Age-Related microRNA Overexpression in Lafora Disease Male Mice Provides Links between Neuroinflammation and Oxidative Stress
Abstract
1. Introduction
2. Results and Discussion
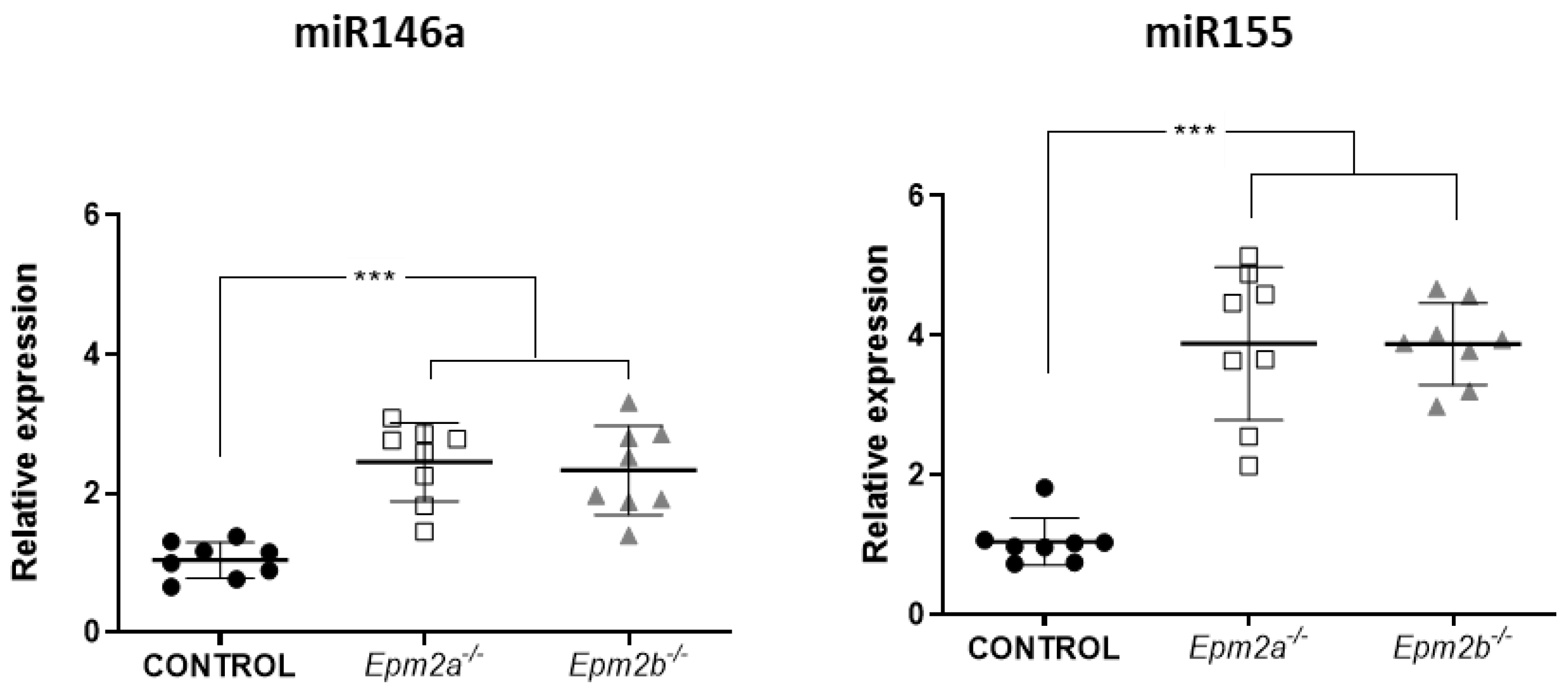
2.1. Differential microRNA Expression in the Brain of 16-Month-Old Epm2a−/− and Epm2b−/− Mice as Compared to Control Mice
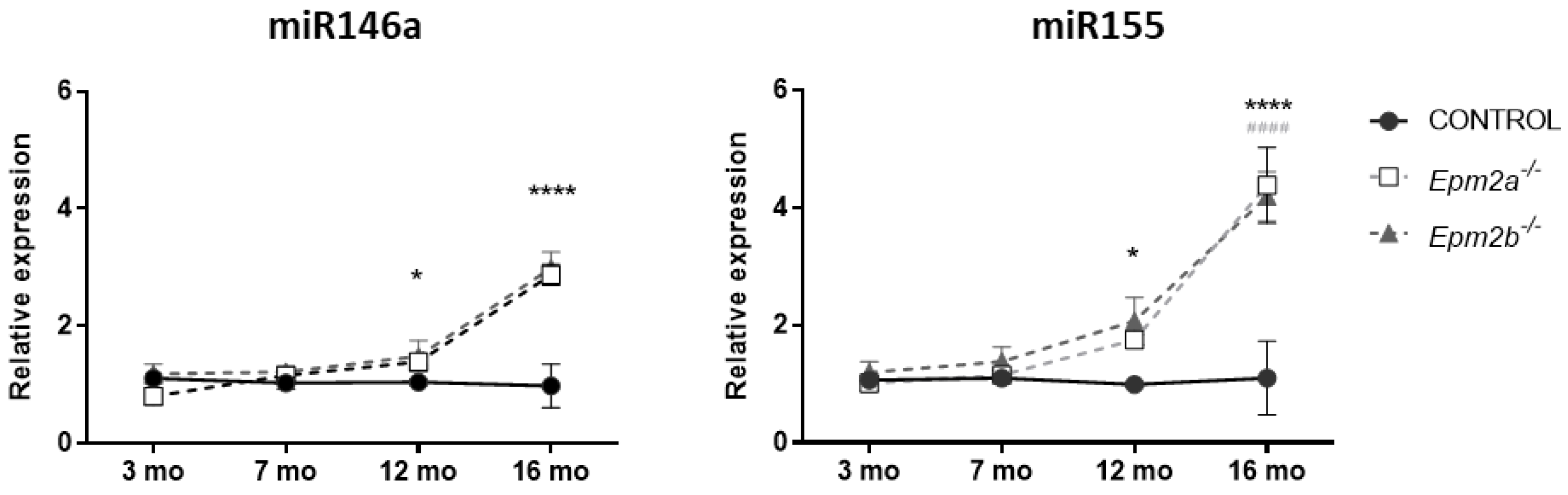
2.2. MicroRNAs miR-155 and miR-146a Are Overexpressed in an Age-Dependent Manner
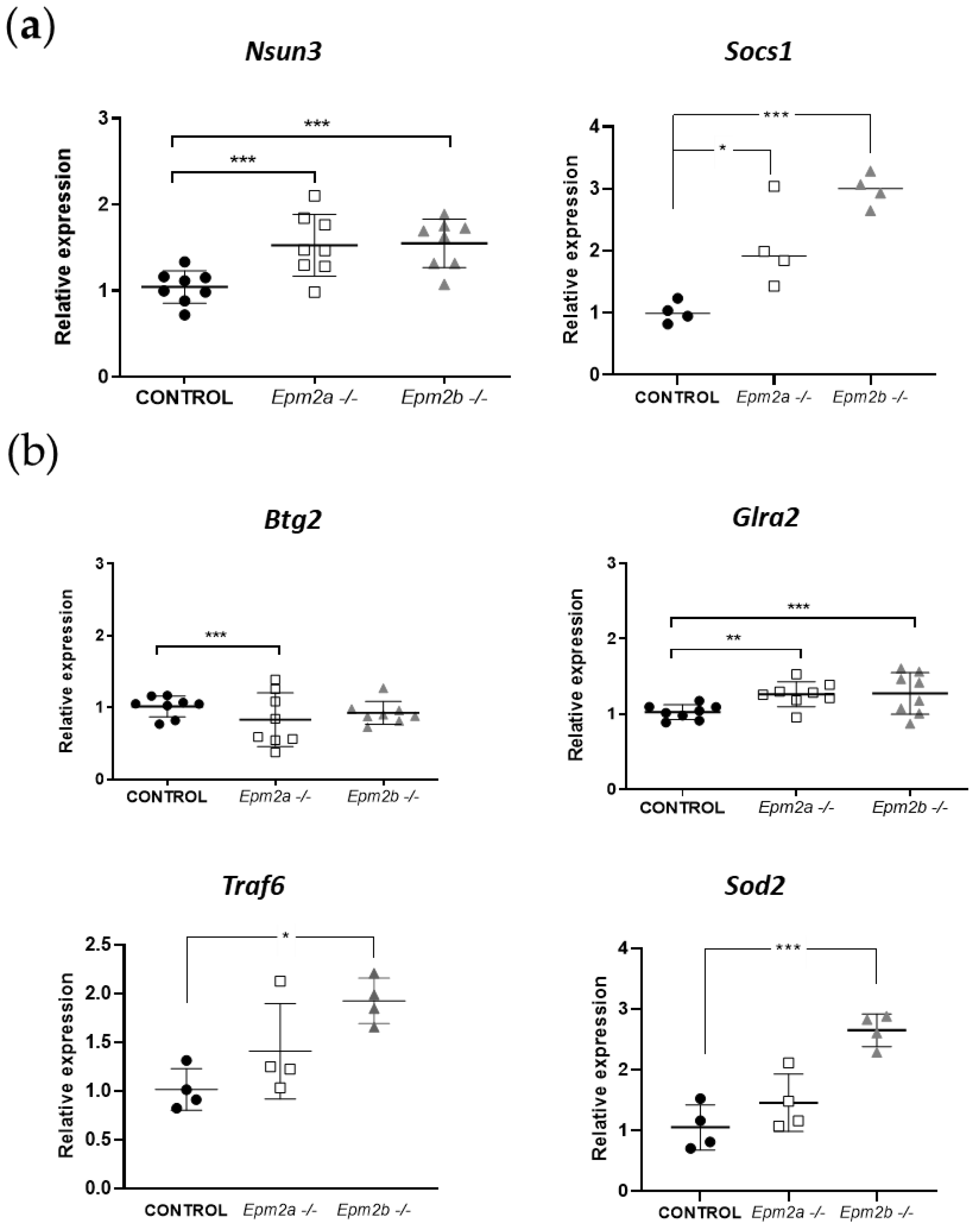
2.3. Analysis of the Expression of Putative Gene Targets of miR-146a and miR-155 in Brain Extracts from Epm2a−/− and Epm2b−/− Mice of 16 Months of Age
3. Materials and Methods
3.1. Animal Care, Mice, and Husbandry
3.2. Whole RNA Extraction from Mouse Brain
3.3. Small RNA-Seq and Data Analysis
3.4. RT-qPCR Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gentry, M.S.; Worby, C.A.; Dixon, J.E. Insights into Lafora Disease: Malin Is an E3 Ubiquitin Ligase That Ubiquitinates and Promotes the Degradation of Laforin. Proc. Natl. Acad. Sci. USA 2005, 102, 8501–8506. [Google Scholar] [CrossRef] [PubMed]
- Worby, C.A.; Gentry, M.S.; Dixon, J.E. Laforin, a Dual Specificity Phosphatase That Dephosphorylates Complex Carbohydrates. J. Biol. Chem. 2006, 281, 30412–30418. [Google Scholar] [CrossRef] [PubMed]
- Pondrelli, F.; Muccioli, L.; Licchetta, L.; Mostacci, B.; Zenesini, C.; Tinuper, P.; Vignatelli, L.; Bisulli, F. Natural History of Lafora Disease: A Prognostic Systematic Review and Individual Participant Data Meta-Analysis. Orphanet J. Rare Dis. 2021, 16, 362. [Google Scholar] [CrossRef] [PubMed]
- Susumu, Y.; Hiroshi, N.; Tasuo, N. Biochemical Studies on Tissues from a Patient with Lafora Disease. Clin. Chim. Acta 1975, 62, 415–423. [Google Scholar] [CrossRef]
- Lafora, G.R.; Glueck, B. Beitrag Zur Histopathologie Der Myoklonischen Epilepsie. Zeitschrift für Die Gesamte Neurologie und Psychiatrie 1911, 6, 1–14. [Google Scholar] [CrossRef]
