Abstract
The process of flowering in plants is a pivotal stage in their life cycle, and the CONSTANS-like (COL) protein family, known for its photoperiod sensing ability, plays a crucial role in regulating plant flowering. Over the past two decades, homologous genes of COL have been identified in various plant species, leading to significant advancements in comprehending their involvement in the flowering pathway and response to abiotic stress. This article presents novel research progress on the structural aspects of COL proteins and their regulatory patterns within transcription complexes. Additionally, we reviewed recent information about their participation in flowering and abiotic stress response, aiming to provide a more comprehensive understanding of the functions of COL proteins.
1. Introduction
The precise coordination of specific developmental processes and appropriate times is crucial for plant survival and reproduction. The timing of flowering, for instance, enables plants to allocate sufficient time for optimal seed development and maturation prior to the onset of winter. In addition, the plant’s ability to respond to photoperiod also enables it to anticipate annual environmental changes and proactively adapt accordingly. This requires plants to possess special mechanisms that enable them to sense seasonal differences by detecting and responding to changes in photoperiod [1]. There are three types of photoperiodic response plants: long-day plants (LDPs), the response of which is induced when the photoperiod surpasses the critical day length (CDL); short-day plants (SDPs), the response of which is induced when the photoperiod is shorter than the CDL; and day-neutral plants (DNPs) that do not respond to photoperiod [2]. The time measurement in plants is achieved through an endogenous time-keeping mechanism known as the circadian rhythm, wherein plants perceive light signals via photoreceptors and regulate the rhythmic expression of CO through circadian components [1,3,4,5]. As a representative member of the COL protein family, CO plays a key role in the regulation of photoperiodic flowering, and is specifically regulated by time and space [6]. Research on the CO domain reveals functional support for the regulatory mechanism of CO transcription factors, and the identification of CO regulatory factors at the transcriptional and post-transcriptional levels enriches our knowledge of the CO-FT pathway [3,7,8].
The first COL protein found in plants was AtCO of Arabidopsis, which is able to promote plant flowering under long-day conditions [9]. Later, researchers located and cloned HEADING DATE 1 (HD1) in rice, which showed significant amino acid sequence similarity to AtCO [10]. Further research revealed that HD1 plays a similar role in the flowering pathway to that of CO in Arabidopsis and has a similar function, confirming that HD1 is a homologous gene of AtCO in rice (Oryza sativa) [11,12]. Referring to the sequences of CO and HD1, CO/COL family genes have been identified sequentially in various plants. Among dicotyledonous plants, 17 members were identified in Arabidopsis (Arabidopsis thaliana) [13], 13 members in tomato (Solanum lycopersicum) [14], 20 members in radish (Raphanus sativus) [15], 10 members in sugar beet (Beta vulgaris ssp. Vulgaris) [16], 11 members each in alfalfa (Medicago truncatula) [17] and soybean (Glycine max [L.] Merr.) [18], and 25 members each in rape (Brassica rapa) [19] and banana (Musa paradisiaca) [20]; meanwhile, among monocotyledonous plants, 16 members have been identified in rice (Oryza sativa) [21], 19 members in maize (Zea mays) [22], and 9 members in barley (Hordeum vulgare L.) [21].
The discovery of these COL members has enhanced our understanding of how they participate in the regulation of flowering based on photoperiods. The research has shown that some COL members may function through interfering with the CO complex [23,24]. Moreover, many COL members show significant changes in expression abundance in response to external stressors [25,26]. Exploring the mechanisms of COL genes responding to stress is important in order to understand how plants coordinate growth and development, especially in terms of flowering under stress conditions [27,28]. This review aims to provide an overview of the functional role of COL members in photoperiod regulation and in abiotic stress response as reported in recent years.
2. Function of COL Protein Domains
The COL proteins typically comprise an N-terminal B-box domain and a C-terminal CCT domain, along with a central region containing glutamine-rich sequences [9]. Based on their conserved B-box and CCT domains, they can be categorized into three subfamilies [13]: group I proteins includes two B-box domains, one CCT domain, and one VP motif; group II proteins has one B-box domain and one CCT domain; and group III proteins has one B-box domain, a divergent zinc finger structure, and a CCT motif.
The B-box domain in COL members is highly conserved, characterized by a single motif consisting of 30–40 amino acid residues that bind zinc ions through Cys, Asp, and His residues to form a zinc finger tertiary structure [29]. In vitro experiments, such as GST pull-down assays, have demonstrated that the B-box domains can interact with each other, forming diverse oligomeric states, predominantly tetramers [24,30]. It has been reported that BBX proteins like AtBBX19, 28, 30, and 31 can physically associate with AtCO via their B-box domains, indicating that COL proteins form oligomeric complexes that function not only through self-interaction but also heterologous interaction of B-box domains [31,32,33].
Proteins are targeted to the nucleus via nuclear localization signal (NLS) sequences [34]. A conserved bipartite NLS (consisting of RK-X11-R sequence) is present in the CCT domain at the C terminus of COL proteins, facilitating their nuclear import [35,36]. Studies on AtCO suggest that the CCT domain plays an essential role in binding downstream motifs [37]. CCT domain can also participate in protein–protein interactions [38].
The central region between the B-box and CCT domains in most COL proteins contains uninterrupted glutamine-rich sequences [39]. Following truncation of the CO gene, it was revealed that the intermediate fragments exhibited transcription activation capability, while the intact protein containing the middle region exhibited a higher transcriptional activation capacity than the truncated protein [37]. Consequently, it is widely postulated that the transcriptional activation capacity of COL protein predominantly originates from this specific region.
3. Assembly of COL Transcription Complex
The CCT domain of CO protein exhibits structural similarity to the NF-YA subunit of NUCLEAR FACTOR-Y A (NF-YA) [38]. Within the trimeric complex formed by NF-YA/YB/YC, NF-YA interacts with the NF-YB/YC dimer through an α-helix [24,40]. This trimeric complex demonstrates specificity in binding to CCAAT sequences [41]. Previous studies postulated that CO protein competes with NF-YA for occupancy of the NF-YB/NF-YC dimer, thereby exerting its transcriptional function through recognizing CCAAT motifs in promoter sequences of downstream target genes [41,42,43].
Currently, research has demonstrated that the formation of NF-YB/NF-YC dimer depends on their HFD (histone fold domain), which facilitates a responsive electrostatic-interaction network, thereby enabling the dimer to adaptively trimerize with various interaction partners [44]. The NF-YB/NF-YC dimer itself does not have DNA binding specificity. The resemblance between CCT and NF-YA enables NF-YB/YC to acts as a scaffold for CCT to bind to DNA [45,46,47]. This interaction is facilitated by the first α-helix of CCT, while sequence-specific binding relies on the second α-helix [30]. As a downstream target gene of CO and several COL members, FLOWERING LOCUS T (FT) has been experimentally shown to harbor CORE1 (TGTGA, −220 bp), CORE2 (TGTGG, −161 bp), and P1/P2 (CCACA/TGTGG, −267 bp/−285 bp) motifs in its promoter, which serve as direct binding sites for CO-CCT complex and, collectively, constitute the CO response region (CORR) of FT promoter [37,48]. The spatial interference (18 bp) hinders the simultaneous binding of P1 and P2.
In order to define the scope of influence of CO on the promoter of FT (pFT), β-glucuronidase (GUS) reporter gene analysis was performed and the −5.7 kb upstream sequence of FT was identified as the minimal functional length, which contains three possible CO regulatory regions [48]. Although CCAAT does not appear to serve as a binding site for CO itself, the CCAAT motif at −5.3 kb of the FT promoter in cruciferous plants is relatively conserved [48,49]. Moreover, chromatin conformation capture (3C) has revealed a dynamic interaction between CCAAT (−5.3 kb) and CORE regions mediated by NF-Y [50]. This finding demonstrates that CCAAT likely acts as a crucial cis-regulatory element facilitating sequential interaction between CO and pFT.
Based on various studies, researchers have proposed a “recruitment” model wherein the NFY complex modulates the DNA structure by binding to conserved regulatory regions within pFT, thereby establishing time-specific chromatin loop structures that facilitate recruitment of CO through interactions between NF-Y and CO-CCT at the proximal pFT region (Figure 1) [48,50]. Additionally, a “silencing relief” model has been proposed, wherein the binding of POLYCOMB GROUP (PcG) proteins to pFT effectively suppresses FT expression. The interaction between the NF-CO and NF-Y complexes synergistically relieves PcG repression and initiates transcription of FT [51]. However, research has revealed that NF-YA and CO compete for occupancy at the same NF-YB/YC HFD dimer binding site, suggesting the potential involvement of unidentified proteins in mediating communication between NF-YA and CO during this process [30].
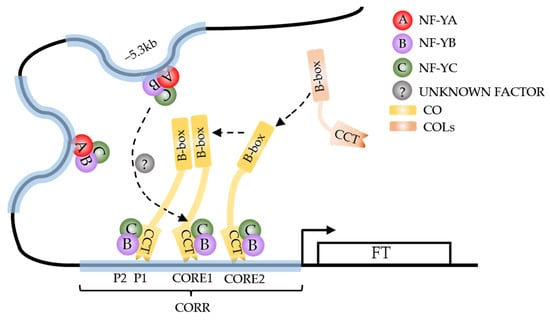
Figure 1.
CONSTANS (CO) transcription complex “recruitment” model. NY-FB and NF-YC physically bind to CO through the CCT domain and provide a scaffold to assist CO binding to the CORR region of pFT. NY-FA/YB/YC trimeric complexes can bind to multiple sites on pFT to promote the formation of chromatin loops at this specific sites, facilitating its proximity to the CORR motif, and ultimately promoting the binding of NF-YB/YC/CO complex to CORR elements through the function of unknown factor (long dashed arrow). Other COL members may participate in the assembly of CO complexes through interactions between B-box domains (short dashed arrow). The solid arrow represents the promotion of FT expression by the CO complex.
Considering the emergence of the CO transcription complex, it is conceivable that COL members sharing similar motifs might participate in this process. Previous investigations demonstrated that certain COL members can modulate FT transcription via physically interacting with CO and depleting its transcriptional activity [52,53]. The CO transcription complex appears to function as a signal center, accommodating the inclusion of COL members to incorporate additional information. However, numerous COL members, despite possessing CCT domains, do not directly target pFT or interfere with CO complexes [54,55,56]. The HD1 protein strictly recognizes the TGTGG motif, in contrast to AtCO, which conservatively recognizes only the four-base sequence TGTG [57]. The specificity of CO/COL in recognizing downstream targets may also determine the differences in their function across different species, providing some theoretical support for precise flowering regulation. Studies conducted using complex models, although not without flaws, have significantly enhanced our comprehension of CO regulatory mechanisms and provide indispensable theoretical support for the precise regulation of flowering processes.
