Genome-Wide Association Studies of Seven Root Traits in Soybean (Glycine max L.) Landraces
Abstract
1. Introduction
2. Results
2.1. Root Trait Variation
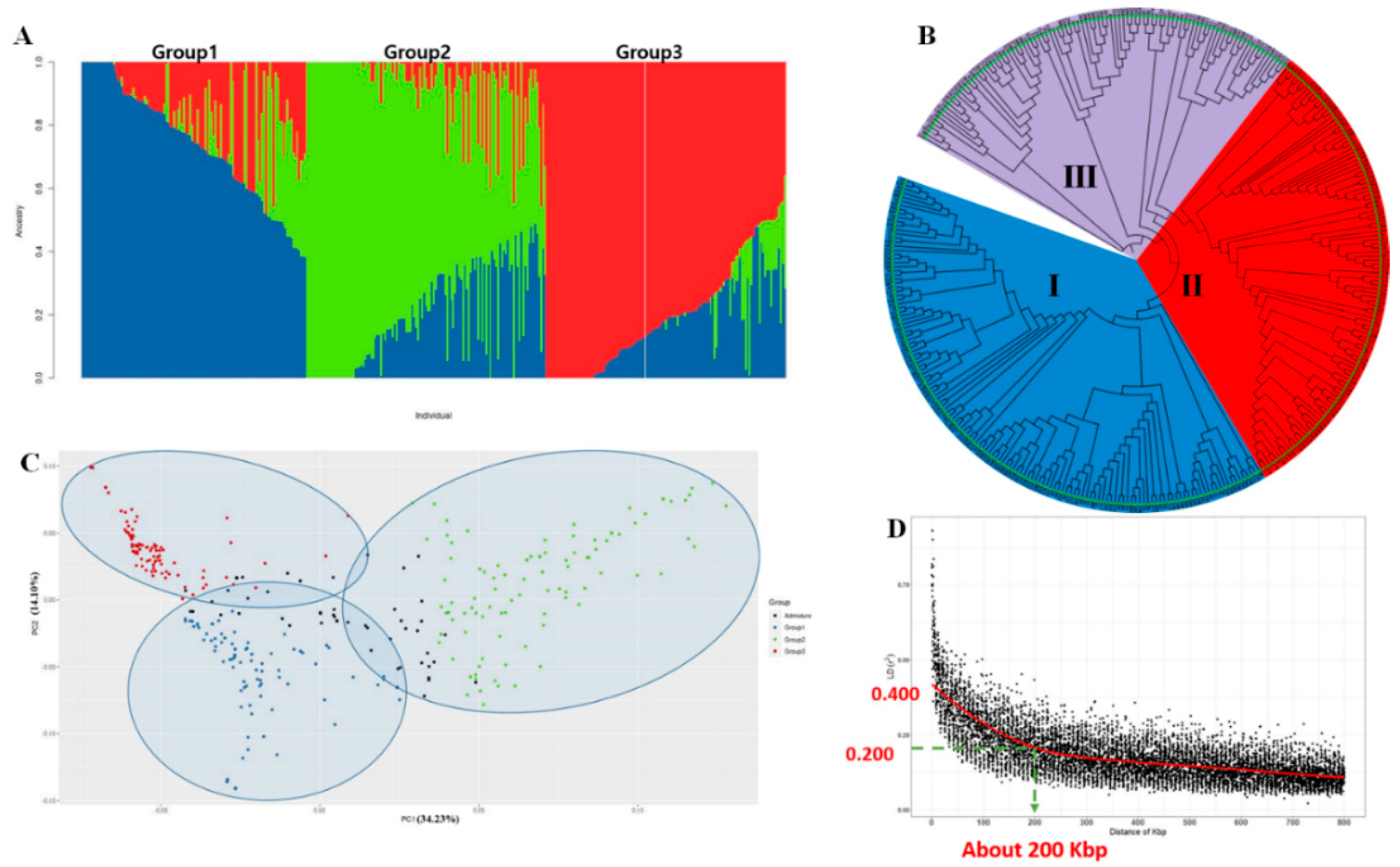
2.2. Population Structure and Principal Component and Phylogenetic Analyses
2.3. Estimation of Linkage Disequilibrium Decay
2.4. Genome-Wide Association Analysis
2.5. Putative Candidate Genes
2.6. Comparative Genome and Orthologous Gene Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Root Phenotype Evaluation
4.3. Genotyping, Population Structure, and Linkage Disequilibrium Analyses
4.4. Phylogenetic and Principal Component Analyses
4.5. Genome-Wide Association Studies
4.6. Candidate Gene Identification
4.7. RNA-Seq Data Analysis of Selected Candidate Genes
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Hameren, B.; Hayashi, S.; Gresshoff, P.M.; Ferguson, B.J. Advances in the identification of novel factors required in soybean nodulation, a process critical to sustainable agriculture and food security. J. Plant Biol. Soil Health 2013, 1, 6. [Google Scholar]
- Marinkovic, J.; Bjelic, D.; Tintor, B.; Miladinovic, J.; Dukic, V.; Dorđevic, V. Effects of soybean co-inoculation with plant growth promoting rhizobacteria in field trial. Rom. Biotechnol. Lett. 2018, 23, 13401. [Google Scholar]
- Department of Economic and Social Affairs. World Population Prospects 2019; Department of Economic and Social Affairs: New York, NY, USA, 2019. [Google Scholar]
- Gitz, V.; Meybeck, A.; Lipper, L.; Young, C.; Braatz, S. Climate Change and Food Security: Risks and Responses; FAO: Rome, Italy, 2016; pp. 1–110. [Google Scholar] [CrossRef]
- Ates, A.M.; Bukowski, M. 2021/22 Global Soybean Stocks Fall in Tandem with South American Production Economic Research Service|Situation and Outlook Report Domestic Outlook International Outlook. 2022. Available online: https://www.ers.usda.gov/webdocs/outlooks/103026/ocs-22a.pdf?v=6071 (accessed on 14 October 2022).
