Jatrophane Diterpenoids from Euphorbia peplus Linn. as Activators of Autophagy and Inhibitors of Tau Pathology
Abstract
1. Introduction
2. Results
2.1. Structure Elucidation
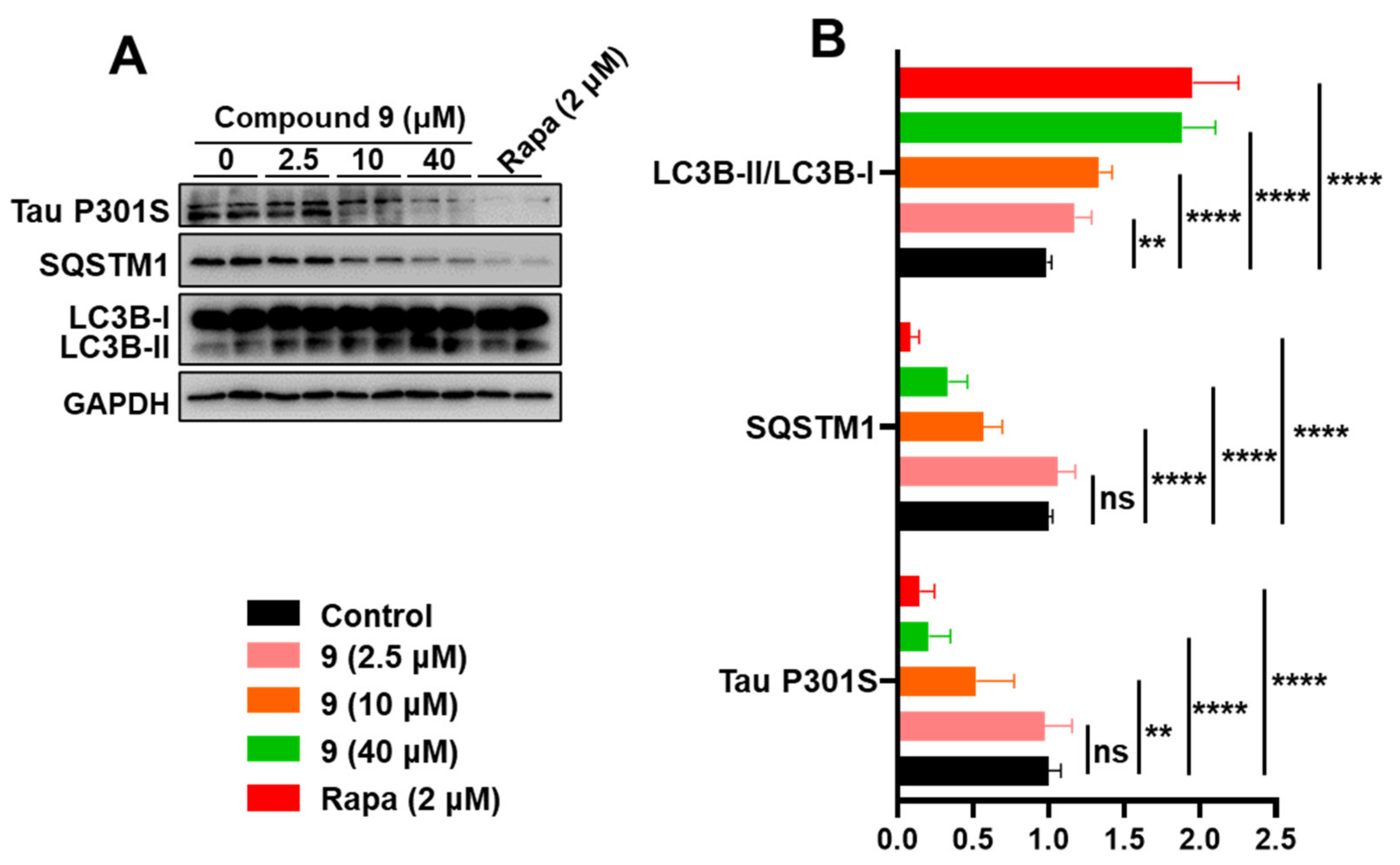
2.2. Bioactivity of the Compounds towards Autophagic Flux
3. Discussion
4. Materials and Methods
4.1. General Experimental
4.2. Plant Material
4.3. Extraction and Isolation
4.3.1. Euphjatrophane A (1)
4.3.2. Euphjatrophane B (2)
4.3.3. Euphpepluone G (3)
4.3.4. Euphjatrophane C (4)
4.3.5. Euphjatrophane D (5)
4.3.6. Euphjatrophane E (6)
4.3.7. Euphjatrophane F (7)
4.3.8. Euphjatrophane G (8)
4.3.9. Euphpepluone K (9)
4.3.10. Euphpepluone L (10)
4.4. Flow Cytometry Analysis
4.5. Tandem mCherry-GFP Fluorescence Microscopy
4.6. Construction of U251 Cells with Stable Expression of the Mutant MAPT (MAPT P301S) Gene
4.7. Western Blot
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.L.; Xiang, Y.; Jin, W.S.; Wang, J.; Shen, L.L.; Huang, Z.L.; Zhang, K.; Liu, Y.H.; Zeng, F.; Liu, J.H.; et al. Blood-derived amyloid-β protein induces Alzheimer’s disease pathologies. Mol. Psychiatry 2017, 23, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.S.; Shen, L.L.; Bu, X.L.; Zhang, W.W.; Chen, S.H.; Huang, Z.L.; Xiong, J.X.; Gao, C.Y.; Dong, Z.; He, Y.N.; et al. Peritoneal dialysis reduces amyloid-beta plasma levels in humans and attenuates Alzheimer-associated phenotypes in an APP/PS1 mouse model. Acta. Neuropathol. 2017, 134, 207–220. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. Addendum: The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2017, 546, 564. [Google Scholar] [CrossRef]
- Pohl, C.; Dikic, I. Cellular quality control by the ubiquitin-proteasome system and autophagy. Science 2019, 366, 818–822. [Google Scholar] [CrossRef]
- Sun-Wang, J.L.; Yarritu-Gallego, A.; Ivanova, S.; Zorzano, A. The ubiquitin-proteasome system and autophagy: Self-digestion for metabolic health. Trends. Endocrinol. Metab. 2021, 32, 594–608. [Google Scholar] [CrossRef]
- Hara, T.; Nakamura, K.; Matsui, M.; Yamamoto, A.; Nakahara, Y.; Suzuki-Migishima, R.; Yokoyama, M.; Mishima, K.; Saito, I.; Okano, H.; et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006, 441, 885–889. [Google Scholar] [CrossRef]
