Abstract
Hidradenitis suppurativa (HS; also designated as acne inversa) is a chronic inflammatory disease characterized by painful skin lesions that occur in the axillary, inguinal, gluteal and perianal areas of the body. These lesions contain recurring deep-seated, inflamed nodules and pus-discharging abscesses and fistulas. Affecting about 1% of the population, this common disease has gained appropriate clinical attention in the last years. Associated with numerous comorbidities including metabolic syndrome, HS is considered a systemic disease that severely impairs the quality of life and shortens life expectancy. Therapeutic options for HS are limited, comprising long-term antibiotic treatment, the surgical removal of affected skin areas, and neutralization of TNF-α, the only approved systemic treatment. Novel treatment options are needed to close the therapeutic gap. HS pathogenesis is increasingly better understood. In fact, neutrophilic granulocytes (neutrophils) seem to be decisive for the development of the purulent destructive skin inflammation in HS. Recent findings suggest a key role of the immune mediators IL-1β, IL-17A and G-CSF in the migration into and activation of neutrophils in the skin. Although phytomedical drugs display potent immunoregulatory properties and have been suggested as complementary therapy in several chronic disorders, their application in HS has not been considered so far. In this review, we describe the IL-1/IL-17/G-CSF axis and evaluate it as potential target for an integrated phytomedical treatment of HS.
1. Hidradenitis Suppurativa—A Debilitating Disease with High Medical Need
Hidradenitis suppurativa (HS; also designated as acne inversa) is a chronic inflammatory skin disease characterized by the recurrent appearance of painful inflamed nodules, and pus-discharging abscesses and tunnels in the intertriginous skin areas [1]. The axillary, inguinal, gluteal and perianal sites are most commonly affected. Usually starting in early adulthood, HS affects both sexes with an overall similar frequency [2,3]. The worldwide prevalence of HS is estimated at about 1% [1]. HS is associated with numerous comorbidities including metabolic syndrome, spondyloarthritis, inflammatory bowel disease as well as non-alcoholic fatty liver disease (NAFLD), and is therefore seen as a systemic disease [4,5,6,7,8,9,10,11]. Importantly, the number of concomitant diseases correlates with the duration from manifestation of first symptoms until HS diagnosis [12]. In Germany, this delay in diagnosis of HS was shown to be ~10 years on average [12].
A closer look at the disease reveals the huge burden HS patients have to carry. With the painful skin lesions secreting malodorous pus in intimate body sites, HS has a large negative impact on the quality of life of those patients [13,14]. In fact, discomfort, an impaired sexual health and body image, anxiety, depression, stigmatization, social exclusion, and passive forms of indirect self-destructiveness are frequently associated with HS [15,16,17,18,19].
From the etiological point of view, genetic factors play a role in about 33% of patients as suggested from a positive family history for HS. In the majority of these patients, the contributing genetic factors are unknown; only in a small proportion of patients they have been elucidated and involve molecules of the Notch signaling pathway [1,20,21]. Moreover, life style factors, such as obesity (due to immunological shift, mechanical friction of skin folds, wetness and microbiome alterations) and in particular smoking habits are suggested to trigger the disease [20,22]. These factors are supposed to induce subclinical inflammation around hair follicles and infundibular hyperkeratosis and acanthosis, leading to follicular plugging, dilatation and rupture of the pilo-sebaceous unit [20]. As a consequence, local immune cells get activated by released components of the pilo-sebaceous microbiome and damaged host cells. Inflammatory cytokines secreted by immune cells then activate tissue cells to drive immune cell infiltration from peripheral blood into the skin [20]. Resulting chronic inflammatory processes lead to destruction of normal skin architecture, with formation of abscesses, fistulas and scarring. Especially neutrophilic granulocytes, by secreting matrix-degrading enzymes and reactive oxygen species, seem to play a role in tissue destruction [23,24,25]. G-CSF, the main regulator of neutrophilic granulocyte survival, was recently shown to be a key cytokine in HS pathogenesis and potential therapeutic target [24].
Despite the high prevalence of HS and severe physical and mental suffering of the patients, the therapeutic options for this disease are still limited [1,26,27]. This is in contrast to other common chronic inflammatory skin diseases, such as psoriasis, for which we have numerous very effective innovative drugs [28]. In fact, HS therapy relies primarily on long-term antibiotic treatment and the surgical excision of affected skin areas. Importantly, these interventions are not associated with long-lasting reduction of the impairment of patients’ quality of life [13]. Furthermore, trapped hair fragments are frequently found in lesioned HS skin, resulting in suggestion of laser epilation as complementary treatment [29]. Moreover, the TNF-α-neutralizing antibody adalimumab is currently the only approved systemic therapy for HS. Since only a part of the patients respond to anti-TNF-α therapy with relevant symptom reduction, there is still an urgent need for novel therapy options [30]. Contraindications to, refusal of certain therapy elements, or adverse effects such as Clostridium difficile infection in context of long-term antibiotic treatment are also relevant factors underlining this need [31].
For several chronic inflammatory diseases, conventional medicine can be complemented by phytomedical drug-based therapy options. For some of them, such as Colitis ulcerosa, Morbus Crohn and early rheumatoid arthritis, phytotherapy is even part of respective S3 guidelines [32]. In contrast, phytomedicals are not considered for complementary HS therapy so far although there is a positive perception of alternative complementary treatment concepts by patients and dermatologists [33,34]. In this review, we describe cytokines that regulate the migration, persistence and activation of neutrophilic granulocytes (neutrophils) in the skin of HS patients and evaluate the potential to influence them by phytomedical drugs.
2. Regulation of Neutrophilic Granulocyte Homeostasis
Neutrophilic granulocytes are the most abundant cell type among leukocytes. They are part of the first-line innate host defense against tissue-invading microbes. These short-lived cells exert their function through reactive oxygen production, the release of molecules from intracellular vesicles (enzymes, proteases, antimicrobial peptides, chemokines, cytokines), phagocytosis, and neutrophil extracellular trap (NET) formation. Neutrophil homeostasis is regulated through balancing granulopoiesis, the retention of produced neutrophils in the bone marrow, and their mobilization and attraction to peripheral tissues [35].
During bone marrow-located granulopoiesis, pluripotent hematopoietic stem cells, via an intermediate myeloid progenitor cell stage, constantly give rise to immature neutrophils, whose retention in or their mobilization from the bone marrow is dependent on a fine-tuned process involving growth factors as well as chemokine–chemokine receptor interactions [35]. Acute and chronic infections as well as chronic mental or physiological stress increase granulopoiesis and the mobilization of neutrophils from the bone marrow [35,36]. The main regulator of neutrophil granulopoiesis and survival, G-CSF, is produced by activated fibroblast and epithelial cells [24]. The cytokines interleukin (IL)-1β and IL-17A are the most potent inducers of G-CSF in dermal fibroblasts and keratinocytes [24]. Additionally, G-CSF is produced upon tissue injury and the recognition of microbial and damaged host cell components by tissue resident immune cells [24]. By upregulating transcription factors that direct the differentiation of myeloid progenitors towards the neutrophil lineage, G-CSF directly impacts the size of the neutrophil reservoir in the bone marrow [35]. Furthermore, G-CSF-mediated downregulation of the bone marrow-homing receptor CXCR4 expression on neutrophils and simultaneous upregulation of the neutrophil-attracting chemokine CXCL1 by endothelial cells provoke neutrophil mobilization and attraction into the blood [35].
The limitation of strengthened granulopoiesis during subsidence of the inflammation is mediated by a negative feedback loop based on the decreased availability of G-CSF-inducing stimuli (e.g., IL-1β/IL-1β-inducing microbial and damage cell components). Moreover, after phagocytosis of apoptotic neutrophils (efferocytosis), monocytes/macrophages and dendritic cells show a reduced IL-23 production [37,38]. As IL-23 plays a key role in the development and maintenance of specific lymphocyte subtypes (Th17/γδ17/Tc17) that produce the G-CSF inducer IL-17 [24,39], the apoptosis of neutrophils may limit G-CSF-dependent granulopoiesis in the resolution phase of an infection. Interestingly, the phagocytosis of apoptotic cells by monocytes/macrophages is strengthened by IL-10 [40], a cytokine whose expression is highly produced in lesioned HS skin [41].
