Abstract
Ethylene Insensitive 2 (EIN2) is an integral membrane protein that regulates ethylene signaling towards plant development and immunity by release of its carboxy-terminal functional portion (EIN2C) into the nucleus. The present study elucidates that the nuclear trafficking of EIN2C is induced by importin β1, which triggers the phloem-based defense (PBD) against aphid infestations in Arabidopsis. In plants, IMPβ1 interacts with EIN2C to facilitate EIN2C trafficking into the nucleus, either by ethylene treatment or by green peach aphid infestation, to confer EIN2-dependent PBD responses, which, in turn, impede the phloem-feeding activity and massive infestation by the aphid. In Arabidopsis, moreover, constitutively expressed EIN2C can complement the impβ1 mutant regarding EIN2C localization to the plant nucleus and the subsequent PBD development in the concomitant presence of IMPβ1 and ethylene. As a result, the phloem-feeding activity and massive infestation by green peach aphid were highly inhibited, indicating the potential value of EIN2C in protecting plants from insect attacks.
1. Introduction
The gaseous phytohormone ethylene (C2H4) regulates plant growth, development, and immunity via a signaling (signal transduction) pathway that uses EIN2 as the central regulator [1,2,3,4,5,6,7,8]. EIN2 is a 141-kD integral membrane protein of 1294 amino acids with structural features suited for ethylene signaling [1]. The N-terminal part of 461 amino acids forms 12 transmembrane domains [1], which enable EIN2 to partake in ethylene signal transduction from the endoplasmic reticulum (ER) membrane towards an intracellular physiological pathway [3]. At the opposite end, the longer C-terminal portion of 833 amino acids [1] contains a putative nuclear localization signal comprising 1262–1269 amino acids, which targets EIN2 or its C-terminal portion into the nucleus [9]. Following translocation, the C-terminal region of EIN2 is sufficient to activate ethylene responses associated with plant growth, development, and immunity [1,7,9,10,11]. Such a functional C-terminal fragment truncated from the EIN2 sequence was designated as EIN2 CEND [1,12] and is called EIN2C for the sake of convenience hereafter.
In response to the ethylene signal, EIN2 is proteolytically cleaved at its C-terminal region to produce length-varied EIN2C species. Many EIN2Cs have been confirmed to be the products of EIN2 protease cleavage [1,7,9,12,13,14]. The first characterized EIN2C, EIN2454–1294, was produced by polymerase chain reaction (PCR) amplification of EIN2 C-terminal partial sequence [1]. EIN2Cs have been shown to function in ethylene signaling [7,9,12,13,14,15,16]. After being expressed de novo in plants, particularly in Arabidopsis and tobacco, these EIN2Cs function to confer ethylene responses that associate with plant growth and immunity [1,9,12,14,15].
EIN2 is involved in plant immunity [1,17,18,19,20], including the Phloem-Based Defense (PBD) mechanism [21,22]. The PBD can effectively counterattack infestations of phloem-feeding insects, which are also called sap-sucking insects and, typically, include aphids [23]. The insects of this category are highly specialized in feeding from the phloem through stylet penetration of plant cells, which presents a unique stress on plant fitness [21,22,23,24,25,26]. In response, the PBD is established by massive production of β-1,3-glucan callose and phloem proteins Phloem Protein 2-like A1 (PP2-A1) and Phloem Protein 2-like A1 (PP2-A2) in sieve tubes [21,27]. Callose coagulation on sieve plates and phloem phase plugging and callose closure of sieve pores provide a strong physical barrier that effectively impedes the phloem-feeding activities of the insects, thereby blocking continued infestation in the plant [10,28,29]. In plants under aphid attack, ethylene signaling is critical for the PBD establishment usually in leaves via deposition of callose, which is produced by the Glucan Synthesis-like (GSL) enzymes [21,22,28], and accumulation of lectin-type phloem proteins [11,22,25]. Lectin-type phloem proteins that have been characterized as essential components of the PBD include PP2-A1 and PP2-A2 in Arabidopsis [11,25]. In essence, both GSL and phloem proteins are produced following expression of the cognate genes GSL5, PP2-A1, and PP2-A2 [11,28]. These genes are regarded as critical PBD response genes, which are expressed upon induction either by plant treatment with ethylene or by plant colonization with aphids, and which are expressed in an EIN2-dependent manner [10,11,21,22,28,30].
EIN2 becomes activated upon ethylene perception by any of the five functionally redundant receptors [4,31,32]. Once ethylene is sensed by an ethylene receptor, EIN2 is dephosphorylated to liberate EIN2Cs [12]. Then, some EIN2Cs move into the nucleus [12] to activate a transcriptional cascade that confers plant immunity and ethylene responses [11,33,34]. EIN2C translocation from the ER into the nucleus is a pivotal step towards the transcriptional cascade. In eukaryotic cells, the import of macromolecules, which are referred to as cargoes and mainly include proteins and RNA, into the nucleus most often requires nuclear import carrier proteins called importins (IMP). IMPs are presented by α and β forms and mediate cargo trafficking most frequently in a complex of either “cargo–IMPβ” or “cargo–IMPα–IMPβ”, wherein the IMPα is a substrate adaptor, or, rarely, in a form of “cargo–IMPα”, wherein the IMPα directly serves as a transporter [35,36,37]. Arabidopsis genome encodes 8 IMPα and 18 IMPβ proteins [38], but none was associated with the nuclear import of EIN2Cs.
This gap has been bridged by the present study demonstrating the IMP loss-of-function imp Arabidopsis mutants with respect to molecular trafficking engaged in phytohormone-mediated basal defense pathways in the plant. By investigating the WT plant, imp mutants including those defected in IMPβ1 (synonym KPNB1), and impβ1-complemented (impβ1/IMPβ1-RFP) transgenic lines, IMPβ1was characterized as an efficient facilitator for the nuclear import of EIN2454–1294, which is the first identified EIN2C [1]. The present study was devised to elucidate if IMPβ1-mediated nuclear import of EIN2C also regulates plant PBD against aphid infestations. IMPβ1 and EIN2C were determined to inhibit the phloem-feeding activity of green peach aphid (Myzus persicae Sulzer), a model species of insects attacking Arabidopsis [10,11,25]. Evidence detailed below will demonstrate that IMPβ1 mediates the nuclear transport of EIN2C to activate PBD responses against aphid infestations in Arabidopsis.
2. Results
2.1. IMPβ1 Strongly Affects Arabidopsis Resistance to Green Peach Aphid
This study began with investigating 13 Arabidopsis IMP genes (Figure 1A), that have loss-of-function mutants generated previously by T-DNA insertion (Supplementary Table S1). To look for functional links between the IMPs and Arabidopsis defenses against green peach aphid, qRT-PCR was performed to quantify IMP transcript levels in plants of the Arabidopsis ecotype Col-3 during the aphid infestation. Expression of IPT, IMPβ1, IMPα1, and IMPα7 were significantly increased by artificial colonization of the plants with aphids (Figure 1A). Quantities of IPT, IMPβ1, IMPα1, and IMPα7 transcripts in aphid-colonized plants were 5.8-, 11.0-, 6.6-, and 4.7-times higher than those in aphid-free control plants, respectively. In contrast, the other nine IMP genes did not show significant transcriptional changes in responses to aphid colonization (Figure 1A).
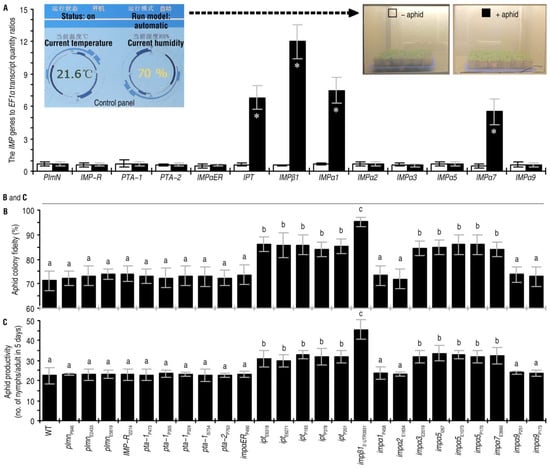
Figure 1.
Five IMPs, including IMPβ1, are related to Arabidopsis response to green peach aphid infestations. (A) Differential expression of 13 IMP genes in Arabidopsis plants in the presence and absence of aphid infestations. Leaves of 20-day-old plants were artificially colonized with 10-day-old nymphs of green peach aphid (+aphid), while plants in the control group remained free from aphids (−aphid). Two days later, RNA was isolated from leaves and analyzed by qRT-PCR using EF1α as a reference gene. Data shown are mean values ± standard deviation (SD) estimates of results from six independent experiments, each involving 15 plants. Asterisks indicate significant differences between plants with and without aphid infestations. (n = 6, * p < 0.001; Student’s t-test.) (B,C) Aphid colony fidelity in two days and productivity rates in five days after placement on leaves of the tested genotypes (listed at bottom). Data shown are mean values ± SD estimates of results obtained from six independent experiments, each involving 200 aphids colonized on 10 plants. The different letters on the graphs indicate significant differences, as assessed using Duncan’s new multiple-range test (p < 0.05).