4. COL Involved in Photoperiod Flowering
4.1. Regulation of CO in Photoperiod Flowering
CO responds to photoperiodic changes and modulates the expression of the flowering gene FT [9,58]. The CO-FT photoperiod pathway has been identified to be conserved across multiple species and has been studied carefully in the long-day plant Arabidopsis [59,60]. In this pathway, the core regulatory factor CO is regulated at transcriptional and post-transcriptional levels. In transcriptional regulation, GIGANTEA (GI), FLAVIN-BINDING-KELCH REPEAT-F-BOX 1 (FKF1), and CYCLING DOF FACTOR 1 (CDF1) are key regulators of CO transcription [61,62]. In post-transcriptional regulation, CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1), HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENE 1 (HOS1), FKBP12 (FK506 BINDING PROTEIN 12 kDa), and PHYTOCHROMES A/B (PHYA/B), CRYPTOCHROME 2 (CRY2) are directly or indirectly involved in the regulation of CO [63,64,65,66,67]. During the whole process, the input of external light information comes from the red-light receptor phytochrome PHYA/B and blue-light receptor FKF1/CRY. The CO photoperiodic flowering pathway in Arabidopsis is shown in Figure 2.
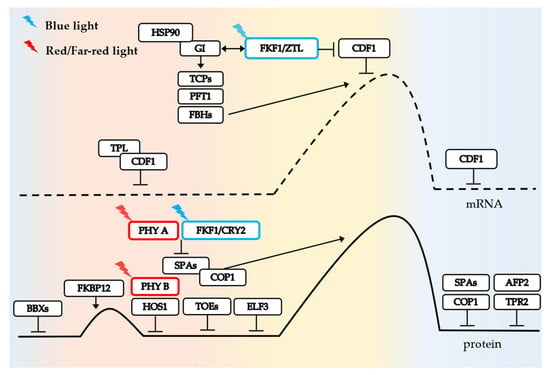
Figure 2.
CONSTANS (CO) photoperiodic flowering pathway. Background color represents change from morning to evening during the day (left to right). In the morning, CO transcription levels are inhibited by the CDF1-TPL complex. In the afternoon, blue light can stabilize FKF1/ZTL, thereby releasing inhibition of CDF1. Positive transcriptional regulators such as TCP also facilitate transcription. CO mRNA levels accumulate to a peak. Similarly, at protein level, CO abundance gradually increases and reaches its highest level in the afternoon, with a small peak in the morning, possibly due to the action of FKBP12. After that, members such as PHYB and HOS1 quickly control CO abundance to avoid its premature accumulation. Until the afternoon, PHYA, FKF1, and CRY2 relieve CO degradation by COP1.
In the morning, the CDF1 transcription factor inhibits CO transcription by binding to DOF binding sites in conjunction with TOPLESS (TPL) co-repressor [62]. Later, FKF1/ZEITLUPE (ZTL) is stabilized by blue light and interacts with GI-HSP90 to alleviate CDF-mediated inhibition of CO [68]. The high expression of FKBP12 protein in the morning can mitigate COP1-SPA(SUPPRESSOR OF PHYA-105) degradation of CO through its interaction with the CO-CCT domain [65]. Additionally, BBXs (BBX19, 30, 31), TARGET OF EAT1/2 (TOE1/2), PHYB, HOS1, and DELLA negatively regulate CO protein activity or abundance in the morning to ensure that CO does not accumulate too early and initiate downstream FT expression to prevent premature flowering in Arabidopsis [31,33,69,70,71,72].
In the afternoon, CO transcription is upregulated by the positive regulatory factors FLOWERING BHLH (FBH) and TEOSINTE BRANCHED 1/CYCLOIDEA/PROLIFERATING CELL NUCLEAR ANTIGEN FACTOR (TCP). PHYTOCHROME AND FLOWERING TIME 1 (PFT1) acts as an intermediary between FBH and TCP proteins, while specific members of TCP, such as TCP4, are activated by GIGANTEA (GI) to stimulate CO transcription [73,74]. PHYA, FKF1, and CRY2 all positively stabilize CO protein through inhibiting COP1 function, resulting in gradual accumulation of CO protein and initiation of FT transcription [67,75,76]. The NF-Y complex enhances the binding affinity between CO protein and FT promoter [37]. Additionally, CO also induces the transcription of SODIUM POTASSIUM ROOT DEFECTIVE 1 (NaKR1) gene encoding FT transport protein [77]. Consequently, both CO and FT exhibit peak abundance later in the day.
Despite the reduction in CDF1 and CDF2 abundance by GI-FKF1 at nighttime, a sufficient amount of CDF protein remains to inhibit CO transcription [78]. The nocturnal peak expression of ABI5-BINDING PROTEIN2 (AFP2) gene facilitates its interaction with CO through its C-terminus, while also interacting with the N-terminus of TOPLESS-RELATED PROTEIN2 (TPR2) to form a CO-AFP2-TPR2 complex [79]. This intricate complex orchestrates CO degradation and concurrently inhibits FT transcription. Moreover, stable factors like FKBP12 are depleted at night, leading to COP1-SPA E3 ubiquitin ligase-mediated ubiquitination and subsequent degradation of CO protein [65].
4.2. Other COL Members Participate in Photoperiod Flowering
A substantial body of genetic and biochemical research has demonstrated that, apart from CO, the COL family encompasses multiple members that actively or negatively regulate flowering through distinct molecular mechanisms (Table 1). Overexpression of AtCOL1 in Arabidopsis can expedite the circadian clock and abbreviate both long-day and short-day rhythms [80]. Overexpression of AtCOL2 induces a delay in flowering, albeit with relatively minor and unstable effects [80]. Through interacting with BBX32 protein via its B-box domain, AtCOL3 facilitates binding to the promoter region of the downstream target gene FT, thereby repressing transcription of the FT gene and retarding flowering under long-day (LD) conditions [81]. As a suppressor of flowering, AtCOL4’s mode of action is influenced by day length; it acts upstream of FT and SUPPRESSOR OF OVEREXPRESSION OF CO 1 (SOC1) under LD conditions [82]. Overexpression of AtCOL5 can induce flowering in Arabidopsis under short-day (SD) conditions, while downregulation of its expression does not directly impact the flowering phenotype, suggesting a potential redundancy in COL5 function. It is noteworthy that evolutionarily, AtCOL5 may have preceded CO and served as its precursor [83]. Interactions between AtCOL6 and AtCOL16 (BBX15)/AtCOL7 (BBX16) protein members have been reported to delay flowering by inhibiting FT transcription mediated by CO [84]. The role of AtCOL7 in flower regulation remains undiscovered; however, research on this protein suggests a possible light signal transduction pathway, where it connects external light information with downstream SUPERROOT2 (SUR2) gene transcription activation through upstream PHYB to coordinate auxin levels [85]. AtCOL8 and AtCOL12 interact with CO to inhibit the transcriptional activation of FT expression, and their degradation is dependent on COP1 [52,53]. AtCOL9 directly suppresses the transcriptional expression of CO, and itself is regulated by the biological clock [86]. The potential impact of AtCOL10 on flowering regulation in Arabidopsis remains unexplored; however, its homologous protein CmBBX8 (CmCOL10) in chrysanthemum promotes flowering by accelerating the transcription of TERMINAL FLOWER 1 (CmFTL1) [87]. AtCOL11 can induce the production of JASMONATE (JA) and may be involved in JA-mediated flower organ development [88]. AtCOL13 plays an important role in plant photomorphogenesis [89,90,91].
Furthermore, COL members from exogenous genetic backgrounds can also influence flowering regulation in Arabidopsis. Transformation experiments with exogenous genes revealed that multiple COL members from mango (Mangifera indica L.; MiCOL1s, MiCOL2s, MiCOL9s, and MiCOL16s) suppress flowering by affecting the expression of AtFT, while PvCO1 in bamboo (Phyllostachys violascens) and VrCOL2 in mungbean (Vigna radiata) also reduce the expression of AtFT in Arabidopsis, leading to delayed flowering [92,93,94,95,96].

Table 1.
Role of Arabidopsis COL in plant flowering and other regulation (ND, not determined).
Table 1.
Role of Arabidopsis COL in plant flowering and other regulation (ND, not determined).
| Gene Name | BBX Name | Gene Locus ID | Biological Process | Function | Target Genes | Interacting Proteins | References |
|---|---|---|---|---|---|---|---|
| CO | BBX1 | AT5G15840 | Flowering | Positive | FT | COP1, HOS1 | [64,97] |
| TOE, DELLA | [69,72] | ||||||
| AFP2, FKBP12 | [65,79] | ||||||
| BBX19, BBX30 | [31,33] | ||||||
| BBX31 | [5] | ||||||
| COL1 | BBX2 | AT5G15850 | Flowering | Positive | FT | ND | [80] |
| COL2 | BBX3 | AT3G02380 | Flowering | Negative | ND | ND | [80] |
| COL3 | BBX4 | AT2G24790 | Flowering | Negative | FT | BBX32, COP1 | [81,98] |
| PIF3, PHYB | [99] | ||||||
| COL4 | BBX5 | AT5G24930 | Flowering | Negative | FT | CO | [82] |
| COL5 | BBX6 | AT5G57660 | Flowering | Negative | FT | ND | [83] |
| COL6 | BBX14 | AT1G68520 | Flowering | Negative | FT | CO | [84] |
| COL7 | BBX16 | AT1G73870 | Branching | Positive | SUR2 | [85] | |
| Flowering | Negative | FT | CO | [84] | |||
| COL8 | BBX17 | AT1G49130 | Flowering | Negative | FT | CO | [52] |
| COL9 | BBX7 | AT3G07650 | Flowering | Negative | CO, FT | ND | [86] |
| COL10 | BBX8 | AT5G48250 | ND | ND | ND | ND | |
| COL11 | BBX9 | AT4G15250 | Flowering | Positive | ND | ND | [88] |
| COL12 | BBX10 | AT3G21880 | Flowering | Positive | FT | CO, COP1, SPA1 | [53] |
| COL13 | BBX11 | AT2G47890 | Photomorphogenesis | Positive | CHLH, HEMA1 | PHYB, PIF4 | [89,91] |
| COL14 | BBX12 | AT2G33500 | ND | ND | ND | ND | |
| COL15 | BBX13 | AT1G28050 | ND | ND | ND | HAPs | [38] |
| COL16 | BBX15 | AT1G25440 | Flowering | Negative | FT | CO | [84] |
4.3. Functional Role of COL in LDPs, SDPs, and NDPs
The plant photoperiod, which was discovered in the last century, is defined as the ability of plants to recognize changes in day length and make adjustments to flower at the appropriate time [100]. Plans are divided into long-day plants (LDPs), short-day plants (SDPs), and day-neutral plants (NDPs) based on differences in their response to photoperiod [2]. The peak expression of CO in Arabidopsis was observed only in the afternoon under long-day conditions. Regulation at the transcriptional and post-transcriptional levels of CO requires sufficient sunlight time for its function. Otherwise, the abundance of CO is not enough to activate the expression of FT, that is, Arabidopsis flowering is delayed under short-day conditions [101].
