Abstract
As a common abiotic stress, drought severely impairs the growth, development, and even survival of plants. Here we report a transcription factor, Caragana korshinskii REVOLUTA(CkREV), which can bidirectionally regulate the expression of the critical enzyme gene CkYUC5 in auxin synthesis according to external environment changes, so as to control the biosynthesis of auxin and further enhance the drought resistance of plants. Quantitative analysis reveals that the expression level of both CkYUC5 and AtYUC5 is down-regulated after C. korshinskii and Arabidopsis thaliana are exposed to drought. Functional verification of CkREV reveals that CkREV up-regulates the expression of AtYUC5 in transgenic A. thaliana under common conditions, while down-regulating it under drought conditions. Meanwhile, the expression of CkYUC5 is also down-regulated in C. korshinskii leaves instantaneously overexpressing CkREV. We apply a dual-luciferase reporter system to discover that CkREV can bind to the promoter of CkYUC5 to regulate its expression, which is further proved by EMSA and Y1H esxperiments. Functional verification of CkREV in C. korshinskii and transgenic A. thaliana shows that CkREV can regulate the expression of CkYUC5 and AtYUC5 in a contrary way, maintaining the equilibrium of plants between growth and drought resisting. CkREV can positively regulate the expression of CkYUC5 to promote auxin synthesis in favor of growth under normal development. However, CkREV can also respond to external signals and negatively regulate the expression of CkYUC5, which inhibits auxin synthesis in order to reduce growth rate, lower water demands, and eventually improve the drought resistance of plants.
1. Introduction
Auxin primarily originates from Greek, meaning growth [1]. It is irreplaceable during plant growth and development, which influences plant apical growth, axillary bud formation, floral organ development, and root development [2,3,4,5]. On the cell level, auxin is competent in changing the plasticity of plant cells, facilitating cells to differentiate and elongate. Besides, auxin can also compose a sophisticated regulatory network together with diverse kinds of plant hormones, collectively accommodating plants to their surroundings [6]. Auxin biosynthesis mainly depends on the tryptophan pathway consisting of four chief branches, each of which can synthesize indoleacetic acid (IAA) catalyzed by different enzymes [2,3,7]. So far, only the IpyA pathway has been clearly proved. It mainly includes two steps. First, tryptophan is reversibly transformed to IpyA through transamination catalyzed by TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS (TAA). Then, IpyA is irreversibly transformed to IAA through oxidative decarboxylation by YUCCA (YUC), a rate-limiting enzyme [8,9]. TAA/YUC pathway has been functionally proved in various plants serving as the chief biosynthesis pathway for endogenous auxin [10,11,12,13,14]. YUC5, one protein from the YUC family, plays an important role in regulating auxin biosynthesis.
ABA, salicylic acid, and ethylene are involved in plants’ responses to drought stress, and so is auxin [15,16]. Under stress conditions, WES1, a gene encoding IAA–amido synthase, from the GH3 gene family is up-regulated to inactivate IAA by binding it to an amino acid, which lowers the level of endogenous auxin and activates the expression of stress-related genes PR-1 and CBF [17]. Aux/IAA and ARF are two significant protein families mediating auxin response, which directly regulate the expression of auxin early response genes. 31 OsIAA genes and 25 OsARF genes have been identified in Oryza sativa, among which OsIAA2 and OsIAA20 are up-regulated under high salinity conditions. The expression level of O. sativa TLD1 from the GH3.13 gene family is inhibited in tissues above ground under normal conditions while it is remarkably induced under drought conditions. The activation of the TLD1 gene leads to the reduction in IAA concentration and the change of plant shape in O. sativa tld1–D gain-of-function mutant, which decreases plant water loss and improves survival rate [18]. During seed germination, ntm2–1 mutant possesses strong salt resistance. Salt stress can induce NTM2 to specifically bind to the promoter of IAA30 and activate its high expression. Nevertheless, the high expression of IAA30 induced by NaCl disappears in the ntm2-1 mutant, which attenuates the inhibition of auxin on seed germination [19]. Moreover, TCPs are able to activate the expression of auxin synthesis genes, for example, YUC8, by facilitating the transcription activity of PIF4 under high-temperature stress [20].
HD-ZIP III family significantly regulates the pattern formation of embryo, root, stem, and vascular bundle together with leaf development [21,22,23]. It was first reported in 1995 that ATHB 8, a member of the HD-ZIP III family in A. thaliana, could express after it was induced by auxin [24]. Further research found that auxin flow induced the expression of MONOPTEROS (MP), the latter would induce the expression of PIN-FORMED 1 (PIN1), and polarly localized PIN1 at a high expression level would promote the polarity flow of auxin. MP directly binds to the promoter of ATHB8 to induce its expression while inducing PIN1. Interestingly, up-regulated AtHB8 reduces the sensitivity of MP–induced PIN1 to auxin, thus limiting auxin flow to a narrow scope [25] and stimulating precursor cells of procambium to differentiate into xylem cells at designated regions. The expression of other HD-ZIP III members, such as ATHB15, PHV, PHB, and REV/IFL, is down-regulated in mp mutants, which can be considered to be regulated by MP [26]. Besides, auxin biosynthesis genes TAR1, TAR2, and YUCCA3, 5, 7, 8, 9 are indispensable for high expression of HD-ZIP III in primary root and the formation of metaxylem in A. thaliana [27]. Meanwhile, the HD-ZIP III family shares the same expression pattern with auxin [28,29,30]. Hence, the achievement of the function of the HD-ZIP family possesses a close relationship with auxin.
Drought is one of the major abiotic stresses. It triggers water deficiency that poses a severe threat to plants’ survival and yield [31]. C.korshinskii is widely distributed in relatively harsh environments. It has a variety of stress tolerance characteristics through adaptive evolution and plays an extremely important role in protecting the ecological environment and completing the ecological restoration [32]. It is an ideal material for studying the formation mechanism of plant adversity adaptation. Previous studies on C. korshinskii mainly focused on morphological anatomy, physiological ecology, etc., and its molecular regulation mechanism in response to abiotic stress needs to be further studied. Research focusing on the relationship between auxin and plant response to stress has been increasingly emphasized in recent years. Studies concentrating on the relationship between drought response genes and plant hormones in A. thaliana unveil that although the ABA-dependent pathway dominates plant response to drought stress, other plant hormones including auxin also have an impact on the expression of genes related to drought resistance [33]. Abundant studies have indicated that the synthesis and critical response genes of auxin are regulated by environmental stress, however, the response to stress of auxin and the regulatory mechanism thereof at the molecular level still requires elucidation. This paper reports a transcription factor called CkREV, a member of the HD-ZIP III family which can bidirectionally regulate the expression of the critical enzyme CkYUC5 in auxin synthesis according to external environment changes in C. korshinskii, a drought-resisting pioneer plant widely spread among the desert area in northwest China, and its mechanism of maintaining the equilibrium of plants between growth and drought resisting by controlling auxin biosynthesis.