To determine if the IMP genes affect Arabidopsis defenses, colony fidelities and reproduction rates of aphids placed on leaves of the WT and imp plants were conveyed. If the aphid colony fidelity was higher on an imp mutant compared with the WT plant, the IMP was thought to be a positive regulator of Arabidopsis resistance to aphid colonization. The aphid colony fidelity rates on the ipt, impβ1, impα3, impα5, and impα7 mutants were significantly increased compared with the WT plants. Thus, IPT, IMPβ1, IMPα3, IMPα5, and IMPα7 contributed substantially to plant resistance (Figure 1B). Meanwhile, an IMP was thought inhibitive to aphid reproduction if the reproduction rate was greater on the imp mutant than on the WT plant. The imp mutants that favored the colonization of aphid (Figure 1B) also supported aphid reproduction (Figure 1C). The highest rate of aphid reproduction occurred on the impβ1 mutant (Figure 1C). These data suggested that IPT, IMPβ1, IMPα3, IMPα5, and IMPα7 contribute to Arabidopsis resistance against green peach aphid infestation and that IMPβ1 is the most influential resistance constituent.
2.2. IMPβ1 Supports PBD Defense Gene Expression but Does Not Affect Bacterial Infection
Defense response genes PP2-A1, PP2-A2, and GSL5 have been shown to be essential constituents of the PBD [10,11,21,22,25,28]. To determine whether IPT, IMPβ1, IMPα3, IMPα5, or IMPα7 affects Arabidopsis PBD, we conducted qRT-PCR analysis on the leaves of both WT and imp mutant plants. These plants were either free from aphids or artificially colonized with 10-day-old nymphs of green peach aphids. After 24 h, we compared the expression levels of PP2-A1, PP2-A2, and GSL5 in the leaves. The qRT-PCR data indicated that PP2-A1, PP2-A2, and GSL5 were expressed at the steady-state levels in leaves of all plants without aphid colonization, but these genes considerably increased their transcript quantities after aphid colonization in the WT plant (Figure 2A). Aphid-induced enhancements of PP2-A1, PP2-A2, and GSL5 expression were also found in the ipt, impα3, impα5, and impα7 mutants (Figure 2A). However, colonization by aphid did not have substantial effects on expression levels of PP2-A1, PP2-A2, and GSL5 in the impβ1 mutant, and did not cause significant changes in transcript amounts of the three genes from the steady-state levels (Figure 2A).
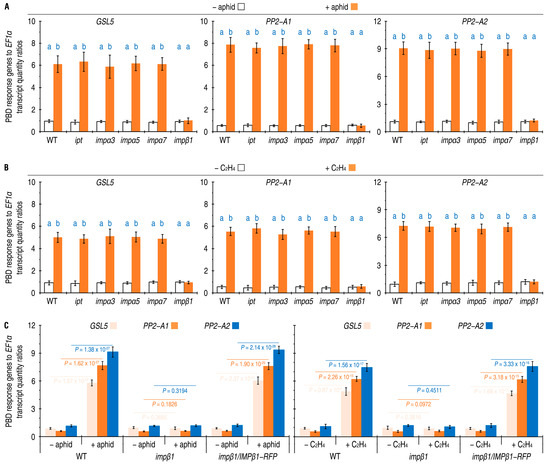
Figure 2.
The five IMPs, including IMPβ1, affect PBD response gene expression in Arabidopsis plants responding to aphid infestation or ethylene (C2H4) treatment. (A) The gene expression in leaves of the WT and imp mutant plants that remained free from or artificially colonized with aphids. (B) The gene expression in leaves of the WT and imp mutant plants that were incubated in air or in 10 µL/L ethylene. (C) The gene expression in leaves of the WT, impβ1 mutant, and impβ1-complemented (impβ1/IMPβ1-RFP) transgenic plants that remained free from or artificially colonized with aphids. In (A,B), 20-day-old plants were treated differently as designed, and, 24 h later, RNA was isolated from the treated or equivalent leaves and analyzed by qRT-PCR, which used EF1α as a reference gene. Data shown are mean values ± SD estimates of results obtained from six independent experiments, each involving 200 aphids colonized on 10 plants. In (A,B) The different letters on the graphs indicate significant differences, as assessed using Duncan’s new multiple-range test (p < 0.01). In (C), p-values obtained in the two-sided student’s t-test are provided.
Similar results were obtained from plants incubated in air and in 10 µL/L ethylene. The ipt, impα3, impα5, and impα7 mutants resembled the WT plants in response to the externally applied ethylene, which highly enhanced expression of PP2-A1, PP2-A2, and GSL5 in leaves of these plants (Figure 2B). In 24 h after ethylene treatment, expression levels of GSL5, PP2-A1, and PP2-A2 were increased accordingly by five, nine, and six times on average in comparison to the ready-state expression extents. On the contrary, the impβ1 mutant failed to display ethylene-enhanced expression of the PBD response genes. Instead, the expression of PP2-A1, PP2-A2, and GSL5 in leaves of ethylene-treated impβ1 plants remained around the steady-state levels as found in the absence of ethylene treatment (Figure 2B).
The effects of IMPβ1 on PP2-A1, PP2-A2, and GSL5 expression were confirmed by investigating impβ1-complemented (impβ1/IMPβ1-RFP) transgenic Arabidopsis lines. The impβ1/IMPβ1-RFP lines were generated by transformation of the impβ1 mutant blossoms with the plant binary vector pCAMB1301 [10] that was constructed to carry a recombinant of the native IMPβ1 promoter (IMPβ1P), IMPβ1 coding sequence (IMPβ1), and red-fluorescent protein (RFP) gene (Supplementary Figure S1A). Well-characterized four impβ1/IMPβ1-RFP lines (#1 to #4) shared common characters, resembling the WT plant in growth and development (Supplementary Figure S1B). Here, impβ1/IMPβ1-RFP #1 (simply called impβ1/IMPβ1 hereafter) was compared with the mutant and WT plants in terms of PP2-A1, PP2-A2, and GSL5 expression after plant colonization by aphids or ethylene treatment. Based on qRT-PCR analyses carried out 24 h later, quantities of PP2-A1, PP2-A2, and GSL5 transcripts detected in leaves were significantly increased by aphid colonization or ethylene treatment in impβ1/IMPβ1-RFP plants, as well as in WT plants (Figure 2C). In both the WT and impβ1/IMPβ1-RFP plants, expression levels of GSL5, PP2-A1, and PP2-A2 gained approximately six-, twelve-, and seven-fold increases by aphid colonization, and about five, nine, and six times by ethylene treatment, respectively. In the impβ1 mutant, however, neither aphid colonization nor ethylene treatment provided evident noticeable in GSL5, PP2-A1, and PP2-A2 expression, which instead remained around the steady-state levels (Figure 2C).
Taken together, these analyses suggest that IMPβ1 is essential, but IPT, IMPα3, IMPα5, and IMPα7 are not, for the PBD response gene expression induced either by aphid colonization or by ethylene treatment in Arabidopsis. In essence, IMPβ1 is likely to partake in ethylene signaling for the PBD regulation. Thus, IMPβ1 is focused in further studies stated hereafter.
We have confirmed that IMPβ1 supports the resistance to aphids in Arabidopsis, and we are interested in exploring whether IMPβ1 affects the resistance to pathogens. To our surprise, IMPβ1 did not affect the plant defense against the bacterial pathogen Pseudomonas syringae pv. tomato (Pst). The WT, impβ1, and impβ1/IMPβ1-RFP plants displayed similar sensitivities to Pst, allowing it to vigorously propagate in leaf tissues and finally cause disease with visible symptoms (Figure S2A,B). IMPβ1 was also unrelated to the plant resistance against Pectobaterium carotovora subsp. carotovora (Pcc), the bacterial pathogen that causes soft rot in cruciferous plants. Pcc causes necrosis in Arabidopsis leaves after spray inoculation in the absence of water films. Indeed, Pcc bacteria propagated in leaf tissues and caused severe necrosis symptoms irrespectively of the plant genotypes (Supplementary Figure S2C,D). Therefore, IMPβ1 has no visible function in the plant resistance against these virulent bacterial pathogens.
