.; Yuan, J.; Liu, L.; Zhou, Q.; Ren, X.; Chen, Q.; Zhang, G.; Ruan, Q.; Chen, Y.H.; et al. DcR3 Combined with Hematological Traits Serves as a Valuable Biomarker for the Diagnosis of Cancer Metastasis. Oncotarget 2017, 8, 107612–107620. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, W.-F.; Nie, C.-F.; Wu, G.; Zhang, J.-C.; Zhu, Y.-Z.; Zhang, W.; Sun, P.-C. Soluble Interleukin-2 Receptor as a Factor Associated with Angiogenesis in Gastric Cancer. Mol. Med. Rep. 2017, 16, 6916–6919. [Google Scholar] [CrossRef]
- Yang, H.; Han, Y.; Wu, L.; Wu, C. Diagnostic and Prognostic Value of Serum Interleukin-16 in Patients with Gastric Cancer. Mol. Med. Rep. 2017, 16, 9143–9148. [Google Scholar] [CrossRef]
- Yoo, M.-W.; Park, J.; Han, H.-S.; Yun, Y.-M.; Kang, J.W.; Choi, D.-Y.; Lee, J.W.; Jung, J.H.; Lee, K.-Y.; Kim, K.P. Discovery of Gastric Cancer Specific Biomarkers by the Application of Serum Proteomics. Proteomics 2017, 17, 1600332. [Google Scholar] [CrossRef]
- Liu, W.-L.; Liu, D.; Cheng, K.; Liu, Y.-J.; Xing, S.; Chi, P.-D.; Liu, X.-H.; Xue, N.; Lai, Y.-Z.; Guo, L.; et al. Evaluating the Diagnostic and Prognostic Value of Circulating Cathepsin S in Gastric Cancer. Oncotarget 2016, 7, 28124–28138. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Li, J.; Zhang, H.; Guo, S.; Yan, M.; Zhu, Z.; Lan, B.; Ding, Y.; Xu, M.; et al. Identification of Serum Biomarkers for Gastric Cancer Diagnosis Using a Human Proteome Microarray. Mol. Cell. Proteom. 2016, 15, 614–623. [Google Scholar] [CrossRef]
- Tong, W.; Ye, F.; He, L.; Cui, L.; Cui, M.; Hu, Y.; Li, W.; Jiang, J.; Zhang, D.Y.; Suo, J. Serum Biomarker Panels for Diagnosis of Gastric Cancer. Onco Targets Ther. 2016, 9, 2455–2463. [Google Scholar] [CrossRef]
- Wu, C.; Luo, Z.; Tang, D.; Liu, L.; Yao, D.; Zhu, L.; Wang, Z. Identification of Carboxyl Terminal Peptide of Fibrinogen as a Potential Serum Biomarker for Gastric Cancer. Tumour Biol. 2016, 37, 6963–6970. [Google Scholar] [CrossRef]
- Subbannayya, Y.; Mir, S.A.; Renuse, S.; Manda, S.S.; Pinto, S.M.; Puttamallesh, V.N.; Solanki, H.S.; Manju, H.C.; Syed, N.; Sharma, R.; et al. Identification of Differentially Expressed Serum Proteins in Gastric Adenocarcinoma. J. Proteom. 2015, 127, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Tas, F.; Tilgen Yasasever, C.; Karabulut, S.; Tastekin, D.; Duranyildiz, D. Clinical Significance of Serum Interleukin-18 (IL-18) Levels in Patients with Gastric Cancer. Biomed. Pharmacother. 2015, 70, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Tas, F.; Tilgen Yasasever, C.; Karabulut, S.; Tastekin, D.; Duranyildiz, D. Circulating Annexin A2 as a Biomarker in Gastric Cancer Patients: Correlation with Clinical Variables. Biomed. Pharmacother. 2015, 69, 237–241. [Google Scholar] [CrossRef]
- Toiyama, Y.; Tanaka, K.; Kitajima, T.; Shimura, T.; Imaoka, H.; Mori, K.; Okigami, M.; Yasuda, H.; Okugawa, Y.; Saigusa, S.; et al. Serum Angiopoietin-like Protein 2 as a Potential Biomarker for Diagnosis, Early Recurrence and Prognosis in Gastric Cancer Patients. Carcinogenesis 2015, 36, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiong, X.; Wang, X.; Guo, B.; He, K.; Huang, C. Identification of Peptide Regions of SERPINA1 and ENOSF1 and Their Protein Expression as Potential Serum Biomarkers for Gastric Cancer. Tumour Biol. 2015, 36, 5109–5118. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Son, M.; Kim, H.; Kim, H.; Kong, S.-H.; Kim, H.K.; Kim, Y.; Han, D. Comparative Proteomic Analysis of Human Malignant Ascitic Fluids for the Development of Gastric Cancer Biomarkers. Clin. Biochem. 2018, 56, 55–61. [Google Scholar] [CrossRef]
- Wu, W.; Yong, W.W.; Chung, M.C.M. A Simple Biomarker Scoring Matrix for Early Gastric Cancer Detection. Proteomics 2016, 16, 2921–2930. [Google Scholar] [CrossRef]
- Wu, W.; Juan, W.C.; Liang, C.R.M.Y.; Yeoh, K.G.; So, J.; Chung, M.C.M. S100A9, GIF and AAT as Potential Combinatorial Biomarkers in Gastric Cancer Diagnosis and Prognosis. Proteom. Clin. Appl. 2012, 6, 152–162. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, Y.; Kim, Y.; Kim, S.; Kim, J.J.; Kim, K.M.; Yoshizawa, J.; Fan, L.-Y.; Cao, C.-X.; Wong, D.T.W. Differential Proteomic Analysis of Human Saliva Using Tandem Mass Tags Quantification for Gastric Cancer Detection. Sci. Rep. 2016, 6, 22165. [Google Scholar] [CrossRef]
- Joshi, N.; Bhat, F.; Bellad, A.; Sathe, G.; Jain, A.; Chavan, S.; Sirdeshmukh, R.; Pandey, A. Urinary Proteomics for Discovery of Gastric Cancer Biomarkers to Enable Precision Clinical Oncology. OMICS 2023, 27, 361–371. [Google Scholar] [CrossRef]
- Fan, H.; Li, X.; Li, Z.-W.; Zheng, N.-R.; Cao, L.-H.; Liu, Z.-C.; Liu, M.-W.; Li, K.; Wu, W.-H.; Li, Z.-X.; et al. Urine Proteomic Signatures Predicting the Progression from Premalignancy to Malignant Gastric Cancer. eBioMedicine 2022, 86, 104340. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Dayde, D.; Wang, H.; Okuda, Y.; Iwasaki, H.; Ebi, M.; Kitagawa, M.; Yamada, T.; Yamada, T.; Hanash, S.M.; et al. Novel Urinary Protein Biomarker Panel for Early Diagnosis of Gastric Cancer. Br. J. Cancer 2020, 123, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Dagher, A.; Sachdev, M.; Ebi, M.; Yamada, T.; Yamada, T.; Joh, T.; Moses, M.A. Urinary ADAM12 and MMP-9/NGAL Complex Detect the Presence of Gastric Cancer. Cancer Prev. Res. 2015, 8, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, G.; Zhang, G.; Ni, Z.; Suo, J.; Cui, J.; Cui, A.; Yang, Q.; Xu, Y.; Li, F. The Endothelial Lipase Protein Is Promising Urinary Biomarker for Diagnosis of Gastric Cancer. Diagn. Pathol. 2013, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Nakayasu, E.S.; Gritsenko, M.; Piehowski, P.D.; Gao, Y.; Orton, D.J.; Schepmoes, A.A.; Fillmore, T.L.; Frohnert, B.I.; Rewers, M.; Krischer, J.P.; et al. Tutorial: Best Practices and Considerations for Mass-Spectrometry-Based Protein Biomarker Discovery and Validation. Nat. Protoc. 2021, 16, 3737–3760. [Google Scholar] [CrossRef]
- Diamandis, E.P.; van der Merwe, D.-E. Plasma Protein Profiling by Mass Spectrometry for Cancer Diagnosis: Opportunities and Limitations. Clin. Cancer Res. 2005, 11, 963–965. [Google Scholar] [CrossRef]
- Palstrøm, N.B.; Rasmussen, L.M.; Beck, H.C. Affinity Capture Enrichment versus Affinity Depletion: A Comparison of Strategies for Increasing Coverage of Low-Abundant Human Plasma Proteins. Int. J. Mol. Sci. 2020, 21, 5903. [Google Scholar] [CrossRef]
- Paul, J.; Veenstra, T.D. Separation of Serum and Plasma Proteins for In-Depth Proteomic Analysis. Separations 2022, 9, 89. [Google Scholar] [CrossRef]
- Macklin, A.; Khan, S.; Kislinger, T. Recent Advances in Mass Spectrometry Based Clinical Proteomics: Applications to Cancer Research. Clin. Proteom. 2020, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Bernt, M.; Chambers, M.; Fahrner, M.; Föll, M.C.; Gruening, B.; Horro, C.; Johnson, J.E.; Loux, V.; Rajczewski, A.T.; et al. A Galaxy of Informatics Resources for MS-Based Proteomics. Expert. Rev. Proteom. 2023, 20, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhang, F.; Xu, L.; Yue, L.; Kon, O.L.; Zhu, Y.; Guo, T. High-Throughput Proteomics and AI for Cancer Biomarker Discovery. Adv. Drug Deliv. Rev. 2021, 176, 113844. [Google Scholar] [CrossRef] [PubMed]
