The Effect of Angiogenesis-Based Scaffold of MesoporousBioactive Glass Nanofiber on Osteogenesis
Abstract
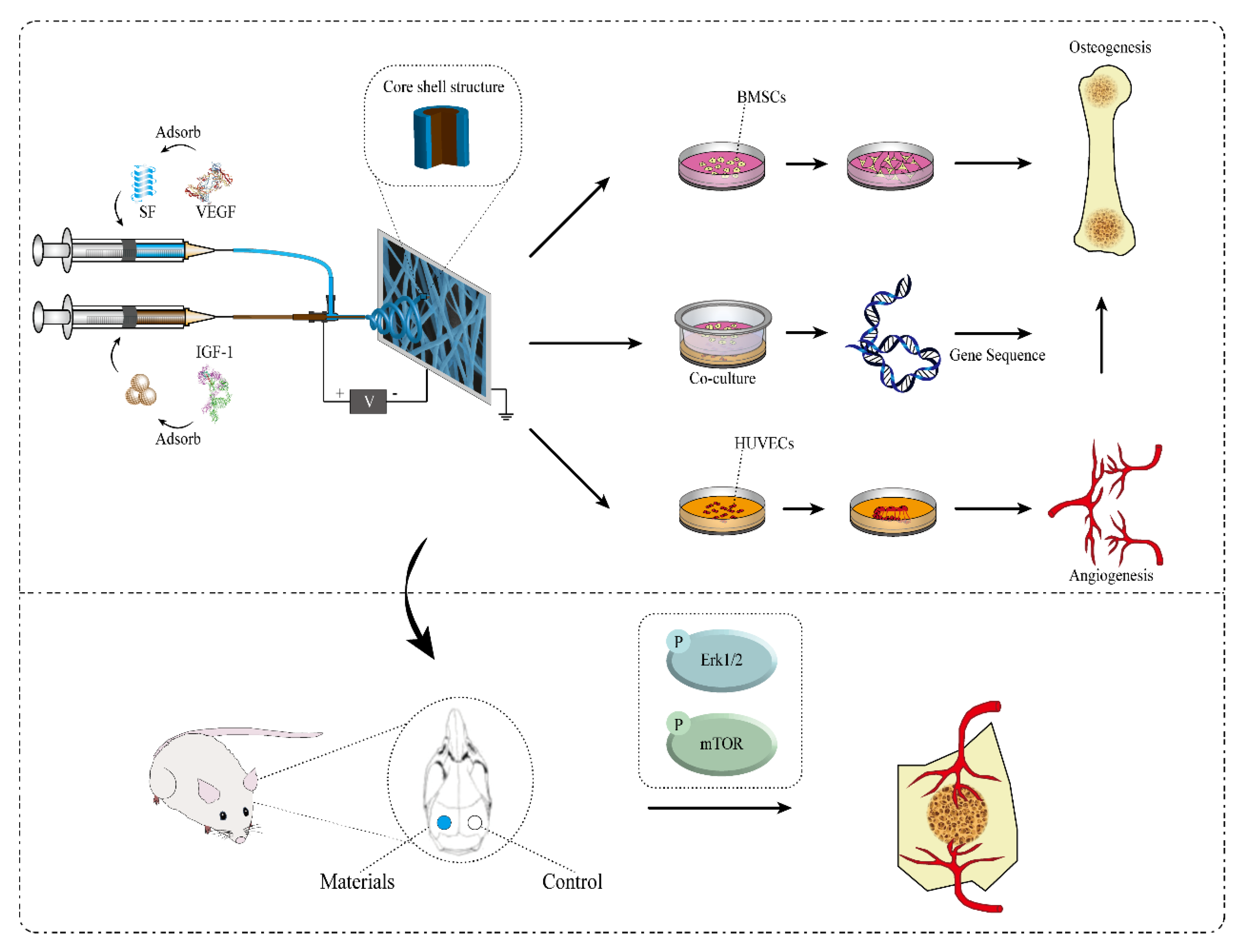
1. Introduction
2. Results
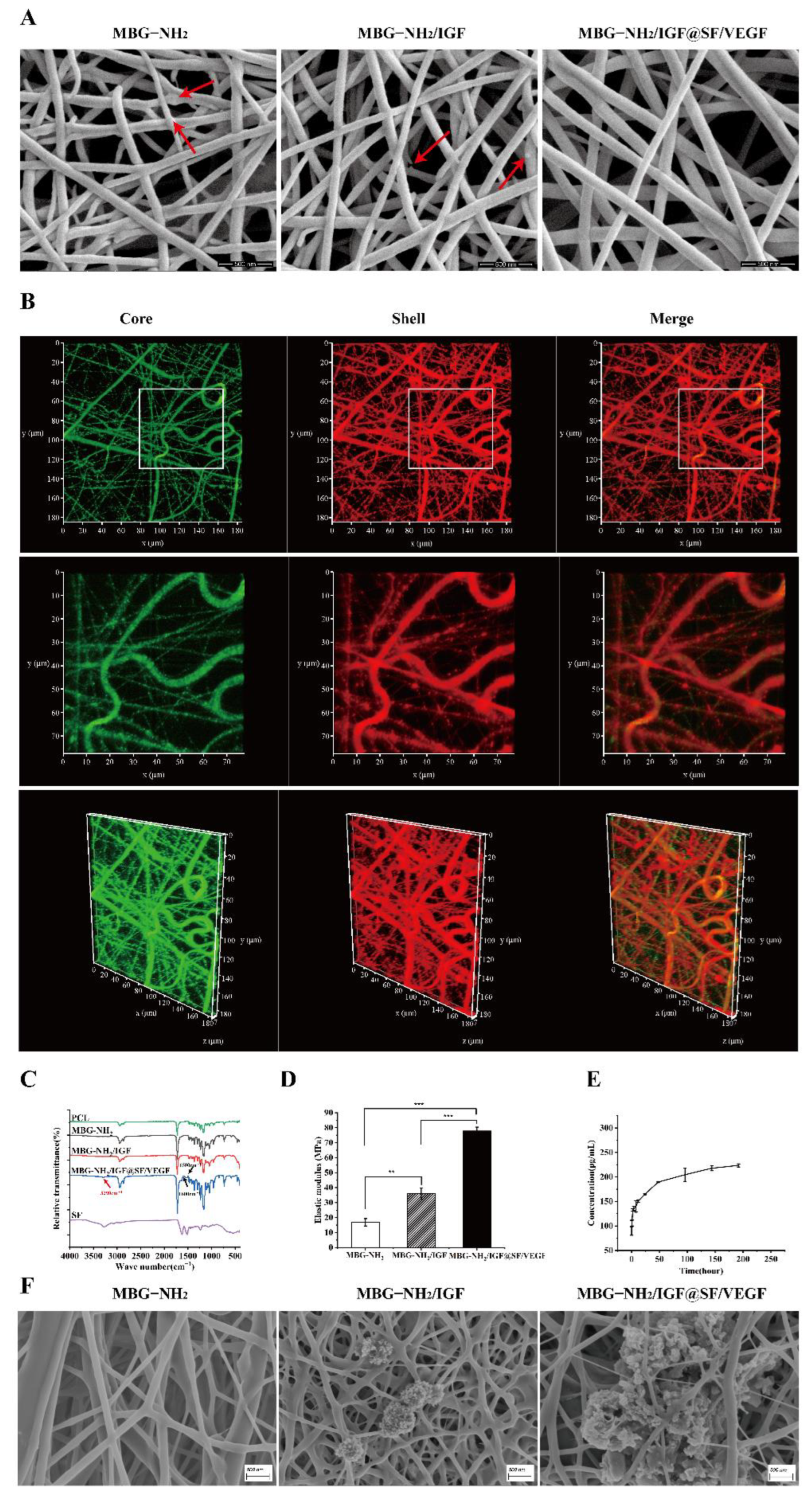
2.1. Characterization and Bioactivity of Scaffolds
2.1.1. Characterization of Scaffolds
2.1.2. Bioactivity of Scaffolds
2.2. BMSCs Proliferation, Differentiation and Mineralization Cultured on These Scaffolds
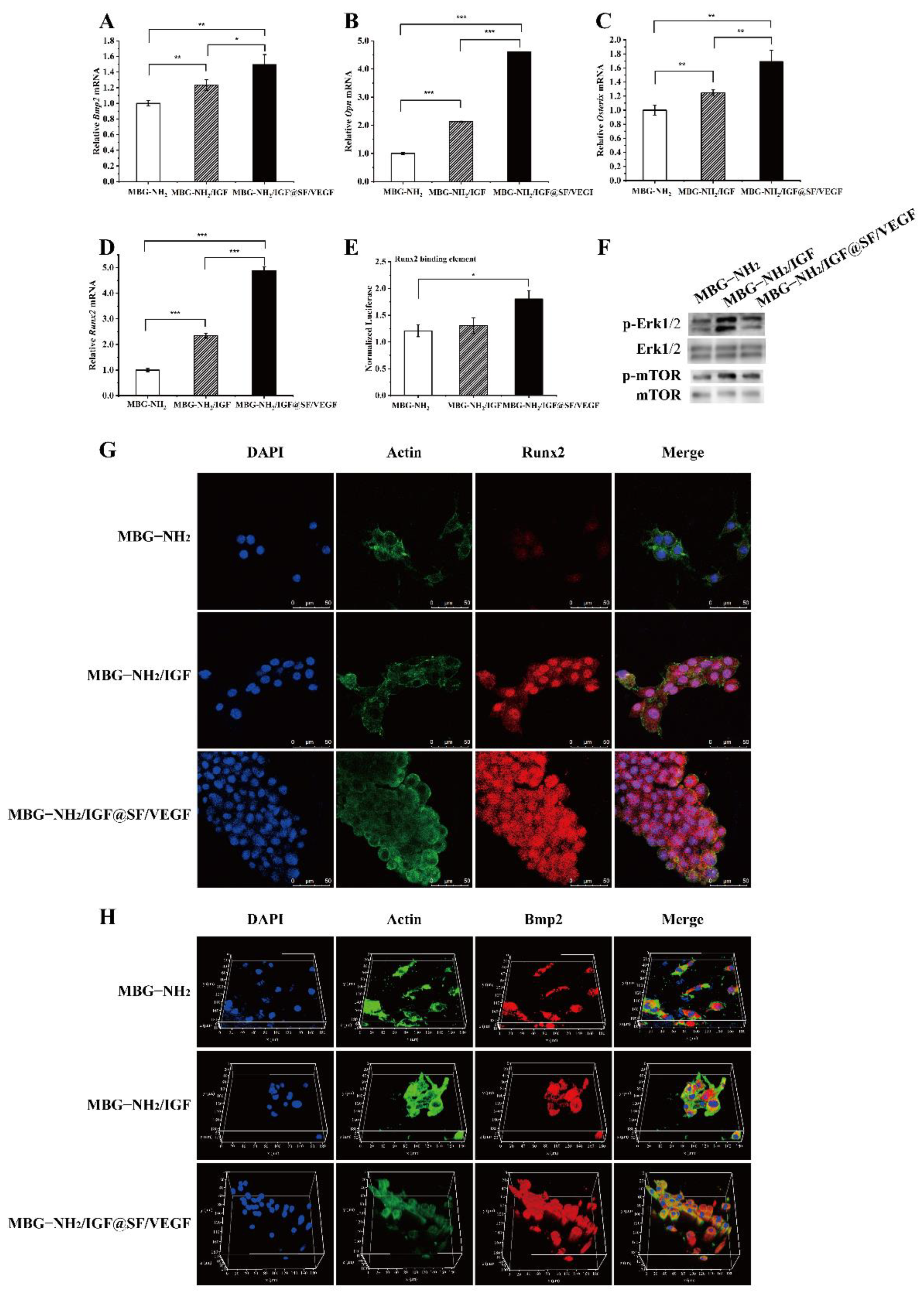
2.3. The Effect of Scaffolds on Osteogenesis In Vitro
2.3.1. Expression of Osteogenic Genes and Runx2 Transcriptional Activity
2.3.2. Expression of Osteogenic Proteins
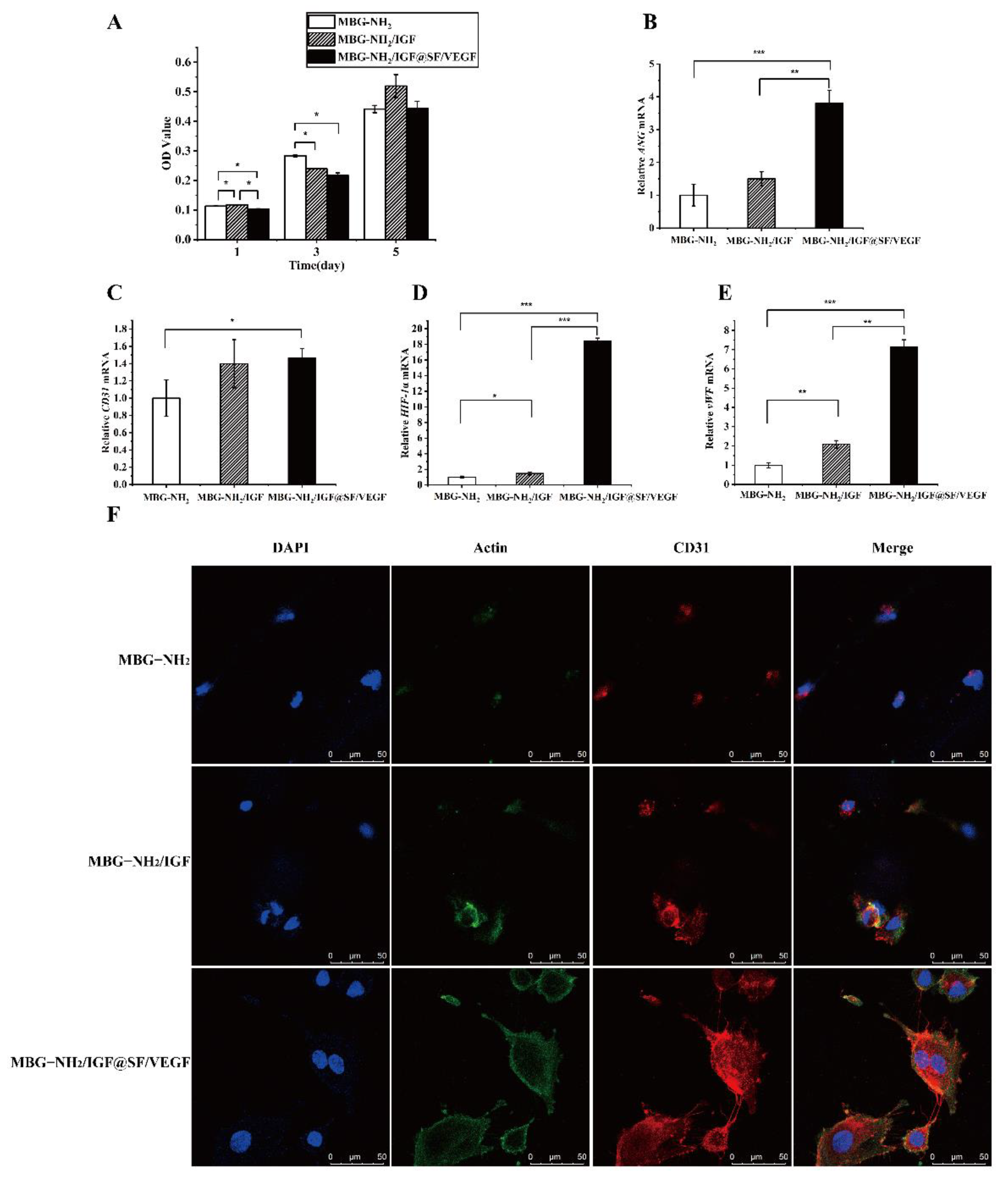
2.4. The Effect of Scaffolds on Angiogenesis In Vitro
2.4.1. Proliferation in HUVECs during Angiogenesis
2.4.2. Expression of Angiogenic Genes
2.4.3. Expression of Angiogenic Proteins
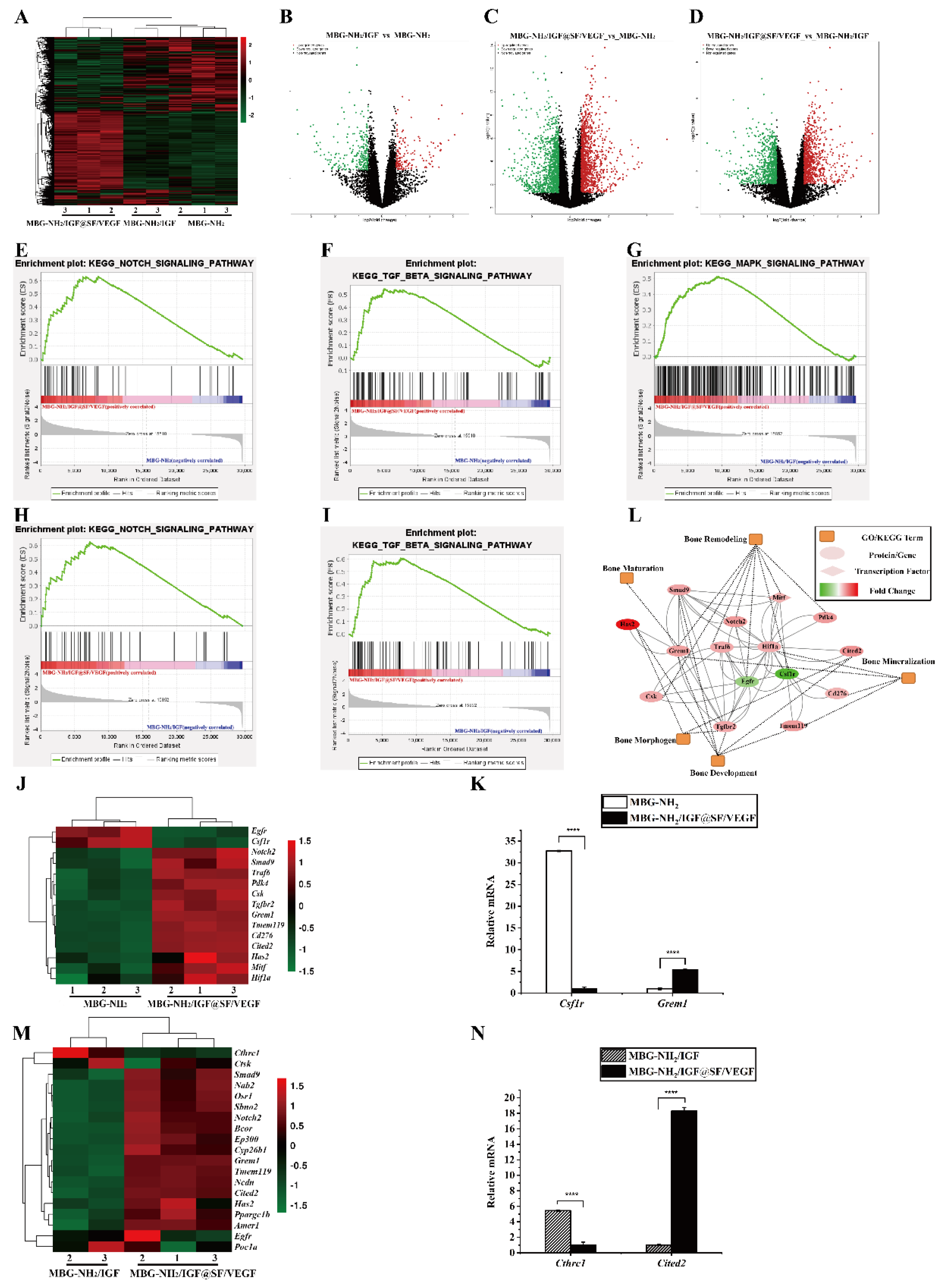
2.5. Synergistic Effect of Osteogenesis and Angiogenesis
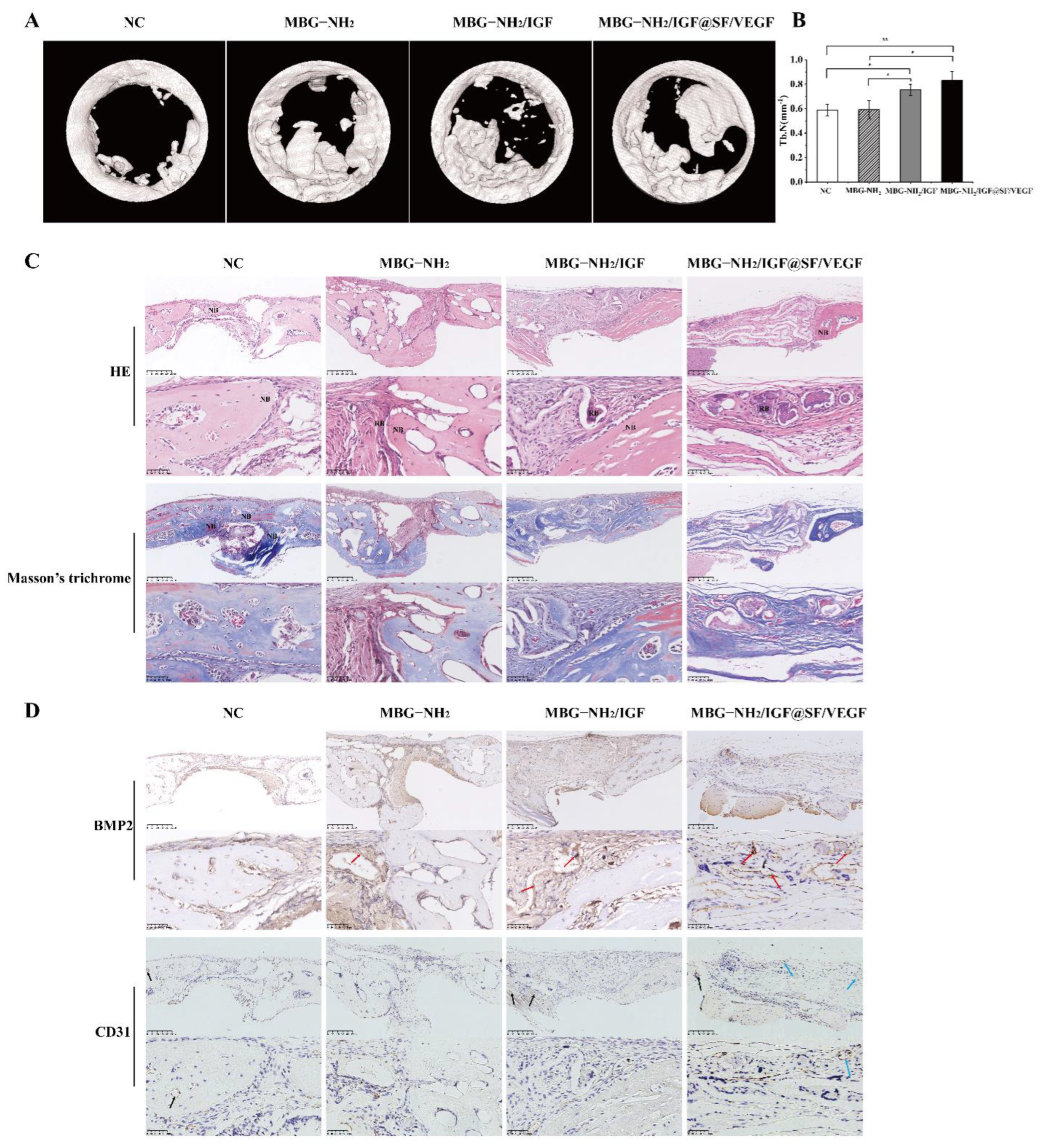
2.6. In Vivo Evaluation of Bone Defect Repair Effects of Scaffolds
3. Discussion
4. Materials and Methods
4.1. Preparation of MBG-NH2 and SF
4.2. Preparation of Electrospun Scaffolds
4.3. Morphology and Structure Characterization
4.4. Mineralization Deposits in Simulated Body Fluid
4.5. Cells and Cell Culture
4.5.1. BMSCs
4.5.2. HUVECs
4.6. Cell Proliferation
4.7. Cell Differentiation
4.8. Cell Mineralization
4.9. Transfection and Luciferase Assay
4.10. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.11. Western Blot
4.12. Cell Immunofluorescence
4.13. Gene Sequencing
4.14. In Vivo Studies
4.15. Statistics Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HA | Hydroxyapatite |
| TCP | Tricalcium phosphate |
| PLA | Polylactic acid |
| CS | Chitosan |
| SF | Silk fibroin |
| LBL | Layer-by-layer |
| MBG | Mesoporous bioactive glass |
| MBG-NH2 | MBG modified with amino |
| ECM | Extracellular matrix |
| DC | Direct current |
| Bmp2 | Bone morphogenetic protein 2 |
| Ocn | Osteocalcin |
| MAPK | Mitogen-activated protein kinase |
| BMSCs | Bone marrow mesenchymal stem cells |
| IGF-1 | Insulin-like growth factor-1 |
| VEGF | Vascular endothelial growth factor |
| HUVECs | Human umbilical vein endothelial cells |
| SBF | Simulated body fluid |
| RNA-seq | RNA sequencing |
| Tb. N | The number of trabeculae |
| HE | Hematoxylin–eosin staining |
| p-mTOR | Phosphorylated mammalian target of rapamycin |
| PEO20-PPO70-PEO20 | P123 |
| TMB | 1,3,5-trimethylbenzene |
| TEOS | Tetraethyl orthosilicate |
| TEP | Triethyl phosphate |
| APTES | Aminopropyltriethoxysilane |
| PEG | Polyethylene glycol |
| PBS | Phosphate-buffered saline |