- Harris, H.; Rubinsztein, D.C. Control of autophagy as a therapy for neurodegenerative disease. Nat. Rev. Neurol. 2011, 8, 108–117. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Chiba, T.; Murata, S.; Iwata, J.I.; Tanida, I.; Ueno, T.; Koike, M.; Uchiyama, Y.; Kominami, E.; et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006, 441, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.C.; Fan, Y.; Yang, J.; Ye, M.; Zhang, D.F.; Guo, K.; Li, X.; Bi, R.; Xu, M.; Yang, L.X.; et al. A novel missense variant in ACAA1 contributes to early-onset Alzheimer’s disease, impairs lysosomal function, and facilitates amyloid-β pathology and cognitive decline. Signal. Transduct. Target. Ther. 2021, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Menzies, F.M.; Fleming, A.; Rubinsztein, D.C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 2015, 16, 345–357. [Google Scholar] [CrossRef]
- Schaeffer, V.; Goedert, M. Stimulation of autophagy is neuroprotective in a mouse model of human tauopathy. Autophagy 2014, 8, 1686–1687. [Google Scholar] [CrossRef] [PubMed]
- Di Meco, A.; Li, J.-G.; Blass, B.E.; Abou-Gharbia, M.; Lauretti, E.; Praticò, D. 12/15-Lipoxygenase Inhibition Reverses Cognitive Impairment, Brain Amyloidosis, and Tau Pathology by Stimulating Autophagy in Aged Triple Transgenic Mice. Biol. Psychiatry 2017, 81, 92–100. [Google Scholar] [CrossRef]
- Luo, R.C.; Su, L.Y.; Li, G.; Yang, J.; Liu, Q.; Yang, L.X.; Zhang, D.F.; Zhou, H.; Xu, M.; Fan, Y.; et al. Activation of PPARA-mediated autophagy reduces Alzheimer disease-like pathology and cognitive decline in a murine model. Autophagy 2020, 16, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Hohmann, J. Euphorbia diterpenes: Isolation, structure, biological activity, and synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef]
- Fattahian, M.; Ghanadian, M.; Ali, Z.; Khan, I.A. Jatrophane and rearranged jatrophane-type diterpenes: Biogenesis, structure, isolation, biological activity and SARs (1984–2019). Phytochem. Rev. 2020, 19, 265–336. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Ding, X.; Yan, C.; Song, Z.; Chen, L.; Huang, X.; Wang, X.; Jian, Y.; Tang, G.; et al. Protein kinase C controls lysosome biogenesis independently of mTORC1. Nat. Cell. Biol. 2016, 18, 1065–1077. [Google Scholar] [CrossRef]
- Hohmann, J.; Gunther, G.; Vasas, A.; Kalman, A.; Argay, G. Isolation and Structure Revision of Pepluane Diterpenoids from Euphorbia peplus. J. Nat. Prod. 1999, 62, 107–109. [Google Scholar] [CrossRef]
- Jakupovic, J.; Morgenstern, T.; Bittner, M.; Silva, M. Diterpenes from Euphorbia peplus. Phytochemistry 1998, 47, 1601–1609. [Google Scholar] [CrossRef]
