Abstract
Aiming at expanding the portfolio of Old Yellow Enzymes (OYEs), which have been systematically studied to be employed in the chemical and pharmaceutical industries as useful biocatalysts, we decided to explore the immense reservoir of filamentous fungi. We drew from the genome of the two Ascomycetes Aspergillus niger and Botryotinia fuckeliana four new members of the OYE superfamily belonging to the classical and thermophilic-like subfamilies. The two BfOYEs show wider substrate spectra than the AnOYE homologues, which appear as more specialized biocatalysts. According to their mesophilic origins, the new enzymes neither show high thermostability nor extreme pH optimums. The crystal structures of BfOYE4 and AnOYE8 have been determined, revealing the conserved features of the thermophilic-like subclass as well as unique properties, such as a peculiar N-terminal loop involved in dimer surface interactions. For the classical representatives BfOYE1 and AnOYE2, model structures were built and analyzed, showing surprisingly wide open access to the active site cavities due to a shorter β6-loop and a disordered capping subdomain.
1. Introduction
Old Yellow Enzymes (OYEs, EC 1.6.99.1) are a class of flavin-dependent ene-reductases (ERs) catalyzing the asymmetric hydrogenation of electronically activated C=C bonds in the presence of nicotinamide cofactors. As such, they are biocatalysts sought for effective synthesis methods designed and exploited in the pharmaceutical industry. They have been ubiquitously described and characterized at the genomic, structural, and catalytical levels in bacteria, animals, plants, and some yeast species [1]. In 2014, 60 genomes from the clades of Ascomycota and Basidiomycota were widely screened by Nizam et al. for the systematic research of OYE homologues, in which 424 putative OYE proteins were identified, and intriguingly, the number of OYE homologues found in each genome varied from 1 to 22 [2]. Such an evolutionary diversification was interpreted as a mechanism of fungal adaptation to different environments and carbon sources. The different isoenzymes may have evolved toward the recognition and reduction of specific substrates, as their expression is generally triggered by the surrounding environment (e.g., nutrients, presence of noxious substrates, or chemical-physical parameters) [2,3].
Filamentous fungi have recently attracted increasing attention as OYE reservoirs due to their unique features, and different whole-cell functional screenings have been reported. Carballeira et al. [4] analyzed the ability of 241 fungi to reduce the C=C bond of carvone. Out of the considered 241 fungi, just 3 of them (i.e., Gongronella butleri, Schizosaccharomyces octosporus, and Diplogelasinospora grovesii) were capable of reducing the model substrate with high productivity and stereoselectivity. Another functional screening was performed by Romagnolo et al. [3], evaluating the ability of 28 filamentous fungi belonging to different phyla (Ascomycota, Basidiomycota, and Zygomycota) to reduce 3 representative standard OYE substrates (cyclohex-2-en-1-one, α-methylnitrostyrene, and α-methylcinnamaldehyde). ER activity was widespread among all the screened organisms, as 27 fungi catalyzed the reduction of at least 1 of the 3 substrates tested. Mucor circinelloides, Mucor plumbeus, and Gliomastix masseei were the most versatile strains, showing the highest conversions for all the analyzed substrates. Avoiding an extensive review here, several other works showed the chemoselective reduction of α,β-unsaturated compounds using marine-derived fungi [5,6,7,8,9,10,11,12].
Fungi therefore appear to be valuable sources for ERs. Nevertheless, the number of isolated and characterized enzymes from these eukaryotic organisms is limited. These few examples are the OYE homologues FgaOX3 (or EasA) from Aspergillus fumigatus [13,14], FgaOX3Pc from Penicillium commune [15], and FgaOX3Pr3 from Penicillium roqueforti [16], which were produced as recombinant proteins, and their activity as ene-reductases was demonstrated in vitro in the reduction of chanoclavine-I aldehyde, an intermediate of the ergot alkaloid biosynthesis. Other studies have demonstrated the potential of fungal ERs to catalyze critical steps in the production of active pharmaceutical ingredients. Two examples are the synthesis of (R)-flurbiprofen, achieved at the semi-preparative scale by KYE2 from Kluyveromyces marxianus CBS4857 [17], and the asymmetric reduction of β-activated vinylphosphonates (fosfomycin and fosmidomycin analogues), carried out at the preparative scale by OYE3 from Saccharomyces cerevisiae [18].
Apart from the few examples reported in the literature, fungi are still a source of OYEs that is mostly unexplored. We have identified and produced four new putative ERs from the filamentous fungi Aspergillus niger CBS 513.88 and Botryotinia fuckeliana B05.10. We have characterized their substrate scope and biochemical properties, and obtained the crystal structure for one of them (a second crystal structure, the one for BfOYE4, was anticipated in a submitted article by our group) [19]. This study thus broadens the present landscape of ene-reductases.
2. Results and Discussion
2.1. Identification of the New Putative ERs and Sequence Analysis
The Ascomycetes Aspergillus niger and Botryotinia fuckeliana were identified as promising sources for new putative ene-reductases by the genome-wide bioinformatic screening of Nizam et al. [2] and by the functional activity screening of Romagnolo et al. [3]. In the genomes of these fungi, different OYE homologues (i.e., 12 and 4) are present, and they belong to different OYE classes (Table S1). Furthermore, A. niger cells were shown to moderately reduce cyclohex-2-en-1-one and completely convert α-methyl-β-nitrostyrene within 2 days, while the B. fuckeliana cells were able to completely reduce cyclohex-2-en-1-one, and moderate activity was detected with α-methylcinnamaldehyde [3].
AnOYE2 (XM_001393007) and AnOYE8 (NT_166519) were chosen among the 12 OYE homologues present in the A. niger genome, being 42% and 30% identical to the OYE1 probe, respectively. Furthermore, BfOYE1 (XP_001558622) and BfOYE4 (XP_001554780) were chosen among the 4 OYE homologues present in the B. fuckeliana genome, as BfOYE1 shows 43% identity and BfOYE4 shows 27% identity with the OYE1 sequence.
Based on the most recent classification [20], AnOYE2 and BfOYE1 belong to Class II OYEs, as they share all the fingerprint motifs described for “classical” OYE homologues identified in fungi. The alignments in Figure S1 show that both enzymes have the conserved residues involved in the FMN binding site architecture reported for OYE1 (P36, T38, G73, Q115, R244, G325, N326, F327, G346, G348, and R349) [21]. Notably, up to the present update, Class II is exclusively populated by members of the Ascomycota phylum of fungi (Figure 1).
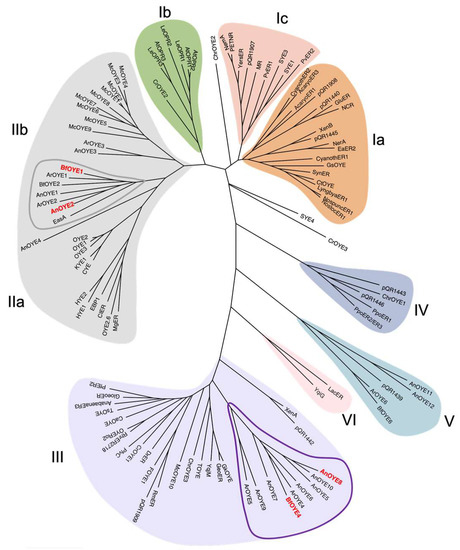
Figure 1.
Phylogenetic analysis of fungal and previously described Old Yellow Enzymes. The evolutionary history conducted in MEGA11 was inferred by using the maximum likelihood method based on the JTT matrix-based model. The unrooted tree with the highest log likelihood (−68,148.01) is shown. Accession codes of all sequences used are reported in Table S2.
