The Mechanisms Underlying Salt Resistance Mediated by Exogenous Application of 24-Epibrassinolide in Peanut
Abstract
:1. Introduction
2. Results
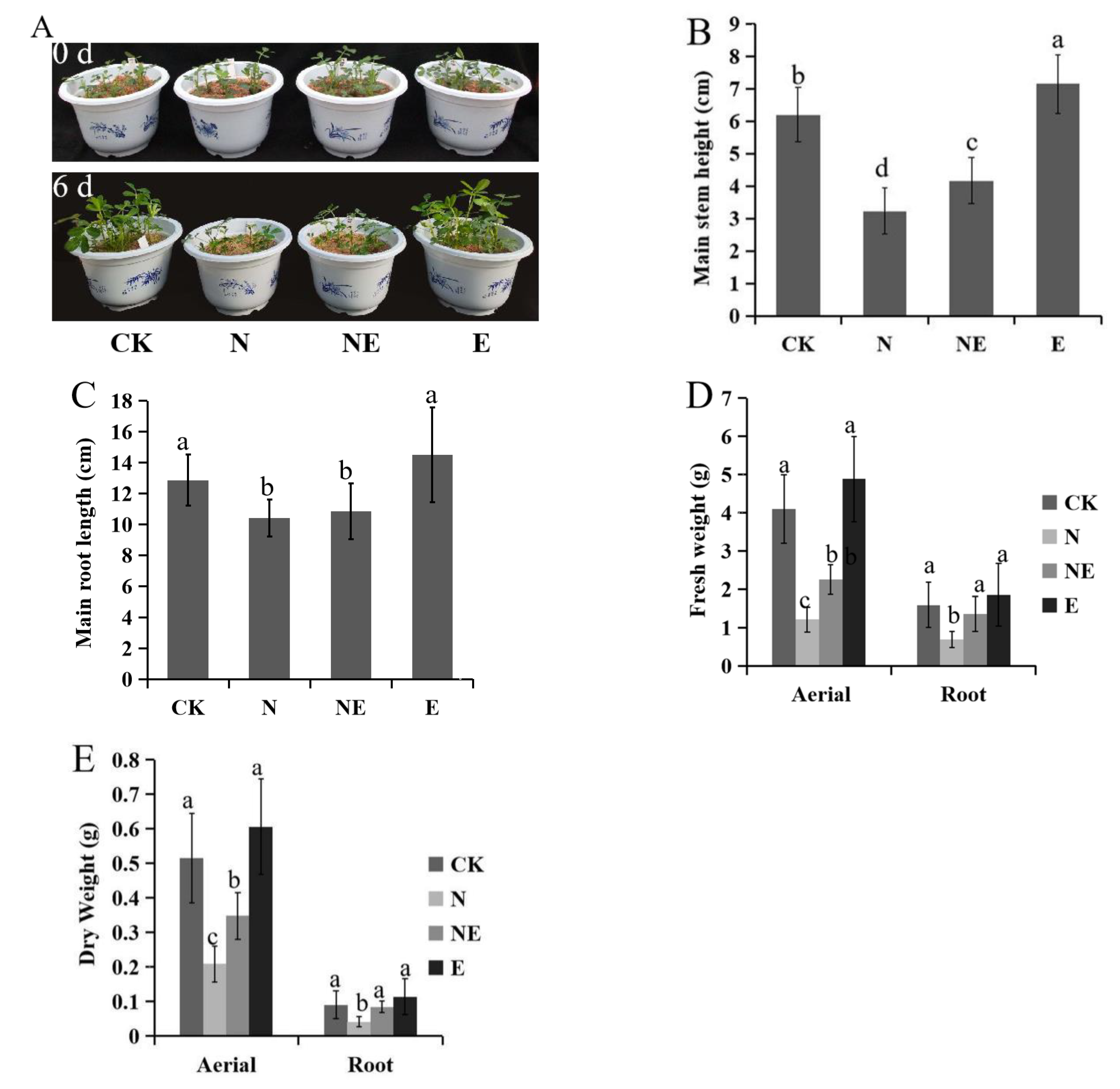
2.1. Effects of EBL Treatments on the Growth of Peanut Seedling
2.2. Effects of EBL Treatments on Production of O2− and H2O2 of Peanut Seedling
2.3. Effects of EBL Treatments on MDA Content and Antioxidant Enzyme Activities
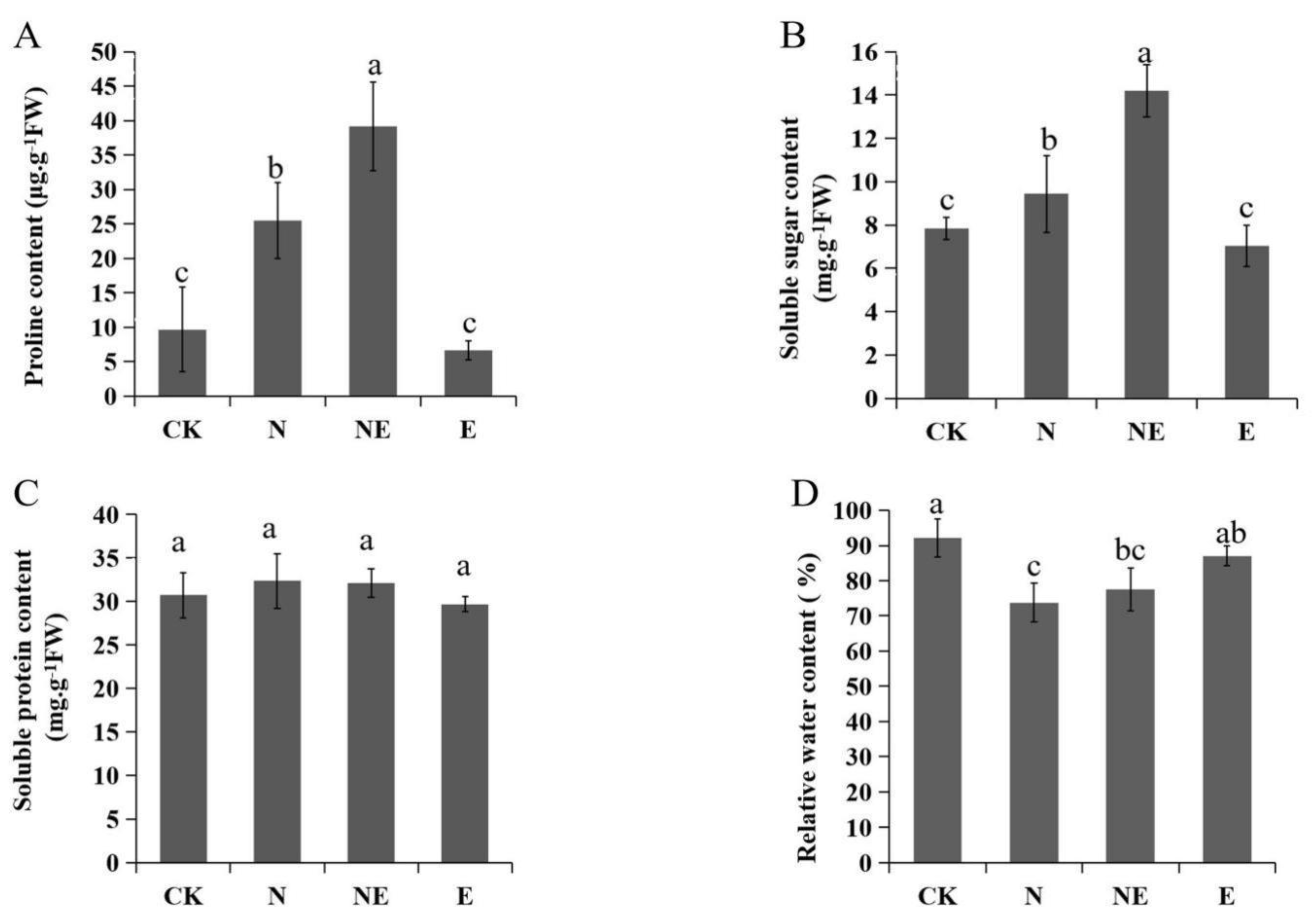
2.4. Effects of EBL Treatments on Osmolytes Accumulation and Leaf Relative Water Content (RWC)
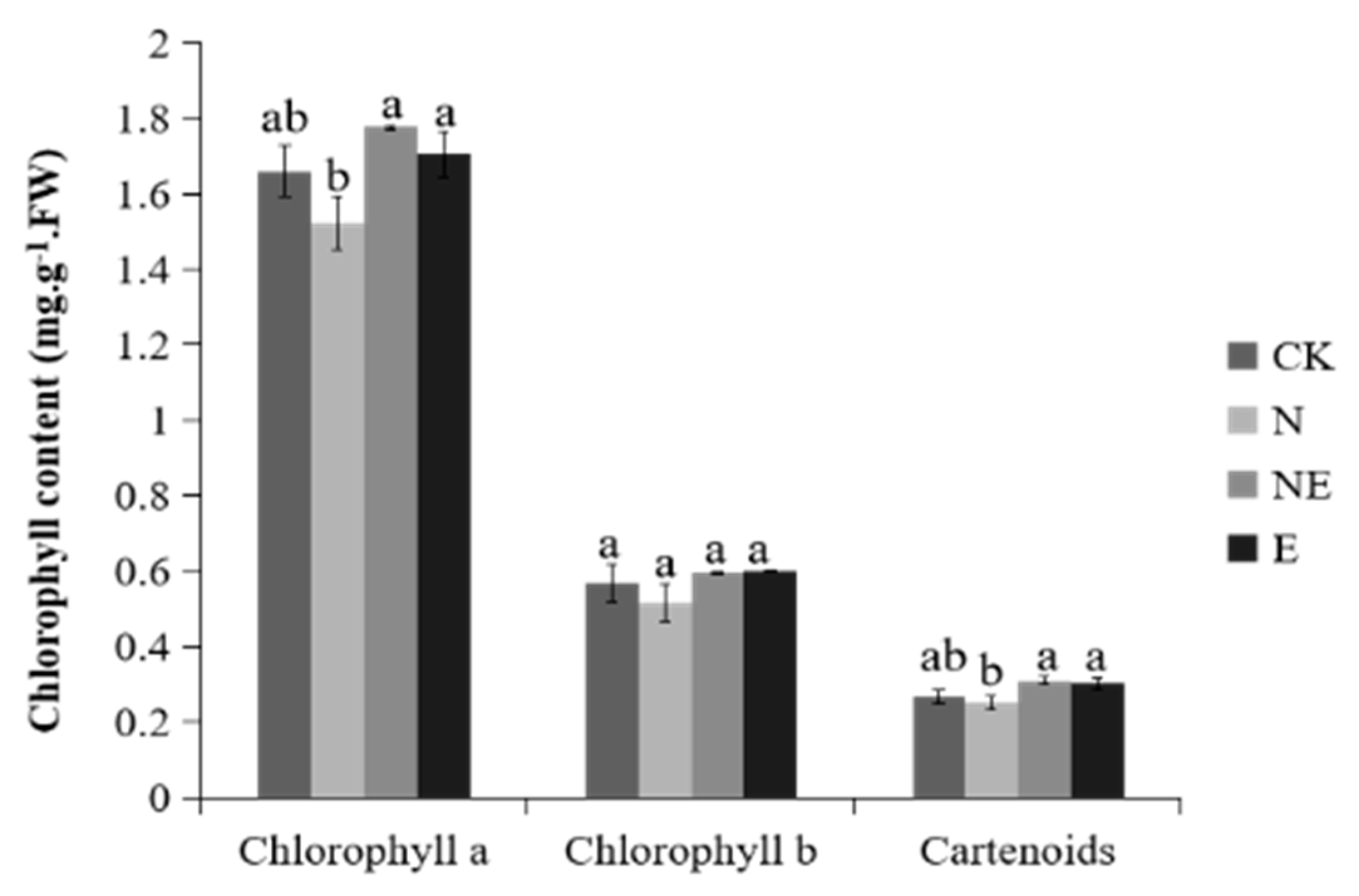
2.5. Effects of EBL Treatments on Chlorophyll and Carotenoids Content
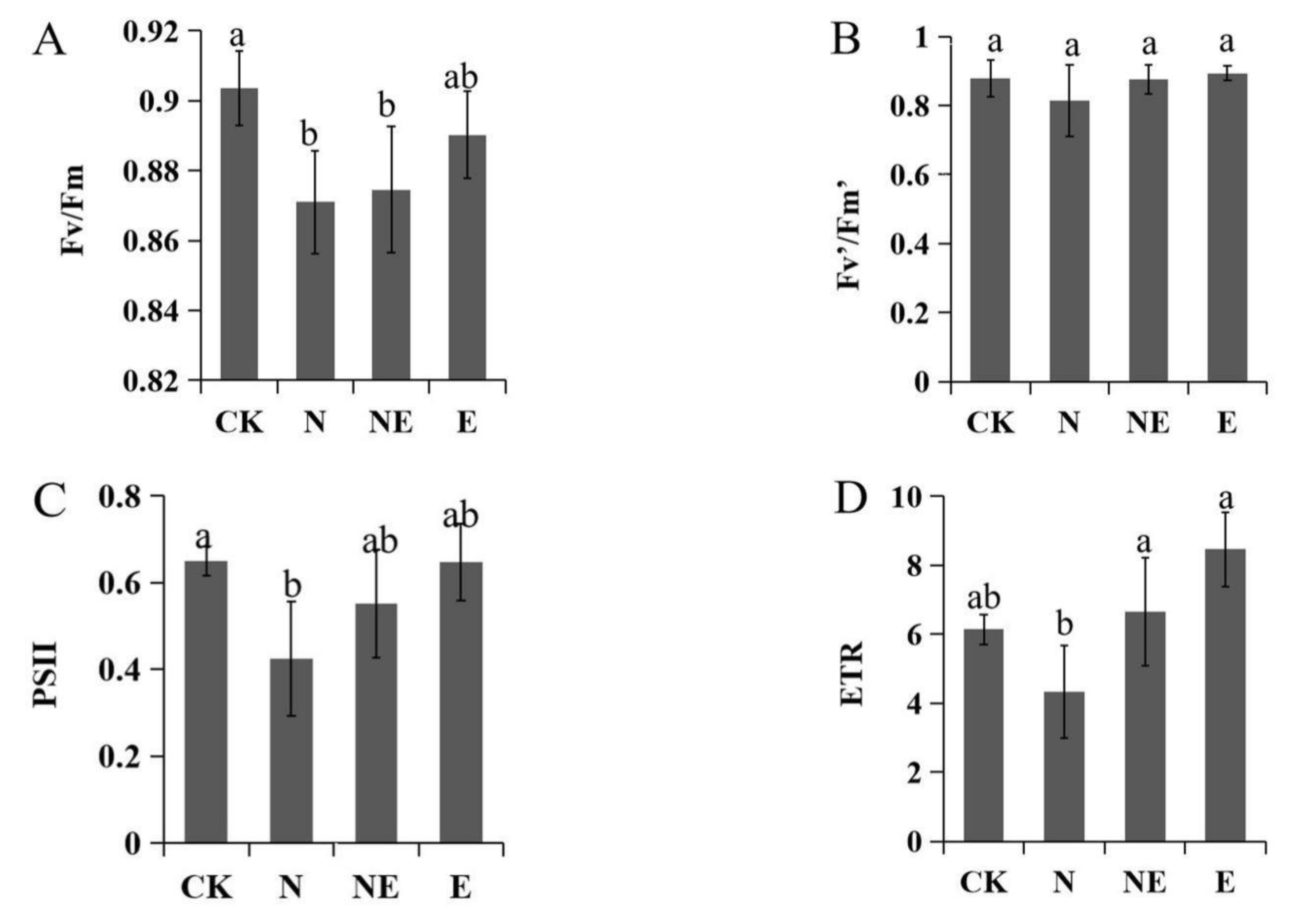
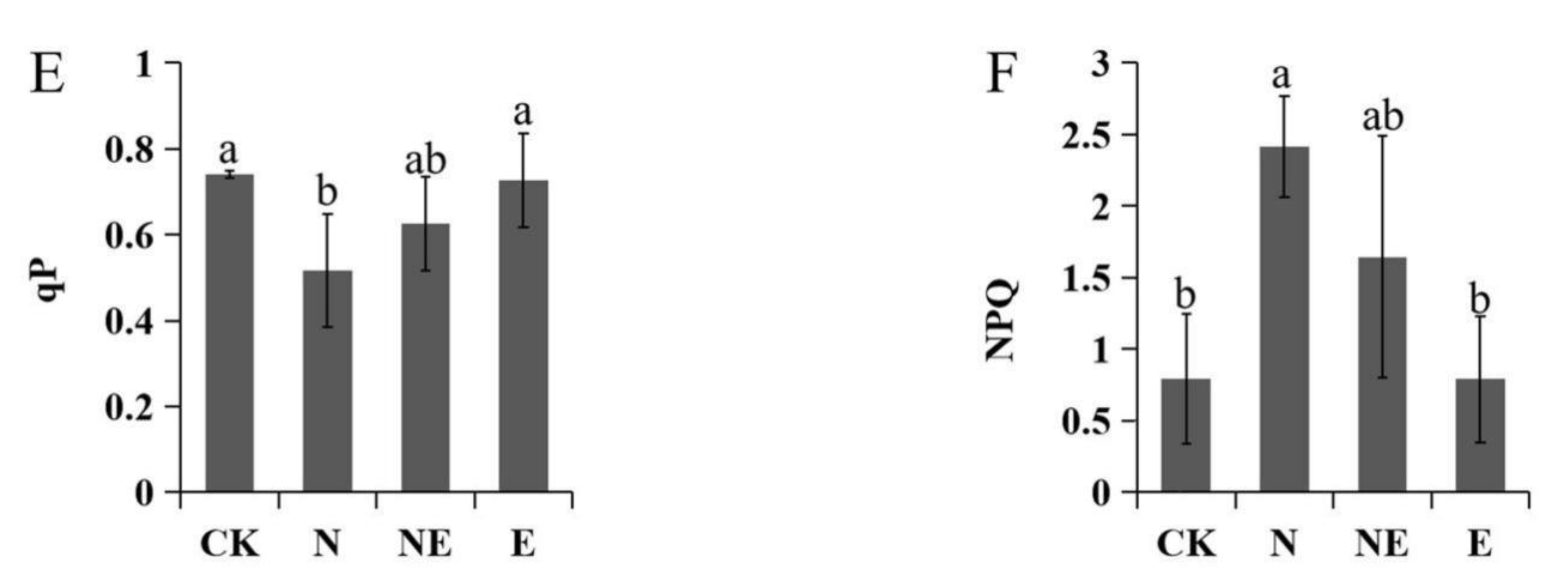
2.6. Effects of EBL Treatments on Chlorophyll Fluorescence
2.7. Effects of EBL Treatments on Key Gene Expression
2.8. Transcriptome Sequencing and Analysis of Differentially Expressed Genes (DEGs)
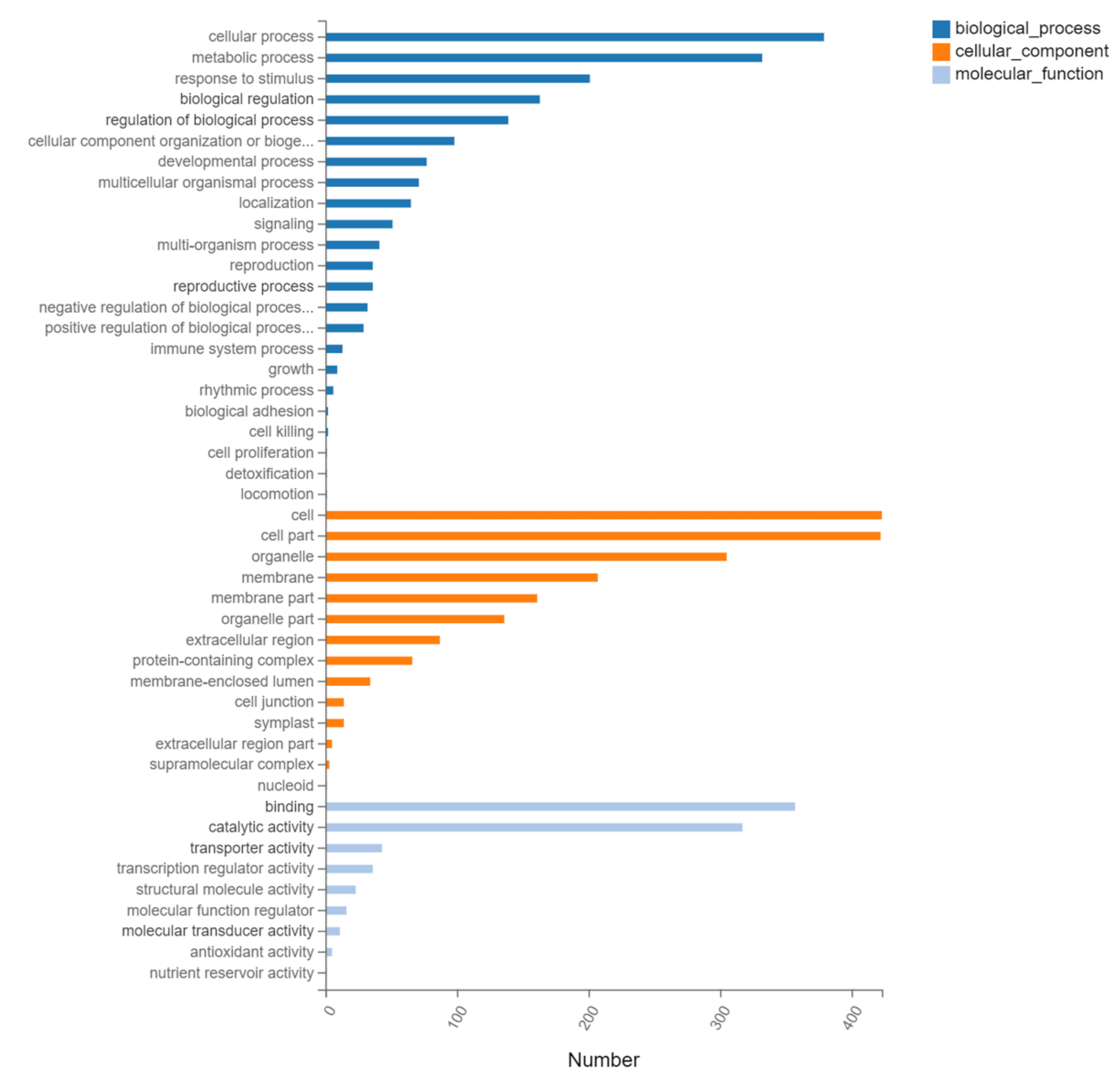
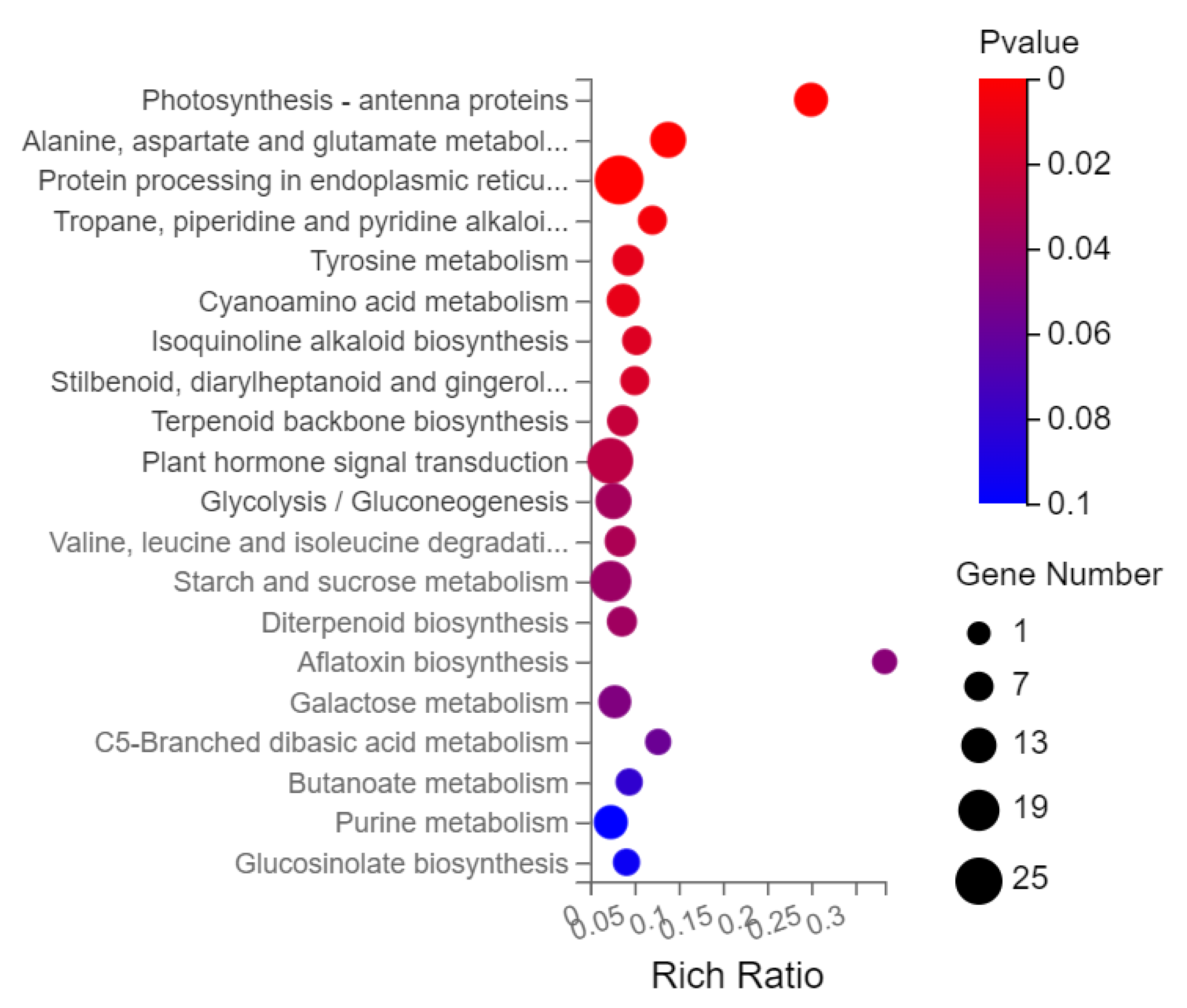
2.9. Gene Ontology (GO) and KEGG Pathway Analysis of DEGs of N12-vs.-NE12
2.10. EBL Regulates Salt Tolerance Genes in Peanut Seedlings Exposed to Salt Stress
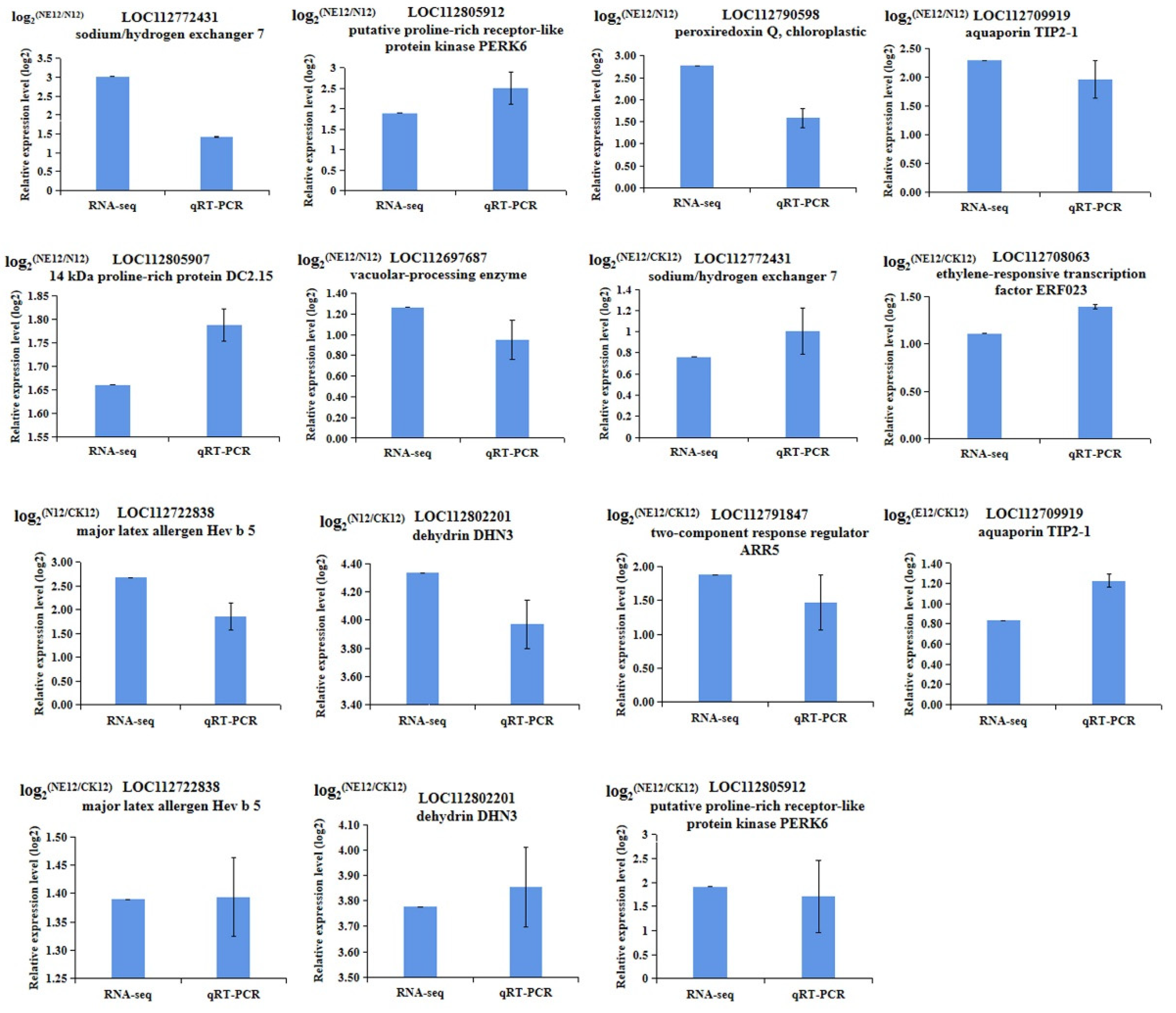
2.11. qRT-PCR Validation of Transcriptome Data
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.1.1. Plant Material
4.1.2. Experimental Design
4.2. Determination of Seedling Growth and Relative Water Content (RWC) of Leaves
4.3. Measurements of ROS and Malondialdehyde (MDA) Content
4.4. Determination of Antioxidant Enzyme Activities
4.5. Determination of Osmolytes Substance Content
4.6. Measurements of Chl Content and Fluorescence Parameters
4.7. RNA Extraction and RNA Sequencing
4.8. Validation of DEGs by Quantitative Real-Time PCR (qRT-PCR)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Wei, L.Q.; Wang, Z.Z.; Wang, T. Physiological and molecular features of Puccinellia tenuiflora tolerating salt and alkaline-salt stress. J. Integr. Plant Biol. 2013, 55, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Bhattacharjee, S. ROS and oxidative stress: Origin and implication. In Reactive Oxygen Species in Plant Biology; Springer: New Delhi, India, 2019; pp. 1–31. [Google Scholar]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.S.; Zhang, Q.K.; Liu, M.Y.; Zhou, H.P.; Ma, C.L.; Wang, P.P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Sun, J.K.; He, L.; Li, T. Response of seedling growth and physiology of Sorghum bicolor (L.) Moench to saline-alkali stress. PLoS ONE 2019, 14, e0220340. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Tao, H.; Zhang, X.; Pang, H.; Fu, J. Exogenous glycine betaine ameliorates the adverse effect of salt stress on perennial ryegrass. J. Am. Soc. Hort. Sci. 2012, 137, 38–46. [Google Scholar] [CrossRef]
