Abstract
Asthma is a major global health issue. Over 300 million people worldwide suffer from this chronic inflammatory airway disease. Typical clinical symptoms of asthma are characterized by a recurrent wheezy cough, chest tightness, and shortness of breath. The main goals of asthma management are to alleviate asthma symptoms, reduce the risk of asthma exacerbations, and minimize long-term medicinal adverse effects. However, currently available type 2 T helper cells (Th2)-directed treatments are often ineffective due to the heterogeneity of the asthma subgroups, which manifests clinically with variable and poor treatment responses. Personalized precision therapy of asthma according to individualized clinical characteristics (phenotype) and laboratory biomarkers (endotype) is the future prospect. This mini review discusses the molecular mechanisms underlying asthma pathogenesis, including the hot sought-after topic of microbiota, add-on therapies and the potential application of probiotics in the management of asthma.
1. Pathophysiology of Asthma
Asthma is one of the most common chronic diseases in industrialized countries. According to the 2016 Global Burden of Disease Study, it was estimated that more than 339 million people worldwide suffer from asthma, representing a notable 3.6% increase in age-standardized prevalence since 2006 [1]. Asthma is not just a public health problem for high-income countries; it occurs in all countries regardless of the level of economic development. There were 417,918 deaths due to asthma at the global level, and most asthma-related deaths occur in low- and lower-middle-income countries [1]. Asthma symptoms most commonly develop for the first time in early childhood, but no more than half of them go on to have characteristic asthma at school age. The prevalence of asthma-like symptoms in children are varied widely between countries. The global prevalence of current wheeze in adolescents and children was estimated to be 14.1% and 11.7%, with a mean increase of 0.06% and 0.13% per year, respectively, whilst the highest prevalence of over 20% was observed in higher-income countries in both age groups [2,3]. New data on asthma prevalence and severity in children, adolescents and adults around the world are pending to be reported this year by the Global Asthma Network.
Wheezy respiration, coughing, chest tightness and shortness of breath are characteristic symptoms during asthma exacerbations. The dogma of asthma pathogenesis is that the aberrant airway epithelial sensing of environmental harmless antigens, such as pollens, mites or cockroaches or certain occupational exposures, triggers release of inflammatory mediators from the epithelia, such as thymic stromal lymphopoietin (TSLP), interleukin (IL)-25 and IL-33, which, through a cascade of mucosal immune activation involving dendritic cells (DCs), innate lymphoid cells, eosinophils, mast cells, the adaptive type 2 helper T cells (Th2) and the nerves innervating the airways, lead to tissue structural cell activation and remodeling of the endothelial cells, the goblet cells and airway smooth muscles. Recurrent allergen stimulations elicit chronic allergic inflammation of the airways, and result in irreversible structural changes and permanent lung function loss [4]. Most childhood asthma is caused by Th2-mediated airway inflammation. Th2-cell activation produces a series of cytokines. Among them, the most investigated are IL-4, IL-5, IL-9 and IL-13. IL-4 induces B-cell immunoglobulin (Ig)G to IgE class switch and IgE synthesis [5]. IL-5 enhances eosinophil proliferation and differentiation in the bone marrow, and promotes eosinophil tissue trafficking, activation as well as survival [6,7]. IL-9 supports mast-cell growth and modulates the property of type 2-driven inflammation [8,9], whilst IL-13 activates epithelial expression of inducible nitric oxide synthase (iNOs) [10], induces goblet cell hyperplasia [11] and mucus production [12], airway hyper-responsiveness (AHR) and fibrosis, bridging allergic inflammatory cells to structural non-immune cells [13].
The Th2-allergy paradigm came from observations predominantly made in western high-income countries. The association between allergy and asthma is not as strong in low- or middle-income countries. Occupational causes of asthma often do not involve allergy. It is now widely conceived that no more than half of asthma is caused by allergic mechanisms. In many people, asthma is caused by non-allergic inflammation of the airways, although the mechanisms involved are not yet completely understood (Table 1).

Table 1.
Clinical and laboratory characteristics differentiating allergic vs. non-allergic asthma.