- Tagliabracci, V.S.; Turnbull, J.; Wang, W.; Girard, J.M.; Zhao, X.; Skurat, A.V.; Delgado-Escueta, A.V.; Minassian, B.A.; Depaoli-Roach, A.A.; Roach, P.J. Laforin Is a Glycogen Phosphatase, Deficiency of Which Leads to Elevated Phosphorylation of Glycogen in Vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 19262–19266. [Google Scholar] [CrossRef]
- Romá-Mateo, C.; Sanz, P.; Gentry, M.S. Deciphering the Role of Malin in the Lafora Progressive Myoclonus Epilepsy. IUBMB Life 2012, 64, 801–808. [Google Scholar] [CrossRef]
- Romá-Mateo, C.; Aguado, C.; García-Giménez, J.L.; Ibáñez-Cabellos, J.S.; Seco-Cervera, M.; Pallardó, F.V.; Knecht, E.; Sanz, P. Increased Oxidative Stress and Impaired Antioxidant Response in Lafora Disease. Mol. Neurobiol. 2015, 51, 932–946. [Google Scholar] [CrossRef]
- Muñoz-Ballester, C.; Berthier, A.; Viana, R.; Sanz, P. Homeostasis of the Astrocytic Glutamate Transporter GLT-1 Is Altered in Mouse Models of Lafora Disease. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 1074–1083. [Google Scholar] [CrossRef]
- Lahuerta, M.; Gonzalez, D.; Aguado, C.; Fathinajafabadi, A.; García-Giménez, J.L.; Moreno-Estellés, M.; Romá-Mateo, C.; Knecht, E.; Pallardó, F.V.; Sanz, P. Reactive Glia-Derived Neuroinflammation: A Novel Hallmark in Lafora Progressive Myoclonus Epilepsy That Progresses with Age. Mol. Neurobiol. 2020, 57, 1607–1621. [Google Scholar] [CrossRef]
- López-González, I.; Viana, R.; Sanz, P.; Ferrer, I. Inflammation in Lafora Disease: Evolution with Disease Progression in Laforin and Malin Knock-out Mouse Models. Mol. Neurobiol. 2017, 54, 3119–3130. [Google Scholar] [CrossRef] [PubMed]
- Berthier, A.; Payá, M.; García-Cabrero, A.M.; Ballester, M.I.; Heredia, M.; Serratosa, J.M.; Sánchez, M.P.; Sanz, P. Pharmacological Interventions to Ameliorate Neuropathological Symptoms in a Mouse Model of Lafora Disease. Mol. Neurobiol. 2015, 53, 1296–1309. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P.K.; Bhadauriya, P.; Ganesh, S. Lafora Disease E3 Ubiquitin Ligase Malin Is Recruited to the Processing Bodies and Regulates the MicroRNA-Mediated Gene Silencing Process via the Decapping Enzyme Dcp1a. RNA Biol. 2012, 9, 1440–1449. [Google Scholar] [CrossRef]