- Gu, M.; Li, N.; Shao, T.; Long, X.; Brestic, M.; Shao, H.; Li, J.; Mbarki, S. Accumulation capacity of ions in cabbage (Brassica oleracea L.) supplied with sea water. Plant Soil. Environ. 2016, 62, 314–320. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.S.; Guo, Y.; Quintero, F.J.; Pardo, J.M.; Schumaker, K.S.; Zhu, J.K. Regulation of vacuolar Na+/H+ exchange in Arabidopsis thaliana by the Salt-Overly-Sensitive (SOS) pathway. J. Biol. Chem. 2004, 279, 207–215. [Google Scholar] [CrossRef]
- Planas-Riverola, A.; Gupta, A.; Betegón-Putze, I.; Bosch, N.; Ibañes, M.; Caño-Delgado, A.I. Brassinosteroid signaling in plant development and adaptation to stress. Development 2019, 146, dev151894. [Google Scholar] [CrossRef]
- Li, J.M.; Chory, J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 1997, 90, 929–938. [Google Scholar] [CrossRef]
- Peres, A.L.G.L.; Soares, J.S.; Tavares, R.G.; Righetto, G.; Zullo, M.A.T.; Mandava, N.B.; Menossi, M. Brassinosteroids, the sixth class of phytohormones: A molecular view from the discovery to hormonal interactions in plant development and stress adaptation. Int. J. Mol. Sci. 2019, 20, 331. [Google Scholar] [CrossRef]
- Alam, P.; Albalawi, T.H.; Altalayan, F.H.; Bakht, M.A.; Ahanger, M.A.; Raja, V.; Ashraf, M.; Ahmad, P. 24-Epibrassinolide (EBR) confers tolerance against NaCl Stress in soybean plants by up-regulating antioxidant system, sscorbate-glutathione cycle, and glyoxalase system. Biomolecules 2019, 9, 640. [Google Scholar] [CrossRef]
- Liu, J.; Gao, H.; Wang, X.; Zheng, Q.; Wang, C. Effects of 24-epibrassinolide on plant growth, osmotic regulation and ion homeostasis of salt-stressed canola. Plant Biol. 2013, 16, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Q.; Xia, S.T.; Su, Y.; Wang, H.Q.; Luo, W.G.; Su, S.Y.; Xiao, L.T. Brassinolide increases potato root growth in vitro in a dose-dependent way and alleviates salinity stress. Biomed. Res. Int. 2016, 2016, 8231873. [Google Scholar] [CrossRef]
- Wu, W.L.; Zhang, Q.; Ervin, E.H.; Yang, Z.P.; Zhang, X.Z. Physiological mechanism of enhancing salt stress tolerance of perennial ryegrass by 24-Epibrassinolide. Front. Plant Sci. 2017, 8, 1017. [Google Scholar] [CrossRef] [PubMed]