2. Asthma Endotypes
Eosinophils have been long considered pathognomonic in Th2-mediated asthma; however, clinical trials have found weak correlation between eosinophilic inflammation and AHR [21,22]. For example, although anti-IgE antibody omalizumab [23] and anti-IL-5 antibody mepolizumab [24,25] reduced airway eosinophilia, they failed to show a significant effect on AHR. In contrast, anti-TNF antibody etanercept improved lung functions and reduced AHR in refractory asthma without affecting eosinophils or neutrophils [26]. In addition, patients with severe asthma may present with persistent eosinophilic inflammation in the absence of specific IgE [27,28]. Phenotypic heterogeneity of asthma and the variable treatment responses to Th2-directed trials and therapies have brought out the speculation of non-Th2 inflammation in the pathogenesis of allergic airway inflammation; the categorization of asthma based on distinct mechanistic pathways was proposed as an algorithm to tailor treatment strategies [29,30,31,32]. With the intention to leverage laboratory evidence for precision asthma treatment, two major asthma endotypes have been described (Table 1). The long-known Th2-high endotype is characterized by increased eosinophils in the sputum and airways of patients [17], whereas the Th2-low endotype manifests with increased neutrophils or a pauci-granulocytic profile [18,19,20].
Asthma endotype classifications combined with specific biomarkers hold great potential for new therapeutic modalities and better treatment efficacies [33]. Whilst a standardized protocol is still lacking, endotype classification is often performed according to the absolute blood/sputum eosinophil counts, serum total IgE, fractional excretion of nitric oxide (FeNO), and various allergen sensitization tests. For example, high levels of IgE, FeNO and eosinophils are biomarkers indicative of Th2-high asthma [34,35,36]. In accordance with this protocol of stratification, most of the archetypal childhood asthma falls into the Th2-high endotype, characterized by atopy, elevated IgE and FeNO levels, as well as increased sputum and blood eosinophils [37,38]. In contrast, Th2-high airway inflammation is observed in only half of adult patients with mild to moderate asthma [29], whilst among patients with moderate to severe disease, only one third of them are driven by Th2-type inflammation [39]. Th2-low endotype is characterized by neutrophil-dominated or pauci-granulocytic inflammation with high levels of IFN-γ, IL-17A/F and IL-17A/IL-22 cytokines released from Th1, Th17 or type 3 innate lymphoid cells (ILC3) [40,41]. Patients with Th2-low asthma are prone to respond poorly to corticosteroid therapy. The cause of neutrophilic inflammation in Th2-low asthma is still unclear. The nature of the inciting agents/allergens and the immediate downstream signaling pathways as elicited by the inciting agents, such as differential Notch receptor-ligand pair signaling, are tentative regulators for Th2-low inflammation [42,43]. Nonetheless, the off-target effect of high-dose corticosteroid on neutrophilia and the masking of eosinophilic inflammation by steroid therapy should be taken into consideration in Th2-low asthma presented as neutrophilic inflammation. Biomarkers for Th2-low asthma have not been widely investigated. Neutrophils, matrix metallopeptidase 9 (MMP-9) and IL-6 have all been proposed as potential candidates, but none have been shown to represent all phenotypic subgroups of the Th2-low asthma endotype [29,32]. Application of multi-omics approaches encompassing transcriptomics, epigenomics, metagenomics, metabolomics, and proteomics, in combination with clinical features and laboratory data will enable asthma endotyping to be more informative and allow the designation of precision treatment strategies [44,45].
3. Add-On Therapy
A stepwise adjustment for asthma medication on an as-needed basis is suggested by the Global Initiative for Asthma (GINA) guideline [46]. The use of an inhaled corticosteroid (ICS) is an effective controller strategy in long-term management of asthma. However, the prevalence of severe or uncontrolled asthma despite good adherence to GINA-guided treatments are still as high as 10–19.8% [47,48]. For patients with poorly controlled asthma despite high-dose ICS in combination with add-on long-acting beta-agonists (LABAs), an alternative approach guided by the underlying inflammatory pathways, i.e., the endotypes, is mandatory. Add-on therapies using pharmacological non-biologic agents or biologics have been shown to improve symptom control and provide a dose-reduction strategy to limit the side effects of corticosteroid therapy [49,50,51]. Conventional add-on therapies include LABAs, long-acting muscarinic antagonists, leukotriene receptor antagonists, anti-fungal agents, macrolides and theophylline. The more recent and still rapidly progressing add-on therapies are biological agents, such as monoclonal antibodies specifically targeting inflammatory molecules. In this article, we review the add-on biologics currently available and the promising ones under clinical trials.