- Chen, Y.N.; Ding, X.; Lu, Q.Y.; Li, D.M.; Li, B.T.; Liu, S.; Yang, L.; Zhang, Y.; Di, Y.T.; Fang, X.; et al. Macrocyclic diterpenoids from the seeds of Euphorbia peplus with potential activity in inducing lysosomal biogenesis. Bioorg. Chem. 2020, 105, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Ding, X.; Li, D.M.; Sun, M.; Yang, L.; Zhang, Y.; Di, Y.T.; Fang, X.; Hao, X.J. Diterpenoids with an unprecedented ring system from Euphorbia peplus and their activities in the lysosomal-autophagy pathway. Org. Biomol. Chem. 2021, 19, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Rizk, A.M.; Hammouda, F.M.; Elmissiry, M.M.; Radwan, H.M.; Evans, F.J. Biologically active diterpene esters from Euphorbia peplus. Phytochemistry 1985, 24, 1605–1606. [Google Scholar] [CrossRef]
- Hua, J.; Liu, Y.; Xiao, C.J.; Jing, S.X.; Luo, S.H.; Li, S.H. Chemical profile and defensive function of the latex of Euphorbia peplus. Phytochemistry 2017, 136, 56–64. [Google Scholar] [CrossRef]
- Duarte, N.; Lage, H.; Ferreira, M.J. Three new jatrophane polyesters and antiproliferative constituents from Euphorbia tuckeyana. Planta. Med. 2008, 74, 61–68. [Google Scholar] [CrossRef]
- Corea, G.; Fattorusso, E.; Lanzotti, V.; Motti, R.; Simon, P.N.; Dumontet, C.; Di Pietro, A. Jatrophane diterpenes as modulators of multidrug resistance. Advances of structure-activity relationships and discovery of the potent lead pepluanin A. J. Med. Chem. 2004, 47, 988–992. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Cuervo, A.M.; Ravikumar, B.; Sarkar, S.; Korolchuk, V.; Kaushik, S.; Klionsky, D.J. In search of an “autophagomometer”. Autophagy 2009, 5, 585–589. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, M.; Wang, D.; Liu, X.; Ye, X.; Wang, G.; Lin, T.; Sun, C.; Ding, R.; Tian, W.; et al. Jatrophane Diterpenoids from Euphorbia peplus as Multidrug Resistance Modulators with Inhibitory Effects on the ATR-Chk-1 Pathway. J. Nat. Prod. 2021, 84, 339–351. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, M.S. Autophagy-a key player in cellular and body metabolism. Nat. Rev. Endocrinol. 2014, 10, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.A. The role of autophagy in neurodegenerative disease. Nat. Med. 2013, 19, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, B.; Xu, Y.; Yang, M.; Wang, C.; Song, M.; Liu, J.; Wang, W.; You, J.; Sun, F.; et al. Activation of CREB-mediated autophagy by thioperamide ameliorates beta-amyloid pathology and cognition in Alzheimer’s disease. Aging Cell. 2021, 20, 1–20. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Song, Y.Q.; Tu, J. Autophagy in Alzheimer’s disease pathogenesis: Therapeutic potential and future perspectives. Ageing Res. Rev. 2021, 72, 1–15. [Google Scholar] [CrossRef]