| ELISA | Enzyme-linked immunosorbent assay kit |
| PCL | Poly (ε-caprolactone) |
| HFIP | 1,1,1,3,3,3-hexafluoro-2-propanol |
| SEM | Scanning electron microscope |
| FITC | Fluorescein-5-isothiocyannate |
| CLSM | Confocal laser scanning microscope |
| FTIR | Fourier transform infrared |
| DMEM | Dulbecco’s modified eagle medium |
| FBS | Fetal bovine serum |
| VC | Ascorbic acid |
| DEX | Dexamethasone |
| β-Gly | β-glycerophosphate |
| CCK8 | Cell Counting Kit-8 |
| ALP | Alkaline phosphatase |
| PNPP | p-nitrophenol inorganic phosphate |
| PNP | p-nitrophenol |
| RT-qPCR | Real-time polymerase chain reaction |
| Opn | Osteopontin |
| Runx2 | Runt-related transcription factor 2 |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| ANG | Angiotensin |
| CD31 | Platelet endothelial cell adhesion molecule-1 |
| HIF-1a | Hypoxia-inducible factor-1α |
| vWF | Von Willebrand factor |
| Csf1r | Colony-Stimulating Factor 1 Receptor |
| Grem1 | Gremlin 1 |
| Cthrc1 | Collagen Triple Helix Repeat Containing Protein 1 |
| Cited2 | Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain 2 |
| PVDF | Polyvinylidene fluoride |
| Erk | Extracellular-regulated protein kinases |
| DAPI | 4′,6-diamidino-2-phenylindole |
| GSEA | Gene set enrichment analysis |
| micro-CT | Microcomputed tomography |
References
- Qi, X.; Pei, P.; Zhu, M.; Du, X.; Xin, C.; Zhao, S.; Li, X.; Zhu, Y. Three dimensional printing of calcium sulfate and mesoporous bioactive glass scaffolds for improving bone regeneration in vitro and in vivo. Sci. Rep. 2017, 7, srep42556. [Google Scholar] [CrossRef] [PubMed]
- Tschon, M.; Boanini, E.; Sartori, M.; Salamanna, F.; Panzavolta, S.; Bigi, A.; Fini, M. Antiosteoporotic Nanohydroxyapatite Zoledronate Scaffold Seeded with Bone Marrow Mesenchymal Stromal Cells for Bone Regeneration: A 3D In Vitro Model. Int. J. Mol. Sci. 2022, 23, 5988. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, C.R.; Guda, T.; Singleton, B.M.; Oh, D.S.; Appleford, M.R.; Ong, J.L.; Wenke, J.C. Effect of cell-seeded hydroxyapatite scaffolds on rabbit radius bone regeneration. J. Biomed. Mater. Res. Part A 2014, 102, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yan, Y.; Gao, J.; Li, Y.; Wang, R.; Wang, J.; Zou, Q.; Zuo, Y.; Zhu, M.; Li, J. 3D-printed hydroxyapatite microspheres reinforced PLGA scaffolds for bone regeneration. Biomater. Adv. 2022, 133, 112618. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, W.; Liu, C.; Tan, J.; Wang, H.; Hai, B.; Cai, H.; Leng, H.-J.; Liu, Z.-J.; Song, C.-L. Incorporating simvastatin/poloxamer 407 hydrogel into 3D-printed porous Ti6Al4V scaffolds for the promotion of angiogenesis, osseointegration and bone ingrowth. Biofabrication 2016, 8, 045012. [Google Scholar] [CrossRef]
