Abstract
Putrescine (Put) is the starting point of the polyamines (PAs) pathway and the most common PA in higher plants. It is synthesized by two main pathways (from ornithine and arginine), but recently a third pathway from citrulline was reported in sesame plants. There is strong evidence that Put may play a crucial role not only in plant growth and development but also in the tolerance responses to the major stresses affecting crop production. The main strategies to investigate the involvement of PA in plant systems are based on the application of competitive inhibitors, exogenous PAs treatments, and the most efficient approaches based on mutant and transgenic plants. Thus, in this article, the recent advances in understanding the role of this metabolite in plant growth promotion and protection against abiotic and biotic stresses will be discussed to provide an overview for future research.
1. Introduction
Polyamines (PAs) are small, low molecular weight, and ubiquitous polycations found in eukaryotic and prokaryotic cells [1,2]. In higher plants, they could be found not only in the free form but also as conjugates bound to phenolic acids (hydroxycinnamic, coumaric, caffeic, or ferulic acid) or to biomacromolecules such as proteins and nucleic acids in order to regulate the free PAs intracellular levels or control enzyme activity, DNA replication, gene transcription, cell division, and membrane stability [3,4]. The most common PAs in higher plants are diamine putrescine (Put), triamine spermidine (Spd), tetramine spermine (Spm), thermospermine (Tspm), and cadaverine (Cad) [5,6,7,8] (Figure 1). Among them, Put is the central product of the PA biosynthetic pathway and the most abundant PA in nature, being mainly synthesized by two pathways derived from ornithine (Orn) or from arginine (Arg) as a result of the activity of ornithine decarboxylase (ODC, EC 4.1.1.17) or arginine decarboxylase (ADC, EC 4.1.1.19), respectively [9]. Orn is produced from Arg by arginase, and then ODC eliminates a carboxyl group of Orn to generate Put and CO2 [10]. The Arg pathway includes the following steps: (i) arginine decarboxylation to agmatine catalyzed by ADC; (ii) deimination of agmatine by agmatine iminohydrolase (AIH, EC 3.5.3.12) to form N-carbamoylputrescine (NCP) and NH3, and (iii) hydrolysis of NCP to Put by N-carbamoylputrescine amidohydrolase (CPA, EC 3.5.1.53), releasing NH3 and CO2 [11]. It should be noted that the ODC gene is not present in Arabidopsis thaliana and other plants of the Brassicaceae family [12]. In A. thaliana, two genes encoding ADC, ADC1 and ADC2, were identified and are expressed in a tissue-specific manner [13]. This gene duplication seems to be related to the differential regulation of gene responsiveness [14]. A third pathway uncovered to date only in sesame involves the conversion of Arg to citrulline (Cit) and subsequent decarboxylation catalyzed by citrulline decarboxylase (CDC) to generate Put [4]. Once Put is formed, the Spd and Spm are synthesized from Put and aminopropyl residues by the activity of Spd synthase (SPDS) and Spm synthase (SPMS), respectively [15], whereas the Tspm is then produced through the isomerization of the Spm by the enzyme thermospermine synthase (tSPMS) called ACAULIS5 (ACL5) [16,17]. The breakdown of PAs is mediated by amine oxidases, including the diamine oxidase (DAO) and polyamine oxidase (PAO) [4,18,19].
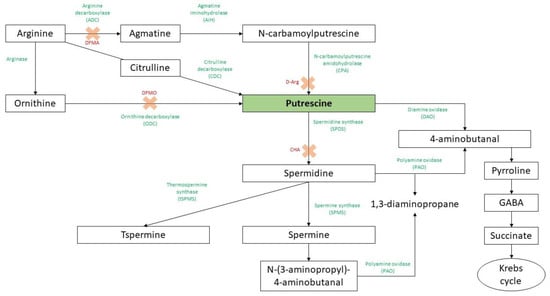
Figure 1.
Putrescine biosynthesis and catabolism in plants. The green words indicate the enzyme activities, and the red ones followed by a cross are referred to competitive enzyme inhibitors (DFMA: difluoromethylarginine; DFMO: difluoromethylornithine; D-Arg: D-Arginine; CHA: cyclohexylamine). Adapted from Wojtasik et al. [20] and Chen et al. [4].
The activity of ADC and ODC can be inhibited by the irreversible competitive inhibitors difluoromethylarginine (DFMA) and difluoromethylornithine (DFMO), respectively [21,22], as well as by the reversible inhibitor of ADC, D-Arginine (D-Arg) [23] (Figure 1). Intriguingly, plants treated with biosynthetic inhibitors displayed a reduction in stress tolerance, which was reversed when PAs were applied exogenously [24]. However, the use of these chemical inhibitors seems to be limited due to their stability and specificity, and it is influenced by the concentration, the plant system, and the induction of compensatory mechanisms [25]. Therefore, the use of most sustainable approaches such as mutants or transgenic plants will allow a deeper understanding of the direct implication of PAs in plant systems [26].
PAs play a role in physiological processes such as embryogenic competence, root growth, organogenesis, flower development, fruit ripening, or programmed cell death [27,28], as well as plant defense responses [29,30]. Their cationic nature explains most of their biological activity. However, the numerous biological interactions where PAs are involved makes it difficult to determine their role in plant growth and development. Put is not only a signal molecule by itself, but also interacts with numerous molecules such as phytohormones and gas molecules, among others. Put mostly displayed the opposite effects of Spd and Spm, which suggests that each PA may play a distinctive role in plant metabolome and transcriptome, as it was reviewed by Anwar et al. [31]. Generally, Put was positively linked with the gene expression for abscisic acid (ABA) biosynthesis and indole acetic acid (IAA) and salicylic acid (SA) levels, albeit downregulating those of ethylene, jasmonates (JA), and gibberellin (GA) biosynthesis. Moreover, Put seems to play a neutral to positive role in regulating the JA or brassinosteroids signaling pathways [31]. However, Put does not appear to affect cytokinin (CK) biosynthesis or signaling. Likewise, ABA seems to crosstalk with PAs in regulating abiotic stress responses, including reactive oxygen species (ROS), nitric oxide (NO), and changing ion homeostasis [31,32]. NO production could be mediated by H2O2 as the result of PAs oxidation by DAO and PAO or by other unknown mechanisms that could be related to the PAs pathway [33]. The positive regulatory role of NO in the increase of the expression of genes involved in PA biosynthesis and the decrease of PAO enzyme activity was previously reviewed, confirming the role of NO in PA homeostasis [34]. It should also be noted that H2S, an endogenous gas transmitter, could also play a negative and positive role in plants, acting as a toxic intermediate of cellular metabolism or as a signaling molecule, respectively [35,36]. Its role in seed germination, adventitious rooting, senescence, and protection against abiotic stresses was previously reported [37]. Thus, it could be speculated that both gas molecules could behave as a link messenger in stress responses mediated by PAs, filling a gap between many known physiological effects of Put and stress tolerance.
In addition, plants are often subjected to various abiotic and biotic constraints that limit plant growth and productivity. PAs are one of the involved pathways protecting plants, even though their catabolic products could damage plants [38]. Nonetheless, the regulation of plant growth and stress responses by PAs is not fully understood. Thus, the main goal of this review is to present an overview of the recent research about the role of Put in plant growth and development, tolerance, and resistance against the major abiotic and biotic stresses to provide a basis for future research on Put action in plant systems.
2. Plant Growth Responses
PAs are considered a class of plant growth regulators [39]. In general, enhanced plant growth and metabolism are associated with greater PA biosynthesis and higher PA content [40,41]. PAs show specific tissue and organ distribution and different localization patterns within cells, which are related to their unique functions. Among the main PAs, Put is the most abundant in leaves, and it is found to accumulate in the cytoplasm [40]. It is also demonstrated that the chemical or genetic depletion of Put is lethal for many organisms, not only for plants, suggesting that Put may play an essential role in growth and development [42,43]. However, the molecular mechanisms behind these roles remain ambiguous. It was suggested that the enhancement of plant growth might be due to the fact that PAs act as hormonal second-messengers of cell proliferation and differentiation in many processes or regulate plant sensitivity to auxins/CKs ratio. In addition, the metabolism of PAs was related to the production of NO, which is considered an essential signaling component for plant growth [44].
In this regard, many studies showed that modifications in Put content can affect root growth and development. For example, the depletion of Put due to a decrease of ADC activity led to a reduction of root length in Phaseolus vulgaris plants [45]. Moreover, Lee [46] showed that Put treatment in concentrations varying from 0.01 to 1 mM enhanced root elongation in the excised root of Oryza sativa grown under in vitro conditions at 25 °C. Likewise, DFMO was found to inhibit root elongation and PAs levels in roots, and these effects were reversed by DFMO plus Put co-treatment, or 1 mM Put exogenous treatment. Similarly, Tarenghi et al. [47] showed that 1 mM Put exogenous treatment led to an increase of Put level in roots and to an increase of root length in strawberry microcuttings. In the same way, Wu et al. [48], when investigating the effect of an arbuscular mycorrhizal fungus (AMF) and Put on root development, plant growth, and biomass production of 4 months-old trifoliate orange, observed that total root length, projected area, surface area, and root volume were significantly increased by the Put treatment compared to the sole AMF treatment. However, DFMO treatment was found to cause an increase in root system length in parallel with a decrease in Put levels in excised roots from plants of Nicotiana tabacum [49]. These changes were reversed when 1 mM Put was added to the DFMO treatment. In accordance with previous observations, in Pringlea antiscorbutica, a decrease of the Put pool seemed to enhance primary root growth in a concentration-dependent manner. Similar results were obtained by Tang et al. [50] in Virginia pine plantlets. They demonstrated that Put application at 0.001 mM improved rooting frequency and promoted root elongation while Put treatment at 0.01–1 mM decreased rooting frequency and reduced root elongation. Likewise, simultaneous silencing of the two ADC genes in A. thaliana led to a significant reduction in primary root length [51]. Put also plays a crucial role in rooting of Decalepis hamiltonii since it is shown that supplementing Put in the rooting medium enhances the quantity and quality of roots [52]. Moreover, spraying Antirrhinum majus with 200 mg L−1 of Put has a significant effect on root length and fresh (FW) and dry weight (DW) [53]. Studies on Basil plants revealed that the application of Put, Spd, or Spm at different concentrations increased root FW and DW when compared with the control plants [54]. Furthermore, Hashem et al. [23] progressively reduced Put biosynthesis by inhibiting ADC1/2 enzyme activity using the competitive inhibitor D-Arg, leading to increased root growth at low D-Arg concentrations and progressively decreased root growth at higher ones. A similar trend was also observed for the meristematic zone size. They next investigated whether reduced Put affects auxin and CK signaling since both hormones are involved in the regulation of root meristem size. Auxin signaling displayed a U-shaped trend as D-Arg increased, whereas CK progressively decreased with increasing concentrations of the inhibitor. Taken together, all of these results highlight the fact that there are important inter-species differences, which might also depend on culture conditions.
The root system plays an important role in water and nutrient uptake. It was observed that nitrogen sources such as ammonium (NH4+) and nitrate (NO3−) impact differently on some physiological and biochemical processes in higher plants. In fact, detailed analysis from Houdusse et al. [55] revealed that the foliar free Put content was well correlated with the intensity of the negative effects of NH4+ as the sole N source on the development of wheat (Triticum aestivum) and pepper (Capsicum annuum) plants. In the same study, it was observed that plants supplied with NH4NO3 exhibited a decline in free Put content when compared with those fed with NH4+ alone, in both roots and leaves of wheat and pepper plants. Moreover, the effect of different concentrations of Put on the activity of enzymes of N assimilation was examined in maize seedlings. It was observed that both glutamate dehydrogenase (GDH) and glutamine synthetase (GS) activities were enhanced at low concentrations of Put while glutamate synthase (GOGAT) activity increased with increasing Put concentration [56]. In a recent study, González-Hernández et al. [57] tested the effect of defective ADC and ODC gene expression on the root architecture development of tomato plants (Solanum lycopersicum) under both NO3− and NH4+ nutrition. The ADC transgenic silenced tomato seedlings showed an increase in FW, shoot length, lateral root number and shoot:root ratio under NO3− supply and an enhancement in FW, and shoot and root length under NH4+ supply. However, ODC transgenic silenced tomato seedlings displayed greater weight and shoot length with NO3−, whereas a decrease in lateral root density was found with NH4+.
Put also modulates shoot growth. Related to this, Nahed and Lobna [58] showed that foliar application of Put significantly increased plant height, number of leaves per plant, and FW and DW of leaves per plant when compared with untreated plants. Similarly, Youssef et al. [59] reported that foliar application of Put to Matthiola incana plants significantly promoted plant height, the number of leaves per plant, and FW and DW of leaves per plant at the vegetative growth stage. In periwinkle plants (Catharanthus roseus), Talaat et al. [60] observed that foliar application of Put had beneficial effects on different growth parameters of shoots and leaves at successive developmental stages, which were correlated with an increase in the endogenous GA3, IAA, CKs, and ABA levels. Moreover, leaf spraying with Put and thiamine showed that Put treatment significantly increased plant height, number of shoots, number of leaves, leaf, stem FW and DW, and stem diameter in Dahlia pinnata plants [61]. In Antirrhinum majus, foliar Put spray also led to an increase in growth parameters such as plant height, number of shoots, number of leaves, leaf area, and stem FW and DW [53]. In a study on the effect of humic acid and Put on rose, it was found that humic acid with Put treatment increased stem FW and DW, leaf area, and plant height [62]. Tomato seedlings exposed to cinnamic acid decreased root and shoot lengths and FW and DW. These parameters increased when Put was applied in combination with cinnamic acid or alone [63]. A study on cucumber reported that root application of humic acid caused a significant increase in shoot growth that was associated with an enhancement in the shoot concentration of several CKs and PAs (principally Put), concomitant with a decrease in roots [64]. In addition, Krizek et al. [65] showed that the treatment of cucumber plants with Put increased the DW of shoots and leaf area but had no effect on root DW. Oryza sativa seed treatments with Put resulted in earlier and enhanced germination along with improved shoot and root lengths and seedling FW and DW [66].