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Atkinson, J.A.; Pound, M.P.; Bennett, M.J.; Wells, D.M. Uncovering the hidden half of plants using new advances in root phenotyping. Curr. Opin. Biotechnol. 2019, 55, 1–8. [Google Scholar] [CrossRef]
- Tayade, R.; Kim, S.-H.; Tripathi, P.; Choi, Y.-D.; Yoon, J.-B.; Kim, Y.-H. High-Throughput Root Imaging Analysis Reveals Wide Variation in Root Morphology of Wild Adzuki bean (Vigna angularis) Accessions. Plants 2022, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Tayade, R.; Mun, B.-G.; Yun, B.-W.; Kim, Y. Silicon Application Differentially Modulates Root Morphology and Expression of PIN and YUCCA Family Genes in Soybean (Glycine max L.). Front. Plant Sci. 2022, 13, 842832. [Google Scholar] [CrossRef]
- McGrail, R.K.; Van Sanford, D.A.; McNear, D.H. Trait-Based Root Phenotyping as a Necessary Tool for Crop Selection and Improvement. Agronomy 2020, 10, 1328. [Google Scholar] [CrossRef]
- Guimarães, P.H.R.; de Lima, I.P.; de Castro, A.P.; Lanna, A.C.; Guimarães Santos Melo, P.; de Raïssac, M. Phenotyping Root Systems in a Set of Japonica Rice Accessions: Can Structural Traits Predict the Response to Drought? Rice 2020, 13, 67. [Google Scholar] [CrossRef]
- Marone, D.; Russo, M.A.; Mores, A.; Ficco, D.B.M.; Laidò, G.; Mastrangelo, A.M.; Borrelli, G.M. Importance of Landraces in Cereal Breeding for Stress Tolerance. Plants 2021, 10, 1267. [Google Scholar] [CrossRef]
- Arca, M.; Mary-Huard, T.; Gouesnard, B.; Bérard, A.; Bauland, C.; Combes, V.; Madur, D.; Charcosset, A.; Nicolas, S.D. Deciphering the Genetic Diversity of Landraces With High-Throughput SNP Genotyping of DNA Bulks: Methodology and Application to the Maize 50k Array. Front. Plant Sci. 2021, 11, 1926. [Google Scholar] [CrossRef]
- Hyten David, L.; Song, Q.; Zhu, Y.; Choi, I.-Y.; Nelson Randall, L.; Costa Jose, M.; Specht James, E.; Shoemaker Randy, C.; Cregan Perry, B. Impacts of genetic bottlenecks on soybean genome diversity. Proc. Natl. Acad. Sci. USA 2006, 103, 16666–16671. [Google Scholar] [CrossRef] [PubMed]
- Mandozai, A.; Moussa, A.A.; Zhang, Q.; Qu, J.; Du, Y.; Anwari, G.; Al Amin, N.; Wang, P. Genome-Wide Association Study of Root and Shoot Related Traits in Spring Soybean (Glycine max L.) at Seedling Stages Using SLAF-Seq. Front. Plant Sci. 2021, 12, 1598. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.J.; Song, L.; Qiu, D.; Maldonado Dos Santos, J.V.; Chai, C.; Joshi, T.; Patil, G.; Valliyodan, B.; Vuong, T.D.; Murphy, M.; et al. Genetic variants in root architecture-related genes in a Glycine soja accession, a potential resource to improve cultivated soybean. BMC Genom. 2015, 16, 132. [Google Scholar] [CrossRef]