3. Role of Neutrophilic Granulocytes in HS Lesions
In HS lesions, the limited epidermal upregulation of antibacterial proteins enables the propagation of the microbes in the skin [20,41,42]. The persistent presence of bacterial components supports the chronic inflammation through the stimulation of monocytes/macrophages/dendritic cells via their innate immune receptors (i.e., pattern recognition receptors, PRRs) [41,43]. Cytokines, secreted by these cells (e.g., IL-1β, TNF-α) promote the skin infiltration (induction of specific chemokines such as CXCL1, CXCL6, CXCL8 [43] as well as LCN2 [25]), survival and activity (induction of G-CSF [24,43]) of neutrophils.
The expression of G-CSF is strongly upregulated in HS skin lesions compared not only to healthy donor skin, but also to lesioned skin from other chronic, inflammatory skin diseases such as psoriasis and atopic dermatitis [24]. Even though quantification of systemic G-CSF level reveals only a trend of increase in HS patients, a correlation with disease severity expressed by the Sartorius score was found [24]. G-CSF strengthens the expression of several transmembrane receptors in neutrophils that contribute to the prolonged activation of these cells by components of bacteria and the mitochondria of disrupted host cells (e.g., formyl peptide receptor 1 (FPR1), FPR2 and free fatty acid receptor 2 (FFAR2)) [24]. Furthermore, G-CSF upregulates the expression of the decoy receptor TNFRSF10C/TRAIL-R3 and TNFRSF6B, which prevent the effect of TRAIL and TNFSF6, respectively, and protect cells from apoptosis [24]. The most downstream elements of the G-CSF pathway in HS include proteases (e.g., ADAM8, MMP8, MMP9, MMP25) [24]. Thus, extracellular matrix-damaging and -degrading proteins (e.g., MMPs, myeloperoxidase, neutrophil elastase, cathepsins), secreted by neutrophils are assumed to contribute to the substantial tissue destruction observed in HS [20,23]. By supporting the rupture of dilated hair follicles, MMPs might promote abscess and tunnel formation in the chronic stage of HS. G-CSF could further drive those processes as it was shown to provoke an upregulation of ADAM8 and MMP8 in toll-like receptor 4-activated neutrophils [24]. It should also be mentioned that TNF-α is also able to directly activate neutrophils to produce MMP8 and LCN2 [23,25]. LCN2 is a soluble multifunctional glycoprotein. It transports small hydrophobic molecules and is involved in the induction of inflammatory pain. LCN2 also acts as a chemoattractant for neutrophils, promotes adhesion and extravasation of these cells, and may therefore contribute to purulent inflammation in HS [20,44].
Like G-CSF itself, the expression of the known G-CSF inducers IL-1β and IL-17 is also strongly increased in lesioned HS skin [41,43]. These cytokines induce G-CSF in fibroblasts (IL-1β) and keratinocytes (IL-17A) [24,45]. Accordingly, cutaneous IL-1β and IL-17A expression levels clearly correlate with the expression of G-CSF in lesioned HS areas [24]. Additionally, IL-1β and IL-17A are major inducers of neutrophil-attracting chemokines in fibroblasts and keratinocytes, respectively [43,45].
4. IL-1/IL-17/G-CSF Axis as Potential Target of Phytotherapy in HS
The field of phytotherapy comprises several candidates for the modulation of the purulent destructive inflammation characteristic of HS. Targets may include the immune mediators that are involved in the immigration of neutrophils into the skin, the G-CSF inducers, G-CSF itself, or other cytokines activating neutrophils in the skin. Within the wide variety of secondary phytochemicals, polyphenols are substances with high anti-inflammatory potential. Mechanisms underlying their anti-inflammatory effects comprise the inhibition of proinflammatory cytokine production, the interference with cytokine-induced signal transduction pathways, as well as the modulation of lymphocyte lineage development [46].
The transcriptional regulation of G-CSF is known to be mainly dependent on NF-κB signaling [47]. Accordingly, the signal transduction profile of the G-CSF inducers IL-1β and IL-17A includes activation of the NF-κB pathway [48]. IL-1β exerts its effects through binding to a heterodimeric receptor comprising IL-1R1/IL-1RAcp, while IL-17A mediates its biological effects through the heterodimeric IL-17RA/IL17RC receptor complex, which is shared by IL-17F as well as by IL-17A/IL-17F homo-/heterodimers [49,50]. Among phytochemicals, polyphenols derived from Camellia sinensis (epigallocatechine-3-gallate, EGCG), Withania somnifera (withaferin A) and red berries (cyanidin) are potential candidates for the therapeutic modulation of the IL-1β/IL-17A/G-CSF axis (Figure 1).

Figure 1.
Botanical pictures of Camellia sinensis (A), Withania somnifera (B) and Sambucus nigra (C).
The tea plant (Camellia sinensis) is found in tropical and subtropical areas with a history in its agricultural use for tea preparation that spans more than 1500 years. The main polyphenolic constituents of Camellia sinensis are catechins (flavan-3-ols) and their derivatives. Among those, epigallocatechine-3-gallate (EGCG) as well as a whole polyphenol mixture prepared as green tea extract are the most studied phytochemicals of Camellia sinensis. It is assumed that EGCG is a natural ligand of the 67 kDa laminin receptor (67LR) [51]. The winter cherry (Withania somnifera) is a common plant predominantly found in Mediterranean regions, with a long history of its use in ayurvedic medicine. Among the withanolides, the secondary phytochemicals present in the roots of Withania somnifera, withaferin A (steroidal lactone) are the most studied one. Cyanidin as well as its derivatives (e.g., cyanidin-3-glucoside) belong to the group of anthocyanidins and are commonly found in red berries and some fruits, e.g., black elderberries (Sambucus nigra), chokeberries (Aronia melanocarpa), or blackberries (Rubus fruticosus). The authors note that phytochemical substances mediate differential effects on primary and tumor cells [52,53]. This review therefore primarily focuses on the scientific work based on primary cells.
5. IL-1β Pathway Modulators
A potential candidate for the modulation of the IL-1β pathway is the polyphenol EGCG, derived from the tea plant (Camellia sinensis), as well as withaferin A from Withania somnifera (Figure 2).
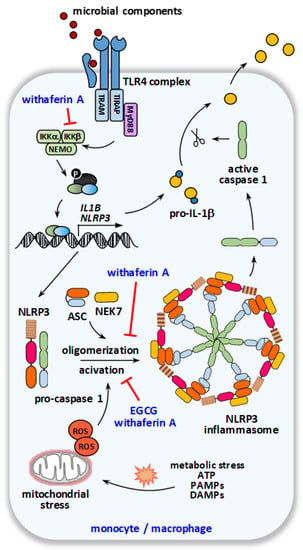
Figure 2.
Schematic overview on the molecular targets of EGCG and withaferin A within the IL-1β pathway. Recognition of microbial components by pattern recognition receptors (priming) on monocytes/macrophages leads to upregulation of NLRP3 inflammasome components and their subsequent oligomerization resulting in NLRP3 inflammasome assembly. As a second signal, e.g., metabolic stress and mitochondrial dysfunction lead to activation of the NLRP3 inflammasome, autoactivation of pro-caspase-1, and thereby enables caspase-1-mediated cleavage of pro-IL-1β to IL-1β as its active form.
As IL-1β is secreted in an inactive form (pro-IL-1β), it has to be activated by the inflammasome to confer its biological activity, a mechanism that enables the fine-tuned regulation of immune activation to prevent excessive inflammation and inflammatory cell death (pyroptosis) [54]. Thereby, the conversion of pro-IL-1β to active IL-1β is initiated by the oligomerization and activation of the NLRP3 (NLR family pyrin domain containing 3) inflammasome, a multi-protein complex consisting of NLRP3, caspase-1 and the adaptor accessory protein ASC (apoptosis-associated speck-like protein containing a CARD) and NEK7 (NIMA-related kinase 7), and subsequent caspase-1 mediated cleavage. Components of the NLRP3 inflammasome have been shown to be upregulated in HS lesions [43,55].
In murine macrophages, EGCG was described to prevent IL-1β induction, possibly by inhibiting NLRP3 activation [56]. A potent NLRP3 inhibitory property of EGCG and the subsequent prevention of caspase-1 activation and IL-1β secretion was also found by Zhang et al. [57]. In line with these data, EGCG limited ex vivo lymphocyte-derived, as well as systemic, IL-1β levels using different in vivo models [58,59]. Moreover, the PPR (AIM2)-dependent IL-1β secretion of keratinocytes in response to a dsDNA analog was suppressed by EGCG in vitro [60].