On the other hand, AnOYE8 and BfOYE4 share most of the typical conserved residues reported in Class III OYEs (S23, P24, C26, A60, Q102, R215, S249, Q265, G284, M285, F305, G307, R308, and R312; YqjM numbering) (Figure S1). However, some differences can be found in the C-terminal sequence involved in monomer-monomer interaction and dimer formation. The arginine finger (R336 in YqjM) that protrudes into the active site of each monomer and contributes to defining the key features of the catalytic pocket is substituted, both in AnOYE8 and BfOYE4, by a bulkier hydrophobic amino acid such as tryptophan, as also seen in Ppo-ER3 (W376) (Figure S1) and XenA (W358). Generally, Class III OYEs isolated from extremophile bacteria have shorter amino acid sequences (337–371 residues) than Class I or Class II homologues (349–412) and a higher content of proline residues in their loops and turns [22]. AnOYE8 and BfOYE4 are 439 and 421 amino acids long, respectively, and they have a lower proline content (<7%) if compared with thermophilic Class III homologues, especially the one isolated from the Thermus species. The longer N-terminal loops (blue stretch in Figure S1) can be a common feature of fungal enzymes (i.e., putative signal peptides targeting the enzyme to the appropriate subcellular compartment). Nizam et al. [2] reported a mitochondrial localization for BfOYE4 protein and a cytoplasmatic one for AnOYE8. Longer sequences, longer N-termina, and a low proline content (see forward, Table S3) are peculiar characteristics that make the new members cluster in an isolated fungal (Ascomycota) sub-clade of Class III, which exclusively groups OYEs of a bacterial origin (Figure 1).
Based on the genome-wide screening performed by Nizam et al. [2] on a great variety of OYE sequences present in fungi, a third class of OYE homologues was identified (Class V in Figure 1) with peculiar FMN interactions and active site organization [21]. Two OYE homologues from A. niger (AnOYE11 and AnOYE12) and one homologue from B. fuckeliana (BfOYE6) belong to this new class (see Table S1). However, to our knowledge, this class of OYEs is still unexplored.
2.2. Production and Purification of the Recombinant Proteins
All the proteins were produced in the heterologous host E. coli BL21 (DE3) with an N-terminal His6-tag. BfOYE1 was the only protein to be handily purified with very good yields (40 mg/L) and homogeneity (Figure S2) when 25 µM of riboflavin was added to the cell culture media. In the absence of this FMN precursor, the protein was obtained in lower amounts (Figure S3). The addition of the cofactor precursor was also employed for the production of the other OYE homologues. However, both lowering the growth temperature after induction and trying different E. coli strains endowed with bacterial chaperons were necessary to increase the yield (BfOYE4, as already reported in Robescu et al. [19]) or improve solubility (AnOYE2 and AnOYE8).
Initially, the expression of the AnOYE2 gene was attempted in BL21 (DE3) cells at 25 °C and 16 °C. The protein was highly over-expressed but completely insoluble at 25 °C, and only a small fraction was soluble at 16 °C (Figure S4, panel A). The E. coli Arctic® strain resulted in being the most convenient, with a high amount of soluble protein obtained at 16 °C (Figure S4, panel B). AnOYE8 was synthesized at similarly high levels in BL21 (DE3) cells at 25 °C, but its solubility was very low (Figure S5, panel A). In the E. coli BL21 (DE3) Arctic® strain grown at 12 °C, its solubility was clearly enhanced, but a major insoluble fraction was still present (Figure S5, panel B). Both proteins were finally purified at a low temperature from the E. coli BL21 (DE3) Arctic® cells in moderate yields (13 mg/L AnOYE2 and 9 mg/L AnOYE8). The chaperonin Cpn60 co-expressed by the Arctic® strain was co-purified as a major contaminant of AnOYE2 and AnOYE8. Even though most of the undesired contaminant was removed (Figures S4 and S5, panel C, lane Chp) following a standard protocol [23], some contaminant chaperonin molecules remained in the final preparation. With the goal of favoring the folding of these proteins in vivo and detaching them from the undesired Cpn60, for further crystallization trials, we increased the concentration of riboflavin added to the culture media up to 100 μM. With this strategy, the solubilities of both proteins in the E. coli BL21 (DE3) cells were enhanced, with yields of 18 mg/L for AnOYE2 and 10 mg/L for AnOYE8 (Figure S2, panel A and Figure S2, panel B, respectively).
The UV–visible absorbance spectra of the purified proteins displayed a shift in the maximum peak absorbance upon thermal denaturation. After boiling the samples for 15 min at 90 °C, the supernatant turned bright yellow, and the maximum of its absorbance spectrum shifted to 446 nm (Figure S6), corresponding to the maximum of the released free FMN.
2.3. Oligomeric State Determination
Following examination of the size and purity by SDS-PAGE, the oligomeric states of the recombinant AnOYE2, AnOYE8, and BfOYE1 proteins were investigated by analytical gel filtration chromatography (Figure 2A; see calibration curve in Figure S7). BfOYE1 (43.5 kDa) was eluted as a single peak in solution with an apparent molecular weight of 57 kDa, corresponding to a retained dimer (as confirmed by BN-PAGE, Figure 2B). AnOYE2 (43.3 kDa) showed the presence of both retained monomeric and dimeric or oligomeric species (as suggested by BN-PAGE, Figure 2B).
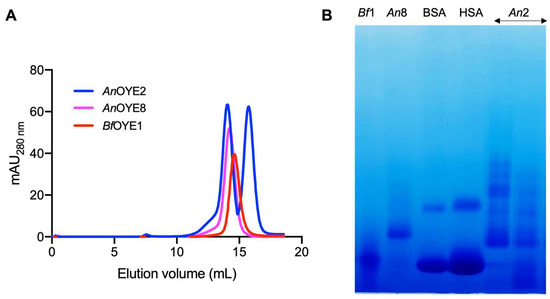
Figure 2.
Analytical gel filtration of purified protein samples (150 µg) eluted from a Superdex 200 10/300 GL column (A) and blue native gel (BN-PAGE) of samples (12.5 µg) from each observed peak (B). BSA = bovine serum albumin (25 µg); HSA = human serum albumin (30 µg).
For the Class III AnOYE8 enzyme (47.9 kDa), analytical gel filtration analysis revealed a single peak with an apparent molecular weight of roughly 1.3-fold the monomer, once again in between the dimeric and monomeric expected retention times. This result can be interpreted again as the retention by the matrix of dimeric fractions (as observed in BN-PAGE, too; Figure 2B). Indeed, in the crystal structure determined in this work, AnOYE8 formed a dimeric species whose architecture agreed with the typical quaternary organization of Class III OYEs.
For BfOYE4, a heterogeneous profile in size exclusion chromatography suggesting the presence of different oligomeric species in solution was already reported [19]. Analogous to AnOYE8, in the crystal structure of BfOYE4, the typical dimeric architecture could be observed [19].
Generally speaking, members of the Class I and Class II OYEs (“classical homologues”) were found to occur in solution exclusively as monomers or homodimers. On the other hand, class III OYEs (“thermophilic-like homologues”) usually occurred as homodimers or homotetramers in solution. Higher species (octamers and dodecamers) were observed in some thermophilic homologues (e.g., TOYE [24] and TsOYE [25]), and monomeric species were found in non-thermostable homologues as well (e.g., RmER [26] and CaOYE [27]) (Table S3). It has been reported that the equilibrium between different oligomeric species in OYEs depends on the protein concentration as well as the flavin redox state [28].
2.4. Substrate Spectra
To explore the biocatalytic potential of the newly identified ene-reductases, different standard substrates were tested spectrophotometrically by measuring the consumption of NADPH at 340 nm. The standard substrates belong to different types of molecular classes activated by different electron-withdrawing groups (e.g., linear and cyclic ketones, as well as aldehydes and maleimides). The results from such an evaluation are summarized in Table 1.

Table 1.
Spectrophotometric substrate screening 1.