- Dhanapal, A.P.; York, L.M.; Hames, K.A.; Fritschi, F.B. Genome-Wide Association Study of Topsoil Root System Architecture in Field-Grown Soybean [Glycine max (L.) Merr.]. Front. Plant Sci. 2021, 11, 590179. [Google Scholar] [CrossRef]
- Li, L.; Peng, Z.; Mao, X.; Wang, J.; Chang, X.; Reynolds, M.; Jing, R. Genome-wide association study reveals genomic regions controlling root and shoot traits at late growth stages in wheat. Ann. Bot. 2019, 124, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haleem, H.; Lee, G.-J.; Boerma, R.H. Identification of QTL for increased fibrous roots in soybean. Theor. Appl. Genet. 2011, 122, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Brensha, W.; Kantartzi, S.K.; Meksem, K.; Grier IV, R.L.; Barakat, A.; Lightfoot, D.A.; Kassem, M.A. Genetic Analysis of Root and Shoot Traits in the ‘ Essex’ By ‘ Forrest’ Recombinant Inbred Line (RIL) Population of Soybean [Glycine max (L.) Merr.]. Plant Genet. Genom. Biotechnol. 2012, 1, 1–9. [Google Scholar]
- Seck, W.; Torkamaneh, D.; Belzile, F. Comprehensive Genome-Wide Association Analysis Reveals the Genetic Basis of Root System Architecture in Soybean. Front. Plant Sci. 2020, 11, 590740. [Google Scholar] [CrossRef]
- Manavalan, L.P.; Prince, S.J.; Musket, T.A.; Chaky, J.; Deshmukh, R.; Vuong, T.D.; Song, L.; Cregan, P.B.; Nelson, J.C.; Shannon, J.G.; et al. Identification of Novel QTL Governing Root Architectural Traits in an Interspecific Soybean Population. PLoS ONE 2015, 10, e0120490. [Google Scholar] [CrossRef]
- Prince, S.J.; Valliyodan, B.; Ye, H.; Yang, M.; Tai, S.; Hu, W.; Murphy, M.; Durnell, L.A.; Song, L.; Joshi, T.; et al. Understanding genetic control of root system architecture in soybean: Insights into the genetic basis of lateral root number. Plant Cell Environ. 2019, 42, 212–229. [Google Scholar] [CrossRef]
- Lynch, J.P. Roots of the Second Green Revolution. Aust. J. Bot. 2007, 55, 493–512. [Google Scholar] [CrossRef]
- Jo, H.; Lee, J.Y.; Cho, H.; Choi, H.J.; Son, C.K.; Bae, J.S.; Bilyeu, K.; Song, J.T.; Lee, J.-D. Genetic Diversity of Soybeans (Glycine max (L.) Merr.) with Black Seed Coats and Green Cotyledons in Korean Germplasm. Agronomy 2021, 11, 581. [Google Scholar] [CrossRef]
- Chung, W.H.; Jeong, N.; Kim, J.; Lee, W.K.; Lee, Y.G.; Lee, S.H.; Yoon, W.; Kim, J.H.; Choi, I.Y.; Choi, H.K.; et al. Population structure and domestication revealed by high-depth resequencing of korean cultivated and wild soybean genomes. DNA Res. 2014, 21, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.; Kim, K.S.; Jeong, S.; Kim, J.Y.; Park, S.K.; Lee, J.S.; Jeong, S.C.; Kang, S.T.; Ha, B.K.; Kim, D.Y.; et al. Korean soybean core collection: Genotypic and phenotypic diversity population structure and genome-wide association study. PLoS ONE 2019, 14, e0224074. [Google Scholar] [CrossRef] [PubMed]