As a short-day plant, rice (Oryza sativa) has a flowering pathway similar to that in Arabidopsis. OSGI (GI), Hd1 (CO), Hd3a (FT), and RFT1 (FT) in rice correspond to the homologous genes GI, CO, and FT, respectively, in Arabidopsis [10,102,103]. What is not identical is that there are two pathways regulating flowering in rice [104,105]. In the OSGI-Hd1-Hd3a pathway of rice, similar to Arabidopsis, Hd1 is transcriptionally regulated by upstream OSGI and reaches its peak at dusk (LD) or at night (SD) [106]. Notably, Hd1 (CO) has dual functions, and its function changes under the influence of phytochrome; it converts into an inhibitor of Hd3a during the day, so it only promotes the expression of Hd3a at night [107,108]. The properties of Hd1 prompt rice to recognize short-day photoperiods and initiate the flowering process. In addition, there is a unique long-day suppression pathway in rice, the GRAIN NUMBER, PLANT HEIGHT AND HEADING DATE 7 (Ghd7)-EARLY HEADING DATE 1 (Ehd1)-Hd3a/RFT1 pathway [109]. Ghd7 and Ehd1 are new photoperiod regulators that have emerged in rice evolution. Ehd1 promotes flowering by promoting downstream Hd3a and RFT1 expression. Ghd7 is expressed under long-day conditions and inhibits the transcription of Ehd1, inhibiting flowering under LD conditions [110,111,112].
There are many possible reasons for plant photoperiod insensitivity. In day-neutral plants, such as roses (R. chinensis), RcCO is highly expressed only under long-day conditions and promotes flowering by regulating the expression of downstream RcFT. However, under short days, rose flowering was not significantly affected. RcCOL4, one of the Rose COL members, can enhance the ability of RcCO to bind RcFT promoter in SD by physically interacting [113]. In tomato (Solanum lycopersicum L.), although COL members SlCOL, SlCOL4a, and SlCOL4b were identified as potential flowering promoters, it is unclear whether they determine photoperiod insensitivity [14]. Tomato flowering inhibitor SELF PRUNING 5G (SP5G) can inhibit the expression of tomato FT (SFT). In wild varieties, SP5G is highly expressed under long days, but due to cis-regulatory variation, expression is lower in cultivated tomatoes, which may explain the genetic changes in tomatoes from SDP to NDP [114]. In cucumber (Cucumis sativus), the upstream region of the FT (CsFT) gene differs among cultivars grown at different latitudes. The lack of photoperiod sensitivity may result from the loss of upstream regulatory elements of FT after long-term artificial selection, resulting in a lack of precise control of CsFT expression [115].
5. COL Is Involved in Abiotic Stress Response
5.1. CO-FT Pathways and Abiotic Stress
The response of flowering to environmental signals has long been a subject of significant interest. Under unfavorable circumstances, plants possess the ability to regulate their flowering time by either advancing or delaying it, thereby optimizing seed survival [116,117]. Although the mechanisms underlying the integration of flowering and stress responses may be highly intricate, numerous protein members within the flowering pathway have demonstrated associations with external stress [118]. The GI-CO-FT pathway in leaves can respond to diverse stress signals, while shoot apical meristem (SAM) differentiation is influenced by various environmental cues (Figure 3).
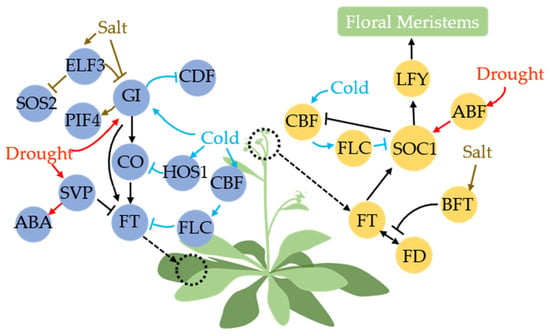
Figure 3.
Effects of abiotic stress (drought, cold, salt) on the photoperiodic pathway in Arabidopsis. Blue and yellow circles represent photoperiodic members in leaf and shoot apical meristem (SAM), respectively (these two positions are marked with dashed circles). Black arrows represent transmission of photoperiodic signals. Drought stress signals can directly affect GI, FT, and SOC1 in the photoperiodic pathway through SVP and ABF to promote plant flowering (red arrow). Salt stress signals can promote degradation of GI and interfere with FT function by inducing BFT/TFL1 to hinder plant flowering (brown arrow). Cold stress signals can indirectly block CO, FT, and SOC1 in the photoperiodic pathway to delay plant flowering. Cold can also directly induce GI and release CDF to improve plant cold tolerance (blue arrow).
Stress signals such as drought, salt, and cold can modulate the abundance of GI and thereby influence the initiation of the flowering pathway. This highlights the multifunctionality of GI as a central signaling hub [28,119,120]. Drought stress can upregulate GI transcription, and by promoting early flowering through the GI-CO-FT pathway but not solely relying on it, GI can also directly bind to the pFT without CO [121,122]. Previous studies demonstrated that cold stress induces independent expression of GI, unrelated to C-REPEAT/DREB BINDING FACTOR (CBF) [123]. Subsequent studies found that GI can participate in the regulation of various processes through CDF, and enhances cold tolerance by releasing CDF [124]. GI is also considered a pivotal component of the salt stress adaptation pathway. GI and SALT OVERLY SENSITIVE (SOS2) kinases form protein complexes, in which, under salt stress conditions, GI undergoes degradation by 26S proteasome, leading to the release of SOS2. Subsequently, SOS2 activates other Na+/H+ antiporters (SOS1, SOS3) to maintain cellular homeostasis [125,126,127,128].
EARLY FLOWERING3 (ELF3) acts as a constituent of the ELF3-ELF4-LUX ARRHYTHMO (LUX) complex, governing light input to the circadian clock in the flowering pathway [129]. ELF3 potentially participates in GI-SOS2 by modulating GI levels, likely accomplished through COP1-ELF3 complex-mediated degradation of GI protein [130]. Moreover, serving as an upstream regulator of PIF4, a pivotal regulatory factor for abiotic stress tolerance, ELF3 controls PIF4 transcription, thereby enhancing plant salt tolerance [131,132,133]. CO is regulated not only by GI, but also by the protein HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENES1 (HOS1), leading to degradation under cold stress conditions [134]. SHORT VEGETATIVE PHASE (SVP), a crucial regulatory factor in flower development, acts as a negative regulator of flowering upstream of FT and TWIN SISTER OF FT (TSF) [135]. Drought stress induces upregulation of SVP, which subsequently triggers downstream AtBG1 protein expression to facilitate ABA accumulation and enhance plant drought tolerance [136]. Cold stress can enhance the expression of CBF members, which in turn activate the transcription of FLOWERING LOCUS C (FLC) [137]. Under cold stress conditions, FLC acts as a repressor of two key flowering pathway integrators, FT and SUPPRESSOR OF OVEREXPRESSION OF CO1 (SOC1), thereby causing a delay in flowering time [138]. Upon relief from cold signaling, SOC1, GI, and other factors reciprocally suppress the CBF-mediated cold response pathway [139].
The function of the CO-activated FT protein is mediated through its transport via the phloem to the shoot apical meristem (SAM) [140]. Subsequently, FT requires assistance from a bZIP protein called FD for its flowering-promoting activity [141,142]. Upon FD induction, SOC1, the downstream protein of FT, can activate LEAFY (LFY), a floral meristem identity gene [143]. LFY and other floral formation factors initiate flower development at the primordium in the SAM [144,145]. The Arabidopsis flower inhibitory factor BROTHER OF FT AND TFL1 (BFT) is induced by high salinity and delays flowering by competitively binding with FD-FT to disrupt FT’s function [146]. ABA-RESPONSIVE ELEMENT-BINDING FACTOR (ABF), a bZIP transcription factor, plays a crucial role in ABA signal transduction during drought and osmotic stress [147]. Drought induces an increase in ABA content, and ABA-dependent ABF members (ABF3 and ABF4) directly promote early flowering and a drought-escape (DE) response via transcriptional regulation of SOC1 [148].
5.2. COL Participates in Abiotic Stress Response
The COL family governs the photoperiodic flowering pathway, and numerous studies have reported the responses of its members to abiotic stress (Figure 4). A study on the significant induction of MaCOL1 expression in response to cold and pathogen infection stress in banana (Musa acuminate) may be the first report of COL members involved in abiotic stress (2012) [26].
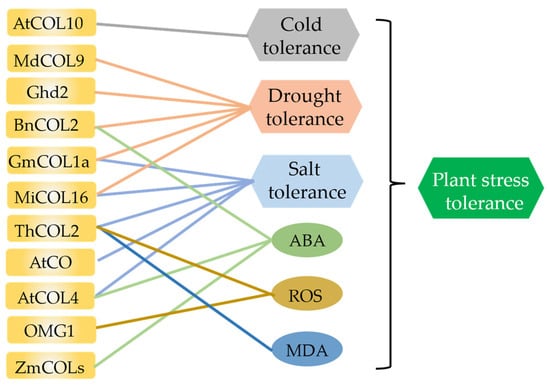
Figure 4.
Involvement of COLs from different species in stress responses. Among them, AtCOL10 is involved in cold response; MdCOL9, Ghd2, BnCOL2, GmCOL1a, and MiCOL16 are involved in drought response; GmCOL1a, MiCOL16, ThCOL2, AtCO, and AtCOL4 are involved in salt response; BnCOL2, AtCOL4, ZmCOL, and HaCOL are involved in ABA regulation; and ThCOL2 and OMG1 are involved in ROS regulation. ThCOL2 is also involved in MDA regulation, and indirectly involved in plant stress response.