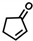
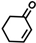
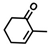
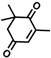
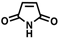
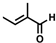
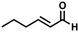
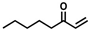
The non-substituted five-member ring cyclopent-2-en-1-one (1) was reduced with lower reaction rates by BfOYE enzymes compared with the non-substituted six-member ring cyclohex-2-en-1-one (2) (3.3–10.3 U/mg vs. 2.2–3.8 U/mg). The same enzymes converted the α-substituted 2-methyl-cyclohex-2-en-1-one (3), with BfOYE1 showing fourfold higher activity (5.7 vs. 1.4 U/mg) than BfOYE4. The reduction of ketoisophorone (4) was detected just in the presence of BfOYE1 with a rate comparable to those of the other accepted substrates (10.3 U/mg). BfOYE1, BfOYE4, and AnOYE2 demonstrated themselves to be active in the presence of maleimide (5) (11.3, 22.5, and 16.6 U/mg, respectively). Curiously, AnOYE8 appeared to be totally inactive on this substrate, which is generally considered a typical substrate of ene-reductases. The activity of all four enzymes with 5 was also tested in the presence of NADH, but a maleimide reduction was undetectable, suggesting a strong preference for the NADPH cofactor. Three linear substrates were also taken in the exam: (E)-2-methylpent-2-enal (6), (E)-hex-2-enal (7), and oct-1-en-3-one (8). Among all measures, significant activities were registered for BfOYE1, with BfOYE4 showing a singularly high activity only with 8.
Overall, the enzymes isolated from B. fuckeliana had a wider substrate spectrum and higher specific activities compared with the ones isolated from A. niger. Even if the A. niger cells were shown to be active in the reduction of cyclohex-2-en-1-one (2) and (Z)-β-methyl-nitrostyrene [3], in our hands, AnOYE2 and AnOYE8 seemed to have a restricted substrate spectrum. Particularly, the former enzymes appeared to be the most selective one among those studied here. Since the genome of this Ascomycete encodes 12 putative OYEs, high substrate specialization for each isoenzyme and expression triggered by different substrates or conditions can be conceivably envisaged. Indeed, such a hypothesis has already been put forward for two other fungal species: Ascochyta rabiei (Ascomycete) and Mucor circinelloides (Mucoromycete). In both cases, genome analysis found 6 [21] and 10 [29] putative OYEs, respectively, for which the modeling-predicted structural differences suggested specific substrate-enzyme matching. Afterward, the expression profiles of the genes encoding the putative isoenzymes were analyzed for both species and clearly showed differential activation in response to environmental stimuli (plant infection or oxidative stress for A. rabiei) [2,21] or exposure to different substrates (for M. circinelloides) [30]. Thus, the low activities registered for our two AnOYEs could be ascribed to the fact that standard substrates generally used during screenings of OYEs may not mimic the natural substrates (still unknown) of these enzymes, leading to a limited activity spectrum and low specific activities being recorded.
2.5. Steady State Kinetic Parameters
Given the substrate preferences and specific activities observed during the preliminary screening, the kinetic constants were determined for the best substrates (Table 2). The enzymatic properties of three out of the four new OYEs were rapidly measured by spectrophotometric assays. This could not be managed with AnOYE8 due to the very modest activity registered for all the substrates tested.

Table 2.
Steady state kinetic parameters of AnOYE2, BfOYE1, and BfOYE4 1.
For AnOYE2, the kinetic constants were determined, with just the preferred substrate maleimide (5) showing good catalytic efficiency (738 mM−1 s−1). In addition, with maleimide, the highest affinities (KM 0.01 mM and 0.07 mM) and activities (kcat = 9.91 s−1 and 36.35 s−1) were registered for both BfOYE1 and BfOYE4, respectively, resulting in high catalytic efficiencies. Cyclohex-2-en-1-one (2) was reduced by both the Bf enzymes with catalytic efficiencies in the range of 3.9–11.4 mM−1 s−1. The introduction of the methyl group in the α position (3) of cyclohex-2-en-1-one reduced their activity toward the substrate, as already found in the preliminary screening. In particular, BfOYE1 could better tolerate the methyl substitution on the ring than BfOYE4, with a catalytic efficiency of 11.4 vs. 3.9 mM−1 s−1. Other bulkier substitutions, such as that in ketoisophorone (4), reduced the catalytic efficiency almost fourfold compared with 2 for BfOYE1 and zero for BfOYE4. The steric hindrances appeared to be significant for the activity on the aromatic compounds. Indeed, the ring size also had a meaningful effect on the kinetic parameters, with the catalytic efficiencies observed for both enzymes with cyclopent-2-en-1-one (1) being very low (1.0–2.1 mM−1 s−1) compared with the ones for cyclohex-2-en-1-one (2). BfOYE1 accepted as substrates the aliphatic enals (E)-2-methylpent-2-enal (6) and (E)-hex-2-enal (7) (112.0 and 23.6 mM−1 s−1, respectively) but not the aliphatic enone oct-1-en-3-one (8). The latter compound was instead transformed with discrete efficiency by BfOYE4 (8.3 mM−1 s−1). Finally, for AnOYE2, BfOYE1, and BfOYE4 the kinetic parameters for the preferred cofactor NADPH were also determined. AnOYE2 and BfOYE1 showed very good catalytic efficiencies (216.2 mM−1 s−1 and 233.2 mM−1 s−1, respectively). BfOYE4 showed a lower affinity (KM = 0.13 mM) but high activity (kcat = 89 s−1) for NADPH.
2.6. pH Optimum and Thermal Stability
Other relevant parameters such as the pH optimum and melting temperature were investigated.
BfOYE1 and AnOYE8 showed their maximum activity in a limited pH range of between 6.0 and 7.0 and between 7.0 and 8.0, respectively, compared with BfOYE4 [19] and AnOYE2 (100% activity from pH 6.0 to pH 10.0) (Figure S8). While BfOYE1 was still active at extremely acidic pH values (e.g., 35% of activity was retained at pH 4.0), AnOYE8 seemed sensitive to acidic and basic pH values.
The stability of all proteins was evaluated by detecting the apparent melting temperature (Tm) using the Thermofluor method in the presence of various co-solvents: ethanol, acetone, acetonitrile, dimethyl sulfoxide, and dioxane. As reported in Figure S9, AnOYE2 and BfOYE1 showed Tm values in aqueous buffer at pH 8.0 of 41.5 °C and 42 °C, respectively. Both enzymes could tolerate low percentages of organic co-solvents (5% v/v), even if AnOYE2 showed good stability in up to 40% v/v dimethyl sulfoxide. A decrease in stability was observed in high percentages (10–40% v/v) for the other co-solvents. Although clustering in the thermophilic-like Class III, AnOYE8, and BfOYE4 showed Tm values of 44 °C and 43 °C, respectively, similar to other enzymes categorized into the same subclass but isolated from mesophilic organisms and reported in the literature as non-thermostable (Table S3). AnOYE8 was very stable up to 50% v/v dimethyl sulfoxide, but a decrease in its stability was measured in all the other co-solvents even in low percentages. Conversely, BfOYE4 could tolerate up to 30% v/v of acetone, acetonitrile, and dimethyl sulfoxide well.