- Flint-Garcia, S.A.; Thornsberry, J.M.; Buckler, E.S. Structure of Linkage Disequilibrium in Plants. Annu. Rev. Plant Biol. 2003, 54, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Tan, R.; Yuan, J.; Bales, C.; Du, W.; Zhang, S.; Chilvers, M.I.; Schmidt, C.; Song, Q.; Cregan, P.B.; et al. Genome-wide association mapping of quantitative resistance to sudden death syndrome in soybean. BMC Genom. 2014, 15, 809. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Tao, Y.; Xu, C.; Li, X.; Zhang, X.; Han, Y.; Yang, X.; Sun, J.; Li, W.; et al. Identification of genetic loci and candidate genes related to soybean flowering through genome wide association study. BMC Genom. 2019, 20, 987. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Fan, X.; Huang, W.; Yang, J.; Li, C.; Wen, Z.; Li, Y.; Guan, R.; Guo, Y.; et al. Phenotypic Characterization and Genetic Dissection of Growth Period Traits in Soybean (Glycine max) Using Association Mapping. PLoS ONE 2016, 11, e0158602. [Google Scholar] [CrossRef]
- Huang, X.; Wei, X.; Sang, T.; Zhao, Q.; Feng, Q.; Zhao, Y.; Li, C.; Zhu, C.; Lu, T.; Zhang, Z.; et al. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 2010, 42, 961–967. [Google Scholar] [CrossRef]
- Morris, G.P.; Ramu, P.; Deshpande, S.P.; Hash, C.T.; Shah, T.; Upadhyaya, H.D.; Riera-Lizarazu, O.; Brown, P.J.; Acharya, C.B.; Mitchell, S.E.; et al. Population genomic and genome-wide association studies of agroclimatic traits in sorghum. Proc. Natl. Acad. Sci. USA 2013, 110, 453–458. [Google Scholar] [CrossRef]
- Liang, H.; Yu, Y.; Yang, H.; Xu, L.; Dong, W.; Du, H.; Cui, W.; Zhang, H. Inheritance and QTL mapping of related root traits in soybean at the seedling stage. Theor. Appl. Genet. 2014, 127, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, L.; Takahashi, R.; Githiri, S.M.; Rodriguez, T.O.; Tsutsumi, N.; Kajihara, S.; Sayama, T.; Ishimoto, M.; Harada, K.; Suematsu, K.; et al. Mapping quantitative trait loci for root development under hypoxia conditions in soybean (Glycine max L. Merr.). Theor. Appl. Genet. 2017, 130, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gai, J.; Lu, H. Identification of rhizosphere abiotic stress tolerance and related root traits in soybean [Glycine max (L.) Merr.]. Acta Agron. Sin. 2005, 31, 1132. [Google Scholar]
- Liu, B.; Fujita, T.; Yan, Z.H.; Sakamoto, S.; Xu, D.; Abe, J. QTL mapping of domestication-related traits in soybean (Glycine max). Ann. Bot. 2007, 100, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.J.; Vuong, T.D.; Wu, X.; Bai, Y.; Lu, F.; Kumpatla, S.P.; Valliyodan, B.; Shannon, J.G.; Nguyen, H.T. Mapping Quantitative Trait Loci for Soybean Seedling Shoot and Root Architecture Traits in an Inter-Specific Genetic Population. Front. Plant Sci. 2020, 11, 1284. [Google Scholar] [CrossRef] [PubMed]