2. Results
2.1. HD-ZIP III TFs Phylogenetic Analysis
We constructed a phylogenetic tree including transcription factors of the HD-ZIP III family in C. korshinskii, Glycine max, Cicer arietinum, Medicago truncatula, Cajanus cajan, Camellia sinensis, and A. thaliana (Figure 1). 31 HD-ZIP III proteins derived from different species were categorized into four branches, among which the members of the HD-ZIP III family in C. korshinskii exhibited a relatively close relationship with those in G. max and M. truncatula, located in the same branch. On the contrary, REV exhibited a relatively distant relationship with other members of the HD-ZIP III family, implying that it probably possessed unknown regulatory functions different from others. We analyzed the conserved domain of the transcription factors of the HD-ZIP III family members of the above species through the pfam (http://pfam.xfam.org/) (accessed on 19 August 2021). The HD-ZIP III family of C. korshinskii including CkREV, has basically the same number and distribution of motifs as other species (Figure 2), and CkREV subcellular localization is also in the nucleus (Figure 3), which indicates that they may have similar biological functions.
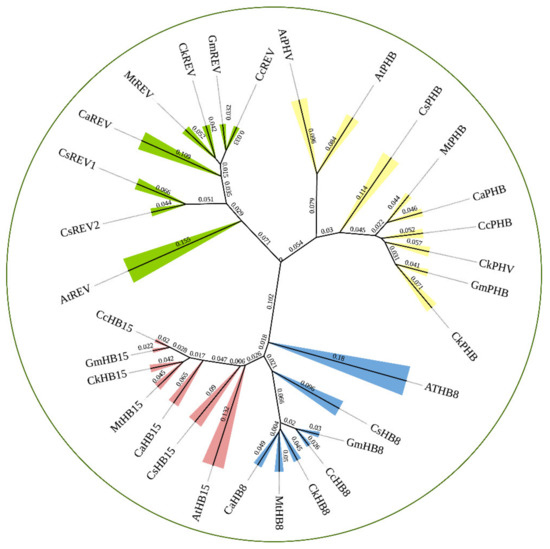
Figure 1.
Phylogenetic analysis of HD-ZIP III family between C. korshinskii, G. max, C. arietinum, M. truncatula, C. cajan, C. sinensis, and A. thaliana. Bootstrap support (1000 repetitions) is shown for each node.
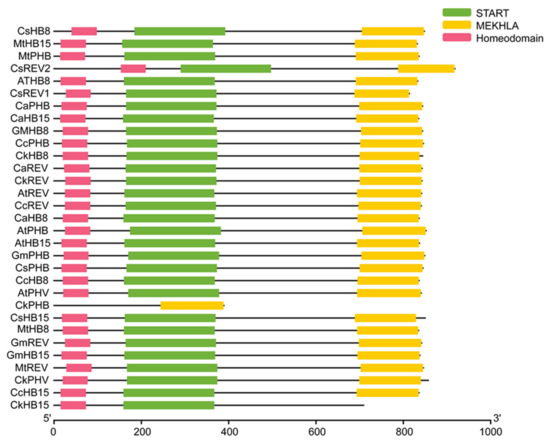
Figure 2.
Conserved domain analysis of HD-ZIP III family between C. korshinskii, G. max, C. arietinum, M. truncatula, C. cajan, C. sinensis, and A. thaliana.
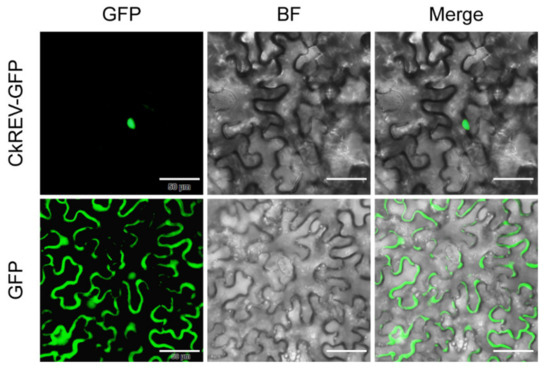
Figure 3.
CkREV subcellular localization observation. Scale bars, 50 μm.
2.2. CkREV Balances Plant Growth and Stress Resistance by Regulating the Expression of CkYUC5
Under stress conditions, WES1, a gene encoding IAA–amido synthase, from the GH3 family is up-regulated to inactivate IAA by binding it to an amino acid, which lowers the level of endogenous auxin [16]. In addition to scavenging the existing IAA, can plants enhance their stress resistance by reducing their biosynthesis under stress? First, we treated C. korshinskii with drought conditions in order to explore the expression of the critical enzyme gene CkYUC5 in auxin synthesis. We performed qRT–PCR to detect the expression level of CkYUC5 (Figure 4a), which suggested that CkYUC5 was down-regulated under drought conditions and auxin synthesis may be inhibited. The same outcomes were proved in A. thaliana, as it dropped precipitously with increasingly severe drought treatment (Figure 4b). In A. thaliana, the HD-ZIP III family has the same expression pattern as auxin [27,29,30]. Auxin biosynthesis genes TAR1, TAR2, and YUCCA3, 5, 7, 8 and 9 are necessary for the high expression of the HD-ZIP III family and the formation of metaxylem in A. thaliana primary roots [26], nevertheless, a member of the HD-ZIP III family called REV can directly bind to the promoter region of YUC5 [34] and LAX2, 3 to regulate the synthesis and transport of auxin, respectively [35]. These two processes are closely related to the HD-ZIP III family, but the crosstalk between them under stress conditions has not been reported. In this study, we discovered that the expression level of CkREV, a member of the HD-ZIP family, was continuously up-regulated with increased drought levels (Figure 4c). Does CkREV mediate the negative regulation of CkYUC5 or not?
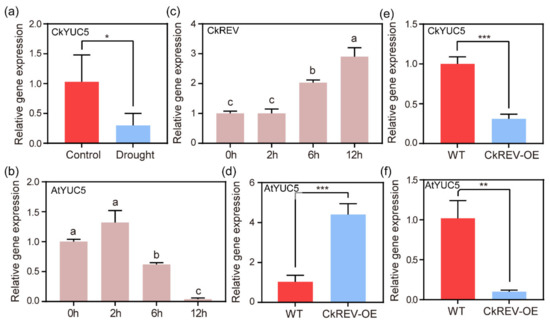
Figure 4.
qRT–PCR analysis on the expression level of relevant genes. (a) Analysis of the expression level of CkYUC5 in C. korshinskii leaves after natural drought treatment; (b) Analysis of the expression level of AtYUC5 in A. thaliana seedlings after cultured on 1/2 MS medium for 7 days and transferred to PEG medium for simulated drought treatment; (c) Analysis of the expression level of CkREV in hydroponic C. korshinskii after drought simulation on PEG medium; (d) Analysis of the expression level of AtYUC5 in A. thaliana CkREV–OE lines at the age of 4 weeks; (e) Analysis of the expression level of CkYUC5 in C. korshinskii leaves instantaneously overexpressing CkREV; (f) Analysis of the expression level of AtYUC5 in wild type and transgenic A. thaliana after drought treatment. (a,d–f) Data are shown as the mean ± SD of three independent experiments. Student’s t–test is employed to measure statistical significance between two samples with confidence level at 0.95 (*, p < 0.05; **, p < 0.01; ***, p < 0.001). (b,c) Data are shown as the mean ± SD of three independent experiments. One–way ANOVA was performed for the statistical analysis, where different letters represent significant differences (p < 0.05).