- Eshraghi, M.; Ahmadi, M.; Afshar, S.; Lorzadeh, S.; Adlimoghaddam, A.; Rezvani Jalal, N.; West, R.; Dastghaib, S.; Igder, S.; Torshizi, S.R.N.; et al. Enhancing autophagy in Alzheimer’s disease through drug repositioning. Pharmaco.l Ther. 2022, 237, 1–37. [Google Scholar] [CrossRef]
- Levine, B.; Packer, M.; Codogno, P. Development of autophagy inducers in clinical medicine. J. Clin. Investig. 2015, 125, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, J.; Pan, H.; Hu, P.; Hao, Y.; Cai, W.; Zhu, H.; Yu, A.D.; Xie, X.; Ma, D.; et al. Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc. Natl. Acad. Sci. USA 2007, 104, 19023–19028. [Google Scholar] [CrossRef]
- Williams, A.; Sarkar, S.; Cuddon, P.; Ttofi, E.K.; Saiki, S.; Siddiqi, F.H.; Jahreiss, L.; Fleming, A.; Pask, D.; Goldsmith, P.; et al. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol. 2008, 4, 295–305. [Google Scholar] [CrossRef]
- Katti, S.; Igumenova, T.I. Structural insights into C1-ligand interactions: Filling the gaps by in silico methods. Adv. Biol. Regul. 2021, 79, 1–20. [Google Scholar] [CrossRef]
- Bi, R.; Zhang, W.; Yu, D.; Li, X.; Wang, H.Z.; Hu, Q.X.; Zhang, C.; Lu, W.; Ni, J.; Fang, Y.; et al. Mitochondrial DNA haplogroup B5 confers genetic susceptibility to Alzheimer’s disease in Han Chinese. Neurobiol. Aging 2015, 36, 1604.e7–1604.e1.604E16. [Google Scholar] [CrossRef]
- Tang, X.H.; Luo, R.C.; Ye, M.S.; Tang, H.Y.; Ma, Y.L.; Chen, Y.N.; Wang, X.M.; Lu, Q.Y.; Liu, S.; Li, X.N.; et al. Harpertrioate A, an A,B,D-seco-Limonoid with promising biological activity against Alzheimer’s disease from twigs of Harrisonia perforata (Blanco) Merr. Org. Lett. 2021, 23, 262–267. [Google Scholar] [CrossRef] [PubMed]
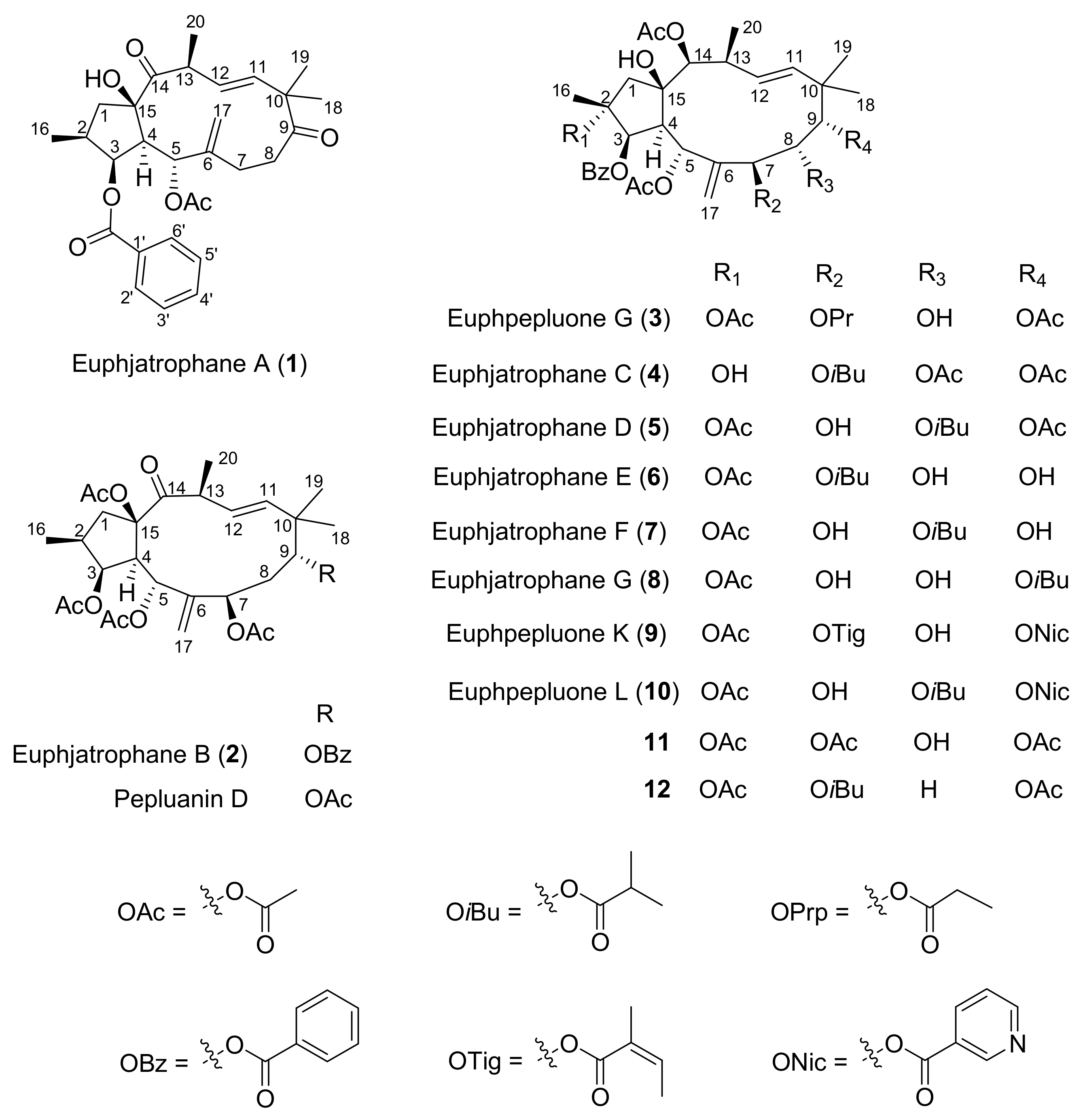

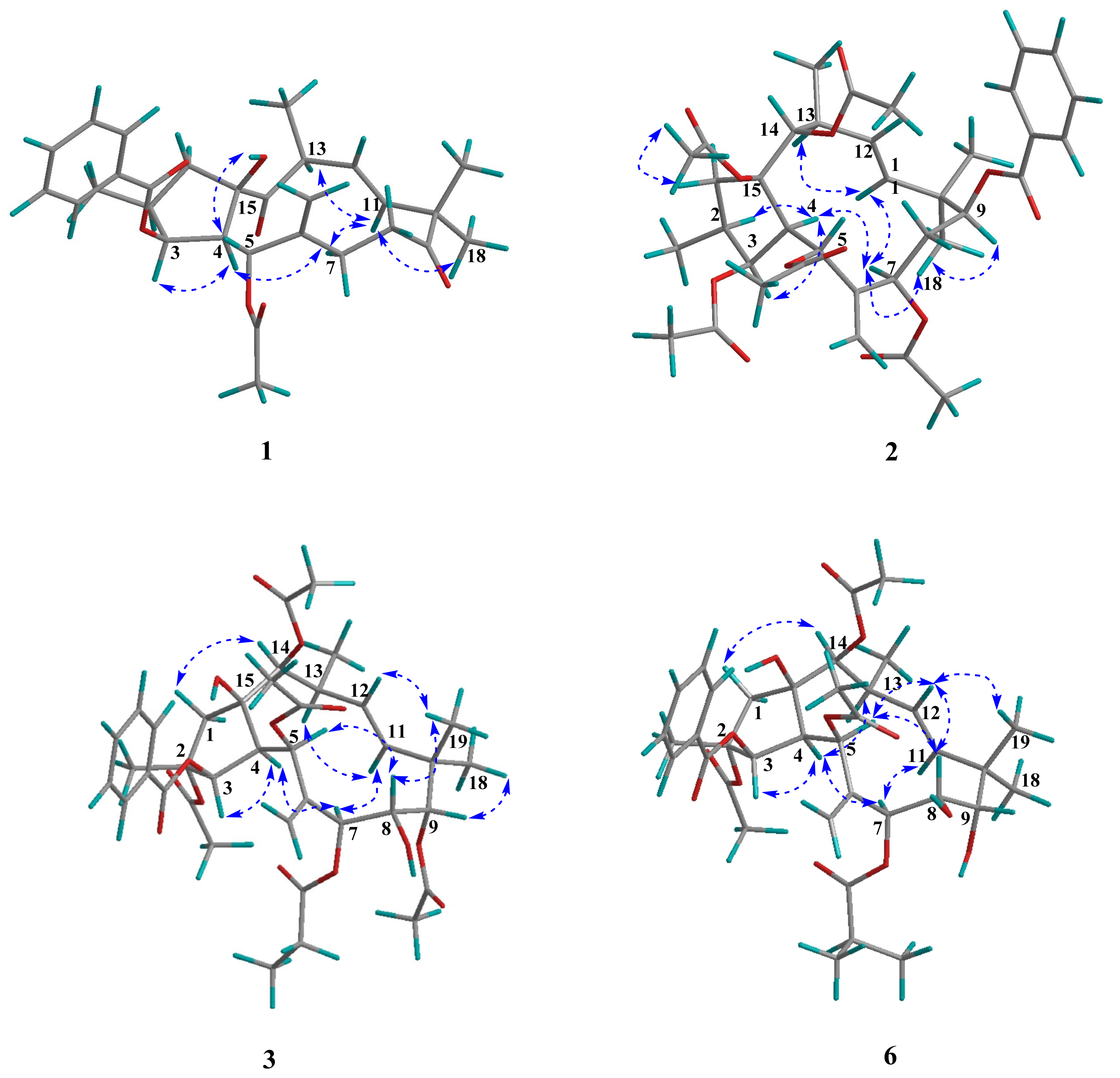
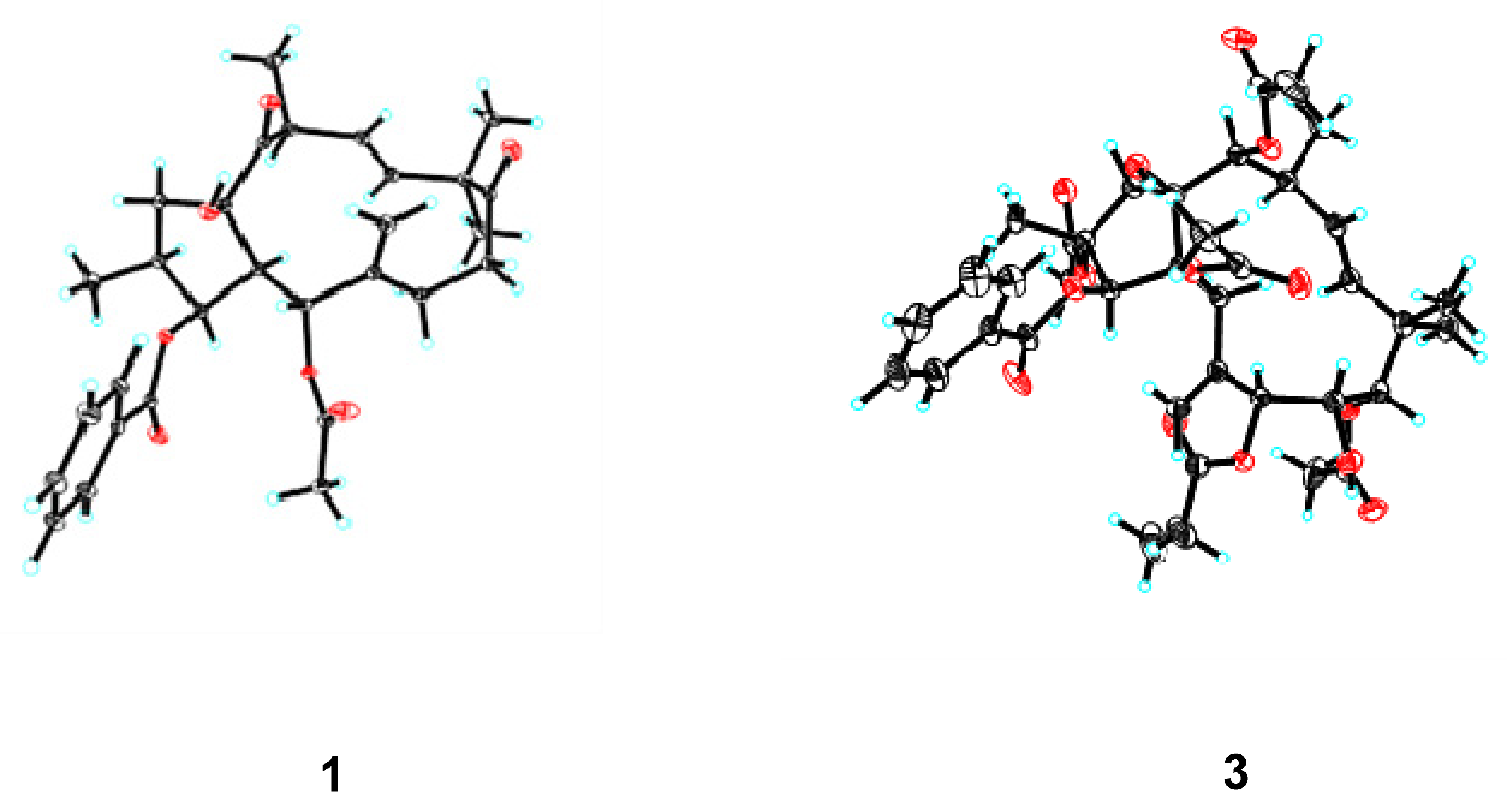



| Euphjatrophane A (1) | Euphjatrophane B (2) | |||
|---|---|---|---|---|
| Position | δH (J in Hz) | δC | δH (J in Hz) | δC |
| 1α | 2.41 dd (14.2, 8.4) | 46.8 | 2.99 dd (14.0, 7.6) | 46.4 |
| 1β | 1.93 dd (14.2, 12.2) | 1.65 dd (14.0, 12.4) | ||
| 2 | 2.32 m | 38.7 | 2.26 m | 38.3 |
| 3 | 5.84 t (3.6) | 78.0 | 5.42 t (3.0) | 76.6 |
| 4 | 2.76 dd (10.2, 3.6) | 50.5 | 2.78 br d (3.0) | 53.0 |
| 5 | 5.75 d (10.2) | 72.9 | 5.81 br s | 68.5 |
| 6 | 142.4 | 147.4 | ||
