Abstract
Regular exercise, especially aerobic exercise, is beneficial for increasing serum high-density lipoprotein-cholesterol (HDL-C) levels in the general population. In addition to the HDL-C quantity, exercise enhances HDL functionality, antioxidants, and cholesterol efflux. On the other hand, the optimal intensity and frequency of exercise to increase HDL quantity and enhance HDL quality in middle-aged women need to be determined. The current study was designed to compare the changes in HDL quantity and quality among middle-aged women depending on exercise intensity, frequency, and duration; participants were divided into a sedentary group (group 1), a middle-intensity group (group 2), and a high-intensity group (group 3). There were no differences in anthropometric parameters among the groups, including blood pressure, muscle mass, and handgrip strength. Although there was no difference in serum total cholesterol (TC) among the groups, the serum HDL-C and apolipoprotein (apo)A-I levels remarkably increased to 17% and 12%, respectively, in group 3. Serum low-density lipoprotein-cholesterol (LDL-C), glucose, triglyceride, and the apo-B/apoA-I ratio were remarkably decreased in the exercise groups depending on the exercise intensity; group 3 showed 13%, 10%, and 45% lower LDL-C, glucose, and triglyceride (TG), respectively, than group 1. The hepatic and muscle damage parameter, aspartate aminotransferase (AST), was significantly decreased in the exercise groups, but high-sensitivity C-reactive protein (CRP), alanine aminotransferase (ALT), and γ-glutamyl transferase (γ-GTP) were similar in the three groups. In LDL, the particle size was increased 1.5-fold (p < 0.001), and the oxidation extent was decreased by 40% with a 23% lower TG content in group 3 than in group 1. In the exercise groups (groups 2 and 3), LDL showed the slowest electromobility with a distinct band intensity compared to the sedentary group (group 1). In HDL2, the particle size was 2.1-fold increased (p < 0.001) in the exercise group (group 3) with a 1.5-fold increase in TC content compared to that in group 1, as well as significantly enhanced antioxidant abilities, paraoxonase (PON) activity, and ferric ion reduction ability (FRA). In HDL3, the particle size was increased 1.2-fold with a 45% reduction in TG in group 3 compared to group 1. With increasing exercise intensity, apoA-I expression was increased in HDL2 and HDL3, and PON activity and FRA were enhanced (p < 0.001). In conclusion, regular exercise in middle-aged women is associated with the elevation of serum HDL-C and apoA-I with the enhancement of HDL quality and functionality and an increase in the TC content, particle size, and antioxidant abilities. With the reduction in TG and oxidized products in LDL and HDL, lipoproteins could have more anti-atherogenic properties through regular exercise in an intensity-dependent manner.
1. Introduction
Sedentary lifestyles are a major risk factor for metabolic syndrome and cardiovascular diseases [1,2]. A sedentary lifestyle is frequently associated with low HDL-C and high triglyceride (TG), insulin resistance, and abdominal obesity [3,4]. Exercise is associated with a reduced risk of cardiovascular disease and reduced overall mortality in a dose-dependent manner [5,6]. Regular exercise is beneficial for increasing HDL-C with enhanced functionality, such as antioxidant activity, anti-inflammatory activity, and cholesterol efflux activity [7,8].
HDL-C quantity and quality can be impaired by sedentary lifestyles and poor dietary habits [9], alcohol consumption [10], and smoking [11]. The production of dysfunctional HDL is intimately associated with a high incidence of metabolic syndrome because impaired qualities of HDL, such as decreased oxidation and glycation properties, are frequently found in patients with obesity, diabetes, and hypertension [12]. Therefore, it is widely accepted that maintaining HDL to retain its healthy and protective effects is associated with the suppression of hypertension, diabetes, and dementia [13]. In general, the quantity of HDL-C gradually decreases with aging [14], which impairs HDL quality [15].
HDL-C quantity and quality can also be enhanced through lifestyle and nonpharmacological interventions, such as exercise [16] and nutritional supplementation [17]. As an enhancement strategy, many human studies have shown that nutrient supplementation, such as policosanol, could enhance HDL-C quantity, quality, and functionality in healthy subjects [18,19] with prehypertension [20,21]. Enhanced HDL functionality was closely associated with increased apoA-I and paraoxonase activity in HDL [16,19,20]. Aerobic sports athletes, such as runners and wrestlers, have the highest HDL-C and the largest particle size with the highest content of apoA-I and paraoxonase (PON) activity in HDL among national representatives of the Beijing Olympics [16]. These results suggest that regular exercise could enhance HDL functionality and increase HDL-C quantity regardless of the exercise type.
Many reports have shown that the pro-atherogenic lipid profile was improved to an anti-atherogenic profile through exercise, as illustrated by decreases in LDL-C and TG and an increase in HDL-C [22]. On the other hand, there are no reports comparing HDL quality and functionality between sedentary and exercise groups in nonsmoking middle-aged women. This study was designed to compare changes in HDL and LDL properties, such as particle shape and size, oxidation and glycation extent, and lipid and apolipoprotein compositions, depending on the exercise intensity in healthy and normolipidemic middle-aged women who carried out regular exercise for at least one year prior to the study.
2. Results
2.1. Anthropometric Profiles
As shown in Table 1, all participants (n = 57) were divided into three groups depending on the metabolic equivalent score based on exercise time and intensity: sedentary (group 1, MET 2.48 ± 0.03), low-exercise (group 2, moderate intensity for 60 min/week, MET 4.55 ± 0.27), and high-exercise (group 3, high intensity for 90 min/week, 9.13 ± 0.35) groups. The three groups comprised females of similar age (around 50 years of age; 35–63 years old) and similar occupations, such as office and indoor work. Regardless of the exercise time and intensity, anthropometric data, such as BMI, blood pressure, muscle mass, fat mass, and handgrip strength (HS), were similar among the groups. All participants showed normal and similar ranges of anthropometric profiles for middle-aged women and were healthy without diagnosed diseases (Table 1).

Table 1.
Anthropometric profiles of women groups depending on exercise intensity (Me (25%; 75%)) *.
Table 1.
Anthropometric profiles of women groups depending on exercise intensity (Me (25%; 75%)) *.