In safflower plants subjected to different Put concentrations, it was found that the maximum shoot regeneration occurred with the highest concentration of Put tested, which also had the highest peroxidase activity [67]. Moreover, the addition of Put (40 mM) resulted in increased shoot proliferation, in vitro flowering, and increased endogenous levels of PAs in Cichorium intybus cv. Lucknow [68].
Several studies with different plant species provided evidence of the effect of Put on photosynthetic pigments. Indeed, Put application increased the content of chlorophyll in several ornamental plants [58,69,70], while in Salvia splendens it not only increased the chlorophyll content but also the anthocyanin and soluble sugars [71]. Total photosynthetic pigments in fresh leaves were significantly promoted because of the application of Put in chickpea plants (Cicer arietinum) [72]. Moreover, tomato seedlings showed higher levels of photosynthetic pigments, protein and sugar, and higher nitrate reductase activity when Put was exogenously applied [63]. It was also reported that foliar application increased the content of chlorophylls (a and b) as well as carotenoids in a concentration-dependent manner in periwinkle plants. These results resemble those reported in wheat after application of either Arg or Put [73]. In a recent study carried out with basil plants (Ocimum basilicum), Danaee and Abdossi [54] showed that the total leaf chlorophyll and vitamin C contents were increased in plants treated with Put at 100 ppm compared to untreated ones. Moreover, El-Bassiuony and Bekheta [74] reported an accumulation of total carbohydrates content in wheat plants treated with Put, suggesting the stimulation of the photosynthetic assimilation of CO2. Regulation of ATP production is one of the most important processes for a photosynthetic organism which determines the amount of energy available for energy-consuming processes. Related to this, Ioannidis et al. [75] revealed that Put is an efficient stimulator of ATP synthesis by demonstrating that Put can increase light energy utilization through stimulation of photophosphorylation.
Put may influence the different stages of plant development, including flowering. In this context, Singh and Bala [76] observed that an increase in Put concentration delayed the bud formation in chrysanthemum cv. ‘Punjab Shyamli’. On the contrary, studies in Dendrobium nobile plants with higher levels of Put and Spd in the leaves showed that these plants had more flower buds, and they also presented not only more flowers but also with a larger average flower diameters [77]. Similarly, the application of 200 ppm of Put increased the number of flowers and their FW and DW in Dianthus caryophyllus and Gladiolus grandflorum [58,78]. Antirrhinum majus plants treated with different concentrations of Put significantly increased the number of inflorescences per plant, yield of spike, and FW and DW of inflorescences per plant compared with untreated plants [79]. Treated plants also showed a decrease in the number of days for flowering. In addition, treatment with Put induced flowering in chicory shoot cultures [68]. However, in Fragaria × ananassa Duch. cv. Selva, Calendula officinalis, and Rosa hybrida cv. ‘Herbert Stevens’ plants, no significant effect of Put treatment on the number of flowers was found [70,80,81]. In other studies, foliar application of Put with α-tocopherol enhanced the anthocyanin content in the inflorescences and the total carbohydrates in shoots and inflorescences of A. majus [53].
Put appears to be involved in a wide range of physiological processes in citrus plants which were recently reviewed by Killiny and Nehela [82]. In summary, Put positively regulated root and shoot growth, increased the number of total flowers per tree, fruit yield per tree, fruit weight, and fruit diameter. Likewise, exogenous Put not only significantly increased chlorophyll a, total carotenoid and total chlorophyll contents, but also the photosynthetic rates.
Interestingly, it has been shown that plant hormones involved in plant growth and development are associated with the metabolism of PAs. Related to this, El-Bassiouny [73] and Bekheta and El-Bassiouny [83] demonstrated that exogenous application of Put on Pisum sativum and wheat increased IAA, GA, and CK levels and decreased ABA content, respectively. Moreover, constitutive overexpression of ADC2 (35S:AtADC2) in A. thaliana led to a more than 16-fold increase in the levels of Put in the transgenic plants when compared to the wild type, whereas the level of Spd and Spm remained unchanged [84]. These transgenic plants were dwarfed with delayed flowering. Transcriptomic and metabolomic analysis revealed that accumulation of Put downregulated the expression of dioxygenase genes (GA20ox1, GA3ox1, and GA3ox3), which are involved in the last step of GA metabolism and decreased the content of bioactive GA4 and GA1, and of GA9 (a precursor of GA4). No changes in the expression of genes encoding earlier enzymes in the GA biosynthesis pathway were detected by microarray analysis. Furthermore, ADC2-overexpression upregulated ATP-binding cassette B4 (ABCB4), which is a root-localised auxin efflux transporter, and auxin-amido synthetase GH3.4, GH3.6, and GH3.17 in A. thaliana leaves [31]. However, no change in transcript levels of CK biosynthetic genes was found in A. thaliana leaves overexpressing ADC2 [84]. Moreover, the expression levels of CK dehydrogenase (CKX) that cleaves the CK side chain, producing aldehydes and adenine derivatives, remained unaffected in ADC2-overexpressing A. thaliana plants, suggesting that Put does not appear to affect CK biosynthesis or signaling [84]. However, it seems that CKs favor Put biosynthesis and inhibit Spd and Spm accumulation in etiolated cucumber cotyledons since treatment with kinetin increased PAO activity, decreased SAMDC activity, along with a decrease in Spd levels, and increased Put content [85]. Other studies performed on cucumber cotyledon to determine the possible relationship between CK and PAs revealed that kinetin application to excised cotyledons caused a significant increase in the activity of ADC, which was accompanied by an increase in Put content and a decrease in Spd and Spm levels [86]. However, the inhibition of Put biosynthesis with D-Arg did not affect CK-induced expansion of cotyledons and applied alone; Put had no significant effect on growth. In contrast, PAs and ethylene have antagonistic roles. For example, Put treatment decreased ethylene production [87], and this phytohormone was shown to be an effective inhibitor of ADC and SAMDC [88]. The effects of Put on plant growth and development are summarized in Figure 2.
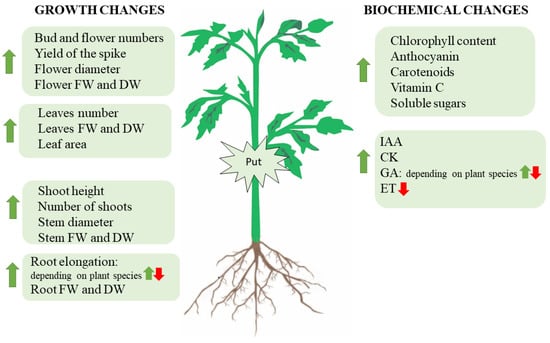
Figure 2.
Developmental and biochemical changes produced in plants under Put treatment. FW: fresh weight; DW: dry weight; IAA: indole acetic acid; GA: gibberellins; CK: cytokinins; ET: ethylene.
3. Tolerance Responses to Abiotic Stresses
The general effect of Put has long been known; it not only participates in plant growth and developmental processes but also contributes to the tolerance to different abiotic stresses such as salinity, drought, high temperatures, and cold. The main described mechanisms are associated with scavenging free radicals, regulating ABA levels, preventing lipid peroxidation, maintaining cellular pH and ionic balance, and regulating cationic channels, among others [89]. These different mechanisms could be induced simultaneously or separately in order to reduce membrane damage, promote cell growth, or enhance cell survival under stress constraints [90]. Furthermore, as previously described, the exogenous application of Put to normal and stressed conditions or the use of transgenic plants overexpressing genes responsible for PAs biosynthesis as well as the loss of function mutants are the main tools used to identify PA-dependent stress responses. Thus, in recent decades, the research community has investigated the effect of PAs when plants are exposed to sole or combined stressors. For example, ADC1 is mostly activated by cold [91], and ADC2 expression is induced under drought, salt, and mechanical injury stresses [91,92,93,94,95]. Thus, the effect of the Put pathway on plants affected by the major abiotic stresses (salinity, drought, cold, heat and cold), which negatively influence plant performance [96], will be addressed.
One of the most detrimental environmental stressors is salinity, which produces an osmotic imbalance, ionic toxicity, and oxidative stress [97]. Concerning the PAs role, Zapata et al. [98] described that salt stress might lead to increased (Spd+Spm)/Put ratio, and specifically, the plants more tolerant to salt stress displayed a reduction in Put accumulation. In fact, the increase of Put levels that produces a reduction in this ratio could even cause plant damage. In addition, alfalfa plants under salinity stress exhibited an increase in Spm content at the expense of the decrease of Put and Spd, indicating a deviation of PAs metabolism towards the synthesis of increasing polycationic forms [99]. Similar results were reported by Krishnamurthy and Bhagwat [100] and Santa Cruz et al. [101], showing that rice and tomato tolerant cultivars accumulated Spd and Spm, whereas salt-sensitive cultivars accumulated Put. However, the effect seems to be dependent on the type of stress, time of exposure, and plant species [102]. Leaves of Lupinus luteus seedlings accumulated Put and Spd in response to salt stress [103]. Moreover, Quinet et al. [104] showed that the exogenous application of Put diminished Na+ levels in roots of a salt-sensitive rice cultivar, leading to an increase in Put and conjugated PAs biosynthesis. In A. thaliana, the role of Put in the alleviation of the deleterious effects of salt stress seems to be produced by the induction of AtADC2 [105]. Support is provided by evidence that the characterization of the adc2-1 mutant under salt stress is more sensitive than the wild type, but they were recovered by the addition of exogenous Put. Likewise, Camellia sinensis cultivars displayed an alleviation of salinity stress through the reduction in the antioxidant enzymes superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) and the positive effect on photosynthetic efficiency when treated with Put [106]. In line with this, Ekinci et al. [107] indicated that the exogenous application of Put improved plant height, number of leaves, stem diameter, FW, tissue electrical conductivity, and the activity of CAT of pepper seedlings grown under salt stress conditions. Put application also had an effect on reducing CAT and POD activities and increasing carotenoid levels in Psidium guajava under salt stress, which seems to be related to the involvement of Put in plant growth via photosynthesis [108]. A study in Cucumis sativus showed that Put alleviated stress not only by inducing thylakoid membrane lipid peroxidation by increasing unsaturated fatty acid content but also upregulating ATPase facilitating the Na+ efflux [109]. Altogether, this suggests that Put application enhances the ability of PSII-repairing reaction centers and regulates protein expression at transcriptional levels by increasing endogenous PAs content in thylakoid membranes, thereby stabilizing the photosynthetic apparatus under salinity [109]. In addition, Put seems to be actively involved in the glycolytic pathway and the Krebs cycle by means of the inhibition of carbohydrates over-accumulation in leaves and improving the energy formation to face up the salinity damage [110]. Focusing on the biosynthesis pathways of Put, it should be noted that exogenous application of Put could increase endogenous PA levels through ADC pathways in cucumber seedlings under salt stress [111]. pRD29A:oat ADC transgenic lines showed a smaller reduction in shoot biomass and a slight enhancement in root growth and were healthier than the wild type in response to stress [112]. In addition, ADC overexpression led to an osmotic adjustment via the release of proline (Pro) and the increase in K+ uptake by roots with a concomitant reduction of Na+ accumulation, maintaining Na+/K+ ratio [112]. A decrease in Na+/K+ and Na+/Ca2+ ratios were found in roots when exogenous Put was added, which was associated with root growth promotion [113]. Leaves of Prosopis strombulifera treated with Na2SO4 displayed low Put levels accompanied by the reduction of shoot growth [114]. Nevertheless, roots treated with Na2SO4 showed an increase in the Put content which was correlated with the formation of adventitious and lateral roots [115,116]. Moreover, an upregulation in ADC and ODC was observed when NO was exogenously applied in order to synthetize more Put and, in turn, Spd and Spm by NO-induced expression of S-adenosyl methionine decarboxylase (SAMDC), SPDS, and SPMS in tomato plants under sodic alkaline stress [117]. In addition to this statement, Recalde et al. [118] showed that CuAO8 A. thaliana mutants reported lower NO levels in response to salt stress, which was probably due to a higher arginase activity preventing Arg availability from affecting the production of NO via nitric oxide synthase-like pathway. On the other hand, García-Jiménez et al. [119] reported that hyposaline shock led to an accumulation in free Put, Spd, and Spm due to a decline in the transglutaminase (TGase) activity, which was accompanied by an increase in the L-Arg pathway.