- Marcus, K.; Lelong, C.; Rabilloud, T. What Room for Two-Dimensional Gel-Based Proteomics in a Shotgun Proteomics World? Proteomes 2020, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Dowling, P. DIGE Analysis of ProteoMinerTM Fractionated Serum/Plasma Samples. Methods Mol. Biol. 2023, 2596, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Angel, T.E.; Aryal, U.K.; Hengel, S.M.; Baker, E.S.; Kelly, R.T.; Robinson, E.W.; Smith, R.D. Mass Spectrometry-Based Proteomics: Existing Capabilities and Future Directions. Chem. Soc. Rev. 2012, 41, 3912–3928. [Google Scholar] [CrossRef]
- Kay, R.G.; Galvin, S.; Larraufie, P.; Reimann, F.; Gribble, F.M. Liquid Chromatography/Mass Spectrometry Based Detection and Semi-Quantitative Analysis of INSL5 in Human and Murine Tissues. Rapid Commun. Mass. Spectrom. 2017, 31, 1963–1973. [Google Scholar] [CrossRef]
- Zecha, J.; Satpathy, S.; Kanashova, T.; Avanessian, S.C.; Kane, M.H.; Clauser, K.R.; Mertins, P.; Carr, S.A.; Kuster, B. TMT Labeling for the Masses: A Robust and Cost-Efficient, In-Solution Labeling Approach. Mol. Cell. Proteom. 2019, 18, 1468–1478. [Google Scholar] [CrossRef]
- Bai, M.; Deng, J.; Dai, C.; Pfeuffer, J.; Sachsenberg, T.; Perez-Riverol, Y. LFQ-Based Peptide and Protein Intensity Differential Expression Analysis. J. Proteome Res. 2023, 22, 2114–2123. [Google Scholar] [CrossRef]
- Zhang, Y.; Birru, R.; Di, Y.P. Analysis of Clinical and Biological Samples Using Microsphere-Based Multiplexing Luminex System. Methods Mol. Biol. 2014, 1105, 43–57. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Lu, M.; Shen, L. Predictive Value of Serum CEA, CA19-9 and CA72.4 in Early Diagnosis of Recurrence after Radical Resection of Gastric Cancer. Hepatogastroenterology 2011, 58, 2166–2170. [Google Scholar] [CrossRef]
- Shimada, H.; Noie, T.; Ohashi, M.; Oba, K.; Takahashi, Y. Clinical Significance of Serum Tumor Markers for Gastric Cancer: A Systematic Review of Literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer 2014, 17, 26–33. [Google Scholar] [CrossRef]
- He, Q.-Y.; Cheung, Y.H.; Leung, S.Y.; Yuen, S.T.; Chu, K.-M.; Chiu, J.-F. Diverse Proteomic Alterations in Gastric Adenocarcinoma. Proteomics 2004, 4, 3276–3287. [Google Scholar] [CrossRef]
- Emoto, S.; Ishigami, H.; Yamashita, H.; Yamaguchi, H.; Kaisaki, S.; Kitayama, J. Clinical Significance of CA125 and CA72-4 in Gastric Cancer with Peritoneal Dissemination. Gastric Cancer 2012, 15, 154–161. [Google Scholar] [CrossRef]
- Lei, Z.-N.; Teng, Q.-X.; Tian, Q.; Chen, W.; Xie, Y.; Wu, K.; Zeng, Q.; Zeng, L.; Pan, Y.; Chen, Z.-S.; et al. Signaling Pathways and Therapeutic Interventions in Gastric Cancer. Signal Transduct. Target. Ther. 2022, 7, 358. [Google Scholar] [CrossRef] [PubMed]
- Jaroenlapnopparat, A.; Bhatia, K.; Coban, S. Inflammation and Gastric Cancer. Diseases 2022, 10, 35. [Google Scholar] [CrossRef]
- Wang, H.; Liu, M.; Zeng, X.; Zheng, Y.; Wang, Y.; Zhou, Y. Cell Death Affecting the Progression of Gastric Cancer. Cell Death Discov. 2022, 8, 377. [Google Scholar] [CrossRef] [PubMed]
- Forma, A.; Tyczyńska, M.; Kędzierawski, P.; Gietka, K.; Sitarz, M. Gastric Carcinogenesis: A Comprehensive Review of the Angiogenic Pathways. Clin. J. Gastroenterol. 2021, 14, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Senol, K.; Ozkan, M.B.; Vural, S.; Tez, M. The Role of Inflammation in Gastric Cancer. Adv. Exp. Med. Biol. 2014, 816, 235–257. [Google Scholar] [CrossRef]
- Ford, C.E.; Werner, B.; Hacker, N.F.; Warton, K. The Untapped Potential of Ascites in Ovarian Cancer Research and Treatment. Br. J. Cancer 2020, 123, 9–16. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Wang, C.; Xia, L.; Xu, J.; Xie, X.; Lu, W. Microenvironment Remodeled by Tumor and Stromal Cells Elevates Fibroblast-Derived COL1A1 and Facilitates Ovarian Cancer Metastasis. Exp. Cell Res. 2020, 394, 112153. [Google Scholar] [CrossRef] [PubMed]
- Han, W.K.; Alinani, A.; Wu, C.-L.; Michaelson, D.; Loda, M.; McGovern, F.J.; Thadhani, R.; Bonventre, J.V. Human Kidney Injury Molecule-1 Is a Tissue and Urinary Tumor Marker of Renal Cell Carcinoma. J. Am. Soc. Nephrol. 2005, 16, 1126–1134. [Google Scholar] [CrossRef]
- Fernández, C.A.; Yan, L.; Louis, G.; Yang, J.; Kutok, J.L.; Moses, M.A. The Matrix Metalloproteinase-9/Neutrophil Gelatinase-Associated Lipocalin Complex Plays a Role in Breast Tumor Growth and Is Present in the Urine of Breast Cancer Patients. Clin. Cancer Res. 2005, 11, 5390–5395. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xun, D.; Li, L.; Wang, B.; Lv, J.; Liu, H.; Zhu, L.; Ma, F.; Chen, X.; Tian, S.; et al. Deep Dive on the Proteome of Human Body Fluids: A Valuable Data Resource for Biomarker Discovery. Cancer Genom. Proteom. 2021, 18, 549–568. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.A.; Adam, Y.G. Malignant Ascites: Past, Present, and Future. J. Am. Coll. Surg. 2004, 198, 999–1011. [Google Scholar] [CrossRef]
- Liu, F.; Kong, X.; Dou, Q.; Ye, J.; Xu, D.; Shang, H.; Xu, K.; Song, Y. Evaluation of Tumor Markers for the Differential Diagnosis of Benign and Malignant Ascites. Ann. Hepatol. 2014, 13, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Streckfus, C.F.; Dubinsky, W.P. Proteomic Analysis of Saliva for Cancer Diagnosis. Expert Rev. Proteom. 2007, 4, 329–332. [Google Scholar] [CrossRef][Green Version]
- Kalantari, S.; Jafari, A.; Moradpoor, R.; Ghasemi, E.; Khalkhal, E. Human Urine Proteomics: Analytical Techniques and Clinical Applications in Renal Diseases. Int. J. Proteom. 2015, 2015, 782798. [Google Scholar] [CrossRef]
- Marimuthu, A.; O’Meally, R.N.; Chaerkady, R.; Subbannayya, Y.; Nanjappa, V.; Kumar, P.; Kelkar, D.S.; Pinto, S.M.; Sharma, R.; Renuse, S.; et al. A Comprehensive Map of the Human Urinary Proteome. J. Proteome Res. 2011, 10, 2734–2743. [Google Scholar] [CrossRef]
- Bones, J.; Byrne, J.C.; O’Donoghue, N.; McManus, C.; Scaife, C.; Boissin, H.; Nastase, A.; Rudd, P.M. Glycomic and Glycoproteomic Analysis of Serum from Patients with Stomach Cancer Reveals Potential Markers Arising from Host Defense Response Mechanisms. J. Proteome Res. 2011, 10, 1246–1265. [Google Scholar] [CrossRef]
- Ozcan, S.; Barkauskas, D.A.; Renee Ruhaak, L.; Torres, J.; Cooke, C.L.; An, H.J.; Hua, S.; Williams, C.C.; Dimapasoc, L.M.; Han Kim, J.; et al. Serum Glycan Signatures of Gastric Cancer. Cancer Prev. Res. 2014, 7, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Ma, J.; Zhu, Y.; Wang, T.; Yang, Y.; Sun, Y.; Chen, Y.; Song, H.; Huo, X.; Zhang, J. The Role and Potential Mechanism of O-Glycosylation in Gastrointestinal Tumors. Pharmacol. Res. 2022, 184, 106420. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Isaji, T.; Xu, Q.; Kariya, Y.; Gu, W.; Fukuda, T.; Du, Y. Potential Roles of N-Glycosylation in Cell Adhesion. Glycoconj. J. 2012, 29, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Carvalho, S.; Marcos-Pinto, R.; Magalhães, A.; Oliveira, C.; Gu, J.; Dinis-Ribeiro, M.; Carneiro, F.; Seruca, R.; Reis, C.A. Gastric Cancer: Adding Glycosylation to the Equation. Trends Mol. Med. 2013, 19, 664–676. [Google Scholar] [CrossRef]