- Lü, H.; Yang, Y.; Li, H.; Liu, Q.; Zhang, J.; Yin, J.; Chu, S.; Zhang, X.; Yu, K.; Lv, L.; et al. Genome-Wide Association Studies of Photosynthetic Traits Related to Phosphorus Efficiency in Soybean. Front. Plant Sci. 2018, 9, 1226. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Miao, H.; Liu, H.; Zhou, J.; Sui, M.; Zhan, Y.; Xia, N.; Zhao, X.; Han, Y. Genome-wide association studies reveal novel QTLs, QTL-by-environment interactions and their candidate genes for tocopherol content in soybean seed. Front. Plant Sci. 2022, 13, 1026581. [Google Scholar] [CrossRef]
- Kim, S.-H.; Subramanian, P.; Hahn, B.-S.; Ha, B.-K. High-Throughput Phenotypic Characterization and Diversity Analysis of Soybean Roots (Glycine max L.). Plants 2022, 11, 2017. [Google Scholar] [CrossRef]
- Lee, Y.G.; Jeong, N.; Kim, J.H.; Lee, K.; Kim, K.H.; Pirani, A.; Ha, B.K.; Kang, S.T.; Park, B.S.; Moon, J.K. Development, validation and genetic analysis of a large soybean SNP genotyping array. Plant J. 2015, 81, 625–636. [Google Scholar] [CrossRef]
- Alexander, D.H.; Lange, K. Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinform. 2011, 12, 246. [Google Scholar] [CrossRef]
- Porras-Hurtado, L.; Ruiz, Y.; Santos, C.; Phillips, C.; Carracedo, Á.; Lareu, M. An overview of STRUCTURE: Applications, parameter settings, and supporting software. Front. Genet. 2013, 4, 98. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.A.M.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience 2015, 4, s13742-015. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Dai, X.; Zhang, W.; Zhuang, Z.; Sanchez, D.L.; Lübberstedt, T.; Kang, Y.; Udvardi, M.K.; Beavis, W.D.; Xu, S.; et al. GWASpro: A high-performance genome-wide association analysis server. Bioinformatics 2019, 35, 2512–2514. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, H.; Long, Y.; Shu, Y.; Zhai, J. Plant Public RNA-seq Database: A comprehensive online database for expression analysis of ~45 000 plant public RNA-Seq libraries. Plant Biotechnol. J. 2022, 20, 806–808. [Google Scholar] [CrossRef]
- Xu, L.; Dong, Z.; Fang, L.; Luo, Y.; Wei, Z.; Guo, H.; Zhang, G.; Gu, Y.Q.; Coleman-Derr, D.; Xia, Q.; et al. OrthoVenn2: A web server for whole-genome comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 2019, 47, W52–W58. [Google Scholar] [CrossRef]
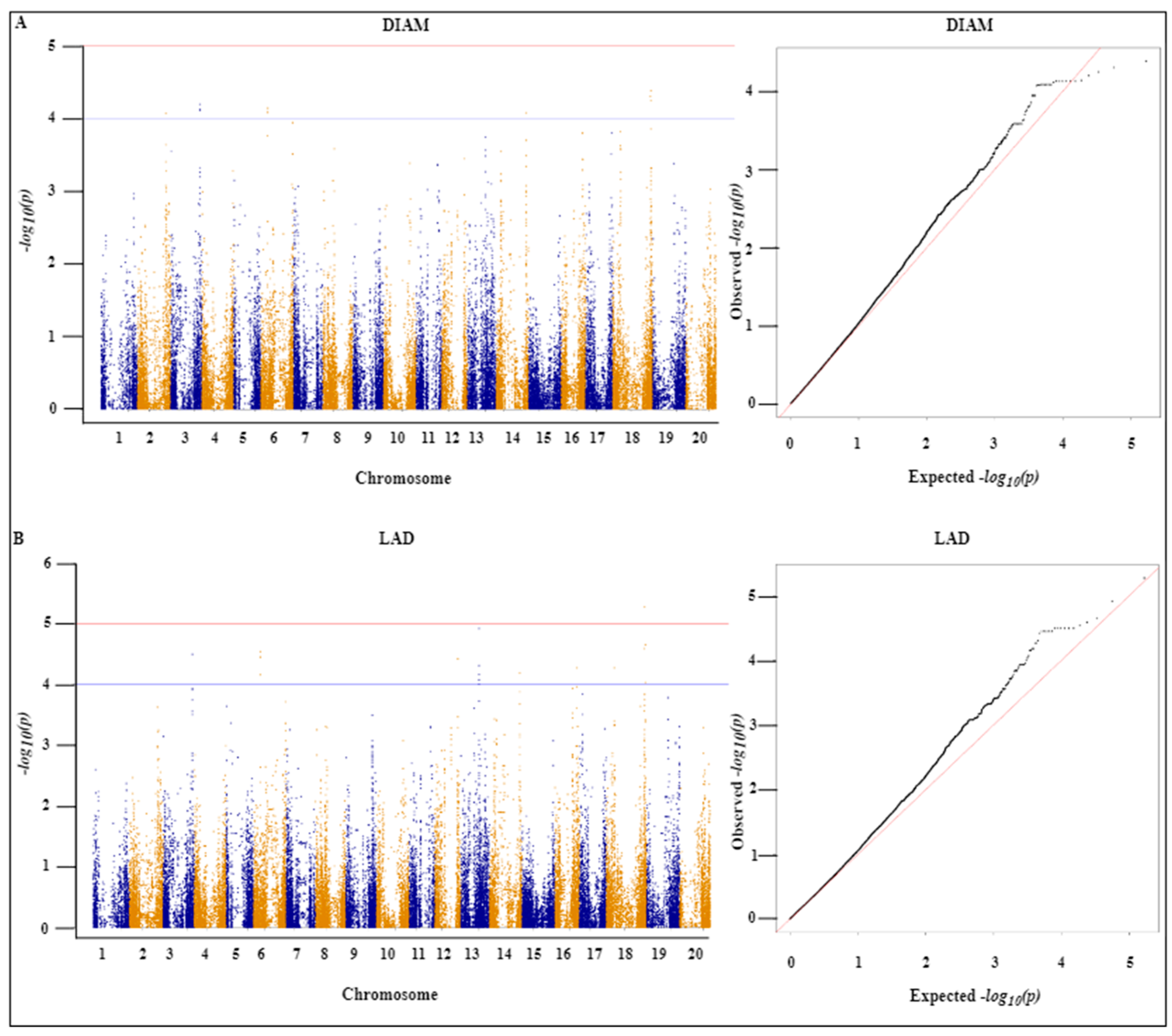
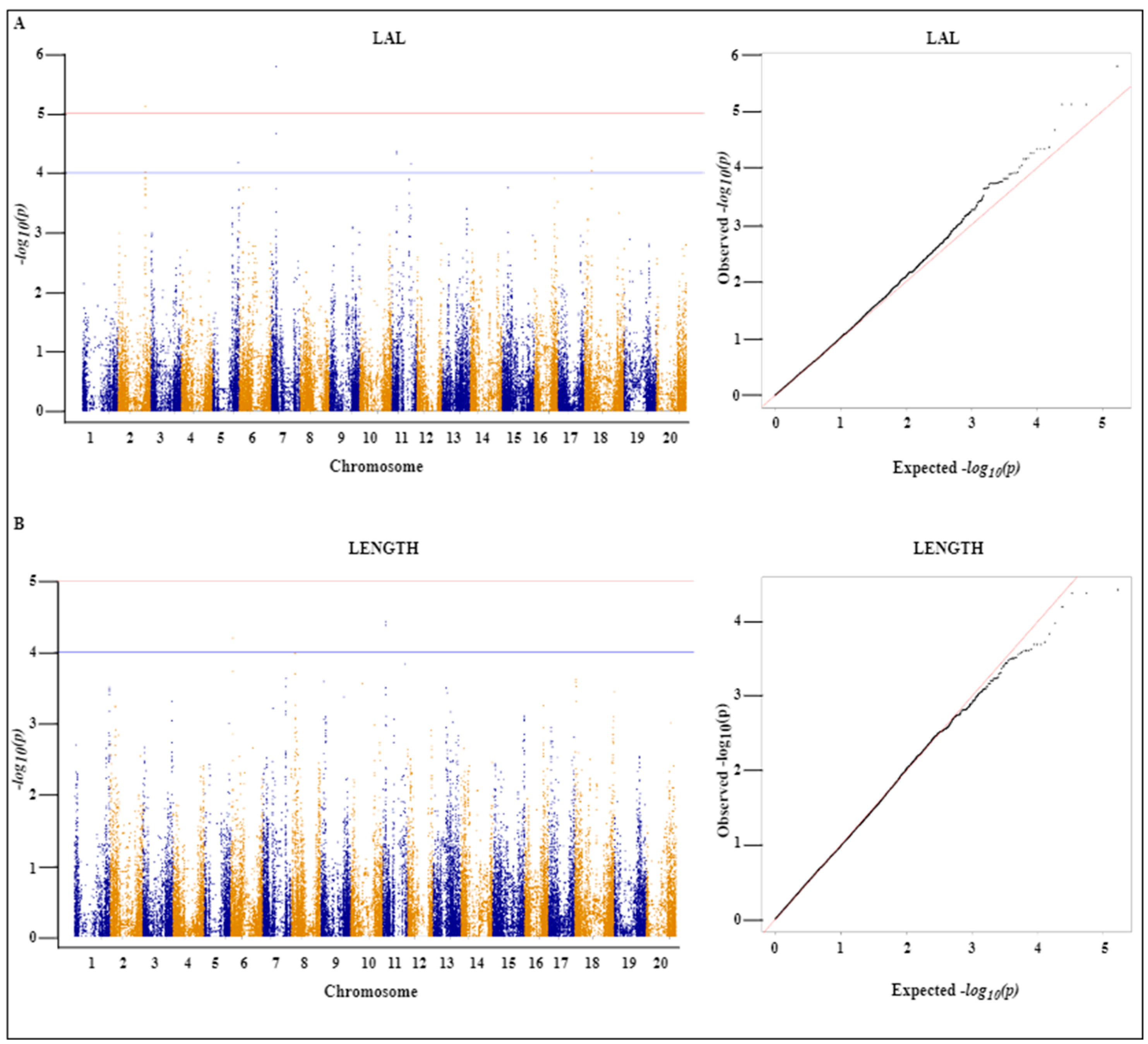
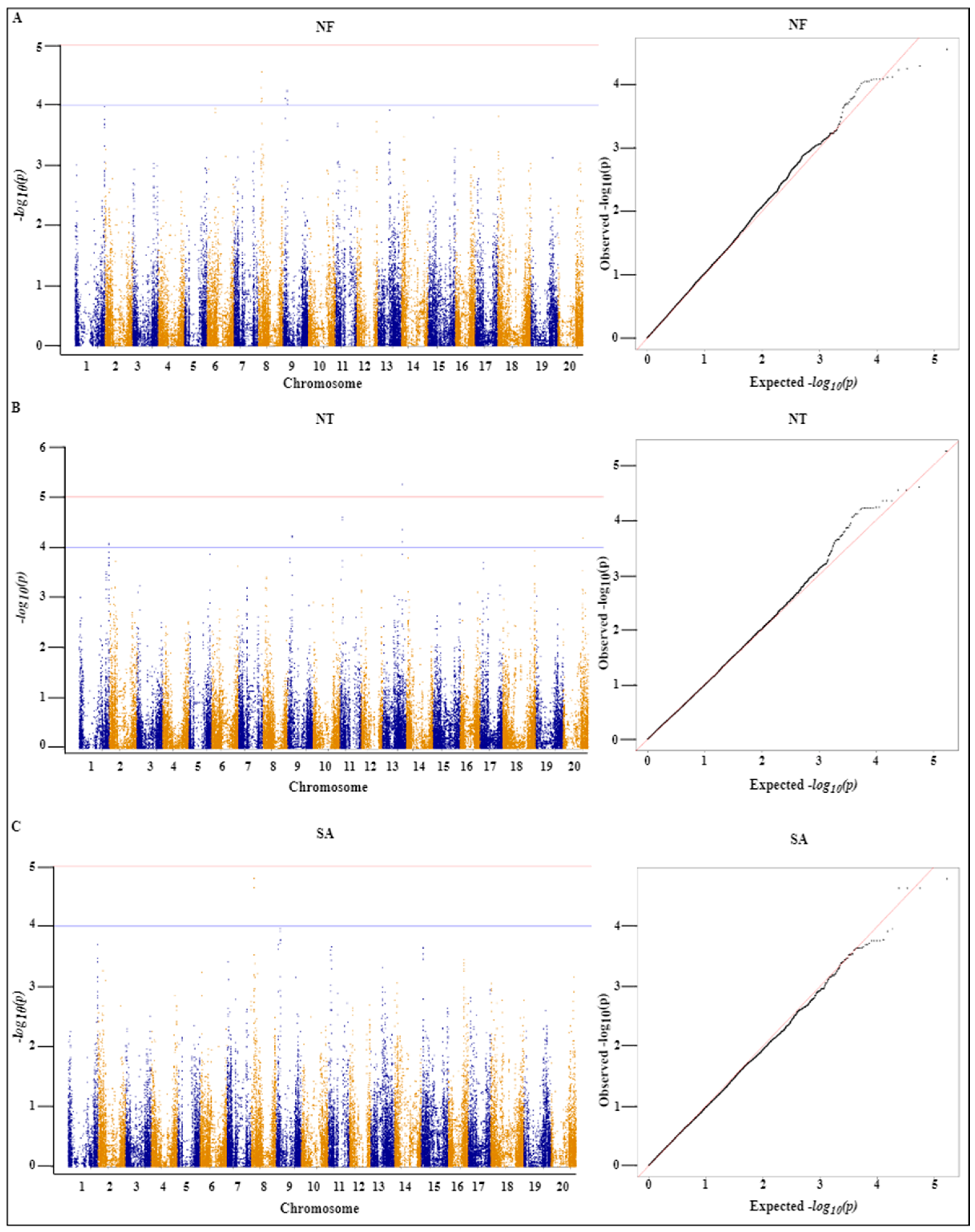
| Trait | SNP | Chr | Position | Ref | Alt | p Value | −log10(p) |