2.7. AnOYE8 X-ray Crystal Structure
The crystal structure of AnOYE8 (PDB 7QFX) was determined to a maximum resolution of 2.8 Å. AnOYE8 was solved and refined in the C121 space group with four molecules per asymmetric unit (Table S4). The enzyme presented both the typical features of Class III OYE homologues and peculiar properties. The overall structure of a single subunit showed the typical conserved α8/β8-TIM barrel fold (Figure 3A). In agreement with the general behavior of Class III OYEs, the four molecules within the asymmetric unit assembled into dimers with the functional quaternary architecture peculiar of these enzymes. AnOYE8 is an elongated dimer composed of two protomers (Figure 3A) with an interacting surface of 2807 Å2 (data obtained by EMBL-EBI-PISA analysis [31]). Both the α1-helix and loop 3 (capping subdomain), as well as the peculiar long N-terminal and C-terminal loops of each protomer, were involved in the dimerization surface, which contained 71 interfacing residues that established 18 hydrogen bonds and 8 salt bridges. The contribution to solvation free energy upon complex formation due to van der Waals contacts and hydrophobic interactions amounted to −30.9 kcal/mol (average values within the asymmetric unit). The C-terminus of each subunit (from Gly393 to Lys416) protruded through an “amino acid finger” (Trp397) that reached the active site of the adjacent subunit, shaping the entrance and the catalytic cavity of each subunit (Figure 3A). AnOYE8 accommodated a non-covalently bound FMN cofactor molecule lying at the bottom of the active site pocket. As expected, the si-face of the FMN was exposed to the solvent while the re-face was in contact with the protein backbone. The cofactor was bound via extensive hydrogen bonding and hydrophobic interactions with side as well as main chain elements (Figure S10). Similar to other Class III OYEs, AnOYE8 contained the highly conserved proton donor Tyr220 and the histidine pair (His215 and His218) involved in the binding and proper orientation of the substrate through hydrogen bonding with the electron-withdrawing group of the substrate (Figure 3B). The omit electronic density map revealed the presence of a small organic molecule, most likely SO4 anion, present within the crystallization precipitant agents, lying on top of the isoalloxazine ring of FMN (Figure 3B). In the complex with SO4, two oxygens of the compound formed two hydrogen bonds with the side chain of His215 and Tyr220, thus confirming the importance of these residues in substrate orientation and catalysis.
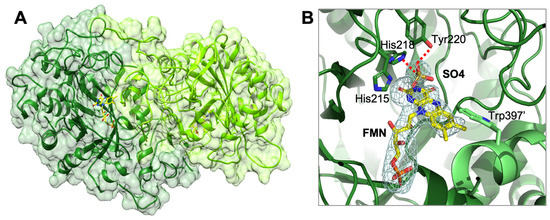
Figure 3.
Cartoon representation of the crystal structure of AnOYE8 (PDB: 7QFX): (A) surface representation showing the two protein chains forming the dimeric architecture; (B) details of AnOYE8 active site, where the ligands omit map (FMN and SO4) was calculated by Phenix and is shown contoured at 2.5 σ. The two protomers of AnOYE8 are colored in forest green and light green. FMN cofactor and sulphate anion (SO4) bound in the active site are shown with C atoms in yellow and orange, respectively. Most relevant residues of the catalytic cavity are shown with forest green C atoms, while Trp397′ of the adjacent protomer is shown with light green C atoms, red for O, and blue for N. Hydrogen bonds are shown as red dashed lines.
To our knowledge, a single crystal structure of Class III OYEs isolated from filamentous fungi has been reported so far: BfOYE4, crystalized by our group and described elsewhere [19]. As previously shown in the phylogenetic tree (Figure 1), AnOYE8 and BfOYE4 were grouped along with other putative fungal OYEs into a sub-clade of Class III, distinct from other well-known bacterial enzymes. Indeed, the two structures show high structural similarity with an r.m.s.d. of 0.712 Å (Figure S11), and both are characterized by protein sequences longer than those of the other enzymes of the clade (421 and 439 amino acids, respectively), as underlined above. In both cases, the longer N-terminal and C-terminal loops contribute to forming the dimer interface and shaping the active site architecture, respectively (Figure S12). In particular, the C-terminus of both proteins protrudes through a Trp finger (and not an Arg finger, as reported for the majority of bacterial Class III homologues) into the adjacent protomer, partially reducing the accessibility of the active site pocket (this effect being more pronounced in AnOYE8 than BfOYE4). CASTp analysis confirmed that the volume of the active site cavity was slightly smaller in AnOYE8 (861.24 Å3) compared with BfOYE4 (1117.2 Å3), which indeed has a more exposed catalytic pocket (Figure S12).
When the electrostatic surface potential was considered, the two structures appeared to be quite similar (Figure 4), as well as their theoretical isoelectric points (Table S1). In both cases, the inner surface of the barrel was found to be heavily positive, in agreement with its affinity to the negatively charged FMN molecule. The outer surface of both enzymes showed some differences, with BfOYE4 presenting negatively charged spots more pronounced than those in AnOYE8. Around the active site cavities, the surface seemed to be slightly positively charged in both proteins. Distinctive features in the charge distributions of AnOYE8 and BfOYE4 with respect to other members of ClassIII can be further appreciated in Figure S13.
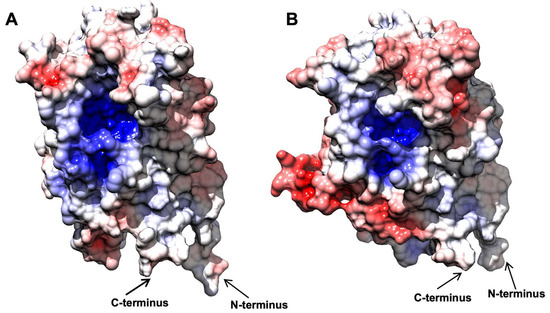
Figure 4.
Comparative analysis of surface charge distribution, calculated at 150 mM NaCl, pH 8.0, and 310 K. AnOYE8 is on the left (A), and BfOYE4 is on the right (B). Density of negative potential is red, positive is blue, and neutral is white.
2.8. AnOYE2 and BfOYE1 Model Structures
Different crystallization conditions and experimental strategies were attempted in order to determine the 3D crystal structures of AnOYE2 and BfOYE1. Even if we managed to obtain crystals for both proteins, no diffraction was observed. To obtain some insights from these Class II homologues, we decided to model their structures. To this aim, we used the highly homologous EasA crystal structure (PDB 4QNW) as a template (50% and 52% sequence similarity, respectively). The values in Table S5 indicate an overall good quality of the models as, in both cases, the GMQE index (which indicates the Global Model Quality Estimation) was close to 1, and almost 95% of the residues displayed Ramachandran favored orientation. The high identity and coverage with the template allowed the modeling of highly homologous structures, as indicated by the low r.m.s.d. between the models and the template. Overall, the models shared a conserved architecture where eight α-helices surrounded a TIM barrel domain composed by eight twisted β-strands. Both proteins were endowed with an N-terminal β-hairpin loop which closed one side of the barrel and defined a cavity inside both molecules (Figure S14). CASTp analysis revealed that this cavity was slightly smaller in BfOYE1 (506.49 Å3) than in AnOYE2 (578.96 Å3). Docking simulations performed with COFACTOR showed that both pockets were able to fit an FMN cofactor molecule allocated as observed with other OYEs. Sequence alignment (Figure S1) and a Ligplot analysis of the homology models (Figures S15 and S16) showed that the residues involved in H-bonds, charge-charge, and van der Waals interactions were highly conserved. The cofactor molecule was roughly perpendicular to the barrel axis, with its si-face exposed to the solvent and the re-face buried inside the protein core (Figure 5). A comparison with the template structure EasA also highlighted that the orientation of the catalytically active residues His173, Asn176, and Tyr178 (EasA numbering) was conserved in both models, suggesting that both proteins can favorably catalyze a hydride transfer from N5 of the reduced flavin cofactor to a substrate with an activated C=C-bond (Figure 5). Interestingly, due to a shorter loop 6 (which could not protrude into the active site cavity until the FMN binding core) and a disordered loop 3 (capping subdomain, which also showed an open orientation compared with other Class II homologues), both proteins showed a highly exposed active site cavity that left wide access to the substrates and solvent (Figure S17). Such wide open access was measured by the pseudo-atom distance between the loop β2 “bounding” residues and the C-terminus extension [32] involved in shaping the bottom of the binding pocket (e.g., Tyr82 and Tyr375 in OYE1). This was significantly larger in AnOYE2 and BfOYE1 as well as in EasA (about 13–14 Å) than in other Class II enzymes (around 8–9 Å) (Figure S18).