| 7α | 2.09 m | 25.0 | 4.78 br d (6.8) | 69.4 |
| 7β | 1.87 m | |||
| 8α | 2.57 m | 34.1 | 2.18 m | 33.5 |
| 8β | 2.23 m | 2.05 m | ||
| 9 | 211.6 | 5.04 m | 75.4 | |
| 10 | 5.63 d (16.2) | 50.4 | 40.7 | |
| 11 | 5.62 d (16.2) | 135.7 | 5.93 d (16.2) | 137.9 |
| 12 | 3.45 m | 130.9 | 5.70 dd (16.2, 9.0) | 129.9 |
| 13 | 44.2 | 3.52 m | 42.9 | |
| 14 | 212.2 | 212.7 | ||
| 15 | 4.28 s | 84.7 | 93.0 | |
| 16 | 1.05 d (6.4) | 14.0 | 0.88 d (6.6) | 13.4 |
| 17a | 5.18 s | 114.8 | 5.01 m | 109.6 |
| 17b | 4.84 s | |||
| 18 | 1.22 s | 23.5 | 1.13 s | 26.2 |
| 19 | 1.21 s | 23.7 | 1.07 s | 24.1 |
| 20 | 1.37 d (6.6) | 20.9 | 1.19 d (6.8) | 19.5 |
| OAc-2 | ||||
| C=O | ||||
| OAc-3 | ||||
| C=O | 169.8 | |||
| 2.11 s | 21.2 | |||
| OAc-5 | ||||
| C=O | 169.2 | 169.6 | ||
| 1.84 s | 20.7 | 2.13 s | 21.3 | |
| OAc-7 | ||||
| C=O | 170.2 | |||
| 1.37 s | 20.2 | |||
| OAc-8 | ||||
| C=O | ||||
| OAc-9 | ||||
| C=O | ||||
| OAc-14 | ||||
| C=O | ||||
| OAc-15 | ||||
| C=O | 170.5 | |||
| 2.13 s | 21.3 | |||
| OBz-3 | ||||
| C=O | 166.0 | |||
| 1′ | 129.7 | |||
| 2′,6′ | 8.11 d (7.8) | 129.9 | ||
| 3′,5′ | 7.45 t (7.6) | 128.5 | ||
| 4′ | 7.56 t (7.2) | 134.0 | ||
| OBz-9 | ||||
| C=O | 165.4 | |||
| 1′ | 129.8 | |||
| 2′,6′ | 7.99 d (7.8) | 129.6 | ||
| 3′,5′ | 7.46 t (7.8) | 128.7 | ||
| 4′ | 7.56 t (7.4) | 133.2 | ||
| OPrp-7 | ||||
| C=O | ||||
| 1″ | ||||
| 2″ | ||||
| OiBu-7 | ||||
| C=O | ||||
| 1″ | ||||
| 2″ | ||||
| 3″ | ||||
| OH-15 | 4.31 s |
| Euphpepluone G (3) | Euphjatrophane C (4) | |||
|---|---|---|---|---|
| Position | δH (J in Hz) | δC | δH (J in Hz) | δC |
| 1α | 2.67 d (15.0) | 50.2 | 2.24 d (15.0) | 53.3 |
| 1β | 2.15 d (15.0) | 2.14 d (15.0) | ||
| 2 | 88.4 | 78.5 | ||
| 3 | 5.87 d (5.8) | 80.4 | 5.48 d (3.8) | 82.4 |
| 4 | 3.36 dd (5.8, 3.6) | 44.6 | 3.37 t (3.6) | 44.1 |
| 5 | 5.75 d (3.6) | 71.8 | 5.83 d (3.2) | 71.2 |
| 6 | 144 | 144.1 | ||
| 7α | 5.24 s | 68.4 | 5.60 s | 67.9 |
| 7β | ||||
| 8α | 4.03 d (11.0) | 69.9 | 4.97 s | 80.5 |
| 8β | ||||
| 9 | 4.72 s | 86.0 | 5.23s | 70.5 |
| 10 | 40.0 | 40.7 | ||
| 11 | 5.92 d (16.0) | 133.9 | 5.83 d (16.0) | 133.9 |
| 12 | 5.58 dd (16.0, 9.6) | 131.0 | 5.70 dd (16.0, 9.6) | 131.6 |
| 13 | 2.68 m | 37.5 | 2.83 m | 36.6 |
| 14 | 5.10 s | 79.3 | 5.13 s | 79.7 |
| 15 | 84.0 | 83.5 | ||
| 16 | 1.51 s | 22.6 | 1.34 s | 24.3 |
| 17a | 4.87 s | 109.4 | 4.95 s | 110.2 |
| 17b | 4.50 s | 4.57 s | ||
| 18 | 1.01 s | 27.2 | 0.92 s | 26.3 |
| 19 | 1.28 s | 23.2 | 1.33 s | 23.8 |
| 20 | 1.14 d (7.2) | 23.6 | 1.15 d (7.0) | 23.4 |