We overexpressed CkREV in A. thaliana and performed qRT–PCR to test the expression level of AtYUC5 in transgenic A. thaliana, the outcome of which indicated that the expression level of AtYUC5 in CkREV–OE lines was conspicuously up-regulated (Figure 4d). Meanwhile, we instantaneously transformed CkREV into C. korshinskii leaves through plasmolysis and deplasmolysis to test the expression level of endogenous auxin synthetase gene CkYUC5 after CkREV was overexpressed. Intriguingly, the expression level of CkYUC5 is considerably down-regulated by CkREV (Figure 4e). The method we used to transform CkREV into C. korshinskii leaves caused osmotic stress similar to drought stress, which brought about the question of whether the contrary regulation mechanisms in CkREV stably transformed A. thaliana and instantaneously transformed the original plant were the consequences of osmotic stress during the transformation process. We made further efforts to raise wild type and CkREV–transformed A. thaliana under drought conditions and perceived that the expression of AtYUC5 was remarkably down-regulated by CkREV under drought response (Figure 4f) while it was exceedingly up-regulated in normally cultured A. thaliana CkREV–OE lines.
2.3. CkREV Affects Auxin Biosynthesis by Regulating CkYUC5 and Inhibiting A. thaliana Root Length under Stress
The promoter of CkYUC5 was constructed using the pCambia1305 vector, and the expression pattern of CkYUC5 gene was observed by the expression of the β–glucuronidase (GUS) gene. Under normal culture conditions, CkYUC5 was abundantly expressed in the tip of tobacco leaves, which could be further up-regulated by the overexpression of CkREV. Consistent with the previous qRT–PCR results, the accumulation of GUS signals guided by the CkYUC5 promoter was significantly inhibited after PEG treatment (Figure 5a–d,h). The root length of transgenic A. thaliana after PEG treatment was further analyzed. Under normal culture conditions, the A. thaliana CkREV–OE line showed no significant difference in root length compared with the wild–type, but after PEG treatment, the root length of A. thaliana CkREV–OE line was inhibited (Figure 5e,f). In A. thaliana, the balance between cell division and differentiation depends on the mutual regulation of the hormone cytokinin and auxin [36]. A previous study found that the free IAA content in the roots of A. thaliana yucQ mutants decreased by 55% compared to the wild type, and the lack of auxin significantly inhibited root elongation [37]. In order to further verify whether the inhibition of root length of the A. thaliana CkREV–OE line after PEG treatment was related to auxin deficiency, the transgenic A. thaliana was treated with PEG while adding 0.05 mg/L NAA, and the root length of A. thaliana CkREV–OE line returned to the level of wild type (Figure 5g,i). At the same time, the auxin content was detected in the A. thaliana seedlings treated as above. Not exactly as expected, under normal culture conditions, the IAA content in the A. thaliana CkREV–OE line did not show a significant difference from WT, but after drought stress treatment, the IAA content in the CkREV–OE line was decreased and significantly lower than WT (Figure 5j and Figure S1). In summary, CkREV affects the biosynthesis of auxin on the expression of CkYUC5 in a different environment and plays an important role in regulating plant growth and stress adaptation.
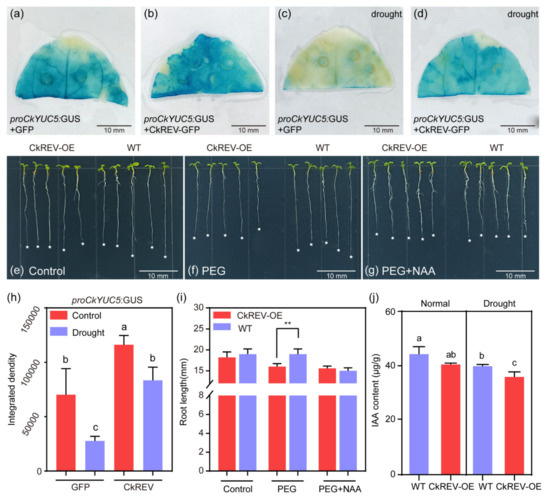
Figure 5.
CkREV bidirectionally regulates the expression of CkYUC5 and inhibits the A. thaliana root length under drought stress. (a–d) Observe the expression changes of CkYUC5 under different treatment conditions through tobacco leaves cultured for 28 days (n = 3 biologically independent samples); (a) Localization of CkYUC5 in the tip of tobacco leaf under normal culture conditions; (b) Localization of CkYUC5 in the tip of tobacco leaf after CkREV overexpression; (c) Localization of CkYUC5 in the tip of tobacco leaf after PEG treatment; (d) Localization of CkYUC5 in the tip of tobacco leaf after overexpression of CkREV treated with PEG; (e–g) Four days after germination on 1/2MS plates, the phenotype of root length change of A. thaliana CkREV–OE strain and wild type under normal culture conditions, PEG treatment and PEG treatment with NAA added for 3 days (n = 5 biologically independent samples); (h) GUS staining statistics of A. thaliana CkREV–OE strain and wild type under different treatments; (i) Root length statistics of A. thaliana CkREV–OE strain and wild type under different treatments; (j) IAA content determination. Scale bars in (a–g), 10 mm. (i) Data are shown as the mean ± SD of five independent experiments; Student’s t–test is employed to measure statistical significance between two samples with a confidence level of 0.95 (**, p < 0.01). (h,j) Data are shown as the mean ± SD of three independent experiments. One–way ANOVA was performed for the statistical analysis, where different letters represent significant differences (p < 0.05).
2.4. CkREV Interacts with the Promoter of CkYUC5 to Regulate Its Expression
The following critical question consists of whether CkREV directly or indirectly regulates CkYUC5. Since there was no public genome information about C. korshinskii, we firstly cloned the promoter of CkYUC5 by genome walking and successfully obtained the unknown promoter sequence for 1144 bp in total upstream from CkYUC5 after two rounds of nested PCR (Figure S2). We predicted transcription factor families probably regulating CkYUC5 promoter region by PlantTFDB (http://planttfdb.gao-lab.org/) (accessed on 23 March 2021) and found 27 appropriate ones in aggregate, among which members of ERF and WRKY family extensively regulated that region (Table S1), in which a large number of light response elements were predicted by PlantCare as well (Figure S3). However, we also discovered that transcription factors from the HD-ZIP family potentially regulated it. We found that ATGAT is necessary for the binding of AtREV by searching its binding site on PLANT PAN (http://plantpan.itps.ncku.edu.tw/) (accessed on 26 March 2021) (Figure 6a). Being a homologous gene of AtREV, CkREV possesses a relatively conservative binding site. We constructed a Dual-luciferase reporter system with cloned CkYUC5 promoter (Figure 6b) and injected it into Nicotiana tabacum to detect the activity of firefly luciferase and renilla luciferase. It turned out that CkREV could interact with CkYUC5 and the former negatively regulated the expression of the latter remarkably (Figure 6c). We also discovered that the promoter region of CkYUC5 possessed an ATGAT element, namely the binding site of CkREV. Based on that, we synthesized probes containing the core element and adjacent sequence (Figure 6d) with a biotin label linked to its 3′ end and cold probes without a biotin label for EMSA experiments in order to prove their interaction (Figure 6e). It turned out that CkREV could bind to the probes with a biotin label while cold probes competed with them. Therefore, it is proved that CkREV can bind to the ATGAT element in the promoter region of CkYUC5 and regulate its expression. At the same time, we also used the yeast one-hybrid method for supplementary verification (Figure 6f), which further confirmed the regulation of CkREV on CkYUC5.