In the course of scientific investigations on the therapeutic effects and mode of action of withaferin A, this phytochemical has been shown to be an effective inhibitor of NLRP3 activation. In fact, the Helicobacter pylori-induced upregulation of NLRP3 and IL-1β in bone marrow-derived dendritic cells was limited by withaferin A treatment [61]. Xia et al. also reported a dose-dependent decrease of LPS-induced NLRP3 and IL-1β mRNA expression in primary murine macrophages, whereby this effect was diminished in NLRP3-/- macrophages [62]. In THP1 macrophages, NLRP3 activation was counteracted by withaferin A, associated with a dose-dependent suppression of the bacterial component-induced upregulation of IL-1β at the mRNA and protein level [63]. The authors suggested an impaired co-localization of NLRP3 inflammasome elements to be a possible mechanism underlying this withaferin A effect [63]. Withaferin A also prevented NLRP3 activator-induced cleavage of inactive pro-caspase-1 and pro-IL-1β in a dose-dependent manner [61]. Furthermore, in a chronic pancreatitis model, withaferin A was observed to prevent cerulein-induced pancreatic NLRP3 and ASC mRNA upregulation [64]. The inhibitory effect of withaferin A on NLRP3 activation and IL-1β secretion was also confirmed using an ovalbumin-induced airway inflammation model [65]. Moreover, the suppressing effect of withaferin A on hepatic NLRP3 inflammasome activation and systemic IL-1β induction was observed using an in vivo hepatitis model [62].
6. IL-17 Pathway Modulators
Within the field of phytotherapeuticals, potential IL-17A pathway modulators include the polyphenols EGCG from Camellia sinensis and cyanidin from red berries (Figure 3 and Figure 4). Cyanidin was first identified as a potent inhibitor of IL-17A based on a bioinformatic approach, followed by in vitro and in vivo evaluations [66]. Molecular interaction analyses revealed that cyanidin binds to the IL-17A-binding site of its IL-17RA receptor chain [66]. Cyanidin prevented IL-17A-induced chemokine production associated with reduced phosphorylation of IκBα in vitro. Furthermore, IL-17A-mediated skin hyperplasia was attenuated by cyanidin in vivo [66]. Using an in vivo asthma model, cyanidin was also shown to prevent ovalbumin-specific Th17- but not Th2-mediated inflammation and neutrophilia [66]. Additionally, in an experimental autoimmune encephalomyelitis model, cyanidin treatment attenuated the disease activity score in myelin oligodendrocyte glycoprotein-specific Th17, but not Th1 adopted mice [66].
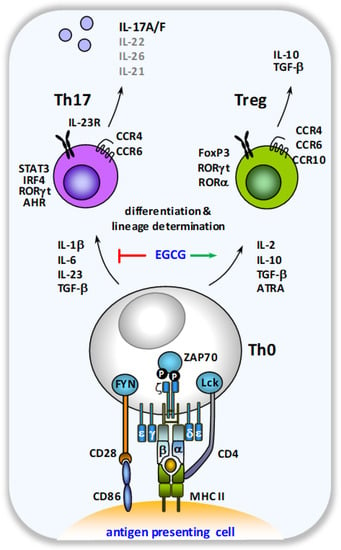
Figure 3.
Schematic overview of the modulation of Th cell lineage development by EGCG. When activated by antigen-presenting cells, naïve CD4+ T cells can differentiate into various effector cell subtypes, depending on the local cytokine milieu. In the presence of an inflammatory cytokine milieu, dominated by the presence of IL-1β, IL-6, IL-23 and TGF-β, Th17 lineage determination is favored with the concomitant production of the Th17 cell signature cytokine IL-17A.
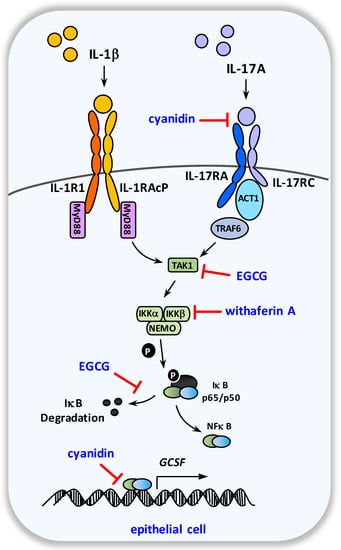
Figure 4.
Schematic overview of the molecular targets of EGCG, withaferin A, and cyanidin within the G-CSF pathway. After binding to and signaling through their specific heterodimeric receptors, the G-CSF-inducers IL-1β and IL-17A provoke NF-κB activation and the subsequent upregulation of G-CSF expression in epithelial cells.
In different preclinical models of neutrophilic airway disease, asthma and COPD, cyanidin-3-glucoside (a cyanidin derivative with similar IL-17A/IL-17R blocking capacity) substantially suppressed neutrophilic inflammation and restored dexamethasone sensitivity [67]. In monocytes and synoviocytes obtained from arthritis rats, the IL-17A-mediated migration and target gene expression was alleviated by cyanidin [68,69]. In a further study using this model, respective mice also showed a normalized IL-17 and IL-10 blood level indicating an influence on the Th17/regulatory T cell balance [70]. However, further studies are needed to validate these findings.
Decreased Th17 cell numbers in favor of enhanced proportions of regulatory T cells, accompanied by reduced lymphocyte IL-17A secretion and disease severity reduction, were also detected after EGCG treatment in murine models of autoimmune encephalomyelitis, arthritis, colitis, and obesity [59,71,72,73,74]. In line with these data, EGCG impeded Th17 lineage development by the modulation of murine-naïve T helper cell differentiation [74,75]. Furthermore, Th17 polarization of murine splenocytes was reduced by EGCG treatment [74]. The underlying mode of action is suggested to involve the EGCG-mediated suppression of mTOR activation and the subsequent abrogation of hypoxia-inducible factor 1 (HIF-1a)-dependent RORγt activation, a molecular checkpoint for Th17/Treg lineage development described earlier [73,74,76,77].
EGCG may also directly interfere with IL-17A target gene expression. The suggested mechanism is the modulation of respective signal transduction pathways. In fact, by inhibiting the IL-17-dependent activation of mitogen-activated protein kinases (p38 and ERK), EGCG decreased IL-17A target gene expression in fibroblasts [78]. However, these data have to be validated in further studies.
7. TNF-α/NF-κB Pathway Modulators
Phytochemicals targeting the NF-κB pathway include the polyphenols EGCG, withaferin A and cyanidin (Figure 4). Inhibition of NF-κB pathway activation by EGCG was shown in various in vitro cell culture models [79,80,81,82]. Analysis on the underlying mechanisms revealed that EGCG targets specific key points within the NF-κB signaling pathway. In Fact, EGCG was reported to prevent the degradation of IRAK and thereby inhibited NF-κB activation in respiratory epithelial cells in vitro [82]. In contrast, Singh et al. did not find prevention of IRAK degradation by EGCG in rheumatoid synovial fibroblasts cells but instead found inhibition of IRAK activity [81]. Furthermore, using synovial fibroblasts, EGCG was found to represent a potent inhibitor of TAK1 kinase, a signaling molecule upstream of NF-κB, activated by preventing its phosphorylation at Thr(184/187) [80,81]. Accordingly, EGCG effectively prevented IL-1β-induced NF-κB p65 nuclear translocation in the study by Fechtner et al. [80]. Beside its ability to interfere with canonical NF-κB (IKKα/β) signaling, in silico analysis further revealed that EGCG might also block the non-canonical NF-κB pathway molecule NF-κB induced kinase (NIK) [83].
