Old and New Blood Markers in Human Colorectal Cancer
Abstract
1. Introduction
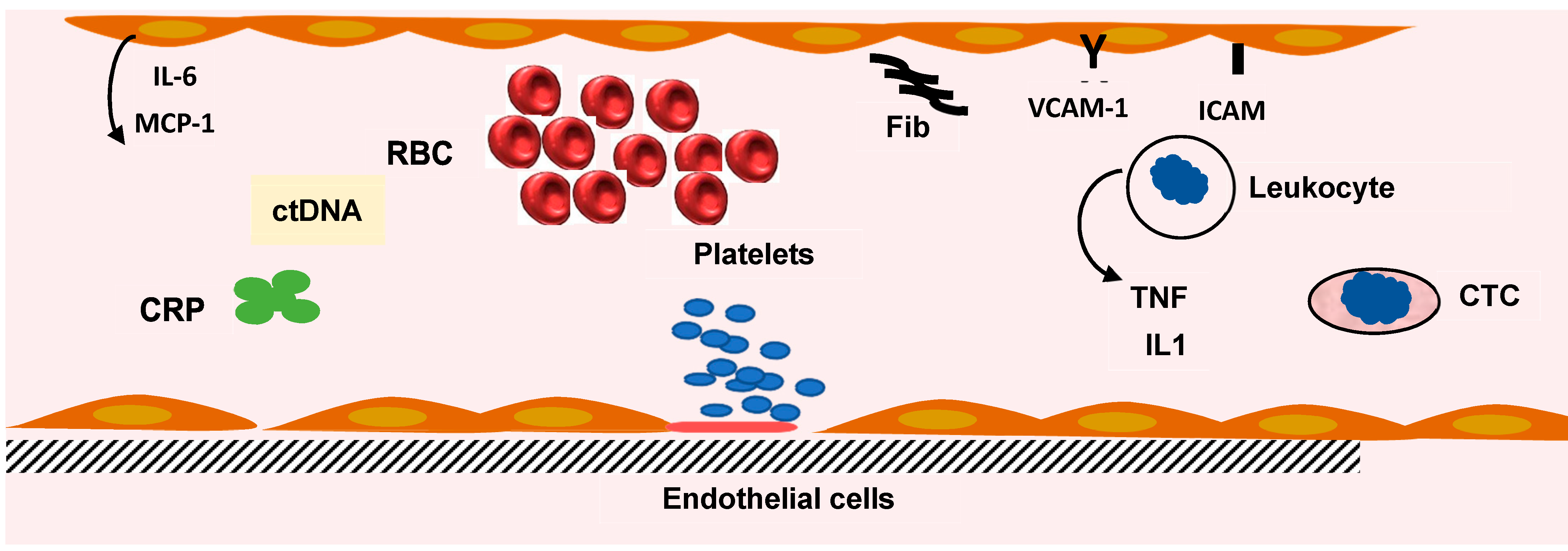
2. Inflammatory Markers in CRC
2.1. Erythrocyte Sedimentation Rate (ESR) and Hemogram
2.2. C-Reactive Protein (CRP)
2.3. Systemic Inflammatory Score or Index
2.4. Cytokines
3. Tumor Markers
3.1. Carcinoembryonic Antigen (CEA)
3.2. CEA Structure
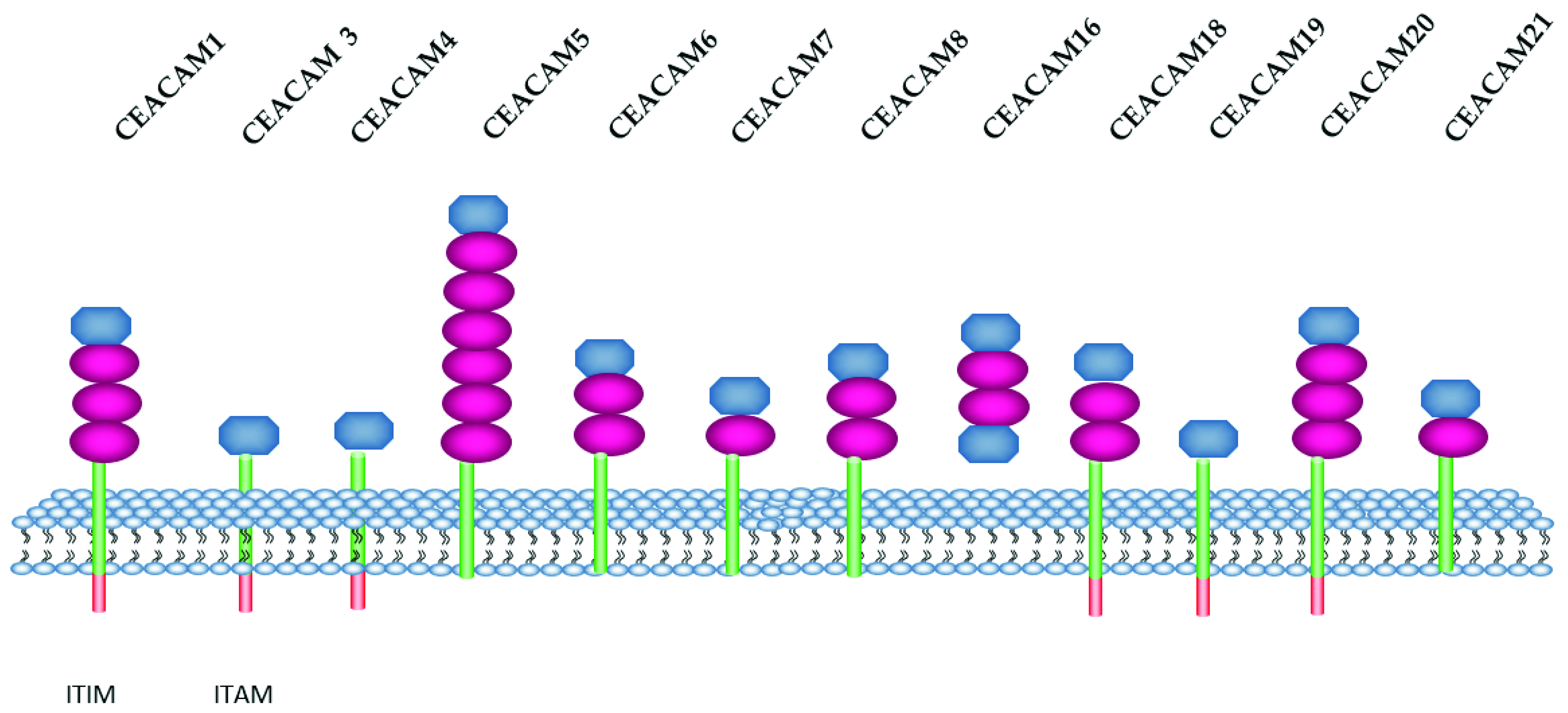
3.3. CEACAM Family
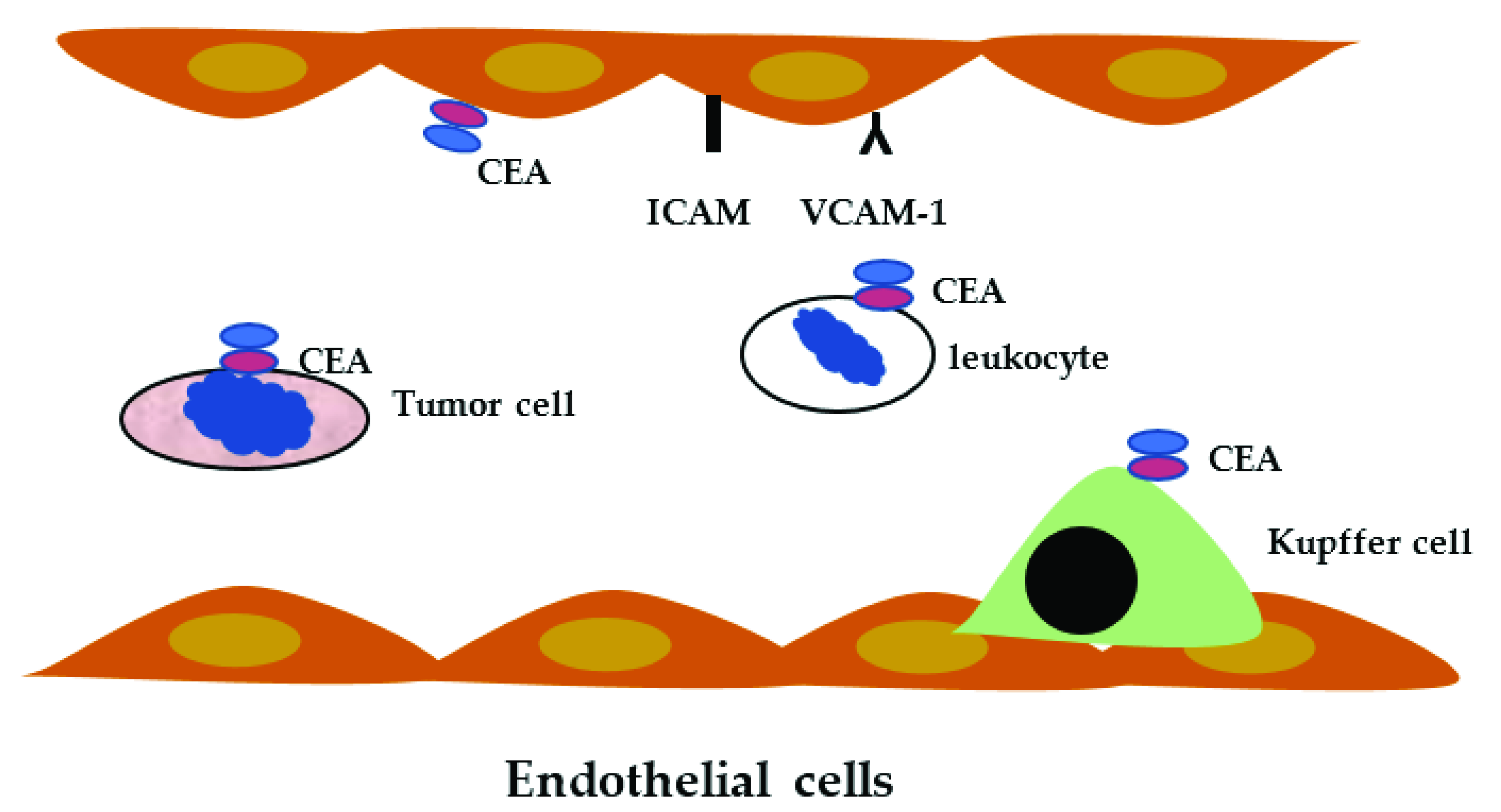
3.4. CEACAM and Leukocytes
4. CEACAM and Colorectal Cancer
5. Biomarkers in CRC Patients
5.1. Carbohydrate Antigen 19-9 (CA 19-9)
5.2. Tumor-Associated Glycoprotein-72 (TAG-72)
5.3. Tissue Polypeptide Specific Antigen (TPS)
5.4. Hematopoietic Growth Factors
5.5. Tumor Associated Auto and Specific Antigens
5.6. Matrix Metalloproteinases (MMPs)
6. Detection of Circulating Tumor Cells in CRC Patients
7. Micro-RNA and Circulating Tumor DNA (ctDNA) in CRC Patients
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poste, G.; Fidler, I.J. The pathogenesis of cancer metastasis. Nature 1980, 283, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Ismael, G.F.; Rosa, D.D.; Mano, M.S.; Awada, A. Novel cytotoxic drugs: Old challenges, new solutions. Cancer Treat. Rev. 2008, 34, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Kimiz-Gebologlu, I.; Gulce-Iz, S.; Biray-Avci, C. Monoclonal antibodies in cancer immunotherapy. Mol. Biol. Rep. 2018, 45, 2935–2940. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Laubli, H.; Borsig, L. Altered Cell Adhesion and Glycosylation Promote Cancer Immune Suppression and Metastasis. Front. Immunol. 2019, 10, 2120. [Google Scholar] [CrossRef]
- Favoriti, P.; Carbone, G.; Greco, M.; Pirozzi, F.; Pirozzi, R.E.; Corcione, F. Worldwide burden of colorectal cancer: A review. Updates Surg. 2016, 68, 7–11. [Google Scholar] [CrossRef]
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar] [CrossRef]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef]
- Navratilova, J.; Hankeova, T.; Benes, P.; Smarda, J. Acidic pH of tumor microenvironment enhances cytotoxicity of the disulfiram/Cu2+ complex to breast and colon cancer cells. Chemotherapy 2013, 59, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Huntington, K.E.; Louie, A.; Zhou, L.; Seyhan, A.A.; Maxwell, A.W.; El-Deiry, W.S. Colorectal cancer extracellular acidosis decreases immune cell killing and is partially ameliorated by pH-modulating agents that modify tumor cell cytokine profiles. Am. J. Cancer Res. 2022, 12, 138–151. [Google Scholar] [PubMed]
- Park, G.; Song, S.Y.; Ahn, J.H.; Kim, W.L.; Lee, J.S.; Jeong, S.Y.; Park, J.W.; Choi, E.K.; Choi, W.; Jung, I.H. The pretreatment erythrocyte sedimentation rate predicts survival outcomes after surgery and adjuvant radiotherapy for extremity soft tissue sarcoma. Radiat. Oncol. 2019, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Alexandrakis, M.G.; Passam, F.H.; Ganotakis, E.S.; Sfiridaki, K.; Xilouri, I.; Perisinakis, K.; Kyriakou, D.S. The clinical and prognostic significance of erythrocyte sedimentation rate (ESR), serum interleukin-6 (IL-6) and acute phase protein levels in multiple myeloma. Clin. Lab. Haematol. 2003, 25, 41–46. [Google Scholar] [CrossRef]