4. FDA-Approved Monoclonal Antibodies
There are currently five biologics licensed for severe asthma in adults: omalizumab binds to IgE at Fc𝛆RI binding site; mepolizumab and reslizumab both bind to IL-5; and benralizumab binds to IL-5 receptor α subunit and dupilumab, which binds to IL4 receptor α subunit, thus blocking both IL-4 and IL-13 signaling (Table 2).

Table 2.
Monoclonal antibodies and small molecule drugs for allergic asthma treatment.
Omalizumab is the first biologic approved for the treatment of asthma in the U.S. and European Union. Omalizumab is a humanized recombinant monoclonal antibody with binding specificity at the FcεRI binding site of IgE, thus preventing IgE binding to Fc𝛆RI on mast cells, basophils and DCs, and the subsequent release of inflammatory mediators from these cells. It reduces asthma exacerbations and the maintenance doses of ICS and improves quality-of life scores in clinical trials [18,69,70]. Omalizumab is currently specifically indicated for moderate-to-severe persistent asthma with serum IgE greater than 30 IU/L in both adults and children 6 years of age and older. In adolescent and adult severe asthmatic patients, omalizumab has shown beneficial real-world short-term effectiveness at 1 year and strong evidence of long-term effectiveness for up to 4 years and beyond [71].
There are three licensed biologics targeting IL-5-mediated inflammatory pathway, including mepolizumab, reslizumab, and benralizumab. Mepolizumab and reslizumab are humanized monoclonal antibodies specifically targeting IL-5. Whilst reslizumab is administered by intravenous infusion with weight-based dosing [56], mepolizumab is delivered in a fixed dose of 100 mg subcutaneously to patients aged 12 years and above. In young children 6–11 years of age, mepolizumab is used at a lower dose of 40 mg [72,73,74]. In oral corticosteroid-dependent patients, mepolizumab reduces the need for oral corticosteroid therapy [75]. Reslizumab reduces the exacerbation frequency and improves lung function [76,77]. The other biologic targeting IL-5 signaling pathway is benralizumab. It binds to the IL-5 receptor on eosinophils and basophils, and thus prevents binding of the IL-5 receptor by IL-5 [78]. A systematic review and network meta-analysis showed that reslizumab may be more effective than benralizumab in patients with eosinophilic asthma receiving GINA step4/5 treatment [79].
Dupilumab was approved by the FDA for the treatment of adult moderate-to-severe atopic dermatitis in 2017, and later for moderate-to-severe asthma in 2018 [80]. It inhibits both IL-4 and IL-13 signaling and reduces Th2 response through direct binding to the IL-4R⍺, the shared subunit for IL-4 and IL-13 receptors, hence preventing IL-4 and IL-13 interaction with the receptors. Dupilumab notably reduced serum total IgE levels and FeNO and increased blood eosinophil counts. The increase in blood eosinophils is plausibly attributed to the blockade of IL-4 and IL-13 effects on eosinophil survival, activation, and tissues trafficking by dupilumab, but not mobilization of eosinophils from bone marrow, which is influenced by IL-5. Dupilumab reduced the exacerbation risk of severe asthma, and improved FEV1 without an increased risk of adverse effects [81]. Comorbidities, including atopic dermatitis, chronic rhinosinusitis, and allergic rhinitis, may also respond to treatment with dupilumab [82].
The data on biologic therapies are mostly derived from studies on adults; it is extremely limited in the pediatric population, and even more limited in children younger than 12 years of age. As of March 2021, the FDA has approved four biologic drugs for use in pediatric patients with severe asthma: omalizumab, mepolizumab, benralizumab and dupilumab. Whilst omalizumab and mepolizumab are the only two biologics approved for children 6–18 years of age, benralizumab and dupilumab are licensed for use in adolescents aged 12 years and older [83,84].