- Readhead, B.; Haure-Mirande, J.-V.; Mastroeni, D.; Audrain, M.; Fanutza, T.; Kim, S.H.; Blitzer, R.D.; Gandy, S.; Dudley, J.T.; Ehrlich, M.E. MiR155 Regulation of Behavior, Neuropathology, and Cortical Transcriptomics in Alzheimer’s Disease. Acta Neuropathol. 2020, 140, 295–315. [Google Scholar] [CrossRef]
- Brennan, G.P.; Henshall, D.C. MicroRNAs as Regulators of Brain Function and Targets for Treatment of Epilepsy. Nat. Rev. Neurol. 2020, 16, 506–519. [Google Scholar] [CrossRef]
- Testa, U.; Pelosi, E.; Castelli, G.; Labbaye, C. MiR-146 and MiR-155: Two Key Modulators of Immune Response and Tumor Development. Noncoding RNA 2017, 3, 22. [Google Scholar] [CrossRef]
- Sun, X.; Song, M.; Song, H.; Wang, Y.; Luo, M.; Yin, L. MiR-155 Mediates Inflammatory Injury of Hippocampal Neuronal Cells via the Activation of Microglia. Mol. Med. Rep. 2019, 19, 2627–2635. [Google Scholar] [CrossRef]
- Paramasivam, A.; Meena, A.K.; Venkatapathi, C.; Pitceathly, R.D.S.; Thangaraj, K. Novel Biallelic NSUN3 Variants Cause Early-Onset Mitochondrial Encephalomyopathy and Seizures. J. Mol. Neurosci. 2020, 70, 1962–1965. [Google Scholar] [CrossRef]
- Chen, X.; Wilson, K.A.; Schaefer, N.; de Hayr, L.; Windsor, M.; Scalais, E.; van Rijckevorsel, G.; Stouffs, K.; Villmann, C.; O’Mara, M.L.; et al. Loss, Gain and Altered Function of GlyR A2 Subunit Mutations in Neurodevelopmental Disorders. Front. Mol. Neurosci. 2022, 15, 886729. [Google Scholar] [CrossRef]
- Yildirim, F.; Foddis, M.; Blumenau, S.; Müller, S.; Kajetan, B.; Holtgrewe, M.; Kola, V.; Beule, D.; Sassi, C. Shared and Oppositely Regulated Transcriptomic Signatures in Huntington’s Disease and Brain Ischemia Confirm Known and Unveil Novel Potential Neuroprotective Genes. Neurobiol. Aging 2021, 104, 122.e1–122.e17. [Google Scholar] [CrossRef]