- Henry-Amar, M.; Friedman, S.; Hayat, M.; Somers, R.; Meerwaldt, J.H.; Carde, P.; Burgers, J.M.; Thomas, J.; Monconduit, M.; Noordijk, E.M.; et al. Erythrocyte sedimentation rate predicts early relapse and survival in early-stage Hodgkin disease. The EORTC Lymphoma Cooperative Group. Ann. Intern. Med. 1991, 114, 361–365. [Google Scholar] [CrossRef]
- Hart, P.C.; Rajab, I.M.; Alebraheem, M.; Potempa, L.A. C-Reactive Protein and Cancer-Diagnostic and Therapeutic Insights. Front. Immunol. 2020, 11, 595835. [Google Scholar] [CrossRef]
- Cesana, C.; Klersy, C.; Barbarano, L.; Nosari, A.M.; Crugnola, M.; Pungolino, E.; Gargantini, L.; Granata, S.; Valentini, M.; Morra, E. Prognostic factors for malignant transformation in monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. J. Clin. Oncol. 2002, 20, 1625–1634. [Google Scholar] [CrossRef]
- Proctor, M.J.; Horgan, P.G.; Talwar, D.; Fletcher, C.D.; Morrison, D.S.; McMillan, D.C. Optimization of the systemic inflammation-based Glasgow prognostic score: A Glasgow Inflammation Outcome Study. Cancer 2013, 119, 2325–2332. [Google Scholar] [CrossRef]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Emir, S.; Aydin, M.; Can, G.; Bali, I.; Yildirim, O.; Oznur, M.; Yildiz, Z.D.; Sozen, S.; Gurel, A. Comparison of colorectal neoplastic polyps and adenocarcinoma with regard to NLR and PLR. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3613–3618. [Google Scholar]
- Haram, A.; Boland, M.R.; Kelly, M.E.; Bolger, J.C.; Waldron, R.M.; Kerin, M.J. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review. J. Surg. Oncol. 2017, 115, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Stotz, M.; Pichler, M.; Absenger, G.; Szkandera, J.; Arminger, F.; Schaberl-Moser, R.; Samonigg, H.; Stojakovic, T.; Gerger, A. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br. J. Cancer 2014, 110, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, M.; Maeda, K.; Nagahara, H.; Ohtani, H.; Iseki, Y.; Ikeya, T.; Sugano, K.; Hirakawa, K. The prognostic significance of the postoperative prognostic nutritional index in patients with colorectal cancer. BMC Cancer 2015, 15, 521. [Google Scholar] [CrossRef]
- Climent, M.; Ryan, E.J.; Stakelum, A.; Khaw, Y.L.; Creavin, B.; Lloyd, A.; Alhassan, D.; Mohan, H.M.; Kennelly, R.; Sheahan, K.; et al. Systemic inflammatory response predicts oncological outcomes in patients undergoing elective surgery for mismatch repair-deficient colorectal cancer. Int. J. Colorectal. Dis. 2019, 34, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Lin, Z.Y.; Ma, D.D.; Shang, Z.H.; Shen, Y.B.; Zhang, T.; Zhang, J.X.; Jin, W.D. A preoperative prediction model based on Lymphocyte-C-reactive protein ratio predicts postoperative anastomotic leakage in patients with colorectal carcinoma: A retrospective study. BMC Surg. 2022, 22, 283. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Fu, Y.; Su, Q.; Wang, H. Prognostic role of platelet-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Medicine 2016, 95, e3837. [Google Scholar] [CrossRef]