- Pei, X.; Wu, L.; Zhou, C.; Fan, H.; Gou, M.; Li, Z.; Zhang, B.; Lei, H.; Sun, H.; Liang, J.; et al. 3D printed titanium scaffolds with homogeneous diamond-like structures mimicking that of the osteocyte microenvironment and its bone regeneration study. Biofabrication 2021, 13, 015008. [Google Scholar] [CrossRef]
- Tarafder, S.; Bose, S. Polycaprolactone-Coated 3D Printed Tricalcium Phosphate Scaffolds for Bone Tissue Engineering: In Vitro Alendronate Release Behavior and Local Delivery Effect on In Vivo Osteogenesis. Acs Appl. Mater. Interfaces 2014, 6, 9955–9965. [Google Scholar] [CrossRef]
- Xu, S.; Liu, J.; Zhang, L.; Yang, F.; Tang, P.; Wu, D. Effects of HAp and TCP in constructing tissue engineering scaffolds for bone repair. J. Mater. Chem. B 2017, 5, 6110–6118. [Google Scholar] [CrossRef]
- Liu, Z.; Chu, W.; Zhang, L.; Wang, Y.; Zhai, Z.; Liu, F. The effect of enhanced bone marrow in conjunction with 3D-printed PLA-HA in the repair of critical-sized bone defects in a rabbit model. Ann. Transl. Med. 2021, 9, 1134. [Google Scholar] [CrossRef]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Wang, C.-H.; Chen, H.-C.; Cherng, J.-H.; Chang, S.-J.; Wang, Y.-W.; Chang, A.; Yeh, J.-Z.; Huang, Y.-H.; Liu, C.-C. Combination of resveratrol-containing collagen with adipose stem cells for craniofacial tissue-engineering applications. Int. Wound J. 2018, 15, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Vepari, C.; Jin, H.J.; Kim, H.J.; Kaplan, D.L. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3115–3124. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xia, J.; Wu, T.; Gao, B.; Zhang, Y.; Wang, X.; Cheng, G.; Zhu, Y. Preparation, Characterization, and Implantation of Porous Fibroin/Hydroxyapatite Scaffolds for Bone Tissue Engineering. Sci. Adv. Mater. 2018, 10, 1601–1607. [Google Scholar] [CrossRef]
- Kim, U.J.; Park, J.; Kim, H.J.; Wada, M.; Kaplan, D.L. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials 2005, 26, 2775–2785. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.; Thirumurugan, S.; Hu, C.-C.; Wu, C.-J.; Shih, S.-J.; Chung, R.-J. Preparation and characterization of polyelectrolyte multilayer coatings on 316L stainless steel for antibacterial and bone regeneration applications. Surf. Coat. Technol. 2022, 435, 128254. [Google Scholar] [CrossRef]