Withaferin A was also reported to possess NF-κB-inhibiting activity in various cell types [61,64,84,85,86,87]. Kaileh et al. found that this phytochemical is able to prevent IκBa degradation by inhibiting IKKβ activity in vitro [84]. Subsequently, the work of Heynink et al. further elucidated the mode of action of withaferin A-mediated IKKβ inactivation [88]. This study revealed that withaferin A specifically targets the Cys179 residue of IKKβ, thus inhibiting its catalytic activity [88]. In line with its described NF-κB pathway-modulating property, withaferin A suppressed the spontaneous and bacterial component-induced IL-1β production in immune cells obtained from rheumatoid arthritis patients [87]. Moreover, paw swelling was effectively prevented by withaferin A in vivo, using a zymosan-induced paw inflammation model [84]. In a chronic pancreatitis model, withaferin A suppressed pancreatic neutrophil infiltration and the nuclear translocation of the NF-κB p65 subunit in acinar cells [63]. The therapeutic suitability of Withania somnifera as a potential NF-κB modulator has also been of clinical interest. A respective randomized phase II clinical trial is currently ongoing (clinicalTrials.gov Identifier: NCT05031351; accessed on 1 August 2022).
Cyanidin represents a further phytochemical modulator of the NF-κB pathway. In fact, cyanidin was found to be a very potent small molecule activator of sirtuin-6, a histone H3 deacetylase [89]. Sirtuin-6 itself was shown to interact with NF-κB p65 subunit (RelA) and to deacetylate the histone H3 lysine 9 position (H3K9) at NF-κB target gene promoters, thereby impeding transcription of NF-κB dependent genes [90,91]. Furthermore, cyanidin also inhibited NF-κB signaling by the suppression of IκBα degradation and NF-kB translocation [92,93,94,95]. Accordingly, the attenuation of NF-κB mediated inflammation by cyanidin and cyanidin-3-glucoside in vivo was observed [92,93,94,96].
8. Conclusions
Considering the massive burden HS patients have to carry, including the substantial negative impact on the patients’ personal life, professional life and life expectancy, HS is a huge clinical challenge for dermatology [30].
Complementary therapy options are already an established part in dermatology. However, phytochemicals are not taken into account as complementary approaches so far, though their therapeutic potential could offer an important contribution to an integrated HS management. There are substantial data suggesting the clinical use of phytochemicals derived from Withania somnifera, Camellia sinensis as well as cyanidin (from red berries) for the modulation of the IL-1/IL-17/G-CSF axis in HS. Overall, data from respective preclinical studies on these substances reveal several interesting findings and contribute to our understanding of underlying mechanisms of their action.
Before starting an integrative HS management, a detailed and careful analysis of potential drug interactions, also considering the concomitant medications of the patient, should be made. In general, phytomedical therapy should only be performed under strict medical supervision and monitoring, considering the individual clinical situation of the patients and the evaluation of current data.
Safety and Drug Interactions of Phytomedicals for Integrated HS Therapy
In general, the clinical use of phytochemicals derived from Withania somnifera was found to be tolerable and safe, indicated by pharmacokinetics and safety data obtained from clinical trials [97,98,99,100,101,102,103]. The clinical use of Camellia sinensis-derived phytochemicals was extensively studied in clinical trials and respective pharmacokinetic and safety data are available [104]. Based on these data, the upper safe dosage limits of 338 mg EGCG (in form of an extract) or 704 mg EGCG (consumed as beverage) for the clinical use of these substances are recommended [104]. For cyanidin and its derivatives, no safety concerns are indicated from clinical studies evaluating pharmacokinetics, tolerability and safety so far, whereby these data have to be substantiated in further studies [105,106,107,108].
Among the phytomedical drugs evaluated here, most reservations were raised about the clinical use of catechins derived from Camellia sinensis. This relates partly to their interaction with cytochrome P450-metabolizing/detoxifying enzymes, having the potential to modify the efficacy of cytochrome P450 metabolization of concomitantly given drugs [109,110,111]. Additionally, EGCG and other Camellia sinensis catechins are natural inhibitors as well as substrates of the enzyme catechol-o-methyltransferase (COMT), which is required for the detoxification and metabolization of xenobiotics (i.g. levodopa, isoprenaline, benserazide), catecholamines and catechol estrogens, promoting their excretion and preventing oxidative stress or carcinogenesis [112]. As catechol-containing flavonoids from Camellia sinensis might compete in vivo with those substrates for COMT-mediated metabolization, possible interactions with current medication should be considered, especially for those patients carrying the low-activity COMT genotype [112]. Where appropriate, assessment of the COMPT genotype is therefore suggested before starting green tea extract or ECGC treatment. Furthermore, as liver toxicity has been proposed as a rare adverse reaction, treatment of patients showing hepatic dysfunction with Camellia sinsensis catechins should be avoided as safety precaution.
Special caution should also be made for diabetic patients before staring Camellia sinensis-derived catechins. In fact, these substances should be administered only postprandially, as the achievement of protective effects on glucose metabolism is probably limited to their gastrointestinal effects [113]. In fact, a negative impact on glucose metabolism was reported when EGCG was applied preprandially, but whether this might be a result of inhibited tissue glucose uptake is still under debate [113,114]. However, data on the preprandial effects of tea catechins need to be confirmed in larger studies. Moreover, for EGCG, a histone deacetylase-inhibitory potential has been proposed, but remains to be proven in vivo [115].
For Withania somnifera root extract it is not finally clarified as to whether there is an interaction with cytochrome P450 enzymes or choline esterases [116,117,118,119]. However, precaution regarding respective possible adverse effects or drug interactions with concomitant medication including modulation of drug efficacy is indicated.
Furthermore, it has to be noted that an improvement of thyroid function after therapy with Withania somnifera-derived root extract has been observed in subclinical hypothyroid participants, including a significant reduction in thyroid-stimulating hormone levels (TSH) and a concomitant increase of triiodothyronine (T3) and thyroxine (T4) levels [102]. A constant monitoring of thyroid parameters is thereby recommended during the Withania somnifera-based treatment of hypothyroid patients with concomitant L-thyroxin, as well as of hyperthyroid patients. On the other hand, subclinical hypothyroid patients might benefit from the described effect on thyroid function. Of note, using an obesity mouse model, withaferin A was shown to represent a potent leptin sensitizer [120]. Therefore, changes in lipid/glucose parameters should be considered as possible effects of Withania somnifera preparations and carefully monitored under treatment.
In summary, the evaluated data indicate that phytomedical drugs derived from Withania somnifera, Camellia sinensis and red berries may represent potential modulators of cytokines that play a decisive role in promoting the granulocyte-associated pathogenetic destructive inflammation of HS skin. Respective clinical trials are now needed to evaluate the potential and suitability of these substances as an element of an integrated therapeutic strategy for HS. Based on the clinical trial by Czank et al. and the so far reported safety profile, especially cyanidin and its derivatives might be an appropriate candidate for setting up a first clinical pilot trial in this regard [105].