- El-Mashad, A.A.A.; Mohamed, H.I. Brassinolide alleviates salt stress and increases antioxidant activity of cowpea plants (Vigna sinensis). Protoplasma 2012, 249, 625–635. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Khalil, R.R.A.E.; Mir, B.A.; Yusuf, M.; Ahmad, A. 24-Epibrassinolide regulates photosynthesis, antioxidant enzyme activities and proline content of Cucumis sativus under salt and/or copper stress. Environ. Monit. Assess. 2013, 185, 7845–7856. [Google Scholar] [CrossRef] [PubMed]
- Otie, V.; Udo, I.; Shao, Y.; Itam, M.O.; Okamoto, H.; An, P.; Eneji, E.A. Salinity effects on morpho-physiological and yield traits of soybean (Glycine max L.) as mediated by foliar spray with brassinolide. Plants 2021, 10, 541. [Google Scholar] [CrossRef]
- Cui, F.; Liu, L.J.; Zhao, Q.Z.; Zhang, Z.H.; Li, Q.L.; Lin, B.Y.; Wu, Y.R.; Tang, S.Y.; Xie, Q. Arabidopsis ubiquitin conjugase UBC32 is an ERAD component that functions in brassinosteroid-mediated salt stress tolerance. Plant Cell 2012, 24, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.G.; Zhao, S.K.; Bao, T.T.; Zhao, P.Q.; Peng, K.; Guo, Q.X.; Gao, X.; Qin, J.C.; Qin, J.Q. Tomato BZR/BES transcription factor SlBZR1 positively regulates BR signaling and salt stress tolerance in tomato and Arabidopsis. Plant Sci. 2020, 302, 110719. [Google Scholar] [CrossRef]
- Li, J.F.; Zhou, H.P.; Zhang, Y.; Li, Z.; Yang, Y.Q.; Guo, Y. The GSK3-like Kinase BIN2 is a molecular switch between the salt stress response and growth recovery in Arabidopsis thaliana. Dev. Cell 2020, 55, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.S.; Reichelt, M.; Vadassery, J.; Signaling, A. Calmodulin-like protein CML37 is a positive regulator of ABA during drought stress in Arabidopsis. Plant Signal. Behav. 2015, 10, e1011951. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Campos, M.L.; Azevedo, R.A. The role of phytochrome in stress tolerance. J. Integr. Plant Biol. 2011, 53, 920–929. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Zhang, H.; Song, C.P.; Zhu, J.P.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Sharma, I.; Ching, E.; Saini, S.; Bhardwaj, R.; Pati, P.K. Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol. Biochem. 2013, 69, 17–26. [Google Scholar] [CrossRef]
- Wani, A.S.; Ahmad, A.; Hayat, S.; Tahir, I. Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiol. Biochem. 2019, 135, 85–394. [Google Scholar] [CrossRef]