In model plant Arabidopsis, AtCOL4 participates in ABA pathway and salt-stress responses through an ABA-dependent signaling pathway to positively regulate non-biological stress tolerance [149]. Conversely, AtCO negatively mediates salt tolerance in Arabidopsis by interacting with four ABSCISIC ACID-RESPONSIVE ELEMENT BINDING FACTORS (ABF1, ABF2, ABF3, and ABF4) [150]. Additionally, CO is also a JAZ-binding factor that participates in jasmonic acid signal transduction through protein interactions [151]. OMG1, an uncharacterized Arabidopsis III class COL protein, functions as a regulator of the ROS pathway by modulating the expression of ROS pathway-related genes MYB77 and GRX480 [152]. The blue light receptor CRYPTOCHROME 2 (CRY2) stabilizes at low temperatures and inhibits COP1-mediated degradation of LONG HYPOCOTYL 5 (HY5) through its interaction with COP1. As a downstream target gene of HY5, AtCOL10 positively regulates cold tolerance by modulating the expression of cold-responsive COR (cold-regulated) genes [153].
In addition, overexpression of Ghd2 in rice (Oryza sativa) significantly impairs drought resistance and plays a crucial role in drought-induced leaf senescence [154]. In the halophyte Tamarix hispida, ThCOL2 protein enhances the activity of protective enzymes, reduces ROS and MDA accumulation in plants, and mitigates cell damage to enhance salt tolerance in transgenic plants [155]. In maize (Zea mays), eight members of group I ZmCOL possess ABA-responsive cis-elements, and their expression levels are modulated by ABA, indicating their widespread involvement in ABA response [25]. BnCOL2 in rapeseed (Brassica napus) regulates plant tolerance to drought stress by modulating ABA response and regulating the expression of drought-related genes. Heterologous overexpression of BnCOL2 significantly compromises drought tolerance in Arabidopsis under drought stress conditions [156]. Overexpression of mango (Mangifera indica L.) COL members MiCOL16A and MiCOl16B in Arabidopsis enhances the salt tolerance and drought resistance [94]. In soybean (Glycine max [L.] Merr.), GmCOL1a has been found to enhance salt tolerance through promoting the expression of salt transport-related proteins, thereby effectively reducing the Na+/K+ ratio in plants. Moreover, GmCOL1a positively regulates the transcription of GmLEA and GmP5CS to improve drought resistance in soybeans [157]. In apple (Malus × domestica), MdCOL9 is regulated by ubiquitination through its interaction with the drought-responsive protein MdMIEL1. This interaction activates downstream positive regulatory factors such as MdERF1, MdGLK1, and MdERD15 to enhance drought resistance [158].
Furthermore, several studies have reported dynamic transcriptional changes in COL genes under diverse abiotic stress conditions. For instance, under drought stress, GhCOL3, 4, 14, 16, 17, and 20 in cotton (Gossypium hirsutum) were upregulated and under salt stress GhCOL6, 8, 9, 10, 11, 13, 18, 19, 21 were downregulated [25]. In response to high-temperature stress, CaCOL1, 6, 7, 8, and 9 in pepper (Capsicum annuum) were upregulated while CaCOL2, 3, and 5 were downregulated. Similarly, under osmotic stress conditions, the expression levels of CaCOL2, 3, 7, and 8 were observed to be upregulated following 2 h treatment [159]. In sunflower (Helianthus annuus L.), under drought stress, the expression of HaCOL3 was significantly downregulated, while the HaCOL19 and HaCOL22 were significantly upregulated [160]. Although the functions and molecular mechanisms of these COLs have not been investigated, promoter element analysis or similar methods can be used to quickly explore the possible upstream members. For example, through yeast one-hybrid library screening, it was found that ABA-induced BrABF3 can directly activate BrCO transcription [161]. These investigations will serve as valuable references for further elucidating the mechanisms of COL proteins responding to non-biological stress and exploring the intricate relationship between flowering regulation and stress response.
6. Future Perspectives
The transition from vegetative growth to reproductive growth is a pivotal event in plant development, where flowering serves to initiate reproductive growth. Plants possess the ability to perceive light and regulate the onset of flowering. This study provides a comprehensive understanding about the role and molecular mechanisms underlying COL proteins in governing flowering regulation, although many intricacies remain undisclosed. The assembly and regulatory mechanism of the COL transcriptional complex binding to the FT promoter needs to be further elucidated. Currently, the conjunction of NY-F complex with CO requires the participation of some unknown members. Uncovering the intrinsic characteristics of COLs and identifying these key factors remain subjects of research. COL actively participates throughout the entire flowering process beyond merely activating/inhibiting FT transcription regulatory functions. The fact that multiple COL members can participate in the regulation of plant photoperiodic flowering at multiple pathways highlights the intricate complexity of COL regulation mechanisms.
Investigating the COL family is important, not only to elucidate the mechanisms underlying plant growth and development, but also to enhance crop yields. As a pivotal gene family governing flowering through photoperiodic regulation, the COL family can be regarded as having a central role in integrating signals during light-mediated flowering in plants. In agricultural production, the timing of flowering directly affects crop yields. The COL genes have significant potential in enhancing crop productivity. In crops, various COL genes exhibit pleiotropic effects on traits associated with yield, plant height, and resistance against environmental stresses. For instance, OsCOL9, OsCOL10, and OsCOL16 have the capacity to positively modulate characteristics including grain count per panicle, length of inflorescence, and overall plant stature, resulting in improved rice grain output irrespective of photoperiodic constraints [162,163,164]. In wheat, TaCOL-B5 overexpression was reported to induce greater tillering and spike formation, resulting in an approximately 12% increase in productivity [165]. Moreover, it has been reported that all eight ThCOL genes in Tamarix hispida exhibit responsiveness to stress [155]. Even though numerous COL genes across various species have been identified and characterized, several members of the COL family have not yet been extensively investigated, and their biological functions and molecular mechanisms remain poorly elucidated. Currently, the COL protein shows potential as an integrated regulator of flowering, stress response, and yield formation. But how COL proteins integrate various environmental and developmental signals to coordinate the appropriate plant response will be a fascinating topic.
Author Contributions
B.Z. outlined and wrote the manuscript and Z.S. acquired funding. All authors reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Shandong Provincial Natural Science Fund Project (ZR2020MC108), Shandong Provincial Natural Science Fund Project (ZR2021MC184), and Agricultural Scientific and Technological Innovation Project of Shandong Academy of Agricultural Sciences (CXGC2023F06).
Acknowledgments
A special thanks to Furong Wang (wangfurong@shandong.cn) for the article modification comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jackson, S.D. Plant responses to photoperiod. New Phytol. 2009, 181, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Zeevaart, J.A. Florigen coming of age after 70 years. Plant Cell 2006, 18, 1783–1789. [Google Scholar] [CrossRef]
- Imaizumi, T. Arabidopsis circadian clock and photoperiodism: Time to think about location. Curr. Opin. Plant Biol. 2010, 13, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Zeevaart, J.A. Leaf-produced floral signals. Curr. Opin. Plant Biol. 2008, 11, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Suárez-López, P.; Wheatley, K.; Robson, F.; Onouchi, H.; Valverde, F.; Coupland, G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 2001, 410, 1116–1120. [Google Scholar] [CrossRef]
- Shim, J.S.; Imaizumi, T. Circadian clock and photoperiodic response in Arabidopsis: From seasonal flowering to redox homeostasis. Biochemistry 2015, 54, 157–170. [Google Scholar] [CrossRef]
- Shim, J.S.; Kubota, A.; Imaizumi, T. Circadian clock and photoperiodic flowering in Arabidopsis: CONSTANS is a hub for signal integration. Plant Physiol. 2017, 173, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Putterill, J.; Laurie, R.; Macknight, R. It’s time to flower: The genetic control of flowering time. Bioessays 2004, 26, 363–373. [Google Scholar] [CrossRef]
- Putterill, J.; Robson, F.; Lee, K.; Simon, R.; Coupland, G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 1995, 80, 847–857. [Google Scholar] [CrossRef]
- Yano, M.; Katayose, Y.; Ashikari, M.; Yamanouchi, U.; Monna, L.; Fuse, T.; Baba, T.; Yamamoto, K.; Umehara, Y.; Nagamura, Y. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 2000, 12, 2473–2483. [Google Scholar] [CrossRef]