Another natural abiotic factor that is expected to become more prevalent in terms of frequency and intensity as a result of climate change is drought, being one of the main abiotic factors affecting crop productivity in the Mediterranean regions [120]. Drought causes a reduction in plant growth due to modifications in photosynthesis, nutrient metabolism, ion uptake and translocation, respiration, and carbohydrates metabolism [121]. Focusing these effects on PAs metabolism, it should be noted that Put application by spraying increased leaf area, height, leaf area, and grain yield of wheat plants owing to the increase in chlorophyll, water status, and the content of Pro, amino acids, and soluble sugars [122]. Zhu et al. [123] showed that foliar Put application to lettuce subjected to drought conditions triggered a reduction in stomatal density, keeping chloroplast structure and cell turgor. Similarly, Shallan et al. [124] described that Put application as pretreatment in cotton plants improved root to shoot ratio, leaf area, number and setting of bolls, seed cotton yield, total soluble sugars, pigments content, Pro content, total free amino acids, total phenols, total soluble proteins, total antioxidant capacity, and antioxidant enzyme activities. Put treatment also reduces the sensitivity of Medicago sativa plants to PEG-induced drought stress by reducing the activity of the hydrolytic enzymes and increasing the polysaccharide, protein and photosynthetic pigment contents, and photosynthetic activity [125]. Put has the ability to improve anatomical features, retaining chlorophyll concentrations and accumulating total soluble phenolic compounds in Thymus vulgaris plants, which leads to improved oil yield under drought conditions [126]. Following this line, Put application promoted drought tolerance in Cabernet Sauvignon seedlings by increasing net photosynthesis rate, the activities of SOD, POD and CAT, levels of ascorbic acid and glutathione, and PA pool [127], as well as in safflower plants via increasing antioxidant enzyme activities, anthocyanin and soluble protein contents, and decreasing lipid peroxidation, electrolyte leakage, and H2O2 contents [128]. Put-sprayed sugar beet plants suffered less oxidative stress than those not treated, as indicated by lower H2O2 and MDA accumulation, which is mostly due to the enhanced antioxidant enzyme activities that regulated ROS homeostasis [129]. NO is a key messenger in plant responses, and the interplay with PAs metabolism under drought conditions was studied in transgenic barley plants overexpressing the non-symbiotic hemoglobin gene HvHb1, which oxidizes NO to NO3− [130]. These plants displayed an increase in Put and Spd, which were correlated with amino acid precursors of PAs and with the expression of specific PA biosynthesis genes. In addition, exogenous Put treatment led to an enhancement of the phospholipase D activity, an enzyme that plays a role in drought stress mitigation at early stages [131]. Foliar application of Put (especially at 150 ppm) prevented the degradation of leaf proteins and chlorophyll and decreased Pro by reducing water deficit stress [132]. Alcázar et al. [133] showed that A. thaliana ADC2 overexpressor lines displayed a reduction in transpiration rate and stomatal conductance, which indicates that one of the mechanisms involved in the drought tolerance is associated with a reduction of water loss by transpiration. Thus, Put accumulation seems to be an ABA-dependent metabolic response under drought stress, which was revealed due to the impaired Put levels in ABA-deficient and ABA-insensitive A. thaliana mutants subjected to this stress [95]. These results resemble those reported in other studies, suggesting that Put acts directly as a protective compound through the activation of the antioxidant machinery to scavenge ROS and prevent lipid peroxidation, thereby contributing to the maintenance of membrane integrity, as well as the accumulation of secondary metabolites under water deficit [134]. Nevertheless, Put application not only improves the drought resistance at vegetative developmental stages but also as seed priming pretreatment leading to an improvement of seed germination, vigor, and enhanced tolerance in maize plants [135]. The correlation between Put content and the degree of resistance to drought seems to be dependent on plant species since a correlation was found in A. thaliana, but no correlation was found in rice plants [133,136]. Nonetheless, ADC expression was much more induced than ODC in response to drought, but the fold change in ODC1 transcript abundance was linearly correlated with the drought tolerance of the cultivars [136]. Similar results were found by Espasandin et al. [112], showing a direct correlation between ADC expression levels and drought tolerance in Lotus tenuis transgenic plants overexpressing the oat ADC gene. These plants also revealed an upregulation of the 9-cis-epoxycarotenoid dioxygenase (NCED) gene under drought conditions, indicating an interrelationship between Put and ABA. It is also worth mentioning that drought stress led to an increase in Put content and PAO and ODC activities in the roots of tolerant maize cultivars compared to susceptible ones [137]. It should be noted that exogenous H2S increased the total free PAs, together with an upregulation of SoADC, SoCPA, and SoODC genes in Spinacia oleracea seedlings under drought stress, suggesting that H2S enhanced tolerance by regulating PA biosynthesis [138].
Plant performance is also negatively affected by soil flooding, an environmental factor that occurs seasonally. However, climate change models predict an increase in the frequency of flooding events worldwide [139]. Flooding stress produces a reduction in relative water content, chlorophyll content, stomatal conductance, and photosynthetic efficiency [140]. The accumulation of Put induced plasma membrane H(+)-ATPase activity in flooded roots, helping to the maintenance of plant cell homeostasis and nutrient uptake [141]. When Carrizo Citrange (Citrus sinensis × Poncirus trifoliata) and Volkameriana (Citrus volkameriana) rootstocks were treated with Put, an improvement of growth and physiological parameters and a reduction of oxidative damage were observed under flooding conditions [142]. Similar results were observed in Put-treated welsh onion plants resulting in the alleviation of flooding stress by the reduction of relative water content, plant growth, chlorophyll fluorescence, and ROS, and the upgrade in the antioxidant system [143].
The progressive increase in the mean global air temperature associated with climate change may adversely affect plant growth and productivity [144,145]. Photosynthesis is one of the most sensitive physiological processes to warmer temperatures. PAs protect plants from high temperatures affecting photosynthesis through the maintenance of thermostability of thylakoid membranes [146,147]. In wheat plants exposed to heat stress during 4 h and 8 h, Hassanein et al. [148] reported a reduction in the growth parameters yield components, as well as in the level of Put, total PAs, and amino acids. In contrast, pretreatment with Arg or Put before exposure to heat stress led to improved tolerance via enhancing the content of Put, Spd, total PAs, and amino acids and decreasing ethylene and NH4+ content. In accordance with these results, Mostafa et al. [149] showed that foliar application of Arg or Put (1.25 and 2.5 mM, respectively) improved growth and all yield parameters of late sowing wheat plants under heat stress. However, the foliar application of Put combined with the supply of the same proportion of NO3−/NH4+ in the nutrient solution alleviated the negative effects of the thermal stress, increasing the content of various sugars, total phenolic compounds, PAs, and the activity of antioxidant enzymes in cauliflower plants (Brassica oleracea) [150]. It was also reported that the treatment with PAs 2 h before the application of heat stress at 45 °C for 2 h, as well as the combined treatment of PAs with DFMO, increased the recovery growth of root and hypocotyls in soybean seedlings, whereas when DFMO was supplied alone plants were more vulnerable to heat shock [151]. Additionally, PAs were found to be involved in the reduction of electrolyte leakage and MDA levels, reflecting their role in protecting membrane integrity. These findings also suggest that PAs may replace Ca2+ and contribute to the maintenance of membrane integrity by binding to membrane phospholipids under the studied conditions. In line with the previous observation, plants treated with combined heat shock and Arg or Put at varying concentrations showed a significant increase of the antioxidant machinery, in particular SOD and CAT activities [152]. Moreover, PAs can also influence heat-shock protein (HSP) synthesis, which plays an important role in the integrity of cell membranes under high-temperature stress [153]. Melatonin pretreatment increased heat tolerance of tomato seedlings by enhancing the antioxidant defense mechanism and reprogramming the PAs metabolic and NO biosynthesis pathways, which allowed us to scavenge the excess of ROS and improve cellular membrane stability. Melatonin induced respiratory burst oxidase (RBOH), heat shock transcription factors A2 (HsfA2), heat shock protein 90 (HSP90), and delta 1-pyrroline-5-carboxylate synthetase (P5CS) gene expression, aiding ROS detoxification [154]. Furthermore, the exogenous application of Put increased HSP17 transcript levels, and the impact was more marked in thermotolerant cultivars than in the susceptible ones [155]. Moreover, microarray analysis showed that ADC overexpression up- and down-regulated several genes involved in hormone and signaling pathways, such as the genes encoding transcription factors belonging to the APETALA2/ethylene-responsive factor domain family [156]. Altogether, this reflects the duality of Put by its direct role and the indirect participation in the acclimation processes [44].
As outlined above, temperature is one of the main abiotic factors that limits plant performance, distribution, productivity, and survival [157]. In this review, the role of PAs under high-temperature conditions were already described, but the role of low temperatures should also be addressed. Put levels increased within 12 h after exposure to 4 °C in A. thaliana plants [158]. The exogenous application of Put improved tolerance to chilling in tomato plants by reducing H2O2 and MDA levels and modulating the antioxidant machinery [159]. The involvement of ABA was also described in Put-induced tolerance to chilling stress in tomato seedlings [160]. ABA treatment could alleviate the electrolyte leakage induced by D-Arg. Hummel et al. [161] showed that ADC seemed to play a more important role than the ODC pathway in PAs biosynthesis in P. antiscorbutica seedlings at low temperatures. A positive correlation was observed between agmatine content and primary root growth rate, whereas no correlation was found with Put, or they were even negatively correlated. In this regard, the expression of BrrADC2.2 followed a cumulative pattern in accordance with Put levels in Tibetan turnip (Brassica rapa) under freezing conditions [162]. It was found that BrrICE1.1 (Inducer of CBF Expression 1) could directly interact with the BrrADC2.2 promoter, activating BrrADC2.2 to stimulate the accumulation of Put levels. Furthermore, knock-out mutants for A. thaliana ADC1 showed increased sensitivity to freezing, but the exogenous application of Put reversed that phenotype [158]. It should be noted that Put also induced seed priming, improving germination, seedling growth, and alleviation to low temperatures [163]. Concerning fruit quality, Abbasi et al. [164] showed that Put application reduced the rate of fruit softening, fruit weight losses, total soluble solids, titratability, ascorbic acid content, and fading of skin colour during storage in peach fruit during low- temperature storage, regardless of the doses of Put applied, or the time of application in Antirrhinum Majus. Finally, Put may also play a role in plant tolerance to other abiotic stresses, including heavy metal toxicity or UV radiations, which can lead to yield losses and hamper food security as recently reviewed [25,165]. Several studies demonstrated an accumulation of different PAs accompanied by an increase of ADC when plants were exposed to heavy metals [2,166], i.e., Cd effects on Put accumulation by the activation of ADC was previously described in Phaseolus vulgaris seedlings by means of the application of DFMA and DFMO [167]. Concerning UV radiations, which could affect DNA or damage the physiological processes, it should be mentioned that tobacco callus subjected to UV-C radiation displayed higher concentrations of Put in the upper layers, especially in the first 6 h of exposure [168]. Put also protected hulless barley from damage due to UV-B stress via H2S- and H2O2-mediated signaling pathways and their interaction increased plant tolerance by maintaining redox homeostasis and enhancing the accumulation of UV-absorbing molecules [169].
In general, exogenous Put application or the use of ADC/ODC-overexpressed plants displayed enhanced tolerance to abiotic stresses and improved the parameters associated with growth, photosynthetic capacity, and antioxidant activity. Several examples of ADC and ODC transgenic plants subjected to different abiotic stresses are shown in Table 1.

Table 1.
ADC and ODC transgenic plants showed enhanced tolerance to different abiotic stresses.
4. Resistance Responses to Biotic Stresses
PAs not only play important roles in abiotic stress but also in the regulation of plant defense responses against pathogens [177]. In many cases, the effect of PAs in plant defense is explained by the basis of the production of H2O2 through PA oxidation [178]. Put is biosynthesized in plants by ADC and ODC routes but Put content is also conditioned by the catabolism activity of copper amine oxidase (CuAO), which oxidizes the primary amino groups of Put, generating the corresponding aldehyde, H2O2, and NH4+ [179,180]. ROS production through PA oxidation is proposed to underlie many of the PA functions, including defense signaling [181]. Thus, in A. thaliana, AMINE OXIDASE1 (ATAO1/CuAOβ) and CuAO γ1, α3, and ζ exhibited high affinity for Put and loss-of-function mutations in CuAO compromise basal defenses. Treatment with Put (500 μM) or the avirulent SAR-inducing Pseudomonas syringae pv. tomato DC3000 AvrRpm1 triggered systemic resistance in CuAO mutants (atao1-3, cuao1-3, cuao2-1, and cuao3-1), suggesting that the different CuAOs additively contribute to Put-triggered systemic responses [182,183]. However, plants not only activate PAs catabolism pathways against pathogens but the activation of the biosynthetic routes are also observed. There is evidence that the ADC, ODC, SAMDC, and DAO activities were upregulated in a TMV resistant tobacco line during viral infection, while no changes were observed in the susceptible line [184]. Similarly, barley (Hordeum vulgare) infected with the powdery mildew fungus Blumeria graminis f. sp. hordei displayed an increase in Put, Spd, and Spm concentrations in infected leaves, while the ADC and ODC activities were also induced [9]. In maize, tumor formation during the interaction with the biotrophic pathogenic fungus Ustilago maydis increased the levels of free and conjugated Put [185]. Moreover, the ODC seems to be responsible for the PA increase in wheat leaves infected by Puccinia graminis f. sp. tritici [186]. In this case, a reduction of the ADC activity was found, whereas the ODC activity significantly increased in the pustules.
The accumulation of Put and Spm was related to an increase in the resistance of tobacco plants infected with Sclerotinia sclerotiorum [187]. In addition, the overexpression of arginase 2 in A. thaliana produced an increase in the Put and Pro contents, which is involved in a major resistance to Botrytis cinerea infection [188]. The ADC gene induction in transgenic eggplants (Solanum melongena) with a constitutive promoter of cauliflower mosaic virus-induced resistance against fungal wilt disease caused by Fusarium oxysporium and different abiotic stresses [172]. In A. thaliana, the expression of both ADC isoforms was reported to increase during the hypersensitive response triggered by the avirulent cucumber mosaic virus [189]. Despite having evidence of the protective effect of the Put during defense, signaling pathways underlying PA functions were not elucidated. Kim et al. [14] showed that Put is involved in MAPK cascades regulation producing an increase in AtADC2 expression during A. thaliana response to P. syringae infection. In addition, a reduction in Put content in the adc2 mutant led to an increase in susceptibility to P. syringae inoculation. Liu et al. [190] also showed that Put was accumulated in response to flg22, a well-characterized pathogen-associated molecular pattern (PAMP). Through the analysis of adc1 and adc2 loss-of-function mutants deficient in Put biosynthesis, it was found that the ADC2 isoform was the major contributor to Put biosynthesis triggered by flg22. Moreover, exogenous Put application induced defense responses, such as callose deposition and up-regulation of several PAMP triggered immunity (PTI) marker genes. Put could be involved in amplifying PTI responses through ROS production, enhancing disease resistance against bacterial pathogens. In addition, Liu et al. [183] reported that defense signaling triggered by Put partly depends on SA accumulation in A. thaliana. Indeed, Put elicits ROS-dependent local SA accumulation and produces a local and systemic reprogramming of genes involved in systemic acquired resistance (SAR). Recently, Rossi et al. [191] demonstrated that Put supplementation reduces plant susceptibility to P. syringae in A. thaliana, showing an interconnection between SA signaling and plant PA metabolism. This was also supported by the finding that SA treatment led to the upregulation of ADC1 and ADC2 genes, whereas the SA treatment of adc1 and adc2 loss of function mutants showed that adc2 had no effect on PA levels. Therefore, ADC2 seems to be the ADC isoform that makes the major contribution to Put accumulation in A. thaliana regulated by SA signaling. Avirulent Xanthomonas campestris pv. vesicatoria was shown to trigger hypersensitive cell death in Capsicum annuum via ADC. By binding CaADC1, AvrBsT promoted ROS production, defense gene expression, and cell death [192]. On the contrary, this effector acts as a defense suppressor in tomato plants [193], but no studies about its interaction with tomato ADCs were reported. Recently, tomato ADC1 and ADC2 genes were found to be targeted by the Brg11 effector protein produced by Ralstonia solanacearum [194]. Brg11 induced the production of ADC transcripts with higher translational activity than the native mRNA, promoting Put accumulation. However, this perturbation of plant PA metabolism induced by Brg11 did not seem to affect the infection by R. solanacearum, but reduced host susceptibility to P. syringae, suggesting that Brg11 allows R. solanacearum to manipulate host defense, obtaining an advantage over other pathogen competitors [194].