- Tan, D.; Fu, Y.; Tong, W.; Li, F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: A meta-analysis. Int. J. Surg. 2018, 55, 128–138. [Google Scholar] [CrossRef]
- Rossi, S.; Basso, M.; Strippoli, A.; Schinzari, G.; D’Argento, E.; Larocca, M.; Cassano, A.; Barone, C. Are Markers of Systemic Inflammation Good Prognostic Indicators in Colorectal Cancer? Clin. Colorectal. Cancer 2017, 16, 264–274. [Google Scholar] [CrossRef]
- Ullman, T.A.; Itzkowitz, S.H. Intestinal inflammation and cancer. Gastroenterology 2011, 140, 1807–1816. [Google Scholar] [CrossRef]
- Waldner, M.J.; Foersch, S.; Neurath, M.F. Interleukin-6--a key regulator of colorectal cancer development. Int. J. Biol. Sci. 2012, 8, 1248–1253. [Google Scholar] [CrossRef]
- Xu, X.; Fu, X.Y.; Plate, J.; Chong, A.S. IFN-gamma induces cell growth inhibition by Fas-mediated apoptosis: Requirement of STAT1 protein for up-regulation of Fas and FasL expression. Cancer Res. 1998, 58, 2832–2837. [Google Scholar] [PubMed]
- Tannenbaum, C.S.; Hamilton, T.A. Immune-inflammatory mechanisms in IFNgamma-mediated anti-tumor activity. Semin. Cancer Biol. 2000, 10, 113–123. [Google Scholar] [CrossRef]
- Street, S.E.; Trapani, J.A.; MacGregor, D.; Smyth, M.J. Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J. Exp. Med. 2002, 196, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Galon, J.; Pages, F.; Tartour, E.; Sautes-Fridman, C.; Kroemer, G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011, 71, 5601–5605. [Google Scholar] [CrossRef] [PubMed]
- Tosolini, M.; Kirilovsky, A.; Mlecnik, B.; Fredriksen, T.; Mauger, S.; Bindea, G.; Berger, A.; Bruneval, P.; Fridman, W.H.; Pages, F.; et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011, 71, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- De Simone, V.; Franze, E.; Ronchetti, G.; Colantoni, A.; Fantini, M.C.; Di Fusco, D.; Sica, G.S.; Sileri, P.; MacDonald, T.T.; Pallone, F.; et al. Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 2015, 34, 3493–3503. [Google Scholar] [CrossRef]
- Ueda, T.; Shimada, E.; Urakawa, T. Serum levels of cytokines in patients with colorectal cancer: Possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J. Gastroenterol. 1994, 29, 423–429. [Google Scholar] [CrossRef]
- Jin, W.J.; Xu, J.M.; Xu, W.L.; Gu, D.H.; Li, P.W. Diagnostic value of interleukin-8 in colorectal cancer: A case-control study and meta-analysis. World J. Gastroenterol. 2014, 20, 16334–16342. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, F.; Wang, B.; Liu, S.; Niu, W.; Liu, E.; Peng, C.; Wang, J.; Gao, H.; Liang, B.; et al. Interleukin-8 promotes cell migration through integrin alphavbeta6 upregulation in colorectal cancer. Cancer Lett. 2014, 354, 245–253. [Google Scholar] [CrossRef]
- Xia, W.; Chen, W.; Zhang, Z.; Wu, D.; Wu, P.; Chen, Z.; Li, C.; Huang, J. Prognostic value, clinicopathologic features and diagnostic accuracy of interleukin-8 in colorectal cancer: A meta-analysis. PLoS ONE 2015, 10, e0123484. [Google Scholar] [CrossRef]