- You, J.; Chan, Z.L. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Mir, B.A.; Yusuf, M.; Ahmad, A. 24-epibrassinolide and/or putrescine trigger physiological and biochemical responses for the salt stress mitigation in Cucumis sativus L. Photosynthetica 2014, 52, 464–474. [Google Scholar] [CrossRef]
- Sun, S.; An, M.; Han, L.; Yin, S. Foliar application of 24-Epibrassinolide improved salt stress tolerance of perennial ryegrass. HortScience 2015, 50, 1518–1523. [Google Scholar] [CrossRef]
- Khalid, A.; Aftab, F. Effect of exogenous application of 24-epibrassinolide on growth, protein contents, and antioxidant enzyme activities of in vitro-grown Solanum tuberosum L. under salt stress. In Vitr. Cell. Dev. Biol.—Plant 2016, 52, 81–91. [Google Scholar] [CrossRef]
- Veldhoven, P.P.; Schryver, E.D.; Young, S.G.; An, Z.; Ael, M.B.E.V. Slc25a17 Gene Trapped Mice: PMP34 Plays a Role in the Peroxisomal Degradation of Phytanic and Pristanic Acid. Front. Cell Dev. Biol. 2020, 8, 144. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zolman, B.K. Metabolic alterations in the Enoyl-CoA Hydratase 2 mutant disrupt peroxisomal pathways in seedlings. Plant Physiol. 2019, 180, 1860–1876. [Google Scholar] [CrossRef]
- Rouhier, N.; Gelhaye, E.; Gualberto, J.M.; Jordy, M.N.; De Fay, E.; Hirasawa, M.; Duplessis, S.; Lemaire, S.D.; Frey, P.; Martin, F.; et al. Poplar peroxiredoxin Q. A thioredoxin-linked chloroplast antioxidant functional in pathogen defense. Plant Physiol. 2004, 134, 1027–1038. [Google Scholar] [CrossRef]
- Yusuf, M.; Fariduddin, Q.; Khan, T.A.; Hayat, S. Epibrassinolide reverses the stress generated by combination of excess aluminum and salt in two wheat cultivars through altered proline metabolism and antioxidants. S. Afr. J. Bot. 2017, 112, 391–398. [Google Scholar] [CrossRef]
- Su, Q.F.; Zheng, X.D.; Tian, Y.K.; Wang, C.H. Exogenous brassinolide alleviates salt stress in Malus hupehensis Rehd. by regulating the transcription of NHX-Type Na+(K+)/H+ antiporters. Front. Plant Sci. 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, X.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Enhanced photosynthetic capacity and antioxidant potential mediate brassinosteriod-induced phenanthrene stress tolerance in tomato. Environ. Pollut. 2015, 201, 58–66. [Google Scholar] [CrossRef]