- Gomes, C.; Almeida, A.; Ferreira, J.A.; Silva, L.; Santos-Sousa, H.; Pinto-de-Sousa, J.; Santos, L.L.; Amado, F.; Schwientek, T.; Levery, S.B.; et al. Glycoproteomic Analysis of Serum from Patients with Gastric Precancerous Lesions. J. Proteome Res. 2013, 12, 1454–1466. [Google Scholar] [CrossRef]
- Lee, J.; Hua, S.; Lee, S.H.; Oh, M.J.; Yun, J.; Kim, J.Y.; Kim, J.-H.; Kim, J.H.; An, H.J. Designation of Fingerprint Glycopeptides for Targeted Glycoproteomic Analysis of Serum Haptoglobin: Insights into Gastric Cancer Biomarker Discovery. Anal. Bioanal. Chem. 2018, 410, 1617–1629. [Google Scholar] [CrossRef]
- Lee, H.W.; Choe, Y.H.; Kim, D.K.; Jung, S.Y.; Lee, N.G. Proteomic Analysis of a Ferric Uptake Regulator Mutant ofHelicobacter Pylori: Regulation ofHelicobacter Pylori Gene Expression by Ferric Uptake Regulator and Iron. Proteomics 2004, 4, 2014–2027. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, S.H.; Choi, S.; Kim, U.; Yeo, I.S.; Kim, S.H.; Oh, M.J.; Moon, H.; Lee, J.; Jeong, S.; et al. Direct Analysis of Aberrant Glycosylation on Haptoglobin in Patients with Gastric Cancer. Oncotarget 2017, 8, 11094–11104. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeong, S.; Lee, J.; Yeo, I.S.; Oh, M.J.; Kim, U.; Kim, S.; Kim, S.H.; Park, S.-Y.; Kim, J.-H.; et al. Glycomic Profiling of Targeted Serum Haptoglobin for Gastric Cancer Using Nano LC/MS and LC/MS/MS. Mol. Biosyst. 2016, 12, 3611–3621. [Google Scholar] [CrossRef]
- Jeong, S.; Oh, M.J.; Kim, U.; Lee, J.; Kim, J.-H.; An, H.J. Glycosylation of Serum Haptoglobin as a Marker of Gastric Cancer: An Overview for Clinicians. Expert Rev. Proteom. 2020, 17, 109–117. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, B.; Wang, Y.; Xia, P.; He, C.; Liu, Y.; Zhang, R.; Zhang, M.; Li, Z. Disease-Specific IgG Fc N-Glycosylation as Personalized Biomarkers to Differentiate Gastric Cancer from Benign Gastric Diseases. Sci. Rep. 2016, 6, 25957. [Google Scholar] [CrossRef]
- Ruhaak, L.R.; Barkauskas, D.A.; Torres, J.; Cooke, C.L.; Wu, L.D.; Stroble, C.; Ozcan, S.; Williams, C.C.; Camorlinga, M.; Rocke, D.M.; et al. The Serum Immunoglobulin G Glycosylation Signature of Gastric Cancer. EuPA Open Proteom. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Lin, J.; Wang, Y.; Li, D.; Xie, G.-Y.; Guo, A.-Y.; Liu, B.-F.; Cheng, L.; Liu, X. Three Major Gastrointestinal Cancers Could Be Distinguished through Subclass-Specific IgG Glycosylation. J. Proteome Res. 2022, 21, 2771–2782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qin, Y.; Jiang, Q.; Li, F.; Jing, X.; Cao, L.; Cai, S.; Wu, F.; Li, Q.; Lian, J.; et al. Glycopattern Alteration of Glycoproteins in Gastrointestinal Cancer Cell Lines and Their Cell-Derived Exosomes. J. Proteome Res. 2022, 21, 1876–1893. [Google Scholar] [CrossRef] [PubMed]
- Vermassen, T.; D’Herde, K.; Jacobus, D.; Van Praet, C.; Poelaert, F.; Lumen, N.; Callewaert, N.; Decaestecker, K.; Villeirs, G.; Hoebeke, P.; et al. Release of Urinary Extracellular Vesicles in Prostate Cancer Is Associated with Altered Urinary N-Glycosylation Profile. J. Clin. Pathol. 2017, 70, 838–846. [Google Scholar] [CrossRef]
- Garza-Campos, A.; Prieto-Correa, J.R.; Domínguez-Rosales, J.A.; Hernández-Nazará, Z.H. Implications of Receptor for Advanced Glycation End Products for Progression from Obesity to Diabetes and from Diabetes to Cancer. World J. Diabetes 2023, 14, 977–994. [Google Scholar] [CrossRef]
- Kishi, S.; Nishiguchi, Y.; Honoki, K.; Mori, S.; Fujiwara-Tani, R.; Sasaki, T.; Fujii, K.; Kawahara, I.; Goto, K.; Nakashima, C.; et al. Role of Glycated High Mobility Group Box-1 in Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 5185. [Google Scholar] [CrossRef]
- Rojas, A.; Lindner, C.; Schneider, I.; González, I.; Morales, M.A. Contributions of the Receptor for Advanced Glycation End Products Axis Activation in Gastric Cancer. World J. Gastroenterol. 2023, 29, 997–1010. [Google Scholar] [CrossRef]
- Dorf, J.; Pryczynicz, A.; Matowicka-Karna, J.; Zaręba, K.; Żukowski, P.; Zalewska, A.; Maciejczyk, M. Could Circulating Biomarkers of Nitrosative Stress and Protein Glycoxidation Be Useful in Patients with Gastric Cancer? Front. Oncol. 2023, 13, 1213802. [Google Scholar] [CrossRef]
- Shu, J.; Yu, H.; Li, X.; Zhang, D.; Liu, X.; Du, H.; Zhang, J.; Yang, Z.; Xie, H.; Li, Z. Salivary Glycopatterns as Potential Biomarkers for Diagnosis of Gastric Cancer. Oncotarget 2017, 8, 35718–35727. [Google Scholar] [CrossRef]
- Demirhan, D.B.; Yılmaz, H.; Erol, H.; Kayili, H.M.; Salih, B. Prediction of Gastric Cancer by Machine Learning Integrated with Mass Spectrometry-Based N-Glycomics. Analyst 2023, 148, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Polom, K.; Adamczyk, B.; Afshar, M.; D’Ignazio, A.; Kamali-Moghaddam, M.; Karlsson, N.G.; Guergova-Kuras, M.; Lisacek, F.; Marrelli, D.; et al. Machine Learning Methodology Applied to Characterize Subgroups of Gastric Cancer Patients Using an Integrated Large Biomarker Dataset. Eur. J. Surg. Oncol. 2019, 45, e79. [Google Scholar] [CrossRef]
- De Re, V.; Orzes, E.; Canzonieri, V.; Maiero, S.; Fornasarig, M.; Alessandrini, L.; Cervo, S.; Steffan, A.; Zanette, G.; Mazzon, C.; et al. Pepsinogens to Distinguish Patients With Gastric Intestinal Metaplasia and Helicobacter Pylori Infection Among Populations at Risk for Gastric Cancer. Clin. Transl. Gastroenterol. 2016, 7, e183. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Sun, L.; Dong, X.; Gong, Y.; Xu, Q.; Jing, J.; Bostick, R.M.; Wu, X.; Yuan, Y. A Serological Biopsy Using Five Stomach-Specific Circulating Biomarkers for Gastric Cancer Risk Assessment: A Multi-Phase Study. Am. J. Gastroenterol. 2017, 112, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, G.X.; Wang, H.G.; Mo, F.F.; Tang, B.B. The Value of Detecting Pepsinogen and Gastrin-17 Levels in Serum for Pre-Cancerous Lesion Screening in Gastric Cancer. Neoplasma 2019, 66, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Trivanovic, D.; Plestina, S.; Honovic, L.; Dobrila-Dintinjana, R.; Vlasic Tanaskovic, J.; Vrbanec, D. Gastric Cancer Detection Using the Serum Pepsinogen Test Method. Tumori 2022, 108, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Yanan, Z.; Juan, W.; Jun, W.; Xin, M.; Kejian, W.; Fangyu, W. Application of Serum Gastric Function Markers and Digestive Tumor Indices to the Diagnosis of Early Gastric Cancer and Precancerous Lesions. Saudi Med. J. 2023, 44, 795–800. [Google Scholar] [CrossRef]
- Sasakabe, T.; Obata, Y.; Kawai, S.; Lin, Y.; Kikuchi, S. Comparison of Gastric Cancer Risk Classifications Using Conventional and New Pepsinogen Criteria. Gastroenterol. Res. Pract. 2023, 2023, 7646536. [Google Scholar] [CrossRef]
- De Re, V.; Realdon, S.; Vettori, R.; Zaramella, A.; Maiero, S.; Repetto, O.; Canzonieri, V.; Steffan, A.; Cannizzaro, R. A DSC Test for the Early Detection of Neoplastic Gastric Lesions in a Medium-Risk Gastric Cancer Area. Int. J. Mol. Sci. 2023, 24, 3290. [Google Scholar] [CrossRef]
- GBD 2017 Stomach Cancer Collaborators. The Global, Regional, and National Burden of Stomach Cancer in 195 Countries, 1990-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, Y.; Pan, J.; Zhou, L.; Lin, J. Application of a Novel Scoring System for Gastric Cancer Opportunistic Screening in Hospital Visits. BMC Gastroenterol. 2022, 22, 223. [Google Scholar] [CrossRef]