2.3. IMPβ1 Directly Interacts with EIN2C in Plant Nuclei
Our study found that IMPβ1 may be involved in the regulation of PBD by ethylene signaling. EIN2 is a critical factor in the positive regulation of the ethylene signaling pathway. To infer a functional relationship between EIN2 and IMPβ1, the IMPβ1 was subjected to protein–protein interaction assays with EIN2C (EIN2454–1294) using a split-ubiquitin-based yeast-two hybrid (SUB-Y2H) system. An interaction occurred specifically between EIN2C and IMPβ1, but not between EIN2C and any of the other IMPs (IPT, IMPα3, IMPα5, and IMPα7) (Figure 3A). The specificity was further evidenced by the positive control using KAT1 and the negative control using SUC2 (Figure 3A). To confirm and locate the IMPβ1 and EIN2C interaction, we carried out bimolecular fluorescence complementation (BiFC) assays. IMPβ1 was fused to the N-terminal half of YFP (YFPN), generating the IMPβ1:YFPN fusion protein, while EIN2N (EIN2N1−453) and EIN2C were fused to the YFP C-terminal half (YFPC), forming the EIN2N:YFPC and EIN2C:YFPC fusion protein (Figure 3B). An interaction was observed only between IMPβ1:YFPN and EIN2C:YFPC but not EIN2N:YFPC, and the interaction was found in the nucleus (Figure 3B and Figure S3). The IMPβ1-EIN2C interaction was corroborated by the luciferase assay performed on leaves of tobacco leaves (Figure 3C). Clearly, IMPβ1 interaction with EIN2C, creating a molecular basis for the possibility that IMPβ1 mediates EIN2C trafficking into the nucleus.
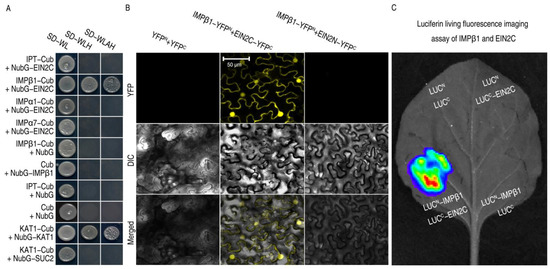
Figure 3.
IMPβ1 directly interacts with EIN2C in plant nuclei. (A) SUB-Y2H assays of EIN2C and the IMPs. Multiple positive and negative controls are partly shown here. The positive control was provided by combination of the potassium ion (K+) channel protein KAT1 (K+ Arabidopsis thaliana 1). One of the negative controls was achieved using SUC2 (sucrose transport protein 2). Results shown represent three independent experiments. (B) The YFP BiFC of IMPβ1 and Ein2C expressed in Nicotiana benthamiana leaves was observed using confocal laser-scanning microscope (CLSM). (C) Luciferin (Luc) living fluorescence imaging (LLFI) assays performed on N. benthamiana leaves transformed with IMPβ1 and Ein2C proteins. LucN and LucC represent the N-terminal and C-terminal halves of the Luc fusion constructs of the tested proteins. The leaf image represents six leaves from two plants tested in three experimental replicates.
2.4. IMPβ1 Targets EIN2C into Plant Nuclei in Response to Ethylene
Subcellular localization of IMPβ1 protein is affected by ethylene. In the assay, the impβ1/IMPβ1-RFP seedlings were growing continuously in air and were shifted into ethylene, respectively. Twelve h later, leaves were excised from the plants and stained with 4,6-diamidino-2-phenylindole (DAPI), a blue-fluorescent compound that is permeable to membranes, has a high affinity with DNA, and is widely used as a molecular marker of nuclei [39,40]. CLSM clearly visualized the IMPβ1:RFP fusion protein presented in leaf epidermal cells (Supplementary Figure S4A). In those cells, IMPβ1:RFP was found in cytoplasmic and nuclei, before plant treatment with ethylene. After ethylene was applied to the plants, IMPβ1:RFP decreased its localization in the plasma membrane and cytoplasm and more co-localized with DAPI on the nucleus (Supplementary Figure S4A).
Ethylene enhanced the nuclear localization of IMPβ1 protein, which was further confirmed by transient expression assays. In the assay, leaves of WT tobacco plants were transformed with the 35S:IMPβ1:YFP construct. These plants were incubated in air and ethylene, respectively. Leaves of these transformed plants were stained with DAPI, and observed by CLSM. In CLSM imaging, the IMPβ1:YFP fusion protein was localized in cell membranes and nuclei, in the leaves of transformed plants growing in air (Supplementary Figure S4B). In the presence of ethylene, IMPβ1:YFP fusion protein was abundantly expressed, and co-localized with DAPI were colocalized to nuclei in the leaves of transformed plants (Supplementary Figure S4B). Clearly, ethylene facilitates the localization of IMPβ1 in plant nucleus.
Consistent with ethylene-facilitated nuclear localization of IMPβ1, EIN2C was found to move into the nucleus in the concomitant presence of ethylene and a functional IMPβ1. In the assay, EIN2C-YFP was linked to P35S (Figure 4A) and transferred into the WT, impβ1, and impβ1/IMPβ1-RFP plants (Figure 4B inset 1). These transformed plants were incubated in 10 µL/L ethylene for 40 h. In the subsequent 3 h, EIN2C was constitutively expressed to similar levels in all plants no matter if they were incubated in air or ethylene, but IMPβ1 was expressed only in the WT and impβ1/IMPβ1-RFP plants incubated in ethylene (Supplementary Figure S5). In the WT and impβ1/IMPβ1-RFP plants, the EIN2C-YFP fusion protein started to move into nuclei after 40 min of incubation in ethylene and displayed sharp increases in amounts of the nuclear localization subsequently in 50–170 min (Figure 4B). Consistently, CLSM of the impβ1/IMPβ1-RFP leaves, the EIN2C-YFP protein was localized to the endoplasmic reticulum (ER) membrane and nuclear envelope, and a minor amount of this protein was found in the nucleus, which was clearly visualized by DAPI, when the plants were incubated in air (Figure 4B inset 2 and Supplementary Figure S6). On the contrary, EIN2C-YFP colocalized to nuclei with DAPI in the plants as observed at the 3rd h after ethylene application (Figure 4B inset 3 and Supplementary Figure S6). During the period of observation, the impβ1 mutant did not support the nuclear trafficking of EIN2C-YFP in comparison to the background readings (Figure 4B blue curve). We examined the co-localization of Impβ1-RFP and EIN2C-YFP. In air, IMPβ1 and EIN2C were co-located in cell membrane. However, IMPβ1 and EIN2C were co-located in nuclei in plant leaves treated with ethylene (Supplementary Figure S7). Clearly, IMPβ1 targets EIN2C into the nucleus in response to the exogenous ethylene.
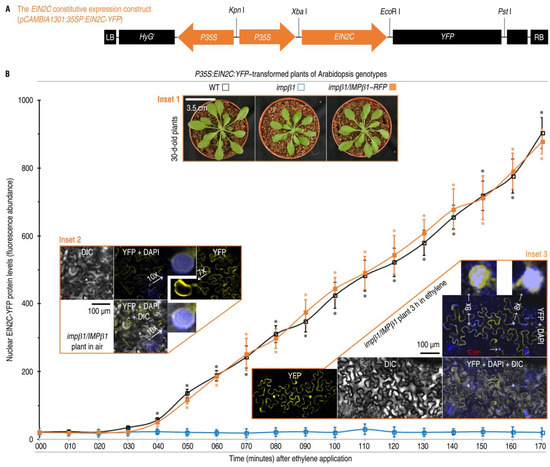
Figure 4.
The EIN2C-YFP fusion protein moves into nuclei of Arabidopsis plants in the concomitant presence of IMPβ1 and ethylene. (A) Diagram of the fusion gene expression construct. (B) Chronological changes in relative concentrations of the fusion protein localized to plant nuclei. Leaves of the indicated plants (inset 1) were transformed with the construct shown in (A). These plants were shifted into 10 µL/L ethylene to grow for 40 h. Excised leaves were stained with DAPI and observed 10 min later by CLSM. Blue, orange, and black curves indicate the fluorescence abundance of EIN2C-YFP in the nucleus in WT, impβ1, and impβ1/IMPβ1-RFP plants, respectively. Each CLSM image (insets 2 and 3) represents six leaves from three plants. The quantitative data are shown as mean values ± SD estimates (n = six leaves). Asterisks indicate significant differences in WT, impβ1/IMPβ1-RFP compared with impβ1 plants (* p < 0.05; Student’s t-test).
2.5. IMPβ1-Mediated Nuclear Import of EIN2C Confers PBD Defense Responses
As with the exogenous ethylene, green peach aphid infestation also facilitated the EIN2C-YFP fusion protein localized in the nuclei of WT and impβ1/IMPβ1-RFP plants (Figure 5A). In WT and impβ1/IMPβ1-RFP plants that were not colonized with aphids, EIN2C-YFP was mostly located in ER membranes, instead of nuclei (Figure 5A). The nuclear localization of EIN2C-YFP was not detected in impβ1 mutant plants with and without aphid colonization (Figure 5A). Taken together, these analyses elucidate that IMPβ1 transports EIN2C into the nucleus in response to aphid colonization in Arabidopsis.