| Group 1 n = 20 | Group 2 n = 16 | Group 3 n = 21 | p | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |
|---|---|---|---|---|---|---|---|
| Moderate-intensity exercise/week (times) | 0.0 (0.0; 0.0) | 1.5 (1.5; 3.5) | 3.5 (1.5, 5.5) | <0.001 | 0.000 | 0.000 | 0.671 |
| High-intensity exercise/week (times) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 1.5 (1.5, 3.5) | <0.001 | 1.000 | <0.001 | <0.001 |
| Total number of exercises/week (times) | 0.0 (0.0; 0.0) | 1.5 (1.5; 3.5) | 5.0 (3.0, 7.0) | <0.001 | <0.001 | <0.001 | 0.009 |
| Total time of exercise/week (minutes) | 0 (0; 0) | 60 (60; 150) | 90 (75; 150) | <0.001 | <0.001 | <0.001 | 0.357 |
| MET equivalents of common physical activities (score) | 2.5 (2.5; 2.5) | 4.8 (3.3; 5.5) | 9.5 (8.0; 10.0) | 0.000 | 0.000 | 0.000 | 0.000 |
| Alcohol intake/month (g) | 63 (5; 210) | 21 (0; 94.5) | 21 (0, 137) | 0.458 | 0.801 | 0.901 | 1.000 |
| Age (year), (min., max.) | 53.5 (50.0; 56.0) (41, 58) | 45.0 (40.8; 54.8) (35, 63) | 52.0 (43.0, 55.5) (36, 62) | 0.132 | 0.171 | 0.301 | 0.924 |
| Height (cm) | 160.0 (157; 163.8) | 161.0 (158.3; 165.0) | 159.0 (156.0, 164.5) | 0.804 | 1.000 | 1.000 | 1.000 |
| Weight (kg) | 55.7 (50.9; 59.9) | 56.9 (53.3; 66.8) | 54.5 (51.2; 61.1) | 0.116 | 0.242 | 1.000 | 0.169 |
| BMI (kg/m2), (min., max.) | 21.4 (20.1; 22.8) (19.5, 26.1) | 23.0 (20.4; 24.2) (18.4, 30.9) | 21.4 (20.2, 23.0) (17.0, 26.8) | 0.446 | 0.797 | 1.000 | 0.773 |
| Heart rate (BPM) | 70.0 (66.8; 75.8) | 73.0 (66.0; 84.8) | 75.0 (72.0, 81.0) | 0.142 | 0.549 | 0.167 | 1.000 |
| SBP (mmHg) | 120.5 (110.0; 129.8) | 123.0 (108.5; 127.5) | 120.0 (117.0, 124.0) | 0.930 | 1.000 | 1.000 | 1.000 |
| DBP (mmHg) | 75.5 (63.3; 87.8) | 68.0 (62.8; 77.0) | 69.0 (65.5; 78.0) | 0.207 | 0.262 | 0.673 | 1.000 |
| Muscle mass (kg) | 37.5 (35.8; 38.8) | 39.7 (35.4; 42.8) | 38.1 (34.5; 40.1) | 0.172 | 0.248 | 1.000 | 0.357 |
| Fat mass_subcutaneous (kg) | 13.6 (12.2; 15.9) | 14.4 (12.7; 18.1) | 13.7 (11.0; 16.4) | 0.240 | 0.860 | 1.000 | 0.282 |
| Fat mass_visceral (kg) | 1.5 (1.2; 2.0) | 1.8 (1.2; 2.4) | 1.5 (1.1; 2.0) | 0.406 | 1.000 | 1.000 | 0.542 |
| HS (kg), (min., max.) | 26.5 (23.0; 28.0) (16.0, 30.0) | 25.0 (23.0; 27.8) (22.0, 37.0) | 28.0 (25.5; 30.0) (13.0, 32.0) | 0.158 | 1.000 | 0.275 | 0.334 |
| Body water (kg) | 29.2 (27.4; 30.3) | 30.9 (27.6; 33.5) | 29.9 (31.5) | 0.087 | 0.103 | 1.000 | 0.263 |
BMI, body mass index; BPM, beat per minute, DBP, diastolic blood pressure; HS, handgrip strength; SBP, systolic blood pressure. * Data are presented as median (25th; 75th percentiles). The metabolic equivalent (MET) score was calculated based on the survey results of the participants and can be classified as previously described [23]: Light < 3.0 METs; Moderate 3.0~6.0 METs; Vigorous > 6.0 METs.
2.2. Blood Parameters
In the blood lipid profiles, all groups showed similar total cholesterol (TC) and low-density lipoprotein-cholesterol (LDL-C) levels of 202–222 mg/dL and 134–153 mg/dL, respectively (Table 2), with 66–68% of LDL-C in TC. However, HDL-C increased significantly with exercise intensity (p = 0.030); group 3 showed 15% higher HDL-C than group 1 (p = 0.040), while groups 1 and 2 did not show a difference. In the same context, the % of HDL-C in TC was more elevated in the exercise groups than the nonexercise group (p = 0.002) and showed the highest ratio of HDL-C in TC, around 28% (p < 0.001 vs. group 1), while groups 1 and 2 showed 22% and 26% of the %HDL-C/TC level in group 3. The serum TG levels remarkably decreased in the exercise groups compared to the nonexercise group (p < 0.001); group 3 showed a 45% lower TG level than group 1 (p < 0.001), while group 2 showed 23% lower TG than group 1 with no significance (p = 0.165). Concomitantly, the TG/HDL-C ratio and LDL-C/HDL-C ratio were decreased in the exercise groups; group 3 showed significantly lower levels than group 1 (p = 0.001).

Table 2.
Blood lipid and inflammatory parameters of women groups depending on exercise (Me (25%; 75%)) *.
Among apolipoprotein levels, apoA-I was increased and apo-B was decreased in the exercise groups, but the difference was not significant. On the other hand, the apo-B/apoA-I ratio was significantly lower in the exercise groups than in the nonexercise group (p = 0.014); group 3 showed a 23% lower apo-B/apoA-I ratio (0.52 ± 0.02) than group 1 (0.67 ± 0.04, p = 0.009). The serum glucose level was also lower in the exercise groups than in the nonexercise group (p = 0.003); group 3 showed a 10% lower serum glucose level than group 1 (p = 0.003). These results suggest that high-intensity exercise was associated with an increase in apoA-I, a decrease in apo-B, and a decrease in glucose in serum.
For hepatic functions, the determination of hepatic enzymes and acute inflammation parameters showed that the exercise groups had a significant reduction in aspartate aminotransferase (AST, p = 0.002) (Table 2). In contrast, none of the groups showed differences in alanine aminotransferase (ALT, p = 0.088), gamma-glutamyl transferase (γ-GTP, p = 0.064), or high-sensitivity C-reactive protein (hs-CRP, p = 0.896). Groups 3 and 2 showed 25% (p = 0.003) and 21% (p = 0.028) lower AST levels, respectively, than group 1. Group 3 showed 23% lower ALT and 35% lower γ-GTP levels than group 1, although there was no significance among any of the groups. These results suggest that exercise is specifically associated with a reduction in AST, but not ALT, because AST is more specifically influenced by muscle function and its damage than ALT, which is more specific to hepatic damage. The other enzymes, ALT and γ-GTP, are more related to hepatic-specific inflammation and alcoholic damage, respectively.
2.3. LDL Compositions and Particle Analysis
As shown in Table 3, the determination of the glycation extent using fluorescence spectroscopy showed that group 3 showed 7% lower fluorescence intensity (FI) than group 1, while group 2 showed a similar level to group 1. This result suggests that only high-intensity exercise, not low-intensity exercise, lowered the glycation of LDL compared to the sedentary group. The quantification of the TC content showed that group 1 had the lowest TC content in LDL, while groups 2 and 3 had 10% and 13%, respectively, higher TC content than group 1, but no significance was detected in the group comparison. The quantification of TG in LDL showed that the exercise groups had lower TG levels than the sedentary group (p = 0.023); group 3 had 23% lower TG content in LDL than group 1 (p = 0.041). These results suggest that high-intensity exercise is associated with an increase in TC and a decrease in TG in LDL.

Table 3.
Lipid compositions, oxidation extent, and glycation extent of lipoproteins from participants.