| OAc-2 | ||||
| C=O | 170.7 | |||
| 2.15 s | 22.3 | |||
| OAc-3 | ||||
| C=O | ||||
| OAc-5 | ||||
| C=O | 168.5 | 168.3 | ||
| 2.00 s | 21.1 | 1.80 s | 20.6 | |
| OAc-7 | ||||
| C=O | ||||
| OAc-8 | ||||
| C=O | 169.7 | |||
| 2.04 s | 20.6 | |||
| OAc-9 | ||||
| C=O | 171.9 | 169.7, | ||
| 2.05 s | 20.6 | 2.02 s | 20.6 | |
| OAc-14 | ||||
| C=O | 170.4 | 171.0 | ||
| 2.08 s | 20.5 | 2.10 s | 20.8 | |
| OAc-15 | ||||
| C=O | ||||
| OBz-3 | ||||
| C=O | 164.9 | 165.2 | ||
| 1′ | 130.0 | 130.0 | ||
| 2′,6′ | 8.07 d (8.4) | 129.7 | 8.04 d (8.4) | 129.6 |
| 3′,5′ | 7.43 t (7.8) | 128.5 | 7.43 t (7.8) | 128.5 |
| 4′ | 7.57 t (7.4) | 133.2 | 7.56 t (7.4) | 133.3 |
| OBz-9 | ||||
| C=O | ||||
| 1′ | ||||
| 2′,6′ | ||||
| 3′,5′ | ||||
| 4′ | ||||
| OPrp-7 | ||||
| C=O | 171.9 | |||
| 1″ | 2.36 m | 27.5 | ||
| 2″ | 1.17 t (7.6) | 9.0 | ||
| OiBu-7 | ||||
| C=O | 176.3 | |||
| 1″ | 2.66 m | 34.1 | ||
| 2″ | 1.28 d (7.0) | 19.3 | ||
| 3″ | 1.24 d (7.0) | 18.5 |
| Euphjatrophane D (5) | Euphjatrophane E (6) | Euphjatrophane F (7) | ||||
|---|---|---|---|---|---|---|
| Position | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC |
| 1α | 2.70 d (14.2) | 49.1 | 2.61 d (14.6) | 50.7 | 2.67 d (14.6) | 49.5 |
| 1β | 2.07 d (14.2) | 2.18 d (14.6) | 2.10 d (14.6) | |||
| 2 | 88.7 | 88.1 | 88.6 | |||
| 3 | 5.74 d (5.8) | 79.9 | 5.82 d (5.6) | 80.2 | 5.76 d (5.8) | 79.7 |
| 4 | 3.51 m | 44.3 | 3.29 dd (5.6, 3.8) | 44.6 | 3.40 m | 44.5 |
| 5 | 5.78, br d (1.8) | 72.1 | 5.73 br d (3.2) | 72.0 | 5.79 br d (3.2) | 71.9 |
| 6 | 146.4 | 144.7 | 145.3 | |||
| 7 | 4.33 s | 67.9 | 5.40 s | 67.8 | 4.48 br d (5.0) | 68.3 |
| 8 | 5.14 s | 71.7 | 3.96 br d (5.5) | 70.7 | 5.09 s | 72.3 |
| 9 | 4.84 s | 80.8 | 3.62 s | 84.0 | 3.61 s | 82.2 |
| 10 | 40.4 | 40.7 | 41.2 | |||
| 11 | 5.87 d (16.0) | 134.5 | 5.91 d (16.2) | 134.5 | 5.86 d (16.0) | 135.3 |
| 12 | 5.62 dd (16.0, 9.4) | 131.2 | 5.52 dd (16.2, 9.4) | 129.9 | 5.54 dd (16.0, 9.6) | 130.2 |
| 13 | 2.61 m | 37.7 | 2.62 m | 37.4 | 2.58 m | 37.6 |
| 14 | 5.08 s | 79.6 | 5.08 s | 79.6 | 5.06 s | 79.9 |
| 15 | 83.9 | 83.8 | 83.7 | |||
| 16 | 1.50 s | 23.3 | 1.53 s | 22.1 | 1.50 s | 22.6 |
| 17a | 4.93 s | 109.2 | 4.94 s | 109.1 | 5.05 s | 109.3 |
| 17b | 4.53 s | 4.52 s | 4.54 s | |||
| 18 | 1.01 s | 26.9 | 1.07 s | 26.9 | 1.12 s | 26.4 |
| 19 | 1.36 s | 23.8 | 1.19 s | 23.8 | 1.21 s | 24.0 |
| 20 | 1.13 d (6.8) | 23.3 | 1.12 d (7.2) | 23.6 | 1.14 d (6.2) | 23.5 |
| OAc-2 | ||||||
| C=O | 170.6, | 170.7 | 170.9 | |||