- Tao, S.-C.; Li, X.-R.; Wei, W.-J.; Wei, Z.-Y.; Zhang, C.-R.; Wang, F.; Dawes, H.; Guo, S.-C. Polymeric coating on β-TCP scaffolds provides immobilization of small extracellular vesicles with surface-functionalization and ZEB1-Loading for bone defect repair in diabetes mellitus. Biomaterials 2022, 283, 121465. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, L.; Huang, S.; Zheng, W.; Liu, H.; Bai, Z.; Jiang, K.; Wang, X. PCL nanofibrous incorporating unique matrix fusion protein adsorbed mesoporous bioactive glass for bone tissue engineering. Int. J. Biol. Macromol. 2022, 208, 136–148. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Chang, J.; Xiao, Y. Delivery of dimethyloxallyl glycine in mesoporous bioactive glass scaffolds to improve angiogenesis and osteogenesis of human bone marrow stromal cells. Acta Biomater. 2013, 9, 9159–9168. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Shu, Y.; Wu, Z.; Cao, W.; Huang, W. Amino-functionalized mesoporous bioactive glass for drug delivery. Biomed. Mater. 2017, 12, 025017. [Google Scholar] [CrossRef]
- Lin, D.; Chai, Y.; Ma, Y.; Duan, B.; Yuan, Y.; Liu, C. Rapid initiation of guided bone regeneration driven by spatiotemporal delivery of IL-8 and BMP-2 from hierarchical MBG-based scaffold. Biomaterials 2019, 196, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Lin, D.; Chai, Y.; Yuan, Y.; Liu, C. MBG scaffolds containing chitosan microspheres for binary delivery of IL-8 and BMP-2 for bone regeneration. J. Mater. Chem. B 2018, 6, 4453–4465. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, W.; Liu, Q.; Gao, K.; Wang, G.; Gao, L.; Liu, L. Function and mechanism of mesoporous bioactive glass adsorbed epidermal growth factor for accelerating bone tissue regeneration. Biomed. Mater. 2017, 12, 025020. [Google Scholar] [CrossRef] [PubMed]
- Migneco, C.; Fiume, E.; Verné, E.; Baino, F. A Guided Walk through the World of Mesoporous Bioactive Glasses (MBGs): Fundamentals, Processing, and Applications. Nanomaterials 2020, 10, 2571. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.X.; Huang, X.H.; Yu, C.Z.; Deng, H.X.; Wang, Y.; Zhang, Z.D.; Qiao, S.Z.; Lu, G.Q.; Zhao, D.Y. The in-vitro bioactivity of mesoporous bioactive glasses. Biomaterials 2006, 27, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- Fiume, E.; Tulyaganov, D.U.; Akbarov, A.; Ziyadullaeva, N.; Cochis, A.; Scalia, A.C.; Rimondini, L.; Verné, E.; Baino, F. Biological Evaluation of a New Sodium-Potassium Silico-Phosphate Glass for Bone Regeneration: In Vitro and In Vivo Studies. Materials 2021, 14, 4546. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Zhou, Y.; Fan, W.; Xiao, Y. A comparative study of mesoporous glass/silk and non-mesoporous glass/silk scaffolds: Physiochemistry and in vivo osteogenesis. Acta Biomater. 2011, 7, 2229–2236. [Google Scholar] [CrossRef]