- Hurwitz, A.; Brady, D.A.; Schaal, S.E.; Samloff, I.M.; Dedon, J.; Ruhl, C.E. Gastric Acidity in Older Adults. JAMA 1997, 278, 659–662. [Google Scholar] [CrossRef]
- Rodriguez, K.; Franceschi, M.; Ferronato, A.; Brozzi, L.; Antico, A.; Panozzo, M.P.; Massella, A.; Pertoldi, B.; Morini, A.; Barchi, A.; et al. A Non-Invasive Combined Strategy to Improve the Appropriateness of Upper Gastrointestinal Endoscopy. Acta Biomed. 2022, 93, e2022210. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter Pylori Infection: The Maastricht VI/Florence Consensus Report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Annibale, B.; Esposito, G.; Lahner, E. A Current Clinical Overview of Atrophic Gastritis. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Kishikawa, H.; Nakamura, K.; Ojiro, K.; Katayama, T.; Arahata, K.; Takarabe, S.; Sasaki, A.; Miura, S.; Hayashi, Y.; Hoshi, H.; et al. Relevance of Pepsinogen, Gastrin, and Endoscopic Atrophy in the Diagnosis of Autoimmune Gastritis. Sci. Rep. 2022, 12, 4202. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Gu, Y.; Du, Y.; Liu, J. Exosomes: Diagnostic Biomarkers and Therapeutic Delivery Vehicles for Cancer. Mol. Pharm. 2019, 16, 3333–3349. [Google Scholar] [CrossRef]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a New Frontier of Cancer Liquid Biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J.; Chen, W.; Li, G.; Li, Z.; Lei, J. The Updated Role of Exosomal Proteins in the Diagnosis, Prognosis, and Treatment of Cancer. Exp. Mol. Med. 2022, 54, 1390–1400. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular Vesicles in Physiological and Pathological Conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Wortzel, I.; Dror, S.; Kenific, C.M.; Lyden, D. Exosome-Mediated Metastasis: Communication from a Distance. Dev. Cell 2019, 49, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.-L.; Qu, X.-J.; Zhao, M.-F.; Teng, Y.-E.; Zhang, Y.; Hou, K.-Z.; Jiang, Y.-H.; Yang, X.-H.; Liu, Y.-P. Gastric Cancer Exosomes Promote Tumour Cell Proliferation through PI3K/Akt and MAPK/ERK Activation. Dig. Liver Dis. 2009, 41, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Gu, J.; Jiang, P.; Qian, H.; Xu, W.; Zhang, X. Exosomes in Gastric Cancer: Roles, Mechanisms, and Applications. Mol. Cancer 2019, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.-D.; Pang, J.-R.; Bi, Y.-P.; Zhao, L.-F.; Li, Y.-R.; Zhao, L.-J.; Gao, Y.; Wang, B.; Wang, N.; Wei, L.; et al. LSD1 Deletion Decreases Exosomal PD-L1 and Restores T-Cell Response in Gastric Cancer. Mol. Cancer 2022, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, T.; Liu, R.; Bai, M.; Zhou, L.; Wang, X.; Li, S.; Wang, X.; Yang, H.; Li, J.; et al. Exosome-Delivered EGFR Regulates Liver Microenvironment to Promote Gastric Cancer Liver Metastasis. Nat. Commun. 2017, 8, 15016. [Google Scholar] [CrossRef]
- Zheng, P.; Luo, Q.; Wang, W.; Li, J.; Wang, T.; Wang, P.; Chen, L.; Zhang, P.; Chen, H.; Liu, Y.; et al. Tumor-Associated Macrophages-Derived Exosomes Promote the Migration of Gastric Cancer Cells by Transfer of Functional Apolipoprotein E. Cell Death Dis. 2018, 9, 434. [Google Scholar] [CrossRef]
- Li, R.; Jiang, J.; Shi, H.; Qian, H.; Zhang, X.; Xu, W. CircRNA: A Rising Star in Gastric Cancer. Cell. Mol. Life Sci. 2020, 77, 1661–1680. [Google Scholar] [CrossRef]
- Tang, X.-H.; Guo, T.; Gao, X.-Y.; Wu, X.-L.; Xing, X.-F.; Ji, J.-F.; Li, Z.-Y. Exosome-Derived Noncoding RNAs in Gastric Cancer: Functions and Clinical Applications. Mol. Cancer 2021, 20, 99. [Google Scholar] [CrossRef]
- Fu, H.; Yang, H.; Zhang, X.; Wang, B.; Mao, J.; Li, X.; Wang, M.; Zhang, B.; Sun, Z.; Qian, H.; et al. Exosomal TRIM3 Is a Novel Marker and Therapy Target for Gastric Cancer. J. Exp. Clin. Cancer Res. 2018, 37, 162. [Google Scholar] [CrossRef]
- Yoon, J.H.; Ham, I.-H.; Kim, O.; Ashktorab, H.; Smoot, D.T.; Nam, S.W.; Lee, J.Y.; Hur, H.; Park, W.S. Gastrokine 1 Protein Is a Potential Theragnostic Target for Gastric Cancer. Gastric Cancer 2018, 21, 956–967. [Google Scholar] [CrossRef]
- Yoon, J.H.; Ashktorab, H.; Smoot, D.T.; Nam, S.W.; Hur, H.; Park, W.S. Uptake and Tumor-Suppressive Pathways of Exosome-Associated GKN1 Protein in Gastric Epithelial Cells. Gastric Cancer 2020, 23, 848–862. [Google Scholar] [CrossRef]
- Guo, X.-Y.; Dong, L.; Qin, B.; Jiang, J.; Shi, A.-M. Decreased Expression of Gastrokine 1 in Gastric Mucosa of Gastric Cancer Patients. World J. Gastroenterol. 2014, 20, 16702–16706. [Google Scholar] [CrossRef]
- Farjadian, S.; Tabebordbar, M.; Mokhtari, M.; Safaei, A.; Malekzadeh, M.; Ghaderi, A. HLA-G Expression in Tumor Tissues and Soluble HLA-G Plasma Levels in Patients with Gastrointestinal Cancer. Asian Pac. J. Cancer Prev. 2018, 19, 2731–2735. [Google Scholar] [CrossRef]
- Coban, S.; Yüksel, O.; Koçkar, M.C.; Köklü, S.; Basar, O.; Tutkak, H.; Ormeci, N. The Significance of Serum Transforming Growth Factor Beta 1 in Detecting of Gastric and Colon Cancers. Hepatogastroenterology 2007, 54, 1472–1476. [Google Scholar]
- Shimoda, A.; Ueda, K.; Nishiumi, S.; Murata-Kamiya, N.; Mukai, S.-A.; Sawada, S.; Azuma, T.; Hatakeyama, M.; Akiyoshi, K. Exosomes as Nanocarriers for Systemic Delivery of the Helicobacter Pylori Virulence Factor CagA. Sci. Rep. 2016, 6, 18346. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lv, M.; Lv, L.; Cao, N.; Zhao, A.; Chen, J.; Tang, X.; Luo, R.; Yu, S.; Zhou, Y.; et al. Identifying HER2 from Serum-Derived Exosomes in Advanced Gastric Cancer as a Promising Biomarker for Assessing Tissue HER2 Status and Predicting the Efficacy of Trastuzumab-Based Therapy. Cancer Med. 2023, 12, 4110–4124. [Google Scholar] [CrossRef] [PubMed]
- Hartl, J.; Kurth, F.; Kappert, K.; Horst, D.; Mülleder, M.; Hartmann, G.; Ralser, M. Quantitative Protein Biomarker Panels: A Path to Improved Clinical Practice through Proteomics. EMBO Mol. Med. 2023, 15, e16061. [Google Scholar] [CrossRef]
- Nolen, B.M.; Lokshin, A.E. Protein Biomarkers of Ovarian Cancer: The Forest and the Trees. Future Oncol. 2012, 8, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Poulos, R.C.; Liu, J.; Zhong, Q. Machine Learning for Multi-Omics Data Integration in Cancer. iScience 2022, 25, 103798. [Google Scholar] [CrossRef] [PubMed]

| Biomarker(s) (a) | GO Biological Process (b) | Proteomic Technology (c) | Patients | Major Findings | Ref. | |
|---|---|---|---|---|---|---|
| Tumor Characteristics (d) | Group (nr) (e) | |||||
| Plasma | ||||||
| sHLA-G [P17693] (plasma and exosomal) | Immune response | • ELISA | • Histology: 58.4% intestinal, 26.0% single ring cell adenocarcinoma, 15.6% other • clinical stages: 24.9% I–II, 56.6 III–IV • 39.9% poorly differentiated | GC (173), benign gastric disease (307; 86.3% chronic gastritis) | A higher sHLA-G concentration was found in GC vs. benign pathologies in GC-affected women vs. men, but no significant differences were found among the GC stages. sHLA-G was proposed as a potential diagnostic marker, although not as an adequate marker for staging GC. HLA-G was found in exosome membranes. | [43] |