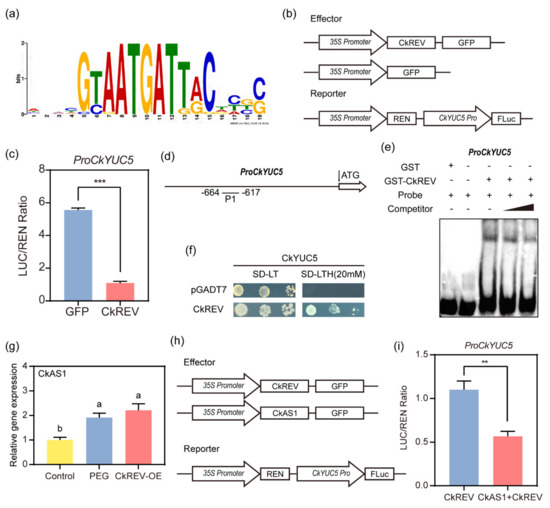
Figure 6.
Interaction proof between CkREV and CkYUC5. (a) Recognition site of downstream genes of REV; (b) Dual–LUC experimental mode diagram; (c) Results of Dual–LUC experiment CkREV interacted with the promoter region of CkYUC5 and negatively regulated its expression compared with GFP control group; (d) Sketch map of probe binding site in EMSA experiment; (e) Results of EMSA experiment indicated that CkREV–GST could directly bind to ATGAT element in the promoter region of critical enzyme gene CkYUC5 in auxin synthesis; (f) The results of yeast one–hybrid further verified the binding of CkREV to the promoter region of CkYUC5; (g) qRT–PCR detection on CkAS1 expression level in C. korshinskii leaves under different treatments; (h) Dual–LUC experimental mode diagram; (i) The Dual–LUC experiment was used to detect the effect of CkAS1 as an effector on the regulation of CkREV on CkYUC5. (c,e,g,i) Data are shown as the mean ± SD of three independent experiments. (c,i) Data are shown as the mean ± SD of three independent experiments. Student’s t–test is employed to measure statistical significance between two samples with a confidence level of 0.95 (**, p < 0.01; ***, p < 0.001). (g) Data are shown as the mean ± SD of three independent experiments. One–way ANOVA was performed for the statistical analysis, where different letters represent significant differences (p < 0.05).
Interestingly, as a transcriptional activator, CkREV can negatively regulate the expression of CkYUC5. We speculate that there are other transcription factors involved in the regulation of CkREV. CkAS1, which is closely related to the function of CkREV, encodes a R2–R3 MYB domain protein that inhibits transcription. The results of qRT–PCR found that under PEG–simulated drought conditions CkAS1 in C. korshinskii leaves was significantly up-regulated, which is seen as CkREV was transiently overexpressed as well (Figure 6g). Therefore, CkAS1 may be involved in the negative regulation of CkREV on CkYUC5. Based on the previous Dual–LUC experiment, we injected two effectors CkAS1 and CkREV into tobacco and detected the expression of the reporter gene. Compared with CkREV alone CkREV+CkAS1 further down-regulated the expression of CkYUC5 (Figure 6h,i).
2.5. CkREV Down–Regulates the Expression of YUC5 to Enhance the Drought-Resisting Ability of Plants under Drought Response
Based on the results above, we discovered that CkREV would respond to external signals with improved expression levels when plants were confronted with drought stress. Its regulation on the expression of CkYUC5 would transfer from positive to negative, which participates in the process of auxin reduction under stress conditions. So, what contributions do negative regulation of CkREV on auxin synthesis make to the adaptation of plants to drought stress? We treated transgenic A. thaliana with drought and detected the level of ROS in leaves by DAB (Figure 7a) and NBT (Figure 7b) staining. It turned out that A. thaliana CkREV–OE lines possessed ROS at a considerably lower level under drought stress. We also determined several drought resistance indexes for transgenic A. thaliana, such as proline content (Figure 7c) and relative water content (Figure 7d), the results of which indicated that CkREV–OE lines possessed rather exceptional traits for drought resistance, exhibiting excellent capability to adapt to drought stress. In summary, CkREV is conducive to the biosynthesis of auxin and the acceleration of plant growth by positively regulating the expression of the CkYUC5 gene during normal development. However, when plants are subjected to drought stress, CkREV negatively regulates the expression of CkYUC5 by sensing external signals and inhibits the biosynthesis of auxin, thereby slowing the growth rate of plants, reducing their demand for water, and enhancing the ability of plants to adapt to drought.
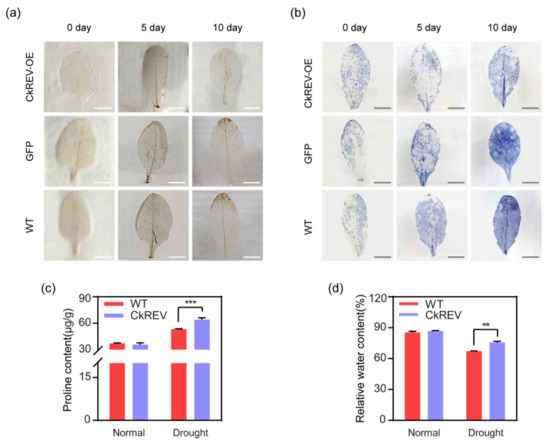
Figure 7.
Phenotype analysis on transgenic A. thaliana. (a) DAB staining on rosette leaves at the same position after natural drought treatment for 0, 5, and 10 days (n = 3 biologically independent samples); (b) NBT staining on rosette leaves at the same position after natural drought treatment for 0, 5, and 10 days (n = 3 biologically independent samples); (c) Results of proline content determination; (d) Results of relative water content determination. (c,d) Data are shown as the mean ± SD of three independent experiments. Scale bars in (a,b), 5 mm. (c,d) Data are shown as the mean ± SD of three independent experiments. Student’s t–test is employed to measure statistical significance between two samples with a confidence level of 0.95 (**, p < 0.01; ***, p < 0.001).
3. Discussion
The synthesis and metabolism, homeostasis regulation, polar transport, and signal transduction of auxin collectively influence its distributing gradient and how plants respond to it, which plays an indispensable role throughout the growth and development of plants. Auxin was primarily discovered to facilitate the growth of stems and roots [1]. Gradually, people perceived that it impacted a variety of physiological activities in plants, including senescence controlling, responses to abiotic stress and pathogens, formation of fruits, establishment and maintenance of cell polarity, apical dominance, phototropism, geotropism, etc. [4,5,38,39,40,41]. Auxin is synthesized in immature tissues such as tender leaves, cotyledons, and roots [42,43,44,45,46]. For roots, the synthesis of auxin aids in the maintenance of its concentration gradient, which is tremendously significant for normal growth and development [3].