Generally, reduced Put levels, in adc loss of function mutants and adc-silenced lines, result in pathogen susceptibility [183,195]. Sánchez-Rangel et al. [51] generated a transgenic line of A. thaliana that silences ADC1 and ADC2 genes; this adc-silenced line is a non-lethal line with reduced ADC gene expression and low Put levels and high levels of ROS. Chávez-Martínez et al. [195] examined the response of this adc-silenced line against two pathogens with different lifestyles. This silenced line was more susceptible to Botrytis cinerea, and the expression of PR1 was upregulated, while the jasmonic acid-related genes LOX3 and PDF1.2 and PAD3 involved in camalexin biosynthesis were downregulated. On the other hand, the ADC-silenced line increased their resistance to P. syringae infection associated with the upregulation of PR1, ZAT1.2, WRKY54, and WRKY70 genes. The differences in plant defense responses against the two pathogens could be related to the accumulation of ROS previously reported for this ADC-silenced line, in which the deregulation of SA- and JA-response genes occurred.
The protective effect of Put was also reported in plants infected by nematodes. A. thaliana root infection by the cyst nematode Heteodera schachtii increased expression of ADC1 and ADC2 genes [196]. The exogenous application of Put can suppress nematode development in tomato plants [197]. Further research is needed to better understand the involvement of Put in plant response to nematode infection.
As previously mentioned, free and conjugated Put levels are enhanced in plant tissues infected by fungi and bacteria [185,198]. In most cases, Put levels in infected tissues are the result of de novo biosynthesis [192], although it was also proposed that Put is excreted by the pathogen during plant tissue colonisation [198]. Vilas et al. [198] explained the accumulation of Put in the whole leaf tissues, as well as in the apoplast of tomato plants infected with the bacterial P. syringae by the induction of its synthesis in plant cells and also by the excretion of bacteria. The excretion of Put by P. syringae was stimulated under virulence inducing conditions, but no activation of bacterial virulence traits or induction of plant invasion were observed after the exogenous addition of Put. However, the possibility that Put could elicit plant defense response cannot be ruled out because the experiment was conducted in plants unchallenged with the pathogen. In some cases, pathogens can modulate plant PA metabolism for their own benefit. In this sense, Stes et al. [199] demonstrated that Rhodococcus fascians produces CKs that induce Put accumulation in A. thaliana by activating ADC expression, increasing symptom development. In addition, the N source can promote an accumulation of Put enhancing resistance to biotic stress. In tomato plants, Fernández-Crespo et al. [200] demonstrated that NH4+ nutrition confers resistance to P. syringae by enhancing H2O2 accumulation that acts as a signal for activation of systemic acquired acclimation (SAA) mediated by ABA and Put. The high level of the Put precursor Arg hints towards the importance of the glutamate pathway as a key metabolic checkpoint in this pathosystem under NH4+ nutrition [201]. In addition, Wimalasekera et al. [33] suggested that PA could induce NO production by an unknown pathway. The occurrence of the interplay among PAs, NO, and H2O2 in stress responses is an interesting phenomenon that requires further investigation.
In plant–pathogen interactions, not only free PAs are involved. PAs are precursors for secondary metabolites and conjugated with phenolic acids which are linked with plant–pathogen defense responses [9,202]. Plant phenolamides, hydroxycinnamic acid amides, or phenylamides identified from the phenylpropanoid derivative pathway are involved in plant defense against bacteria, viruses, fungi, and insects [203,204,205]. Concretely, Put derivatives as caffeoylputrescine increased dramatically in local and systemic tissues of Nicotiana attenuata after herbivore attack [206], and p-coumaroylputrescine and feruloylputrescine were strongly accumulated in rice (Oryza sativa cv. Nipponbare) leaves subjected to the attack of chewing and sucking herbivores [207]. An accumulation of these phenolamides was also observed in response to Alternaria brassicicola challenge in A. thaliana rosette leaves [208]. However, further studies are required to identify the mechanisms of these compounds for inducing plant immunity against pathogens.
Several examples of ADC and ODC transgenic plants subjected to different biotic stresses are shown in Table 2.

Table 2.
Increased expression of ADC and ODC genes in response to biotic stress in different plant species.
Other studies showed that the exogenous application of phytohormones associated with plant defense modified PA metabolism. SA can induce the accumulation of PAs by activating the expression of ADC and ODC in maize, tobacco, and tomato [209,210,211]. The exogenous application of SA induced PA metabolism in A. thaliana, increasing Put levels by ADC activity induction. These changes were NPR1-independent and partially dependent on MPK6 activity [191]. In contrast, Liu et al. [183] found that exogenous SA did not affect PA levels in A. thaliana. However, this discrepancy can probably be attributed to different experimental conditions used in both studies. The application of the methyl-SA (a SA derivative) to cherry tomato plants also contributed to the accumulation of Put, Spd, and Spm, which was associated with the upregulation of ADC and ODC genes [209]. The treatment of barley primary leaves with methyl-jasmonate (MeJA) induced the accumulation of free and conjugated Put and Spd, as well as the ADC, ODC, SAMDC, and DAO activities [212]. Similarly, increased PA levels and the activity of enzymes involved both in PA biosynthesis and oxidation were also observed in wheat [213]. MeJA treatment increased the ADC2 gene expression, while ADC1 remained unaltered in A. thaliana, suggesting a possible different regulatory pathway for both genes [92]. Two ODC genes were also induced in tobacco in response to MeJA, but the effect in plant defense is not yet studied [214]. However, in rice, MeJA produced a transient inhibition of ADC, SAMDC, and SPDS gene expression [215]. ABA was also able to induce Put oxidation at the apoplast of Vicia faba [216]. This was demonstrated to be important for stomatal closure, an important mechanism to prevent bacterial entry in plants. Nevertheless, more research is necessary to understand the complex connection between SA and ABA metabolism with PAs in plant biotic stress responses.
5. Conclusions and Perspectives
This comprehensive review of PAs metabolism is mainly focused on the findings that were made about the involvement of Put in plant growth and development as well as adaptation responses to both biotic and abiotic stresses. There is enough evidence indicating that agriculture will be impaired by global climate change, which could compromise food security in the next future. According to FAO predictions, food production needs to increase 70% more by 2050 for feeding an increasing world population. Future growing conditions will bring the concurrence of different biotic and abiotic stresses. To deal with these upcoming problems and to ensure food security, different approaches to develop stress-tolerant crops will be required. Thus, the role of PAs in the regulation of plant promotion and protection mechanisms might have future implications in agriculture. Although PAs are widely studied across different plant species, further studies are still required to better understand the role of Put and other PAs in the intricate molecular mechanisms underlying plant growth and development and tolerance to abiotic and biotic stresses. Even though most of the reviewed studies examined the effects of exogenous Put, the increase of endogenous Put production by genetic manipulation is becoming of great interest. Thus, metabolic engineering, together with the current development of different high throughput techniques, will be very useful tools to throw light on the complex interactions of PAs with different metabolic pathways, including primary and secondary metabolism, hormones, etc., under different environmental cues. It is also largely unknown how the PAs pathway is regulated at the transcriptional, translational, and post-translational levels. Finally, it should be noted that the use of external applications of these compounds constitutes a good approach to be exploited in agriculture.
Funding
This work was funded by the regional government of Castilla y León, grant number CSI260P20. A.I. González was the recipient of a contract supported by Project CSI260P20, with European Regional Development Fund, ERD. Project “CLU-2019-05—IRNASA/CSIC Unit of Excellence”, funded by the Junta de Castilla y León and co-financed by the European Union (ERDF “Europe drives our growth”). This work was also supported by “Projectes d’Investigació científica i desenvolupament tecnològic. Pla de promoció de la Investigació de Universitat Jaume I (UJI-B2020-24)”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources of Research (URICI) and CSIC Interdisciplinary Thematic Platform (PTI) Optimization of agricultural and forestry systems (PTI-AGROFOR). Due to the limited length of this manuscript, the authors apologize for not citing all the literature related to the topic.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alcázar, R.; Marco, F.; Cuevas, J.C.; Patron, M.; Ferrando, A.; Carrasco, P.; Tiburcio, A.F.; Altabella, T. Involvement of Polyamines in Plant Response to Abiotic Stress. Biotechnol. Lett. 2006, 28, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Groppa, M.D.; Benavides, M.P. Polyamines and Abiotic Stress: Recent Advances. Amino Acids 2008, 34, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kaur-Sawhney, R.; Tiburcio, A.F.; Altabella, T.; Galston, A.W. Polyamines in Plants: An Overview. J. Cell Mol. Biol. 2003, 2, 1–12. [Google Scholar]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Watanabe, K.; Murayama, C.; Izawa, S.; Niitsu, M.; Michael, A.J.; Berberich, T.; Kusano, T. Polyamine Oxidase5 Regulates Arabidopsis Growth through Thermospermine Oxidase Activity. Plant Physiol. 2014, 165, 1575–1590. [Google Scholar] [CrossRef]
- Sobieszczuk-Nowicka, E. Polyamine Catabolism Adds Fuel to Leaf Senescence. Amino Acids 2017, 49, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Regla-Márquez, C.F.; Canto-Flick, A.; Avilés-Viñas, S.A.; Valle-Gough, R.E.; Pérez-Pastrana, J.; García-Villalobos, F.J.; Santana-Buzzy, N. Cadaverine: A Common Polyamine in Zygotic Embryos and Somatic Embryos of the Species Capsicum chinense Jacq. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 124, 253–264. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Rahman, A.; Alam, M.M.; Mahmud, J.-A.; Suzuki, T.; Fujita, M. Polyamines Confer Salt Tolerance in Mung Bean (Vigna radiata L.) by Reducing Sodium Uptake, Improving Nutrient Homeostasis, Antioxidant Defense, and Methylglyoxal Detoxification Systems. Front. Plant Sci. 2016, 7, 1104. [Google Scholar] [CrossRef]
- Walters, D. Resistance to Plant Pathogens: Possible Roles for Free Polyamines and Polyamine Catabolism. New Phytol. 2003, 159, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Docimo, T.; Reichelt, M.; Schneider, B.; Kai, M.; Kunert, G.; Gershenzon, J.; D’Auria, J.C. The First Step in the Biosynthesis of Cocaine in Erythroxylum Coca: The Characterization of Arginine and Ornithine Decarboxylases. Plant Mol. Biol. 2012, 78, 599–615. [Google Scholar] [CrossRef]
- Sekula, B.; Ruszkowski, M.; Malinska, M.; Dauter, Z. Structural Investigations of N-Carbamoylputrescine Amidohydrolase from Medicago Truncatula: Insights into the Ultimate Step of Putrescine Biosynthesis in Plants. Front. Plant Sci. 2016, 7, 350. [Google Scholar] [CrossRef] [PubMed]