- Sharma, I.; Bhardwaj, R.; Pati, P.K. Exogenous Application of 28-Homobrassinolide modulates the dynamics of salt and pesticides induced stress responses in an elite rice variety pusa basmati-1. J. Plant Growth Regul. 2015, 34, 509–518. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Kang, J.G.; Gan, Y.T.; Yu, J.H.; Calderón-Urrea, A.; Lyu, J.; Zhang, G.B.; Feng, Z.; Xie, J.J. Transcriptome analysis of pepper (Capsicum annuum) revealed a role of 24-Epibrassinolide in response to chilling. Front. Plant Sci. 2016, 7, 1281. [Google Scholar] [CrossRef]
- Anwar, A.; Bai, L.Q.; Miao, L.; Liu, Y.M.; Li, S.Z.; Yu, X.C.; Li, Y.S. 24-epibrassinolide ameliorates endogenous hormone levels to enhance low-temperature stress tolerance in cucumber seedlings. Int. J. Mol. Sci. 2018, 19, 2497. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.J.; Haider, M.S.; Chai, P.P.; Guo, J.J.; Du, P.; Li, H.Y.; Dong, W.Z.; Huang, B.Y.; Zheng, Z.; Shi, L.; et al. Genome-wide identification and expression analysis of AP2/ERF transcription factors in sugarcane (Saccharum spontaneum L.). Front. Genet. 2020, 12, 750761. [Google Scholar] [CrossRef] [PubMed]
- Ghoulam, C.; Foursy, A.; Fares, K. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Exp. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- Zheng, X.D.; Zhou, J.Z.; Tan, D.X.; Wang, N.; Wang, L. Melatonin iImproves waterlogging tolerance of Malus baccata (Linn.) borkh. seedlings by maintaining aerobic respiration, photosynthesis and ROS migration. Front. Plant Sci. 2017, 8, 483. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Prange, F.R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Henry, L.; Hall, D.O. Superoxide dismutase in green algae: An evolutionary survey. In Photosynthetic Organelles; Japanese Society of Plant Physiologists: Kyoto, Japan, 1977; pp. 377–382. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods. Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochemical 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wu, X.X.; He, J.; Zhu, Z.W.; Yang, S.J.; Zha, D.S. Protection of photosynthesis and antioxidative system by 24-epibrassinolide in Solanum melongena under cold stress. Biol. Plant. 2013, 58, 185–188. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Hu, L.; Huang, Z.; Liu, S.; Fu, J. Growth response and gene expression in antioxidant-related enzymes in two bermudagrass genotypes differing in salt tolerance. J. Am. Soc. Hort. Sci. 2012, 137, 134–143. [Google Scholar] [CrossRef]