|---|---|---|---|---|---|---|---|
| DIAM | AX−90460045 | 2 | 43709318 | G | A | 8.60 × 10−5 | 4.07 |
| AX−90505093 | 3 | 44598254 | C | G | 6.41 × 10−5 | 4.19 | |
| AX−90311611 | 3 | 44782942 | G | A | 7.53 × 10−5 | 4.12 | |
| AX−90308307 | 3 | 44785822 | G | A | 7.53 × 10−5 | 4.12 | |
| AX−90385325 | 3 | 44786068 | A | G | 7.53 × 10−5 | 4.12 | |
| AX−90442177 | 3 | 44786975 | T | C | 7.53 × 10−5 | 4.12 | |
| AX−90503377 | 3 | 44787733 | A | G | 7.53 × 10−5 | 4.12 | |
| AX−90345460 | 3 | 44799953 | T | A | 7.53 × 10−5 | 4.12 | |
| AX−90385554 | 3 | 44815582 | C | T | 7.76 × 10−5 | 4.11 | |
| AX−90513850 | 6 | 10393414 | G | A | 8.32 × 10−5 | 4.08 | |
| AX−90502675 | 6 | 10469343 | G | A | 8.32 × 10−5 | 4.08 | |
| AX−90329829 | 6 | 10471655 | A | G | 7.33 × 10−5 | 4.14 | |
| AX−90504776 | 6 | 10472539 | T | A | 8.32 × 10−5 | 4.08 | |
| AX−90434642 | 6 | 10478559 | A | G | 8.32 × 10−5 | 4.08 | |
| AX−90416589 | 6 | 10495842 | A | G | 8.32 × 10−5 | 4.08 | |
| AX−90426968 | 6 | 10501950 | A | G | 8.32 × 10−5 | 4.08 | |
| AX−90524509 | 14 | 46332487 | T | C | 8.41 × 10−5 | 4.08 | |
| AX−90332250 | 14 | 46340213 | G | T | 8.41 × 10−5 | 4.08 | |
| AX−90367887 | 18 | 58632433 | T | G | 5.02 × 10−5 | 4.3 | |
| AX−90402282 | 18 | 58817483 | A | G | 4.19 × 10−5 | 4.38 | |
| AX−90468047 | 18 | 59089827 | A | C | 5.72 × 10−5 | 4.24 | |
| LAD | AX−90311611 | 3 | 44782942 | G | A | 3.18 × 10−5 | 4.5 |
| AX−90308307 | 3 | 44785822 | G | A | 3.18 × 10−5 | 4.5 | |
| AX−90385325 | 3 | 44786068 | A | G | 3.18 × 10−5 | 4.5 | |
| AX−90442177 | 3 | 44786975 | T | C | 3.18 × 10−5 | 4.5 | |
| AX−90503377 | 3 | 44787733 | A | G | 3.18 × 10−5 | 4.5 | |
| AX−90345460 | 3 | 44799953 | T | A | 3.18 × 10−5 | 4.5 | |
| AX−90513850 | 6 | 10393414 | G | A | 3.53 × 10−5 | 4.45 | |
| AX−90454547 | 6 | 10396428 | A | C | 6.94 × 10−5 | 4.16 | |
| AX−90502675 | 6 | 10469343 | G | A | 3.53 × 10−5 | 4.45 | |
| AX−90329829 | 6 | 10471655 | A | G | 2.86 × 10−5 | 4.54 | |
| AX−90504776 | 6 | 10472539 | T | A | 3.53 × 10−5 | 4.45 | |
| AX−90434642 | 6 | 10478559 | A | G | 3.53 × 10−5 | 4.45 | |
| AX−90416589 | 6 | 10495842 | A | G | 3.53 × 10−5 | 4.45 | |
| AX−90426968 | 6 | 10501950 | A | G | 3.53 × 10−5 | 4.45 | |
| AX−90481233 | 12 | 35529714 | A | G | 3.77 × 10−5 | 4.42 | |
| AX−90405818 | 13 | 27859216 | T | C | 4.91 × 10−5 | 4.31 | |
| AX−90405799 | 13 | 27870065 | G | A | 1.21 × 10−5 | 4.92 | |
| AX−90496747 | 13 | 27877321 | G | A | 8.44 × 10−5 | 4.07 | |
| AX−90521967 | 13 | 27901991 | T | G | 6.88 × 10−5 | 4.16 | |
| AX−90336511 | 13 | 27939975 | C | T | 9.79 × 10−5 | 4.01 | |
| AX−90524509 | 14 | 46332487 | T | C | 6.53 × 10−5 | 4.18 | |
| AX−90332250 | 14 | 46340213 | G | T | 6.53 × 10−5 | 4.18 | |