Author Contributions
Conceptualization, K.W. (Katrin Witte), R.S., E.W.-H. and K.W. (Kerstin Wolk); writing—original draft preparation, K.W. (Katrin Witte); writing—review and editing, K.W. (Katrin Witte), R.S., K.G. and K.W. (Kerstin Wolk); visualization, K.W. (Katrin Witte), E.W.-H., K.W. (Kerstin Wolk). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the financial support from the Open Access Publication Fund of Charité—Universitätsmedizin Berlin and the German Research Foundation (DFG). We thank Yakov Oskanov, Lubomír Jílek and Thalabhula for botanical pictures that were obtained via 123RF.com (accessed on 8 August 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sabat, R.; Jemec, G.B.E.; Matusiak, L.; Kimball, A.B.; Prens, E.; Wolk, K. Hidradenitis suppurativa. Nat. Rev. Dis. Primers 2020, 6, 18. [Google Scholar] [CrossRef]
- Liang, Y.T.; Yeh, C.J.; Huang, J.Y.; Wei, J.C. Epidemiology of hidradenitis suppurativa in Taiwan: A 14-year nationwide population-based study. J. Dermatol. 2021, 48, 613–619. [Google Scholar] [CrossRef]
- Schneider-Burrus, S.; Lux, G.; van der Linde, K.; Barbus, S.; Huss-Marp, J.; Tsaousi, A.; Wasem, J.; Wolff, B.; Sabat, R. Hidradenitis suppurativa—Prevalence analyses of German statutory health insurance data. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e32–e35. [Google Scholar] [CrossRef]
- Damiani, G.; Leone, S.; Fajgenbaum, K.; Bragazzi, N.L.; Pacifico, A.; Conic, R.R.; Pigatto, P.D.; Maiorana, C.; Poli, P.; Berti, E.; et al. Nonalcoholic fatty liver disease prevalence in an Italian cohort of patients with hidradenitis suppurativa: A multi-center retrospective analysis. World J. Hepatol. 2019, 11, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Duran-Vian, C.; Arias-Loste, M.T.; Hernandez, J.L.; Fernandez, V.; Gonzalez, M.; Iruzubieta, P.; Rasines, L.; Gonzalez-Vela, C.; Vaque, J.P.; Blanco, R.; et al. High prevalence of non-alcoholic fatty liver disease among hidradenitis suppurativa patients independent of classic metabolic risk factors. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2131–2136. [Google Scholar] [CrossRef]
- Gonzalez-Villanueva, I.; DeGracia, C.; Planells, M.; Poveda, I.; Alvarez, P.; Schneller-Pavalescu, L.; Betlloch, I.; Jemec, G.B.E.; Ramos, J.M.; Pascual, J.C. Hidradenitis Suppurativa is Associated with Non-alcoholic Fatty Liver Disease: A Cross-sectional Study. Acta Derm. Venereol. 2020, 100, adv00239. [Google Scholar] [CrossRef] [PubMed]
- Almuhanna, N.; Finstad, A.; Alhusayen, R. Association between Hidradenitis Suppurativa and Inflammatory Arthritis: A Systematic Review and Meta-Analysis. Dermatology 2021, 237, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Sabat, R.; Chanwangpong, A.; Schneider-Burrus, S.; Metternich, D.; Kokolakis, G.; Kurek, A.; Philipp, S.; Uribe, D.; Wolk, K.; Sterry, W. Increased prevalence of metabolic syndrome in patients with acne inversa. PLoS ONE 2012, 7, e31810. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, M.C.; Kirchgesner, J.; Wyss, R.; Jin, Y.; York, C.; Merola, J.F.; Mostaghimi, A.; Silverberg, J.I.; Schneeweiss, S.; Glynn, R.J. Occurrence of inflammatory bowel disease in patients with chronic inflammatory skin diseases: A cohort study. Br. J. Dermatol. 2022. [Google Scholar] [CrossRef]
- Van der Zee, H.H.; van der Woude, C.J.; Florencia, E.F.; Prens, E.P. Hidradenitis suppurativa and inflammatory bowel disease: Are they associated? Results of a pilot study. Br. J. Dermatol. 2010, 162, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Burrus, S.; Witte-Haendel, E.; Christou, D.; Rigoni, B.; Sabat, R.; Diederichs, G. High Prevalence of Back Pain and Axial Spondyloarthropathy in Patients with Hidradenitis Suppurativa. Dermatology 2016, 232, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Kokolakis, G.; Wolk, K.; Schneider-Burrus, S.; Kalus, S.; Barbus, S.; Gomis-Kleindienst, S.; Sabat, R. Delayed Diagnosis of Hidradenitis Suppurativa and Its Effect on Patients and Healthcare System. Dermatology 2020, 236, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Burrus, S.; Tsaousi, A.; Barbus, S.; Huss-Marp, J.; Witte, K.; Wolk, K.; Fritz, B.; Sabat, R. Features Associated with Quality of Life Impairment in Hidradenitis Suppurativa Patients. Front. Med. 2021, 8, 676241. [Google Scholar] [CrossRef]
- Krajewski, P.K.; Matusiak, L.; von Stebut, E.; Schultheis, M.; Kirschner, U.; Nikolakis, G.; Szepietowski, J.C. Quality-of-Life Impairment among Patients with Hidradenitis Suppurativa: A Cross-Sectional Study of 1795 Patients. Life 2021, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Glowaczewska, A.; Reszke, R.; Szepietowski, J.C.; Matusiak, L. Indirect Self-Destructiveness in Hidradenitis Suppurativa Patients. J. Clin. Med. 2021, 10, 4194. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.; Lancaster, N.; McPhee, M.; Bundy, C.; Ingram, J.R.; Leighton, P.; Henaghan-Sykes, K.; Thomas, K.S. Thematic synthesis of the experiences of people with hidradenitis suppurativa: A systematic review. Br. J. Dermatol. 2021, 185, 921–934. [Google Scholar] [CrossRef]
- Kurek, A.; Johanne Peters, E.M.; Sabat, R.; Sterry, W.; Schneider-Burrus, S. Depression is a frequent co-morbidity in patients with acne inversa. J. Dtsch. Dermatol. Ges. 2013, 11, 743–750. [Google Scholar] [CrossRef]
- Kurek, A.; Peters, E.M.; Chanwangpong, A.; Sabat, R.; Sterry, W.; Schneider-Burrus, S. Profound disturbances of sexual health in patients with acne inversa. J. Am. Acad. Dermatol. 2012, 67, 422–428. [Google Scholar] [CrossRef]
- Schneider-Burrus, S.; Jost, A.; Peters, E.M.J.; Witte-Haendel, E.; Sterry, W.; Sabat, R. Association of Hidradenitis Suppurativa with Body Image. JAMA Dermatol. 2018, 154, 447–451. [Google Scholar] [CrossRef]
- Wolk, K.; Join-Lambert, O.; Sabat, R. Aetiology and pathogenesis of hidradenitis suppurativa. Br. J. Dermatol. 2020, 183, 999–1010. [Google Scholar] [CrossRef]
- Gratton, R.; Tricarico, P.M.; Moltrasio, C.; Lima Estevao de Oliveira, A.S.; Brandao, L.; Marzano, A.V.; Zupin, L.; Crovella, S. Pleiotropic Role of Notch Signaling in Human Skin Diseases. Int. J. Mol. Sci. 2020, 21, 4214. [Google Scholar] [CrossRef] [PubMed]
- Hana, A.; Booken, D.; Henrich, C.; Gratchev, A.; Maas-Szabowski, N.; Goerdt, S.; Kurzen, H. Functional significance of non-neuronal acetylcholine in skin epithelia. Life Sci. 2007, 80, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Tsaousi, A.; Witte, E.; Witte, K.; Rowert-Huber, H.J.; Volk, H.D.; Sterry, W.; Wolk, K.; Schneider-Burrus, S.; Sabat, R. MMP8 Is Increased in Lesions and Blood of Acne Inversa Patients: A Potential Link to Skin Destruction and Metabolic Alterations. Mediat. Inflamm 2016, 2016, 4097574. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Brembach, T.C.; Simaite, D.; Bartnik, E.; Cucinotta, S.; Pokrywka, A.; Irmer, M.L.; Triebus, J.; Witte-Handel, E.; Salinas, G.; et al. Activity and components of the granulocyte colony-stimulating factor pathway in hidradenitis suppurativa. Br. J. Dermatol. 2021, 185, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Wenzel, J.; Tsaousi, A.; Witte-Handel, E.; Babel, N.; Zelenak, C.; Volk, H.D.; Sterry, W.; Schneider-Burrus, S.; Sabat, R. Lipocalin-2 is expressed by activated granulocytes and keratinocytes in affected skin and reflects disease activity in acne inversa/hidradenitis suppurativa. Br. J. Dermatol. 2017, 177, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Burrus, S.; Arpa, E.; Kors, C.; Stavermann, T.; Sabat, R.; Kokolakis, G. Drug therapy of acne inversa. Hautarzt 2018, 69, 58–63. [Google Scholar] [CrossRef]