- Nemoto, Y.; Nonoue, Y.; Yano, M.; Izawa, T. Hd1, a CONSTANS ortholog in rice, functions as an Ehd1 repressor through interaction with monocot-specific CCT-domain protein Ghd7. Plant J. 2016, 86, 221–233. [Google Scholar] [CrossRef]
- Tamaki, S.; Matsuo, S.; Wong, H.L.; Yokoi, S.; Shimamoto, K. Hd3a protein is a mobile flowering signal in rice. Science 2007, 316, 1033–1036. [Google Scholar] [CrossRef]
- Robson, F.; Costa, M.M.R.; Hepworth, S.R.; Vizir, I.; Piñeiro, M.; Reeves, P.H.; Putterill, J.; Coupland, G. Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J. 2001, 28, 619–631. [Google Scholar] [CrossRef]
- Yang, T.; He, Y.; Niu, S.; Yan, S.; Zhang, Y. Identification and characterization of the CONSTANS (CO)/CONSTANS-like (COL) genes related to photoperiodic signaling and flowering in tomato. Plant Sci. 2020, 301, 110653. [Google Scholar] [CrossRef]
- Hu, T.; Wei, Q.; Wang, W.; Hu, H.; Mao, W.; Zhu, Q.; Bao, C. Genome-wide identification and characterization of CONSTANS-like gene family in radish (Raphanus sativus). PLoS ONE 2018, 13, e0204137. [Google Scholar] [CrossRef] [PubMed]
- Chia, T.; Müller, A.; Jung, C.; Mutasa-Göttgens, E. Sugar beet contains a large CONSTANS-LIKE gene family including a CO homologue that is independent of the early-bolting (B) gene locus. J. Exp. Bot. 2008, 59, 2735–2748. [Google Scholar] [CrossRef]
- Herrmann, D.; Barre, P.; Santoni, S.; Julier, B. Association of a CONSTANS-LIKE gene to flowering and height in autotetraploid alfalfa. Theor. Appl. Genet. 2010, 121, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Price, B.W.; Haider, W.; Seufferheld, G.; Nelson, R.; Hanzawa, Y. Functional and evolutionary characterization of the CONSTANS gene family in short-day photoperiodic flowering in soybean. PLoS ONE 2014, 9, e85754. [Google Scholar] [CrossRef] [PubMed]
- Robert, L.S.; Robson, F.; Sharpe, A.; Lydiate, D.; Coupland, G. Conserved structure and function of the Arabidopsis flowering time gene CONSTANS in Brassica napus. Plant Mol. Biol. 1998, 37, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, A.K.; Patil, H.B.; Azeez, A.; Subramaniam, V.R.; Krishna, B.; Sane, A.P.; Sane, P.V. Molecular characterization of CONSTANS-Like (COL) genes in banana (Musa acuminata L. AAA Group, cv. Grand Nain). Physiol. Mol. Biol. Plants 2016, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, S.; Dunford, R.P.; Coupland, G.; Laurie, D.A. The evolution of CONSTANS-like gene families in barley, rice, and Arabidopsis. Plant Physiol. 2003, 131, 1855–1867. [Google Scholar] [CrossRef]
- Miller, T.A.; Muslin, E.H.; Dorweiler, J.E. A maize CONSTANS-like gene, conz1, exhibits distinct diurnal expression patterns in varied photoperiods. Planta 2008, 227, 1377–1388. [Google Scholar] [CrossRef]
- Song, Z.; Bian, Y.; Liu, J.; Sun, Y.; Xu, D. B-box proteins: Pivotal players in light-mediated development in plants. J. Integr. Plant Biol. 2020, 62, 1293–1309. [Google Scholar] [CrossRef]
- Zeng, X.; Lv, X.; Liu, R.; He, H.; Liang, S.; Chen, L.; Zhang, F.; Chen, L.; He, Y.; Du, J. Molecular basis of CONSTANS oligomerization in FLOWERING LOCUS T activation. J. Integr. Plant Biol. 2022, 64, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Xu, Z.; Wang, J.; Qin, Q.; Jiang, H.; Si, W.; Li, X. Genome-wide analysis of maize CONSTANS-LIKE gene family and expression profiling under light/dark and abscisic acid treatment. Gene 2018, 673, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.-Y.; Wang, J.-N.; Kuang, J.-F.; Shan, W.; Lu, W.-J. Molecular characterization and expression profiles of MaCOL1, a CONSTANS-like gene in banana fruit. Gene 2012, 496, 110–117. [Google Scholar] [CrossRef]
- Takeno, K. Stress-induced flowering: The third category of flowering response. J. Exp. Bot. 2016, 67, 4925–4934. [Google Scholar] [CrossRef]
- Kazan, K.; Lyons, R. The link between flowering time and stress tolerance. J. Exp. Bot. 2016, 67, 47–60. [Google Scholar] [CrossRef]
- Khanna, R.; Kronmiller, B.; Maszle, D.R.; Coupland, G.; Holm, M.; Mizuno, T.; Wu, S.-H. The Arabidopsis B-Box Zinc Finger Family. Plant Cell 2009, 21, 3416–3420. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zeng, X.; Hu, H.; Chen, L.; Zhang, F.; Liu, R.; Liu, Y.; Zhou, X.; Wang, C.; Wu, Z. Structural insights into the multivalent binding of the Arabidopsis FLOWERING LOCUS T promoter by the CO–NF–Y master transcription factor complex. Plant Cell 2021, 33, 1182–1195. [Google Scholar] [CrossRef]
- Wang, C.-Q.; Guthrie, C.; Sarmast, M.K.; Dehesh, K. BBX19 interacts with CONSTANS to repress FLOWERING LOCUS T transcription, defining a flowering time checkpoint in Arabidopsis. Plant Cell 2014, 26, 3589–3602. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, G.; Yin, C.; Fang, Y. B-box transcription factor 28 regulates flowering by interacting with constans. Sci. Rep. 2020, 10, 17789. [Google Scholar] [CrossRef] [PubMed]
- Graeff, M.; Straub, D.; Eguen, T.; Dolde, U.; Rodrigues, V.; Brandt, R.; Wenkel, S. MicroProtein-mediated recruitment of CONSTANS into a TOPLESS trimeric complex represses flowering in Arabidopsis. PLoS Genet. 2016, 12, e1005959. [Google Scholar] [CrossRef]
- Lange, A.; Mills, R.E.; Lange, C.J.; Stewart, M.; Devine, S.E.; Corbett, A.H. Classical nuclear localization signals: Definition, function, and interaction with importin α. J. Biol. Chem. 2007, 282, 5101–5105. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.; McLane, L.M.; Mills, R.E.; Devine, S.E.; Corbett, A.H. Expanding the definition of the classical bipartite nuclear localization signal. Traffic 2010, 11, 311–323. [Google Scholar] [CrossRef]
- Crocco, C.D.; Botto, J.F. BBX proteins in green plants: Insights into their evolution, structure, feature and functional diversification. Gene 2013, 531, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.B.; Shen, Y.; Chang, H.C.; Hou, Y.; Harris, A.; Ma, S.F.; McPartland, M.; Hymus, G.J.; Adam, L.; Marion, C. The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element. New Phytol. 2010, 187, 57–66. [Google Scholar] [CrossRef]
- Wenkel, S.; Turck, F.; Singer, K.; Gissot, L.; Le Gourrierec, J.; Samach, A.; Coupland, G. CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell 2006, 18, 2971–2984. [Google Scholar] [CrossRef]
- Tanaka, M.; Clouston, W.M.; Herr, W. The Oct-2 glutamine-rich and proline-rich activation domains can synergize with each other or duplicates of themselves to activate transcription. Mol. Cell. Biol. 1994, 14, 6046–6055. [Google Scholar]
- Bi, W.; Wu, L.; Coustry, F.; De Crombrugghe, B.; Maity, S.N. DNA binding specificity of the CCAAT-binding factor CBF/NF-Y. J. Biol. Chem. 1997, 272, 26562–26572. [Google Scholar] [CrossRef]
- Ben-Naim, O.; Eshed, R.; Parnis, A.; Teper-Bamnolker, P.; Shalit, A.; Coupland, G.; Samach, A.; Lifschitz, E. The CCAAT binding factor can mediate interactions between CONSTANS-like proteins and DNA. Plant J. 2006, 46, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Kumimoto, R.W.; Adam, L.; Hymus, G.J.; Repetti, P.P.; Reuber, T.L.; Marion, C.M.; Hempel, F.D.; Ratcliffe, O.J. The Nuclear Factor Y subunits NF-YB2 and NF-YB3 play additive roles in the promotion of flowering by inductive long-day photoperiods in Arabidopsis. Planta 2008, 228, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Kumimoto, R.W.; Zhang, Y.; Siefers, N.; Holt III, B.F. NF–YC3, NF–YC4 and NF–YC9 are required for CONSTANS-mediated, photoperiod-dependent flowering in Arabidopsis thaliana. Plant J. 2010, 63, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Sanjuan, A.; Gnesutta, N.; Gobbini, A.; Martignago, D.; Bernardini, A.; Fornara, F.; Mantovani, R.; Nardini, M. Structural determinants for NF-Y subunit organization and NF-Y/DNA association in plants. Plant J. 2021, 105, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Gnesutta, N.; Donati, G.; Gatta, R.; Forni, C.; Fossati, A.; Vonrhein, C.; Moras, D.; Romier, C.; Bolognesi, M. Sequence-specific transcription factor NF-Y displays histone-like DNA binding and H2B-like ubiquitination. Cell 2013, 152, 132–143. [Google Scholar] [CrossRef]
- Gnesutta, N.; Saad, D.; Chaves-Sanjuan, A.; Mantovani, R.; Nardini, M. Crystal structure of the Arabidopsis thaliana L1L/NF-YC3 histone-fold dimer reveals specificities of the LEC1 family of NF-Y subunits in plants. Mol. Plant 2017, 10, 645–648. [Google Scholar] [CrossRef]
- Nardone, V.; Chaves-Sanjuan, A.; Nardini, M. Structural determinants for NF-Y/DNA interaction at the CCAAT box. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2017, 1860, 571–580. [Google Scholar] [CrossRef]
- Adrian, J.; Farrona, S.; Reimer, J.J.; Albani, M.C.; Coupland, G.; Turck, F. cis-Regulatory elements and chromatin state coordinately control temporal and spatial expression of FLOWERING LOCUS T in Arabidopsis. Plant Cell 2010, 22, 1425–1440. [Google Scholar] [CrossRef]
- Gnesutta, N.; Kumimoto, R.W.; Swain, S.; Chiara, M.; Siriwardana, C.; Horner, D.S.; Holt III, B.F.; Mantovani, R. CONSTANS imparts DNA sequence specificity to the histone fold NF-YB/NF-YC dimer. Plant Cell 2017, 29, 1516–1532. [Google Scholar] [CrossRef]