- Li, B.; Wang, F.; Ma, C.; Hao, T.; Geng, L.; Jiang, H. Predictive value of IL-18 and IL-10 in the prognosis of patients with colorectal cancer. Oncol. Lett. 2019, 18, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.C.; Freimuth, J.; Akhurst, R.J. Complexities of TGF-beta targeted cancer therapy. Int. J. Biol. Sci. 2012, 8, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Gotzmann, J.; Mikula, M.; Eger, A.; Schulte-Hermann, R.; Foisner, R.; Beug, H.; Mikulits, W. Molecular aspects of epithelial cell plasticity: Implications for local tumor invasion and metastasis. Mutat. Res. 2004, 566, 9–20. [Google Scholar] [CrossRef]
- Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef] [PubMed]
- Sabeh, F.; Ota, I.; Holmbeck, K.; Birkedal-Hansen, H.; Soloway, P.; Balbin, M.; Lopez-Otin, C.; Shapiro, S.; Inada, M.; Krane, S.; et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J. Cell Biol. 2004, 167, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Wautier, J.L.; Wautier, M.P. Vascular Permeability in Diseases. Int. J. Mol. Sci. 2022, 23, 3645. [Google Scholar] [CrossRef]
- Gold, P.; Freedman, S.O. Demonstration of Tumor-Specific Antigens in Human Colonic Carcinomata by Immunological Tolerance and Absorption Techniques. J. Exp. Med. 1965, 121, 439–462. [Google Scholar] [CrossRef]
- Beauchemin, N.; Arabzadeh, A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013, 32, 643–671. [Google Scholar] [CrossRef]
- Kuroki, M.; Matsuo, Y.; Kuroki, M.; Matsuoka, Y. Nonspecific cross-reacting antigen (NCA) expressed by human granulocytes: Six species with different peptide sizes and membrane anchoring forms. Biochem. Biophys. Res. Commun. 1990, 166, 701–708. [Google Scholar] [CrossRef]
- van Gisbergen, K.P.; Aarnoudse, C.A.; Meijer, G.A.; Geijtenbeek, T.B.; van Kooyk, Y. Dendritic cells recognize tumor-specific glycosylation of carcinoembryonic antigen on colorectal cancer cells through dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin. Cancer Res. 2005, 65, 5935–5944. [Google Scholar] [CrossRef]
- Zid, M.; Drouin, G. Gene conversions are under purifying selection in the carcinoembryonic antigen immunoglobulin gene families of primates. Genomics 2013, 102, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, N.; Draber, P.; Dveksler, G.; Gold, P.; Gray-Owen, S.; Grunert, F.; Hammarstrom, S.; Holmes, K.V.; Karlsson, A.; Kuroki, M.; et al. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp. Cell Res. 1999, 252, 243–249. [Google Scholar] [CrossRef]