| Sample | Total Raw Reads (M) | Total Clean Reads (M) | Total Clean Bases (Gb) | Clean Reads Q20 (%) | Clean Reads Q30 (%) |
|---|---|---|---|---|---|
| CK12_1 | 23.92 | 23.8 | 1.19 | 98.42 | 95.18 |
| CK12_2 | 23.92 | 23.83 | 1.19 | 98.28 | 94.72 |
| CK12_3 | 23.92 | 23.8 | 1.19 | 98.3 | 94.8 |
| N12_1 | 23.92 | 23.78 | 1.19 | 98.38 | 95.03 |
| N12_2 | 23.92 | 23.6 | 1.18 | 98.45 | 95.26 |
| N12_3 | 23.92 | 23.76 | 1.19 | 98.3 | 94.86 |
| NE12_1 | 23.92 | 23.81 | 1.19 | 98.38 | 95.06 |
| NE12_2 | 23.92 | 23.78 | 1.19 | 98.39 | 95.12 |
| NE12_3 | 23.92 | 23.8 | 1.19 | 98.39 | 95.06 |
| E12_1 | 23.92 | 23.81 | 1.19 | 98.43 | 95.2 |
| E12_2 | 23.92 | 23.78 | 1.19 | 98.38 | 95.05 |
| E12_3 | 23.92 | 23.78 | 1.19 | 98.39 | 95.09 |
| Gene ID | log2 FC (NE12/N12) | Q Value | Symbol | Gene Bank Description |
|---|---|---|---|---|
| Na+/H+ antiporters (NHXs) | ||||
| 112772431 | 3.02 | 1.39 × 10−10 | NHX7 | sodium/hydrogen exchanger 7 |
| 112732776 | 5.59 | 4.18 × 10−19 | NHX8 | sodium/hydrogen exchanger 8 |
| ROS scavenging related genes | ||||
| 112696745 | 2.52 | 7.43 × 10−6 | PMP34 | peroxisomal nicotinamide adenine dinucleotide carrier |
| 112733788 | 5.91 | 5.07 × 10−3 | AAE11 | butyrate-CoA ligase AAE11, peroxisomal |
| 112766271 | 9.13 | 2.49 × 10−11 | ECH2 | enoyl-CoA hydratase 2, peroxisomal |
| 112790598 | 2.78 | 1.3 × 10−26 | PRXQ | peroxiredoxin Q, chloroplastic |
| 112701439 | 1.58 | 7.05 × 10−3 | LAPX | L-ascorbate peroxidase T, chloroplastic |
| 112706364 | 1.76 | 1.49 × 10−2 | glutaredoxin-C6 | |
| osmotic stress related genes | ||||
| 112797943 | 1.10 | 1.31 × 10−4 | PERK9 | proline-rich receptor-like protein kinase PERK9 |
| 112805912 | 1.90 | 3.86 × 10−5 | PERK6 | putative proline-rich receptor-like protein kinase PERK6 |
| 112744691 | −1.44 | 2.56 × 10−2 | PRODH | proline dehydrogenase 2, mitochondrial |
| 112797983 | −1.36 | 1.86 × 10−5 | PRODH | proline dehydrogenase 2, mitochondrial |
| 112697841 | −1.69 | 5.96 × 10−4 | AMY | alpha-amylase-like |
| 112697429 | 1.04 | 6.37 × 10−5 | SWEET1 | bidirectional sugar transporter SWEET1 |
| 112724011 | 5.81 | 7.7 × 10−3 | SUC | sugar carrier protein C-like |
| 112741002 | 1.13 | 7.17 × 10−7 | SPS | probable sucrose-phosphate synthase 2 |
| 112776149 | 3.77 | 4.28 × 10−44 | BFUCT | beta-fructofuranosidase, cell wall isozyme |
| phytohormones related genes | ||||
| 112715407 | 1.94 | 1.17 × 10−2 | CPD | cytochrome P450 90A1 |