- Semmler, S.; Gagné, M.; Garg, P.; Pickles, S.R.; Baudouin, C.; Hamon-Keromen, E.; Destroismaisons, L.; Khalfallah, Y.; Chaineau, M.; Caron, E.; et al. TNF Receptor–Associated Factor 6 Interacts with ALS-Linked Misfolded Superoxide Dismutase 1 and Promotes Aggregation. J. Biol. Chem. 2020, 295, 3808–3825. [Google Scholar] [CrossRef] [PubMed]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An Important Regulator of Neuroinflammation. Int. J. Mol. Sci. 2021, 23, 90. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, J.; Li, W.; Wang, Y.; Wu, F.; Xi, Y.; Zhang, L.; Ding, C.; Luo, H.; Li, Y.; et al. MicroRNAs Activate Gene Transcription Epigenetically as an Enhancer Trigger. RNA Biol. 2017, 14, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Tong, Y.; Steitz, J.A. Switching from Repression to Activation: MicroRNAs Can Up-Regulate Translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.; Guo, J.; Cui, L.; Yang, L.; Li, Y.; Mou, Y.; Jia, C.; Zhang, L.; Song, X. MiR-146a Enhances Regulatory T-cell Differentiation and Function in Allergic Rhinitis by Targeting STAT5b. Allergy 2022, 77, 550–558. [Google Scholar] [CrossRef]
- Chen, J.; Chen, T.; Zhou, J.; Zhao, X.; Sheng, Q.; Lv, Z. MiR-146a-5p Mimic Inhibits NLRP3 Inflammasome Downstream Inflammatory Factors and CLIC4 in Neonatal Necrotizing Enterocolitis. Front. Cell Dev. Biol. 2021, 8, 594143. [Google Scholar] [CrossRef]
- Tong, J.; Duan, Z.; Zeng, R.; Du, L.; Xu, S.; Wang, L.; Liu, Y.; Chen, Q.; Chen, X.; Li, M. MiR-146a Negatively Regulates Aspergillus Fumigatus-Induced TNF-α and IL-6 Secretion in THP-1 Macrophages. Mycopathologia 2021, 186, 341–354. [Google Scholar] [CrossRef]
- Chen, L.; Ming, X.; Li, W.; Bi, M.; Yan, B.; Wang, X.; Yang, P.; Yang, B. The MicroRNA-155 Mediates Hepatitis B Virus Replication by Reinforcing SOCS1 Signalling–Induced Autophagy. Cell Biochem. Funct. 2020, 38, 436–442. [Google Scholar] [CrossRef]
- Babuta, M.; Furi, I.; Bala, S.; Bukong, T.N.; Lowe, P.; Catalano, D.; Calenda, C.; Kodys, K.; Szabo, G. Dysregulated Autophagy and Lysosome Function Are Linked to Exosome Production by Micro-RNA 155 in Alcoholic Liver Disease. Hepatology 2019, 70, 2123–2141. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Mori, T.; Houri, K.; Onodera, Y.; Takehara, T.; Shigi, K.; Nakao, S.; Teramura, T.; Fukuda, K. MiR-155 Inhibits Mitophagy through Suppression of BAG5, a Partner Protein of PINK1. Biochem. Biophys. Res. Commun. 2020, 523, 707–712. [Google Scholar] [CrossRef]