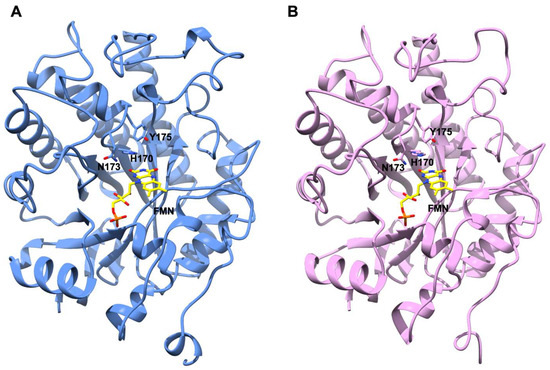
Figure 5.
Model structures of AnOYE2 (cornflower blue) (A) and BfOYE1 (pink) (B). FMN cofactor docked in the active site is shown with C atoms in yellow. The conserved catalytic residues His170, Asn173, and Tyr175 are shown as sticks in both models.
BfOYE1’s isoelectric point was slightly more acidic (5.48) than that of AnOYE2 (6.02) (Table S1). However, both models showed a quite similar distribution of the electrostatic surface potential (Figure 6). In both enzymes, the outer surface was slightly negative, with a roughly neutral patch surrounding the highly positive inner surface of the β-barrel, which indeed bound the negatively charged FMN molecule. Given the high sequence identity, similar predicted 3D folding, and surface electrostatics, the very different substrate spectrum observed with these two enzymes was likely to depend on a few relevant changes at the substrate binding site.
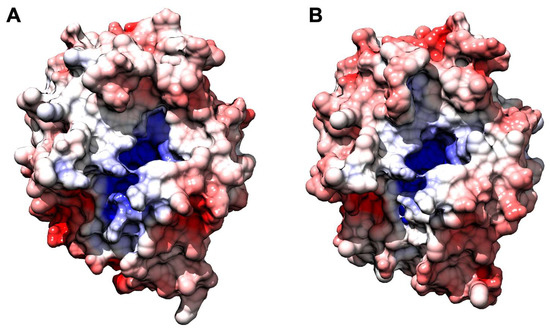
Figure 6.
Comparative analysis of surface charge distribution calculated at 150 mM NaCl, pH 8.0, and 310 K. AnOYE2 is on the left (A), and BfOYE1 is on the right (B). Density of negative potential is red, positive is blue, and neutral is white.
3. Materials and Methods
3.1. Organisms and Culture Conditions
The strain Aspergillus niger CBS 513.88 was purchased from the CBS (Centraalbureau voor Schimmelculture; Central Bureau of Fungal Cultures) strain collection (The Netherlands). The cells were grown on a solid malt extract broth medium (Sigma Aldrich, Milano, Italy) at room temperature for 3 days.
3.2. Sequence Analysis and Cloning
The NCBI database was used for DNA sequence analysis. Searches and multiple alignments of fungal OYE sequences were performed using tBLASTn [33] and Clustal Omega [34], respectively. The genomic DNA of the fungus Botryotinia fuckeliana B05.10 was kindly provided by Professor Paul Tudzynski from Münster University, while Aspergillus niger genomic DNA was extracted in house following an established protocol [35]. The cloning strategy for BfOYE4 was reported elsewhere [19]. Similarly, the other coding sequences were obtained by PCR amplifications from genomic DNA, introducing the restriction sites NdeI and BamHI (HindIII for AnOYE8) at the 5′ and 3′ ends of the open reading frames, respectively. The sequence of BfOYE1 was also mutagenized in order to suppress a predicted N-glycosylation site (in light of its eventual expression in yeast), while for AnOYE8, an additional PCR reaction was performed in order to remove an intronic sequence. The sequences of the synthetic oligonucleotides used for PCR amplifications are reported in Table S6. The expression vectors were produced by digestion of pET-28a(+) (Novagen, San Diego, CA, USA) with NdeI/BamHI (HindIII for AnOYE8) (New England Biolabs, Ipswich, MA, USA) and ligation of the amplified OYE sequences cut by the same enzymes.
3.3. Production, Analysis, and Purification of Recombinant Proteins
The recombinant enzymes were produced in different strains of E. coli BL21 (DE3) (Novagen, San Diego, CA, USA) (see Table S7 for optimized conditions). Pre-cultures were carried out in a lysogeny broth medium (LB) (50 mL) containing kanamycin (50 μg/mL) at 37 °C. Preparative cultures were carried out in LB medium (1 L), and the cells were grown in a shaking incubator (180 rpm) at 37 °C to an optical density at 600 nm (OD600) of 0.4–0.6. Then, riboflavin (25–100 μM final concentration) and isopropyl-β-D-1-thiogalactopyranoside (IPTG, 0.2 mM final concentration) were added. After induction, the cells were cultured at the optimum temperature overnight (Table S7). The cells were harvested by centrifugation (4 °C, 10 min, 5000× g) and washed with Tris-HCl buffer (pH 8.0, 50 mM). Cell disruption was obtained by a French Press (Constant Systems Cell Disruptor OneShot; Constant Systems, Kennesaw, GA, USA), and crude extract was centrifuged (4 °C, 30 min, 18,000× g) to separate the soluble and insoluble fractions. To enhance flavination, FMN cofactor (at a 100-μM final concentration) was added to the crude extract before cell disruption. Synthesis of the recombinant proteins was checked by SDS-PAGE. Overexpressed proteins were purified by immobilized metal affinity chromatography (IMAC). The soluble fractions obtained from 1 L culture were incubated with Ni-NTA resin (Sigma Aldrich, Milano, Italy) for 30 min at 4 °C and then loaded and packed in a 10-mL empty Poly-prep® column (Bio-Rad, Milano, Italy). The column was washed by gravity flow with five column volumes of Tris-HCl buffer (pH 8.0, 50 mM). Elution was performed by five column volumes of Tris-HCl (pH 8.0, 50 mM) and imidazole solution (250 mM). Alternatively, proteins were purified by fast protein liquid chromatography (FPLC) using a GE ÄKTA Purifier 100 FPLC System w/ UPC-900 (HisTrap™ High Performance 1-mL pre-packed columns (Cytiva, Global Life Sciences Solutions, Marlborough, MA, USA)). The soluble fraction was eluted twice and washed with 10 column volumes with Tris-HCl buffer (pH 8.0, 50 mM) and imidazole (10 mM). Elution was performed by a 0–100% gradient in 40 min (flow 1 mL/min). For the crystallization trials, an additional step of purification by size exclusion chromatography (SEC) was performed.
The concentration of the enzyme preparations was evaluated by spectrophotometric measurement of the concentration of free flavin in a solution of thermal denatured protein and calculated as previously reported [36].
3.4. Oligomeric State Determination by Analytical Gel Filtration and BN-PAGE
Analytical size exclusion chromatography analysis of BfOYE1, AnOYE2, and AnOYE8 (150 µg of purified protein) was performed with an ÄKTA purifier system using a Superdex 200 10/300 GL column (GE Healthcare, Milano, Italy). The matrix was equilibrated with Tris–HCl buffer (pH 8.0, 50 mM) and NaCl (150 mM). The flow rate was kept constant at 1 mL/min. The apparent Kr values of the eluted peaks were determined by comparison to a calibration curve obtained with four standard proteins—carbonic anhydrase (30 kDa and Kr 0.49), ovalbumine (45 kDa and Kr 0.43), bovine serum albumin (66.5 kDa and Kr 0.36), and ferritin (440 kDa and Kr 0.17)—as reported in Figure S3. BN-PAGE was performed by using NativePAGE™ 3 to 12%, Bis-Tris, 1.0 mm, Mini Protein Gels from Thermo Fischer Scientific (Waltham, MA, USA). Then, 12.5 µg of purified protein samples were run at 150 V for 2 h using NativePAGE™ Anode and Cathode Buffer Additives from Thermo Fischer Scientific (Waltham, MA, USA). Bovine serum albumin (BSA; monomeric species 66.5 kDa and dimeric species 133 kDa) and human serum albumin (HSA; monomeric species 66.37 kDa and dimeric species 132.74 kDa) (25 and 30 µg, respectively) were used for comparison of the MWs.
