Trastuzumab and Doxorubicin Sequential Administration Increases Oxidative Stress and Phosphorylation of Connexin 43 on Ser368
Abstract
1. Introduction
2. Results
2.1. Effect of Doxorubicin, Trastuzumab and Their Association on Cytosolic and Mitochondrial ROS Production
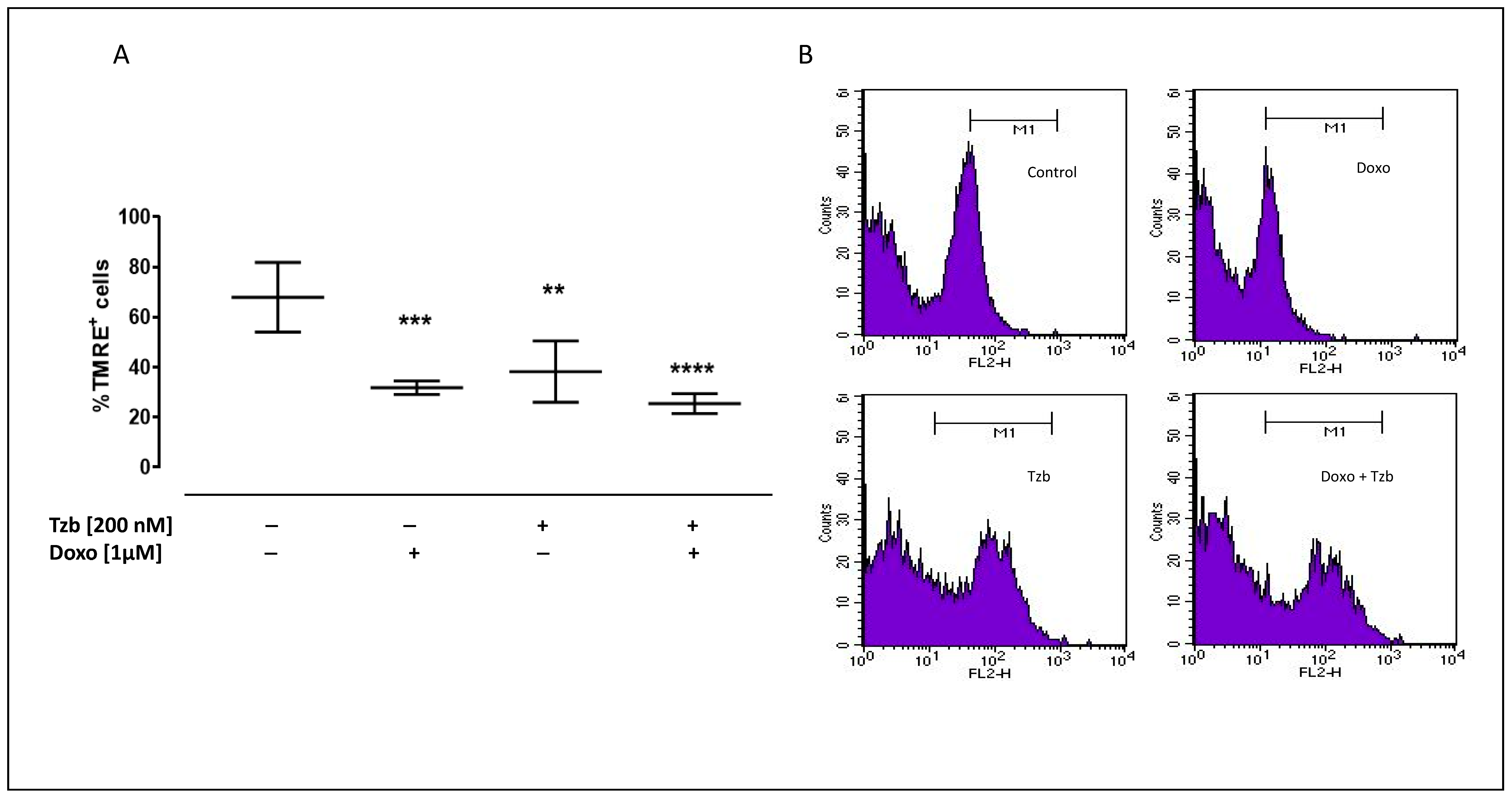
2.2. Effect of Doxorubicin, Trastuzumab and Their Association on Mitochondrial Membrane Depolarization
2.3. Effect of Doxorubicin, Trastuzumab and Their Association on Calcium Homeostasis
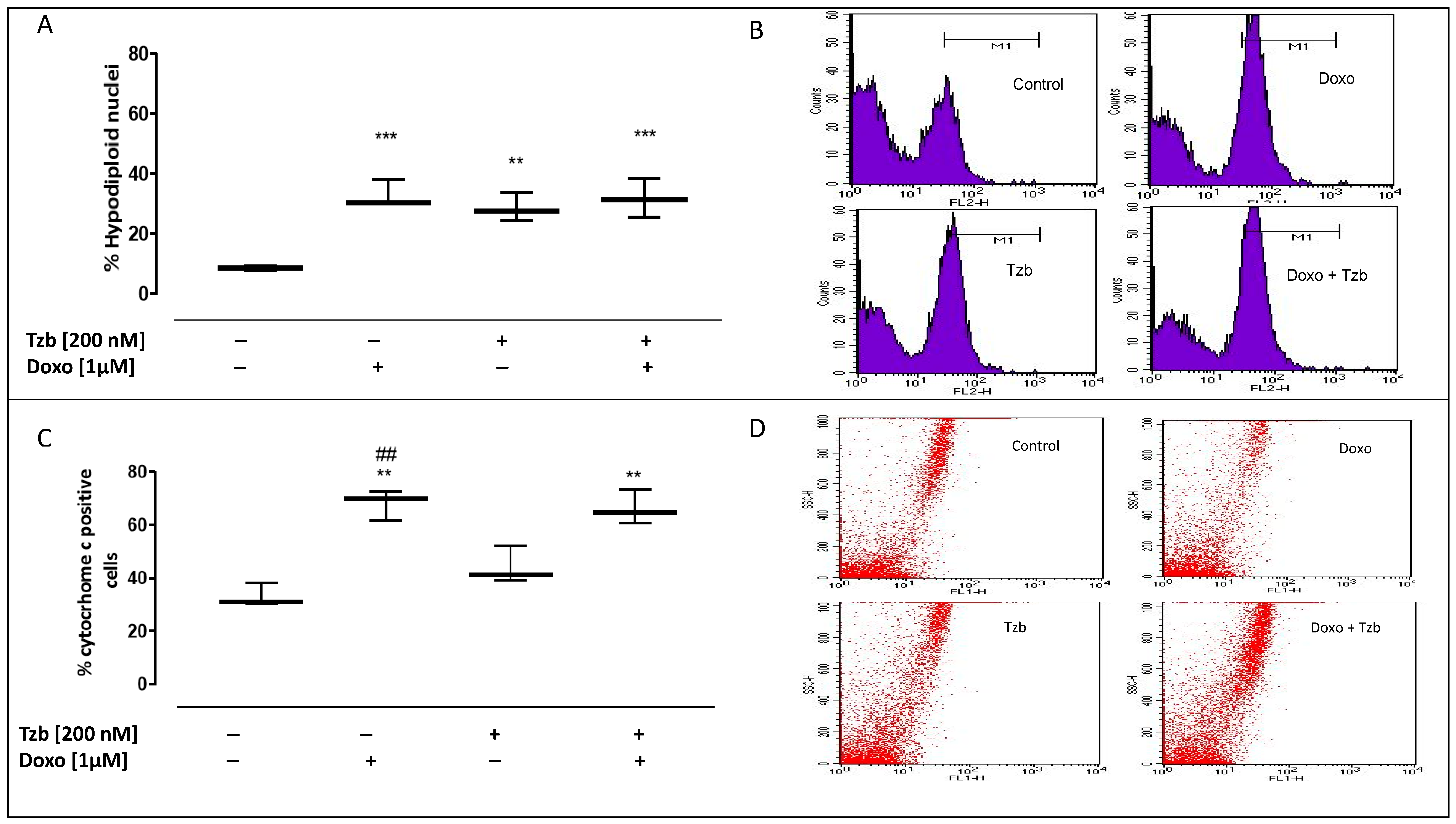
2.4. Effect of Doxorubicin, Trastuzumab and Their Association on Apoptosis
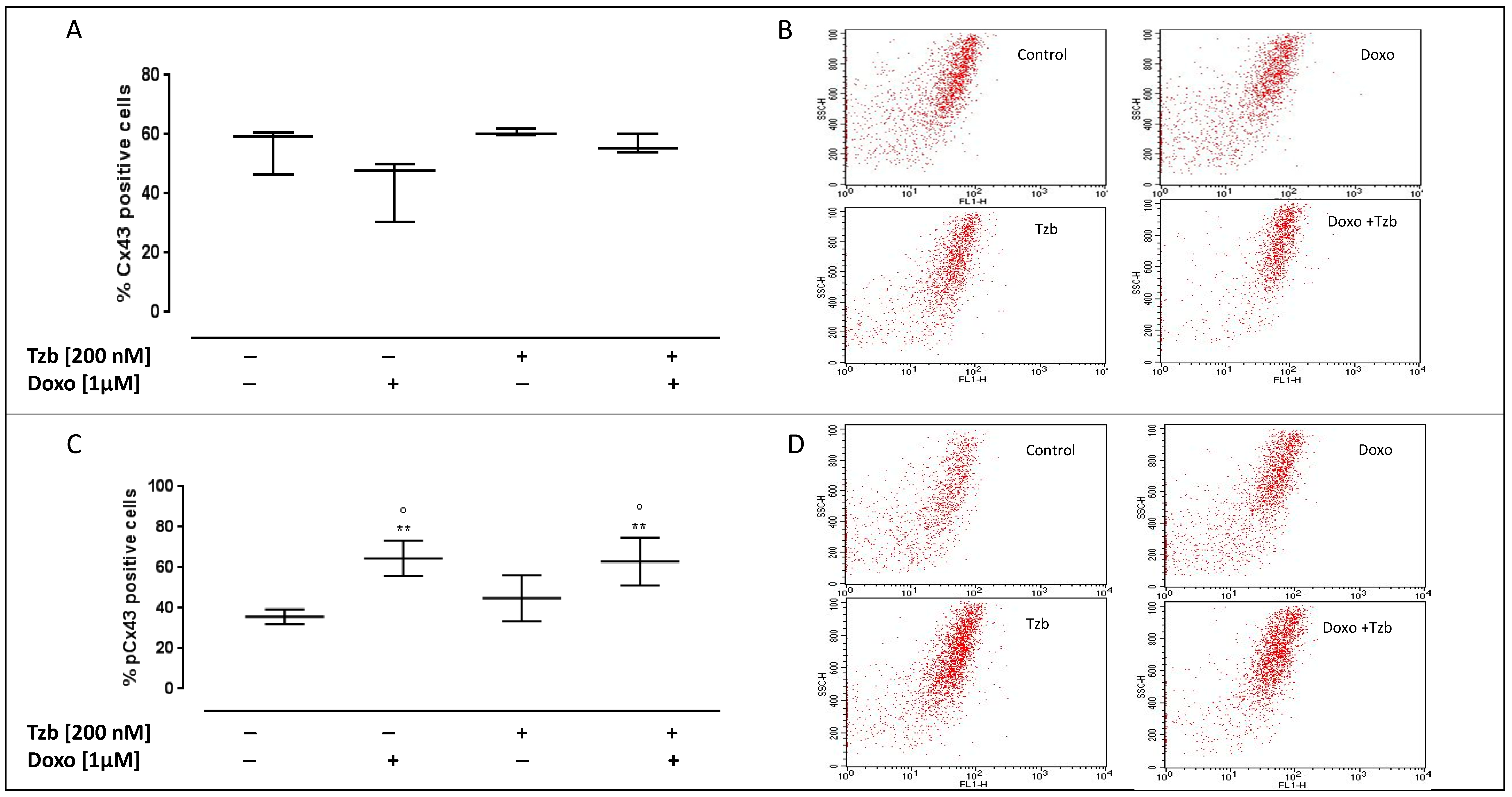
2.5. Effect of Doxorubicin, Trastuzumab and Their Association on Cx43 and pCx43 Expression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Protocol
4.3. Measurement of Intracellular Reactive Oxygen Species (ROS)
4.4. Measurement of Mitochondrial Superoxide Formation
4.5. Measurement of Mitochondrial Membrane Depolarization
4.6. Measurement of Intracellular Calcium Signalling
4.7. Analysis of Apoptosis
4.8. Flow Cytometry Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef]
- Coughlin, S.S.; Ekwueme, D.U. Breast cancer as a global health concern. Cancer Epidemiol. 2009, 33, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.A.; Cronin, K.A.; Plevritis, S.K.; Fryback, D.G.; Clarke, L.; Zelen, M.; Mandelblatt, J.S.; Yakovlev, A.Y.; Habbema, J.D.; Feuer, E.J.; et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N. Engl. J. Med. 2005, 27, 353. [Google Scholar] [CrossRef]
- Nicolazzi, M.A.; Carnicelli, A.; Fuorlo, M.; Scaldaferri, A.; Masetti, R.; Landolfi, R.; Favuzzi, A.M.R. Anthracycline and trastuzumab-induced cardiotoxicity in breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2175–2185. [Google Scholar]
- Akbari, V.; Chou, C.P.; Abedi, D. New insights into affinity proteins for HER2-targeted therapy: Beyond trastuzumab. Biochim. Biophys. Acta Rev. Cancer. 2020, 1874, 188448. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.R. Myocyte survival pathways and cardiomyopathy: Implications for trastuzumab cardiotoxicity. Semin. Oncol. 2000, 27, 92–100. [Google Scholar]