- Criado, O.; Aguado, C.; Gayarre, J.; Duran-Trio, L.; Garcia-Cabrero, A.M.; Vernia, S.; San Millan, B.; Heredia, M.; Romá-Mateo, C.; Mouron, S.; et al. Lafora Bodies and Neurological Defects in Malin-Deficient Mice Correlate with Impaired Autophagy. Hum. Mol. Genet. 2012, 21, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Knecht, E.; Aguado, C.; Sarkar, S.; Korolchuk, V.I.; Criado-Garcia, O.; Vernia, S.; Boya, P.; Sanz, P.; Rodriguez de Cordoba, S.; Rubinsztein, D.C. Impaired Autophagy in Lafora Disease. Autophagy 2010, 6, 991–993. [Google Scholar] [CrossRef]
- Aguado, C.; Bovolenta, P.; Criado-García, O.; de Córdoba, S.R.; Dominguez, M.; Duran-Trio, L.; Garcia-Cabrero, A.M.; Gayarre, J.; Heredia, M.; Juana-López, L.; et al. Malin Knockout Mice Support a Primary Role of Autophagy in the Pathogenesis of Lafora Disease. Autophagy 2012, 8, 701–703. [Google Scholar] [CrossRef][Green Version]
- Lahuerta, M.; Aguado, C.; Sánchez-Martín, P.; Sanz, P.; Knecht, E. Degradation of Altered Mitochondria by Autophagy Is Impaired in Lafora Disease. FEBS J. 2018, 285, 2071–2090. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.K.; Roncon, P.; Lukasiuk, K.; Gorter, J.A.; Aronica, E.; Pitkänen, A.; Petretto, E.; Johnson, M.R.; Simonato, M. Meta-Analysis of MicroRNAs Dysregulated in the Hippocampal Dentate Gyrus of Animal Models of Epilepsy. eNeuro 2017, 4, ENEURO.0152-17.2017. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.; Peariso, K.; Gross, C. MicroRNA-Induced Silencing in Epilepsy: Opportunities and Challenges for Clinical Application. Dev. Dyn. 2018, 247, 94–110. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, J.; Tao, H.; Cai, Y.; Huang, L.; Zhou, H.; Chen, Y.; Cui, L.; Zhong, W.; Li, K. Intranasal Delivery of MiR-155-5p Antagomir Alleviates Acute Seizures Likely by Inhibiting Hippocampal Inflammation. Neuropsychiatr Dis. Treat 2020, 16, 1295–1307. [Google Scholar] [CrossRef]