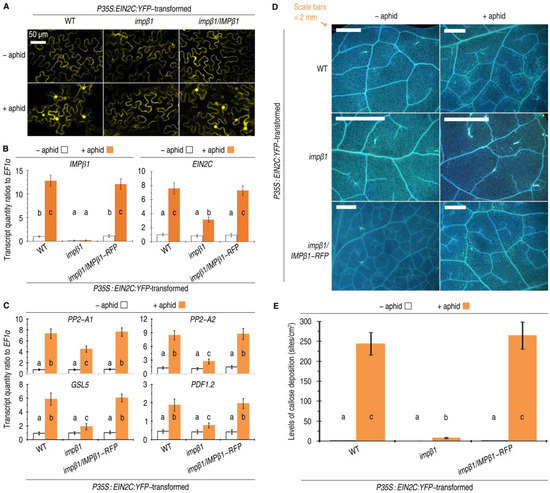
Figure 5.
Overproduced EIN2C localizes to nuclei and confers PBD responses in Arabidopsis plants in the concomitant presence of IMPβ1 and ethylene. (A) CLSM images of leaves from the indicated plants 60 h after transformation with the P35S:EIN2C:YFP construct and 24 h after artificial colonization with aphids. Each image represents 18 leaves from nine plants tested in three independent experiments. (B,C) Analyses by qRT-PCR to quantify the gene expression in leaves of the indicated plants 24 h after colonization with aphids or remained free from aphids. (D,E) Images showing callose deposition (blue dots) on leaves from plants 24 h after colonization with aphids or remained free from aphids. Each image represents 18 leaves randomly excised from 30 plants investigated in six independent experiments. Extents of callose deposition. (B,C,E) Data have been expressed as the mean ± SD (n = six); the different letters on the graphs indicate significant differences, as assessed using Duncan’s new multiple-range test (p < 0.05).
The aphid-induced nuclear import of EIN2C in the presence of a functional IMPβ1 provides the molecular basis for IMPβ1 to regulate EIN2-dependent insect-deterrent defense responses, which mainly include callose deposition and PBD response gene expression [10,21,22]. We found that these responses were induced in coincidence with constitutive expression of the EIN2C gene and aphid-induced expression of the IMPβ1 gene and the innate EIN2 gene in P35S:EIN2C:YFP-transformed WT and impβ1/IMPβ1-RFP plants. In both plants, the expression of IMPβ1 was significantly increased by artificial colonization with aphids in contrast to the ready-state expression levels detected in the aphid-free control (Figure 5B). Aphid-induced expression of the EIN2C was significantly inhibited in impβ1 plants compared with WT and impβ1/IMPβ1-RFP plants colonized with aphids (Figure 5B).
In leaves of the WT and impβ1/IMPβ1-RFP plants, but not in the impβ1 mutant, infestation with green peach aphid induced strong expression of EIN2-dependent insect-deterrent defense response genes (Figure 5C). The PDF1.2 gene is a molecular marker of the ethylene-mediated insect-deterrent plant-defense mechanism [41]. The expression of PDF1.2 is subject to ethylene signaling and provides a broad spectrum of resistance to herbivores, including leaf-eating and sap-sucking insects [11,42]. In contrast, the PBD response genes PP2-A1, PP2-A2, and GSL5 are more specific in function against phloem-feeding insects, typically including aphids [21,22,25,28,41,43]. The aphid-induced expression of these PBD response genes (Figure 5C) coincided with enhanced callose deposition in leaves of the plants carrying a functional IMPβ1 (Figure 5D,E). Taken together, these results suggest that the PBD activation is dependent on IMPβ1.
2.6. IMPβ1-Conferred PBD Inhibits Phloem Feeding and Massive Infestation by Aphids
The PBD induced by green peach aphid began to impede aphid feeding activities on Arabidopsis (Figure 6A). Feeding from plants by sap-sucking insects undergoes several phases that can be monitored by an electrical penetration graph (EPG) instrument in real time as distinct waveforms [21,22,25,44,45]. EPG-monitoring over 4 h (Supplementary Table S4) showed that green peach aphid feeding did undergo these major phases (Figure 6A), and that the aphids fed longer and more often from the phloem of the impβ1 mutant than from the WT and impβ1/IMPβ1-RFP plants (Figure 6B). Over 4 h, the aphids fed in the phloem phase (Ph2) for a total of 97.4 ± 17.7 min from the impβ1 mutant, but only 11.8 ± 0.8 min on WT and 4.3 ± 0.3 min on the complemented line.
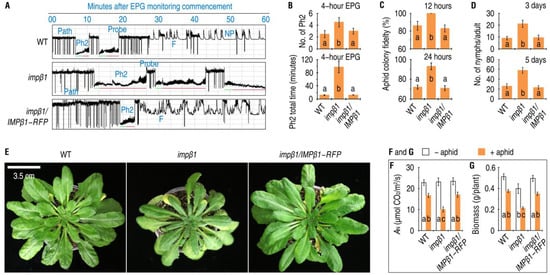
Figure 6.
IMPβ1 contributes to Arabidopsis resistance against phloem-feeding activities and massive infestations by aphids. (A) The 2 h records from 4 h EPG monitoring of aphid feeding on leaves of the different plants. Uniform aphids were placed on upper surfaces of leaves living on 30-day-old plants and monitored with the EPG device. The EPG waveform patterns shown here represent feeding behaviors of eight aphids placed on each of the three plant genotypes. The non-puncturing phase (NP) indicates that the stylet remained outside the cuticle. Cell puncturing (probe) usually leads to the pathway phase (path) in which the stylet penetrates between cells en route to the vascular tissue [45]. A successful path navigates the stylet to the phloem phase (Ph2) to absorb the phloem sap. In order to prevent protein clogging inside the sieve element, E1 salivation (green lines) first ejects watery saliva [45,46]. Second, E2 saliva (red lines) is added to the ingested sap, thought to prevent phloem proteins, mainly phloem protein 1 (PP1) and phloem protein 2 (PP2), from clogging inside the capillary food canal [44]. During the feeding process, mechanical problems with stylet penetration into the plant tissues, namely, derailed stylet mechanics shown as F, may occur due to a deficient saliva composition [22,47]. Both path and F delay the time to the Ph2 and prevent ingestion of phloem sap [47]. Following a smooth Ph2, the xylem phase (XP) may proceed while aphids try to suck water from the xylem [10,25] to reduce osmotic pressure caused by increased sucrose concentrations in ingested phloem sap [44]. (B) Summation of Ph2 time as mean values ± SDs of results from three independent experiments, each involving eight aphids (n = 24 aphids). (C) Aphid colony fidelity (means ± SDs, n = nine biological repeats). (D) Aphid productivity rates (means ± SDs, n = nine biological repeats). (E) Symptoms of aphid infestation in the different plants 10 days after artificial colonization. Each photo represents nine plants. (F) Leaf net photosynthesis rate (AN) measurements (means ± SDs, n = six leaves). (G) Plant biomass (means ± SDs, n = 15 plants). In F and G, 30-day-old plants were colonized with or remained free from aphids and the measurements were performed two weeks later. In (B–D,F,G), the different letters on the graphs indicate significant differences, as assessed using Duncan’s new multiple-range test (p < 0.05).
Assessment of aphid colony fidelity (Figure 6C) and reproductivity rates (Figure 6D) showed that the WT and impβ1/IMPβ1-RFP plants were more resistant than the impβ1 mutant to infestation by the aphid. Colony fidelity is the number of aphids remaining in the leaf colonies within 24 h, while reproductivity rate is the number of nymphs produced by an adult within five days after artificial colonization [21,22]. The aphid colony fidelity (Figure 6C) and reproductivity rates (Figure 6D) were significantly increased in the impβ1 mutant compared with the WT and impβ1/IMPβ1-RFP plants. Over 24 h, the aphid fidelity averaged 72% in the WT, 71% in impβ1/IMPβ1-RFP, and 92% in impβ1 plants, with about 22% higher in the mutant. In five days, totally 26, 23, and 58 nymphs, on average, were produced by an aphid adult individual in leaf colonies of the WT, impβ1/IMPβ1-RFP, and impβ1 plants, respectively. In other words, the presence of a functional IMPβ1 gene in the plants provided >55% resistance against aphid population growth. As a result of altered feeding, colonizing, and reproductive behaviors of the aphids, the impβ1 mutant incurred more severe infestations and displayed leaf chlorosis and necrosis (Figure 6E). The leaf infestations by aphids caused substantial reductions in net photosynthesis rates shown as AN variant (Figure 6F) and plant biomass (Figure 6G). These results demonstrate the critical role of IMPβ1 in the establishment of aphid-induced PBD, which develops in relevance to aphid-induced nuclear import of EIN2, impedes phloem-feeding activities, and reduces infestation of the plant.