- Yago, T.; Leppanen, A.; Qiu, H.; Marcus, W.D.; Nollert, M.U.; Zhu, C.; Cummings, R.D.; McEver, R.P. Distinct molecular and cellular contributions to stabilizing selectin-mediated rolling under flow. J. Cell Biol. 2002, 158, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Borsig, L. Selectins in cancer immunity. Glycobiology 2018, 28, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Bonsor, D.A.; Zhao, Q.; Schmidinger, B.; Weiss, E.; Wang, J.; Deredge, D.; Beadenkopf, R.; Dow, B.; Fischer, W.; Beckett, D.; et al. The Helicobacter pylori adhesin protein HopQ exploits the dimer interface of human CEACAMs to facilitate translocation of the oncoprotein CagA. EMBO J. 2018, 37, e98664. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, P.; Kuiper, J.W.P.; Adrian, J.; Goob, G.; Hauck, C.R. CEACAM3-A Prim(at)e Invention for Opsonin-Independent Phagocytosis of Bacteria. Front. Immunol. 2019, 10, 3160. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, C.; Screaton, R.A.; Ilantzis, C.; Stanners, C.P. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res. 2000, 60, 3419–3424. [Google Scholar]
- Samara, R.N.; Laguinge, L.M.; Jessup, J.M. Carcinoembryonic antigen inhibits anoikis in colorectal carcinoma cells by interfering with TRAIL-R2 (DR5) signaling. Cancer Res. 2007, 67, 4774–4782. [Google Scholar] [CrossRef]
- Zheng, J.; Miller, K.K.; Yang, T.; Hildebrand, M.S.; Shearer, A.E.; DeLuca, A.P.; Scheetz, T.E.; Drummond, J.; Scherer, S.E.; Legan, P.K.; et al. Carcinoembryonic antigen-related cell adhesion molecule 16 interacts with alpha-tectorin and is mutated in autosomal dominant hearing loss (DFNA4). Proc. Natl. Acad. Sci. USA 2011, 108, 4218–4223. [Google Scholar] [CrossRef]
- Kuespert, K.; Pils, S.; Hauck, C.R. CEACAMs: Their role in physiology and pathophysiology. Curr. Opin. Cell Biol. 2006, 18, 565–571. [Google Scholar] [CrossRef]
- Skubitz, K.M.; Skubitz, A.P. Interdependency of CEACAM-1, -3, -6, and -8 induced human neutrophil adhesion to endothelial cells. J. Transl. Med. 2008, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Hastings, W.D.; Anderson, D.E.; Kassam, N.; Koguchi, K.; Greenfield, E.A.; Kent, S.C.; Zheng, X.X.; Strom, T.B.; Hafler, D.A.; Kuchroo, V.K. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 2009, 39, 2492–2501. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Neurath, M.F.; Nagaishi, T.; Glickman, J.N.; Nieuwenhuis, E.E.; Nakajima, A.; Chen, D.; Fuss, I.J.; Utku, N.; Lewicki, D.N.; et al. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J. Exp. Med. 2004, 199, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, P.; Li, L.; Shi, L.; Chang, P.; Liang, T.; Yang, Q.; Liu, Y.; Wang, L.; Hu, L. Co-expression of TIM-3 and CEACAM1 promotes T cell exhaustion in colorectal cancer patients. Int. Immunopharmacol. 2017, 43, 210–218. [Google Scholar] [CrossRef]
- Piancone, F.; Saresella, M.; Marventano, I.; La Rosa, F.; Caputo, D.; Mendozzi, L.; Rovaris, M.; Clerici, M. A Deficit of CEACAM-1-Expressing T Lymphocytes Supports Inflammation in Primary Progressive Multiple Sclerosis. J. Immunol. 2019, 203, 76–83. [Google Scholar] [CrossRef]
- Hall, C.; Clarke, L.; Pal, A.; Buchwald, P.; Eglinton, T.; Wakeman, C.; Frizelle, F. A Review of the Role of Carcinoembryonic Antigen in Clinical Practice. Ann. Coloproctol. 2019, 35, 294–305. [Google Scholar] [CrossRef]