| AX−90502945 | 16 | 33527990 | A | G | 5.38 × 10−5 | 4.27 | |
| AX−90422071 | 18 | 12128963 | G | T | 5.35 × 10−5 | 4.27 | |
| AX−90367887 | 18 | 58632433 | G | A | 2.55 × 10−5 | 4.59 | |
| AX−90402282 | 18 | 58817483 | A | C | 5.28 × 10−6 | 5.28 | |
| AX−90365160 | 18 | 60026624 | G | A | 2.23 × 10−5 | 4.65 | |
| AX−90495375 | 18 | 60153725 | A | C | 9.16 × 10−5 | 4.04 | |
| LAL | AX−90523253 | 2 | 42676896 | A | C | 9.88 × 10−5 | 4.01 |
| AX−90367890 | 2 | 42742102 | T | G | 7.71 × 10−6 | 5.11 | |
| AX−90407903 | 2 | 42742896 | G | A | 7.71 × 10−6 | 5.11 | |
| AX−90372917 | 2 | 42754007 | T | C | 7.71 × 10−6 | 5.11 | |
| AX−90456562 | 5 | 40343307 | C | A | 6.75 × 10−5 | 4.17 | |
| AX−90365252 | 7 | 7177786 | A | G | 2.20 × 10−5 | 4.66 | |
| AX−90477018 | 7 | 7419551 | A | G | 1.65 × 10−6 | 5.78 | |
| AX−90491184 | 11 | 7212890 | G | T | 4.76 × 10−5 | 4.32 | |
| AX−90348822 | 11 | 7234607 | T | G | 4.76 × 10−5 | 4.32 | |
| AX−90337775 | 11 | 7234726 | T | G | 4.36 × 10−5 | 4.36 | |
| AX−90498877 | 11 | 7234867 | A | G | 4.76 × 10−5 | 4.32 | |
| AX−90443985 | 11 | 30156429 | C | T | 7.06 × 10−5 | 4.15 | |
| AX−90340335 | 11 | 30159162 | A | G | 7.06 × 10−5 | 4.15 | |
| AX−90362827 | 18 | 11624535 | G | T | 9.16 × 10−5 | 4.04 | |
| AX−90513611 | 18 | 11665000 | T | C | 5.60 × 10−5 | 4.25 | |
| AX−90396575 | 18 | 11670146 | G | T | 5.60 × 10−5 | 4.25 | |
| LENGTH | AX−90431861 | 6 | 3169898 | G | A | 6.34 × 10−5 | 4.2 |
| AX−90446460 | 11 | 4302823 | C | T | 4.20 × 10−5 | 4.38 | |
| AX−90414551 | 11 | 4333105 | C | G | 4.20 × 10−5 | 4.38 | |
| AX−90472866 | 11 | 4337689 | T | G | 3.75 × 10−5 | 4.43 | |
| NF | AX−90428520 | 8 | 6172141 | G | A | 5.20 × 10−5 | 4.28 |
| AX−90467878 | 8 | 6174931 | T | C | 9.11 × 10−5 | 4.04 | |
| AX−90468823 | 8 | 6176238 | C | T | 9.11 × 10−5 | 4.04 | |
| AX−90412442 | 8 | 6176371 | A | G | 9.11 × 10−5 | 4.04 | |
| AX−90493535 | 8 | 7168291 | C | A | 8.54 × 10−5 | 4.07 | |
| AX−90455156 | 8 | 7168816 | G | T | 7.81 × 10−5 | 4.11 | |
| AX−90331992 | 8 | 7170112 | C | A | 2.84 × 10−5 | 4.55 | |
| AX−90365804 | 9 | 4467094 | T | C | 7.79 × 10−5 | 4.11 | |
| AX−90495283 | 9 | 7691924 | A | C | 9.53 × 10−5 | 4.02 | |
| AX−90449059 | 9 | 7699360 | G | T | 5.73 × 10−5 | 4.24 | |
| AX−90520390 | 9 | 7709441 | T | G | 8.40 × 10−5 | 4.08 | |
| AX−90434197 | 9 | 7713796 | G | A | 8.40 × 10−5 | 4.08 | |
| AX−90498061 | 9 | 7719800 | A | G | 6.01 × 10−5 | 4.22 | |
| AX−90419697 | 9 | 7726552 | G | A | 8.40 × 10−5 | 4.08 | |
| AX−90492156 | 9 | 7734112 | A | G | 8.40 × 10−5 | 4.08 | |
| AX−90493039 | 9 | 7740050 | A | G | 9.67 × 10−5 | 4.01 | |
| NT | AX−90522644 | 1 | 54922179 | G | T | 8.83 × 10−5 | 4.05 |