2.7. EIN2C Complements the ein2-1 Mutant in PBD Responses
To look for the functional connection between IMPβ1-mediated nuclear import of EIN2C and ethylene-induced EIN2-regulated PBD responses, EIN2C was transformed in plants of the Arabidopsis WT, impβ1/IMPβ1-RFP, ein2-1, and impβ1 single mutants, and ein2-1 impβ1 double mutant. These plants were transformed with P35S:EIN2C:YFP or remained untransformed in control and then incubated in air and in 10 µL/L ethylene, respectively. Analyses by qRT-PCR performed 24 h later of the ready-state and ethylene-induced expression of the genomic EIN2C (Figure 7A) and total expression of the genomic and introduced EIN2C fragments (Figure 7B) in untransformed and transformed WT, impβ1, and impβ1/IMPβ1-RFP plants. The qRT-PCR data also confirmed the ready-state and ethylene-induced expression of the genomic EIN2 in untransformed (Figure 7C) and transformed (Figure 7D) plants of WT, impβ1, and impβ1/IMPβ1-RFP. In particular, the introduced EIN2C was well expressed in the transformed plants of the ein2-1 and ein2-1 impβ1 mutants as in the other genotypes (Figure 7B).
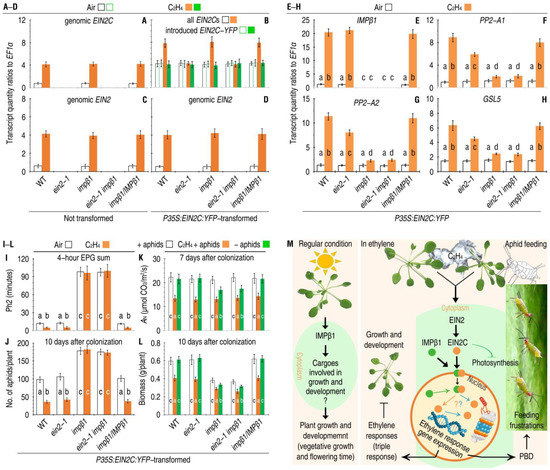
Figure 7.
IMPβ1-mediated nuclear import of EIN2C is indispensable to EIN2-regulated PBD activation and is a crucial component in the proposed model of IMPβ1 functions. (A–H) EIN2C produced by transform expression complements the ein2-1 mutant in PBD-defense-response gene expression. Leaves of the indicated plant genotypes were transformed or not. These plants were incubated in air or in 10 µL/L ethylene. Two days later, expression of the indicated genes in the transformed leaves was analyzed by qRT-PCR. (I–L) The combined effects of ethylene and EIN2C on phloem feeding and massive infestations of aphids and plant photosynthesis and growth. Plants were treated similarly as in (B). The displayed paragraphs were scored at the indicated times. (A–L) Data shown are mean values ± SDs, and the different letters on the graphs indicate significant differences, as assessed using Duncan’s new multiple-range test (n = 6, p < 0.05). (M) Model of IMPβ1 functions in plant growth and defense regulation. IMPβ1 at least has three functions depending on plant growth conditions. First, in plants growing under regular conditions, IMPβ1 participates in vegetative growth, possibly by targeting specific cargoes related to growth and development, but this hypothesis remains to be verified. Second, in plants treated with ethylene or incurring aphid infestations, IMPβ1 mediates the nuclear entry of EIN2C to confer ethylene response. Third, IMPβ1-guided nuclear import of EIN2C is also critical for activation of PBD, which effectively inhibits the phloem-feeding activities and massive infestations of aphids on the plants. Question marks (in the nucleus) indicate unanswered questions, namely, to what extent EIN2C is associated with the regulation of expression of ethylene-regulated transcription factors and defense genes [2,3,5,7,11,28,31,48], or with the proteasome activities that degrade ethylene-signaling repressors to facilitate signal transduction [14,21,22,25,28,33,34,49].
Foliar expression levels of IMPβ1 and PBD response genes were quantified. In the absence of ethylene, the expression of IMPβ1 (Figure 7E) and PBD response genes, including PP2-A1, PP2-A2, and GSL5 (Figure 7F–H), remained around the steady-state levels all plants. In the presence of ethylene, P35S:EIN2C:YFP-transformed WT, impβ1/IMPβ1-RFP, and ein2-1 plants well supported the PBD-response gene expression (Figure 7F–H). However, ethylene displayed higher extents to induce the gene expression in the WT and impβ1/IMPβ1-RFP plants than in the ein2 mutant (Figure 7F–H). Furthermore, the gene expression was considerably inhibited in the impβ1 single mutant and impβ1 ein2-1 double mutant, even in the presence of ethylene (Figure 7F–H). These differences suggest that transient constitutive expression of EIN2C:YFP temporarily compensates the ein2-1 mutant defects in sufficient expression of the PBD-response genes only when ethylene and a functional IMPβ1 are concomitantly present in the plants. This compensation caused strong inhibitions to the phloem-feeding activities of aphid (Figure 7I), resulting in marked reductions in the aphid infestations (Figure 7J) in ein2-1 plants incubated with ethylene in contrast to the incubation in air. In this mutant, the introduced EIN2C provided inhibitions to aphid feeding and population growth as strong as in the WT and impβ1/IMPβ1-RFP plants (Figure 7I,J). On the contrary, the engineering EIN2C introduced into the impβ1 and impβ1 ein2-1 plants failed to execute any inhibitory effects on aphids, indicating that EIN2C functions downstream of IMPβ1 in ethylene signal transduction towards defense responses.
While aphid infestations substantially reduced leaf photosynthesis rates, photosynthesis was further inhibited by the exogenous ethylene and introduced EIN2C, as evidenced by variations in the AN values detected from the plants with and without ethylene treatment, EIN2C introduction, and aphid infestations (Figure 7K). In contrast, IMPβ1 did not evidently affected photosynthesis, as evidenced by equivalent AN levels in the plants that carry or lack a functional IMPβ1 (Figure 7K). While growth of the impβ1 mutant was considerably impaired as compared with that of the WT plant, growth inhibition was further caused by the exogenous ethylene (Figure 7L). These analyses suggest that de novo expression of EIN2C can complement the ein2-1 mutant also in photosynthesis and vegetative growth in addition to PBD responses.
3. Discussion
This study has focused on the function of IMPβ1 in nuclear import of EIN2C linked to plant PBD against aphid infestations (Figure 7M), a distinct model of biological interactions characterized by stylet penetration of plant cells and the cellularly specified responses to this unique stress on plant fitness [21,22,23,24,25,26]. Such a functional relationship between IMPβ1 and EIN2C is also recognized as a fascinating question for the central role that EIN2 bears in ethylene signal transduction towards plant growth, development, and immunity [1,3,4,5,6,7]. Now the scientific community commonly appreciates that EIN2C liberation from the full-length EIN2 sequence and subsequent trafficking to the nucleus represent pivotal events in the intricate ethylene signaling networks [2,7,9,12,13].
Since EIN2454–1294 was demonstrated to be a sufficient regulator of ethylene signaling in Arabidopsis [1], many EIN2C fragments have been characterized [7,12], and some fragments verified to regulate ethylene responses associated with plant growth and development [7,9,12,13,14,15]. Increasing studies have been devised to elucidate the functional relationship between subcellular localizations and biological performance of the length-varied EIN2Cs and even the full-length sequence of EIN2 in plants [7,9,12,13,14,15]. The present study extends the functional scope of the first identified EIN2C, namely, EIN2454–1294 [1], to plant defenses against insect infestations. Genetic, molecular, and cytological data obtained in this study clearly demonstrate that this EIN2C is navigated by IMPβ1 into plant nuclei to confer EIN2-dependent PBD responses under induction either by the exogenous ethylene or by aphid infestation (Figure 7M). Especially, EIN2C can complement the impβ1 mutant, restoring it to the WT in EIN2-dependent PBD responses. Considering these findings, researchers in the scientific community would not find it difficult to comprehend that via nuclear import of EIN2C, IMPβ1 are imported via nuclear transport for regular plant growth and development, physiological responses, and immunological responses, respectively (Figure 7M). IMPβ1 may be a constituent of normal growth and development (Supplemental Figure S1B), at least affecting vegetative growth. The transition to flowering marks a key adaptive developmental switch in plants which has an impact on their survival and fitness. It is necessary to further identify any other physiological processes affected by IMPβ1. It is particularly necessary to characterize the functional relationship between IMPβ1 or IMPβ1-guided EIN2C trafficking and any regulators of the floral transition pathways that interplay with plant hormones to determine flowering time [50].