- Lee, J.H.; Hong, S.P.; Jeon, T.J.; Kang, G.H.; Choi, W.C.; Jeon, S.M.; Moon, C.M.; Park, J.J.; Cheon, J.H.; Kim, T.I.; et al. Should a colonoscopy be recommended for healthy individuals with increased carcinoembryonic antigen levels? A case-control study. Dig. Dis. Sci. 2011, 56, 2396–2403. [Google Scholar] [CrossRef]
- Michl, M.; Stintzing, S.; Fischer von Weikersthal, L.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmueller, C.; Kahl, C.; et al. CEA response is associated with tumor response and survival in patients with KRAS exon 2 wild-type and extended RAS wild-type metastatic colorectal cancer receiving first-line FOLFIRI plus cetuximab or bevacizumab (FIRE-3 trial). Ann. Oncol. 2016, 27, 1565–1572. [Google Scholar] [CrossRef]
- Pakdel, A.; Malekzadeh, M.; Naghibalhossaini, F. The association between preoperative serum CEA concentrations and synchronous liver metastasis in colorectal cancer patients. Cancer Biomark 2016, 16, 245–252. [Google Scholar] [CrossRef]
- Gangopadhyay, A.; Thomas, P. Processing of carcinoembryonic antigen by Kupffer cells: Recognition of a penta-peptide sequence. Arch. Biochem. Biophys. 1996, 334, 151–157. [Google Scholar] [CrossRef]
- Wautier, J.L.; Wautier, M.P. Endothelial Cell Participation in Inflammatory Reaction. Int. J. Mol. Sci. 2021, 22, 6341. [Google Scholar] [CrossRef] [PubMed]
- Neumaier, M.; Paululat, S.; Chan, A.; Matthaes, P.; Wagener, C. Biliary glycoprotein, a potential human cell adhesion molecule, is down-regulated in colorectal carcinomas. Proc. Natl. Acad. Sci. USA 1993, 90, 10744–10748. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Nedellec, P.; Jothy, S.; Fleiszer, D.; Turbide, C.; Beauchemin, N. The expression of mouse biliary glycoprotein, a carcinoembryonic antigen-related gene, is down-regulated in malignant mouse tissues. Cancer Res. 1993, 53, 4938–4945. [Google Scholar] [PubMed]
- Kim, K.S.; Kim, J.T.; Lee, S.J.; Kang, M.A.; Choe, I.S.; Kang, Y.H.; Kim, S.Y.; Yeom, Y.I.; Lee, Y.H.; Kim, J.H.; et al. Overexpression and clinical significance of carcinoembryonic antigen-related cell adhesion molecule 6 in colorectal cancer. Clin. Chim. Acta 2013, 415, 12–19. [Google Scholar] [CrossRef]
- Duxbury, M.S.; Matros, E.; Clancy, T.; Bailey, G.; Doff, M.; Zinner, M.J.; Ashley, S.W.; Maitra, A.; Redston, M.; Whang, E.E. CEACAM6 is a novel biomarker in pancreatic adenocarcinoma and PanIN lesions. Ann. Surg. 2005, 241, 491–496. [Google Scholar] [CrossRef]
- Thompson, J.; Zimmermann, W.; Nollau, P.; Neumaier, M.; Weber-Arden, J.; Schrewe, H.; Craig, I.; Willcocks, T. CGM2, a member of the carcinoembryonic antigen gene family is down-regulated in colorectal carcinomas. J. Biol. Chem. 1994, 269, 32924–32931. [Google Scholar] [CrossRef]
- Messick, C.A.; Sanchez, J.; Dejulius, K.L.; Hammel, J.; Ishwaran, H.; Kalady, M.F. CEACAM-7: A predictive marker for rectal cancer recurrence. Surgery 2010, 147, 713–719. [Google Scholar] [CrossRef]
- Bian, Q.; Chen, J.; Qiu, W.; Peng, C.; Song, M.; Sun, X.; Liu, Y.; Ding, F.; Chen, J.; Zhang, L. Four targeted genes for predicting the prognosis of colorectal cancer: A bioinformatics analysis case. Oncol. Lett. 2019, 18, 5043–5054. [Google Scholar] [CrossRef]