5. Biological Therapies under Clinical Trials
Currently available biologic therapies, including anti-IgE, anti-IL-5, anti-IL-5R⍺ and anti-IL-4R⍺, reduce asthma exacerbation rates in patients with Th2-high asthma. However, there are no effective treatments for patients with severe Th2-low asthma. The airway epithelium acts as the first line of defense against airborne substances. The classic features of asthma exacerbations are initiated by the releasing of alarmins, including TSLP, IL-33 and IL-25, from the airway epithelium in response to inflammation or injury [85]. These cytokines activate group 2 innate lymphoid cells (ILC2), which produce large amounts of IL-5, IL-13, and a small amount of IL-4 without production of specific IgE [13]. These findings may partially explain why patients with severe asthma lack an allergen-induced Th2 response, but manifest with persistent eosinophilic inflammation. Currently, clinical trials for biologics antagonizing alarmins include tezepelumab against TSLP, etokimab for inhibiting IL-33, and lebrikizumab for IL-13 blockage (Table 1).
Additionally, for alleviating airflow obstruction by preventing airway smooth muscle contraction, a new biologic GDC-0334 is under trial for its effects in inhibiting transient receptor potential cation channel member A1 (TRPA1) activation. TRPA1 is a nonselective cation channel, monitoring changes in the chemical environment, and responds to physical stimuli, such as mechanical stress or changes in temperature [86]. The conditional deletion of TRPA1 in neuronal cells resulted in reduced inflammatory cell infiltration and IL-5 production [87]. These findings indicate that neuronal TRPA1 is critical in asthmatic inflammation. Recent preclinical studies showed that TRPA1 blockage with a small molecule inhibitor GDC-0334 suppressed inflammation and airway smooth muscle contraction [88]. Instead of direct blockage of the immunologic factors mediating asthma pathogenesis, alternative approaches targeting pathogenic factors, such as those involved in neurogenic inflammation in asthma, hold great potential for the treatment of Th2-low asthma.
6. Microbiota and Allergic Asthma
6.1. Hygiene Hypothesis
The seminal study linking microbial exposures with the tendency of developing allergic diseases was conducted by the British epidemiologist Professor David Strachan over 30 years ago. The theory he proposed is nowadays widely known as the “hygiene hypothesis” [89]. According to the theory, reduced exposures to environmental bacteria in early life, including birth by cesarean section, being bottle-fed, growing up in the city, fewer family members or contacts to various persons and less infections due to vaccinations, are associated with an increased risk of developing allergies and asthma later in life. The mechanistic thinking derived from the hygiene hypothesis is that microbial exposures during the perinatal stage influence the establishment of a child’s gut microbiota. Microbial alterations, i.e., dysbiosis, driven by these “hygienic” factors, acting through affecting the infant immune development and responses, are causally related to the increased risks of allergic diseases [90,91].
In line with this hypothesis, studies have shown that children growing up in developed countries or in urban areas, where allergies are more prevalent, host different gut microbiota compared to children growing up in underdeveloped countries or in farm fields, where allergies are relatively rare [92,93,94]. In addition, phylogenetic differences in the home microbiota in early life were associated with a subsequent risk of childhood asthma [95]; farm-like indoor microbiota has been shown to protect children living in non-farm homes from developing asthma, suggesting that the indoor dust microbiota composition could not only be a predictor of asthma risk, but also pose as a potential modifiable target for asthma prevention [93].