- Jang, J.-H.; Castano, O.; Kim, H.-W. Electrospun materials as potential platforms for bone tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1065–1083. [Google Scholar] [CrossRef]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Srouji, S.; Rachmiel, A.; Blumenfeld, I.; Livne, E. Mandibular defect repair by TGF-β and IGF-1 released from a biodegradable osteoconductive hydrogel. J. Cranio-Maxillofac. Surg. 2005, 33, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, Z.; Li, L.; Shi, X.; Jia, E.; Ji, Q.; Wang, Y.; Ito, Y.; Wei, Y.; Zhang, P. DOPA-derived electroactive copolymer and IGF-1 immobilized poly (lactic-co-glycolic acid)/hydroxyapatite biodegradable microspheres for synergistic bone repair. Chem. Eng. J. 2021, 416, 129129. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Jia, G.; Jiang, Y.; Liu, Q.; Yang, X.; Pan, S. Improving osteogenesis of PLGA/HA porous scaffolds based on dual delivery of BMP-2 and IGF-1 via a polydopamine coating. Rsc Adv. 2017, 7, 56732–56742. [Google Scholar] [CrossRef]
- Feng, X.; Huang, D.; Lu, X.; Feng, G.; Xing, J.; Lu, J.; Xu, K.; Xia, W.; Meng, Y.; Tao, T.; et al. Insulin-like growth factor 1 can promote proliferation and osteogenic differentiation of human dental pulp stem cells via mTOR pathway. Dev. Growth Differ. 2014, 56, 615–624. [Google Scholar] [CrossRef]
- Xu, G.-J.; Cai, S.; Wu, J.-B. Effect of Insulin-like Growth Factor-1 on Bone Morphogenetic Protein-2 Expression in Hepatic Carcinoma SMMC7721 Cells through the p38 MAPK Signaling Pathway. Asian Pac. J. Cancer Prev. 2012, 13, 1183–1186. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; Shen, X.; Wan, C.; Zhao, Q.; Zhang, L.; Zhou, Q.; Deng, L. Effects of insulin and insulin-like growth factor 1 on osteoblast proliferation and differentiation: Differential signalling via Akt and ERK. Cell Biochem. Funct. 2012, 30, 297–302. [Google Scholar] [CrossRef]
- Xian, L.; Wu, X.; Pang, L.; Lou, M.; Rosen, C.J.; Qiu, T.; Crane, J.; Frassica, F.; Zhang, L.; Pablo Rodriguez, J.; et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 2012, 18, 1095–1101. [Google Scholar] [CrossRef]
- Keramaris, N.C.; Calori, G.M.; Nikolaou, V.S.; Schemitsch, E.H.; Giannoudis, P.V. Fracture vascularity and bone healing: A systematic review of the role of VEGF. Injury 2008, 39, S45–S57. [Google Scholar] [CrossRef]
- Mazor, R.; Alsaigh, T.; Shaked, H.; Altshuler, A.E.; Pocock, E.S.; Kistler, E.B.; Karin, M.; Schmid-Schönbein, G.W. Matrix Metalloproteinase-1-mediated Up-regulation of Vascular Endothelial Growth Factor-2 in Endothelial Cells. J. Biol. Chem. 2013, 288, 598–607. [Google Scholar] [CrossRef]