- Hanfrey, C.; Sommer, S.; Mayer, M.J.; Burtin, D.; Michael, A.J. Arabidopsis Polyamine Biosynthesis: Absence of Ornithine Decarboxylase and the Mechanism of Arginine Decarboxylase Activity. Plant J. 2001, 27, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Soyka, S.; Heyer, A.G. Arabidopsis Knockout Mutation of ADC2 Gene Reveals Inducibility by Osmotic Stress. FEBS Lett. 1999, 458, 219–223. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, S.-H.; Yoo, S.-J.; Min, K.-H.; Nam, S.-H.; Cho, B.H.; Yang, K.-Y. Putrescine Regulating by Stress-Responsive MAPK Cascade Contributes to Bacterial Pathogen Defense in Arabidopsis. Biochem. Biophys. Res. Commun. 2013, 437, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Vuosku, J.; Karppinen, K.; Muilu-Mäkelä, R.; Kusano, T.; Sagor, G.H.M.; Avia, K.; Alakärppä, E.; Kestilä, J.; Suokas, M.; Nickolov, K.; et al. Scots Pine Aminopropyltransferases Shed New Light on Evolution of the Polyamine Biosynthesis Pathway in Seed Plants. Ann. Bot. 2018, 121, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Knott, J.M.; Römer, P.; Sumper, M. Putative Spermine Synthases from Thalassiosira Pseudonana and Arabidopsis Thaliana Synthesize Thermospermine Rather than Spermine. FEBS Lett. 2007, 581, 3081–3086. [Google Scholar] [CrossRef] [PubMed]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-Molecules with Diverse Functions in Plant and Human Health and Disease. Front. Chem. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Moschou, P.N.; Paschalidis, K.A.; Delis, I.D.; Andriopoulou, A.H.; Lagiotis, G.D.; Yakoumakis, D.I.; Roubelakis-Angelakis, K.A. Spermidine Exodus and Oxidation in the Apoplast Induced by Abiotic Stress Is Responsible for H2O2 Signatures That Direct Tolerance Responses in Tobacco. Plant Cell 2008, 20, 1708–1724. [Google Scholar] [CrossRef] [PubMed]
- Farvardin, A.; González-Hernández, A.I.; Llorens, E.; García-Agustín, P.; Scalschi, L.; Vicedo, B. The Apoplast: A Key Player in Plant Survival. Antioxidants 2020, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- Wojtasik, W.; Kulma, A.; Namysł, K.; Preisner, M.; Szopa, J. Polyamine Metabolism in Flax in Response to Treatment with Pathogenic and Non–Pathogenic Fusarium Strains. Front. Plant Sci. 2015, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Grossi, M.; Phanstiel, O.; Rippe, C.; Swärd, K.; Alajbegovic, A.; Albinsson, S.; Forte, A.; Persson, L.; Hellstrand, P.; Nilsson, B.-O. Inhibition of Polyamine Uptake Potentiates the Anti-Proliferative Effect of Polyamine Synthesis Inhibition and Preserves the Contractile Phenotype of Vascular Smooth Muscle Cells. J. Cell. Physiol. 2016, 231, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Shim, I.-S.; Fujihara, S. Inhibition of Putrescine Biosynthesis Enhanced Salt Stress Sensitivity and Decreased Spermidine Content in Rice Seedlings. Biol. Plant. 2017, 61, 385–388. [Google Scholar] [CrossRef]
- Hashem, A.M.; Moore, S.; Chen, S.; Hu, C.; Zhao, Q.; Elesawi, I.E.; Feng, Y.; Topping, J.F.; Liu, J.; Lindsey, K.; et al. Putrescine Depletion Affects Arabidopsis Root Meristem Size by Modulating Auxin and Cytokinin Signaling and ROS Accumulation. Int. J. Mol. Sci. 2021, 22, 4094. [Google Scholar] [CrossRef]
- He, L.; Nada, K.; Kasukabe, Y.; Tachibana, S. Enhanced Susceptibility of Photosynthesis to Low-Temperature Photoinhibition Due to Interruption of Chill-Induced Increase of S-Adenosylmethionine Decarboxylase Activity in Leaves of Spinach (Spinacia oleracea L.). Plant Cell Physiol. 2002, 43, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Navakoudis, E.; Lütz, C.; Langebartels, C.; Lütz-Meindl, U.; Kotzabasis, K. Ozone Impact on the Photosynthetic Apparatus and the Protective Role of Polyamines. Biochim. Biophys. Acta (BBA) Gen. Subj. 2003, 1621, 160–169. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Varshney, P.; Yusuf, M.; Ahmad, A. Polyamines: Potent Modulators of Plant Responses to Stress. J. Plant Interact. 2013, 8, 1–16. [Google Scholar] [CrossRef]
- Couée, I.; Hummel, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of Polyamines in Root Development. Plant Cell Tissue Organ Cult. (PCTOC) 2004, 76, 1–10. [Google Scholar] [CrossRef]
- Liu, J.-H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines Function in Stress Tolerance: From Synthesis to Regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H.M. Polyamines: Natural and Engineered Abiotic and Biotic Stress Tolerance in Plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Mustafavi, S.H.; Naghdi Badi, H.; Sękara, A.; Mehrafarin, A.; Janda, T.; Ghorbanpour, M.; Rafiee, H. Polyamines and Their Possible Mechanisms Involved in Plant Physiological Processes and Elicitation of Secondary Metabolites. Acta Physiol. Plant. 2018, 40, 102. [Google Scholar] [CrossRef]
- Anwar, R.; Mattoo, A.K.; Handa, A.K. Polyamine Interactions with Plant Hormones: Crosstalk at Several Levels. In Polyamines; Springer: Tokyo, Japan, 2015; pp. 267–302. [Google Scholar]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with Regulatory Functions in Plant Abiotic Stress Tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Wimalasekera, R.; Villar, C.; Begum, T.; Scherer, G.F.E. COPPER AMINE OXIDASE1 (CuAO1) of Arabidopsis Thaliana Contributes to Abscisic Acid-and Polyamine-Induced Nitric Oxide Biosynthesis and Abscisic Acid Signal Transduction. Mol. Plant 2011, 4, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Wani, K.I.; Naeem, M.; Khan, M.M.A.; Aftab, T. Cellular Responses, Osmotic Adjustments, and Role of Osmolytes in Providing Salt Stress Resilience in Higher Plants: Polyamines and Nitric Oxide Crosstalk. J. Plant Growth Regul. 2022, 7, 347. [Google Scholar] [CrossRef]
- Khanna, K.; Sharma, N.; Kour, S.; Ali, M.; Ohri, P.; Bhardwaj, R. Hydrogen Sulfide: A Robust Combatant against Abiotic Stresses in Plants. Hydrogen 2021, 2, 319–342. [Google Scholar] [CrossRef]
- Deng, G.; Zhou, L.; Wang, Y.; Zhang, G.; Chen, X. Hydrogen Sulfide Acts Downstream of Jasmonic Acid to Inhibit Stomatal Development in Arabidopsis. Planta 2020, 251, 42. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Huo, J.; Liao, W. Hydrogen Sulfide: Roles in Plant Abiotic Stress Response and Crosstalk with Other Signals. Plant Sci. 2021, 302, 110733. [Google Scholar] [CrossRef] [PubMed]
- Minocha, R.; Majumdar, R.; Minocha, S.C. Polyamines and Abiotic Stress in Plants: A Complex Relationship1. Front. Plant Sci. 2014, 5, 175. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xing, S.T.; Sun, X. Effects of Polyamines on Hormones Contents and the Relationship with the Flower Bud Differentiation in Chrysanthemum. Plant Physiol. J. 2014, 50, 1195–1202. [Google Scholar]
- Cai, Q.; Zhang, J.; Guo, C.; Al, E. Reviews of the Physiological Roles and Molecular Biology of Polyamines in Higher Plants. J. Fujian Educ. Coll. 2006, 7, 118–124. [Google Scholar]
- Zhao, W.; Sun, G.; Li, S. Polyamines and Plant Stress Resistance. J. South. Agric. 2004, 35, 443–447. [Google Scholar]
- Imai, A.; Akiyama, T.; Kato, T.; Sato, S.; Tabata, S.; Yamamoto, K.T.; Takahashi, T. Spermine Is Not Essential for Survival of Arabidopsis. FEBS Lett. 2004, 556, 148–152. [Google Scholar] [CrossRef]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential Factors for Growth and Survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef]
- Pál, M.; Szalai, G.; Janda, T. Speculation: Polyamines Are Important in Abiotic Stress Signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Palavan-Ünsal, N. Polyamine Metabolism in the Roots of Phaseolus vulgaris Interaction of the Inhibitors of Polyamine Biosynthesis with Putrescine in Growth and Polyamine Biosynthesis. Plant Cell Physiol. 1987, 28, 565–572. [Google Scholar]
- Lee, T.-M. Polyamine Regulation of Growth and Chilling Tolerance of Rice (Oryza sativa L.) Roots Cultured in Vitro. Plant Sci. 1997, 122, 111–117. [Google Scholar] [CrossRef]
- Tarenghi, E.; Carré, M.; Martin-Tanguy, J. Effects of Inhibitors of Polyamine Biosynthesis and of Polyamines on Strawberry Microcutting Growth and Development. Plant Cell Tissue Organ Cult. (PCTOC) 1995, 42, 47–55. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; He, X.H. Exogenous Putrescine, Not Spermine or Spermidine, Enhances Root Mycorrhizal Development and Plant Growth of Trifoliate Orange (Poncirus trifoliata) Seedlings. Int. J. Agric. Biol. 2010, 12, 576–580. [Google Scholar]
- Ben-Hayyim, G.; Martin-Tanguy, J.; Tepfer, D. Changing Root and Shoot Architecture with the RolA Gene from Agrobacterium Rhizogenes: Interactions with Gibberellic Acid and Polyamine Metabolism. Physiol. Plant. 1996, 96, 237–243. [Google Scholar] [CrossRef]
- Tang, W.; Newton, R.J. Polyamines Promote Root Elongation and Growth by Increasing Root Cell Division in Regenerated Virginia Pine (Pinus virginiana Mill.) Plantlets. Plant Cell Rep. 2005, 24, 581–589. [Google Scholar] [CrossRef]
- Sánchez-Rangel, D.; Chávez-Martínez, A.I.; Rodríguez-Hernández, A.A.; Maruri-López, I.; Urano, K.; Shinozaki, K.; Jiménez-Bremont, J.F. Simultaneous Silencing of Two Arginine Decarboxylase Genes Alters Development in Arabidopsis. Front. Plant Sci. 2016, 7, 300. [Google Scholar] [CrossRef] [PubMed]
- Matam, P.; Parvatam, G. Putrescine and Polyamine Inhibitors in Culture Medium Alter in Vitro Rooting Response of Decalepis Hamiltonii Wight & Arn. Plant Cell Tissue Organ Cult. (PCTOC) 2017, 128, 273–282. [Google Scholar]
- Badawy, E.S.M.; Kandil, M.M.; Habib, A.M.; El-Sayed, I.M. Influence of Diatomite, Putrescine and Alpha-Tocopherol on Some Vegetative Growth and Flowering of Antirrhinum majus L. Plants. J. Hortic. Sci. Ornam. Plants 2015, 7, 7–18. [Google Scholar]
- Danaee, E.; Abdossi, V. Phytochemical And Morphophysiological Responses In Basil (Ocimum basilicum L.) Plant To Application Of Polyamines. J. Med. Plants 2019, 18, 125–133. [Google Scholar]
- Houdusse, F.; Zamarreño, A.M.; Garnica, M.; García-Mina, J. The Importance of Nitrate in Ameliorating the Effects of Ammonium and Urea Nutrition on Plant Development: The Relationships with Free Polyamines and Plant Proline Contents. Funct. Plant Biol. 2005, 32, 1057. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, V.; Gautam, I.K.; Sengar, R.S.; Garg, S.K. Influence of Putrescine on Enzymes of Ammonium Assimilation in Maize Seedling. Am. J. Plant Sci. 2013, 04, 297–301. [Google Scholar] [CrossRef]
- González-Hernández, A.I.; Scalschi, L.; Troncho, P.; García-Agustín, P.; Camañes, G. Putrescine Biosynthetic Pathways Modulate Root Growth Differently in Tomato Seedlings Grown under Different N Sources. J. Plant Physiol. 2022, 268, 153560. [Google Scholar] [CrossRef]
- Nahed, G.A.A.; Lobna, S.T.; Soad, M.I. Some Studies on The Effect Of Putrescine, Ascorbic Acid And Thiamine On Growth, Flowering And Some Chemical Constituents Of Gladiolus Plants At Nubaria. Ozean J. Appl. Sci. 2009, 2, 169–179. [Google Scholar]
- Youssef, A.A.; Mahgoub, M.H.; Talaat, I.M. Physiological and Biochemical Aspects of Matthiola incana L. Plants under the Effect of Putrescine and Kinetin Treatments. Egypt. J. Appl. Sci. 2004, 19, 492–510. [Google Scholar]
- Talaat, I.M.; Bekheta, M.A.; Mahgoubi, M.H. Physiological Response of Periwinkle Plants (Catharanthus roseus L.) to Tryptophan and Putrescine. Int. J. Agric. Biol. 2005, 7, 210–213. [Google Scholar]
- Mahgoub, M.; el Aziz, N.G.A.; Mazhar, A.M.A. Response of Dahlia pinnata L. Plant to Foliar Spray with Putrescine and Thiamine on Growth, Flowering and Photosynthetic Pigments. Am. Eurasian J. Agric. Environ. Sci. 2011, 10, 769–775. [Google Scholar]
- Dastyaran, M.; Hosseini Farahi, M. The Effect of Humic Acid and Putrescin on Vegetative Properties and Vase Life of Rose in a Soilless System. J. Soil Plant Interact. 2014, 5, 241–250. [Google Scholar]
- Yadav, V.; Singh, N.B.; Singh, H.; Singh, A.; Hussain, I. Putrescine Affects Tomato Growth and Response of Antioxidant Defense System Due to Exposure to Cinnamic Acid. Int. J. Veg. Sci. 2019, 25, 259–277. [Google Scholar] [CrossRef]