| 112709363 | 6.72 | 1.44 × 10−4 | BRI1 | squamosa promoter-binding-like protein 6 |
| 112792599 | −1.03 | 3.39 × 10−2 | BRI1 | squamosa promoter-binding-like protein 12 |
| 112788794 | 1.43 | 1.04 × 10−2 | BSK5 | serine/threonine-protein kinase BSK5 |
| 112697317 | −2.30 | 4.5 × 10−10 | TCH4 | probable xyloglucan endotransglucosylase/hydrolase protein 23 |
| 112698421 | 1.85 | 1.86 × 10−5 | TCH4 | xyloglucan endotransglucosylase/hydrolase protein 22 |
| 112727310 | 1.41 | 8.28 × 10−4 | TCH4 | probable xyloglucan endotransglucosylase/hydrolase protein 23 |
| 112736986 | 2.18 | 3.12 × 10−13 | X10A | auxin-induced protein X10A |
| 112767282 | 2.84 | 4 × 10−2 | SAUR50 | auxin-responsive protein SAUR50 |
| 112709815 | 1.38 | 6.63 × 10−7 | gibberellin 3-beta-dioxygenase 1 | |
| 112755883 | −1.18 | 6.96 × 10−5 | cytokinin dehydrogenase 7 | |
| 112749719 | −1.26 | 4 × 10−3 | PP2C51 | protein phosphatase 2C 51 |
| 112708063 | 4.34 | 1 × 10−2 | ERF023 | ethylene-responsive transcription factor ERF023 |
| 112738367 | 4.95 | 1 × 10−2 | ERF017 | ethylene-responsive transcription factor ERF017 |
| the others | ||||
| 112708162 | 2.00 | 1.41 × 10−2 | CML37 | calcium-binding protein CML37 |
| 112710657 | 3.36 | 1.21 × 10−29 | PhyA | phytochrome A |
| 112709919 | 2.29 | 2.49 × 10−5 | TIP2-1 | aquaporin TIP2-1 |
| Gene ID | Primer Name | Primer Sequence (5′–3′) |
|---|---|---|
| 112772431 | 1-NHX7-1F | GTTCGCTTTACACTACCTTGAC |
| 1-NHX7-1R | TTCTCTAAGTTCCTCATCATCTCC | |
| 112805907 | 3-DC2.15-3F | AATTCTTGTTGTGTCACTCC |
| 3-DC2.15-3R | CAAGTCCTAAGACATCAGCA | |
| 112805912 | 4-PERK6-4F | ATTCTCAAACTGGTTCATCCAG |
| 4-PERK6-4R | AAACAGGTAATCCAAAGCCA | |
| 112790598 | 9-PERQ-9F | TTTCTATCCTGCTGATGAGTCC |
| 9-PERQ-9R | TCACTACTGATTCCAACAACCT | |
| 112709919 | 6-TIP2-1-6F | ACTTTCTGGAATCTTCTACTGG |
| 6-TIP2-1-6R | TCACTACTCCTTCAAATGCTC | |
| 112708063 | 15-ERF023-15F | CATGGAGCTACAAACAACGG |
| 15-ERF023-15R | AATCTCCGAAACCCATTTCC | |
| 112779663 | 1-GPS3-1F | GAAGCCAATCGTACATTTCC |
| 1-GPS3-1R | AAGTTGTGGATAAGAGAAGTGG | |
| 112801730 | 2-CP26-2F | ATTCCACACCCTTACTACTTCC |
| 2-CP26-2R | GTTTCCAAGCATCTCAGACAC | |
| 112736176 | 3-CP4-3F | GAAAGGACGAACAAGAACCA |
| 3-CP4-3R | GTTGTTACAGTCGCCATCTC | |
| 112767125 | 4-CP215-4F | CTTTCTACTCTCAACCTTCACTC |
| 4-CP215-4R | TGCGGAGAAGTTCATTTCCT | |
| 112697687 | 5-VPE-5F | GTCCCTTCTAAGGATCACCC |
| 5-VPE-5R | TGGCTCATGGAAGAATCTGG | |
| 112707104 | 6-R7OM-6F | TCGTGCATTACCTGAATACC |
| 6-R7OM-6R | GAACCTTGTGAACCATTGAG | |