Auxin also possesses a close relationship with stress. Recent research has indicated that growth retardation is the direct consequence of osmotic stress [47]. Abundant studies reveal that asymmetrical distribution of auxin is critical for plant development [48]. Overexpression of ZmPIN1a in Zea mays lowers the height of maize, increases the number of lateral roots, and inhibits their elongation, which assists in forming a well-developed root system and which improves its resistance to drought, lodging, and low phosphate environment [49]. Under stress conditions, the expression of the WES1 gene encoding IAA-amido synthase from the GH3 family is up-regulated, deactivating IAA by catalyzing its binding to an amino acid, which enhances plant resistance through activating the expression of stress-related genes PR-1 and CBF by lowering the level of endogenous auxin [17]. Direct determination of the content of endogenous IAA in leaves and roots indicates that plants living under salt stress and water deficiency conditions possess IAA at an apparently low level [50,51]. Further studies reveal that O. Sativa possesses seven YUC genes for auxin biosynthesis, six of which exhibit low expression levels under dry conditions [50]. The HD-ZIP III family shares the same expression pattern with auxin [28,29,30], the function of which is closely related to the synthesis and transport of auxin. In contrast to previous studies, we are surprised to find that CkREV, a member of the HD-ZIP III family, can bidirectionally regulate the expression of genes critical for auxin biosynthesis under normal and drought conditions (Figure 8). In the A. thaliana CkREV–OE line, the expression level of AtYUC5 was significantly up-regulated, while after drought treatment, the expression level of AtYUC5 shifted to be significantly down-regulated by CkREV (Figure 4d,f).
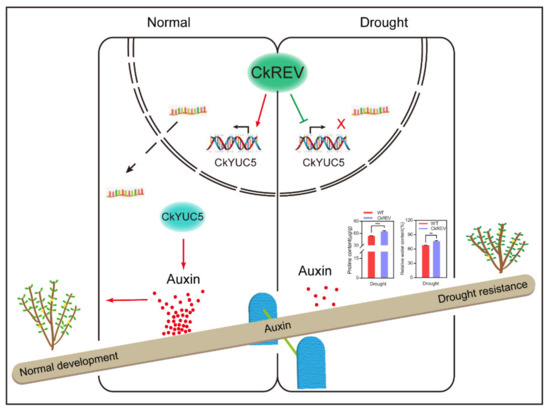
Figure 8.
Model of regulation of CkREV on the expression of CkYUC5. The model concludes our research and exhibits that CkREV bidirectionally regulates the expression of CkYUC5 critical in auxin synthesis depending on changes in external environmental signals, balancing the growth and drought-resistance of plants by influencing auxin synthesis.
Studies focusing on the relationship between drought response genes in A. thaliana and plant hormones uncover that nearly 100 genes can respond to drought stress while responding to IAA [33]. The expression of TLD1 from the GH3.13 gene family in O. Sativa is tremendously induced under drought stress. In O. Sativa tld1–D gain–of–function mutant, the activation of the TLD1 gene leads to the decrease of IAA concentration and the change of plant shape, which abates water loss and improves survival rate [18].
Contrary to expectations, although the expression of AtYUC5 was up-regulated, CkREV did not promote auxin accumulation under normal culture conditions. This might be related to the fact that the biosynthesis of auxin was tightly regulated in plants [52]. Conversely, drought treatment revealed that the IAA content in the CkREV–OE line was significantly lower than that in WT, while low levels of IAA facilitated plant resistance against drought stress [18]. The same pattern of regulation was also applied to C. korshinskii CkYUC5 (Figure 5). C. korshinskii is a woody plant and is significantly different from A. thaliana in many aspects, including but not limited to responses toward biotic and abiotic stresses. In Arabidopsis, IAA can be synthesized by both tryptophan (Trp) -dependent and -independent ways, and the Trp–dependent way is much better characterized compared to the other one [14]. Although it is uncertain whether the remaining synthetic pathways are conserved in different species, as the major endogenous auxin biosynthesis pathway in plants, the conservation of the TAA/YUC pathway in the plant kingdom has been functionally checked in many plant species [10,11,12,13,14,53,54]. Therefore, under drought stress, CkREV downregulates auxin biosynthesis by negatively regulating the expression of YUC5, a key enzyme gene in the TAA/YUC pathway, and promotes plant adaptation under drought stress. This regulatory pattern may be conserved in many plant species.
Early in the 1970s, studies showed that plants with smaller cells possessed a stronger capability of resisting low water potential and water deficiency. Hence, rapid reaction to environmental changes and self-restriction on growth rate probably assist plants in surviving dry periods [55]. We utilized DAB and NBT staining to detect the level of ROS in A. thaliana leaves after drought treatment while determining relevant physiological indexes, the results of which indicated that A. thaliana CkREV–OE lines suffered less under drought conditions, exhibiting capability for drought adaptation (Figure 7).
4. Materials and Methods
4.1. Plant Materials
C. korshinskii samples employed originated from the experimental plot of Northwest A&F University, which was cultivated in soil after germination. Healthy and plump seeds were selected and washed with clean water. seeds were then wrapped up with wet gauze and left in a dark environment for germination for 3–5 days under room temperature before being transplanted into flowerpots.
A. thaliana samples employed were all Col-0 ecotype. The p35S:CkREV-GFP and p35S:GFP plasmids were transformed into Agrobacterium tumefaciens GV3101, which was used to infect A. thaliana Col-0 ecotype. Seeds were disinfected with 10% (v/v) sodium hypochlorite for 5 min and were sifted on 1/2 MS medium with hygromycin until homozygous T3 generation.
4.2. qRT–PCR Measurement
The total RNA of plant tissue was extracted by Plant RNA Isolation Kit (Beibei Bio, Zhengzhou, China). For each sample, we accurately absorbed 1 μg RNA according to RNA concentration of different treatments and repetitions. The first strand of cDNA was amplified by PrimeScriptTM RT reagent Kit with gDNA Eraser (Takara, Tokyo, Japan), and cDNA solution was diluted 3–5 times as the template of qRT-PCR. Quantitative analysis of the expression of relevant genes was performed on 2 × M5 HiPer Realtime PCR Super mix (Mei5bio, Beijing, China) and quantitative PCR Amplifier LightCycler 480 (Roche, Basel, Switzerland). Quantitative primers are listed in Table S2.
4.3. Genome Walking
We designed 3 specific R primers (SP1: TACCTAGCAGACTGAACACACTCGT, SP2: GAAGATGAAGCTTTAATCGGTCGTA, and SP3: TCTCTTAGGCGTACTGCTGTGGCTA), among which SP2 should be designed within SP1 and SP3 within SP2. There were no stringent requirements on the distance between every two primers, as 60–100 bp is preferable normally. Genome walking usually consists of nested PCR reactions for three rounds, each of which is thermal asymmetric interlaced PCR in two different annealing temperatures. A higher annealing temperature encourages the binding of specific primers while a lower one benefits that of universal primers. The sequence of universal primer and amplification program employed in genome walking complies with that in the literature cited [56].