- Cao, S.; Kumimoto, R.W.; Gnesutta, N.; Calogero, A.M.; Mantovani, R.; Holt III, B.F. A distal CCAAT/NUCLEAR FACTOR Y complex promotes chromatin looping at the FLOWERING LOCUS T promoter and regulates the timing of flowering in Arabidopsis. Plant Cell 2014, 26, 1009–1017. [Google Scholar] [CrossRef]
- Luo, X.; Gao, Z.; Wang, Y.; Chen, Z.; Zhang, W.; Huang, J.; Yu, H.; He, Y. The NUCLEAR FACTOR-CONSTANS complex antagonizes Polycomb repression to de-repress FLOWERING LOCUS T expression in response to inductive long days in Arabidopsis. Plant J. 2018, 95, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, J.; Yuan, C.; Chen, Q.; Liu, Q.; Wang, X.; Qin, C. BBX17 interacts with CO and negatively regulates flowering time in Arabidopsis thaliana. Plant Cell Physiol. 2022, 63, 401–409. [Google Scholar] [CrossRef]
- Ordoñez-Herrera, N.; Trimborn, L.; Menje, M.; Henschel, M.; Robers, L.; Kaufholdt, D.; Hänsch, R.; Adrian, J.; Ponnu, J.; Hoecker, U. The transcription factor COL12 is a substrate of the COP1/SPA E3 ligase and regulates flowering time and plant architecture. Plant Physiol. 2018, 176, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, B.J.; Henry-Kirk, R.; Friend, A.; Diack, R.; Helbig, S.; Mouhu, K.; Tomes, S.; Dare, A.P.; Espley, R.V.; Putterill, J. Apple B-box factors regulate light-responsive anthocyanin biosynthesis genes. Sci. Rep. 2019, 9, 17762. [Google Scholar] [CrossRef]
- Sheng, P.; Wu, F.; Tan, J.; Zhang, H.; Ma, W.; Chen, L.; Wang, J.; Wang, J.; Zhu, S.; Guo, X. A CONSTANS-like transcriptional activator, OsCOL13, functions as a negative regulator of flowering downstream of OsphyB and upstream of Ehd1 in rice. Plant Mol. Biol. 2016, 92, 209–222. [Google Scholar] [CrossRef]
- Wong, A.C.; Hecht, V.F.; Picard, K.; Diwadkar, P.; Laurie, R.E.; Wen, J.; Mysore, K.; Macknight, R.C.; Weller, J.L. Isolation and functional analysis of CONSTANS-LIKE genes suggests that a central role for CONSTANS in flowering time control is not evolutionarily conserved in Medicago truncatula. Front. Plant Sci. 2014, 5, 486. [Google Scholar] [CrossRef]
- Shen, C.; Liu, H.; Guan, Z.; Yan, J.; Zheng, T.; Yan, W.; Wu, C.; Zhang, Q.; Yin, P.; Xing, Y. Structural insight into DNA recognition by CCT/NF-YB/YC complexes in plant photoperiodic flowering. Plant Cell 2020, 32, 3469–3484. [Google Scholar] [CrossRef]
- Yoo, S.K.; Chung, K.S.; Kim, J.; Lee, J.H.; Hong, S.M.; Yoo, S.J.; Yoo, S.Y.; Lee, J.S.; Ahn, J.H. Constans activates suppressor of overexpression of constans 1 through Flowering Locus T to promote flowering in Arabidopsis. Plant Physiol. 2005, 139, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Böhlenius, H.; Huang, T.; Charbonnel-Campaa, L.; Brunner, A.M.; Jansson, S.; Strauss, S.H.; Nilsson, O. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 2006, 312, 1040–1043. [Google Scholar] [CrossRef]
- Fan, C.; Hu, R.; Zhang, X.; Wang, X.; Zhang, W.; Zhang, Q.; Ma, J.; Fu, Y.-F. Conserved CO-FT regulons contribute to the photoperiod flowering control in soybean. BMC Plant Biol. 2014, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Sawa, M.; Nusinow, D.A.; Kay, S.A.; Imaizumi, T. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 2007, 318, 261–265. [Google Scholar] [CrossRef]
- Goralogia, G.S.; Liu, T.K.; Zhao, L.; Panipinto, P.M.; Groover, E.D.; Bains, Y.S.; Imaizumi, T. CYCLING DOF FACTOR 1 represses transcription through the TOPLESS co-repressor to control photoperiodic flowering in Arabidopsis. Plant J. 2017, 92, 244–262. [Google Scholar] [CrossRef]
- Liu, L.-J.; Zhang, Y.-C.; Li, Q.-H.; Sang, Y.; Mao, J.; Lian, H.-L.; Wang, L.; Yang, H.-Q. COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis. Plant Cell 2008, 20, 292–306. [Google Scholar] [CrossRef]
- Lazaro, A.; Valverde, F.; Piñeiro, M.; Jarillo, J.A. The Arabidopsis E3 Ubiquitin Ligase HOS1 Negatively Regulates CONSTANS Abundance in the Photoperiodic Control of Flowering. Plant Cell 2012, 24, 982–999. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Bueno, G.; Said, F.E.; de Los Reyes, P.; Lucas-Reina, E.I.; Ortiz-Marchena, M.I.; Romero, J.M.; Valverde, F. CONSTANS–FKBP12 interaction contributes to modulation of photoperiodic flowering in Arabidopsis. Plant J. 2020, 101, 1287–1302. [Google Scholar] [CrossRef] [PubMed]
- Valverde, F.; Mouradov, A.; Soppe, W.; Ravenscroft, D.; Samach, A.; Coupland, G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 2004, 303, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Ponnu, J.; Riedel, T.; Penner, E.; Schrader, A.; Hoecker, U. Cryptochrome 2 competes with COP1 substrates to repress COP1 ubiquitin ligase activity during Arabidopsis photomorphogenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 27133–27141. [Google Scholar] [CrossRef]
- Hwang, D.Y.; Park, S.; Lee, S.; Lee, S.S.; Imaizumi, T.; Song, Y.H. GIGANTEA regulates the timing stabilization of CONSTANS by altering the interaction between FKF1 and ZEITLUPE. Mol. Cells 2019, 42, 693. [Google Scholar]
- Zhang, B.; Wang, L.; Zeng, L.; Zhang, C.; Ma, H. Arabidopsis TOE proteins convey a photoperiodic signal to antagonize CONSTANS and regulate flowering time. Genes Dev. 2015, 29, 975–987. [Google Scholar] [CrossRef]
- Lazaro, A.; Mouriz, A.; Piñeiro, M.; Jarillo, J.A. Red light-mediated degradation of CONSTANS by the E3 ubiquitin ligase HOS1 regulates photoperiodic flowering in Arabidopsis. Plant Cell 2015, 27, 2437–2454. [Google Scholar] [CrossRef]
- Wang, H.; Pan, J.; Li, Y.; Lou, D.; Hu, Y.; Yu, D. The DELLA-CONSTANS transcription factor cascade integrates gibberellic acid and photoperiod signaling to regulate flowering. Plant Physiol. 2016, 172, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, T.; Xu, P.B.; Li, L.; Du, S.S.; Lian, H.L.; Yang, H.Q. DELLA proteins physically interact with CONSTANS to regulate flowering under long days in Arabidopsis. FEBS Lett. 2016, 590, 541–549. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, X.; Liu, P.; Li, D.; Chen, T.; Gu, X.; Sun, J. MicroRNA319-regulated TCPs interact with FBHs and PFT1 to activate CO transcription and control flowering time in Arabidopsis. PLoS Genet. 2017, 13, e1006833. [Google Scholar] [CrossRef] [PubMed]
- Kubota, A.; Ito, S.; Shim, J.S.; Johnson, R.S.; Song, Y.H.; Breton, G.; Goralogia, G.S.; Kwon, M.S.; Laboy Cintrón, D.; Koyama, T. TCP4-dependent induction of CONSTANS transcription requires GIGANTEA in photoperiodic flowering in Arabidopsis. PLoS Genet. 2017, 13, e1006856. [Google Scholar] [CrossRef]
- Lee, B.-D.; Kim, M.R.; Kang, M.-Y.; Cha, J.-Y.; Han, S.-H.; Nawkar, G.M.; Sakuraba, Y.; Lee, S.Y.; Imaizumi, T.; McClung, C.R. The F-box protein FKF1 inhibits dimerization of COP1 in the control of photoperiodic flowering. Nat. Commun. 2017, 8, 2259. [Google Scholar] [CrossRef]
- Ponnu, J. Molecular mechanisms suppressing COP1/SPA E3 ubiquitin ligase activity in blue light. Physiol. Plant. 2020, 169, 418–429. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, L.; Shen, L.; Yu, H. NaKR1 regulates long-distance movement of FLOWERING LOCUS T in Arabidopsis. Nat. Plants 2016, 2, 16075. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T.; Schultz, T.F.; Harmon, F.G.; Ho, L.A.; Kay, S.A. FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 2005, 309, 293–297. [Google Scholar] [CrossRef]
- Chang, G.; Yang, W.; Zhang, Q.; Huang, J.; Yang, Y.; Hu, X. ABI5-BINDING PROTEIN2 coordinates CONSTANS to delay flowering by recruiting the transcriptional corepressor TPR2. Plant Physiol. 2019, 179, 477–490. [Google Scholar] [CrossRef]
- Ledger, S.; Strayer, C.; Ashton, F.; Kay, S.A.; Putterill, J. Analysis of the function of two circadian-regulated CONSTANS-LIKE genes. Plant J. 2001, 26, 15–22. [Google Scholar] [CrossRef]
- Tripathi, P.; Carvallo, M.; Hamilton, E.E.; Preuss, S.; Kay, S.A. Arabidopsis B-BOX32 interacts with CONSTANS-LIKE3 to regulate flowering. Proc. Natl. Acad. Sci. USA 2017, 114, 172–177. [Google Scholar] [CrossRef]
- Steinbach, Y. The Arabidopsis thaliana CONSTANS-LIKE 4 (COL4)—A modulator of flowering time. Front. Plant Sci. 2019, 10, 651. [Google Scholar] [CrossRef]
- Hassidim, M.; Harir, Y.; Yakir, E.; Kron, I.; Green, R.M. Over-expression of CONSTANS-LIKE 5 can induce flowering in short-day grown Arabidopsis. Planta 2009, 230, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Susila, H.; Nasim, Z.; Gawarecka, K.; Jung, J.-Y.; Jin, S.; Youn, G.; Ahn, J.H. Chloroplasts prevent precocious flowering through a GOLDEN2-LIKE–B-BOX DOMAIN PROTEIN module. Plant Commun. 2023, 4, 100515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ji, R.; Li, H.; Zhao, T.; Liu, J.; Lin, C.; Liu, B. CONSTANS-LIKE 7 (COL7) is involved in phytochrome B (phyB)-mediated light-quality regulation of auxin homeostasis. Mol. Plant 2014, 7, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.F.; Wang, Z.Y. Overexpression of COL9, a CONSTANS-LIKE gene, delays flowering by reducing expression of CO and FT in Arabidopsis thaliana. Plant J. 2005, 43, 758–768. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Ren, L.; Zhou, M.; Han, X.; Ding, L.; Zhang, F.; Guan, Z.; Fang, W.; Chen, S. CmBBX8 accelerates flowering by targeting CmFTL1 directly in summer chrysanthemum. Plant Biotechnol. J. 2020, 18, 1562–1572. [Google Scholar] [CrossRef]