2.4. Electron Microscopic Analysis of LDL
Transmission electron microscopy (TEM) showed that LDL from group 3 had the largest diameter of approximately 27.9 ± 0.5 nm (Table 3) and the largest size of 711 ± 13 nm2 (Figure 1A) with the most distinct shape (Figure 1B), while LDL from group 1 showed the smallest diameter, 23.9 ± 0.4 nm, and the smallest size, around 462 ± 8 nm2 (p < 0.001), with a more aggregated and ambiguous particle shape. Groups 2 and 3 showed larger diameters, 24.6 ± 0.3 nm and 27.9 ± 0.5 nm, respectively, and 1.2-fold (p < 0.001) and 1.5-fold (p < 0.001) larger LDL particle sizes, respectively, than group 1, indicating that higher-intensity exercise caused a larger LDL size with a more distinct morphology. Cupric-ion-mediated oxidized LDL (oxLDL) showed the most severe aggregated particle shape and unclear morphology with a diminished particle number (Figure 1). oxLDL showed the most aggregated and obscure image with the smallest particle size, around 402 ± 13 nm2. These results suggest that higher-intensity exercise was associated with a larger LDL particle size with a larger increase in cholesterol and a larger decrease in the TG content, glycation extent, and oxidation extent.
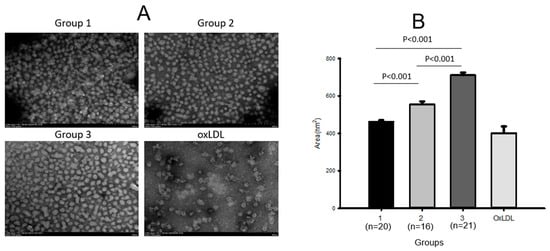
Figure 1.
Transmission electron microscopy (TEM) images (A) and area analysis (B) of LDL from each group.
2.5. Electromobility of LDL
As shown in Figure 2A, LDL electrophoresis on agarose gel showed that the high-exercise group (group 3) had the strongest and distinct band intensity without aggregation in the loading position (lane 3). In contrast, group 1 (no exercise) showed the fastest electromobility and the highest smeared band intensity (lane 1). The low-exercise group (lane 2) showed a lower smeared band intensity and slower electromobility than the sedentary group (lane 1). LDL oxidized by the cupric ion treatment (lane 4) showed the fastest electromobility with the largest smeared band pattern and aggregation at the loading position, as indicated by the red arrowhead. Interestingly, the LDL band intensity was stronger and more distinct depending on the exercise intensity and the increase in MET. More oxidized LDL was prone to aggregation in the loading position due to increased apo-B fragmentation in LDL with the fastest electromobility, as indicated by the blue arrow and red arrowhead in Figure 1A.
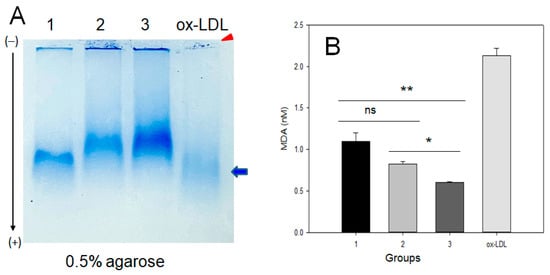
Figure 2.
Native electrophoresis of LDL on agarose from each group and quantification of oxidized species. (A) Comparison of electromobility and aggregation extent of LDL (10 μg) band on 0.5% agarose gel without denaturation. Lane 1, group 1 (n = 20); lane 2, group 2 (n = 16); lane 3, group 3 (n = 21); lane 4, oxLDL, Cu2+-treated for 4 h. Red arrowhead indicates an aggregated band of oxLDL at the loading position. The blue arrow indicates the oxLDL band position and smeared intensity. (B) Quantification of oxidized species using malondialdehyde standard by the thiobarbituric acid-reactive substances (TBARS) assay. MDA, malondialdehyde; oxLDL, oxidized LDL. *, p < 0.05 between group 2 and 3; **, p < 0.01 between group 1 and 3.
The quantification of oxidized species in LDL showed that group 1 had the highest oxidized species level, around 1.1 ± 0.1 nM malondialdehyde (MDA), while groups 2 and 3 showed a lower MDA level, around 0.8 ± 0.1 nM and 0.6 ± 0.1 nM MDA, respectively. These results suggest that LDL oxidation was attenuated by exercise in an intensity-dependent manner; LDL from groups 2 and 3 showed a 28% and 45%, respectively, lower oxidation extent than group 1.
2.6. HDL2 Compositions and Particle Morphology
In HDL2, as shown in Table 3, the TC content was increased (p = 0.031) depending on the exercise intensity; group 3 showed the highest TC (p = 0.042) and the lowest TG content in HDL2. Groups 2 and 3 showed 1.4-fold and 1.7-fold higher TC contents than group 1, respectively, while group 3 showed 1.2-fold higher TC than group 2. Although no significance was detected, the TG content was lower in the exercise groups than in the sedentary group; group 3 showed 29% lower TG than group 1. There was no difference in the TG content in HDL2 between groups 1 and 2, suggesting that low-intensity exercise did not affect the change in TG content in HDL2.
TEM image analysis revealed that the HDL2 particle diameter in group 3 was 1.4 times (p < 0.001) and 1.2 times larger than those in groups 1 and 2, respectively, as shown in Figure 3. The particle size showed an increasing tendency in the exercise groups depending on intensity (p < 0.001); group 3 showed the largest size (234 ± 5 nm2), which was twice that of group 1 (114 ± 4 nm2, p < 0.001), with a distinct shape and clear morphology. Group 2 (162 ± 4 nm2) showed a 1.4 times larger HDL2 level than group 1, p < 0.001, while HDL2 glycated by fructose treatment (final 250 mM) for 24 h showed the smallest particle size with the most ambiguous morphology.
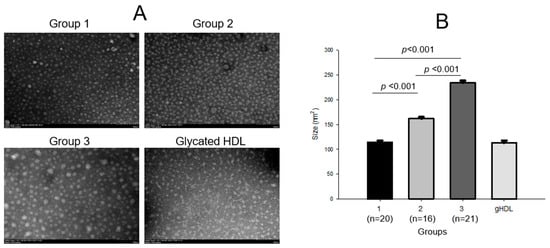
Figure 3.
Transmission electron microscopy (TEM) images and area analysis of HDL2 from each group (A) and size comparison between the groups (B). gHDL, glycated HDL.
Fluorescence spectroscopy showed that the glycation extent was lower in the exercise groups; groups 2 and 3 showed 8% and 10% lower glycation than group 1. The extent of oxidation was lower in the exercise groups than in the sedentary groups, up to 23% lower in group 2 than in group 1, but the difference was not significant.
2.7. HDL3 Compositions and Particle Analysis
Although there was no difference in TC content in HDL3 among any of the groups, the exercise groups showed a lower TG content (p = 0.022) than the sedentary group; groups 2 and 3 showed up to 34% (p = 0.051) and 45% (p = 0.017) lower TG levels, respectively, than group 1. These results suggest that exercise helps reduce the TG content in HDL3, even though there was no accumulation of TC in HDL3. On the other hand, there was no difference in the oxidation extent or glycation extent among the groups.
The HDL3 particle diameter increased significantly in an exercise-intensity-dependent manner (p < 0.001); group 3 showed a 33% and 18% larger diameter than groups 1 and 2, respectively. The HDL size also increased with exercise intensity, as shown in Figure 4; group 3 showed the largest particle size, around 110 ± 13 nm2, whereas group 1 showed the smallest HDL3 size, around 92 ± 2 nm2. HDL3 in groups 2 and 3 showed an 11% (p = 0.004) and 20% (p < 0.001), respectively, bigger size than that in group 1, while group 3 showed an 8% bigger (p = 0.041) particle size than that in group 2. Glycated HDL3, which was fructose-treated (final 250 mM), showed the most significant decrease, around 58 ± 4 nm2. The extent of oxidation and glycation in HDL3 were similar between the groups, as shown in Table 3.