- Frew, J.W.; Marzano, A.V.; Wolk, K.; Join-Lambert, O.; Alavi, A.; Lowes, M.A.; Piguet, V. A Systematic Review of Promising Therapeutic Targets in Hidradenitis Suppurativa: A Critical Evaluation of Mechanistic and Clinical Relevance. J. Investig. Dermatol. 2021, 141, 316–324.e312. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Balato, A.; Enerback, C.; Sabat, R. Therapeutics targeting the, I.L-23 and, I.L-17 pathway in psoriasis. Lancet 2021, 397, 754–766. [Google Scholar] [CrossRef]
- Nazzaro, G.; Zerboni, R.; Passoni, E.; Barbareschi, M.; Marzano, A.V.; Muratori, S.; Veraldi, S. High-frequency ultrasound in hidradenitis suppurativa as rationale for permanent hair laser removal. Skin Res. Technol. 2019, 25, 587–588. [Google Scholar] [CrossRef]
- Ujiie, H.; Rosmarin, D.; Schon, M.P.; Stander, S.; Boch, K.; Metz, M.; Maurer, M.; Thaci, D.; Schmidt, E.; Cole, C.; et al. Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases. Front. Med. 2022, 9, 875492. [Google Scholar] [CrossRef]
- Sanghvi, A.P.; Miles, J.A.; Sayed, C. Clostridium difficile infection risk in hidradenitis suppurativa patients. Br. J. Dermatol. 2022, 87, 406–407. [Google Scholar] [CrossRef]
- Klose, P.; Kraft, K.; Cramer, H.; Lauche, R.; Dobos, G.; Langhorst, J. Phytotherapy in the German Medical AWMF S3 guidelines—A systematic overview. Komplementmed 2014, 21, 388–400. [Google Scholar]
- Price, K.N.; Collier, E.K.; Grogan, T.; Fernandez, J.M.; Alhusayen, R.; Alavi, A.; Hamzavi, I.H.; Lowes, M.A.; Porter, M.J.; Hsiao, J.L.; et al. Physician perspectives on complementary and alternative medicine in hidradenitis suppurativa. Dermatol. Ther. 2021, 34, e14851. [Google Scholar] [CrossRef] [PubMed]
- Price, K.N.; Thompson, A.M.; Rizvi, O.; Hendricks, A.J.; Alavi, A.; Hsiao, J.L.; Shi, V.Y. Complementary and Alternative Medicine Use in Patients With Hidradenitis Suppurativa. JAMA Dermatol. 2020, 156, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Steffens, S.; Hidalgo, A.; Weber, C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 2017, 17, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Heidt, T.; Sager, H.B.; Courties, G.; Dutta, P.; Iwamoto, Y.; Zaltsman, A.; von Zur Muhlen, C.; Bode, C.; Fricchione, G.L.; Denninger, J.; et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 2014, 20, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.A.; Huo, Y.; Burcin, T.L.; Morris, M.A.; Olson, T.S.; Ley, K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via, IL-23 and, IL-17. Immunity 2005, 22, 285–294. [Google Scholar] [CrossRef]
- Von Vietinghoff, S.; Ley, K. Homeostatic regulation of blood neutrophil counts. J. Immunol. 2008, 181, 5183–5188. [Google Scholar] [CrossRef]
- Sabat, R.; Wolk, K.; Loyal, L.; Docke, W.D.; Ghoreschi, K. T cell pathology in skin inflammation. Semin. Immunopathol. 2019, 41, 359–377. [Google Scholar] [CrossRef]
- Lingnau, M.; Hoflich, C.; Volk, H.D.; Sabat, R.; Docke, W.D. Interleukin-10 enhances the CD14-dependent phagocytosis of bacteria and apoptotic cells by human monocytes. Hum. Immunol. 2007, 68, 730–738. [Google Scholar] [CrossRef]
- Wolk, K.; Warszawska, K.; Hoeflich, C.; Witte, E.; Schneider-Burrus, S.; Witte, K.; Kunz, S.; Buss, A.; Roewert, H.J.; Krause, M.; et al. Deficiency of, IL-22 contributes to a chronic inflammatory disease: Pathogenetic mechanisms in acne inversa. J. Immunol. 2011, 186, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Barmatz, S.; Fisch-Gilad, S.; Hackett, A.; Barak Levitt, J.; Dalal, A.; Taieb, Y.; Kremer, N.; Levi, A.; Pavlovsky, L.; Hodak, E.; et al. The Bacteriology of Skin Lesions in Patients with Hidradenitis Suppurativa Is Associated with Previous Antibiotic Treatment in the Community Setting: A Referral Center Experience. Dermatology 2022, 238, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Witte-Handel, E.; Wolk, K.; Tsaousi, A.; Irmer, M.L.; Mossner, R.; Shomroni, O.; Lingner, T.; Witte, K.; Kunkel, D.; Salinas, G.; et al. The, IL-1 Pathway Is Hyperactive in Hidradenitis Suppurativa and Contributes to Skin Infiltration and Destruction. J. Investig. Dermatol. 2019, 139, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Jeon, S.; Jin, M.; Ock, J.; Kim, J.H.; Lee, W.H.; Suk, K. The pivotal role played by lipocalin-2 in chronic inflammatory pain. Exp. Neurol. 2014, 254, 41–53. [Google Scholar] [CrossRef]
- Witte, E.; Kokolakis, G.; Witte, K.; Philipp, S.; Doecke, W.D.; Babel, N.; Wittig, B.M.; Warszawska, K.; Kurek, A.; Erdmann-Keding, M.; et al. IL-19 is a component of the pathogenetic, IL-23/IL-17 cascade in psoriasis. J. Investig. Dermatol. 2014, 134, 2757–2767. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.F.; Coles, L.S.; Fielke, R.K.; Goodall, G.J.; Lagnado, C.A.; Vadas, M.A. Three essential promoter elements mediate tumour necrosis factor and interleukin-1 activation of the granulocyte-colony stimulating factor gene. Growth Factors 1992, 7, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal. Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the, IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The, IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Kumazoe, M.; Sugihara, K.; Tsukamoto, S.; Huang, Y.; Tsurudome, Y.; Suzuki, T.; Suemasu, Y.; Ueda, N.; Yamashita, S.; Kim, Y.; et al. 67-kDa laminin receptor increases cGMP to induce cancer-selective apoptosis. J. Clin. Investig. 2013, 123, 787–799. [Google Scholar] [CrossRef]
- Leon-Gonzalez, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef]
- Stevens, J.F.; Revel, J.S.; Maier, C.S. Mitochondria-Centric Review of Polyphenol Bioactivity in Cancer Models. Antioxid. Redox Signal. 2018, 29, 1589–1611. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Lima, A.L.; Karl, I.; Giner, T.; Poppe, H.; Schmidt, M.; Presser, D.; Goebeler, M.; Bauer, B. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br. J. Dermatol. 2016, 174, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Yang, G.; Park, Y.B.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Epigallocatechin-3-Gallate Prevents Acute Gout by Suppressing NLRP3 Inflammasome Activation and Mitochondrial, DNA Synthesis. Molecules 2019, 24, 2138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, X.; Hu, X.; Xu, Q.; Zhang, Y.; Liu, H.; Diao, Y.; Zhang, X.; Li, L.; Yu, J.; et al. Epigallocatechin-3-gallate prevents inflammation and diabetes -Induced glucose tolerance through inhibition of NLRP3 inflammasome activation. Int. Immunopharmacol. 2021, 93, 107412. [Google Scholar] [CrossRef]