6.2. Gut–Lung Axis and Microbial Mechanisms
With intensive research in the field of microbiota over the past decade, including the completion of the NIH-funded Human Microbiota Project (HMP) [96,97], we have come to appreciate more the role of microbiota in maintaining health and that alterations in the gut microbiota, i.e., dysbiosis, not only causes perturbations of the immune responses within the guts, but it also impacts on the well-being of distant organs, such as the lungs [98]. The concept for the intricate and reciprocal interactions between the gut and lungs, i.e., the gut–lung axis, was prompted by the observation that changes in the intestinal milieu influenced or primed the progress of different lung diseases and vice versa. Whilst how communications between the gut and lungs are achieved are still not completely understood, it has been suggested and well-accepted that mediators derived from intestinal epithelial cells, immune cells, the microbial structural components and/or microbial metabolites traffic through circulation and elicit changes in immune response in the lungs. One good example demonstrating the role of microbiota in the gut–lung axis is the studies on abnormal secretory IgA (SIgA) microbial binding. SIgA is the first line of defense of the mucosa against tissue invasion by pathogens and commensal bacteria. SIgA limits the overgrowth of microbial species and hence guards the compositions and properties of the microbiota. In this regard, studies have found that children with a lower IgA binding to fecal bacteria at 12 months of age are more likely to develop asthma and allergic diseases [99]. Interestingly, altered IgA recognition patterns in children with allergies were observed at ages as early as 1 month old, when IgA in breast-fed children are predominantly maternally derived. Whether it indicates a dysbiotic state of the mothers warrants more investigation.
Mammals harbor over 100 trillion gut bacteria from over 1000 different species. Commensal gut floras have been shown to induce the differentiation of particular CD4+ T cell subsets. Examples include the induction of Th17 cells in the intestinal lamina propria by segmented filamentous bacteria (SFB) [100], the development of systemic Th1 cells [101] and local IL-10-producing Tregs [102] by Bacteroides fragilis and the induction of colonic Treg cells by indigenous Clostridium species [103]. Whilst cumulating data point to a crucial role of the commensal microbes in shaping and regulating the immune system [104,105,106,107,108], the mechanisms underpinning this function are only gradually being uncovered.
In the context of the gut–lung axis in pathogenesis of allergic airway diseases, a series of studies have elegantly demonstrated that short-chain fatty acids (SCFAs), acetate, propionate, and butyrate, the metabolic products of microbial fermentation of indigestible dietary fibers, promoted not only colonic but also peripheral Treg expansion [109,110,111,112].
Metabolites derived from gut microbial functions circulate systematically to distant organs, including the lungs, and regulate the pathophysiological status therein, and vice versa. In a murine asthma model, high-fiber diets increased circulating levels of SCFAs and protected the mice against allergic inflammation in the lungs [113]. Treating the mice with the SCFA propionate recapitulated the protective effect of a high-fiber diet against allergic airway inflammation. In vivo, propionate enhanced the generation of macrophage and DC precursors and subsequent trafficking of these cells to the lungs. However, propionate-induced DCs were ineffective in promoting Th2 effector function [113]. In agreement with these findings, others have shown that butyrate inhibited pulmonary ILC2 functions and the subsequent development of airway hyperreactivity (AHR) through modulation of GATA3 expression and metabolic pathways of pulmonary ILC2s. Association of germfree mice with butyrate-producing gut bacteria effectively suppressed ILC2-driven AHR [114]. These studies highlighted the beneficial effects of high-fiber diets and SCFAs in the prevention of asthma.
SCFAs are pleiotropic metabolites implicated in an array of physiological processes, including the production of satiety hormones, GLP-1, PYY, and leptin, energy expenditure, epithelial proliferation and epithelial barrier function [115]. In addition, SCFAs inhibit LPS-induced NF-kB activation in neutrophils and macrophages by binding to receptors GPR41, GPR43, and GPR109A and by inhibition of histone deacetylase [109,110,111]. With the various beneficial effects of the SCFAs, development of microbiota-directed food or fiber-based interventions to promote growth of SCFA-producing microbiotas provides an alternative preventive and/or therapeutic modality for asthma.
6.3. Relation of Microbial Taxa and Asthma
The burgeoning of microbial studies in recent years is in a large part attributed to the revolutionary advances in metagenomic sequencing, bioinformatics and multi-omics technologies, which allow detailed analysis and identification of the non-culturable microorganisms, in addition to the culturable ones, and their biological products [92]. Previously unrecognized and underappreciated functions of the microbiota in shaping the immune systems and in the pathogenesis of diseases, not only of the gastrointestinal tract, but also of the distant organs, including the lungs and the brain, have been uncovered. Recent studies have elegantly shown a critical window of life, during which the microbiota contributes to education and maturation of the immune system, facilitating the establishment of tolerance to environmental harmless exposures or perpetuate the development of disease later in life [116].