| 18 proteins | Miscellaneous | • LC-MS/MS combined with TMT labeling | • Early-GC • adenocarcinoma (87%) and high-grade intraepithelial neoplasia (13%) • adenocarcinoma mainly well or moderately differentiated with invasive depth mainly limited to the mucosa | early-GC (15) and C (15) | From a total of 2040 proteins identified, 11 proteins were differentially abundant between early-GC patients and C (7 increased and 4 decreased). These proteins distinguished early-GC from healthy C (sensitivity = 66.7%, and specificity = 86.7%). | [44] |
| PD-1 [Q15116] PD-L1 [Q9NZQ7] macrophage and B-cell markers | Adaptive immune response Adaptive immune response | • ELISA • IHC | • Histology: 82.5% adenocarcinoma, 16% signet ring cell carcinoma, 1.5% undifferentiated cancer • clinical stages: 40% I–II, 60% III–IV • depth of invasion: 21% T1–T2, 79% T3–T4 • metastasis: 86% M0, 14% M1 | GC (63) | The plasma content of the sPD-1 receptor was significantly lower in GC vs. C; it inversely correlates with plasma sPD-L1 content and directly correlates with the tissue PD-L1 expression in stromal cells. Levels of sPD-L1 in GC vs. C were similar. | [45] |
| TrxR [Q86VQ6] | Cell differentiation | • Ultraviolet spectrophotometry • ECLA | • Adenocarcinoma | GC (896), benign gastric disease (322; e.g., stomach ulcer, stomach polyps, and gastritis) and C (228) | TrxR activity in GC [8.4 U/mL] was significantly higher than that in benign disease [6.1 U/mL] or C [3.7 U/mL]. ROC analysis of TrxR [AUC = 0.945; sensitivity = 95.6%; specificity = 76.3%] showed a better capacity of GC diagnosis than that of routine tumor markers (AFP, CA50, CA72-4, CA19-9, CA242, CEA). | [46] |
| sEGFR [P00533] TSLP [Q969D9] | Cell morphogenesis/adhesion Positive regulation of chemokine production | • 5 Luminex bead-based multiplex assay panels | • Clinical stages: 52.5% early/localized, 34.1% advanced, 13.4% unknown | GC (446) and individuals (774) as random subcohort. | Levels of sEGFR (Ptrends = 0.017) and TSLP (Ptrends = 0.034) were associated with GC risk. However, none of the Ptrends remained statistically significant after FDR correction. | [47] |
| DEK [P35659] | Chromatin remodeling | • WB • ELISA | • Histology: 72% diffuse, 28% intestinal • depth of invasion: 35% T1–T2, 65% T3–T4 • GC undoing gastrectomy • 39% localized, 61% infiltrative • 67% lymph node metastasis, 18% distant metastasis. | GC (92) and C (120) | Data from ROC curve analysis highlighted a better diagnostic accuracy (AUC = 0.797) and sensitivity (70.4) than CEA, CA 19-9, and CRP. | [48] |
| ApoC1 [P02654] GSN [P06396] SHBG [P04278] C4-A [P0C0L4] | Cholesterol efflux Actin filament capping Androgen binding Complement activation | • Label-free quantitative LC-MS/MS • bioinformatics • WB • ELISA | • Adenocarcinoma • histology: discovery cohort→25% diffuse, 71% intestinal, 4% mixed; verification cohort→29% diffuse, 71% intestinal; validation cohort→52% diffuse, 40% intestinal, 8% mixed • depth of invasion: discovery and verification cohorts→50% T1–T2, 50% T3–T4; validation cohort→34% T1–T2, 66% T3–T4 | Discovery cohort: GC (24) and C (9); verification cohort: GC (24) and C (9); validation cohort: GC (50) and C (68) | Four proteins (apolipoprotein C-1, gelsolin, SHBG, and complement component C4-A) increased in content in GC (p < 0.05). WB and ELISA confirmed higher SHBG levels in GC. Plasma SHBG levels were proposed as a potential early diagnostic biomarker for GC. | [49] |
| sHLA-G [P17693] | Immune response | • ELISA | • Histology: 37% diffuse, 49.3% intestinal, 13.7 mixed • clinical stages: 38% I–II, 62% III–IV • depth of invasion: 25.9% T1–T2, 74.1% T3–T4 • distant metastasis: 95% M0, 5% M1 | 81 GC, benign gastric disease (53, e.g., ulcer, gastritis, polypus) and C (77) | Plasma sHLA-G concentration was significantly higher in GC compared with both benign gastric disease and C. sHLA-G was proposed as a GC diagnostic marker, especially when combined with other GC markers (CA125, CA19-9, and CA72-4). | [50] |
| Serum | ||||||
| TNFα [P01375] IL-8 [P10145] | Acute inflammatory response Angiogenesis | • ELISA | • Histology: 10% diffuse, 48% intestinal, 22% signet ring cell, 12% mixed, 8% other types • various clinical stages | GC (82), CG (94), and C (53) | Moderate levels of TNF-α were detected in the C group (19.9 ± 19.5 pg/mL), which were significantly higher in CG patients (35.7 ± 28.0 pg/mL) but drastically decreased in GC (1.8 ± 5.9 pg/mL). TNF-α was proposed to behave as an inflammatory marker. IL-8 concentrations did not vary among patients. | [51] |
| ITGB6 [P18564] GPX3 [P22352] CRP [P02741] S100A9 [P06702] SERPINA4 [P29622] | Cell adhesion/morphogenesis hydrogen peroxide catabolic process Acute-phase response Apoptotic process Negative regulation of endopeptidase activity | • LC-MS/MS | • Histology: diffuse • WHO classification: 61% tubular, 39% poorly cohesive | 219 H. pylori positive and negative patients diagnosed with GC, gastritis, and ulcers | Two GC serum marker panels, 29preGC-P (with ITGB6 and GPX3) and 10GC-P (with CRP, S100A9, and SERPINA4), were proposed for the diagnosis of early stage and advanced GC independently on H. pylori status, respectively. | [52] |
| G-17 [P01350] PGI [P0DJD8] PGII [P20142] PGR [P06401] | Response to food Digestion Digestion Cell–cell signaling | • GastroPanel ELISA kit | • 75.0% adenocarcinoma, 11.1% mucinous carcinoma, 8.3% poorly cohesive, 2.8% tubular carcinoma, 2.8% papillary carcinoma | GC (36), AG (40), and C (40) | PGI levels significantly decreased in GC and AG compared to C groups (p < 0.05). No significant differences in PGII and G-17 levels between study groups. For GC, the optimal cut-off values of PGI and PGR (PGI to PGII ratio) were ≤35.25 ng/mL (sensitivity = 47.2%; specificity = 86.8%) and ≤5.27 ng/mL (sensitivity = 75%; specificity = 60.5%), respectively. The PGR was significantly lower in GC vs. C (p < 0.01). The combinations of PGI and PGR with risk factors were proposed to improve diagnostic accuracy (AUC for AG 74.8, 95% CI 64.0–85.7, p < 0.001; AUC for GC 75.5, 95% CI 64.2–86.8, p < 0.001). | [53] |
| IGF-1 [P08069] | Insulin receptor signaling pathway | • ELISA • IHC • real-time PCR assay | • Clinical stages: 39.3% I–II, 60.7% III–IV • invasion depth: 30% T1–T2, 38.4% T3–T4, any T 31.6% • distance metastasis: 68.4% M0, 31.6% M1 • H. pylori status: 69.6 pos, 30.4% neg | GC (60) and C (30) | Early GC stages showed a significantly low IHC score for IGF-1R and phosphorylated AKT, mTOR, and ERK proteins compared to the advanced stages. IGF-1 serum levels and the expression of candidate genes increased in advanced vs. early GC and positive vs. negative H. pylori status (p < 0.05). | [54] |
| IL-6 [P05231] PGI [P0DJD8] PGII [P20142] PGR [P06401] TNF-α [P01375] | Acute-phase response Digestion Digestion Cell–cell signaling Acute inflammatory response | • ELISA • IMMU-NITE1000 | Not detailed | Observation group: GC (50) with H. pylori; comparison group: GC (50) without H. pylori. | In the “observation” group, PGI and PGII were lower, while TNF-α, IL-18, and IL-6 were significantly higher than those in the “comparison” group (p < 0.05). | [55] |
| CEA [P06731] SAA [P0DJI8] IL-6 [P05231] | Apoptotic process Acute-phase response Acute-phase response | • Commercial kits | Not detailed | GC (122), gastric benign disease (37), and C (30) | SAA and IL-6 levels were higher in GC vs. C. The ROC curve for the combined detection of SAA, IL-6, and CEA showed AUC = 0.948, sensitivity = 91.0%, and specificity = 89.2%. | [56] |