- Othman, A.I.; El-Sawi, M.R.; El-Missiry, M.A.; Abukhalil, M.H. Epigallocatechin-3-gallate protects against diabetic cardiomyopathy through modulating the cardiometabolic risk factors, oxidative stress, inflammation, cell death and fibrosis in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 2017, 94, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ren, Z.; Xu, Y.; Xiao, S.; Meydani, S.N.; Wu, D. Epigallocatechin-3-gallate ameliorates experimental autoimmune encephalomyelitis by altering balance among CD4+ T-cell subsets. Am. J. Pathol. 2012, 180, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.; Seo, G.; Lee, J.Y.; Chae, G.T.; Lee, S.B. Epigallocatechin-3-gallate attenuates the, A.I.; M2-induced secretion of IL-1beta in human epidermal keratinocytes. Biochem. Biophys. Res. Commun. 2015, 467, 723–729. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, J.Y.; Kang, M.J.; Jeong, Y.J.; Choi, J.A.; Oh, S.M.; Lee, K.B.; Park, J.H. Withaferin A Inhibits Helicobacter pylori-induced Production of IL-1beta in Dendritic Cells by Regulating NF-kappaB and NLRP3 Inflammasome Activation. Immune Netw. 2015, 15, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, P.; Yan, N.; Gonzalez, F.J.; Yan, T. Withaferin A alleviates fulminant hepatitis by targeting macrophage and NLRP3. Cell Death Dis. 2021, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Yoon, H.; Cohen, M.S.; Nagarkatti, P.; Nagarkatti, M.; Karan, D. Withaferin A Associated Differential Regulation of Inflammatory Cytokines. Front. Immunol. 2018, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Kanak, M.A.; Shahbazov, R.; Yoshimatsu, G.; Levy, M.F.; Lawrence, M.C.; Naziruddin, B. A small molecule inhibitor of NFkappaB blocks ER stress and the NLRP3 inflammasome and prevents progression of pancreatitis. J. Gastroenterol. 2017, 52, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.M.; Gao, Z.W.; Xie, S.X.; Han, X.; Sun, Q.S. Withaferin A attenuates ovalbumin induced airway inflammation. Front. Biosci. Landmark Ed. 2019, 24, 576–596. [Google Scholar]
- Liu, C.; Zhu, L.; Fukuda, K.; Ouyang, S.; Chen, X.; Wang, C.; Zhang, C.J.; Martin, B.; Gu, C.; Qin, L.; et al. The flavonoid cyanidin blocks binding of the cytokine interleukin-17A to the IL-17RA subunit to alleviate inflammation in vivo. Sci. Signal. 2017, 10, eaaf8823. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Liu, C.; Xiao, J.; Chen, X.; Lui, A.C.; Li, X. Targeting IL-17A/glucocorticoid synergy to CSF3 expression in neutrophilic airway diseases. JCI Insight 2020, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Samarpita, S.; Ganesan, R.; Rasool, M. Cyanidin prevents the hyperproliferative potential of fibroblast-like synoviocytes and disease progression via targeting IL-17A cytokine signalling in rheumatoid arthritis. Toxicol. Appl. Pharmacol. 2020, 391, 114917. [Google Scholar] [CrossRef] [PubMed]
- Samarpita, S.; Rasool, M. Cyanidin attenuates IL-17A cytokine signaling mediated monocyte migration and differentiation into mature osteoclasts in rheumatoid arthritis. Cytokine 2021, 142, 155502. [Google Scholar] [CrossRef]
- Samarpita, S.; Rasool, M. Cyanidin restores Th17/Treg balance and inhibits T follicular helper cell differentiation via modulation of ROCK2 signaling in an experimental model of rheumatoid arthritis. Int. Immunopharmacol. 2021, 101, 108359. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.K.; Yoon, B.Y.; Jhun, J.Y.; Oh, H.J.; Kim, E.K.; Min, J.K.; Cho, M.L. Epigallocatechin-3-gallate ameliorates both obesity and autoinflammatory arthritis aggravated by obesity by altering the balance among CD4+ T-cell subsets. Immunol. Lett. 2014, 157, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jung, Y.O.; Ryu, J.G.; Oh, H.J.; Son, H.J.; Lee, S.H.; Kwon, J.E.; Kim, E.K.; Park, M.K.; Park, S.H.; et al. Epigallocatechin-3-gallate ameliorates autoimmune arthritis by reciprocal regulation of T helper-17 regulatory T cells and inhibition of osteoclastogenesis by inhibiting STAT3 signaling. J. Leukoc. Biol. 2016, 100, 559–568. [Google Scholar] [CrossRef]
- Xu, Z.; Wei, C.; Zhang, R.U.; Yao, J.; Zhang, D.; Wang, L. Epigallocatechin-3-gallate-induced inhibition of interleukin-6 release and adjustment of the regulatory T/T helper 17 cell balance in the treatment of colitis in mice. Exp. Ther. Med. 2015, 10, 2231–2238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, E.J.; Lee, J.; Lee, S.Y.; Kim, E.K.; Moon, Y.M.; Jung, Y.O.; Park, S.H.; Cho, M.L. EGCG attenuates autoimmune arthritis by inhibition of STAT3 and HIF-1alpha with Th17/Treg control. PLoS ONE 2014, 9, e86062. [Google Scholar]
- Wang, J.; Pae, M.; Meydani, S.N.; Wu, D. Green tea epigallocatechin-3-gallate modulates differentiation of naive CD4+ T cells into specific lineage effector cells. J. Mol. Med. Berl. 2013, 91, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Dang, E.V.; Barbi, J.; Yang, H.Y.; Jinasena, D.; Yu, H.; Zheng, Y.; Bordman, Z.; Fu, J.; Kim, Y.; Yen, H.R.; et al. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell 2011, 146, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.Z.; Wang, R.; Huang, G.; Vogel, P.; Neale, G.; Green, D.R.; Chi, H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 2011, 208, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, Y.; Hosokawa, I.; Ozaki, K.; Nakanishi, T.; Nakae, H.; Matsuo, T. Catechins inhibit CCL20 production in IL-17A-stimulated human gingival fibroblasts. Cell. Physiol. Biochem. 2009, 24, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Haqqi, T.M. Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis Res. Ther. 2011, 13, R93. [Google Scholar] [CrossRef] [PubMed]
- Fechtner, S.; Singh, A.; Chourasia, M.; Ahmed, S. Molecular insights into the differences in anti-inflammatory activities of green tea catechins on IL-1beta signaling in rheumatoid arthritis synovial fibroblasts. Toxicol. Appl. Pharmacol. 2017, 329, 112–120. [Google Scholar] [CrossRef]
- Singh, A.K.; Umar, S.; Riegsecker, S.; Chourasia, M.; Ahmed, S. Regulation of Transforming Growth Factor beta-Activated Kinase Activation by Epigallocatechin-3-Gallate in Rheumatoid Arthritis Synovial Fibroblasts: Suppression of K63 -Linked Autoubiquitination of Tumor Necrosis Factor Receptor-Associated Factor 6. Arthritis Rheumatol. 2016, 68, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.S.; Catravas, J.D.; Odoms, K.; Denenberg, A.; Malhotra, V.; Wong, H.R. Epigallocatechin-3-gallate, a green tea-derived polyphenol, inhibits IL-1 beta-dependent proinflammatory signal transduction in cultured respiratory epithelial cells. J. Nutr. 2004, 134, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Varthya, S.B.; Sarma, P.; Bhatia, A.; Shekhar, N.; Prajapat, M.; Kaur, H.; Thangaraju, P.; Kumar, S.; Singh, R.; Siingh, A.; et al. Efficacy of green tea, its polyphenols and nanoformulation in experimental colitis and the role of non-canonical and canonical nuclear factor kappa beta NF-kB pathway: A preclinical in-vivo and in-silico exploratory study. J. Biomol. Struct. Dyn. 2021, 39, 5314–5326. [Google Scholar] [CrossRef] [PubMed]
- Kaileh, M.; Vanden Berghe, W.; Heyerick, A.; Horion, J.; Piette, J.; Libert, C.; De Keukeleire, D.; Essawi, T.; Haegeman, G. Withaferin a strongly elicits IkappaB kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity. J. Biol. Chem. 2007, 282, 4253–4264. [Google Scholar] [CrossRef]
- Maitra, R.; Porter, M.A.; Huang, S.; Gilmour, B.P. Inhibition of NFkappaB by the natural product Withaferin A in cellular models of Cystic Fibrosis inflammation. J. Inflamm. 2009, 6, 15. [Google Scholar] [CrossRef]
- Oh, J.H.; Lee, T.J.; Park, J.W.; Kwon, T.K. Withaferin A inhibits iNOS expression and nitric oxide production by Akt inactivation and down-regulating LPS-induced activity of NF-kappaB in RAW 264.7 cells. Eur. J. Pharmacol. 2008, 599, 11–17. [Google Scholar] [CrossRef]
- Singh, D.; Aggarwal, A.; Maurya, R.; Naik, S. Withania somnifera inhibits NF-kappaB and AP-1 transcription factors in human peripheral blood and synovial fluid mononuclear cells. Phytother. Res. 2007, 21, 905–913. [Google Scholar] [CrossRef]
- Heyninck, K.; Lahtela-Kakkonen, M.; Van der Veken, P.; Haegeman, G.; Vanden Berghe, W. Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKbeta. Biochem. Pharmacol. 2014, 91, 501–509. [Google Scholar] [CrossRef]
- Rahnasto-Rilla, M.; Tyni, J.; Huovinen, M.; Jarho, E.; Kulikowicz, T.; Ravichandran, S.; Ferrucci, L.; Lahtela-Kakkonen, M.; Moaddel, R. Natural polyphenols as sirtuin 6 modulators. Sci. Rep. 2018, 8, 4163. [Google Scholar] [CrossRef]