- Mora, V.; Bacaicoa, E.; Zamarreño, A.-M.; Aguirre, E.; Garnica, M.; Fuentes, M.; García-Mina, J.-M. Action of Humic Acid on Promotion of Cucumber Shoot Growth Involves Nitrate-Related Changes Associated with the Root-to-Shoot Distribution of Cytokinins, Polyamines and Mineral Nutrients. J. Plant Physiol. 2010, 167, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Krizek, D.T.; Kramer, G.F.; Mirecki, R.M. Influence of UV-B Radiation and Putrescine on Shoot and Root Growth of Cucumber Seedlings Grown in Nutrient Solution. J. Plant Nutr. 1997, 20, 613–623. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Rehman, H.; Hussain, M. Seed Priming with Polyamines Improves the Germination and Early Seedling Growth in Fine Rice. J. New Seeds 2008, 9, 145–155. [Google Scholar] [CrossRef]
- Fazilati, M.; Hossein Forghani, A.; Fazilati, M. The Role of Polyamine to Increasing Growth of Plant: As a Key Factor in Health Crisis. Int. J. Health Syst. Disaster Manag. 2015, 3, 89–94. [Google Scholar]
- Bais, H.P.; Sudha, G.S.; Ravishankar, G.A. Putrescine and Silver Nitrate Influences Shoot Multiplication, In Vitro Flowering and Endogenous Titers of Polyamines in Cichorium intybus L. Cv. Lucknow Local. J. Plant Growth Regul. 2000, 19, 238–248. [Google Scholar] [CrossRef]
- Badawy, E.M.; Kandil, M.M.; Mahgoub, M.; Shanan, N.; Hegazi, N. Chemical Constituents of Celosia argentea var. Cristata L. Plants as Affected by Foliar Application of Putrescine and Alpha-Tocopherol. Int. J. ChemTech Res. 2015, 8, 464–470. [Google Scholar]
- Yousefi, F.; Jabbarzadeh, Z.; Amiri, J.; Rasouli-Sadaghiani, M.H. Response of Roses (Rosa hybrida L. ‘Herbert Stevens’) to Foliar Application of Polyamines on Root Development, Flowering, Photosynthetic Pigments, Antioxidant Enzymes Activity and NPK. Sci. Rep. 2019, 9, 16025. [Google Scholar] [CrossRef]
- Kandil, M.M.; Ibrahaim, S.M.M.; El-Hanafy, S.H.; El-Sabwah, M.M. Effect of Putrescine and Uniconazole on Some Flowering Characteristics, and Some Chemical Constituents of Salvia splendens F. Plant. Int. J. ChemTech Res. 2015, 8, 174–186. [Google Scholar]
- Amin, A.A.; Gharib, F.A.; Abouziena, H.F.; Dawood, M.G. Role of Indole-3-Butyric Acid or/and Putrescine in Improving Productivity of Chickpea (Cicer arientinum L.) Plants. Pak. J. Biol. Sci. 2013, 16, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- El-Bassiouny, H.M.; Mostafa, H.A.; El-Khawas, S.A.; Hassanein, R.A.; Khalil, S.I.; Abd El-Monem, A.A. Physiological Responses of Wheat Plant to Foliar Treatments with Arginine or Putrescine. Aust. J. Basic Appl. Sci. 2008, 2, 1390–1403. [Google Scholar]
- El-Bassiouny, H.M.S.; Bekheta, M.A. Role of Putrescine on Growth, Regulation of Stomatal Aperture, Ionic Contents and Yield by Two Wheat Cultivars under Salinity Stress. Egypt. J. Physiol. Sci. 2001, 23, 235–258. [Google Scholar]
- Ioannidis, N.E.; Sfichi-Duke, L.; Kotzabasis, K. Polyamines Stimulate Non-Photochemical Quenching of Chlorophyll a Fluorescence in Scenedesmus Obliquus. Photosynth. Res. 2011, 107, 169–175. [Google Scholar] [CrossRef]
- Singh, T.; Bala, M. Effect of Putrescine and Benzyl Adenine on Growth, Flowering and Post-Harvest Keeping Quality Parameters in Chrysanthemum (Chrysanthemum morifolium Ramat.). J. Hortic. Sci. 2020, 15, 191–196. [Google Scholar] [CrossRef]
- Li, C.; Pei, Z.X.; Gan, L.Y. Effects of Photoperiod on Flowering and Polyamine Contents of Nobile-Type Dendrobium. Zhiwu Shengli Xuebao/Plant Physiol. J. 2014, 50, 1167–1170. [Google Scholar]
- Mahgoub, M.H.; El-Ghorab, A.H.; Bekheta, M. Effect of Some Bioregulators on the Endogenous Phytohormones, Chemical Composition, Essential Oil and Its Antioxidant Activity of Carnation (Dianthus caryophyllus L.). J. Agric. Sci. Mansoura Univ. 2006, 31, 4229–4245. [Google Scholar]
- Iman, M.E.S.; Kandil, M.M.; Badawy, M.E.S.; Abdalla, M.A.E.F.; Mahgoub, H.M.; Habib, M.A. Effect of Diatomite, Putrescine and Alpha-Tocopherol on Flower Characters and Anatomical Flower Bud Structure of Antirhinum majus L. Plant. Middle East J. Agric. Res. 2018, 7, 1747–1755. [Google Scholar]
- Kazemi, M. Influence of Foliar Application of 5-Sulfosalicylic Acid, Malic Acid, Putrescine and Potassium Nitrate on Vegetative Growth and Reproductive Characteristics of Strawberry Cv. ‘Selva.’ J. Biol. Environ. Sci. 2013, 7, 93–101. [Google Scholar]
- Bani Assadi, F.; Safari, V.R.; Maghsoudi Mod, A.A. Effect of Putrescine and Salinity on the Morphological, Biochemical, and Pigments of English Marigold Plant (Calendula officinalis L.). J. Sci. Technol. Greenh. Cult. 2015, 6, 125–133. [Google Scholar]
- Killiny, N.; Nehela, Y. Citrus Polyamines: Structure, Biosynthesis, and Physiological Functions. Plants 2020, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Bekheta, M.A.; El-Bassiouny, H.M.S. Responses of Two Wheat Cultivars Grown under Salinity Stress to Putrescine Treatment. J. Agric. Sci. 2005, 30, 4505–4521. [Google Scholar]
- Alcázar, R.; García-Martínez, J.L.; Cuevas, J.C.; Tiburcio, A.F.; Altabella, T. Overexpression of ADC2 in Arabidopsis Induces Dwarfism and Late-Flowering through GA Deficiency. Plant J. 2005, 43, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Sobieszczuk-Nowicka, E.; Rorat, T.; Legocka, J. Polyamine Metabolism and S-Adenosylmethionine Decarboxylase Gene Expression during the Cytokinin-Stimulated Greening Process. Acta Physiol. Plant. 2007, 29, 495–502. [Google Scholar] [CrossRef]
- Legocka, J.; Żarnowska, A. Role of Polyamines in the Cytokinin-Dependent Physiological Processes II. Modulation of Polyamine Levels during Cytokinin-Stimulated Expansion of Cucumber Cotyledons. Acta Physiol. Plant. 2000, 22, 395–401. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, C.; Sun, C.; Wang, J.; Ye, Y.; Zhou, W.; Lu, L.; Lin, X. Inhibition of Ethylene Production by Putrescine Alleviates Aluminium-Induced Root Inhibition in Wheat Plants. Sci. Rep. 2016, 6, 18888. [Google Scholar] [CrossRef]
- Takahashi, Y.; Cong, R.; Sagor, G.H.M.; Niitsu, M.; Berberich, T.; Kusano, T. Characterization of Five Polyamine Oxidase Isoforms in Arabidopsis thaliana. Plant Cell Rep. 2010, 29, 955–965. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Polyamines and Abiotic Stress Tolerance in Plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-H.; Kitashiba, H.; Wang, J.; Ban, Y.; Moriguchi, T. Polyamines and Their Ability to Provide Environmental Stress Tolerance to Plants. Plant Biotechnol. 2007, 24, 117–126. [Google Scholar] [CrossRef]
- Hummel, I.; Bourdais, G.; Gouesbet, G.; Couée, I.; Malmberg, R.L.; el Amrani, A. Differential Gene Expression of ARGININE DECARBOXYLASE ADC1 and ADC2 in Arabidopsis thaliana: Characterization of Transcriptional Regulation during Seed Germination and Seedling Development. New Phytol. 2004, 163, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Perez-Amador, M.A.; Leon, J.; Green, P.J.; Carbonell, J. Induction of the Arginine Decarboxylase ADC2 Gene Provides Evidence for the Involvement of Polyamines in the Wound Response in Arabidopsis. Plant Physiol. 2002, 130, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Urano, K.; Yoshiba, Y.; Nanjo, T.; Igarashi, Y.; Seki, M.; Sekiguchi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Characterization of Arabidopsis Genes Involved in Biosynthesis of Polyamines in Abiotic Stress Responses and Developmental Stages. Plant Cell Environ. 2003, 26, 1917–1926. [Google Scholar] [CrossRef]
- Armengaud, P.; Breitling, R.; Amtmann, A. The Potassium-Dependent Transcriptome of Arabidopsis Reveals a Prominent Role of Jasmonic Acid in Nutrient Signaling. Plant Physiol. 2004, 136, 2556–2576. [Google Scholar] [CrossRef]
- Alcázar, R.; Cuevas, J.C.; Patron, M.; Altabella, T.; Tiburcio, A.F. Abscisic Acid Modulates Polyamine Metabolism under Water Stress in Arabidopsis Thaliana. Physiol. Plant. 2006, 128, 448–455. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of Salinity Stress on Plants and Its Tolerance Strategies: A Review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.; Shabala, S. Targeting Redox Regulatory Mechanisms for Salinity Stress Tolerance in Crops. In Salinity Responses and Tolerance in Plants, Volume 1; Springer International Publishing: Cham, Switzerland, 2018; pp. 213–234. [Google Scholar]
- Zapata, P.J.; Serrano, M.; Pretel, M.T.; Amorós, A.; Botella, M.Á. Polyamines and Ethylene Changes during Germination of Different Plant Species under Salinity. Plant Sci. 2004, 167, 781–788. [Google Scholar] [CrossRef]
- Antoniou, C.; Zarza, X.; Gohari, G.; Panahirad, S.; Filippou, P.; Tiburcio, A.F.; Fotopoulos, V. Involvement of Polyamine Metabolism in the Response of Medicago truncatula Genotypes to Salt Stress. Plants 2021, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, R.; Bhagwat, K.A. Polyamines as Modulators of Salt Tolerance in Rice Cultivars. Plant Physiol. 1989, 91, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Santa-Cruz, A.; Acosta, M.; Rus, A.; Bolarin, M.C. Short-Term Salt Tolerance Mechanisms in Differentially Salt Tolerant Tomato Species. Plant Physiol. Biochem. 1999, 37, 65–71. [Google Scholar] [CrossRef]
- Ali, R.M. Role of Putrescine in Salt Tolerance of Atropa belladonna Plant. Plant Sci. 2000, 152, 173–179. [Google Scholar] [CrossRef]
- Legocka, J.; Kluk, A. Effect of Salt and Osmotic Stress on Changes in Polyamine Content and Arginine Decarboxylase Activity in Lupinus Luteus Seedlings. J. Plant Physiol. 2005, 162, 662–668. [Google Scholar] [CrossRef]
- Quinet, M.; Ndayiragije, A.; Lefevre, I.; Lambillotte, B.; Dupont-Gillain, C.C.; Lutts, S. Putrescine Differently Influences the Effect of Salt Stress on Polyamine Metabolism and Ethylene Synthesis in Rice Cultivars Differing in Salt Resistance. J. Exp. Bot. 2010, 61, 2719–2733. [Google Scholar] [CrossRef] [PubMed]
- Urano, K.; Yoshiba, Y.; Nanjo, T.; Ito, T.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Arabidopsis Stress-Inducible Gene for Arginine Decarboxylase AtADC2 Is Required for Accumulation of Putrescine in Salt Tolerance. Biochem. Biophys. Res. Commun. 2004, 313, 369–375. [Google Scholar] [CrossRef]
- Xiong, F.; Liao, J.; Ma, Y.; Wang, Y.; Fang, W.; Zhu, X. The Protective Effect of Exogenous Putrescine in the Response of Tea Plants (Camellia sinensis) to Salt Stress. HortScience 2018, 53, 1640–1646. [Google Scholar] [CrossRef]
- Ekinci, M.; Yildirim, E.; Dursun, A.; Mohamedsrajaden, N. Putresin, Spermin ve Spermidin Uygulamalarının Biber (Capsicum annum L.) Fidesinde Tuz Stresi Zararını Hafifletici Etkisi. Yüzüncü Yıl Üniversitesi Tarım Bilimleri Derg. 2019, 29, 290–299. [Google Scholar] [CrossRef]
- Esfandiari Ghalati, R.; Shamili, M.; Homaei, A. Effect of Putrescine on Biochemical and Physiological Characteristics of Guava (Psidium guajava L.) Seedlings under Salt Stress. Sci. Hortic. 2020, 261, 108961. [Google Scholar] [CrossRef]
- Shu, S.; Yuan, Y.; Chen, J.; Sun, J.; Zhang, W.; Tang, Y.; Zhong, M.; Guo, S. The Role of Putrescine in the Regulation of Proteins and Fatty Acids of Thylakoid Membranes under Salt Stress. Sci. Rep. 2015, 5, 14390. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Yuan, Y.; Shu, S.; Sun, J.; Guo, S.; Yuan, R.; Tang, Y. Effects of Exogenous Putrescine on Glycolysis and Krebs Cycle Metabolism in Cucumber Leaves Subjected to Salt Stress. Plant Growth Regul. 2016, 79, 319–330. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Y.; Shu, S.; Jahan, M.S.; Zhong, M.; Wu, J.; Sun, J.; Guo, S. Exogenous Putrescine Regulates Leaf Starch Overaccumulation in Cucumber under Salt Stress. Sci. Hortic. 2019, 253, 99–110. [Google Scholar] [CrossRef]
- Espasandin, F.D.; Calzadilla, P.I.; Maiale, S.J.; Ruiz, O.A.; Sansberro, P.A. Overexpression of the Arginine Decarboxylase Gene Improves Tolerance to Salt Stress in Lotus Tenuis Plants. J. Plant Growth Regul. 2018, 37, 156–165. [Google Scholar] [CrossRef]
- Shi, K.; Huang, Y.Y.; Xia, X.J.; Zhang, Y.L.; Zhou, Y.H.; Yu, J.Q. Protective Role of Putrescine Against Salt Stress Is Partially Related to the Improvement of Water Relation and Nutritional Imbalance in Cucumber. J. Plant Nutr. 2008, 31, 1820–1831. [Google Scholar] [CrossRef]
- Reginato, M.A.; Abdala, G.I.; Miersch, O.; Ruíz, O.A.; Moschetti, E.; Luna, V. Changes in the Levels of Jasmonates and Free Polyamines Induced by Na2SO4 and NaCl in Roots and Leaves of the Halophyte Prosopis strombulifera. Biologia 2012, 67, 689–697. [Google Scholar] [CrossRef]
- Reinoso, H.; Sosa, L.; Reginato, M.; Luna, V. Histological Alterations Induced by Sodium Sulfate in the Vegetative Anatomy of Prosopis strombulifera (Lam.) Benth. World J. Agric. Sci. 2005, 1, 109–119. [Google Scholar]