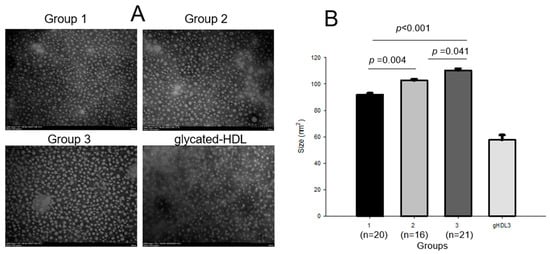
Figure 4.
Transmission electron microscopy (TEM) images and area analysis of HDL3 from each group (A) and comparison of particle sizes (B). gHDL3, glycated HDL3.
2.8. Electrophoretic Patterns of HDL2 and HDL3
As shown in Figure 5A, SDS-PAGE of HDL2 (2 mg/mL) and HDL3 (2 mg/mL) revealed that the exercise groups showed higher apoA-I expression, which increased in an intensity-dependent manner; groups 2 and 3 showed 1.3-fold and 1.6-fold higher band intensities of apoA-I, respectively, than group 1. Interestingly, group 3 showed the lowest band intensity of apoA-II in HDL2, even though group 3 showed the highest apoA-I content. Agarose electrophoresis revealed that the two distinct bands of HDL2 were the strongest in group 3, as shown in Figure 5B, while group 1 showed the two weakest bands with slower electromobility.
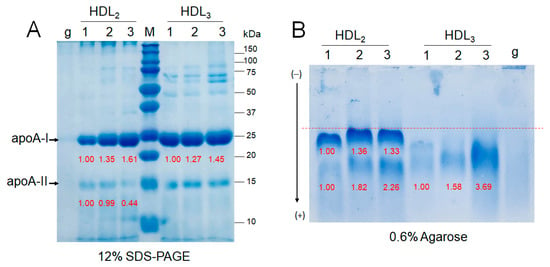
Figure 5.
Electrophoretic patterns of HDL from participants depend on the exercise intensity. Lane 1, group 1 (n = 20); lane 2, group 2 (n = 16); lane 3, group 3 (n = 21). (A) Electrophoresis of HDL (2 mg/mL) under denatured state (12% SDS-PAGE). Lane M, molecular weight standards (Bio-Rad Cat#161-0374); lane g, glycated HDL2. (B) Electrophoresis of HDL (2 mg/mL) under native state (0.6% agarose). Lane g, glycated HDL3.
2.9. Correlation Analysis
As shown in Table 4, Spearman correlation analysis showed that higher-intensity exercise is positively and significantly associated with the serum HDL-C level (r = 0.365, p = 0.005), %HDL-C/TC (r = 0.484, p < 0.001), and the apoA-I level (r = 0.354, p = 0.007). On the other hand, higher-intensity exercise is negatively associated with serum apo-B (r = −0.264, p = 0.047), the apo-B/apoA-I ratio (r = −0.373, p = 0.004), and the serum LDL-C/HDL-C ratio (r = −0.437, p = 0.001). These results suggest that higher-intensity exercise is positively correlated with HDL-related parameters and negatively correlated with LDL-related parameters. Hepatic parameters, AST, ALT, and γ-GTP, were negatively correlated with exercise intensity: AST (r = −0.555, p < 0.001), ALT (r = −0.351, p = 0.007), and γ-GTP (r = −0.365, p = 0.005). On the other hand, hsCRP was not correlated with exercise intensity, suggesting that acute infection or inflammation was not correlated with exercise in healthy subjects.

Table 4.
Spearman correlation (r) and p values of linear regression for all participants (n = 57).
For the lipoprotein level, the MDA content in LDL was negatively (r = −0.238, p < 0.001) correlated and the LDL size (r = 0.271, p < 0.001) was positively correlated with exercise in an intensity-dependent manner. In HDL2, the TC content, particle size, and PON activity were positively correlated with exercise intensity, even though FRA did not show a correlation with exercise intensity. In HDL3, FRA, PON activity, and particle size were positively correlated with exercise intensity, while the TC, TG, and MDA contents did not correlate with exercise intensity.
2.10. Antioxidant Activity in HDL2 and HDL3
Among the three groups, at the same protein concentration (2 mg/mL), HDL3 showed higher paraoxonase (PON) and ferric ion reduction ability (FRA) than HDL2, as shown in Figure 6, suggesting that HDL3 displayed a higher antioxidant capacity than HDL2. As shown in Figure 6A, the HDL-associated paraoxonase (PON) assay revealed that group 3 showed the highest PON activity in both HDL2 and HDL3 with 20.5 ± 1.3 μU/L/min and 41.8 ± 2.3 μU/L/min, respectively. On the other hand, group 1 showed the lowest PON activity in both HDL2 and HDL3 with 15.1 ± 0.7 μU/L/min and 31.6 ±0.8 μU/L/min, respectively, whereas group 2 showed higher PON activity in both HDL2 and HDL3 with 17.1 ± 1.3 μU/L/min and 34.9 ± 1.7 μU/L/min, respectively. These results suggest that the PON activity is significantly and proportionally associated with exercise in an intensity-dependent manner; groups 2 and 3 showed up to 12% and 35% higher HDL3-PON activity, respectively, than group 1.
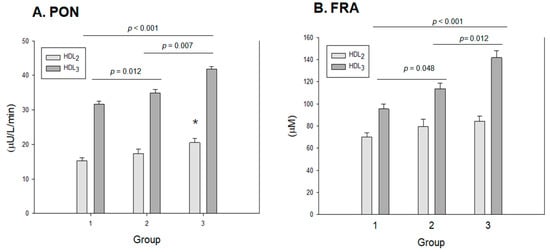
Figure 6.
Antioxidant abilities of HDL2 and HDL3 from each group. Group 1 (n = 20); group 2 (n = 16); group 3 (n = 21). (A) HDL-associated paraoxonase (PON) activity. PON activity is expressed as the initial velocity of p-nitrophenol production per min (μU/L/min) at 37 °C during 60 min incubation. *, p < 0.05 versus group 1. (B) Comparison of HDL-associated ferric ion reduction ability (FRA). FRA is expressed as the concentration of vitamin C (mM), which is equivalent to the amount of ferric ions (μM) reduced per hour.
The FRA of HDL2 and HDL3 was higher in both exercise groups and increased in a dose-dependent manner. In HDL2, group 3 showed the highest FRA of around 89 ± 5 μM, while group 1 showed the lowest FRA of around 69 ± 4 μM, although there were no significant differences in the group comparison. In HDL3, FRA increased significantly with exercise intensity (p < 0.001); groups 2 and 3 showed 14% (p = 0.048) and 40% (p < 0.001) higher FRA, respectively, than group 1. Interestingly, group 3 showed 22% higher FRA than group 2 (p = 0.012), suggesting that the enhancement of FRA is strongly dependent on exercise intensity.
Spearman correlation analysis showed that the PON activity in HDL2 (r = 0.714, p < 0.001) and HDL3 (r = 0.811, p < 0.001) was positively associated with exercise intensity. FRA in HDL2 (r = 0.578, p = 0.004) and HDL3 (r = 0.686, p < 0.001) was also positively correlated with exercise intensity.