| AX−90466067 | 1 | 54927068 | A | C | 8.36 × 10−5 | 4.08 | |
| AX−90467825 | 1 | 54937834 | T | C | 8.83 × 10−5 | 4.05 | |
| AX−90430136 | 9 | 7783387 | A | G | 6.06 × 10−5 | 4.22 | |
| AX−90419830 | 9 | 7795507 | G | T | 6.06 × 10−5 | 4.22 | |
| AX−90317331 | 9 | 7816745 | A | G | 5.91 × 10−5 | 4.23 | |
| AX−90313864 | 9 | 7826748 | C | T | 5.91 × 10−5 | 4.23 | |
| AX−90431604 | 9 | 7832817 | A | G | 6.32 × 10−5 | 4.2 | |
| AX−90382601 | 9 | 7840359 | G | A | 6.06 × 10−5 | 4.22 | |
| AX−90312113 | 9 | 7847844 | T | G | 6.06 × 10−5 | 4.22 | |
| AX−90455909 | 9 | 7848294 | A | C | 6.06 × 10−5 | 4.22 | |
| AX−90306078 | 9 | 7849987 | T | C | 6.06 × 10−5 | 4.22 | |
| AX−90446460 | 11 | 4302823 | C | T | 2.85 × 10−5 | 4.54 | |
| AX−90414551 | 11 | 4333105 | C | G | 2.85 × 10−5 | 4.54 | |
| AX−90472866 | 11 | 4337689 | T | G | 2.52 × 10−5 | 4.6 | |
| AX−90325580 | 13 | 36648560 | A | T | 4.45 × 10−5 | 4.35 | |
| AX−90370286 | 13 | 36650121 | A | G | 4.45 × 10−5 | 4.35 | |
| AX−90457815 | 13 | 36655953 | G | A | 5.60 × 10−6 | 5.25 | |
| AX−90452276 | 13 | 36656131 | G | T | 4.45 × 10−5 | 4.35 | |
| AX−90480597 | 13 | 36667146 | G | A | 7.85 × 10−5 | 4.11 | |
| AX−90305162 | 13 | 36668072 | A | G | 7.85 × 10−5 | 4.11 | |
| AX−90433497 | 13 | 36669907 | G | A | 7.85 × 10−5 | 4.11 | |
| AX−90383650 | 20 | 36657615 | G | T | 6.56 × 10−5 | 4.18 | |
| SA | AX−90428520 | 8 | 6172141 | G | A | 1.61 × 10−5 | 4.79 |
| AX−90467878 | 8 | 6174931 | T | C | 2.30 × 10−5 | 4.64 | |
| AX−90468823 | 8 | 6176238 | C | T | 2.30 × 10−5 | 4.64 | |
| AX−90412442 | 8 | 6176371 | A | G | 2.30 × 10−5 | 4.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Tayade, R.; Kang, B.-H.; Hahn, B.-S.; Ha, B.-K.; Kim, Y.-H. Genome-Wide Association Studies of Seven Root Traits in Soybean (Glycine max L.) Landraces. Int. J. Mol. Sci. 2023, 24, 873. https://doi.org/10.3390/ijms24010873
Kim S-H, Tayade R, Kang B-H, Hahn B-S, Ha B-K, Kim Y-H. Genome-Wide Association Studies of Seven Root Traits in Soybean (Glycine max L.) Landraces. International Journal of Molecular Sciences. 2023; 24(1):873. https://doi.org/10.3390/ijms24010873
Chicago/Turabian StyleKim, Seong-Hoon, Rupesh Tayade, Byeong-Hee Kang, Bum-Soo Hahn, Bo-Keun Ha, and Yoon-Ha Kim. 2023. "Genome-Wide Association Studies of Seven Root Traits in Soybean (Glycine max L.) Landraces" International Journal of Molecular Sciences 24, no. 1: 873. https://doi.org/10.3390/ijms24010873
APA StyleKim, S.-H., Tayade, R., Kang, B.-H., Hahn, B.-S., Ha, B.-K., & Kim, Y.-H. (2023). Genome-Wide Association Studies of Seven Root Traits in Soybean (Glycine max L.) Landraces. International Journal of Molecular Sciences, 24(1), 873. https://doi.org/10.3390/ijms24010873