- Altamura, M.M.; Torrigiani, P.; Capitani, F.; Scaramagli, S.; Bagni, N. De Novo Root Formation in Tobacco Thin Layers Is Affected by Inhibition of Polyamine Biosynthesis. J. Exp. Bot. 1991, 42, 1575–1582. [Google Scholar] [CrossRef]
- Gong, B.; Li, X.; Bloszies, S.; Wen, D.; Sun, S.; Wei, M.; Li, Y.; Yang, F.; Shi, Q.; Wang, X. Sodic Alkaline Stress Mitigation by Interaction of Nitric Oxide and Polyamines Involves Antioxidants and Physiological Strategies in Solanum lycopersicum. Free Radic. Biol. Med. 2014, 71, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Recalde, L.; Gómez Mansur, N.M.; Cabrera, A.v.; Matayoshi, C.L.; Gallego, S.M.; Groppa, M.D.; Benavides, M.P. Unravelling Ties in the Nitrogen Network: Polyamines and Nitric Oxide Emerging as Essential Players in Signalling Roadway. Ann. Appl. Biol. 2021, 178, 192–208. [Google Scholar] [CrossRef]
- García-Jiménez, P.; Just, P.M.; Delgado, A.M.; Robaina, R.R. Transglutaminase Activity Decrease during Acclimation to Hyposaline Conditions in Marine Seaweed Grateloupia Doryphora (Rhodophyta, Halymeniaceae). J. Plant Physiol. 2007, 164, 367–370. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiménez-Donaire, M.d.P.; Giráldez, J.V.; Vanwalleghem, T. Impact of Climate Change on Agricultural Droughts in Spain. Water 2020, 12, 3214. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and Drought Stresses in Crop Plants: Implications, Cross Talk, and Potential Management Opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Gupta, S.; Agarwal, V.P.; Gupta, N.K. Efficacy of Putrescine and Benzyladenine on Photosynthesis and Productivity in Relation to Drought Tolerance in Wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2012, 18, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, L.; Yang, R.; Han, Y.; Hao, J.; Liu, C.; Fan, S. Effects of Exogenous Putrescine on the Ultrastructure of and Calcium Ion Flow Rate in Lettuce Leaf Epidermal Cells under Drought Stress. Hortic. Environ. Biotechnol. 2019, 60, 479–490. [Google Scholar] [CrossRef]
- Shallan, M.A.; Hassan, H.M.M.; Namich, A.A.M.; Ibrahim, A.A. Effect of Sodium Niroprusside, Putrescine and Glycine Betaine on Alleviation of Drought Stress in Cotton Plant. J. Agric. Environ. Sci. 2012, 12, 1252–1265. [Google Scholar]
- Zeid, I.M.; Shedeed, Z.A. Response of Alfalfa to Putrescine Treatment under Drought Stress. Biol. Plant. 2006, 50, 635–640. [Google Scholar] [CrossRef]
- Abd Elbar, O.H.; Farag, R.E.; Shehata, S.A. Effect of Putrescine Application on Some Growth, Biochemical and Anatomical Characteristics of Thymus vulgaris L. under Drought Stress. Ann. Agric. Sci. 2019, 64, 129–137. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Pan, X.; Jiang, Q.; Xi, Z. Exogenous Putrescine Alleviates Drought Stress by Altering Reactive Oxygen Species Scavenging and Biosynthesis of Polyamines in the Seedlings of Cabernet Sauvignon. Front. Plant Sci. 2021, 12, 767992. [Google Scholar] [CrossRef] [PubMed]
- Khosrowshahi, Z.T.; Slehi-Lisar, Y.; Ghassemi-Golezani, K.; Motafakkerazad, R. Physiological Responses of Safolew to Exogenous Putwescine Undew Watew Defcit. J. Stress Physiol. Biochem. 2018, 14, 38–48. [Google Scholar]
- Islam, M.J.; Uddin, M.J.; Hossain, M.A.; Henry, R.; Begum, M.K.; Sohel, M.A.T.; Mou, M.A.; Ahn, J.; Cheong, E.J.; Lim, Y.-S. Exogenous Putrescine Attenuates the Negative Impact of Drought Stress by Modulating Physio-Biochemical Traits and Gene Expression in Sugar Beet (Beta vulgaris L.). PLoS ONE 2022, 17, e0262099. [Google Scholar] [CrossRef] [PubMed]
- Montilla-Bascón, G.; Rubiales, D.; Hebelstrup, K.H.; Mandon, J.; Harren, F.J.M.; Cristescu, S.M.; Mur, L.A.J.; Prats, E. Reduced Nitric Oxide Levels during Drought Stress Promote Drought Tolerance in Barley and Is Associated with Elevated Polyamine Biosynthesis. Sci. Rep. 2017, 7, 13311. [Google Scholar] [CrossRef] [PubMed]
- An, Z.F.; Li, C.Y.; Zhang, L.X.; Alva, A.K. Role of Polyamines and Phospholipase D in Maize (Zea mays L.) Response to Drought Stress. S. Afr. J. Bot. 2012, 83, 145–150. [Google Scholar] [CrossRef][Green Version]
- Karimi, Z. Putrescine Foliar Application Effect on Physiologic and Morphologic Characteristics of Wheat (Triticum aestivum var Sw-82-9) under Water Deficit Stress. Biol. Forum Int. J. 2016, 8, 532–539. [Google Scholar]
- Alcázar, R.; Planas, J.; Saxena, T.; Zarza, X.; Bortolotti, C.; Cuevas, J.; Bitrián, M.; Tiburcio, A.F.; Altabella, T. Putrescine Accumulation Confers Drought Tolerance in Transgenic Arabidopsis Plants Over-Expressing the Homologous Arginine Decarboxylase 2 Gene. Plant Physiol. Biochem. 2010, 48, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Cheraghabadi, M.; Modarres-Sanavy, S.A.M.; Sefidkon, F.; Rashidi-Monfared, S.; Mokhtassi-Bidgoli, A. Improving Water Deficit Tolerance of Salvia officinalis L. Using Putrescine. Sci. Rep. 2021, 11, 21997. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Farooq, M.; Wahid, M.A.; Wahid, A. Seed Priming with Putrescine Improves the Drought Resistance of Maize Hybrids. Int. J. Agric. Biol 2014, 15, 1349–1353. [Google Scholar]
- Do, P.T.; Degenkolbe, T.; Erban, A.; Heyer, A.G.; Kopka, J.; Köhl, K.I.; Hincha, D.K.; Zuther, E. Dissecting Rice Polyamine Metabolism under Controlled Long-Term Drought Stress. PLoS ONE 2013, 8, e60325. [Google Scholar]
- Ahangir, A.; Ghotbi-Ravandi, A.A.; Rezadoost, H.; Bernard, F. Drought Tolerant Maize Cultivar Accumulates Putrescine in Roots. Rhizosphere 2020, 16, 100260. [Google Scholar] [CrossRef]
- Chen, J.; Shang, Y.-T.; Wang, W.-H.; Chen, X.-Y.; He, E.-M.; Zheng, H.-L.; Shangguan, Z. Hydrogen Sulfide-Mediated Polyamines and Sugar Changes Are Involved in Hydrogen Sulfide-Induced Drought Tolerance in Spinacia oleracea Seedlings. Front. Plant Sci. 2016, 7, 1173. [Google Scholar] [CrossRef] [PubMed]
- Pucciariello, C.; Voesenek, L.A.C.J.; Perata, P.; Sasidharan, R. Plant Responses to Flooding. Front. Plant Sci. 2014, 5, 226. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A. Responses to Flooding of Plant Water Relations and Leaf Gas Exchange in Tropical Tolerant Trees of a Black-Water Wetland. Front. Plant Sci. 2013, 4, 106. [Google Scholar] [CrossRef]
- Bertani, A.; Brambilla, I.; Mapelli, S.; Reggiani, R. Elongation Growth in the Absence of Oxygen: The Rice Coleoptile. Russ. J. Plant Physiol. 1997, 44, 543–547. [Google Scholar]
- Mahdavian, M.; Sarikhani, H.; Hadadinejad, M.; Dehestani, A. Putrescine Effect on Physiological, Morphological, and Biochemical Traits of Carrizo Citrange and Volkameriana Rootstocks under Flooding Stress. Int. J. Fruit Sci. 2020, 20, 164–177. [Google Scholar] [CrossRef]
- Yiu, J.-C.; Juang, L.-D.; Fang, D.Y.-T.; Liu, C.-W.; Wu, S.-J. Exogenous Putrescine Reduces Flooding-Induced Oxidative Damage by Increasing the Antioxidant Properties of Welsh Onion. Sci. Hortic. 2009, 120, 306–314. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant Tolerance to High Temperature in a Changing Environment: Scientific Fundamentals and Production of Heat Stress-Tolerant Crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Bhatia, C.R. Bioenergetic Cost of Heat Tolerance in Wheat Crop. Curr. Sci. 2008, 94, 10491053. [Google Scholar]
- Kusano, T.; Yamaguchi, K.; Berberich, T.; Takahashi, Y. Advances in Polyamine Research in 2007. J. Plant Res. 2007, 120, 345–350. [Google Scholar] [CrossRef]
- Hassanein, R.A.; El-Khawas, S.A.; Ibrahim, S.K.; El-Bassiouny, H.M.; Mostafa, H.A.; Abd El-Monem, A.A. Improving the thermo tolerance of wheat plant by foliar application of arginine or putrescine. Pak. J. Bot. 2013, 45, 111–118. [Google Scholar]
- Mostafa, H.A.M.; Hassanein, R.A.; Khalil, S.I.; El-Khawas, S.A.; El-Bassiouny, H.M.S.; El-Monem, A.A.A. Effect of Arginine or Putrescine on Growth, Yield and Yield Components of Late Sowing Wheat. J. Appl. Sci. Res. 2010, 54, 177–183. [Google Scholar]
- Collado-González, J.; Piñero, M.C.; Otálora, G.; López-Marín, J.; Amor, F.M. del The Effect of Foliar Putrescine Application, Ammonium Exposure, and Heat Stress on Antioxidant Compounds in Cauliflower Waste. Antioxidants 2021, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Amooaghaie, R.; Moghym, S. Effect of Polyamines on Thermotolerance and Membrane Stability of Soybean Seedling. Afr. J. Biotechnol. 2011, 10, 9673–9676. [Google Scholar]
- Khalil, S.I.; El-Monem, H.M.S.; Hassanein, R.A.; Mostafa, H.A.; El-Khawas, S.A.; Abd El-Monem, A.A. Antioxidant Defense System in Heat Shocked Wheat Plants Previously Treated with Arginine or Putrescine. Aust. J. Basic Appl. Sci. 2009, 3, 1517–1526. [Google Scholar]
- Königshofer, H.; Lechner, S. Are Polyamines Involved in the Synthesis of Heat-Shock Proteins in Cell Suspension Cultures of Tobacco and Alfalfa in Response to High-Temperature Stress? Plant Physiol. Biochem. 2002, 40, 51–59. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Sun, J.; Guo, S. Melatonin Alleviates Heat-Induced Damage of Tomato Seedlings by Balancing Redox Homeostasis and Modulating Polyamine and Nitric Oxide Biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Singh, G.P.; Sharma, S.K.; Singh, K.; Goswami, S.; Rai, R.D. Molecular Cloning of HSP17 Gene (SHSP) and Their Differential Expression under Exogenous Putrescine and Heat Shock in Wheat (Triticum aestivum). Afr. J. Biotechnol. 2012, 11, 16800–16808. [Google Scholar]
- Marco, F.; Alcázar, R.; Tiburcio, A.F.; Carrasco, P. Interactions between Polyamines and Abiotic Stress Pathway Responses Unraveled by Transcriptome Analysis of Polyamine Overproducers. OMICS J. Integr. Biol. 2011, 15, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.; Clément, C.; Barka, E.A. Physiological and Molecular Changes in Plants Grown at Low Temperatures. Planta 2012, 235, 1091–1105. [Google Scholar] [CrossRef]
- Cuevas, J.C.; López-Cobollo, R.; Alcázar, R.; Zarza, X.; Koncz, C.; Altabella, T.; Salinas, J.; Tiburcio, A.F.; Ferrando, A. Putrescine Is Involved in Arabidopsis Freezing Tolerance and Cold Acclimation by Regulating Abscisic Acid Levels in Response to Low Temperature. Plant Physiol. 2008, 148, 1094–1105. [Google Scholar] [CrossRef]
- Song, Y.; Diao, Q.; Qi, H. Putrescine Enhances Chilling Tolerance of Tomato (Lycopersicon esculentum Mill.) through Modulating Antioxidant Systems. Acta Physiol. Plant. 2014, 36, 3013–3027. [Google Scholar] [CrossRef]
- Diao, Q.; Song, Y.; Shi, D.; Qi, H. Interaction of Polyamines, Abscisic Acid, Nitric Oxide, and Hydrogen Peroxide under Chilling Stress in Tomato (Lycopersicon esculentum Mill.) Seedlings. Front. Plant Sci. 2017, 8, 203. [Google Scholar] [CrossRef]
- Hummel, I.; Couée, I.; el Amrani, A.; Martin-Tanguy, J.; Hennion, F. Involvement of Polyamines in Root Development at Low Temperature in the Subantarctic Cruciferous Species Pringlea antiscorbutica. J. Exp. Bot. 2002, 53, 1463–1473. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yin, X.; Yang, Y.; Lv, Y.; Li, Y.; Yang, D.; Yue, Y.; Yang, Y. BrrICE1.1 Is Associated with Putrescine Synthesis through Regulation of the Arginine Decarboxylase Gene in Freezing Tolerance of Turnip (Brassica rapa Var. Rapa). BMC Plant Biol. 2020, 20, 504. [Google Scholar] [CrossRef] [PubMed]
- Mustafavi, S.H.; Shekari, F.; Abbasi, A. Putrescine Improve Low Temperature Tolerance of Fennel (Foeniculum vulgare Mill.) Seeds. Cercet. Agron. Mold. 2015, 48, 69–76. [Google Scholar] [CrossRef][Green Version]
- Abbasi, N.; Ali, I.; Hafiz, I.; Alenazi, M.; Shafiq, M. Effects of Putrescine Application on Peach Fruit during Storage. Sustainability 2019, 11, 2013. [Google Scholar] [CrossRef]
- Spormann, S.; Soares, C.; Teixeira, J.; Fidalgo, F. Polyamines as Key Regulatory Players in Plants under Metal Stress—A Way for an Enhanced Tolerance. Ann. Appl. Biol. 2021, 178, 209–226. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.-J. The Significance of Amino Acids and Amino Acid-Derived Molecules in Plant Responses and Adaptation to Heavy Metal Stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef]
- Weinstein, L.H.; Kaur-Sawhney, R.; Rajam, M.V.; Wettlaufer, S.H.; Galston, A.W. Cadmium-Induced Accumulation of Putrescine in Oat and Bean Leaves. Plant Physiol. 1986, 82, 641–645. [Google Scholar] [CrossRef]