| SIRT6 [Q8N6T7] | DNA repair-dependent chromatin remodeling | • ELISA | • Histology: 36.3% diffuse, 63.7% intestinal • clinical stages: 38.5% I–II, 60.7% III–IV • invasion depth: 25.9% T1–T2, 74.1% T3–T4 • distance metastasis: 85.9% M0, 14.1% M1 | GC (135), AG (68), and C (60) | Serum SIRT6 levels were lower in GC vs. AG and C. They were positively associated with tumor stage and metastasis and proposed as a diagnostic and predictive biomarker for GC. | [57] |
| AFP [P02771] CA125 [Q8WXI7] CEA [P06731] CA153 [n.a.] CA199 [n.a.] CA242 [n.a.] | Progesterone metabolism Cell adhesion Apoptotic process | • Multi-tumor marker detection kit based on protein chips | Not detailed | GC (268) and C (209) | Serum GC was associated with age, gender, and positive levels of AFP, CEA, CA125, CA199, and CA242. The positive levels of AFP and CA125 were related to distant GC metastasis. | [58] |
| SNCG [O76070] | Regulation of neurotransmitter secretion | • ELISA | • Clinical stages: 45% I–II, 55% III-IV • invasion depth: 34% T1–T2, 66% T3–T4 • distance metastasis: 83% M0, 17% M1 • H. pylori status: 41% positive, 59% negative | GC (87), gastric precancerous lesions (38), and C (44) | Detection of SNCG in serum and gastric juice was a good method for the early diagnosis of GC (cut-off value = 7.716 ng/mL; AUC = 0.924; sensitivity = 95.40%; specificity = 86.36%; p < 0.0001). Serum SNCG was related to TNM stage, lymph node metastasis, and tumor size. | [59] |
| DKK1 [O94907] TK1 [P04183] CA724 [n.a.] | Cell morphogenesis Mitotic DNA replication | • ELISA • ECLIA • ECLA | • GC without any type of treatment • clinical stages: 77.8% I–II, 22.2% III–IV | GC (63) and gastric benign disease (considered as C; 54) | The three serological indexes were higher in GC vs. C (p < 0.001). The ROC analysis for their combined detection showed an AUC = 0.923, with sensitivity and specificity higher than those of separate detection. | [60] |
| PD-1 [Q15116] PD-L1 [Q9NZQ7] | Adaptive immune response Adaptive immune response | • ELISA | • GC without any type of treatment • clinical stages: 66.6% I–II, 33.4% III–IV • patients undergoing gastrectomy and lymph node dissection | GC (30) and C (30) | Preoperative sPD-1 and sPD-L1 were lower in GC vs. C. The ROC analysis showed an AUC equal to 0.675 and 0.885 for sPD-1 and sPD-L1, respectively. | [61] |
| CXCL8 [P10145] CXCR2 [P25025] CEA [P06731] CA19.9 [n.a.] | Angiogenesis Immune response Apoptotic process | • ELISA • CMIA • turbidimetric assay | • Histology: 53% intestinal, 47% diffuse • clinical stages: 28% I–II, 69% III–IV, 3% undefined • invasion depth: 15.6% T1–T2, 84.4% T3–T4 • distant metastasis: 72% M0, 28% M1 | GC (64) and C (34) | Higher levels of CXCL8 and CXCR2 in GC vs. C. Serum CXCL8 was proposed as a promising biomarker for GC diagnosis, especially in combination with CA19-9 (sensitivity = 89%; specificity = 53%). | [62] |
| ITIH4 [Q14624] | Acute-phase response | • LC-MS/MS • WB • IHC | • Clinical stages: 53% I–II, 47% III–IV | Early-GC (38), advanced GC (70), LGN (28, precancerous group), CSG (37), OST (49, patients with other system malignant tumors), and C (178) | ITIH4 abundance in early GC (specificity = 94.44%) was significantly higher than those in the C and other GC groups. ITIH4 was proposed as a diagnostic marker for early-GC. | [63] |
| SOX3 [P41225] | Cell differentiation | • LC-MS/MS combined with TMT/iTRAQ labeling | • Locally advanced GC (55 cases) and early GC (5 cases) • invasion depth: 31.7% T1–T2, 68.3% T3–T4 • clinical stages: 33.3% I–II, 66.7% III–IV • degree of differentiation: 26.7% poorly, 31.7% moderately, 41.6% well • distant metastasis: 90% M0, 10% M1 | GC (60) and C (60) | Among proteins significantly differentially abundant, SOX3 was found to be higher in GC vs. C sera. | [64] |
| 19 proteins | Miscellaneous | • PEA (over 300 proteins tested) | • Clinical stages: 28% I–II, 71% III–IV, 1% undefined | GC (100) and C (50) | In total, 19 serum proteins distinguished GC from C, with a diagnostic sensitivity of 93%, specificity of 100%, and AUC of 0.99 (95% CI: 0.98–1). This protein signature increased diagnostic capacity, particularly in patients at TNM I-II stages (sensitivity = 89%; specificity = 100%; AUC = 0.99) and with high microsatellite instability (MSI) (91%, 98%, and 0.99) compared to individual proteins. | [65] |
| GKN1 [Q9NS71] | Digestion | • ELISA | Not detailed | Early GC (140), advanced GC (360), other cancers (768), and C (200) | Serum GKN1 levels in GC (median: 3.48 ng/μL) were lower than in C (median: 6.34 ng/μL). GKN1 levels were significantly higher in early GC (median: 4.31 ng/μL) than in advanced GC (median: 3.11 ng/μL). The ROC curve analysis distinguished early from advanced GC (AUC = 0.870). Serum GKN1 appeared as a promising and highly specific diagnostic biomarker for both early and advanced GC. | [66] |
| EphA1 [P21709] EREG [O14944] FGF-12 [P61328] FR-β [P14207] Galectin-8 [O00214] GHR [P10912] IFNGR1 [P15260] Integrin α 5 [P08648] Notch-3 [Q9UM47] SLAMF8 [Q9P0V8] TNFRSF19L [Q969Z4] | Angiogenesis Angiogenesis Cell–cell signaling Cell adhesion Lymphatic endothelial cell migration Cytokine-mediated signaling pathway Positive regulation of gene expression Angiogenesis Notch signaling pathway Signaling receptor activity Apoptotic process | • Human cytokine antibody • ELISA | • Clinical stages: IA • undifferentiated • tumor location: mucosa • no metastasis | Antibody array assay: GC (15) and C (10); ELISA: GC (20) and C (20) | In total, 11 serum cytokines increased in GC (p < 0.05) and were proposed as novel biomarkers for the early diagnosis of GC. | [67] |
| BIRC5 [A0A7L8XZM3] | Regulation of apoptotic process | • ELISA | Not detailed | 10.4% together with prostate cancer and glioblastoma from 67 patients with diagnosed cancer | Serum survivin level was high at GC diagnosis (p < 0.05). The optimal cut-off value of serum survivin was determined at >120.8 pg/mL. | [68] |
| CD59 [P13987] COF1 [P23528] S100A8 [P05109] ITIH4 [Q14624] | Blood coagulation Actin cytoskeleton organization Acute inflammatory response | • TMT labeling • LC-MS/MS and bioinformatics • WB | •Adenocarcinoma • stages: I/II | GC (10) and C (10) | A total of 105 proteins differed (p < 0.05) between GC and C, 69 being glycoproteins. The decrease in COF1 and the increase in ITIH4, S100A8, and CD59 could be useful in GC diagnosis. | [69] |
| IL-8 [P10145 ] TNF-α [P01375] CEA [P06731] IL-6 [P05231] CA72-4 [n.a.] | Angiogenesis Acute inflammatory response Apoptotic process Acute phase response | • Luminex 200 bead-based assay for IL-6, IL-8, and TNF-α determination • electrochemiluminescence | • Clinical stages: discovery cohort →36% early stage and 64% advanced stage; validation cohort→33% early stage and 67% advanced stage | Discovery cohort: GC (176) and C (204); validation cohort: GC (58) and C (66) | Serum IL-6 had the best diagnostic value in discriminating GC (AUC of joint analysis = 0.95). The multiparameter model with CEA, CA72-4, IL-6, IL-8. and TNF-α discriminated GC, early GC, and advanced GC from the healthy C one (sensitivity = 89.66%, 84.21%, and 92.31%; specificity = 92.42%, 90.91%, and 90.91%). | [70] |