3. Discussion
Regular exercise is associated with the elevation of serum HDL-C [24], apoA-I content [25], and cholesterol efflux activity with an increase in particle size [26]. A meta-analysis of 10 interventions showed that the number of large HDL particles increased despite the differences in exercise programs [27]. Although it has been widely accepted that HDL quality, including HDL particle size, is increased by exercise, there has been insufficient information on the particle shape, composition, and extent of oxidation and glycation. Moreover, many studies have focused on the short-term physiological effect of exercise, usually 8–24 weeks, which was intentionally forced with the randomization of volunteers [28]. In addition, there have been no studies that compare the effect of regular exercise, for at least 1 year or more, on HDL-C quantity and quality between a sedentary group and an exercise group in the general population. The current study aimed to find differences in HDL quality and functionality specifically in middle-aged women depending on whether they were sedentary or exercised.
The current study showed a difference in physiological parameters, including lipids, lipoproteins, apolipoproteins, hepatic functions, and inflammatory profiles, among healthy middle-aged women who carried out habitual and regular exercise. They were divided into sedentary (group 1), low-intensity exercise (group 2), and high-intensity exercise (group 3) based on a self-reported questionnaire. The exercise groups, particularly group 3, exhibited a significant elevation of serum HDL-C and %HDL-C/TC, with a significant reduction in TG, TG/HDL-C, LDL-C/HDL-C, and apo-B/apoA-I. In addition to improving the lipid profile, lipoprotein profile, and apolipoprotein profile, serum AST was significantly lower in groups 2 (p = 0.028 versus group 1) and 3 (p = 0.003 versus group 1) despite similar serum ALT and γ-GTP. Correlation analysis also showed that serum AST showed the highest negative association (r = −0.555, p < 0.001) with exercise intensity, while serum ALT (r = −0.351, p = 0.007) and γ-GTP (r = −0.365, p = 0.005) showed a less negative association. Similarly, exercise decreased both AST and ALT levels in patients with nonalcoholic fatty liver diseases [29]. From 16 clinical trials, the average reduction in the AST level was −4.93 U/L (95% CI, −7.94 to −1.91), which shows good agreement with the current findings. AST is more specific to muscle damage than ALT, which is produced primarily in the cytoplasm of hepatocytes and is more specific to liver damage [30]. AST is mainly produced in the mitochondria of the muscle, heart, kidney, red blood cells, brain, and small bowel, whereas ALT is present in the liver, muscle, and kidney [29]. The exercise groups showed lower AST (Table 2) with significant negative correlations (Table 4) regardless of age because AST is more specific to muscle damage. To the best of the authors’ knowledge, this is the first report to show that habitual regular exercise for at least one year, not through a forced exercise intervention, could reduce serum AST in middle-aged women.
In group 3, the serum apoA-I level was elevated, and the apoA-I band in HDL2 and HDL3 was increased, while apoA-II in HDL2 was decreased. These reciprocal changes in the expressional levels of apoA-I and apoA-II are similar to a previous report showing that Olympic athletes showed the elevation of apoA-I in HDL2 and HDL3, while their apoA-II was decreased, particularly in HDL2. [16] ApoA-I and apoA-II comprised around 70% and 20% of the total HDL protein content, respectively. Although apoA-II is the second most abundant protein constituent of HDL, apoA-II is still an enigmatic apolipoprotein. HDL containing apoA-I alone (LpA-I) and HDL containing apoA-I and apoA-II (LpA-I:A-II) were distinctly different, both structurally and metabolically [31]; LpA-I:A-II is more pro-atherogenic [32]. LpA-I is more cardioprotective in patients with coronary artery disease than LpA-I-I:A-II. Bioinformatic analysis showed that Lp-A-I is more metabolically active in facilitating the increase in HDL particle size and number [32]. LpA-I:A-II is less metabolically active and usually has a smaller particle size than LpA-I [33]. Overall, the increase in apoA-I and decrease in apoA-II in HDL2 (Table 2 and Figure 5A) in group 3 were linked to an increase in HDL2 particle size (Figure 3).
There have been conflicting data concerning the effect of exercise on improving HDL-C quantity, depending on the type of sport [34]; aerobic exercise effectively improves HDL-C, but resistance exercise does not. Aerobic exercise performed for 12–24 weeks can increase HDL-C more efficiently by approximately 3.8–15.4 mg/dL from the initial level [34]. On the other hand, these data were obtained from short-term or long-term exercise interventions that were carried out for training purposes or as part of mandatory programs. Furthermore, an increase in HDL-C quantity was not accompanied by the enhancement of HDL quality, such as increases in particle size and antioxidant ability. Healthy young men who completed a 12-week moderate-intensity exercise program as military soldiers [35] showed an elevation of HDL-C and apoA-I but without an increase in HDL particle size, even though their cholesterol efflux was enhanced. On the other hand, the anti-inflammatory properties of HDL were enhanced in these soldiers, but the antioxidant ability of HDL was not determined. Interestingly, the HDL particle size was increased with enhanced antioxidant activity in middle-aged women who had performed habitual exercise for at least one year with high intensity. The enhancement of HDL quality was accompanied by increases in the quantities of serum HDL-C and apoA-I that depended on the exercise intensity, suggesting that long-term exercise could improve the quantity and functionality of HDL. These findings can be extrapolated to women with higher BMI, since all groups had overweight subjects, especially group 2, which showed a BMI range of 18.9–30.9.
A limitation of this study was that the data on exercise frequency and intensity were obtained from self-reported questionnaires. The validation of these data for exercise intensity was intricate for distinguishing the borderline between groups 2 and 3. Another concern was the unequal distribution of menopausal women among the groups; groups 1, 2, and 3 contained 14, 5, and 11 postmenopausal women, respectively. These unequal distributions of menopausal status between groups might interfere with the interpretation of the current results. Because menopausal women displayed more atherogenic lipid and lipoprotein profiles with increased dysfunctional HDL [36], this might explain why group 2 showed more LDL aggregation in the loading position (lane 2, Figure 2A) and smaller HDL2 particle size than group 3. In a future study, more details on the in vivo functionality and anti-inflammatory properties of HDL from each participant should be investigated to observe trends in different parameters across participants. It would be useful information if we knew which parameters are more influential on HDL quality and functionality in vivo across participants.
A strength of this study is that the current report is the first report to show that HDL quality could be improved by regular and habitual exercise in middle-aged women (around their fifties) who have a declined metabolic rate and reduced physical activity. It has been reported that HDL-C in women sharply decreased between the ages of 40 and 60 years [37], and the long-term consumption of ethanol in middle-aged women induced the impairment of HDL quality, making it atherogenic [10]. Therefore, it is likely that middle-aged women who drink occasionally and are sedentary would have lower HDL-C and impaired HDL quality and functionality. However, the current study clearly showed that HDL quantity and quality can be remarkably enhanced by regular exercise in an intensity-dependent manner.
In conclusion, this study examined middle-aged women who were sedentary versus those who regularly exercised in their daily lives. The results show that higher exercise intensity is associated with an improved lipid profile, lower LDL-C, higher HDL-C, the amelioration of hepatic functions, and lower AST levels compared to those observed in participants with a sedentary lifestyle. In addition, the lipoprotein and apolipoprotein profiles were improved in the exercise groups (groups 2 and 3) compared to the sedentary group. The LDL properties and cholesterol levels were augmented, and there was less oxidation in the exercise group than in the sedentary lifestyle group. HDL quality and functionality were enhanced, exhibiting a larger particle size, a distinct particle shape, enriched cholesterol content, and elevated antioxidant activities.