- Zacchini, M.; de Agazio, M. Spread of Oxidative Damage and Antioxidative Response through Cell Layers of Tobacco Callus after UV-C Treatment. Plant Physiol. Biochem. 2004, 42, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Z.; Zhao, Y.; Zhang, X.; Zhang, S.; Bo, L.; Wang, Y.; Ding, Y.; An, L. Putrescine Protects Hulless Barley from Damage Due to UV-B Stress via H2S- and H2O2-Mediated Signaling Pathways. Plant Cell Rep. 2016, 35, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Wu, R. Arginine Decarboxylase Transgene Expression and Analysis of Environmental Stress Tolerance in Transgenic Rice. Plant Sci. 2001, 160, 869–875. [Google Scholar] [CrossRef]
- Capell, T.; Bassie, L.; Christou, P. Modulation of the Polyamine Biosynthetic Pathway in Transgenic Rice Confers Tolerance to Drought Stress. Proc. Natl. Acad. Sci. USA 2004, 101, 9909–9914. [Google Scholar] [CrossRef] [PubMed]
- Prabhavathi, V.R.; Rajam, M.V. Polyamine Accumulation in Transgenic Eggplant Enhances Tolerance to Multiple Abiotic Stresses and Fungal Resistance. Plant Biotechnol. 2007, 24, 273–282. [Google Scholar] [CrossRef]
- Alet, A.I.; Sanchez, D.H.; Cuevas, J.C.; del Valle, S.; Altabella, T.; Tiburcio, A.F.; Marco, F.; Ferrando, A.; Espasandín, F.D.; González, M.E.; et al. Putrescine Accumulation in Arabidopsis thaliana Transgenic Lines Enhances Tolerance to Dehydration and Freezing Stress. Plant Signal. Behav. 2011, 6, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-Q.; Zhang, Q.-F.; Liu, J.-H.; Li, G.-H. Overexpression of PtADC Confers Enhanced Dehydration and Drought Tolerance in Transgenic Tobacco and Tomato: Effect on ROS Elimination. Biochem. Biophys. Res. Commun. 2011, 413, 10–16. [Google Scholar] [CrossRef]
- Wang, J.; Sun, P.-P.; Chen, C.-L.; Wang, Y.; Fu, X.-Z.; Liu, J.-H. An Arginine Decarboxylase Gene PtADC from Poncirus trifoliata Confers Abiotic Stress Tolerance and Promotes Primary Root Growth in Arabidopsis. J. Exp. Bot. 2011, 62, 2899–2914. [Google Scholar] [CrossRef]
- Kumria, R.; Rajam, M.V. Ornithine Decarboxylase Transgene in Tobacco Affects Polyamines, In Vitro-Morphogenesis and Response to Salt Stress. J. Plant Physiol. 2002, 159, 983–990. [Google Scholar] [CrossRef]
- Jiménez-Bremont, J.F.; Marina, M.; Guerrero-González, M.d.l.L.; Rossi, F.R.; Sánchez-Rangel, D.; Rodrí guez-Kessler, M.; Ruiz, O.A.; Gárriz, A. Physiological and Molecular Implications of Plant Polyamine Metabolism during Biotic Interactions. Front. Plant Sci. 2014, 5, 95. [Google Scholar] [PubMed]
- Angelini, R.; Cona, A.; Federico, R.; Fincato, P.; Tavladoraki, P.; Tisi, A. Plant Amine Oxidases “on the Move”: An Update. Plant Physiol. Biochem. 2010, 48, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Planas-Portell, J.; Gallart, M.; Tiburcio, A.F.; Altabella, T. Copper-Containing Amine Oxidases Contribute to Terminal Polyamine Oxidation in Peroxisomes and Apoplast of Arabidopsis thaliana. BMC Plant Biol. 2013, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Tavladoraki, P.; Cona, A.; Angelini, R. Copper-Containing Amine Oxidases and FAD-Dependent Polyamine Oxidases Are Key Players in Plant Tissue Differentiation and Organ Development. Front. Plant Sci. 2016, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of Amine Oxidases in Plant Development and Defence. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Alcázar, R. A New Insight into the Contribution of Putrescine to Defense in Arabidopsis thaliana. Plant Signal. Behav. 2021, 16, 1885187. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Atanasov, K.E.; Arafaty, N.; Murillo, E.; Tiburcio, A.F.; Zeier, J.; Alcázar, R. Putrescine Elicits ROS-dependent Activation of the Salicylic Acid Pathway in Arabidopsis thaliana. Plant Cell Environ. 2020, 43, 2755–2768. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Betti, L.; Scaramagli, S.; Biondi, S.; Torrigiani, P. Polyamine Metabolism Is Upregulated in Response to Tobacco Mosaic Virus in Hypersensitive, but Not in Susceptible, Tobacco. New Phytol. 2001, 149, 301–309. [Google Scholar] [CrossRef]
- Rodríguez-Kessler, M.; Ruiz, O.A.; Maiale, S.; Ruiz-Herrera, J.; Jiménez-Bremont, J.F. Polyamine Metabolism in Maize Tumors Induced by Ustilago maydis. Plant Physiol. Biochem. 2008, 46, 805–814. [Google Scholar] [CrossRef]
- Foster, S.A.; Walters, D.R. Polyamine Concentrations and Activities of Ornithine and Arginine Decarboxylase in Wheat Infected with the Stem Rust Fungus. J. Plant Physiol. 1992, 140, 134–136. [Google Scholar] [CrossRef]
- Marina, M.; Maiale, S.J.; Rossi, F.R.; Romero, M.F.; Rivas, E.I.; Gárriz, A.; Ruiz, O.A.; Pieckenstain, F.L. Apoplastic Polyamine Oxidation Plays Different Roles in Local Responses of Tobacco to Infection by the Necrotrophic Fungus Sclerotinia sclerotiorum and the Biotrophic Bacterium Pseudomonas viridiflava. Plant Physiol. 2008, 147, 2164–2178. [Google Scholar] [CrossRef]
- Brauc, S.; de Vooght, E.; Claeys, M.; Geuns, J.M.C.; Höfte, M.; Angenon, G. Overexpression of Arginase in Arabidopsis Thaliana Influences Defence Responses against Botrytis cinerea. Plant Biol. 2012, 14, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, Y.; Takahashi, Y.; Berberich, T.; Miyazaki, A.; Matsumura, H.; Takahashi, H.; Terauchi, R.; Kusano, T. Spermine Signaling Plays a Significant Role in the Defense Response of Arabidopsis thaliana to Cucumber Mosaic Virus. J. Plant Physiol. 2009, 166, 626–643. [Google Scholar] [CrossRef]
- Liu, C.; Atanasov, K.E.; Tiburcio, A.F.; Alcázar, R. The Polyamine Putrescine Contributes to H2O2 and RbohD/F-Dependent Positive Feedback Loop in Arabidopsis PAMP-Triggered Immunity. Front. Plant Sci. 2019, 10, 894. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.R.; Gárriz, A.; Marina, M.; Pieckenstain, F.L. Modulation of Polyamine Metabolism in Arabidopsis thaliana by Salicylic Acid. Physiol. Plant. 2021, 173, 843–855. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, B.S.; Hwang, B.K. Pepper Arginine Decarboxylase Is Required for Polyamine and γ-Aminobutyric Acid Signaling in Cell Death and Defense Response. Plant Physiol. 2013, 162, 2067–2083. [Google Scholar] [CrossRef]
- Kim, N.H.; Choi, H.W.; Hwang, B.K. Xanthomonas campestris Pv. Vesicatoria Effector AvrBsT Induces Cell Death in Pepper, but Suppresses Defense Responses in Tomato. Mol. Plant-Microbe Interact. 2010, 23, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; von Roepenack-Lahaye, E.; Buntru, M.; de Lange, O.; Schandry, N.; Pérez-Quintero, A.L.; Weinberg, Z.; Lowe-Power, T.M.; Szurek, B.; Michael, A.J.; et al. A Plant Pathogen Type III Effector Protein Subverts Translational Regulation to Boost Host Polyamine Levels. Cell Host Microbe 2019, 26, 638–649. [Google Scholar] [CrossRef]
- Chávez-Martínez, A.I.; Ortega-Amaro, M.A.; Torres, M.; Serrano, M.; Jiménez-Bremont, J.F. Arabidopsis Adc-Silenced Line Exhibits Differential Defense Responses to Botrytis cinerea and Pseudomonas syringae Infection. Plant Physiol. Biochem. 2020, 156, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Hewezi, T.; Howe, P.J.; Maier, T.R.; Hussey, R.S.; Mitchum, M.G.; Davis, E.L.; Baum, T.J. Arabidopsis Spermidine Synthase Is Targeted by an Effector Protein of the Cyst Nematode Heterodera schachtii. Plant Physiol. 2010, 152, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, A.; Ohri, P. Exogenously Applied Putrescine Improves the Physiological Responses of Tomato Plant during Nematode Pathogenesis. Sci. Hortic. 2018, 230, 35–42. [Google Scholar] [CrossRef]
- Vilas, J.M.; Romero, F.M.; Rossi, F.R.; Marina, M.; Maiale, S.J.; Calzadilla, P.I.; Pieckenstain, F.L.; Ruiz, O.A.; Gárriz, A. Modulation of Plant and Bacterial Polyamine Metabolism during the Compatible Interaction between Tomato and Pseudomonas syringae. J. Plant Physiol. 2018, 231, 281–290. [Google Scholar] [CrossRef]
- Stes, E.; Vandeputte, O.M.; el Jaziri, M.; Holsters, M.; Vereecke, D. A Successful Bacterial Coup d’État: How Rhodococcus fascians Redirects Plant Development. Annu. Rev. Phytopathol. 2011, 49, 69–86. [Google Scholar] [CrossRef]
- Fernández-Crespo, E.; Scalschi, L.; Llorens, E.; García-Agustín, P.; Camañes, G. NH4+ Protects Tomato Plants against Pseudomonas syringae by Activation of Systemic Acquired Acclimation. J. Exp. Bot. 2015, 66, 6777–6790. [Google Scholar] [CrossRef]
- González-Hernández, A.I.; Fernández-Crespo, E.; Scalschi, L.; Hajirezaei, M.-R.; von Wirén, N.; García-Agustín, P.; Camañes, G. Ammonium Mediated Changes in Carbon and Nitrogen Metabolisms Induce Resistance against Pseudomonas syringae in Tomato Plants. J. Plant Physiol. 2019, 239, 28–37. [Google Scholar] [CrossRef]
- Walters, D.R. Polyamines and Plant Disease. Phytochemistry 2003, 64, 97–107. [Google Scholar] [CrossRef]
- Zeiss, D.R.; Piater, L.A.; Dubery, I.A. Hydroxycinnamate Amides: Intriguing Conjugates of Plant Protective Metabolites. Trends Plant Sci. 2021, 26, 184–195. [Google Scholar] [CrossRef]
- Edreva, A.M.; Velikova, V.B.; Tsonev, T.D. Phenylamides in Plants. Russ. J. Plant Physiol. 2007, 54, 287–301. [Google Scholar] [CrossRef]
- Alamgir, K.M.; Hojo, Y.; Christeller, J.T.; Fukumoto, K.; Isshiki, R.; Shinya, T.; Baldwin, I.T.; Galis, I. Systematic Analysis of Rice (Oryza sativa) Metabolic Responses to Herbivory. Plant Cell Environ. 2016, 39, 453–466. [Google Scholar] [CrossRef]
- Kaur, H.; Heinzel, N.; Schöttner, M.; Baldwin, I.T.; GÁlis, I. R2R3-NaMYB8 Regulates the Accumulation of Phenylpropanoid-Polyamine Conjugates, Which Are Essential for Local and Systemic Defense against Insect Herbivores in Nicotiana Attenuata. Plant Physiol. 2010, 152, 1731–1747. [Google Scholar] [CrossRef]
- Tanabe, K.; Hojo, Y.; Shinya, T.; Galis, I. Molecular Evidence for Biochemical Diversification of Phenolamide Biosynthesis in Rice Plants. J. Integr. Plant Biol. 2016, 58, 903–913. [Google Scholar] [CrossRef]
- Muroi, A.; Ishihara, A.; Tanaka, C.; Ishizuka, A.; Takabayashi, J.; Miyoshi, H.; Nishioka, T. Accumulation of Hydroxycinnamic Acid Amides Induced by Pathogen Infection and Identification of Agmatine Coumaroyltransferase in Arabidopsis thaliana. Planta 2009, 230, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, L.; Li, F.; Meng, D.; Sheng, J. Methyl Salicylate-Induced Arginine Catabolism Is Associated with Up-Regulation of Polyamine and Nitric Oxide Levels and Improves Chilling Tolerance in Cherry Tomato Fruit. J. Agric. Food Chem. 2011, 59, 9351–9357. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.-K.; Min, K.-H.; Kim, S.-H.; Nam, S.-H.; Zhang, S.; Kim, Y.C.; Cho, B.H.; Yang, K.-Y. Mitogen-Activated Protein Kinase Cascade in the Signaling for Polyamine Biosynthesis in Tobacco. Plant Cell Physiol. 2009, 50, 658–664. [Google Scholar] [CrossRef]
- Németh, M.; Janda, T.; Horváth, E.; Páldi, E.; Szalai, G. Exogenous Salicylic Acid Increases Polyamine Content but May Decrease Drought Tolerance in Maize. Plant Sci. 2002, 162, 569–574. [Google Scholar] [CrossRef]
- Walters, D.; Cowley, T.; Mitchell, A. Methyl Jasmonate Alters Polyamine Metabolism and Induces Systemic Protection against Powdery Mildew Infection in Barley Seedlings. J. Exp. Bot. 2002, 53, 747–756. [Google Scholar] [CrossRef]
- Haggag, W.M.; Abd-El-Kareem, F. Methyl Jasmonate Stimulates Polyamines Biosynthesisand Resistance against Leaf Rust in Wheat Plants. Arch. Phytopathol. Plant Prot. 2009, 42, 16–31. [Google Scholar] [CrossRef]
- Xu, B.; Sheehan, M.J.; Timko, M.P. Differential Induction of Ornithine Decarboxylase (ODC) Gene Family Members in Transgenic Tobacco (Nicotiana tabacum L. Cv. Bright Yellow 2) Cell Suspensions by Methyl-Jasmonate Treatment. Plant Growth Regul. 2004, 44, 101–116. [Google Scholar] [CrossRef]
- Peremarti, A.; Bassie, L.; Yuan, D.; Pelacho, A.; Christou, P.; Capell, T. Transcriptional Regulation of the Rice Arginine Decarboxylase (Adc1) and S-Adenosylmethionine Decarboxylase (Samdc) Genes by Methyl Jasmonate. Plant Physiol. Biochem. 2010, 48, 553–559. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Jing, W.; Liu, Y.; Zhang, W. Hydrogen Peroxide Generated by Copper Amine Oxidase Is Involved in Abscisic Acid-Induced Stomatal Closure in Vicia faba. J. Exp. Bot. 2008, 59, 815–825. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).