3.5. Activity Assay and Kinetics
ER activity was determined by monitoring the consumption of NADPH at 340 nm (ε = 6.22 mM−1 cm−1) using an Agilent 8453 or a Cary spectrophotometer against a range of activated alkenes. In the case of ketoisophorone (4), the assay was performed at 365 nm using a molar absorption coefficient of 3.51 mM−1 cm−1 [37]. The standard assay (100 μL) was performed at 25 °C in Tris–HCl buffer (pH 8.0, 50 mM) containing NADPH (100 μM) and the substrate (10 mM) dissolved in 100% ethanol (1% final concentration). The reaction was started by adding the enzyme to the 200-nM final concentration. One unit of ER activity is defined as the amount of protein that reduces 1 μmol of NADPH per minute. The steady state kinetic parameters of the different substrates were determined using substrate concentrations ranging from 0 to 25 mM. The data were fitted using the Michaelis–Menten equation in the program Graph-Pad Prism v5.0 (GraphPad Software, San Diego, CA, USA).
3.6. Determination of pH Optimum
For the determination of the pH optimum, the specific activities (U/mg) were evaluated in a universal buffer of a constant ionic strength (AcOH 50 mM, MES 50 mM, Tris 50 mM, and CAPS 50 mM) adjusted to the desired pH values (4–11) at 25 °C using either NaOH or HCl. The standard assay (100 μL) was performed using NADPH (100 μM), maleimide (5) (for AnOYE2 and AnOYE8) (10 mM), and cyclohex-2-en-1-one (2) (for BfOYE1) as standard substrates. The reactions were started by the addition of purified enzymes (200 nM) and monitored over 1 min.
3.7. Thermofluor Measurements
The apparent unfolding temperatures of the recombinant enzymes Tm in standard conditions (Tris, pH 8.0, 50 mM) and in the presence of different co-solvents (ethanol, acetone, acetonitrile, dimethyl sulfoxide, and dioxane) at different percentages (5–50% v/v) were determined using the Thermofluor method as previously described [36]. All the purified proteins were used and diluted to 5 μM in Tris buffer (pH 8.0, 50 mM). All measurements were performed in triplicate.
3.8. Crystallization and Data Collection
Recombinant AnOYE8 (15 mg/mL in Tris-HCl buffer, pH 8.0, 50 mM) was screened by high-throughput sparse matrix crystallization trials and dispensed by Oryx8 Robot (Douglas Instruments, EastGarston, UK). MRC two-drop 96-well standard plates were adopted both in the initial screenings and the following optimization steps. All the conditions were deposited and left equilibrating by vapor diffusion at 293 K. A panel of 288 crystallization conditions was tested (PACT, LMB, and MORPHEUS screens, Molecular Dimension Ltd., Sheffield, UK). Flat crystals of N-terminal His6-tagged AnOYE8 appeared after one night of incubation. The most regular ones were grown in LMB screen n.69: PEG 5000 MME (18% w/v), MES (pH 6.5, 0.1 M), and ammonium sulphate (0.2 M). The X-ray diffraction data were collected at the ESRF (Grenoble, France) synchrotron radiation source (for beamline and data collection details, see Table S4).
3.9. Model Building and Refinement
All the diffraction data were processed and analyzed by the automated pipelines feasible at the ESRF synchrotron in Grenoble. In particular, for AnOYE8, the data were integrated and scaled by the XDSAPP autoprocessing framework [38]. The obtained data were further cut to an appropriate resolution by running Aimless with the CCP4i2 suite [39]. The same interface was used in combination with Phenix suite for any of the subsequent steps of phasing and refinement. The AnOYE8 structure was determined by molecular replacement using as a template a model of the AnOYE8 enzyme, built by a Swiss model server [40] (PDB 5LNJ was used as a template). The refinement steps were carried out by Refmac5 [41] and Phenix software Refine [42]. Four molecules per asymmetric unit and roughly 45% of the solvent defined the crystal content. The final model was traced and visible from Asp3 to Lys416 in the E and G chains and from Asp3 to Pro417 in the B and C chains, with some gaps between residues 402–404 for chains C, B, and E, residues 404–405 for chain G, between residues 276–278 in chain G, and residues 277–281 in chain B. Flavin cofactor FMN was automatically imported from the Coot dictionary and fitted by a ligand search run. FMN cofactor was easily placed and clearly defined in each of the four molecules present in the asymmetric unit. The final parameters obtained for the best dataset (2.8 Å) reached an Rfactor/Rfree ratio of 0.24/0.26. Structure analysis was performed by PISA [31] and tools feasible in the ccp4i2 package [39].
3.10. Bioinformatic Analyses
The BfOYE1 and AnOYE2 models were modeled at the SwissModel server [40] using the EasA crystal structure (PDB 4NQW) as a template. The model quality was assessed via QMEAN6, QMEANDisCO, and MolProbity [43,44], and the protonation state at pH 8.0 was assigned with PDB2PQR using the CHARMM forcefield [45]. Energy minimization was performed via Gromacs 2021.3 [46] to optimize the side chain packing and interactions. Specifically, the CHARMM36 forcefield [47] was used, and the system energy was minimized by 5000 steps of the steepest descent energy minimization with a tolerance of 1000 kJ/mol/nm. FMN was docked to minimized structures using COFACTOR [48] at the Zhang lab server, and the active site properties were evaluated through the CASTp 3.0 server [49]. Electrostatic maps were obtained via APBS [50] and plotted at ±7 kBT/e using UCSF Chimera 1.15 [51]. Specifically, the dielectric constant values for the protein interior (εp) and solvent (εs) were set as εp = 2 and εs = 78.54 [52,53,54]. The probe radius for the dielectric surface and ion accessibility surface were set as r = 1.4 Å and r = 2.0 Å, respectively, and calculations were run at 310 K for simulating 150 mM NaCl.
4. Conclusions
Since the isolation of the first yellow enzyme from Saccharomyces pastorianus in 1932 by Warburg and Christian, the superfamily of Old Yellow Enzymes has gradually grown, reaching a number of members that is now close to a hundred. Even though the native functionalities of these enzymes are still mostly elusive, they were successfully applied in the asymmetric reduction of a broad variety of “synthetic” substrates, and more recently, reactivities other than the stereospecific addition of hydride to C=C bonds were described.
In general, modeling and structural studies coupled to biochemical characterization represent key elements for guiding protein engineering and, ultimately, the selection of the most appropriate biocatalyst in a peculiar synthetic process. Our study adds four new members to this wide family, providing biochemical and structural details. The catalytic proficiency was determined for a panel of representative substrates in the context of activated C=C double bond reduction catalysis. The enzymes in our hands showed a robust activity toward maleimide and an exquisite dependence from NADPH as an electron donor, except for AnOYE8. The latter refractory behavior toward this and other standard substrates can be partially justified by its structural features. Indeed, the crystal structure of AnOYE8 was described here for the first time, and it was very similar to the one of BfOYE4 as previously determined by our group [19]. This revealed a peculiar small catalytic pocket deeply shaped by the dimeric organization and the very long C-term and N-term extensions of both protomers. On the other hand, in agreement with the spectrum of substrates tested and the reduction activity observed in this preliminary characterization, the AnOYE2 and BfOYE1 structure models suggest a larger and more promiscuous catalytic region that seems to distinguish these enzymes from other “classical” OYEs, populating the Class II branch of the OYE tree.
As for the source of these enzymes, filamentous fungi share with bacteria the proscenium of the phylogenetic classification, which is constantly updated. Considering their ecological biodiversity, metabolic versatility, and provision of a number of different and specific isoforms, filamentous fungi represent a real spring of information for expanding the catalytic potentialities and enlarging the toolbox of OYEs. The chemical and pharmaceutical industries are constantly looking at this wide family to select “in direct line” members or “in-law” (acquired by engineering) relatives to make processes involving stereoselective C=C bond reductions more convenient and greener than the present ones.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms23063050/s1. Reference [55] is cited in the supplementary materials.