- Stiksma, J.; Grootendorst, D.C.; van der Linden, P.W. CA 19-9 as a marker in addition to CEA to monitor colorectal cancer. Clin. Colorectal. Cancer 2014, 13, 239–244. [Google Scholar] [CrossRef]
- Galli, C.; Basso, D.; Plebani, M. CA 19-9: Handle with care. Clin. Chem. Lab. Med. 2013, 51, 1369–1383. [Google Scholar] [CrossRef]
- Guadagni, F.; Roselli, M.; Cosimelli, M.; Ferroni, P.; Spila, A.; Cavaliere, F.; Arcuri, R.; Carlini, S.; Mariotti, S.; Gandolfo, G.M.; et al. TAG-72 expression and its role in the biological evaluation of human colorectal cancer. Anticancer Res. 1996, 16, 2141–2148. [Google Scholar] [PubMed]
- Hitchcock, C.L.; Povoski, S.P.; Mojzisik, C.M.; Martin, E.W., Jr. Survival Advantage Following TAG-72 Antigen-Directed Cancer Surgery in Patients With Colorectal Carcinoma: Proposed Mechanisms of Action. Front. Oncol. 2021, 11, 731350. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, C.; Zhang, F.; Ma, X.; Gai, X. Clinical Value of Combined Determination of Serum B7-H4 with Carcinoembryonic Antigen, Osteopontin, or Tissue Polypeptide-Specific Antigen for the Diagnosis of Colorectal Cancer. Dis. Markers 2018, 2018, 4310790. [Google Scholar] [CrossRef] [PubMed]
- Mroczko, B.; Szmitkowski, M.; Okulczyk, B. Hematopoietic growth factors in colorectal cancer patients. Clin. Chem. Lab. Med. 2003, 41, 646–651. [Google Scholar] [CrossRef]
- Babel, I.; Barderas, R.; Diaz-Uriarte, R.; Martinez-Torrecuadrada, J.L.; Sanchez-Carbayo, M.; Casal, J.I. Identification of tumor-associated autoantigens for the diagnosis of colorectal cancer in serum using high density protein microarrays. Mol. Cell Proteomics 2009, 8, 2382–2395. [Google Scholar] [CrossRef]
- Leman, E.S.; Schoen, R.E.; Weissfeld, J.L.; Cannon, G.W.; Sokoll, L.J.; Chan, D.W.; Getzenberg, R.H. Initial analyses of colon cancer-specific antigen (CCSA)-3 and CCSA-4 as colorectal cancer-associated serum markers. Cancer Res. 2007, 67, 5600–5605. [Google Scholar] [CrossRef]
- Holten-Andersen, M.N.; Christensen, I.J.; Nielsen, H.J.; Stephens, R.W.; Jensen, V.; Nielsen, O.H.; Sorensen, S.; Overgaard, J.; Lilja, H.; Harris, A.; et al. Total levels of tissue inhibitor of metalloproteinases 1 in plasma yield high diagnostic sensitivity and specificity in patients with colon cancer. Clin. Cancer Res. 2002, 8, 156–164. [Google Scholar]
- Said, A.H.; Raufman, J.P.; Xie, G. The role of matrix metalloproteinases in colorectal cancer. Cancers 2014, 6, 366–375. [Google Scholar] [CrossRef]
- Bork, U.; Rahbari, N.N.; Scholch, S.; Reissfelder, C.; Kahlert, C.; Buchler, M.W.; Weitz, J.; Koch, M. Circulating tumour cells and outcome in non-metastatic colorectal cancer: A prospective study. Br. J. Cancer 2015, 112, 1306–1313. [Google Scholar] [CrossRef]
- Cohen, S.J.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 3213–3221. [Google Scholar] [CrossRef]