- Cui, Q.; Dighe, A.S.; Irvine, J.N., Jr. Combined Angiogenic and Osteogenic Factor Delivery for Bone Regenerative Engineering. Curr. Pharm. Des. 2013, 19, 3374–3383. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, S.; Zeng, W.; Yu, J.; Chen, Y.; Yu, B. Controlled in vivo Bone Formation and Vascularization Using Ultrasound-Triggered Release of Recombinant Vascular Endothelial Growth Factor From Poly(D,L-lactic-co-glycolicacid) Microbubbles. Front. Pharmacol. 2019, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Choong, P.; McCarthy, R.; Chou, S.T.; Martin, T.J.; Ng, K.W. In situ hybridization to show sequential expression of osteoblast gene markers during bone formation in vivo. J. Bone Miner. Res. 1994, 9, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Cawthorn, W.P.; Li, Y.; Zhao, G.; Macdougald, O.A.; Franceschi, R.T. Reciprocal Control of Osteogenic and Adipogenic Differentiation by ERK/MAP Kinase Phosphorylation of Runx2 and PPARγ Transcription Factors. J. Cell. Physiol. 2016, 231, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Xiao, G.; Jiang, D.; Yang, Q.; Hatch, N.E.; Roca, H.; Franceschi, R.T. Identification and Functional Characterization of ERK/MAPK Phosphorylation Sites in the Runx2 Transcription Factor. J. Biol. Chem. 2009, 284, 32533–32543. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.A.; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.S.; Clevers, H.; Taketo, M.M.; Long, F.X.; McMahon, A.P.; Lang, R.A.; et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 2005, 8, 751–764. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef]
- Wenk, E.; Merkle, H.P.; Meinel, L. Silk fibroin as a vehicle for drug delivery applications. J. Control. Release 2011, 150, 128–141. [Google Scholar] [CrossRef]
- Lee, K.-S.; Hong, S.-H.; Bae, S.-C. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein. Oncogene 2002, 21, 7156–7163. [Google Scholar] [CrossRef]
- Massagué, J. Integration of Smad and MAPK pathways: A link and a linker revisited. Genes Dev. 2003, 17, 2993–2997. [Google Scholar] [CrossRef]
- Xiao, G.; Jiang, D.; Thomas, P.; Benson, M.D.; Guan, K.; Karsenty, G.; Franceschi, R.T. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J. Biol. Chem. 2000, 275, 4453–4459. [Google Scholar] [CrossRef]
- Murakami, J.; Ishii, M.; Suehiro, F.; Ishihata, K.; Nakamura, N.; Nishimura, M. Vascular endothelial growth factor-C induces osteogenic differentiation of human mesenchymal stem cells through the ERK and RUNX2 pathway. Biochem. Biophys. Res. Commun. 2017, 484, 710–718. [Google Scholar] [CrossRef]