Author Contributions
M.S.R. and E.B. conceived and designed the study; M.S.R., G.L. and F.V. carried out the biochemical investigations; M.G. performed structural modeling and electrostatics analysis; F.F., L.C. and E.B. supervised experiments and validated produced data; M.S.R., G.L., M.G. and E.B. prepared the first draft of the manuscript; M.S.R., F.F., L.C. and E.B. wrote, reviewed, and edited its final form. All authors have read and agreed to the published version of the manuscript.
Funding
M.S.R. was supported by a Ph.D. grant from Fabbrica Italiana Sintetici (F.I.S.) S.p.A. (Alte di Montecchio Maggiore, Italy). M.G. and F.V. were supported by Ph.D. fellowships from the CaRiPaRo (Fondazione Cassa di Risparmio di Padova e Rovigo) Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Paul Tudzynski from Münster University for the kind gift of Botryotinia fuckeliana B05.10 genomic DNA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Toogood, H.S.; Scrutton, N.S. Discovery, Characterization, Engineering, and Applications of Ene-Reductases for Industrial Biocatalysis. ACS Catal. 2018, 8, 3532–3549. [Google Scholar] [CrossRef] [PubMed]
- Nizam, S.; Verma, S.; Borah, N.N.; Gazara, R.K.; Verma, P.K. Comprehensive Genome-Wide Analysis Reveals Different Classes of Enigmatic Old Yellow Enzyme in Fungi. Sci. Rep. 2014, 4, 4013–4024. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, A.; Spina, F.; Brenna, E.; Crotti, M.; Parmeggiani, F.; Varese, G.C. Identification of Fungal Ene-Reductase Activity by Means of a Functional Screening. Fungal Biol. 2015, 119, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Carballeira, J.D.; Valmaseda, M.; Alvarez, E.; Gago, J.V.S. Gongronella butleri, Schizosaccharomyces octosporus and Diplogelasinospora grovesii: Novel Microorganisms Useful for the Stereoselective Reduction of Ketones. Enzyme Microb. Technol. 2004, 34, 611–623. [Google Scholar] [CrossRef]
- Birolli, W.G.; Lima, R.N.; Porto, A.L.M. Applications of Marine-Derived Microorganisms and Their Enzymes in Biocatalysis and Biotransformation, the Underexplored Potentials. Front. Microbiol. 2019, 10, 1453. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.M.; Rocha, L.C.; Yoshioka, S.A.; Nitschke, M.; Jeller, A.H.; Pizzutti, L.; Seleghim, M.H.R.; Porto, A.L.M. Chemoselective Reduction of Chalcones by Whole Hyphae of Marine Fungus Penicillium citrinum CBMAI 1186, Free and Immobilized on Biopolymers. Biocatal. Agric. Biotechnol. 2014, 3, 358–364. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Meira, E.B.; Rosset, I.G.; Porto, A.L.M. Chemoselective Biohydrogenation of α,β- and α,β,γ,δ-Unsaturated Ketones by the Marine-Derived Fungus Penicillium citrinum CBMAI 1186 in a Biphasic System. J. Mol. Catal. B Enzym. 2015, 115, 59–65. [Google Scholar] [CrossRef]
- Ferreira, I.M.; de Vasconcellos, S.P.; da Cruz, J.B.; Comassetto, J.V.; Porto, A.L.M.; Rocha, L.C. Hydrogenation of Bis-α,β-Unsaturated Enones Mediated by Filamentous Fungi. Biocatal. Agric. Biotechnol. 2015, 4, 144–149. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Fiamingo, A.; Campana-Filho, S.P.; Porto, A.L.M. Biotransformation of (E)-2-Methyl-3-Phenylacrylaldehyde Using Mycelia of Penicillium citrinum CBMAI 1186, Both Free and Immobilized on Chitosan. Mar. Biotechnol. 2020, 22, 348–356. [Google Scholar] [CrossRef]
- Jimenez, D.E.Q.; Barreiro, J.C.; Santos, F.M.; Vasconcellos, S.P.; Porto, A.L.M.; Batista, J.M. Enantioselective Ene-Reduction of (E)-2-Cyano-3-(Furan-2-yl) Acrylamide by Marine and Terrestrial Fungi and Absolute Configuration of (R)-2-Cyano-3-(Furan-2-yl) Propanamide Determined by Calculations of Electronic Circular Dichroism (ECD) Spectra. Chirality 2019, 31, 534–542. [Google Scholar] [CrossRef]
- Matos, I.L.; Nitschke, M.; Porto, A.L.M. Hydrogenation of Halogenated 2′-Hydroxychalcones by Mycelia of Marine-Derived Fungus Penicillium raistrickii. Mar. Biotechnol. 2019, 21, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Janicki, I.; Kielbasinski, P.; Szelag, J.; Glebski, A.; Szczesna-Antczak, M. Preparative Scale Application of Mucor circinelloides Ene–Reductase and Alcohol Dehydrogenase Activity for the Asymmetric Bioreduction of α,β-Unsaturated γ-Ketophosphonates. Bioorg. Chem. 2020, 98, 103548. [Google Scholar] [CrossRef] [PubMed]
- Wallwey, C.; Matuschek, M.; Xie, X.-L.; Li, S.-M. Ergot Alkaloid Biosynthesis in Aspergillus fumigatus: Conversion of Chanoclavine-I Aldehyde to Festuclavine by the Festuclavine Synthase FgaFS in the Presence of the Old Yellow Enzyme FgaOx3. Org. Biomol. Chem. 2010, 8, 3500–3508. [Google Scholar] [CrossRef]
- Cheng, J.Z.; Coyle, C.M.; Panaccione, D.G.; O’Connor, S.E. A Role for Old Yellow Enzyme in Ergot Alkaloid Biosynthesis. J. Am. Chem. Soc. 2010, 132, 1776–1777. [Google Scholar] [CrossRef]
- Matuschek, M.; Wallwey, C.; Wollinsky, B.; Xie, X.; Li, S.-M. In Vitro Conversion of Chanoclavine-I Aldehyde to the Stereoisomers Festuclavine and Pyroclavine Controlled by the Second Reduction Step. RSC Adv. 2012, 2, 3662–3669. [Google Scholar] [CrossRef]
- Gerhards, N.; Li, S.-M. A Bifunctional Old Yellow Enzyme from Penicillium roqueforti Is Involved in Ergot Alkaloid Biosynthesis. Org. Biomol. Chem. 2017, 15, 8059. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Meng, G.; Lu, H.; Huang, Z.; Chen, F. Identification of an Ene-Reductase from Yeast Kluyveromyces marxianus and Application in the Asymmetric Synthesis of (R)-profen Esters. Asian J. Org. Chem. 2018, 7, 763–769. [Google Scholar] [CrossRef]
- Janicki, I.; Kielbasinski, P.; Turrini, N.G.; Faber, K.; Hall, M. Asymmetric Bioreduction of β-activated Vinylphosphonate Derivatives Using Ene-reductases. Adv. Synth. Catal. 2017, 359, 4190–4196. [Google Scholar] [CrossRef]
- Robescu, M.S.; Cendron, L.; Bacchin, A.; Wagner, K.; Reiter, T.C.; Janicki, I.; Merusic, K.; Illek, M.; Bergantino, E.; Hall, M. Asymmetric Proton Transfer Catalysis by Stereocomplementary Old Yellow Enzymes for C=C-Bond Isomerization Reaction. 2022; under revision. [Google Scholar]
- Böhmer, S.; Marx, C.; Gomez-Baraibar, A.; Nowaczyk, M.M.; Tischler, D.; Hemschemeier, A.; Happe, T. Evolutionary Diverse Chlamydomonas reinhardtii Old Yellow Enzymes Reveal Distinctive Catalytic Properties and Potential for Whole-Cell Biotransformations. Algal Res. 2020, 50, 101970. [Google Scholar] [CrossRef]
- Nizam, S.; Gazara, R.K.; Verma, S.; Singh, K.; Verma, P.K. Comparative Structural Modeling of Six Old Yellow Enzymes (OYEs) from the Necrotrophic Fungus Ascochyta rabiei: Insight into Novel OYE Classes with Differences in Cofactor Binding, Organization of Active Site Residues and Stereopreferences. PLoS ONE 2014, 9, e95989. [Google Scholar] [CrossRef]