| Epm2a−/−vs. Control | Log2FC/FC | p-value | FDR |
| mmu-miR-155-5p | 3.81 | 1.366 × 10−7 | 8.933 × 10−5 |
| mmu-miR-146a-5p | 2.17 | 8.018 × 10−7 | 2.622 × 10−4 |
| Epm2b−/−vs. control | Log2FC/FC | p-value | FDR |
| mmu-miR-155-5p | 3.41 | 5.194 × 10−7 | 3.397 × 10−4 |
| mmu-miR-146a-5p | 2.13 | 1.138 × 10−6 | 3.720 × 10−4 |
| mmu-miR-10b-5p | 0.42 | 1.933 × 10−4 | 4.213 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romá-Mateo, C.; Lorente-Pozo, S.; Márquez-Thibaut, L.; Moreno-Estellés, M.; Garcés, C.; González, D.; Lahuerta, M.; Aguado, C.; García-Giménez, J.L.; Sanz, P.; et al. Age-Related microRNA Overexpression in Lafora Disease Male Mice Provides Links between Neuroinflammation and Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 1089. https://doi.org/10.3390/ijms24021089
Romá-Mateo C, Lorente-Pozo S, Márquez-Thibaut L, Moreno-Estellés M, Garcés C, González D, Lahuerta M, Aguado C, García-Giménez JL, Sanz P, et al. Age-Related microRNA Overexpression in Lafora Disease Male Mice Provides Links between Neuroinflammation and Oxidative Stress. International Journal of Molecular Sciences. 2023; 24(2):1089. https://doi.org/10.3390/ijms24021089
Chicago/Turabian StyleRomá-Mateo, Carlos, Sheila Lorente-Pozo, Lucía Márquez-Thibaut, Mireia Moreno-Estellés, Concepción Garcés, Daymé González, Marcos Lahuerta, Carmen Aguado, José Luis García-Giménez, Pascual Sanz, and et al. 2023. "Age-Related microRNA Overexpression in Lafora Disease Male Mice Provides Links between Neuroinflammation and Oxidative Stress" International Journal of Molecular Sciences 24, no. 2: 1089. https://doi.org/10.3390/ijms24021089
APA StyleRomá-Mateo, C., Lorente-Pozo, S., Márquez-Thibaut, L., Moreno-Estellés, M., Garcés, C., González, D., Lahuerta, M., Aguado, C., García-Giménez, J. L., Sanz, P., & Pallardó, F. V. (2023). Age-Related microRNA Overexpression in Lafora Disease Male Mice Provides Links between Neuroinflammation and Oxidative Stress. International Journal of Molecular Sciences, 24(2), 1089. https://doi.org/10.3390/ijms24021089