4. Materials and Methods
4.1. Participants
This study was initiated to identify the characteristics of HDL and LDL regarding quantity and quality among a healthy Korean population between 20 and 70 years old through a nationwide advertisement in Korea from 2021 to 2022. Female middle-aged (35–63 years old) volunteers were recruited randomly, and we found that they could be divided into sedentary and exercise groups depending on metabolic equivalents (METs) per week. After recruiting middle-aged women volunteers for the retrospective study, they were divided into three groups: group 1 (sedentary, MET 2.5 ± 0.03), group 2 (low-intensity exercise, MET 4.5 ± 0.3), and group 3 (high-intensity exercise, MET 9.1 ± 0.4)
This study was approved by the Korea National Institute for Bioethics Policy (KoNIBP, approval number P01-202109-31-009) supported by the Ministry of Health Care and Welfare (MOHW) of Korea. All participants were of Korean ethnic origin, and they consumed a typical Korean diet, which is enriched with rice-based carbohydrates (60.8%), total fats (24.2%), and proteins (15.1%), consisting of vegetables, meat, and fish. There were no vegan or kosher diets among the participants.
The exercise frequency, duration, and intensity were estimated from a self-administered questionnaire inquiring about the frequency, time, and intensity of exercise per week during the 1 year prior. Therefore, we assume that participants in groups 2 and 3 had been exercising for at least 1 year or more. METs were calculated using a shorthand method for estimating energy expenditure during physical activity. In accordance with the previous study, MET assigns the intensity values of specific activities [23]. The MET scores were calculated based on the survey results of the participants and were classified as follows: Light < 3.0 METs; Moderate 3.0~6.0 METs; Vigorous > 6.0 METs.
4.2. Anthropometric Analysis
Blood pressure was measured using an Omron HBP-9020 (Kyoto, Japan). Height, body weight, body mass index (BMI), body water, total body fat (%), total body fat mass (kg), and visceral fat mass (VFM) (kg) were measured individually using an X-scan plus II body composition analyzer (Jawon Medical, Gyeongsan, Republic of Korea). Handgrip strength (HS) was measured in the standing position with the arms straight down to the sides. The maximum grip strengths of the right and left hands were measured three times alternatively using a digital hand dynamometer (digital grip strength dynamometer, T.K.K 5401; Takei Scientific Instruments Co., Ltd., Tokyo, Japan). After the handgrip strengths of both hands were measured, a 60 s rest interval was allowed. The maximum grip strength of the dominant hand was used for the analysis [38].
4.3. Blood Analysis
After fasting overnight, blood was collected using a vacutainer (BD Bio Sciences, Franklin Lakes, NJ, USA) without adding an anticoagulant. The serum parameters in Table 2 were determined with an automatic analyzer using CobasC502 (Roche, Germany) at a commercial diagnostic service via SCL healthcare (Seoul, Republic of Korea).
4.4. Isolation of Lipoproteins
Very low-density lipoprotein (VLDL, d < 1.019 g/mL), LDL (1.019 < d < 1.063), HDL2 (1.063 < d < 1.125), and HDL3 (1.125 < d < 1.225) were isolated from individual patient and control sera via sequential ultracentrifugation [14,15], with the density adjusted by adding NaCl and NaBr in accordance with standard protocols [39]. The samples were centrifuged for 24 h at 10 °C and 100,000× g using a Himac NX (Hitachi, Tokyo, Japan) at the Raydel Research Institute (Daegu, Republic of Korea).
For each of the lipoproteins purified individually, the total cholesterol (TC) and TG measurements were obtained using commercially available kits (cholesterol, T-CHO, and TG, Cleantech TS-S; Wako Pure Chemical, Osaka, Japan). The protein concentrations of the lipoproteins were determined using a Lowry protein assay, as modified by Markwell et al. [40], using the Bradford assay reagent (Bio-Rad, Seoul, Republic of Korea) with bovine serum albumin (BSA) as a standard.
4.5. LDL Oxidation and Quantification
The degree of oxidation of individual LDL was assessed by measuring the concentration of oxidized species in LDL according to the thiobarbituric acid-reactive substances (TBARS) method using malondialdehyde (MDA) as a standard [41].
Oxidized LDL (oxLDL) was produced by incubating the LDL fraction with CuSO4 (final concentration, 10 μM) for 4 h at 37 °C. oxLDL was then filtered (0.2 μm) and analyzed using a thiobarbituric acid-reactive substances (TBARS) assay to determine the extent of oxidation, as described elsewhere [41].
4.6. Paraoxonase Assay
The paraoxonase-1 (PON-1) activity in HDL2 and HDL3 toward paraoxon was determined by evaluating the hydrolysis of paraoxon into p-nitrophenol and diethylphosphate, which is catalyzed by the enzyme [42]. The PON-1 activity was then determined by measuring the initial velocity of p-nitrophenol production at 37 °C, as determined by measuring the absorbance at 415 nm (microplate reader, Bio-Rad model 680; Bio-Rad, Hercules, CA, USA), as described previously [42,43].
4.7. Ferric-Ion-Reducing Ability Assay
The ferric-ion-reducing ability (FRA) was determined using the method reported by Benzie and Strain [44]. Briefly, the FRA reagent was freshly prepared by mixing 20 mL of 0.2 M acetate buffer (pH 3.6), 2.5 mL of 10 mM 2,4,6-tripyridyl-S-triazine (Fluka Chemicals, Buchs, Switzerland), and 2.5 mL of 20 mM FeCl3∙6H2O. The antioxidant activities of HDL (2 mg/mL) were estimated by measuring the increase in absorbance induced by the ferrous ions generated. The freshly prepared FRA reagent (300 μL) was mixed with OSO and SO as an antioxidant source. FRA was determined by measuring the absorbance at 593 nm every 2 min over a 60 min period at 25 °C using a UV-2600i spectrophotometer.
4.8. Electromobility of Lipoproteins
The electromobility of the participants’ samples was compared by evaluating the migration of each lipoprotein (LDL, HDL2, and HDL3) by agarose electrophoresis [45]. The relative electrophoretic mobility depends on the intact charge and three-dimensional structure of HDL. Therefore, for each group, agarose gel electrophoresis with HDL2 and HDL3 was carried out in the nondenatured state [46]. The gels were then dried and stained with 0.125% Coomassie Brilliant Blue, after which the relative band intensities were compared by band scanning using Gel Doc® XR (Bio-Rad) with Quantity One software (version 4.5.2).
4.9. Glycation of HDL
Purified HDL (2 mg/mL) was incubated with 250 mM D-fructose in 200 mM potassium phosphate/0.02% sodium azide buffer (pH 7.4) for up to 72 h in air containing 5% CO2 at 37 °C. Fructose can induce a remarkably greater extent of apoA-I glycation than glucose, according to a previous report [47]. The extent of glycation was determined by measuring the fluorometric intensity at 370 nm (excitation) and 440 nm (emission), as described previously [48], using an LS55 spectrofluorometer (PerkinElmer) and a 1 cm path-length suprasil quartz cuvette (Fisher Scientific, Pittsburg, PA, USA).
4.10. Electron Microscopy
Transmission electron microscopy (TEM, Hitachi H-7800; Ibaraki, Japan) was performed at the Raydel Research Institute (Daegu, Republic of Korea) at an acceleration voltage of 80 kV. HDL was negatively stained with 1% sodium phosphotungstate (PTA; pH 7.4) with a final apolipoprotein concentration of 0.3 mg/mL in TBS. A volume of 5 μL of the HDL suspension was blotted with filter paper and replaced immediately with a 5 μL droplet of 1% PTA. After a few seconds, the stained HDL fraction was blotted onto a Formvar carbon-coated 300 mesh copper grid and air-dried. The shape and size of the HDL particles were determined by TEM at 40,000× magnification, according to a previous report [43,46].