- Scholtissek, A.; Tischler, D.; Westphal, A.H.; van Berkel, W.J.H.; Paul, C.E. Old Yellow Enzyme-Catalysed Asymmetric Hydrogenation: Linking Family Roots with Improved Catalysis. Catalysts 2017, 7, 130. [Google Scholar] [CrossRef]
- Joseph, R.E.; Andreotti, A.H. Bacterial Expression and Purification of Interleukin-2 Tyrosine Kinase: Single Step Separation of the Chaperonin Impurity. Protein Expr. Purif. 2008, 60, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Adalbjornsson, B.V.; Toogood, H.S.; Fryszkowska, A.; Pudney, C.R.; Jowitt, T.A.; Leys, D.; Scrutton, N.S. Biocatalysis with Thermostable Enzymes: Structure and Properties of a Thermophilic Ene-Reductase Related to Old Yellow Enzyme. ChemBioChem 2010, 11, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Opperman, D.J.; Piater, L.A.; van Heerden, E. A Novel Chromate Reductase from Thermus scotoductus SA-01 Related to Old Yellow Enzyme. J. Bacteriol. 2008, 190, 3076–3082. [Google Scholar] [CrossRef]
- Litthauer, S.; Gargiulo, S.; van Heerden, E.; Hollmann, F.; Opperman, D.J. Heterologous Expression and Characterization of the Ene-Reductases from Deinococcus radiodurans and Ralstonia metallidurans. J. Mol. Catal. B Enzym. 2014, 99, 89–95. [Google Scholar] [CrossRef]
- Robescu, M.S.; Niero, M.; Loprete, G.; Cendron, L.; Bergantino, E. A New Thermophilic Ene-Reductase from the Filamentous Anoxygenic Phototrophic Bacterium Chloroflexus aggregans. Microorganisms 2021, 9, 953. [Google Scholar] [CrossRef]
- Riedel, A.; Mehnert, M.; Paul, C.E.; Westphal, A.H.; van Berkel, W.J.H.; Tischler, D. Functional Characterization and Stability Improvement of a Thermophilic-like Ene-Reductase from Rhodococcus opacus 1CP. Front. Microbiol. 2015, 6, 1073. [Google Scholar] [CrossRef]
- Catucci, G.; Romagnolo, A.; Spina, F.; Varese, G.C.; Gilardi, G.; Di Nardo, G. Enzyme-Substrate Matching in Biocatalysis: In Silico Studies to Predict Substrate Preference of Ten Putative Ene-Reductases from Mucor circinelloides MUT44. J. Mol. Catal. B Enzym. 2016, 131, 94–100. [Google Scholar] [CrossRef]
- Romagnolo, A.; Spina, F.; Poli, A.; Risso, S.; Serito, B.; Crotti, M.; Monti, D.; Brenna, E.; Lanfranco, L.; Varese, G.C. Old Yellow Enzyme Homologues in Mucor circinelloides: Expression Profile and Biotransformation. Sci. Rep. 2017, 7, 12093. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Inference of Macromolecular Assemblies from Crystalline State. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef]
- Oberdorfer, G.; Steinkellner, G.; Stueckler, C.; Faber, K.; Gruber, K. Stereopreferences of Old Yellow Enzymes: Structure Correlations and Sequence Patterns in Enoate Reductases. ChemCatChem 2011, 3, 1562–1566. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; LI, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Soding, J.; et al. Fast, Scalable Generation of High-quality Protein Multiple Sequence Alignments Using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.; Flores-Vergara, M.; Krasnyanski, S.; Kumar, S.; Thompson, W. A Modified Protocol for Rapid DNA Isolation from Plant Tissues Using Cetyltrimethylammonium Bromide. Nat. Protoc. 2006, 1, 2320–2325. [Google Scholar] [CrossRef]
- Robescu, M.S.; Niero, M.; Hall, M.; Cendron, L.; Bergantino, E. Two New Ene-Reductases from Photosynthetic Extremophiles Enlarge the Panel of Old Yellow Enzymes: CtOYE and GsOYE. Appl. Microbiol. Biotechnol. 2020, 104, 2051–2066. [Google Scholar] [CrossRef]
- Fu, Y.; Castiglione, K.; Weuster-Botz, D. Comparative Characterization of Novel Ene-Reductases from Cyanobacteria. Biotechnol. Bioeng. 2013, 110, 1293–1301. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010, D66, 125–132. [Google Scholar] [CrossRef]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Leslie, A.G.W.; McCoy, A.; McNicholas, S.J.; et al. Overview of the CCP4 Suite and Current Developments. Acta Crystallogr. D Biol. Crystallogr. 2011, D67, 235–242. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling Protein Tertiary and Quaternary Structure Using Evolutionary Information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Murshudov, G.N.; Skubák, P.; Lebedev, A.A.; Pannu, N.S.; Steiner, R.A.; Nicholls, R.A.; Winn, M.D.; Long, F.; Vagin, A.A. REFMAC 5 for the Refinement of Macromolecular Crystal Structures. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 355–367. [Google Scholar] [CrossRef]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.-W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A Comprehensive Python-Based System for Macromolecular Structure Solution. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the Estimation of the Absolute Quality of Individual Protein Structure Models. Bioinformatics 2011, 27, 343–350. [Google Scholar] [CrossRef]
- Chen, V.B.; Bryan, W.; Iii, A.; Headd, J.J.; Keedy, D.A.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-Atom Structure Validation for Macromolecular Crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010, D66, 12–21. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An Automated Pipeline for the Setup of Poisson-Boltzmann Electrostatics Calculations. Nucleic Acids Res. 2004, 32, W665–W667. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Pall, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmuller, H.; MacKerell, A.D., Jr. CHARMM36m: An Improved Force Field for Folded and Intrinsically Disordered Proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef]
- Zhang, C.; Freddolino, P.L.; Zhang, Y. COFACTOR: Improved Protein Function Prediction by Combining Structure, Sequence and Protein–Protein Interaction Information. Nucleic Acids Res. 2017, 45, W291–W299. [Google Scholar] [CrossRef]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed Atlas of Surface Topography of Proteins. Nucleic Acid Res. 2018, 46, W363–W367. [Google Scholar] [CrossRef]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS Biomolecular Solvation Software Suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Schutz, C.N.; Warshel, A. What Are the Dielectric Constants of Proteins and How to Validate Electrostatic Models? Proteins 2001, 44, 400–417. [Google Scholar] [CrossRef] [PubMed]
- Gorham, R.D., Jr.; Kieslich, C.A.; Morikis, D. Electrostatic Clustering and Free Energy Calculations Provide a Foundation for Protein Design and Optimization. Ann. Biomed. Eng. 2010, 39, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Gong, L.-C.; Sui, S.-F. An Electrostatically Preferred Lateral Orientation of SNARE Complex Suggests Novel Mechanisms for Driving Membrane Fusion. PLoS ONE 2010, 5, e8900. [Google Scholar] [CrossRef]
- Dobrijevic, D.; Benhamou, L.; Aliev, A.E.; Mendez-Sanchez, D.; Dawson, N.; Baud, D.; Tappertzhofen, N.; Moody, T.S.; Orengo, C.A.; Hailes, H.C.; et al. Metagenomic ene-reductases for the bioreduction of sterically challenging enones. RSC Adv. 2019, 9, 36608–36614. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).