- Mandaokar, A.; Thines, B.; Shin, B.; Markus Lange, B.; Choi, G.; Koo, Y.J.; Yoo, Y.J.; Choi, Y.D.; Choi, G.; Browse, J. Transcriptional regulators of stamen development in Arabidopsis identified by transcriptional profiling. Plant J. 2006, 46, 984–1008. [Google Scholar] [CrossRef]
- Job, N.; Datta, S. PIF3/HY5 module regulates BBX11 to suppress protochlorophyllide levels in dark and promote photomorphogenesis in light. New Phytol. 2021, 230, 190–204. [Google Scholar] [CrossRef]
- Zhao, X.; Heng, Y.; Wang, X.; Deng, X.W.; Xu, D. A positive feedback loop of BBX11–BBX21–HY5 promotes photomorphogenic development in Arabidopsis. Plant Commun. 2020, 1, 100045. [Google Scholar] [CrossRef]
- Song, Z.; Heng, Y.; Bian, Y.; Xiao, Y.; Liu, J.; Zhao, X.; Jiang, Y.; Deng, X.W.; Xu, D. BBX11 promotes red light-mediated photomorphogenic development by modulating phyB-PIF4 signaling. Abiotech 2021, 2, 117–130. [Google Scholar] [CrossRef]
- Guo, Y.-H.; Luo, C.; Liu, Y.; Liang, R.-Z.; Yu, H.-X.; Lu, X.-X.; Mo, X.; Chen, S.-Q.; He, X.-H. Isolation and functional analysis of two CONSTANS-like 1 genes from mango. Plant Physiol. Biochem. 2022, 172, 125–135. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, C.; Liang, R.; Lan, M.; Yu, H.; Guo, Y.; Chen, S.; Lu, T.; Mo, X.; He, X. Genome-wide identification of the mango CONSTANS (CO) family and functional analysis of two MiCOL9 genes in transgenic Arabidopsis. Front. Plant Sci. 2022, 13, 1028987. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.-Z.; Luo, C.; Liu, Y.; Hu, W.-L.; Guo, Y.-H.; Yu, H.-X.; Lu, T.-T.; Chen, S.-Q.; Zhang, X.-J.; He, X.-H. Overexpression of two CONSTANS-like 2 (MiCOL2) genes from mango delays flowering and enhances tolerance to abiotic stress in transgenic Arabidopsis. Plant Sci. 2023, 327, 111541. [Google Scholar] [CrossRef]
- Xiao, G.; Li, B.; Chen, H.; Chen, W.; Wang, Z.; Mao, B.; Gui, R.; Guo, X. Overexpression of PvCO1, a bamboo CONSTANS-LIKE gene, delays flowering by reducing expression of the FT gene in transgenic Arabidopsis. BMC Plant Biol. 2018, 18, 232. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, Q.; Zhu, H.; Cai, C.; Li, S. Characterization of mungbean CONSTANS-LIKE genes and functional analysis of CONSTANS-LIKE 2 in the regulation of flowering time in Arabidopsis. Front. Plant Sci. 2021, 12, 608603. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Marchal, V.; Panigrahi, K.C.; Wenkel, S.; Soppe, W.; Deng, X.W.; Valverde, F.; Coupland, G. Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO J. 2008, 27, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Hettiarachchi, G.; Deng, X.-W.; Holm, M. Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell 2006, 18, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Heng, Y.; Jiang, Y.; Zhao, X.; Zhou, H.; Wang, X.; Deng, X.W.; Xu, D. BBX4, a phyB-interacting and modulated regulator, directly interacts with PIF3 to fine tune red light-mediated photomorphogenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 26049–26056. [Google Scholar] [CrossRef]
- Garner, W.W.; Allard, H.A. Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. Mon. Weather Rev. 1920, 48, 415. [Google Scholar] [CrossRef]
- Song, Y.H.; Kubota, A.; Kwon, M.S.; Covington, M.F.; Lee, N.; Taagen, E.R.; Laboy Cintrón, D.; Hwang, D.Y.; Akiyama, R.; Hodge, S.K. Molecular basis of flowering under natural long-day conditions in Arabidopsis. Nat. Plants 2018, 4, 824–835. [Google Scholar] [CrossRef]
- Kojima, S.; Takahashi, Y.; Kobayashi, Y.; Monna, L.; Sasaki, T.; Araki, T.; Yano, M. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 2002, 43, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Komiya, R.; Ikegami, A.; Tamaki, S.; Yokoi, S.; Shimamoto, K. Hd3a and RFT1 are essential for flowering in rice. Development 2008, 135, 767–774. [Google Scholar] [CrossRef]
- Chen, R.; Deng, Y.; Ding, Y.; Guo, J.; Qiu, J.; Wang, B.; Wang, C.; Xie, Y.; Zhang, Z.; Chen, J. Rice functional genomics: Decades’ efforts and roads ahead. Sci. China Life Sci. 2022, 65, 33–92. [Google Scholar] [CrossRef]
- Shim, J.S.; Jang, G. Environmental signal-dependent regulation of flowering time in rice. Int. J. Mol. Sci. 2020, 21, 6155. [Google Scholar] [CrossRef]
- Hayama, R.; Yokoi, S.; Tamaki, S.; Yano, M.; Shimamoto, K. Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature 2003, 422, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Izawa, T.; Oikawa, T.; Sugiyama, N.; Tanisaka, T.; Yano, M.; Shimamoto, K. Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes Dev. 2002, 16, 2006–2020. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Tian, W.; Wei, M.; Yan, W.; He, H.; Zhou, D.; Huang, X.; Li, S.; Ouyang, X. The DTH8-Hd1 module mediates day-length-dependent regulation of rice flowering. Mol. Plant 2017, 10, 948–961. [Google Scholar] [CrossRef]
- Sun, C.; Chen, D.; Fang, J.; Wang, P.; Deng, X.; Chu, C. Understanding the genetic and epigenetic architecture in complex network of rice flowering pathways. Protein Cell 2014, 5, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Izawa, T.; Fuse, T.; Yamanouchi, U.; Kubo, T.; Shimatani, Z.; Yano, M.; Yoshimura, A. Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev. 2004, 18, 926–936. [Google Scholar] [CrossRef]
- Xue, W.; Xing, Y.; Weng, X.; Zhao, Y.; Tang, W.; Wang, L.; Zhou, H.; Yu, S.; Xu, C.; Li, X. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat. Genet. 2008, 40, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Osugi, A.; Itoh, H.; Ikeda-Kawakatsu, K.; Takano, M.; Izawa, T. Molecular dissection of the roles of phytochrome in photoperiodic flowering in rice. Plant Physiol. 2011, 157, 1128–1137. [Google Scholar] [CrossRef]
- Lu, J.; Sun, J.; Jiang, A.; Bai, M.; Fan, C.; Liu, J.; Ning, G.; Wang, C. Alternate expression of CONSTANS-LIKE 4 in short days and CONSTANS in long days facilitates day-neutral response in Rosa chinensis. J. Exp. Bot. 2020, 71, 4057–4068. [Google Scholar] [CrossRef]
- Soyk, S.; Müller, N.A.; Park, S.J.; Schmalenbach, I.; Jiang, K.; Hayama, R.; Zhang, L.; Van Eck, J.; Jiménez-Gómez, J.M.; Lippman, Z.B. Variation in the flowering gene SELF PRUNING 5G promotes day-neutrality and early yield in tomato. Nat. Genet. 2017, 49, 162–168. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Li, Y.; Li, Z.; Qi, J.; Lin, T.; Yang, X.; Zhang, Z.; Huang, S. FLOWERING LOCUS T improves cucumber adaptation to higher latitudes. Plant Physiol. 2020, 182, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; MacGregor, D.R.; Dave, A.; Florance, H.; Moore, K.; Paszkiewicz, K.; Smirnoff, N.; Graham, I.A.; Penfield, S. Maternal temperature history activates Flowering Locus T in fruits to control progeny dormancy according to time of year. Proc. Natl. Acad. Sci. USA 2014, 111, 18787–18792. [Google Scholar] [CrossRef]
- Huang, X.; Ding, J.; Effgen, S.; Turck, F.; Koornneef, M. Multiple loci and genetic interactions involving flowering time genes regulate stem branching among natural variants of Arabidopsis. New Phytol. 2013, 199, 843–857. [Google Scholar] [CrossRef]
- Cho, L.H.; Yoon, J.; An, G. The control of flowering time by environmental factors. Plant J. 2017, 90, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, T.; Wright, L.; Fujiwara, S.; Cremer, F.; Lee, K.; Onouchi, H.; Mouradov, A.; Fowler, S.; Kamada, H.; Putterill, J. Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis. Plant Cell 2005, 17, 2255–2270. [Google Scholar] [CrossRef]
- Siemiatkowska, B.; Chiara, M.; Badiger, B.G.; Riboni, M.; D’Avila, F.; Braga, D.; Salem, M.A.A.; Martignago, D.; Colanero, S.; Galbiati, M. GIGANTEA is a negative regulator of abscisic acid transcriptional responses and sensitivity in Arabidopsis. Plant Cell Physiol. 2022, 63, 1285–1297. [Google Scholar] [CrossRef]
- Sawa, M.; Kay, S.A. GIGANTEA directly activates Flowering Locus T in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2011, 108, 11698–11703. [Google Scholar] [CrossRef]
- Riboni, M.; Galbiati, M.; Tonelli, C.; Conti, L. GIGANTEA enables drought escape response via abscisic acid-dependent activation of the florigens and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1. Plant Physiol. 2013, 162, 1706–1719. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Ye, M.; Jiang, S. Involvement of GIGANTEA gene in the regulation of the cold stress response in Arabidopsis. Plant Cell Rep. 2005, 24, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Fornara, F.; de Montaigu, A.; Sánchez-Villarreal, A.; Takahashi, Y.; Ver Loren van Themaat, E.; Huettel, B.; Davis, S.J.; Coupland, G. The GI–CDF module of Arabidopsis affects freezing tolerance and growth as well as flowering. Plant J. 2015, 81, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ishitani, M.; Halfter, U.; Kim, C.-S.; Zhu, J.-K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA 2000, 97, 3730–3734. [Google Scholar] [CrossRef]
- Kim, W.-Y.; Ali, Z.; Park, H.J.; Park, S.J.; Cha, J.-Y.; Perez-Hormaeche, J.; Quintero, F.J.; Shin, G.; Kim, M.R.; Qiang, Z. Release of SOS2 kinase from sequestration with GIGANTEA determines salt tolerance in Arabidopsis. Nat. Commun. 2013, 4, 1352. [Google Scholar] [CrossRef] [PubMed]