4.4. GUS Staining
The CkYUC5 promoter was amplified from C. korshinskii genomic DNA and inserted into pCambia1305 vector. p35s::CkREV-GFP and p35S::GFP as effector plasmids were co-injected with pCkYUC5::GUS into tobacco leaves and cultured for 48 h. After different treatments, the GUS staining kit (Coolaber, Shanghai, China) was used to stain and observe the GUS expression level in the tip of the tobacco leaf.
4.5. IAA Content Detection
Four days after germination on 1/2 MS plates, the A. thaliana seedlings were further cultured for 3 days on plain MS medium and PEG–treated MS medium, respectively. Accurately weighing 0.1 g of A. thaliana seedlings before fully ground with liquid nitrogen, 0.9 mL of PBS (pH 7.4) solution was added to dissolve at a ratio of 1:9 (w/v), which was left at room temperature for 20 min to fully extract the IAA in the sample. Centrifuged at 3000 rpm for 20 min, the supernatant solution was the crude extract of plant IAA, and the IAA content in the plant was determined according to the instructions of the Plant Indole-3-acetic acid (IAA); Auxin ELISA Kit. (Jingmei, Yancheng, China).
4.6. Dual-LUC Assay
The promoters of target genes were amplified from the genome DNA of C. korshinskii and inserted into pGreen II 0800-LUC vector with p35s::CkREV–GFP and p35s::GFP used as effect plasmids. Reporter and effector were transformed into Agrobacterium tumefaciens GV3101 (pSoup-p19) and GV3101, respectively, which were cultured in a shaking incubator under constant temperature until the OD value was around 1.0. Bacteria were resuspended in solution with acetosyringone (AS) to OD 600 value was 0.8. Effector and reporter were combined in the proportion 8:2 and placed in a dark environment for activation for 2–4 h before being injected into leaves of Nicotiana tabacum at the age of 28 d. Samples were collected to test the activity of firefly luciferase and renilla luciferase by GloMax 20/20 Luminometer (Promega, Madison, USA) and Dual luciferase reporter assay system (Promega, Madison, WI, USA) reagents according to their instructions [57].
4.7. Electrophoretic Mobility Shift Assay (EMSA)
First, biotin label was linked to the 3′ end of artificially synthesized single-stranded oligonucleotide probe containing binding site by DNA 3′ end biotin label kit (Beyotime, Shanghai, China). Second, double-stranded DNA probe with biotin label was obtained by annealing with artificially synthesized complimentary chain. Third, purified CkREV protein was incubated with probe with biotin label at a certain proportion while unlabeled double-stranded probe was used as cold probe. Fourth, native-PAGE was employed to separate samples before being transferred onto nylon membrane (Solarbio, Beijing, China) with positive charge through wet transformation method. Fifth, the nylon membrane was placed under ultraviolet crosslinker purple (UVP, Upland, USA) at 254 nm, 120 mJ/cm2 for 60 s. Last, colour development was employed on a completely cross-linked nylon membrane by EMSA chemiluminescence kit (Beyotime, Shanghai, China) for observation under chemiluminescence imager. Detailed steps complied with instructions of EMSA chemiluminescence kit (Beyotime, Shanghai, China).
4.8. Yeast One–Hybrid
The transcription factor was cloned into the pGADT7–rec2 vector, and the promoter fragment to be verified was cloned into the pHIS2 vector. The above plasmids were co-transformed into yeast strain Y187 using the lithium acetate method, and screened by SD/–LT medium. The positive colonies that were successfully transformed were picked out in YPDA liquid medium, cultivated at 30 °C to OD 1.0, and diluted to 1:100 and 1:1000 three concentration gradients. We spotted the bacteria liquid on SD/–LTH plates containing 10-100 mM 3–AT for self-activation verification and performed experiments on the plate with 3–AT, the concentration of which inhibits self-activation.
4.9. DAB and NBT Staining
DAB can be oxidized by hydrogen peroxide into dark brown precipitates. Hence, DAB is employed as a dye to test the existence and distribution of hydrogen peroxide in plant cells. DAB solution was prepared at the concentration of 1 mg/mL and acidated by 0.2 M HCl to pH 3.0. 5 μL TWEEN–20 (0.05% v/v) and 0.5 mL 200 mM Na2HPO4 were added into the DAB solution while stirring, which produced DAB staining solution of 10 mM Na2HPO4 and increased pH again. Leaves were collected and absolutely immersed into DAB staining solution under vacuum and dark conditions for shake incubation for 4–5 h at the speed of 80–100 rpm, after which DAB solution was discarded and samples were bleached by solution (ethanol:acetic acid:glycerol = 3:1:1) before being photographed [58].
For NBT staining, leaves were immersed into 6 mM NBT solution prepared by citrate sodium buffer (pH 6.0) under vacuum and dark conditions for incubation for 5–8 h, after which NBT solution was discarded and samples were bleached by solution (ethanol:acetic acid:glycerol = 3:1:1) before being photographed [59].
5. Conclusions
The distinct expression patterns of YUC5 under diverse environments explained that auxin biosynthesis in plants was stringently regulated. CkREV responded to external environment changes and further influenced the expression of CkYUC5 and AtYUC5 in contrary ways, indicating that the sensitivity of CkREV to the environment determines the regulatory directions of its downstream genes. Accordingly, CkREV can enhance the expression of CkYUC5 in favor of plant growth during normal development while it can sense external signals to function conversely in order to decelerate plant growth and attenuate water demands confronted with drought stress. This research provides a novel pathway for expanding the nature of drought-resisting in C. korshinskii, offering choices of functional and regulatory genes for enhancing the drought-resistance of woody plants through biotechnology in desert areas from now on.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms23115902/s1.
Author Contributions
Experiments in this study were designed by C.-M.G. and J.-Y.L. J.-Y.L. accomplished the majority of experiments and the writing of the manuscript. J.-J.R. fostered plant materials and provided several experimental ideas. T.-X.Z. provided several experimental ideas. J.-H.C. translated the entire paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the National Natural Science Foundation of China (No.31770648 and 31070538), Key R&D Projects in Shaanxi Province (2020NY-190), and Key Scientific Research Projects of China Selenium Industry Research Institute (2019ZKG-6).
Data Availability Statement
The data presented in this study are available in the article or Supplementary Materials.