| 2.14 s | 22.3 | 2.14 s | 22.3 | 2.13 s | 22.3 | |
| OAc-5 | ||||||
| C=O | 168.0, | 168.7 | 168.0 | |||
| 1.93 s | 20.9 | 1.96 s | 20.0 | 1.93 s | 20.9 | |
| OAc-9 | ||||||
| C=O | 170.8, | |||||
| 2.11 s | 20.5 | |||||
| OAc-14 | ||||||
| C=O | 170.8, | 170.4 | 171.0 | |||
| 2.17 s | 21.0 | 2.07 s | 20.5 | 2.10 s | 20.6 | |
| OBz-3 | ||||||
| C=O | 165.3 | 164.8 | 165.5 | |||
| 1′ | 129.8 | 130.0 | 129.7 | |||
| 2′,6′ | 8.11 d (8.0) | 129.7 | 8.08 d (8.2) | 129.7 | 8.10 d (8.4) | 129.8 |
| 3′,5′ | 7.46 t (7.4) | 128.5 | 7.44 t (7.8) | 128.5 | 7.45 t (7.8) | 128.5 |
| 4′ | 7.59 t (7.4) | 133.4 | 7.57 t (7.4) | 133.2 | 7.59 t (7.4) | 133.5 |
| OiBu-7 | ||||||
| C=O | 174.8 | |||||
| 1″ | 2.63 m | 34.0 | ||||
| 2″ | 1.20 d (7.0) | 18.8 | ||||
| 3″ | 1.23 d (7.0) | 19.0 | ||||
| OiBu-8 | ||||||
| C=O | 174.8 | 175.3 | ||||
| 1″ | 2.50 m | 34.0 | 2.49 m | 34.0 | ||
| 2″ | 1.05 d (7.0) | 18.8 | 1.07 d (7.0) | 18.8 | ||
| 3″ | 1.12 d (7.0) | 19.2 | 1.03 d (7.0) | 19.4 |
| Euphjatrophane G (8) | Euphpepluone K (9) | Euphpepluone L (10) | ||||
|---|---|---|---|---|---|---|
| Position | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC |
| 1a | 2.71 d (14.0) | 48.9 | 2.84 d (14.4) | 49.8 | 2.76 d (14.0) | 48.8 |
| 1b | 2.00 d (14.0) | 2.10 d (14.4) | 1.98 d (14.0) | |||
| 2 | 88.8 | 88.5 | 88.8 | |||
| 3 | 5.67 d (6.2) | 80.2 | 5.98 (6.0) | 80.9 | 5.70 d (6.6) | 80.0 |
| 4 | 3.70 m | 44.3 | 3.70 m | 44.9 | 3.94 m | 44.4 |
| 5 | 5.74 d (4.2) | 72.5 | 5.81 br d (3.4) | 72.0 | 5.81 br d (3.8) | 72.3 |
| 6 | 147.4 | 143.8 | 146.5 | |||
| 7 | 4.11 d (8.0) | 67.0 | 5.37 s | 68.8 | 4.52 d (8.8) | 67.4 |
| 8 | 3.94 d (8.0) | 70.5 | 4.20 d (10.8) | 70.3 | 5.21 s | 71.8 |
| 9 | 4.70 s | 84.8 | 5.06 s | 86.8 | 5.07 s | 82.2 |
| 10 | 40.1 | 40.3 | 40.8 | |||
| 11 | 5.94 d (16.0) | 134.6 | 6.15 d (16.0) | 133.7 | 6.14 d (16.0) | 134.6 |
| 12 | 5.52 dd (16.0, 9.8) | 130.5 | 5.67 dd (16.0, 9.8) | 131.6 | 5.67 dd (16.0, 9.8) | 131.4 |
| 13 | 2.62 m | 37.9 | 2.88 m | 37.4 | 2.73 m | 38.1 |
| 14 | 5.08 s | 69.6 | 5.15 s | 79.4 | 5.12 s | 79.6 |
| 15 | 84.1 | 84.3 | 84.2 | |||
| 16 | 1.47 s | 24.0 | 1.48 s | 23.4 | 1.46 s | 24.5 |
| 17a | 4.91 s | 108.2 | 4.78 s | 109.2 | 4.81 s | 108.5 |
| 17b | 4.70 s | 4.49 s | 4.41 s | |||
| 18 | 1.04 s | 27.2 | 1.09 s | 27.3 | 1.07s | 27.3 |
| 19 | 1.28 s | 23.3 | 1.38 s | 23.2 | 1.44 s | 23.6 |
| 20 | 1.12 d (7.2) | 23.3 | 1.18 d (7.2) | 23.6 | 1.14 d (7.0) | 23.3 |
| OAc-2 | ||||||
| C=O | 170.9, | 170.9 | 171.6 | |||
| 2.09 s | 22.2 | 2.17 s | 22.4 | 2.23 s | 22.5 | |