Explaining the relationship between subcellular localization and biological performance of EIN2Cs and full-length EIN2 in plants has been a fascinating problem. The different EIN2C species were found in association with the ER, in the nucleus, or over the cytoplasm [9]. By studying five EIN2Cs, either localizing them to the ER membrane or associating them with the nuclear fraction, Wen and colleagues proposed that the ethylene signal promotes the cleavage of the C-terminal portion from ER-located EIN2, and facilitates its nuclear localization to stabilize the EIN3 protein [9]. They determined that the nuclear localization of the EIN2Cs is sufficient to activate EIN3-mediated transcription and ethylene responses [9,51]. Similar results were obtained by Zhang and colleagues. They determined that an EIN2C was cleaved from EIN2 and moved into the nucleus to facilitate jasmonate-induced leaf senescence [15]. Zhang and colleagues proposed “an alternative model of ethylene signaling” [7]. In this model, EIN2 prevented from activation is targeted by its interacting proteins to the 26S proteasome to be degraded in the absence of ethylene; in the presence of ethylene, EIN2 is released from the inhibition to activate ethylene signaling by trafficking from the ET to the nucleus, while EIN2C species assist EIN2 trafficking and the subsequent ethylene responses [7]. Our study demonstrating that IMPβ1 mediates EIN2C entry to the nucleus in response to ethylene treatment or aphid infestation agrees with the recently proposed “alternative model of ethylene signaling”.
IMPβ1-mediated EIN2-dependent insect-deterrent responses characteristic of PBD effectively impede massive infestations of aphids in Arabidopsis, indicating the potential value of EIN2C in protecting crops from insect attacks. Coincidently, evidence [52,53,54,55] has shown that some physiological regulators function not only in growth and development but also in defense responses, possibly bridging the problem of growth–defense tradeoffs, or the costs to fitness that accompany defense responses [26,55,56]. While nucleocytoplasmic trafficking is the most important transportation route inside plant cells, the mechanisms supporting this trafficking need further exploration [52,53,54,55,56]. The functions of IMPβ1 in EIN2C trafficking link plant growth and defense regulation (Figure 7M) now offer a mechanism that could be used to bridge the growth-defense dichotomy. These molecules could be used to concomitantly improve both defense and productivity in plants, especially crops [55,57]. Since the impβ1 mutant is compromised in both growth and defense, IMPβ1 loss-of-function has negative consequences for both sets of processes, rather than creating a trade-off between them. Future studies will focus on engineering the overexpression of IMPβ1 homologs in crops with the aim of synchronized enhancements of crop productivity and immunity.
4. Materials and Methods
4.1. Plant Material and Growth Conditions
Arabidopsis ecotype Col-3, the ein2-1 single mutant, and 25 imp mutants were previously generated in the Col-3 background. Their seeds were purchased from TAIR (The Arabidopsis Information Resource at www.arabidopsis.org accessed on 12 March 2009). The ein2-1 impβ1 hybrid and impβ1/IMPβ1-RFP transgenic Col-3 lines were generated in the HD lab and used in F6-selfing homozygous generation in this study. Seeds were germinated in flat plastic trays filled with a plant growth substrate. Three days later, germinal seedlings were moved into Φ7-cm pots (1–3 plants per pot) filled with the same substrate. Seed germination and plant growth were accommodated in environmentally controlled plant growth chambers under 24 ± 1 °C, 250 ± 50 μmol quanta/m2/s illumination, and a photoperiod circle of 8 h light and 16 h dark.
4.2. Aphid Cultures
A single isolate of M. persicae was collected from field-grown radish (Raphanus sativus), near Nanjing in China. A clone of apterous agamic females was obtained by acclimatization in WT Arabidopsis grown in the chamber. The subsequently formed colonies were maintained in nursery Arabidopsis seedlings and were transferred to fresh plants every two weeks in the HD lab located at the Nanjing Agricultural University Weigang Campus (2000–2019) and the Shandong Agricultural University North Campus (since 2018). Uniform 10-day-old aphids were used in this study and were transferred to experimental plants with a fine paintbrush.
4.3. Plant Colonization
For Arabidopsis colonization, uniform 10-day-old M. persicae aphids were placed on the top two expanded leaves of plants (10 aphids per leaf). A total of 1200 aphids were monitored in six independent experiments for each genotype and for each single combination of treatment and plant genotype. For each treatment, 200 aphids were placed on 20 leaves (two leaves on 10 plants). Aphid movement from leaf colonies was monitored for five days, and the number of aphids in a leaf colony was scored at 24 h intervals [21,22,25]. The number of nymphs that moved away from colonies was also counted. The proportion of aphids staying in a leaf colony was regarded as colony fidelity. Aphid reproduction was surveyed twice a day by counting newborn nymphs. The reproduction rate was quantified as the ratio between the total number of nymphs produced in five days and the total number of aphid adults that stayed in leaf colonies during the same period [21,22,25].
4.4. Gene Expression and PBD Analyses
Total RNA was extracted via the RNA-easy Isolation Reagent Kit (Vazyme, R701-01, Nanjing, China). Plant gene expression was quantified by real-time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) performed under strict experimental designs (Supplementary Table S2). All the qRT-PCR analyses were conducted using primers (Supplementary Table S3) of high specificities, which were verified by the melting-curve method [22], and using the constitutively expressed EF1α gene as a reference [10]. The qPCR experiments were conducted on the ABI QuantStudio 3a 96 Real-Time PCR system (Thermo Fisher, Waltham, Massachusetts, USA) using the ChamQTM Universal SYBR® qPCR Master Mix (Vazyme, Q711-02, Nanjing, China). Relative expression levels of the tested genes (IMPβ1, EIN2C, related variants, and defense response genes, listed in Supplementary Table S3) were quantified as ratios of their transcript amounts to the EF1α transcript quantity. Callose visualization was performed on leaves as previously described [11].
4.5. Ethylene Treatment
Arabidopsis plants were treated with gaseous ethylene in 15 L glass vacuum chambers using the well-established protocol [58,59]. The container has a dome top cover with a valve in its center that allows vacuum removal of air and administration of ethylene. Before use, all interfaces of the individual components of the container were daubed with petroleum jelly to ensure complete sealing. For treatment, pots containing plants or agar plates containing seeds were placed into the glass container, the container was closed and some air was pumped out. Ethylene gas at the final concentration of 10 µL/L was injected into the container using a syringe and needle through the valve. After ethylene gas was pushed into the container, the valve was left open for a few seconds, allowing the outside air to enter the container so that the total air volume was brought back to the regular level. The valve was closed, and the container was moved into the plant growth chamber to incubate the plants or seeds. Untreated plants were placed in different vacuum containers, but ethylene was not applied.
4.6. Aphid Feeding Behavior Monitoring
Aphid feeding activities were observed by the EPG technique [43,44] using the Giga Amplifier system (EPG Systems, Dillenburg, 12, 6703CJ, Wageningen, The Netherlands). Uniform 10-day-old aphids were placed on the upper side of the upper two expanded leaves of an Arabidopsis or wheat plant. The aphids were monitored in three independent experiments. Each experiment involved a total of eight aphids tested with one aphid per leaf using eight plants. Immediately after aphids were placed on leaves, a 20-mm diameter gold wire was attached to the dorsal surface of each aphid’s abdomen using silver conductive paint. The other end of the wire was connected to an eight-channel Giga-8 direct current amplifier with eight channels and a 109-Ω input resistance in an electrical circuit that is also connected to the plant via an electrode placed in the soil. The behavior of individual aphids was monitored for 4 h. Voltage waveforms were digitized at 100 Hz with an A/D converter USB device. Waveform patterns were identified according to previously described categories [25,44]. Briefly, the nonpuncturing phase (NP) indicates the stylet is outside the cuticle. Cell puncturing (probe) leads to the pathway phase (Ph2) in which the stylet penetrates between cells en route to the vascular tissue [21,22,25,45]. When the phloem is not a favorite source for feeding, the xylem phase (XP) may be observed while aphids try to suck sap from the xylem [21,22,25].
4.7. Gas Exchange Measurements
Gas exchange in the second and third leaves from the top of plants was measured with the LI-6800 photosynthesis system (LI-Corp Biosci, Lincoln, NE, USA). Detailed measurements on single leaves were performed following the manufacturer’s instructions and previously described experimental procedures [39,60]. During measurements, relative humidity in the leaf chamber (2 cm2 for Arabidopsis and 6 cm2 for wheat) was constantly maintained at 45% and the leaf temperature was kept at 25 °C. CO2 concentrations at the inlet and outlet of the leaf chamber were monitored by the non-dispersive infrared gas analyzer installed in the system. Photosynthetically active photon flux density was controlled by adjusting intensities of the lamp-house irradiation. Readings of AN were documented automatically by the LI-6800 monitor system integrated into the LI-6800 system.
4.8. Genetic Complementation
The genetic complement was constructed in the plant binary vector pCAMBIA1301 [10]. The full-length sequence (1–4634) of the canonical WT IMPβ1 gene was linked N-terminally with its own promoter sequence of nucleotides –2000 to –1 and linked C-terminally with the RFP gene sequence. Plant transformation with the recombinant vector and molecular characterization of transgenic lines were performed by conventional protocols [10,25] and T3 homozygous progenies were used in this study.