Early-life airway microbiota may predispose to the development of asthma in childhood through dynamic interactions with the developing immune system [117]. Altered compositions of the airway [118,119,120] and gut microbiota [121,122,123] have both been linked to higher risk of atopy and asthma [124,125,126]. Collectively, increased relative abundance of Bacteroidaceae, Clostridiaceae, and Enterobacteriaceae and a lower abundance of Bifidobacteriaceae and Lactobacillaceae are associated with the development of allergic sensitization, eczema, or asthma [127], whereas members of the Lachnospiraceae family and the genera Faecalibacterium and Dialister are protective of developing atopy [128]. In one study, a group of neonates with highest risk of developing atopy and asthma were identified when the stool microbiome contained lower relative abundance of Bifidobacterium, Akkermansia and Faecalibacterium and higher relative abundance of Candida and Rhodotorula [129]. Fecal metabolome from these high-risk neonates showed enriched pro-inflammatory metabolites, such as 12, 13-DiHOME. Both 12, 13-DiHOME and fecal water from these neonates were able to induce IL-4-producing T cells and concomitantly reduced FoxP3+ regulatory T cell differentiation. These findings plausibly support that dysbiosis perturbs the immune system resulting in pathogenic T cell dysfunction that causes atopy and allergic diseases [129].
In addition to predilection to atopy and allergic diseases, dysbiosis may also hamper therapeutic efficacies [130,131]. An analysis of the bronchoalveolar lavage microbiome found distinct microbial expansions in patients with corticosteroid resistant (CR) asthma [130]. Among them, Haemophilus parainfluenzae was uniquely expanded only in CR asthma airways. Incubation of asthmatic airway macrophages with H. parainfluenzae resulted in TAK1/MAPK activation and corticosteroid resistance.
6.4. Contradictory Data—Take Ruminococcus gnavus as an Example
In a Canadian cohort of infants, bacterial genera Lachnospira, Veillonella, Faecalibacterium, and Rothia were found to be significantly decreased at 3 months of age in children who later developed atopic asthma [122]. The causal effects of these bacterial taxa in preventing asthma development were further verified in an animal model of asthma, wherein offspring of the gnotobiotic mice harboring these four bacterial taxa were able to resist allergen-induced airway inflammation. In a rural Ecuadorian cohort investigated by the same group, increased relative abundance of Streptococcus and Bacteroides species and decreased Bifidobacterium species and Ruminococcus gnavus at 3 months of age were associated with a higher risk of atopy and asthma at 5 years old [121]. The gut microbiota regulates immune responses not only locally but also in distal organs at least partly through microbial metabolites. In a recent study, microbial bile acid metabolism has been linked with Foxp3+ Treg-cell induction [132]. Ruminococcus gnavus is in a particularly important position in bile acid-elicited immune regulation for its capacity to epimerize 3α-hydroxydeoxycholic acid (DCA) to 3β-hydroxydeoxycholic acid (isoDCA), the most potent de-conjugated bile acid to induce colonic Treg differentiation [133]. These findings plausibly provide the mechanistic ground for the association between decreased R. gnavus abundance and increased atopy and asthma risk in previous studies [121,134]. In contradiction to the studies presented above, a twin cohort study has found an association between increased relative abundance of Lachnospiraceae at 2 months of age and a higher risk of developing allergic diseases before the age of 3 [123]. In this study, R. gnavus was the dominant responsible species for increased allergy risk. When conventional naïve mice and allergen-sensitized mice were colonized with R. gnavus, an enhanced allergic airway inflammation was observed.
Whilst experimental settings, geographic, genetic and cultural differences among individual study cohorts may underlie the non-concordant data seen in different studies, interpretation of these data cannot, nonetheless, be too cautious. The quality or performance of the metagenomics platforms as well as the physiological fidelity of the animal models in microbial reconstitution should all be carefully evaluated. In this aspect, colonization of the microbes of interest in conventional specific pathogen-free (SPF) mice may carry the risk of perturbing the gut commensal community and inducing advert immune responses to the microbes by itself. In addition, the timing for colonizing the mice with the microbes, e.g., before allergen sensitization occurs or after, should also be taken into account to justify the rationales underlying the hypothesis with regard to the role and function of the microbiota in the pathogenesis of allergic diseases. To date, the optimal physiological way to avoid these untoward effects is by using the offspring of the gnotobiotic mice inoculated with the microbe of interest; the offspring are borne to parents carrying these microbes and are, therefore, colonized by these microbes in a more physiological way.