- Crone, S.A.; Zhao, Y.Y.; Fan, L.; Gu, Y.; Minamisawa, S.; Liu, Y.; Peterson, K.L.; Chen, J.; Kahn, R.; Condorelli, G.; et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat. Med. 2002, 8, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Force, T.; Krause, D.S.; Van Etten, R.A. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat. Rev. Cancer 2007, 7, 332–344. [Google Scholar] [CrossRef]
- Zhu, S.; Cawley, S.M.; Bloch, K.D.; Huang, P.L. Trastuzumab and lapatinib differ in effects on calcium cycling and HER2 expression in human embryonic stem cell-derived cardiomyocytes. Cardiovasc. Syst. 2013, 25, 1–10. [Google Scholar] [CrossRef][Green Version]
- Andreata, F.; Bonizzi, A.; Sevieri, M.; Truffi, M.; Monieri, M.; Sitia, L.; Silva, F.; Sorrentino, L.; Allevi, R.; Zerbi, P.; et al. Co-administration of H-ferritin-doxorubicin and Trastuzumab in neoadjuvant setting improves efficacy and prevents cardiotoxicity in HER2 + murine breast cancer model. Sci. Rep. 2020, 10, 11425. [Google Scholar] [CrossRef]
- Bria, E.; Cuppone, F.; Fornier, M.; Nisticò, C.; Carlini, P.; Milella, M.; Sperduti, I.; Terzoli, E.; Cognetti, F.; Giannarelli, D. Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: The dark side of the moon? A meta-analysis of the randomized trials. Breast Cancer Res Treat. 2008, 109, 231–239. [Google Scholar] [CrossRef]
- Anjos, M.; Fontes-Oliveira, M.; Costa, V.M.; Santos, M.; Ferreira, R. An update of the molecular mechanisms underlying doxorubicin plus trastuzumab induced cardiotoxicity. Life Sci. 2021, 280, 119760. [Google Scholar] [CrossRef]
- De Korte, M.A.; de Vries, E.G.; Lub-de Hooge, M.N.; Jager, P.L.; Gietema, J.A.; van der Graaf, W.T.; Sluiter, W.J.; van Veldhuisen, D.J.; Suter, T.M.; Sleijfer, D.T.; et al. 111Indium-trastuzumab visualises myocardial human epidermal growth factor receptor 2 expression shortly after anthracycline treatment but not during heart failure: A clue to uncover the mechanisms of trastuzumab-related cardiotoxicity. Eur. J. Cancer 2007, 43, 2046–2051. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, C.; Paciello, R.; Riccio, G.; Rea, D.; Barbieri, A.; Coppola, C.; Maurea, N. Cardiotoxic effects of the novel approved anti-ErbB2 agents and reverse cardioprotective effects of ranolazine. Oncol. Targets Ther. 2018, 11, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Guenancia, C.; Gudjoncik, A.; Hachet, O.; Zeller, M.; Cottin, Y.; Vergely, C. Anthracyclines/trastuzumab: New aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol. Sci. 2015, 36, 326–348. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Pala, B.; Di Marcantonio, M.C.; Muraro, R.; Marzocco, S.; Pinto, A.; Mincione, G.; Popolo, A. Doxorubicin induced oxidative and nitrosative stress: Mitochondrial connexin 43 is at the crossroads. Int. J. Mol. Med. 2020, 46, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Pinto, A.; Popolo, A. Trastuzumab-induced cardiotoxicity and role of mitochondrial connexin43 in the adaptive response. Toxicol. In Vitro 2020, 67, 104926. [Google Scholar] [CrossRef]
- Horie, T.; Ono, K.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Kuwabara, Y.; Nakashima, Y.; Takanabe-Mori, R.; Nishi, E.; et al. Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway. Cardiovasc. Res. 2010, 87, 656–664. [Google Scholar] [CrossRef]
- Kanno, S.; Saffitz, J.E. The role of myocardial gap junctions in electrical conduction and arrhythmogenesis. Cardiovasc Pathol. 2001, 10, 169–177. [Google Scholar] [CrossRef]
- Elhadidy, M.G.; Elmasry, A.; Rabei, M.R.; Eladel, A.E. Effect of ghrelinon VEGF-B and connexin-43 in a rat model of doxorubicin-induced cardiomyopathy. J. Basic Clin. Physiol. Pharmacol 2019, 14, 31. [Google Scholar]
- Tan, R.; Cong, T.; Xu, G.; Hao, Z.; Liao, J.; Xie, Y.; Lin, Y.; Yang, X.; Li, Q.; Liu, Y.; et al. Anthracycline-Induced Atrial Structural and Electrical Remodeling Characterizes Early Cardiotoxicity and Contributes to Atrial Conductive Instability and Dysfunction. Antioxid. Redox. Signal. 2022, 4, 4574. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Moscato, S.; Polizzi, E.; Pieragostino, D.; Cufaro, M.C.; Del Boccio, P.; Bianchi, F.; De Caterina, R.; Mattii, L. Connexin 43 and Connexin 26 Involvement in the Ponatinib-Induced Cardiomyopathy: Sex-Related Differences in a Murine Model. Int. J. Mol. Sci. 2021, 22, 5815. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Shi, A.; Lu, C.; Song, T.; Zhang, Z.; Zhao, J. Breast Cancer: Epidemiology and Etiology. Cell Biochem. Biophys. 2015, 72, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Aikat, S.; Francis, G.S. Trastuzumab therapy and the heart: Palliation at what cost? Congest. Heart Fail. 2001, 7, 188–190. [Google Scholar] [CrossRef][Green Version]
- Gordoa, T.A.; García-Sáenz, J.Á.; Rodríguez Moreno, J.F.; Hernández Pérez, F.J.; Día-Rubio, E. Is trastuzumab-induced cardiotoxicity involved in onco-cardiology outcome? Clin. Transl. Oncol. 2011, 13, 451–459. [Google Scholar] [CrossRef]
- Riccio, G.; Coppola, C.; Piscopo, G.; Capasso, I.; Maurea, C.; Esposito, E.; De Lorenzo, C.; Maurea, N. Trastuzumab and target-therapy side effects: Is still valid to differentiate anthracycline Type I from Type II cardiomyopathies? Hum. Vaccin. Immunother. 2016, 12, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- ElZarrad, M.K.; Mukhopadhyay, P.; Mohan, N.; Hao, E.; Dokmanovic, M.; Hirsch, D.S.; Shen, Y.; Pacher, P.; Wu, W.J. Trastuzumab alters the expression of genes essential for cardiac function and induces ultrastructural changes of cardiomyocytes in mice. PLoS ONE. 2013, 8, e79543. [Google Scholar] [CrossRef] [PubMed]
- Altomare, C.; Lodrini, A.M.; Milano, G.; Biemmi, V.; Lazzarini, E.; Bolis, S.; Pernigoni, N.; Torre, E.; Arici, M.; Ferrandi, M.; et al. Structural and Electrophysiological Changes in a Model of Cardiotoxicity Induced by Anthracycline Combined with Trastuzumab. Front Physiol. 2021, 12, 658790. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, W.; Luo, G.; Alain, L.A. Hypoxia induces connexin 43 dysregulation by modulating matrix metalloproteinases via MAPK signaling. Mol Cell Biochem. 2013, 384, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sysa-Shah, P.; Xu, Y.; Guo, X.; Belmonte, F.; Kang, B.; Bedja, D.; Pin, S.; Tsuchiya, N.; Gabrielson, K. Cardiac-specific over-expression of epidermal growth factor receptor 2 (ErbB2) induces pro-survival pathways and hypertrophic cardiomyopathy in mice. PLoS ONE 2012, 7, e42805. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Sorrentino, R.; Franceschelli, S.; Del Pizzo, M.; Pinto, A.; Popolo, A. Doxorubicin-Mediated Cardiotoxicity: Role of Mitochondrial Connexin 43. Cardiovasc. Toxicol. 2015, 15, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wei, S.; Zhang, B.; Li, W. Molecular Mechanisms of Cardiomyocyte Death in Drug-Induced Cardiotoxicity. Front Cell Dev. Biol. 2020, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Rodríguez-Sinovas, A.; Marzocco, S.; Ciccarelli, M.; Iaccarino, G.; Pinto, A.; Popolo, A. Cardiotoxic Effects of Short-Term Doxorubicin Administration: Involvement of Connexin 43 in Calcium Impairment. Int. J. Mol. Sci. 2017, 18, 2121. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, D.; Patron, M.; Rizzuto, R. Structure and function of the mitochondrial calcium uniporter complex. Biochim. Biophys. Acta 2015, 1853, 2006–2011. [Google Scholar] [CrossRef]
- Negro, A.; Brar, B.K.; Lee, K.F. Essential roles of Her2/erbB2 in cardiac development and function. Recent Prog. Horm. Res. 2004, 59, 1–12. [Google Scholar] [CrossRef]
- Holbro, T.; Hynes, N.E. ErbB receptors: Directing key signaling networks throughout life. Annu. Rev. Pharmacol Toxicol. 2004, 44, 195–217. [Google Scholar] [CrossRef]
- Pecoraro, M.; Ciccarelli, M.; Fiordelisi, A.; Iaccarino, G.; Pinto, A.; Popolo, A. Diazoxide Improves Mitochondrial Connexin 43 Expression in a Mouse Model of Doxorubicin-Induced Cardiotoxicity. Int. J. Mol. Sci. 2018, 19, 757. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Y.; Cai, X.; Gao, Z.; Yu, Z.; Wei, B.; Tang, Y.; Hu, L.; Liu, W.T.; Gu, Y. Uric acid preconditioning alleviated doxorubicin induced JNK activation and Cx43 phosphorylation associated cardiotoxicity via activation of AMPK-SHP2 signaling pathway. Annu. Transl. Med. 2020, 8, 1570. [Google Scholar] [CrossRef]
- Carrizzo, A.; Moltedo, O.; Damato, A.; Martinello, K.; Di Pietro, P.; Oliveti, M.; Acernese, F.; Giugliano, G.; Izzo, R.; Sommella, E.; et al. New Nutraceutical Combination Reduces Blood Pressure and Improves Exercise Capacity in Hypertensive Patients Via a Nitric Oxide-Dependent Mechanism. J. Am. Heart Assoc. 2020, 9, e014923. [Google Scholar] [CrossRef]