| OAc-5 | ||||||
| C=O | 168.6 | 168.6 | 167.9 | |||
| 2.08 s | 21.2 | 2.05 s | 21.1 | 2.07 s | 20.5 | |
| OAc-14 | ||||||
| C=O | 170.4, | 170.4 | 170.6 | |||
| 2.07 s | 20.5 | 2.11 s | 20.5 | 2.11 s | 21.0 | |
| OBz-3 | ||||||
| C=O | 165.6 | 165.0 | 165.5 | |||
| 1′ | 129.7 | 130.0 | 129.7 | |||
| 2′,6′ | 8.09 d (8.4) | 129.8 | 8.05 d (8.4) | 129.7 | 8.07 d (8.4) | 129.7 |
| 3′,5′ | 7.45 t (7.8) | 128.5 | 7.41 t (7.8) | 128.4 | 7.42 t (7.8) | 128.4 |
| 4′ | 7.59 t (7.4) | 133.4 | 7.55 t (7.4) | 133.2 | 7.56 t (7.4) | 133.4 |
| OTig-8 | ||||||
| C=O | 164.2 | |||||
| 1″ | 126.1 | |||||
| 2″ | 5.75 m | 141.7 | ||||
| 3″ | 1.68 d (7.2) | 15.8 | ||||
| 4″ | 1.52 m | 19.5 | ||||
| OiBu-9/8 | OiBu-9 | OiBu-8 | ||||
| C=O | 178.2 | 174.9 | ||||
| 1″ | 2.67 m | 34.4 | 2.53 m | 34.0 | ||
| 2″ | 1.27 d (7.0) | 18.7 | 1.05 d (7.0) | 18.8 | ||
| 3″ | 1.25 d (7.0) | 18.8 | 1.15 d (7.0) | 19.3 | ||
| ONic-9 | ||||||
| C=O | 166.0 | 164.9 | ||||
| 2″ | 9.23 s | 151.4 | 9.39 s | 151.3 | ||
| 3″ | 124.9 | 126.3 | ||||
| 4″ | 8.29 d (8.0) | 137.4 | 8.41 d (8.0) | 137.4 | ||
| 5″ | 7.34 dd (8.0, 4.8) | 123.1 | 7.39 dd (8.0, 4.8) | 123.3 | ||
| 6″ | 8.76 d (4.8) | 153.7 | 8.78 d (4.8) | 153.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Zhou, Q.; Ran, X.; Lu, Q.; Zhang, C.; Peng, M.; Tang, L.; Luo, R.; Di, Y.; Hao, X. Jatrophane Diterpenoids from Euphorbia peplus Linn. as Activators of Autophagy and Inhibitors of Tau Pathology. Int. J. Mol. Sci. 2023, 24, 1088. https://doi.org/10.3390/ijms24021088
Yan Y, Zhou Q, Ran X, Lu Q, Zhang C, Peng M, Tang L, Luo R, Di Y, Hao X. Jatrophane Diterpenoids from Euphorbia peplus Linn. as Activators of Autophagy and Inhibitors of Tau Pathology. International Journal of Molecular Sciences. 2023; 24(2):1088. https://doi.org/10.3390/ijms24021088
Chicago/Turabian StyleYan, Ying, Qi Zhou, Xiaoqian Ran, Qingyun Lu, Cuishan Zhang, Mingyou Peng, Lei Tang, Rongcan Luo, Yingtong Di, and Xiaojiang Hao. 2023. "Jatrophane Diterpenoids from Euphorbia peplus Linn. as Activators of Autophagy and Inhibitors of Tau Pathology" International Journal of Molecular Sciences 24, no. 2: 1088. https://doi.org/10.3390/ijms24021088
APA StyleYan, Y., Zhou, Q., Ran, X., Lu, Q., Zhang, C., Peng, M., Tang, L., Luo, R., Di, Y., & Hao, X. (2023). Jatrophane Diterpenoids from Euphorbia peplus Linn. as Activators of Autophagy and Inhibitors of Tau Pathology. International Journal of Molecular Sciences, 24(2), 1088. https://doi.org/10.3390/ijms24021088