- Kawahara, T.L.; Michishita, E.; Adler, A.S.; Damian, M.; Berber, E.; Lin, M.; McCord, R.A.; Ongaigui, K.C.; Boxer, L.D.; Chang, H.Y.; et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 2009, 136, 62–74. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Z.; Mu, W.; Li, L.; Liang, Y.; Lu, M.; Wang, Z.; Qiu, Y.; Wang, Z. Calorie restriction-induced SIRT6 activation delays aging by suppressing NF-kappaB signaling. Cell. Cycle 2016, 15, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.M.; Li, Y.; Liu, X.Y.; Zhu, W.W.; Ren, X.; Kong, G.Q.; Huang, X.; Wang, L.P.; Luo, L.Q.; Wang, X.Z. Cyanidin-3-O-Glucoside Ameliorates Lipopolysaccharide-Induced Injury Both In Vivo and In Vitro Suppression of NF-kappaB and MAPK Pathways. Inflammation 2015, 38, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Son, Y.O.; Wang, X.; Divya, S.P.; Joseph, B.; Hitron, J.A.; Wang, L.; Kim, D.; Yin, Y.; Roy, R.V.; et al. Cyanidin-3-glucoside inhibits UVB-induced oxidative damage and inflammation by regulating MAP kinase and NF-kappaB signaling pathways in SKH-1 hairless mice skin. Toxicol. Appl. Pharmacol. 2014, 280, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, L. Cyanidin-3-glucoside inhibits inflammatory activities in human fibroblast-like synoviocytes and in mice with collagen-induced arthritis. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1038–1045. [Google Scholar] [CrossRef]
- Thummayot, S.; Tocharus, C.; Jumnongprakhon, P.; Suksamrarn, A.; Tocharus, J. Cyanidin attenuates Abeta25-35-induced neuroinflammation by suppressing NF-kappaB activity downstream of TLR4/NOX4 in human neuroblastoma cells. Acta Pharmacol. Sin. 2018, 39, 1439–1452. [Google Scholar] [CrossRef]
- Yan, X.; Wu, L.; Li, B.; Meng, X.; Dai, H.; Zheng, Y.; Fu, J. Cyanidin-3-O-glucoside attenuates acute lung injury in sepsis rats. J. Surg. Res. 2015, 199, 592–600. [Google Scholar] [CrossRef]
- Ambiye, V.R.; Langade, D.; Dongre, S.; Aptikar, P.; Kulkarni, M.; Dongre, A. Clinical Evaluation of the Spermatogenic Activity of the Root Extract of Ashwagandha Withania somnifera in Oligospermic Males: A Pilot Study. Evid.-Based Complement. Altern. Med. 2013, 2013, 571420. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kapoor, J.; Anishetty, S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J. Psychol. Med. 2012, 34, 255–262. [Google Scholar] [CrossRef]
- Choudhary, D.; Bhattacharyya, S.; Joshi, K. Body Weight Management in Adults Under Chronic Stress Through Treatment With Ashwagandha Root Extract: A Double-Blind, Randomized, Placebo-Controlled Trial. J Evid.-Based Complement. Altern. Med. 2017, 22, 96–106. [Google Scholar] [CrossRef]
- Durg, S.; Bavage, S.; Shivaram, S.B. Withania somnifera Indian ginseng in diabetes mellitus: A systematic review and meta-analysis of scientific evidence from experimental research to clinical application. Phytother. Res. 2020, 34, 1041–1059. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Shukla, K.K.; Ahmad, M.K.; Rajender, S.; Shankhwar, S.N.; Singh, V.; Dalela, D. Withania somnifera Improves Semen Quality in Stress-Related Male Fertility. Evid.-Based Complement. Alternat. Med. 2009, 2011, 576962. [Google Scholar]
- Sharma, A.K.; Basu, I.; Singh, S. Efficacy and Safety of Ashwagandha Root Extract in Subclinical Hypothyroid Patients: A Double-Blind, Randomized Placebo-Controlled Trial. J. Altern. Complement. Med. 2018, 24, 243–248. [Google Scholar] [CrossRef]
- Verma, N.; Gupta, S.K.; Tiwari, S.; Mishra, A.K. Safety of Ashwagandha Root Extract: A Randomized, Placebo-Controlled, study in Healthy Volunteers. Complement. Ther. Med. 2021, 57, 102642. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef] [PubMed]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef]
- Jeon, S.; Han, S.; Lee, J.; Hong, T.; Yim, D.S. The safety and pharmacokinetics of cyanidin-3-glucoside after 2-week administration of black bean seed coat extract in healthy subjects. Korean J. Physiol. Pharmacol. 2012, 16, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Stoner, G.D.; Sardo, C.; Apseloff, G.; Mullet, D.; Wargo, W.; Pound, V.; Singh, A.; Sanders, J.; Aziz, R.; Casto, B.; et al. Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries daily for 7 days. J. Clin. Pharmacol. 2005, 45, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Darweesh, R.S.; El-Elimat, T.; Zayed, A.; Khamis, T.N.; Babaresh, W.M.; Arafat, T.; Al Sharie, A.H. The effect of grape seed and green tea extracts on the pharmacokinetics of imatinib and its main metabolite, N-desmethyl imatinib, in rats. BMC Pharmacol. Toxicol. 2020, 21, 77. [Google Scholar] [CrossRef]
- Mooiman, K.D.; Maas-Bakker, R.F.; Hendrikx, J.J.; Bank, P.C.; Rosing, H.; Beijnen, J.H.; Schellens, J.H.; Meijerman, I. The effect of complementary and alternative medicines on CYP3A4-mediated metabolism of three different substrates: 7-benzyloxy-4-trifluoromethyl-coumarin, midazolam and docetaxel. J. Pharm. Pharmacol. 2014, 66, 865–874. [Google Scholar] [CrossRef]
- Yang, C.S.; Pan, E. The effects of green tea polyphenols on drug metabolism. Expert Opin. Drug. Metab. Toxicol. 2012, 8, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. The Val158Met polymorphism in COMT gene and cancer risk: Role of endogenous and exogenous catechols. Drug. Metab. Rev. 2017, 49, 56–83. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jin, J.Y.; Baek, W.K.; Park, S.H.; Sung, H.Y.; Kim, Y.K.; Lee, J.; Song, D.K. Ambivalent role of gallated catechins in glucose tolerance in humans: A novel insight into non-absorbable gallated catechin-derived inhibitors of glucose absorption. J. Physiol. Pharmacol. 2009, 60, 101–109. [Google Scholar]
- Xiao, X.; Erukainure, O.L.; Sanni, O.; Koorbanally, N.A.; Islam, M.S. Phytochemical properties of black tea Camellia sinensis and rooibos tea (Aspalathus linearis); and their modulatory effects on key hyperglycaemic processes and oxidative stress. J. Food Sci. Technol. 2020, 57, 4345–4354. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, O.; Biesiekierska, M.; Balcerczyk, A. Epigallocatechin-3-gallate (EGCG) Alters Histone Acetylation and Methylation and Impacts Chromatin Architecture Profile in Human Endothelial Cells. Molecules 2020, 25, 2326. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bouic, P.J.; Rosenkranz, B. Investigation of, C.Y.; P2B6, 3A4 and beta-esterase interactions of Withania somnifera, L. dunal in human liver microsomes and HepG2 cells. J. Ethnopharmacol. 2021, 270, 113766. [Google Scholar] [CrossRef] [PubMed]
- Savai, J.; Varghese, A.; Pandita, N.; Chintamaneni, M. Investigation of CYP3A4 and CYP2D6 Interactions of Withania somnifera and Centella asiatica in Human Liver Microsomes. Phytother. Res. 2015, 29, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Savai, J.; Varghese, A.; Pandita, N.; Chintamaneni, M. In vitro assessment of CYP1A2 and 2C9 inhibition potential of Withania somnifera and Centella asiatica in human liver microsomes. Drug. Metab. Pers. Ther. 2015, 30, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Yadav, C.S.; Kumar, V.; Suke, S.G.; Ahmed, R.S.; Mediratta, P.K.; Banerjee, B.D. Propoxur-induced acetylcholine esterase inhibition and impairment of cognitive function: Attenuation by Withania somnifera. Indian J. Biochem. Biophys. 2010, 47, 117–120. [Google Scholar] [PubMed]
- Lee, J.; Liu, J.; Feng, X.; Salazar Hernandez, M.A.; Mucka, P.; Ibi, D.; Choi, J.W.; Ozcan, U. Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nat. Med. 2016, 22, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).