4.11. Data Analysis
All analyses in Table 1, Table 2, Table 3 and Table 4 were normalized using a homogeneity test of variances through Levene’s statistics. Nonparametric statistics were performed using a Kruskal–Wallis test if not normalized. All values are expressed as the median (25th; 75th percentiles) in Table 1 and Table 2 for continuous variables for the middle-aged women groups. Data in Table 3 are expressed as the mean ± SEM (standard error of the mean). Multiple groups were compared using a one-way analysis of variance (ANOVA), and the results are reported in Table 1, Table 2 and Table 3. All tests were two-tailed, and the statistical significance was defined at p < 0.05.
The anthropometric profiles of the middle-aged women groups depend on exercise time and intensity (Table 1). Anthropometric profiles, such as alcohol intake amount, height, weight, heart rate, SBP, DBP, muscle mass, fat mass, and body water, were compared using ANOVA. Age, visceral fat mass, HS, and BMI were compared using a Kruskal–Wallis test.
The blood lipid and inflammatory parameters of the middle-aged women groups depend on the exercise time and intensity (Table 2). Blood lipid and inflammatory parameters, such as HDL-C, HDL-C/TC, LDL-C, LDL-C/HDL-C, apo-B, glucose, hs-CRP, and AST, were compared using ANOVA. TC, TG, TG/HDL-C, apoA-I, apo-B/apoA-I, ALT, and γ-GTP were compared using a Kruskal–Wallis test.
The characteristics of lipoproteins from the middle-aged women groups depend on the exercise time and intensity (Table 3). The characteristics of the lipoproteins, such as LDL-MDA, LDL-TG, HDL2-PON, HDL3-TC, and HDL3-PON, were compared using ANOVA. The LDL size, LDL glycation, LDL-TC, HDL2 size, HDL2 glycation, HDL2-TC, HDL2-TG, HDL3 size, and HDL3-TG were compared using a Kruskal–Wallis test.
A Bonferroni test was used as a post hoc analysis to determine the significance of the differences in continuous variables to identify differences between groups. Spearman correlation analysis was conducted to find a positive or negative association (Table 4). Statistical analyses were carried out using the SPSS statistical package version 28.0 (SPSS Inc., Chicago, IL, USA), incorporating sampling weights and adjusting for the complex survey design.
Author Contributions
Conceptualization, K.-H.C.; methodology, D.-J.K., H.-S.N., M.-H.P. and S.Z.; writing—original draft preparation, K.-H.C.; supervision, K.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The protocol for human blood donation was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Korea National Institute for Bioethics Policy (KoNIBP, approval number P01-202109-31-009) supported by the Ministry of Health Care and Welfare (MOHW) of Korea.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mainous, A.G., III; Tanner, R.J.; Rahmanian, K.P.; Jo, A.; Carek, P.J. Effect of sedentary lifestyle on cardiovascular disease risk among healthy adults with body mass indexes 18.5 to 29.9 kg/m2. Am. J. Cardiol. 2019, 123, 764–768. [Google Scholar] [CrossRef]
- Paffenbarger, R.S., Jr.; Hyde, R.T.; Wing, A.L.; Lee, I.-M.; Jung, D.L.; Kampert, J.B. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N. Engl. J. Med. 1993, 328, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Ozato, N.; Yamaguchi, T.; Sudo, M.; Yamashiro, Y.; Mori, K.; Ishida, M.; Katsuragi, Y.; Sasai, H.; Yasukawa, T. Association of sedentary behaviour and physical activity with cardiometabolic health in Japanese adults. Sci. Rep. 2022, 12, 2262. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Hein, H.O.; Suadicani, P.; Gyntelberg, F. Relation of high TG–low HDL cholesterol and LDL cholesterol to the incidence of ischemic heart disease: An 8-year follow-up in the Copenhagen male study. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Paffenbarger, R.S., Jr.; Hyde, R.; Wing, A.L.; Hsieh, C.-C. Physical activity, all-cause mortality, and longevity of college alumni. N. Engl. J. Med. 1986, 314, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.N.; Kohl, H.W.; Paffenbarger, R.S.; Clark, D.G.; Cooper, K.H.; Gibbons, L.W. Physical fitness and all-cause mortality: A prospective study of healthy men and women. JAMA 1989, 262, 2395–2401. [Google Scholar] [CrossRef]
- Ruiz-Ramie, J.J.; Barber, J.L.; Sarzynski, M.A. Effects of exercise on HDL functionality. Curr. Opin. Lipidol. 2019, 30, 16. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Kim, J.; Choi, I.; Kim, J.-R.; Cho, K.-H. Breast Milk from frequent trans fatty acid consumers shows high triglyceride and glucose levels, but low cholesterol and apolipoprotein AI levels, with resulting impaired in vitro zebrafish embryo growth and survival. Breastfeed. Med. 2016, 11, 239–246. [Google Scholar] [CrossRef]
- Cho, K.-H.; Nam, H.-S.; Kang, D.-J.; Park, M.-H.; Kim, J.-H. Long-Term Alcohol Consumption Caused a Significant Decrease in Serum High-Density Lipoprotein (HDL)-Cholesterol and Apolipoprotein AI with the Atherogenic Changes of HDL in Middle-Aged Korean Women. Int. J. Mol. Sci. 2022, 23, 8623. [Google Scholar] [CrossRef]
- Park, K.H.; Shin, D.G.; Cho, K.H. Dysfunctional lipoproteins from young smokers exacerbate cellular senescence and atherogenesis with smaller particle size and severe oxidation and glycation. Toxicol. Sci. 2014, 140, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D.; Haas, M.J.; Wehmeier, K.R.; Wong, N.C. Obesity-related changes in high-density lipoprotein metabolism. Obesity 2008, 16, 1152–1160. [Google Scholar] [CrossRef]
- Cho, K.-H. The Current Status of Research on High-Density Lipoproteins (HDL): A Paradigm Shift from HDL Quantity to HDL Quality and HDL Functionality. Int. J. Mol. Sci. 2022, 23, 3967. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Shin, D.G.; Kim, J.R.; Cho, K.H. Senescence-related truncation and multimerization of apolipoprotein A-I in high-density lipoprotein with an elevated level of advanced glycated end products and cholesteryl ester transfer activity. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 600–610. [Google Scholar] [CrossRef]
- Park, K.H.; Cho, K.H. High-density lipoprotein (HDL) from elderly and reconstituted HDL containing glycated apolipoproteins A-I share proatherosclerotic and prosenescent properties with increased cholesterol influx. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-H.; Park, J.-E.; Choi, I.-H.; Cho, K.-H. Enhanced functional and structural properties of high-density lipoproteins from runners and wrestlers compared to throwers and lifters. BMB Rep. 2009, 42, 605–610. [Google Scholar] [CrossRef]