- Dizdar, L.; Fluegen, G.; van Dalum, G.; Honisch, E.; Neves, R.P.; Niederacher, D.; Neubauer, H.; Fehm, T.; Rehders, A.; Krieg, A.; et al. Detection of circulating tumor cells in colorectal cancer patients using the GILUPI CellCollector: Results from a prospective, single-center study. Mol. Oncol. 2019, 13, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Stoecklein, N.H.; Fischer, J.C.; Niederacher, D.; Terstappen, L.W. Challenges for CTC-based liquid biopsies: Low CTC frequency and diagnostic leukapheresis as a potential solution. Expert Rev. Mol. Diagn. 2016, 16, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, P.; Yang, J.; Liu, Z.; Yang, Z.; Qin, H. Candidate microRNA biomarkers in human colorectal cancer: Systematic review profiling studies and experimental validation. Int. J. Cancer 2012, 130, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A., Jr.; Bardelli, A. Liquid biopsies: Genotyping circulating tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef]
- Heitzer, E.; Auer, M.; Ulz, P.; Geigl, J.B.; Speicher, M.R. Circulating tumor cells and DNA as liquid biopsies. Genome Med. 2013, 5, 73. [Google Scholar] [CrossRef]
- Jo, P.; Jung, K.; Grade, M.; Conradi, L.C.; Wolff, H.A.; Kitz, J.; Becker, H.; Ruschoff, J.; Hartmann, A.; Beissbarth, T.; et al. CpG island methylator phenotype infers a poor disease-free survival in locally advanced rectal cancer. Surgery 2012, 151, 564–570. [Google Scholar] [CrossRef]
- Wang, C.; Fakih, M. Targeting KRAS in Colorectal Cancer. Curr. Oncol. Rep. 2021, 23, 28. [Google Scholar] [CrossRef]
- Duffy, M.J.; van Dalen, A.; Haglund, C.; Hansson, L.; Klapdor, R.; Lamerz, R.; Nilsson, O.; Sturgeon, C.; Topolcan, O. Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. Eur. J. Cancer 2003, 39, 718–727. [Google Scholar] [CrossRef]
- Tie, J.; Lo, S.N.; Gibbs, P. Circulating Tumor DNA Guiding Adjuvant Therapy in Colon Cancer. Reply. N. Engl. J. Med. 2022, 387, 760. [Google Scholar] [CrossRef]
| Name | Amino Acid | Molecular Weight (kDa) |
|---|---|---|
| CEACAM1, CD66a | 526 | 57.6 |
| CEACAM3, CD66d | 252 | 27.1 |
| CEACAM4, CGM7 | 244 | 28.9 |
| CEACAM5, CD66e | 702 | 76.8 |
| CEACAM6, CD66c | 344 | 37.2 |
| CEACAM7, CGM2 | 265 | 29.3 |
| CEACAM8, CD66b | 349 | 38.1 |
| CEACAM16 | 425 | 45.9 |
| CEACAM18 | 384 | 43.3 |
| CEACAM19 | 300 | 32.6 |
| CEACAM20 | 596 | 65.8 |
| CEACAM21 | 293 | 32.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wautier, J.-L.; Wautier, M.-P. Old and New Blood Markers in Human Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 12968. https://doi.org/10.3390/ijms232112968
Wautier J-L, Wautier M-P. Old and New Blood Markers in Human Colorectal Cancer. International Journal of Molecular Sciences. 2022; 23(21):12968. https://doi.org/10.3390/ijms232112968
Chicago/Turabian StyleWautier, Jean-Luc, and Marie-Paule Wautier. 2022. "Old and New Blood Markers in Human Colorectal Cancer" International Journal of Molecular Sciences 23, no. 21: 12968. https://doi.org/10.3390/ijms232112968
APA StyleWautier, J.-L., & Wautier, M.-P. (2022). Old and New Blood Markers in Human Colorectal Cancer. International Journal of Molecular Sciences, 23(21), 12968. https://doi.org/10.3390/ijms232112968