- Decrock, E.; Vinken, M.; De Vuyst, E.; Krysko, D.V.; D’Herde, K.; Vanhaecke, T.; Vandenabeele, P.; Rogiers, V.; Leybaert, L. Connexin-related signaling in cell death: To live or let die? Cell Death Differ. 2009, 16, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Thevenin, A.F.; Kowal, T.J.; Fong, J.T.; Kells, R.M.; Fisher, C.G.; Falk, M.M. Proteins and mechanisms regulating gap-junction assembly, internalization, and degradation. Physiology 2013, 28, 93–116. [Google Scholar] [CrossRef]
- Pogoda, K.; Kameritsch, P.; Retamal, M.A.; Vega, J.L. Regulation of gap junction channels and hemichannels by phosphorylation and redox changes: A revision. BMC Cell Biol. 2016, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Lampe, P.D.; Lau, A.F. The effects of connexin phosphorylation on gap junctional communication. Int. J. Biochem. Cell Biol. 2004, 36, 1171–1186. [Google Scholar] [CrossRef]
- Vinken, M.; Vanhaecke, T.; Papeleu, P.; Snykers, S.; Henkens, T.; Rogiers, V. Connexins and their channels in cell growth and cell death. Cell Signal. 2006, 5, 592–600. [Google Scholar] [CrossRef]
- Rodríguez-Sinovas, A.; Sánchez, J.A.; Valls-Lacalle, L.; Consegal, M.; Ferreira-González, I. Connexins in the Heart: Regulation, Function and Involvement in Cardiac Disease. Int. J. Mol. Sci. 2021, 22, 4413. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, E.; Yano, T.; Sato, H.; Hagiwara, K.; Yamasaki, H.; Shirai, S.; Fukumoto, K.; Hagiwara, H.; Negishi, E.; Ueno, K. Cytotoxic effect of the Her-2/Her-1 inhibitor PKI-166 on renal cancer cells expressing the connexin 32 gene. J. Pharmacol. Sci. 2005, 97, 294–298. [Google Scholar] [CrossRef]
- Rackauskas, M.; Neverauskas, V.; Skeberdis, V.A. Diversity and properties of connexin gap junction channels. Medicina 2010, 46, 1. [Google Scholar] [CrossRef]
- Engelmann, T.W. Vergleichende Untersuchungen zur Lehre von der Muskel und Nervenelektricität. Pflug. Arch. 1877, 15, 116–148. [Google Scholar] [CrossRef]