- Franceschini, G.; Calabresi, L.; Maderna, P.; Galli, C.; Gianfranceschi, G.; Sirtori, C.R. ω-3 fatty acids selectively raise high-density lipoprotein 2 levels in healthy volunteers. Metabolism 1991, 40, 1283–1286. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, S.-M.; Kim, S.-J.; Lee, E.-Y.; Kim, J.-R.; Cho, K.-H. Consumption of policosanol enhances HDL functionality via CETP inhibition and reduces blood pressure and visceral fat in young and middle-aged subjects. Int. J. Mol. Med. 2017, 39, 889–899. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, S.-J.; Yadav, D.; Kim, J.-Y.; Kim, J.-R. Consumption of cuban policosanol improves blood pressure and lipid profile via enhancement of HDL functionality in healthy women subjects: Randomized, double-blinded, and placebo-controlled study. Oxidative Med. Cell. Longev. 2018, 2018, 4809525. [Google Scholar] [CrossRef]
- Kim, S.-J.; Yadav, D.; Park, H.-J.; Kim, J.-R.; Cho, K.-H. Long-term consumption of cuban policosanol lowers central and brachial blood pressure and improves lipid profile with enhancement of lipoprotein properties in healthy korean participants. Front. Physiol. 2018, 9, 412. [Google Scholar] [CrossRef]
- Park, H.-J.; Yadav, D.; Jeong, D.-J.; Kim, S.-J.; Bae, M.-A.; Kim, J.-R.; Cho, K.-H. Short-term consumption of Cuban policosanol lowers aortic and peripheral blood pressure and ameliorates serum lipid parameters in healthy Korean participants: Randomized, double-blinded, and placebo-controlled study. Int. J. Environ. Res. Public Health 2019, 16, 809. [Google Scholar] [CrossRef]
- Zhao, S.; Zhong, J.; Sun, C.; Zhang, J. Effects of aerobic exercise on TC, HDL-C, LDL-C and TG in patients with hyperlipidemia: A protocol of systematic review and meta-analysis. Medicine 2021, 100, e25103. [Google Scholar] [CrossRef]
- Haskell, W.L.; Lee, I.-M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1081. [Google Scholar] [CrossRef] [PubMed]
- Durstine, J.L.; Grandjean, P.W.; Davis, P.G.; Ferguson, M.A.; Alderson, N.L.; DuBose, K.D. Blood lipid and lipoprotein adaptations to exercise. Sport. Med. 2001, 31, 1033–1062. [Google Scholar] [CrossRef] [PubMed]
- Ben Ounis, O.; Elloumi, M.; Makni, E.; Zouhal, H.; Amri, M.; Tabka, Z.; Lac, G. Exercise improves the ApoB/ApoA-I ratio, a marker of the metabolic syndrome in obese children. Acta Paediatr. 2010, 99, 1679–1685. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M. Comparison of effects of diet versus exercise weight loss regimens on LDL and HDL particle size in obese adults. Lipids Health Dis. 2011, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Sarzynski, M.A.; Burton, J.; Rankinen, T.; Blair, S.N.; Church, T.S.; Després, J.-P.; Hagberg, J.M.; Landers-Ramos, R.; Leon, A.S.; Mikus, C.R. The effects of exercise on the lipoprotein subclass profile: A meta-analysis of 10 interventions. Atherosclerosis 2015, 243, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Liu, Y.; Lebaka, V.R.; Mohammed, A.; Ye, W.; Chen, B.; Korivi, M. Effect of Exercise Training on Serum Transaminases in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 1260. [Google Scholar] [CrossRef]
- Senior, J. Alanine aminotransferase: A clinical and regulatory tool for detecting liver injury–past, present, and future. Clin. Pharmacol. Ther. 2012, 92, 332–339. [Google Scholar] [CrossRef]
- Chalasani, N. Clinical Meaning of Elevated Aminotransferase Activity; Hepatotoxicity Special Interest Group Meeting: Silver Springs, MD, USA, 2008. [Google Scholar]
- Kido, T.; Kondo, K.; Kurata, H.; Fujiwara, Y.; Urata, T.; Itakura, H.; Yokoyama, S. ApoA-I/A-II-HDL positively associates with apoB-lipoproteins as a potential atherogenic indicator. Lipids Health Dis. 2017, 16, 225. [Google Scholar] [CrossRef]
- Kuchta, A.; Strzelecki, A.; Ćwiklińska, A.; Gruchała, M.; Zdrojewski, Z.; Kortas-Stempak, B.; Wieczorek, E.; Gliwińska, A.; Dąbkowski, K.; Jankowski, M. HDL subpopulations containing apoA-I without apoA-II (LpA-I) in patients with angiographically proven coronary artery disease. J. Cardiol. 2017, 69, 523–528. [Google Scholar] [CrossRef]
- Kido, T.; Kurata, H.; Kondo, K.; Itakura, H.; Okazaki, M.; Urata, T.; Yokoyama, S. Bioinformatic analysis of plasma apolipoproteins AI and A-II revealed unique features of AI/A-II HDL particles in human plasma. Sci. Rep. 2016, 6, 31532. [Google Scholar] [CrossRef] [PubMed]
- LeMura, L.M.; von Duvillard, S.P.; Andreacci, J.; Klebez, J.M.; Chelland, S.A.; Russo, J. Lipid and lipoprotein profiles, cardiovascular fitness, body composition, and diet during and after resistance, aerobic and combination training in young women. Eur. J. Appl. Physiol. 2000, 82, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Stanton, K.M.; Kienzle, V.; Dinnes, D.L.M.; Kotchetkov, I.; Jessup, W.; Kritharides, L.; Celermajer, D.S.; Rye, K.A. Moderate- and High-Intensity Exercise Improves Lipoprotein Profile and Cholesterol Efflux Capacity in Healthy Young Men. J. Am. Heart Assoc. 2022, 11, e023386. [Google Scholar] [CrossRef]
- Fonseca, M.I.H.; da Silva, I.T.; Ferreira, S.R.G. Impact of menopause and diabetes on atherogenic lipid profile: Is it worth to analyse lipoprotein subfractions to assess cardiovascular risk in women? Diabetol. Metab. Syndr. 2017, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Park, H.J.; Kim, S.J.; Kim, J.R. Decrease in HDL-C is Associated with Age and Household Income in Adults from the Korean National Health and Nutrition Examination Survey 2017: Correlation Analysis of Low HDL-C and Poverty. Int. J. Environ. Res. Public Health 2019, 16, 3329. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.; Tolbert, N. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Garin, M.-C.B.; Moren, X.; James, R.W. Paraoxonase-1 and serum concentrations of HDL-cholesterol and apoA-I. J. Lipid Res. 2006, 47, 515–520. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-R.; Lee, I.-C.; Kwon, H.-J. Native high-density lipoproteins (HDL) with higher paraoxonase exerts a potent antiviral effect against SARS-CoV-2 (COVID-19), while glycated HDL lost the antiviral activity. Antioxidants 2021, 10, 209. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 15–27. [Google Scholar]
- Noble, R.P. Electrophoretic separation of plasma lipoproteins in agarose gel. J. Lipid Res. 1968, 9, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kang, D.-J.; Nam, H.-S.; Kim, J.-H.; Kim, S.-Y.; Lee, J.-O.; Kim, B.-J. Ozonated sunflower oil exerted protective effect for embryo and cell survival via potent reduction power and antioxidant activity in HDL with strong antimicrobial activity. Antioxidants 2021, 10, 1651. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-H.; Kim, J.-Y.; Choi, I.; Kim, J.-R.; Won, K.C.; Cho, K.-H. Fructated apolipoprotein AI exacerbates cellular senescence in human umbilical vein endothelial cells accompanied by impaired insulin secretion activity and embryo toxicity. Biochem. Cell Biol. 2016, 94, 337–345. [Google Scholar] [CrossRef] [PubMed]
- McPherson, J.D.; Shilton, B.H.; Walton, D.J. Role of fructose in glycation and cross-linking of proteins. Biochemistry 1988, 27, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).