6.5. Therapeutic Potential of Microbiota
6.5.1. Probiotics
Randomized controlled trials (RCTs) for using probiotics or in combination with prebiotics in asthma prevention and control have shown mixed efficacy outcomes. For this reason, several meta-analysis studies have been performed. We searched PubMed for meta-analysis studies with the keywords, probiotics, microbiota, asthma, allergies and atopy from 2010 to 2021. Four meta-analysis studies analyzing published trials from inception to 2013–2018 with detailed descriptions for inclusion/exclusion criteria, methods of analysis and analysis results were selected for review [135,136,137,138]. Collectively, these meta-analyses have pointed to a concordant conclusion that there is no evidence to support prenatal or postnatal administration of probiotics as a standard asthma prevention strategy based on the RCT data published thus far. Although in some analyses, prenatal and/or early-life probiotic supplementations did show protective association with decreased atopic sensitization, IgE production and infantile eczema, they did not necessarily exert beneficial effects in asthma prevention or wheeze risk [136,137,139,140].
One recent study published in 2020 analyzed 30 RCTs dated from 2003 to 2018, investigating the effects of probiotic supplements for asthma risk (primary outcome) or wheeze incidence (secondary outcome) in infants [138]. The probiotics applied in these trials included Lactobacillus (L.) reuteri, L. rhamnosus GG, L. rhamnosus LC705, L. acidophilus, L. paracaseii, L. casei, Bifidobacillus (B.) lactis, B. bifidum, B. breve Bbi99 plus Propionibacterium freudenreichii ssp shermanii, and B. longum BL999. The probiotics were administered either alone or in combination with prebiotics as postnatal interventions or started since the prenatal stage. No significant association of probiotic supplementations with lower asthma risk or wheeze incidence was found [135,140,141,142,143,144,145,146,147,148,149,150,151,152]. In contrast, in subgroup analyses by asthma risk, probiotic supplementations significantly reduced wheeze incidence among infants with atopy, whilst there were still no significant associations in infants with other asthma risk factors, such as family history or a cow’s milk allergy [140,144,145,146,147,153,154,155,156,157,158,159,160,161].
Therefore, despite various studies that have demonstrated a crucial and beneficial role of microbiota in modulating the immune responses and preventing atopy and allergic diseases, the use of probiotics as a therapeutic strategy for asthma is not, as of yet, conclusive. Nonetheless, dietary fiber is nowadays regarded as part of a healthy diet worldwide, and development of dietary fiber-based interventions, which selectively increase the abundance of microbes, that provide metabolic benefits to the host, such as SCFA production, is actively underway [162,163,164].
6.5.2. From Microbial Endotypes to Asthma Endotyping and Precision Medicine for Asthma
The heterogeneity of asthma does not confine to diverse clinical phenotypes and aetiologies; it also manifests with different airway microbiomes. Both bacterial and fungal microbiota signatures were found to correlate with asthma endotypes and clinical features. In recent studies employing the omics approach, decreased airway bacterial and fungal diversity as well as increased relative abundance of Pseudomonas, Trichoderma, Fusarium, Cladosporium and Aspergillus were associated with Th2-high endotype, whereas increased Proteobacteria, Mycosphaerella and Penicillium were clustered with Th2-low type of asthma [119,165,166]. The association of microbiome endotypes with asthma endotypes may further contribute to precision asthma endotyping and selection of treatment regimens; however, it has to be taken into consideration that concurrent steroid treatment may change the microbiome and obscure the true association [167].