Acknowledgments
We earnestly thank everyone in the Tea Plant Development and Stress Biology Laboratory of Northwest A&F University for their assistance. We are grateful to B.H., M.L. and F.Z. (Horticulture Science Research Center, Northwest A&F University, Yangling, China) for their assistance with microscopy and other analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salehin, M.; Bagchi, R.; Estelle, M. SCFTIR1/AFB-based auxin perception: Mechanism and role in plant growth and development. Plant Cell 2015, 27, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olatunji, D.; Geelen, D.; Verstraeten, I. Control of Endogenous Auxin levels in plant root development. Int. J. Mol. Sci. 2017, 18, 2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brumos, J.; Robles, L.M.; Yun, J.; Vu, T.C.; Jackson, S.; Alonso, J.M.; Stepanova, A.N. Local Auxin biosynthesis is a key regulator of plant development. Dev. Cell 2018, 47, 306–318.e5. [Google Scholar] [CrossRef] [Green Version]
- Bu, H.; Yu, W.; Yuan, H.; Yue, P.; Wei, Y.; Wang, A. Endogenous auxin content contributes to larger size of apple fruit. Front. Plant Sci. 2020, 11, 592540. [Google Scholar] [CrossRef]
- Vanstraelen, M.; Benková, E. Hormonal interactions in the regulation of plant development. Annu. Rev. Cell. Dev. Biol. 2012, 28, 463–487. [Google Scholar] [CrossRef]
- Casanova-Sáez, R.; Voß, U. Auxin metabolism controls developmental decisions in land plants. Trends Plant Sci. 2019, 24, 741–754. [Google Scholar] [CrossRef]
- Zhao, Y.; Christensen, S.K.; Fankhauser, C.; Cashman, J.R.; Cohen, J.D.; Weigel, D.; Chory, J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 2001, 291, 306–309. [Google Scholar] [CrossRef]
- Zheng, Z.; Guo, Y.; Novák, O.; Dai, X.; Zhao, Y.; Ljung, K.; Noel, J.P.; Chory, J. Coordination of auxin and ethylene biosynthesis by the aminotransferase VAS1. Nat. Chem. Biol. 2013, 9, 244–246. [Google Scholar] [CrossRef] [Green Version]
- Phillips, K.A.; Skirpan, A.L.; Liu, X.; Christensen, A.; Slewinski, T.L.; Hudson, C.; Barazesh, S.; Cohen, J.D.; Malcomber, S.; McSteen, P. Vanishing tassel2 encodes a grass-specific tryptophan aminotransferase required for vegetative and reproductive development in maize. Plant Cell 2011, 23, 550–566. [Google Scholar] [CrossRef] [Green Version]
- Stepanova, A.N.; Yun, J.; Robles, L.M.; Novak, O.; He, W.; Guo, H.; Ljung, K.; Alonso, J.M. The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell 2011, 23, 3961–3973. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Villalobos, D.; Sankar, M.; Ljung, K.; Hardtke, C.S. Disturbed local auxin homeostasis enhances cellular anisotropy and reveals alternative wiring of auxin-ethylene crosstalk in Brachypodium distachyon seminal roots. PLoS Genet. 2013, 9, e1003564. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, T.; Ito, M.; Sumikura, T.; Nakayama, A.; Nishimura, T.; Kitano, H.; Yamaguchi, I.; Koshiba, T.; Hibara, K.; Nagato, Y.; et al. The rice FISH BONE gene encodes a tryptophan aminotransferase, which affects pleiotropic auxin-related processes. Plant J. 2014, 78, 927–936. [Google Scholar] [CrossRef]
- Zhao, Y. Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu. Rev. Plant. Biol. 2018, 69, 417–435. [Google Scholar] [CrossRef]
- Rowe, J.H.; Topping, J.F.; Liu, J.; Lindsey, K. Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin. New Phytol. 2016, 211, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wang, X.; Jia, H.; Li, F.; Ma, Y.; Liesche, J.; Liao, M.; Ding, X.; Liu, C.; Chen, Y.; et al. Persulfidation-induced structural change in SnRK2.6 establishes intramolecular interaction between phosphorylation and persulfidation. Mol. Plant 2021, 14, 1814–1830. [Google Scholar] [CrossRef]
- Park, J.E.; Park, J.Y.; Kim, Y.S.; Staswick, P.E.; Jeon, J.; Yun, J.; Kim, S.Y.; Kim, J.; Lee, Y.H.; Park, C.M. GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J. Biol. Chem. 2007, 282, 10036–10046. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.W.; Li, C.H.; Cao, J.; Zhang, Y.C.; Zhang, S.Q.; Xia, Y.F.; Sun, D.Y.; Sun, Y. Altered architecture and enhanced drought tolerance in rice via the down-regulation of indole-3-acetic acid by TLD1/OsGH3.13 activation. Plant Physiol. 2009, 151, 1889–1901. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, Y.S.; Kim, S.G.; Jung, J.H.; Woo, J.C.; Park, C.M. Integration of auxin and salt signals by the NAC transcription factor NTM2 during seed germination in Arabidopsis. Plant Physiol. 2011, 156, 537–549. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Yu, H.; Yuan, R.; Yang, Y.; An, F.; Qin, G. Arabidopsis transcription factor TCP5 controls plant thermomorphogenesis by positively regulating PIF4 activity. iScience 2019, 15, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Bowman, J.L.; Floyd, S.K. Patterning and polarity in seed plant shoots. Annu. Rev. Plant. Biol. 2008, 59, 67–88. [Google Scholar] [CrossRef]
- Carlsbecker, A.; Lee, J.Y.; Roberts, C.J.; Dettmer, J.; Lehesranta, S.; Zhou, J.; Lindgren, O.; Moreno-Risueno, M.A.; Vatén, A.; Thitamadee, S.; et al. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 2010, 465, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Smith, Z.R.; Long, J.A. Control of Arabidopsis apical-basal embryo polarity by antagonistic transcription factors. Nature 2010, 464, 423–426. [Google Scholar] [CrossRef] [Green Version]
- Baima, S.; Nobili, F.; Sessa, G.; Lucchetti, S.; Morelli, G. The expression of the Athb-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development 1995, 121, 4171–4182. [Google Scholar] [CrossRef]
- Donner, T.J.; Sherr, I.; Scarpella, E. Regulation of preprocambial cell state acquisition by auxin signaling in Arabidopsis leaves. Development 2009, 136, 3235–3246. [Google Scholar] [CrossRef] [Green Version]
- Mattsson, J.; Ckurshumova, W.; Berleth, T. Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol. 2003, 131, 1327–1339. [Google Scholar] [CrossRef] [Green Version]
- Ursache, R.; Miyashima, S.; Chen, Q.; Vatén, A.; Nakajima, K.; Carlsbecker, A.; Zhao, Y.; Helariutta, Y.; Dettmer, J. Tryptophan-dependent auxin biosynthesis is required for HD-ZIP III-mediated xylem patterning. Development 2014, 141, 1250–1259. [Google Scholar] [CrossRef] [Green Version]
- Heisler, M.G.; Ohno, C.; Das, P.; Sieber, P.; Reddy, G.V.; Long, J.A.; Meyerowitz, E.M. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 2005, 15, 1899–1911. [Google Scholar] [CrossRef] [Green Version]