| TK1 [P04183] CEA [P06731] CA19.9 [P15391] CA72-4 [n.a.] | DNA synthesis Apoptotic process Antigen receptor-mediated signaling pathway | • Cell Cycle Assay Kit • ECLIA assay kits | Not detailed | GC (169) and C (75) | TK1 was a good independent marker for GC. Its combination with CA19.9, CA72-4, and CEA performed better. The combined detection of the 4 markers was proposed to be useful for GC diagnosis. | [71] |
| AHSG [P02765] APOA-I [P02647] FGA [P02671] | Acute-phase response Blood vessel endothelial cell migration Blood coagulation | • MB-IMAC-Cu, MALDI-TOF-MS, and peptide pattern analysis • Nano Acquity UPLC MS/MS • ELISA | • Clinical stages: discovery cohort→50% I/II and 44% III/IV; validation cohort→38% I/II and 62% III/IV | Discovery cohort: GC (32) and C (30); validation cohort: GC (42) and C (28) | Among the 12 differential peptide peaks (p < 0.0001), the serum levels of FGA increased in GC vs. C (AUC = 0.98, p < 0.05), similar to AHSG and APOA-I (AUC = 0.92 and 0.83; p < 0.05), and these 3 proteins were proposed as valuable biomarkers for GC. | [72] |
| Leptin | Energy homeostasis, neuroendocrine function, and metabolism [Kelesidis 2010] | • ELISA | • Clinical stages: 16% I/II and 70% III/IV • invasion depth: 22% T1–T3 and 35% T4 • metastasis: 51% M0 | GC (63) and C (30) | Leptin concentrations were lower in GC vs. C (p = 0.009). A diagnostic role was proposed for serum leptin levels. | [73] |
| CA19.9 [P15391] CEA [P06731] AFP [P02771] CA125 [Q8WXI7] | Antigen receptor-mediated signaling pathway Apoptotic process Progesterone metabolism Cell adhesion | Not reported | • Invasion depth: 43% T1a and 57% T1b | Early GC (587) | The positive rates of CEA, CA19.9, AFP, and CA125 (4.3%, 4.8%, 1.5%, and 1.9%, respectively) were low for early GC. The combination of CEA, CA125, and CA19–9 has been reported to lead to higher sensitivity than CEA alone. | [74] |
| DcR3 [O95407] | Apoptotic process | • ELISA | • Metastasis: 30% M0 and 70% M1 | GC (10) and C (25) | DcR3 levels increased in GC patients (2.04 ± 1.01, p = 0.0061). ROC analysis showed high specificity (90%), sensitivity (85.7%), and AUC (82.3%; threshold = 243.7 pg/mL) to distinguish GC. DcR3 was proposed as a biomarker for GC diagnosis. | [75] |
| IL-2R [P01589] VEGF [P15692] TGF-β1 [P01137] | Activated T cell proliferation Angiogenesis ATP biosynthetic process/cell migration | • ELISA | • Clinical stages: 5.8% II, 94.2% III-IV | GC (35) and C (32) | Serum levels of sIL-2R, VEGF, and TGF-β1 were higher in GC vs. C. Serum sIL-2R levels were also positively associated with VEGF and TGF-β1 levels. | [76] |
| IL-16 [Q14005] | Cytokine-mediated signaling pathway | • ELISA • WB | Not detailed | GC (98) and C (98) | IL-16 levels in GC vs. C were higher (2.59-fold; p < 0.05). It differentiated GC from C (AUC = 0.882, sensitivity = 79.6%, and specificity = 78.6%). IL-16 was proposed as a novel diagnostic marker for GC. | [77] |
| CLU-1 [P10909] SRMS [Q9H3Y6] THB1 [P10828] VN [P04004] | Cell morphogenesis Cell differentiation Cell differentiation Cell adhesion | • Label-free quantitative LC-MS/MS • MS-based MRM • WB | • Discovery cohort: 50% early and 50% advanced; validation cohort: 52% early and 48% advanced | Discovery cohort: GC (6) and C (3); validation cohort: GC (60) and C (29) | In early GC, 119 and 176 proteins were up- and downregulated, respectively. Four proteins (VN, CLU-1, THB1, SRMS) changed in GC vs. C and discriminated GC with sufficient specificity and selectivity. | [78] |
| Cat S [P25774] | Antigen processing and presentation | • ELISA • WB • immunohistochemistry | • Clinical stages: 44.5% I–II, 55.5% III–IV | GC (119) and C (99) | Serum Cat S levels increased in GC (AUC = 0.803, sensitivity = 60.7%, and specificity = 90.0%). | [79] |
| COPS2 [P61201] CTSF [Q9UBX1] NT5E [P21589] TERF1 [P54274] | Negative regulation of transcription by RNA polymerase II Antigen processing and presentation of exogenous peptide antigen via MHC class II Adenosine biosynthetic process Cell division | • Proteome microarray • ELISA | • Invasion depth: discovery phase I→54% T1–T2, 46% T3–T4; training/testing phase→52% T1–T2, 48% T3–T4 • metastasis: 96% M0 and 4% M1 | Discovery phases I and II→GC (37–300) and C (50–300); training set→GC (108) and C (108), testing set→GC (192) and C (192); validation phases I and II→ GC (100–200) and C (100–200) | A final panel of 4 biomarkers (COPS2, CTSF, NT5E, and TERF1) provided high diagnostic power (sensitivity = 95% and specificity = 92%) to differentiate GC from C, and it was proposed as a non-invasive diagnostic index for GC. | [80] |
| ADAM8 [P78325] VEGF [P15692] PGI [P0DJD8] PGII [P20142] IgG to H. pylori | Angiogenesis Angiogenesis Digestion Digestion | • Multiplex assay | • Newly diagnosed primary adenocarcinoma • invasion depth: 36% T1–T2, 64% T3–T4 • clinical stages: 23.1% I, 32.6% II, and 44.2% III; 17% early stage and 83% advanced stage | Training set: GC (228) and C (190); validation set: GC (48) and C (47) | The selected panel of markers differentiated between the majority of GC and C with high accuracy (RF 79.0%, SVM 83.8%, logistic regression 76.2%) in the training set as well as in the validation one (RF 82.5%, SVM 86.1%, logistic regression 78.7%). | [81] |
| FGA carboxyl-terminal fraction [P02671] | Coagulation | • SELDI ProteinChip analysis • LC-MS/MS • immunodepletion • chemiluminescence | • Invasion depth: training set→30% T1–T2, 70% T3–T4; validation set→20% T2, 80% T3–T4 | Training set: GC (30) and C (30); validation set: GC (10) and C (10) | Peak 5910 showed good performance in distinguishing GC from C with high sensitivity and specificity (AUC = 0.89; training set→sensitivity = 86.3% and specificity = 91.3%; validation set→sensitivity = 100% and specificity = 93.3%). | [82] |
| ITIH4 [Q14624] SAA1 [P0DJI8] | Acute-phase response Acute-phase response | • iTRAQ labeling • SCX fractionation • LC-MS/MS • LC-MRM | • Histology: discovery cohort→100% at III; validation cohort→60% at III, 30% at II, 10% signet ring cell | Discovery cohort: GC (10) and C (10); validation cohort: GC (10) and C (10) | In GC, a total of 59 proteins were differentially abundant, 48 being up- (iTRAQ ratios of ≥2) and 11 being downregulated (iTRAQ ratios of ≤0.5). Validation analyses confirmed the increased levels of ITIH4 and SAA1 (p < 0.05) in GC. | [83] |
| IL-18 [Q14116] | Angiogenesis | • ELISA | • Clinical stages: 16% I-II, 70% III, and 14% undetermined • invasion depth: 22% T1–T3, 35% T4, and 43% unknown | GC (63) and C (30) | The baseline IL-18 levels of GC were higher than those of C (p < 0.001), indicating that IL-18 was a good serological diagnostic GC marker. No correlation was observed between IL-18 concentrations and clinical characteristics (p > 0.05). | [84] |
| ANXA2 [P07355] | Angiogenesis | • ELISA | • Clinical stages: 16% I-II, 70% III, and 14% undetermined • invasion depth: 22% T1–T3, 35% T4, and 43% unknown | GC (63) and C (30) | The baseline ANXA2 levels of GC were higher than those of the C group (p < 0.001). The known clinical variables were not correlated with ANXA2 concentrations (p > 0.05). | [85] |