- Wang, F.S.; Wang, C.J.; Chen, Y.J.; Chang, P.R.; Huang, Y.T.; Sun, Y.C.; Huang, H.C.; Yang, Y.J.; Yang, K.D. Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1α and VEGF-A expression in shock wave-stimulated osteoblasts. J. Biol. Chem. 2004, 279, 10331–10337. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, X.; Jiang, Y.; Chu, L.; Hao, H.; Liua, Z.; Verfaillie, C.; Zweier, J.; Gupta, K.; Liu, Z. MAPK/ERK signalling mediates VEGF-induced bone marrow stem cell differentiation into endothelial cell. J. Cell. Mol. Med. 2008, 12, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Wei, D.Y.; Wang, D.; Phimphilai, M.; Krebsbach, P.H.; Franceschi, R.T. In vitro and in vivo synergistic interactions between the Runx2/Cbfa1 transcription factor and bone morphogenetic protein-2 in stimulating osteoblast differentiation. J. Bone Miner. Res. 2003, 18, 705–715. [Google Scholar] [CrossRef]
- Zhang, M.; Xuan, S.H.; Bouxsein, M.L.; von Stechow, D.; Akeno, N.; Faugere, M.C.; Malluche, H.; Zhao, G.S.; Rosen, C.J.; Efstratiadis, A.; et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J. Biol. Chem. 2002, 277, 44005–44012. [Google Scholar] [CrossRef] [PubMed]
- Kopp, H.G.; Avecilla, S.T.; Hooper, A.T.; Shmelkov, S.V.; Ramos, C.A.; Zhang, F.; Rafii, S. Tie2 activation contributes to hemangiogenic regeneration after myelosuppression. Blood 2005, 106, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Albelda, S.M.; Muller, W.A.; Buck, C.A.; Newman, P.J. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): A novel vascular cell-cell adhesion molecule. J. Cell Biol. 1991, 114, 1059–1068. [Google Scholar] [CrossRef]
- Wan, C.; Gilbert, S.R.; Wang, Y.; Cao, X.; Shen, X.; Ramaswamy, G.; Jacobsen, K.A.; Alaql, Z.S.; Eberhardt, A.W.; Gerstenfeld, L.C.; et al. Activation of the hypoxia-inducible factor-1α pathway accelerates bone regeneration. Proc. Natl. Acad. Sci. USA 2008, 105, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Li, B.; Chen, L. Controlled dual delivery of low doses of BMP-2 and VEGF in a silk fibroin–nanohydroxyapatite scaffold for vascularized bone regeneration. J. Mater. Chem. B 2017, 5, 6963–6972. [Google Scholar] [CrossRef]
- Engin, F.; Yao, Z.; Yang, T.; Zhou, G.; Bertin, T.; Jiang, M.M.; Chen, Y.; Wang, L.; Zheng, H.; Sutton, R.E.; et al. Dimorphic effects of Notch signaling in bone homeostasis. Nat. Med. 2008, 14, 299–305. [Google Scholar] [CrossRef]
- Ramasamy, S.K.; Kusumbe, A.P.; Wang, L.; Adams, R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 2014, 507, 376–380. [Google Scholar] [CrossRef]
- Yan, X.X.; Yu, C.Z.; Zhou, X.F.; Tang, J.W.; Zhao, D.Y. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem.-Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef]