- Floyd, S.K.; Bowman, J.L. Distinct developmental mechanisms reflect the independent origins of leaves in vascular plants. Curr. Biol. 2006, 16, 1911–1917. [Google Scholar] [CrossRef] [Green Version]
- Floyd, S.K.; Zalewski, C.S.; Bowman, J.L. Evolution of class III homeodomain-leucine zipper genes in streptophytes. Genetics 2006, 173, 373–388. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wang, L.; Li, L.; Wang, S. De novo assembly of the common bean transcriptome using short reads for the discovery of drought-responsive genes. PLoS ONE 2014, 9, e109262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Yin, J.; Li, G.; Qi, L.; Yang, F.; Wang, R.; Li, G. Reference gene selection for qRT-PCR in Caragana korshinskii Kom. under different stress conditions. Mol. Biol. Rep. 2014, 41, 2325–2334. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wu, W.; Abrams, S.R.; Cutler, A.J. The relationship of drought-related gene expression in Arabidopsis thaliana to hormonal and environmental factors. J. Exp. Bot. 2008, 59, 2991–3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, R.; Salla-Martret, M.; Bou-Torrent, J.; Musielak, T.; Stahl, M.; Lanz, C.; Ott, F.; Schmid, M.; Greb, T.; Schwarz, M.; et al. Genome-wide binding-site analysis of REVOLUTA reveals a link between leaf patterning and light-mediated growth responses. Plant J. 2012, 72, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Baima, S.; Forte, V.; Possenti, M.; Peñalosa, A.; Leoni, G.; Salvi, S.; Felici, B.; Ruberti, I.; Morelli, G. Negative feedback regulation of auxin signaling by ATHB8/ACL5-BUD2 transcription module. Mol. Plant 2014, 7, 1006–1025. [Google Scholar] [CrossRef] [Green Version]
- Dello Ioio, R.; Galinha, C.; Fletcher, A.G.; Grigg, S.P.; Molnar, A.; Willemsen, V.; Scheres, B.; Sabatini, S.; Baulcombe, D.; Maini, P.K.; et al. A PHABULOSA/cytokinin feedback loop controls root growth in Arabidopsis. Curr. Biol. 2012, 22, 1699–1704. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Dai, X.; De-Paoli, H.; Cheng, Y.; Takebayashi, Y.; Kasahara, H.; Kamiya, Y.; Zhao, Y. Auxin overproduction in shoots cannot rescue auxin deficiencies in Arabidopsis roots. Plant Cell Physiol. 2014, 55, 1072–1079. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Bai, Y.; Shen, C.; Wu, Y.; Zhang, S.; Jiang, D.; Guilfoyle, T.J.; Chen, M.; Qi, Y. Auxin-related gene families in abiotic stress response in Sorghum bicolor. Funct. Integr. Genom. 2010, 10, 533–546. [Google Scholar] [CrossRef]
- Leyser, O. Auxin Signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef] [Green Version]
- Ramos Báez, R.; Nemhauser, J.L. Expansion and innovation in auxin signaling: Where do we grow from here? Development 2021, 148, dev187120. [Google Scholar] [CrossRef]
- Retzer, K.; Weckwerth, W. The TOR-Auxin connection upstream of root hair growth. Plants 2021, 10, 150. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Robertson-Hoyt, J.; Yun, J.; Benavente, L.M.; Xie, D.Y.; Dolezal, K.; Schlereth, A.; Jürgens, G.; Alonso, J.M. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 2008, 133, 177–191. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Men, S.; Fischer, U.; Stepanova, A.N.; Alonso, J.M.; Ljung, K.; Grebe, M. Local auxin biosynthesis modulates gradient-directed planar polarity in Arabidopsis. Nat. Cell Biol. 2009, 11, 731–738. [Google Scholar] [CrossRef]
- Yamada, M.; Greenham, K.; Prigge, M.J.; Jensen, P.J.; Estelle, M. The TRANSPORT INHIBITOR RESPONSE2 gene is required for auxin synthesis and diverse aspects of plant development. Plant Physiol. 2009, 151, 168–179. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.B.; Geng, X.; He, C.; Zhang, F.; Wang, R.; Horst, W.J.; Ding, Z. TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis. Plant Cell 2014, 26, 2889–2904. [Google Scholar] [CrossRef] [Green Version]
- Blakeslee, J.J.; Spatola Rossi, T.; Kriechbaumer, V. Auxin biosynthesis: Spatial regulation and adaptation to stress. J. Exp. Bot. 2019, 70, 5041–5049. [Google Scholar] [CrossRef]
- Skirycz, A.; Inzé, D. More from less: Plant growth under limited water. Curr. Opin. Biotechnol. 2010, 21, 197–203. [Google Scholar] [CrossRef]
- Paque, S.; Weijers, D. Q&A: Auxin: The plant molecule that influences almost anything. BMC Biol. 2016, 14, 67. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, X.; Zhao, Y.; Li, Y.; Zhang, G.; Peng, Z.; Zhang, J. Enhancing auxin accumulation in maize root tips improves root growth and dwarfs plant height. Plant Biotechnol. J. 2018, 16, 86–99. [Google Scholar] [CrossRef]
- Du, H.; Liu, H.; Xiong, L. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front. Plant Sci. 2013, 4, 397. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Li, R.J.; Han, T.T.; Cai, W.; Fu, Z.W.; Lu, Y.T. Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol. 2015, 168, 343–356. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Luschnig, C.; Friml, J. Pho-view of auxin: Reversible protein phosphorylation in auxin biosynthesis, transport and signaling. Mol. Plant 2021, 14, 151–165. [Google Scholar] [CrossRef]
- Liu, H.; Xie, W.F.; Zhang, L.; Valpuesta, V.; Ye, Z.W.; Gao, Q.H.; Duan, K. Auxin biosynthesis by the YUCCA6 flavin monooxygenase gene in woodland strawberry. J. Integr. Plant Biol. 2014, 56, 350–363. [Google Scholar] [CrossRef]
- Liu, H.; Ying, Y.Y.; Zhang, L.; Gao, Q.H.; Li, J.; Zhang, Z.; Fang, J.G.; Duan, K. Isolation and characterization of two YUCCA flavin monooxygenase genes from cultivated strawberry (Fragaria × ananassa Duch.). Plant Cell Rep. 2012, 31, 1425–1435. [Google Scholar] [CrossRef]
- Naser, V.; Shani, E. Auxin response under osmotic stress. Plant Mol. Biol. 2016, 91, 661–672. [Google Scholar] [CrossRef]
- Liu, Y.G.; Chen, Y. High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. Biotechniques 2007, 43, 649–650, 652, 654, 656. [Google Scholar] [CrossRef]
- Tu, M.; Wang, X.; Yin, W.; Wang, Y.; Li, Y.; Zhang, G.; Li, Z.; Song, J.; Wang, X. Grapevine VlbZIP30 improves drought resistance by directly activating VvNAC17 and promoting lignin biosynthesis through the regulation of three peroxidase genes. Hortic. Res. 2020, 7, 150. [Google Scholar] [CrossRef]
- Daudi, A.; O’Brien, J.A. Detection of Hydrogen Peroxide by DAB Staining in Arabidopsis Leaves. Bio-Protocol 2012, 2, e263. [Google Scholar] [CrossRef] [Green Version]
- Kaur, N.; Sharma, I.; Kirat, K.; Pati, P.K. Detection of Reactive Oxygen Species in Oryza sativa L. (Rice). Bio-Protocol 2016, 6, e2061. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).