- Kauffman, M.E.; Kauffman, M.K.; Traore, K.; Zhu, H.; Trush, M.A.; Jia, Z.; Li, Y.R. MitoSOX-Based Flow Cytometry for Detecting Mitochondrial ROS. React. Oxyg. Species 2016, 2, 361–370. [Google Scholar]
- Pecoraro, M.; Marzocco, S.; Popolo, A. Diazoxide Needs Mitochondrial Connexin43 to Exert Its Cytoprotective Effect in a Cellular Model of CoCl2-Induced Hypoxia. Int. J. Mol. Sci. 2021, 22, 11599. [Google Scholar] [CrossRef]
- Pecoraro, M.; Pinto, A.; Popolo, A. Inhibition of Connexin 43 translocation on mitochondria accelerates CoCl2-induced apoptotic response in a chemical model of hypoxia. Toxicol. In Vitro 2018, 47, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Popolo, A.; Piccinelli, A.L.; Morello, S.; Sorrentino, R.; Osmany, C.R.; Rastrelli, L.; Pinto, A. Cytotoxic activity of nemorosone in human MCF-7 breast cancer cells. Can. J. Physiol. Pharmacol. 2011, 89, 50–57. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecoraro, M.; Marzocco, S.; Franceschelli, S.; Popolo, A. Trastuzumab and Doxorubicin Sequential Administration Increases Oxidative Stress and Phosphorylation of Connexin 43 on Ser368. Int. J. Mol. Sci. 2022, 23, 6375. https://doi.org/10.3390/ijms23126375
Pecoraro M, Marzocco S, Franceschelli S, Popolo A. Trastuzumab and Doxorubicin Sequential Administration Increases Oxidative Stress and Phosphorylation of Connexin 43 on Ser368. International Journal of Molecular Sciences. 2022; 23(12):6375. https://doi.org/10.3390/ijms23126375
Chicago/Turabian StylePecoraro, Michela, Stefania Marzocco, Silvia Franceschelli, and Ada Popolo. 2022. "Trastuzumab and Doxorubicin Sequential Administration Increases Oxidative Stress and Phosphorylation of Connexin 43 on Ser368" International Journal of Molecular Sciences 23, no. 12: 6375. https://doi.org/10.3390/ijms23126375
APA StylePecoraro, M., Marzocco, S., Franceschelli, S., & Popolo, A. (2022). Trastuzumab and Doxorubicin Sequential Administration Increases Oxidative Stress and Phosphorylation of Connexin 43 on Ser368. International Journal of Molecular Sciences, 23(12), 6375. https://doi.org/10.3390/ijms23126375