- Quintero, F.J.; Martinez-Atienza, J.; Villalta, I.; Jiang, X.; Kim, W.-Y.; Ali, Z.; Fujii, H.; Mendoza, I.; Yun, D.-J.; Zhu, J.-K. Activation of the plasma membrane Na/H antiporter Salt-Overly-Sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc. Natl. Acad. Sci. USA 2011, 108, 2611–2616. [Google Scholar] [CrossRef]
- Zhu, J.-K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Nusinow, D.A.; Helfer, A.; Hamilton, E.E.; King, J.J.; Imaizumi, T.; Schultz, T.F.; Farré, E.M.; Kay, S.A. The ELF4–ELF3–LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature 2011, 475, 398–402. [Google Scholar] [CrossRef]
- Yu, J.-W.; Rubio, V.; Lee, N.-Y.; Bai, S.; Lee, S.-Y.; Kim, S.-S.; Liu, L.; Zhang, Y.; Irigoyen, M.L.; Sullivan, J.A. COP1 and ELF3 control circadian function and photoperiodic flowering by regulating GI stability. Mol. Cell 2008, 32, 617–630. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Bülbül, S.; Piao, W.; Choi, G.; Paek, N.C. Arabidopsis EARLY FLOWERING 3 increases salt tolerance by suppressing salt stress response pathways. Plant J. 2017, 92, 1106–1120. [Google Scholar] [CrossRef]
- Wu, A.; Allu, A.D.; Garapati, P.; Siddiqui, H.; Dortay, H.; Zanor, M.-I.; Asensi-Fabado, M.A.; Munne-Bosch, S.; Antonio, C.; Tohge, T. JUNGBRUNNEN1, a reactive oxygen species–responsive NAC transcription factor, regulates longevity in Arabidopsis. Plant Cell 2012, 24, 482–506. [Google Scholar] [CrossRef]
- Balazadeh, S.; Siddiqui, H.; Allu, A.D.; Matallana-Ramirez, L.P.; Caldana, C.; Mehrnia, M.; Zanor, M.I.; Köhler, B.; Mueller-Roeber, B. A gene regulatory network controlled by the NAC transcription factor ANAC092/AtNAC2/ORE1 during salt-promoted senescence. Plant J. 2010, 62, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-H.; Seo, P.J.; Park, C.-M. The E3 ubiquitin ligase HOS1 regulates Arabidopsis flowering by mediating CONSTANS degradation under cold stress. J. Biol. Chem. 2012, 287, 43277–43287. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Torti, S.; Coupland, G. Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. Plant J. 2009, 60, 614–625. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.; Hong, Y.; Yao, J.; Ren, Z.; Shi, H.; Zhu, J.-K. The flowering repressor SVP confers drought resistance in Arabidopsis by regulating abscisic acid catabolism. Mol. Plant 2018, 11, 1184–1197. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, Y.; Yang, S. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, C.A.; Wood, C.C.; Robertson, M.; James Peacock, W.; Dennis, E.S. The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J. 2006, 46, 183–192. [Google Scholar] [CrossRef]
- Seo, E.; Lee, H.; Jeon, J.; Park, H.; Kim, J.; Noh, Y.-S.; Lee, I. Crosstalk between cold response and flowering in Arabidopsis is mediated through the flowering-time gene SOC1 and its upstream negative regulator FLC. Plant Cell 2009, 21, 3185–3197. [Google Scholar] [CrossRef]
- Corbesier, L.; Vincent, C.; Jang, S.; Fornara, F.; Fan, Q.; Searle, I.; Giakountis, A.; Farrona, S.; Gissot, L.; Turnbull, C. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 2007, 316, 1030–1033. [Google Scholar] [CrossRef]
- Abe, M.; Kobayashi, Y.; Yamamoto, S.; Daimon, Y.; Yamaguchi, A.; Ikeda, Y.; Ichinoki, H.; Notaguchi, M.; Goto, K.; Araki, T. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 2005, 309, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Collani, S.; Neumann, M.; Yant, L.; Schmid, M. FT modulates genome-wide DNA-binding of the bZIP transcription factor FD. Plant Physiol. 2019, 180, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, I. Regulation and function of SOC1, a flowering pathway integrator. J. Exp. Bot. 2010, 61, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Liljegren, S.J.; Gustafson-Brown, C.; Pinyopich, A.; Ditta, G.S.; Yanofsky, M.F. Interactions among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. Plant Cell 1999, 11, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Goslin, K.; Zheng, B.; Serrano-Mislata, A.; Rae, L.; Ryan, P.T.; Kwaśniewska, K.; Thomson, B.; Ó’Maoiléidigh, D.S.; Madueno, F.; Wellmer, F. Transcription factor interplay between LEAFY and APETALA1/CAULIFLOWER during floral initiation. Plant Physiol. 2017, 174, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Lee, H.-J.; Seo, P.J.; Jung, J.-H.; Ahn, J.H.; Park, C.-M. The Arabidopsis floral repressor BFT delays flowering by competing with FT for FD binding under high salinity. Mol. Plant 2014, 7, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y. The role of ABF family bZIP class transcription factors in stress response. Physiol. Plant 2006, 126, 519–527. [Google Scholar] [CrossRef]
- Hwang, K.; Susila, H.; Nasim, Z.; Jung, J.-Y.; Ahn, J.H. Arabidopsis ABF3 and ABF4 transcription factors act with the NF-YC complex to regulate SOC1 expression and mediate drought-accelerated flowering. Mol. Plant 2019, 12, 489–505. [Google Scholar] [CrossRef]
- Min, J.H.; Chung, J.S.; Lee, K.H.; Kim, C.S. The CONSTANS-like 4 transcription factor, AtCOL4, positively regulates abiotic stress tolerance through an abscisic acid-dependent manner in Arabidopsis. J. Integr. Plant Biol. 2015, 57, 313–324. [Google Scholar] [CrossRef]
- Du, J.; Zhu, X.; He, K.; Kui, M.; Zhang, J.; Han, X.; Fu, Q.; Jiang, Y.; Hu, Y. CONSTANS interacts with and antagonizes ABF transcription factors during salt stress under long-day conditions. Plant Physiol. 2023, 193, 1675–1694. [Google Scholar] [CrossRef]
- Han, X.; Kui, M.; Xu, T.; Ye, J.; Du, J.; Yang, M.; Jiang, Y.; Hu, Y. CO interacts with JAZ repressors and bHLH subgroup IIId factors to negatively regulate jasmonate signaling in Arabidopsis seedlings. Plant Cell 2023, 35, 852–873. [Google Scholar] [CrossRef] [PubMed]
- Sng, N.J.; Kolaczkowski, B.; Ferl, R.J.; Paul, A.-L. A member of the CONSTANS-Like protein family is a putative regulator of reactive oxygen species homeostasis and spaceflight physiological adaptation. AoB Plants 2019, 11, ply075. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Li, M.; Fu, D.; Wu, S.; Li, J.; Gong, Z.; Liu, H.; Yang, S. The CRY2–COP1–HY5–BBX7/8 module regulates blue light-dependent cold acclimation in Arabidopsis. Plant Cell 2021, 33, 3555–3573. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, J.; Xu, Y.; Li, X.; Xiao, J.; Xiong, L. Ghd2, a CONSTANS-like gene, confers drought sensitivity through regulation of senescence in rice. J. Exp. Bot. 2016, 67, 5785–5798. [Google Scholar] [CrossRef]
- Lei, X.; Tan, B.; Liu, Z.; Wu, J.; Lv, J.; Gao, C. ThCOL2 improves the salt stress tolerance of Tamarix hispida. Front. Plant Sci. 2021, 12, 653791. [Google Scholar] [CrossRef]
- Liu, L.; Ding, Q.; Liu, J.; Yang, C.; Chen, H.; Zhang, S.; Zhu, J.; Wang, D. Brassica napus COL transcription factor BnCOL2 negatively affects the tolerance of transgenic Arabidopsis to drought stress. Environ. Exp. Bot. 2020, 178, 104171. [Google Scholar] [CrossRef]
- Xu, C.; Shan, J.; Liu, T.; Wang, Q.; Ji, Y.; Zhang, Y.; Wang, M.; Xia, N.; Zhao, L. CONSTANS-LIKE 1a positively regulates salt and drought tolerance in soybean. Plant Physiol. 2023, 191, 2427–2446. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhi, F.; Li, X.; Shen, W.; Yan, M.; He, J.; Bao, C.; Fan, T.; Zhou, S.; Ma, F. Zinc-finger protein MdBBX7/MdCOL9, a target of MdMIEL1 E3 ligase, confers drought tolerance in apple. Plant Physiol. 2022, 188, 540–559. [Google Scholar] [CrossRef]
- Huang, Z.; Bai, X.; Duan, W.; Chen, B.; Chen, G.; Xu, B.; Cheng, R.; Wang, J. Genome-wide identification and expression profiling of CONSTANS-Like genes in Pepper (Capsicum annuum): Gaining an insight to their phylogenetic evolution and stress-specific roles. Front. Plant Sci. 2022, 13, 828209. [Google Scholar] [CrossRef]
- Niu, T.; Wang, X.; Abbas, M.; Shen, J.; Liu, R.; Wang, Z.; Liu, A. Expansion of CONSTANS-like genes in sunflower confers putative neofunctionalization in the adaptation to abiotic stresses. Ind. Crops Prod. 2022, 176, 114400. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Q.; Liu, W.; Wu, X.; Li, Z.; Xu, Y.; Li, Y.; Imaizumi, T.; Hou, X.; Liu, T. BrABF3 promotes flowering through the direct activation of CONSTANS transcription in pak choi. Plant J. 2022, 111, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, F.; Dong, S.; Liu, W.; Wang, H.; Chen, Z.; Wang, J. CONSTANS-like 9 (COL9) delays the flowering time in Oryza sativa by repressing the Ehd1 pathway. Biochem. Biophys. Res. Commun. 2016, 479, 173–178. [Google Scholar] [CrossRef]
- Tan, J.; Jin, M.; Wang, J.; Wu, F.; Sheng, P.; Cheng, Z.; Wang, J.; Zheng, X.; Chen, L.; Wang, M. OsCOL10, a CONSTANS-Like gene, functions as a flowering time repressor downstream of Ghd7 in rice. Plant Cell Physiol. 2016, 57, 798–812. [Google Scholar] [CrossRef]
- Wu, W.; Zheng, X.-M.; Chen, D.; Zhang, Y.; Ma, W.; Zhang, H.; Sun, L.; Yang, Z.; Zhao, C.; Zhan, X. OsCOL16, encoding a CONSTANS-like protein, represses flowering by up-regulating Ghd7 expression in rice. Plant Sci. 2017, 260, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, H.; Li, T.; Wu, J.; Nagarajan, R.; Lei, L.; Powers, C.; Kan, C.-C.; Hua, W.; Liu, Z. TaCol-B5 modifies spike architecture and enhances grain yield in wheat. Science 2022, 376, 180–183. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).