| 112792338 | 7-LHC-7F | CACTCACTCATCAACACCAC |
| 7-LHC-7R | TCTTCCAACTCCAAGCTCAG | |
| 112703831 | 8-LEA5-8F | GGTAAGAGGAAGTTGGTTTATGTG |
| 8-LEA5-8R | CTGGATTCTAATGTTGCCTGTG | |
| 112802201 | 9-DHN3-9F | AGTATGGCAACACTATGAGG |
| 9-DHN3-9R | GTACATCTTGCCGGATTCAG | |
| 112696380 | 10-GIP1-10F | CCTGCCTCATCAACTATAAATACC |
| 10-GIP1-10R | GAGAAGAGAAGCAAGAAGGG | |
| 112722388 | 11-SNA2-11F | GTAGAAGCGAGGAATCACAG |
| 11-SNA2-11R | AAGGCAGAGAAGCAATAACAC | |
| 112730690 | 12-EN75-12F | CATTGCTGATGAATACCCTAAACC |
| 12-EN75-12R | TATGGTGGCTTCTCATGTGG | |
| 112722838 | 13-MLA5-13F | CCCAACCCATTCAATTCTTCC |
| 13-MLA5-13R | TTCTGTGGTTGTTTCCTCTG | |
| 112791847 | 14-ARR5-14F | ATTCCCTCTCAATCTAACGG |
| 14-ARR5-14R | AGGACATAATCACAACTGGA | |
| AhActin | ActinF | GTCATCGTCATCCTCTTCTC |
| ActinR | CATTCCTGTTCCATTGTCAC | |
| AhSOS1 | 1-SOS-F | GAGATTTCCCTTACACTTGCC |
| 1-SOS-R | GAACATTCCCAACGACATGAC | |
| AhNHX1 | 3-NHX1-F | CCGCCTATAATATTCAATGCCG |
| 3-NHX1-R | CCGAAGGTTATGATGGTACAC | |
| AhDWF4 | 4-DWF4-F | TTATTCTGCTACCACCAT |
| 4-DWF4-R | CATCTGCTGACACTATTG | |
| arahy.7MK8W0.1 | 9-BES1-F | AATCCCTCTCCAATGTCTCC |
| 9-BES1-R | GGAAGCATCAGATTCATCACAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Sun, J.; Zhang, X.; Ahmad, N.; Hou, L.; Zhao, C.; Pan, J.; Tian, R.; Wang, X.; Zhao, S. The Mechanisms Underlying Salt Resistance Mediated by Exogenous Application of 24-Epibrassinolide in Peanut. Int. J. Mol. Sci. 2022, 23, 6376. https://doi.org/10.3390/ijms23126376
Li W, Sun J, Zhang X, Ahmad N, Hou L, Zhao C, Pan J, Tian R, Wang X, Zhao S. The Mechanisms Underlying Salt Resistance Mediated by Exogenous Application of 24-Epibrassinolide in Peanut. International Journal of Molecular Sciences. 2022; 23(12):6376. https://doi.org/10.3390/ijms23126376
Chicago/Turabian StyleLi, Wenjiao, Jie Sun, Xiaoqian Zhang, Naveed Ahmad, Lei Hou, Chuanzhi Zhao, Jiaowen Pan, Ruizheng Tian, Xingjun Wang, and Shuzhen Zhao. 2022. "The Mechanisms Underlying Salt Resistance Mediated by Exogenous Application of 24-Epibrassinolide in Peanut" International Journal of Molecular Sciences 23, no. 12: 6376. https://doi.org/10.3390/ijms23126376
APA StyleLi, W., Sun, J., Zhang, X., Ahmad, N., Hou, L., Zhao, C., Pan, J., Tian, R., Wang, X., & Zhao, S. (2022). The Mechanisms Underlying Salt Resistance Mediated by Exogenous Application of 24-Epibrassinolide in Peanut. International Journal of Molecular Sciences, 23(12), 6376. https://doi.org/10.3390/ijms23126376