4.9. Protein-Protein Interaction Assays
As a first step, each of the four IMPs (IMPα1, IMPα7, IMPβ1, and IPT) were cloned into the pGADT7 bait vector, and EIN2C was cloned into the pGBKT7 prey vector of the Dualsystems’ split-ubiquitin yeast two-hybrid system (Dualsystems Biotech, Schlieren, Zurich, Switzerland). For YFP BiFC, previously constructed pCAMBIA1301-YFPN and -YFPC plasmid vectors were used in gene recombination. An IMP gene was fused to YFPN between the KpnI and XbaI restriction sites, whereas EIN2C was linked to YFPC using the KpnI and BamHI recognition sites. Similar operations were used in luciferase assay except for replacing YFPN and YFPC with LucN and LucC, respectively. The presence and absence of molecular interactions were determined using previously described protocols [34,39].
4.10. Subcellular Localization of IMPβ1 and EIN2C
The different chimeric genes were separately constructed in the plant binary vector pCAMBIA1031, transferred into GV3101-bacterial cells, and transiently expressed in plants using a previously described protocol [61]. Alternatively, plants were co-transformed with each of these constructs and the IMPβ1:RFP construct. 48 h after plant transformation, the EIN2C:YFP fusion protein in leaf cells was visualized by CLSM [10,57]. DAPI was used to stain nuclei and applied in an aqueous solution to immerse tested leaves 10 min before observation [61].
4.11. Statistical Analysis
Analysis of variance, student’s t-tests, and Duncan’s new multiple-range tests [62] were performed with GraphPad Prism 8.0 (https://www.graphpad.com/, accessed on 3 August 2019) to determine significance of differences in paired and multiple data from different plants or treatments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24108545/s1.
Author Contributions
K.L. and X.W. performed the experiments, analyzed the data, and participated in the manuscript drafting. L.Z., L.Q., and M.Z. contributed to the data analyses and the manuscript revision. H.D. and X.C. conceived the project, designed the experiments, and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
The Natural Science Foundation of China (31772247) and the Major Science and Technology Innovation Project of Shandong Province (2019JZZY020608) to H.D. and the Natural Science Foundation of Shandong Province (ZR2020QC126) to L.Z.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Ruoxue Liu (Nanjing Normal University) for generating the double mutant and HD lab members for assistance in the experiments.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alonso, J.M.; Hirayama, T.; Roman, G.; Nourizadeh, S.; Ecker, J.R. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 1999, 284, 2148–2152. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Van den Broeck, L.; Inze, D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Aguado, E.; Martinez, C.; Loska, D.; Beltran, S.; Valenzuela, J.L.; Garrido, D.; Jamilena, M. The ethylene receptors CpETR1A and CpETR2B cooperate in the control of sex determination in Cucurbita pepo. J. Exp. Bot. 2020, 71, 154–167. [Google Scholar] [CrossRef]
- Wang, K.L.; Li, H.; Ecker, J.R. Ethylene biosynthesis and signaling networks. Plant Cell 2002, 14 (Suppl. 1), S131–S151. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W.; Yin, Z.; Wen, C.K. Rice CONSTITUTIVE TRIPLE-RESPONSE2 is involved in the ethylene-receptor signaling and regulation of various aspects of rice growth and development. J. Exp. Bot. 2013, 64, 4863–4875. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Lu, J.; Zhang, Y.; Wen, C.K. Uncertainty of EIN2(Ser645/Ser924) Inactivation by CTR1-mediated phosphorylation reveals the complexity of ethylene signaling. Plant Commun. 2020, 1, 100046. [Google Scholar] [CrossRef]
- Zhu, B.S.; Zhu, Y.X.; Zhang, Y.F.; Zhong, X.; Pan, K.Y.; Jiang, Y.; Wen, C.K.; Yang, Z.N.; Yao, X. Ethylene activates the EIN2-EIN3/EIL1 signaling pathway in tapetum and disturbs anther development in Arabidopsis. Cells 2022, 11, 3177. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, C.; Ji, Y.; Zhao, Q.; He, W.; An, F.; Jiang, L.; Guo, H. Activation of ethylene signaling is mediated by nuclear translocation of the cleaved EIN2 carboxyl terminus. Cell Res. 2012, 22, 1613–1616. [Google Scholar] [CrossRef]
- Liu, R.; Chen, L.; Jia, Z.; Lu, B.; Shi, H.; Shao, W.; Dong, H. Transcription factor AtMYB44 regulates induced expression of the ETHYLENE INSENSITIVE2 gene in Arabidopsis responding to a harpin protein. Mol. Plant Microbe. Interact. 2011, 24, 377–389. [Google Scholar] [CrossRef]
- Lü, B.B.; Li, X.J.; Sun, W.W.; Li, L.; Gao, R.; Zhu, Q.; Tian, S.M.; Fu, M.Q.; Yu, H.L.; Tang, X.M.; et al. AtMYB44 regulates resistance to the green peach aphid and diamondback moth by activating EIN2-affected defences in Arabidopsis. Plant Biol. 2013, 15, 841–850. [Google Scholar] [CrossRef]
- Qiao, H.; Shen, Z.; Huang, S.S.; Schmitz, R.J.; Urich, M.A.; Briggs, S.P.; Ecker, J.R. Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science 2012, 338, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Bisson, M.M.; Groth, G. Targeting plant ethylene responses by controlling essential protein-protein interactions in the ethylene pathway. Mol. Plant 2015, 8, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Chang, K.N.; Yazaki, J.; Ecker, J.R. Interplay between ethylene, ETP1/ETP2 F-box proteins, and degradation of EIN2 triggers ethylene responses in Arabidopsis. Genes Dev. 2009, 23, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Chai, J.; Xing, D. Mitogen-activated protein kinase 6 mediates nuclear translocation of ORE3 to promote ORE9 gene expression in methyl jasmonate-induced leaf senescence. J. Exp. Bot. 2016, 67, 83–94. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Li, Z.Q.; Zhao, Y.D.; Shen, W.J.; Chen, M.S.; Gao, H.Q.; Ge, X.M.; Wang, H.Q.; Li, X.; He, J.M. Ethylene-induced stomatal closure is mediated via MKK1/3-MPK3/6 cascade to EIN2 and EIN3. J. Integr. Plant Biol. 2021, 63, 1324–1340. [Google Scholar] [CrossRef]
- Burger, M.; Chory, J. Stressed out about hormones: How plants orchestrate immunity. Cell Host Microbe 2019, 26, 163–172. [Google Scholar] [CrossRef]
- Huang, P.Y.; Catinot, J.; Zimmerli, L. Ethylene response factors in Arabidopsis immunity. J. Exp. Bot. 2016, 67, 1231–1241. [Google Scholar] [CrossRef]
- Salvador-Guirao, R.; Hsing, Y.I.; San Segundo, B. The polycistronic miR166k-166h positively regulates rice immunity via post-transcriptional control of EIN2. Front. Plant Sci. 2018, 9, 337. [Google Scholar] [CrossRef]
- Yang, C.; Li, W.; Cao, J.; Meng, F.; Yu, Y.; Huang, J.; Jiang, L.; Liu, M.; Zhang, Z.; Chen, X.; et al. Activation of ethylene signaling pathways enhances disease resistance by regulating ROS and phytoalexin production in rice. Plant J. 2017, 89, 338–353. [Google Scholar] [CrossRef]
- Fu, M.; Xu, M.; Zhou, T.; Wang, D.; Tian, S.; Han, L.; Dong, H.; Zhang, C. Transgenic expression of a functional fragment of harpin protein Hpa1 in wheat induces the phloem-based defence against English grain aphid. J. Exp. Bot. 2014, 65, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Li, P.; Mei, Y.; Chen, M.; Chen, X.; Xu, H.; Zhou, X.; Dong, H.; Zhang, C.; Jiang, W. Three MYB genes co-regulate the phloem-based defence against English grain aphid in wheat. J. Exp. Bot. 2017, 68, 4153–4169. [Google Scholar] [CrossRef] [PubMed]
- Nalam, V.; Louis, J.; Shah, J. Plant defense against aphids, the pest extraordinaire. Plant Sci. 2019, 279, 96–107. [Google Scholar] [CrossRef]
- Douglas, A.E. Phloem-sap feeding by animals: Problems and solutions. J. Exp. Bot. 2006, 57, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, H.; Chen, L.; Wang, X.; Lu, B.; Zhang, S.; Liang, Y.; Liu, R.; Qian, J.; Sun, W.; et al. Harpin-induced expression and transgenic overexpression of the phloem protein gene AtPP2-A1 in Arabidopsis repress phloem feeding of the green peach aphid Myzus persicae. BMC Plant Biol. 2011, 11, 11. [Google Scholar] [CrossRef]