| ANGPTL2 [Q9UKU9] | Cell–cell signaling | • IHC • ELISA | • Clinical stages: screening phase→50% I and 50% IV; validation phase→51% I, 16% II, 18% III, and 15% IV | Screening phase: GC (16) and C (23); validation phase: GC (194) and C (45) | Serum ANGPTL2 in GC was higher than in C (p < 0.05) and distinguished GC patients from C patients (AUC = 0.865). The validation step confirmed higher ANGPTL2 levels in GC vs. C (p < 0.0001). | [86] |
| SERPINA1 [P01009] ENOSF1 [Q7L5Y1] | Acute-phase response Amino acid/carbohydrate catabolic process | • WCX fractionation and MALDI-TOF MS • LC-MS/MS • ELISA | • Invasion depth: discovery cohort→9% I, 20% II, 46% III, and 25% IV (characteristics of patients belonging to the validation cohort not detailed) | Discovery cohort: GC (70); validation cohort: GC (36) and C (36) | Peptides with m/z values of 1546.02 and 5335.08 showed a higher concentration in the spectra of GC vs. controls (p < 0.001) and were identified as belonging to SERPINA1 and ENOSF1. Only ENOSF1 concentration was higher in GC (1.55-fold, p < 0.001) and it was proposed as a biomarker for GC diagnosis. | [87] |
| Biomarker(s) (a) | Proteomic Technology (b) | Patients | Major Findings | Ref. | |
|---|---|---|---|---|---|
| Tumor Characteristics (c) | Group (nr) (d) | ||||
| Ascitic fluid | |||||
| PGC and POSTN | • LC-MS/MS • ELISA | • Clinical stage IV | GC (85) | In total, 299 differentially expressed proteins were quantified, 81 and 218 of which were up- and downregulated, respectively, in the ascitic fluids of GC. PGC and POSTN proteins distinguished malignant ascites from benign ones and were verified by ELISA, being thus potential candidate biomarkers of disease state. | [88] |
| Gastric juice | |||||
| SNCG (also from serum) | • ELISA | Not reported | GC (87), CPL (38), and C (44) | SNCG levels were higher in GC vs. CPL or C in both gastric juice and serum. The expression of SNCG in GJ and serum was significantly associated with tumor node metastasis stage, lymph node metastasis, tumor size, and drinking. The detection of SNCG in gastric juice and serum was reported as an ideal method of clinical diagnostic value for the early diagnosis of GC, with high specificity (90.91%) and sensitivity (83.91%) (positive predictive value = 94.81%; negative predictive value = 74.07%; 95% CI: 0.869–0.971; p< 0.0001). | [59] |
| Ela3A, PepA, GastL, Gastricsin, and CystD | • iTRAQ labeling • LC-MS/MS • WB | • Histology: 30% diffuse, 33% intestinal, 37% unknown/mix • invasion depth: 54% I–II, 46% III–IV | GC (70) and benign gastritis (17) | An increase in Ela3A together with a decrease in PepA, Gast, Gastricsin, and CystD occurred in gastric fluids of GC patients with high confidence. A three-biomarker panel of CystD + PepA + Ela3A was sufficient for initial GC diagnosis (sensitivity = 95.7%; specificity = 76.5%). | [89] |
| S100A9, GIF, and AAT | • 2DE • MS • WB | • Clinical stages: discovery cohort→33.3% I (early GC), 33.3% III 33.3% IV (late GC); validation cohort→51% early GC, 49% advanced GC | Discovery cohort: GC (9) and gastritis (3); validation cohort: GC (43) and gastritis (17) | Out of the 15 differential proteins identified, levels of S100A9, GIF, and AAT correlated with GC status. S100A9 and AAT (AUC = 0.81; p = 0.0013) were promising biomarker pairs for early GC diagnosis. | [90] |
| Saliva | |||||
| CSTB, TPI1, and DMBT1 | • 1DE • 2D-DIGE • TMT • LC-MS/MS • ELISA | • Clinical stages: 75% I–II, 25% III–IV | Discovery cohort: GC (20) and C (20); validation cohort: GC (20) and C (20) | A total of 48 differential proteins were found (p < 0.05) between GC and C, including 7 up- and 41 downregulated proteins. Three proteins were successfully validated (CSTB, TPI1, and DMBT1). These proteins differentiated GC (p < 0.05) and, combined, showed a sensitivity of 85% and a specificity of 80% in GC detection with an accuracy of 0.93. | [91] |
| Urine | |||||
| SORT1 and VTN | • TMT • LC-MS/MS | • Grade 2 or grade 3 adenocarcinoma | Discovery cohort: GC (5) and C (5); validation cohort: GC (19) and C (12) | A total of 246 proteins were differentially expressed in GC cases. Some proteins more abundant in GC vs. C are already known to play crucial roles in GC progression (ephrin A1, pepsinogen A3, sortilin 1, SORT1, and VTN). Others had not previously been linked to GC (shisa family member 5, mucin-like 1, and leukocyte cell-derived chemotaxin 2). The overexpression of SORT1 and VTN in GC urines was confirmed in an independent set of urine samples. | [92] |
| ANXA11, CDC42, NAPA, and SLC25A4 | • LC-MS/MS | Not detailed | Discovery cohort: GC (14) and patients with gastric lesions (109; SG, CAG, IM, or LGIN); validation cohort: GC (18) and patients with gastric lesions (114; SG, CAG, IM, or LGIN) | Urinary levels of ANXA11, CDC42, NAPA, and SLC25A4 were positively associated with gastric lesion progression; they may potentially predict the progression of gastric lesions and risk of GC occurrence. | [93] |
| ADAM12 and TFF1 | • LC-MS/MS | Not detailed | discovery cohort: 18 patients; training cohort: 176 patients; validation cohort: 88 patients. | A urinary biomarker panel combining TFF1, ADAM12, and H. pylori significantly discriminated early GC vs. C in both training and validation cohorts. | [94] |
| ADAM12 and MMP-9/NGAL complex | • Protein array analysis • substrate gel electrophoresis • ELISA | • Histology: 23% diffuse, 77% intestinal • clinical stages: 75% I–II, 25% III–IV • invasion depth: 69% T1–T2, 31% T3–T4 | GC (35) and C (35) | Urinary levels of the MMP-9/NGAL complex and ADAM12 were higher in GC vs. C (p < 0.001). Both the MMP-9/NGAL complex and ADAM12 were significant, independent diagnostic biomarkers for GC by multivariate analysis and distinguished between GC and C samples (AUC = 0.825, p < 0.001) in an ROC analysis. | [95] |
| EL | • WB | • Histology: 43% intestinal, 57% diffuse • clinical stages: 33% I–II, 67% III–IV • invasion depth: 33% T1–T2, 67% T3–T4 • degree of differentiation: 48% high or moderate, 52% poor or undifferentiated | GC (90) and C (57) | The EL content decreased by a ~9.9-fold average in GC vs. C (p < 0.0001), achieving a 0.967 AUC value for the ROC curve, demonstrating its high accuracy as a promising diagnostic marker for GC. | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Repetto, O.; Vettori, R.; Steffan, A.; Cannizzaro, R.; De Re, V. Circulating Proteins as Diagnostic Markers in Gastric Cancer. Int. J. Mol. Sci. 2023, 24, 16931. https://doi.org/10.3390/ijms242316931
Repetto O, Vettori R, Steffan A, Cannizzaro R, De Re V. Circulating Proteins as Diagnostic Markers in Gastric Cancer. International Journal of Molecular Sciences. 2023; 24(23):16931. https://doi.org/10.3390/ijms242316931
Chicago/Turabian StyleRepetto, Ombretta, Roberto Vettori, Agostino Steffan, Renato Cannizzaro, and Valli De Re. 2023. "Circulating Proteins as Diagnostic Markers in Gastric Cancer" International Journal of Molecular Sciences 24, no. 23: 16931. https://doi.org/10.3390/ijms242316931
APA StyleRepetto, O., Vettori, R., Steffan, A., Cannizzaro, R., & De Re, V. (2023). Circulating Proteins as Diagnostic Markers in Gastric Cancer. International Journal of Molecular Sciences, 24(23), 16931. https://doi.org/10.3390/ijms242316931