- Yan, X.X.; Deng, H.X.; Huang, X.H.; Lu, G.Q.; Qiao, S.Z.; Zhao, D.Y.; Yu, C.Z. Mesoporous bioactive glasses. I. Synthesis and structural characterization. J. Non-Cryst. Solids 2005, 351, 3209–3217. [Google Scholar] [CrossRef]
- Song, W.; Li, X.; Qian, J.; Lv, G.; Yan, Y.; Su, J.; Wei, J. Mesoporous calcium-silicon xerogels with mesopore size and pore volume influence hMSC behaviors by load and sustained release of rhBMP-2. Int. J. Nanomed. 2015, 10, 1715–1726. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Salcedo, S.; García, A.; Vallet-Regí, M. Prevention of bacterial adhesion to zwitterionic biocompatible mesoporous glasses. Acta Biomater. 2017, 57, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bai, Z.; Lin, J.; Jiang, K.; Huang, S.; Zheng, W.; Chen, R.; Xiang, Y.; Wang, X.; Liu, L. 3D printing silk-gelatin-propanediol scaffold with enhanced osteogenesis properties through p-Smad1/5/8 activated Runx2 pathway. J. Biomater. Sci.-Polym. Ed. 2021, 32, 1515–1529. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Gao, J.; Yang, R.; Yuan, C.; Wang, R.; Zou, Q.; Zuo, Y.; Zhu, M.; Li, Y.; Man, Y.; et al. A baicalin-loaded coaxial nanofiber scaffold regulated inflammation and osteoclast differentiation for vascularized bone regeneration. Bioact. Mater. 2022, 8, 559–572. [Google Scholar] [CrossRef]
- Oyane, A.; Kim, H.M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. Part A 2003, 65, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Q.; Chen, W.; Liu, L. FGF adsorbed mesoporous bioactive glass with larger pores in enhancing bone tissue engineering. J. Mater. Sci.-Mater. Med. 2019, 30, 48. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Bai, Z.; Huang, S.; Jiang, K.; Liu, L.; Wang, X. The Effect of Angiogenesis-Based Scaffold of MesoporousBioactive Glass Nanofiber on Osteogenesis. Int. J. Mol. Sci. 2022, 23, 12670. https://doi.org/10.3390/ijms232012670
Zheng W, Bai Z, Huang S, Jiang K, Liu L, Wang X. The Effect of Angiogenesis-Based Scaffold of MesoporousBioactive Glass Nanofiber on Osteogenesis. International Journal of Molecular Sciences. 2022; 23(20):12670. https://doi.org/10.3390/ijms232012670
Chicago/Turabian StyleZheng, Weijia, Zhenzu Bai, Shan Huang, Kai Jiang, Long Liu, and Xiaoyan Wang. 2022. "The Effect of Angiogenesis-Based Scaffold of MesoporousBioactive Glass Nanofiber on Osteogenesis" International Journal of Molecular Sciences 23, no. 20: 12670. https://doi.org/10.3390/ijms232012670
APA StyleZheng, W., Bai, Z., Huang, S., Jiang, K., Liu, L., & Wang, X. (2022). The Effect of Angiogenesis-Based Scaffold of MesoporousBioactive Glass Nanofiber on Osteogenesis. International Journal of Molecular Sciences, 23(20), 12670. https://doi.org/10.3390/ijms232012670