- Züst, T.; Agrawal, A.A. Trade-offs between plant growth and defense against insect herbivory: An emerging mechanistic synthesis. Annu. Rev. Plant Biol. 2017, 68, 513–534. [Google Scholar] [CrossRef]
- Will, T.; van Bel, A.J. Physical and chemical interactions between aphids and plants. J. Exp. Bot. 2006, 57, 729–737. [Google Scholar] [CrossRef]
- Lü, B.; Sun, W.; Zhang, S.; Zhang, C.; Qian, J.; Wang, X.; Gao, R.; Dong, H. HrpN Ea-induced deterrent effect on phloem feeding of the green peach aphid Myzus persicae requires AtGSL5 and AtMYB44 genes in Arabidopsis thaliana. J. Biosci. 2011, 36, 123–137. [Google Scholar] [CrossRef]
- Züst, T.; Agrawal, A.A. Mechanisms and evolution of plant resistance to aphids. Nat. Plants 2016, 2, 15206. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Q.; Zhang, L.; Zhang, M.; Chen, L.; Zou, S.; Zhang, C.; Dong, H. Aphid salivary protein Mp1 facilitates infestation by binding phloem protein 2-A1 in Arabidopsis. Biochem. Biophys. Res. Commun. 2021, 572, 105–111. [Google Scholar] [CrossRef]
- Light, K.M.; Wisniewski, J.A.; Vinyard, W.A.; Kieber-Emmons, M.T. Perception of the plant hormone ethylene: Known-knowns and known-unknowns. J. Biol. Inorg. Chem. 2016, 21, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Solano, R.; Ecker, J.R. Ethylene gas: Perception, signaling and response. Curr. Opin. Plant Biol. 1998, 1, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Dolgikh, V.A.; Pukhovaya, E.M.; Zemlyanskaya, E.V. Shaping ethylene response: The role of EIN3/EIL1 transcription factors. Front. Plant Sci. 2019, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, Z.; Wen, X.; Li, W.; Shi, H.; Yang, L.; Zhu, H.; Guo, H. Salt-induced stabilization of EIN3/EIL1 confers salinity tolerance by deterring ROS accumulation in Arabidopsis. PLoS Genet. 2014, 10, e1004664. [Google Scholar] [CrossRef] [PubMed]
- Lott, K.; Cingolani, G. The importin beta binding domain as a master regulator of nucleocytoplasmic transport. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 1578–1592. [Google Scholar] [CrossRef] [PubMed]
- Mosammaparast, N.; Pemberton, L.F. Karyopherins: From nuclear-transport mediators to nuclear-function regulators. Trends Cell Biol. 2004, 14, 547–556. [Google Scholar] [CrossRef]
- Pemberton, L.F.; Paschal, B.M. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic 2005, 6, 187–198. [Google Scholar] [CrossRef]
- Huang, J.G.; Yang, M.; Liu, P.; Yang, G.D.; Wu, C.A.; Zheng, C.C. Genome-wide profiling of developmental, hormonal or environmental responsiveness of the nucleocytoplasmic transport receptors in Arabidopsis. Gene 2010, 451, 38–44. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Gago, J.; Cui, H.; Qian, Z.; Kodama, N.; Ji, H.; Tian, S.; Shen, D.; Chen, Y.; et al. Harpin Hpa1 interacts with aquaporin PIP1;4 to promote the substrate transport and photosynthesis in Arabidopsis. Sci. Rep. 2015, 5, 17207. [Google Scholar] [CrossRef]
- Asamitsu, S.; Imai, Y.; Yabuki, Y.; Ikenoshita, S.; Takeuchi, M.; Kashiwagi, H.; Tanoue, Y.; Fukuda, T.; Shioda, N. Identification and immunohistochemical characterization of G-quadruplexes in mouse brain. Biochem. Biophys. Res. Commun. 2020, 531, 67–74. [Google Scholar] [CrossRef]
- Dong, H.P.; Peng, J.; Bao, Z.; Meng, X.; Bonasera, J.M.; Chen, G.; Beer, S.V.; Dong, H. Downstream divergence of the ethylene signaling pathway for harpin-stimulated Arabidopsis growth and insect defense. Plant Physiol. 2004, 136, 3628–3638. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, B.; Xu, M.; Han, L.; Zhao, Y.; Liu, Z.; Dong, H.; Zhang, C. Plant growth enhancement and associated physiological responses are coregulated by ethylene and gibberellin in response to harpin protein Hpa1. Planta 2014, 239, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Moran, P.J.; Thompson, G.A. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 2001, 125, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Tjallingii, W.F. Salivary secretions by aphids interacting with proteins of phloem wound responses. J. Exp. Bot. 2006, 57, 739–745. [Google Scholar] [CrossRef]
- Tjallingii, W.F.; Hogen, T.H. Fine-structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiol. Entomol. 1993, 18, 317–328. [Google Scholar] [CrossRef]
- Mutti, N.S.; Louis, J.; Pappan, L.K.; Pappan, K.; Begum, K.; Chen, M.S.; Park, Y.; Dittmer, N.; Marshall, J.; Reese, J.C.; et al. A protein from the salivary glands of the pea aphid, Acyrthosiphon pisum, is essential in feeding on a host plant. Proc. Natl. Acad. Sci. USA 2008, 105, 9965–9969. [Google Scholar] [CrossRef]
- Machado-Assefh, C.R.; Alvarez, A.E. Probing behavior of aposymbiotic green peach aphid (Myzus persicae) on susceptible Solanum tuberosum and resistant Solanum stoloniferum plants. Insect Sci. 2018, 25, 127–136. [Google Scholar] [CrossRef]
- Ecker, J.R. The ethylene signal transduction pathway in plants. Science 1995, 268, 667–675. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Plant cell wall extensibility: Connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 2016, 67, 463–476. [Google Scholar] [CrossRef]
- Conti, L. Hormonal control of the floral transition: Can one catch them all? Dev. Biol. 2017, 430, 288–301. [Google Scholar] [CrossRef]
- An, F.; Zhao, Q.; Ji, Y.; Li, W.; Jiang, Z.; Yu, X.; Zhang, C.; Han, Y.; He, W.; Liu, Y.; et al. Ethylene-induced stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 is mediated by proteasomal degradation of EIN3 binding F-box 1 and 2 that requires EIN2 in Arabidopsis. Plant Cell 2010, 22, 2384–2401. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zavaliev, R.; Dong, X. Membrane trafficking in plant immunity. Mol. Plant 2017, 10, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zebell, S.G.; Liang, Z.; Wang, S.; Kang, B.H.; Dong, X. Nuclear pore permeabilization is a convergent signaling event in effector-triggered immunity. Cell 2016, 166, 1526–1538.e11. [Google Scholar] [CrossRef]
- Tian, S.; Wang, X.; Li, P.; Wang, H.; Ji, H.; Xie, J.; Qiu, Q.; Shen, D.; Dong, H. Plant aquaporin AtPIP1;4 links apoplastic H2O2 induction to disease immunity pathways. Plant Physiol. 2016, 171, 1635–1650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, L.; Dong, H. Plant aquaporins in infection by and immunity against pathogens—A critical review. Front. Plant Sci. 2019, 10, 632. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, R.; Schafer, P. Growth versus immunity--a redirection of the cell cycle? Curr. Opin. Plant Biol. 2015, 26, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Chen, X.; Yao, X.; An, Y.; Wang, X.; Qin, L.; Li, X.; Wang, Z.; Liu, S.; Sun, Z.; et al. Phosphorylation of a wheat aquaporin at two sites enhances both plant growth and defense. Mol. Plant 2022, 15, 1772–1789. [Google Scholar] [CrossRef]
- Merchante, C.; Stepanova, A.N. The triple response assay and its use to characterize ethylene mutants in Arabidopsis. Methods Mol. Biol. 2017, 1573, 163–209. [Google Scholar] [CrossRef]
- Street, I.H.; Aman, S.; Zubo, Y.; Ramzan, A.; Wang, X.; Shakeel, S.N.; Kieber, J.J.; Schaller, G.E. Ethylene inhibits cell proliferation of the Arabidopsis root meristem. Plant Physiol. 2015, 169, 338–350. [Google Scholar] [CrossRef]
- Maurel, C.; Verdoucq, L.; Luu, D.T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant. Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef]
- Li, P.; Zhang, L.; Mo, X.; Ji, H.; Bian, H.; Hu, Y.; Majid, T.; Long, J.; Pang, H.; Tao, Y.; et al. Rice aquaporin PIP1;3 and harpin Hpa1 of bacterial blight pathogen cooperate in a type III effector translocation. J. Exp. Bot. 2019, 70, 3057–3073. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.A. A pocket calculator program for Duncan’s New Multiple Range Test and analysis of variance. Comput. Biol. Med. 1984, 14, 357–362. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).