The microbiome has been shown to affect corticosteroid responsiveness in asthma [130,131]. It is largely unknown whether the microbiome also affects treatment efficacies of the biologics targeting specific features of asthma-related immune mechanisms. In one such study using nasal secretion samples collected from asthmatic children enrolled in an omalizumab trial, nasal Moraxella species was found to associate with increased asthma exacerbations and eosinophil activation. [168]. Therefore, whilst omalizumab successfully reduced asthma exacerbations, the nasal airway microbiota composition might remain largely unaffected. The persistence of pathogenic nasal airway microbiota, such as the Moraxella species, which occurred more often in young children and caused increased epithelial damage and eosinophil activation, is capable of evading targeting biologic therapies.
The findings from these studies indicate that the importance of microbiota goes beyond influencing the development of atopy and asthma at the early life, and the application of microbiota is not restricted to probiotics. Instead, incorporation of microbiome endotypes of individual asthmatic patients can further stratify asthma endotyping and enable the identification of pathogenic microbiome endotypes, which collectively warrant optimal treatment regimen tailoring. Future precision asthma therapies based on microbiome-associated asthma endotypes may potentially comprise treatment targeting pathogenic or dysbiotic microbiota and the biological therapies targeting the underlying inflammatory processes, in addition to pharmacological drugs.
7. Conclusions
With advances in metagenomics, bioinformatics and single-cell platforms, we are witnessing a rapid progress in translational research. In the context of allergic airway diseases, it has become even more clear the heterogeneity of asthma, attributing in a large part to the complex interactions between the host and the environment, including the microbial community: those existing in the environment in which the host lives and those inhabiting the host. Re-classification of asthma into distinct endotypes in a laboratory- and clinical-evidence-based manner contributes to personalized precision medicine. In addition, distinct microbiome endotypes and asthma endotypes’ association suggests the potential utilization of microbiome-targeting as a novel add-on therapy strategy in precision asthma treatment. Whist we understand more of the Th2-high asthma and successfully introduce novel biologics as a Th2-high therapeutic strategy, more investigation is needed for Th2-low asthma before we can leverage the advances in scientific research in designing optimal therapies for these patients (Figure 1).
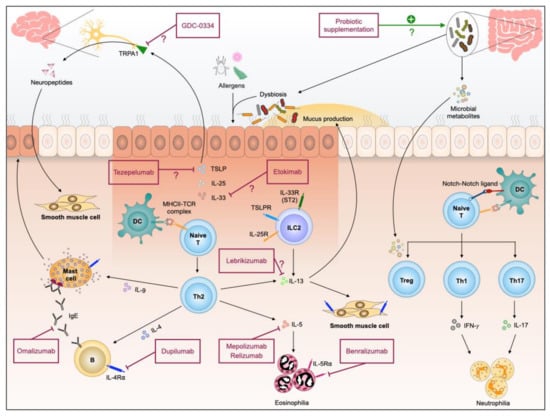
Figure 1.
Pathogenesis of allergic airway inflammation and therapeutic interventions currently available and those with potential.
Author Contributions
C.-J.C.: Wrote the paper and drew the schematic diagram; M.-T.H.: Conceptualization and writing the paper. All authors have read and agreed to the published version of the manuscript.
Funding
C.-J.C. received grant funding from the Ministry of Science and Technology, Taiwan (MOST 108-2321-B-002-057-MY3); M.-T.H. received funding from the Ministry of Science and Technology, Taiwan (MOST 109-2314-B-002-136-MY3).
Institutional Review Board Statement
No human studies involved.
Informed Consent Statement
No human studies involved.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful for the funding of this project from the Ministry of Science and Technology, Taiwan (MOST 108-2321-B-002-057-MY3 and MOST 109-2314-B-002-136-MY3).
Conflicts of Interest
The authors declare no commercial or financial conflict of interest.
References
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Pearce, N.; Aït-Khaled, N.; Beasley, R.; Mallol, J.; Keil, U.; Mitchell, E.; Robertson, C. Worldwide trends in the prevalence of asthma symptoms: Phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2007, 62, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.K.W.; Beasley, R.; Crane, J.; Foliaki, S.; Shah, J.; Weiland, S. Global variation in the prevalence and severity of asthma symptoms: Phase Three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009, 64, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Poulsen, L.K.; Hummelshoj, L. Triggers of IgE class switching and allergy development. Ann. Med. 2007, 39, 440–456. [Google Scholar] [CrossRef]
- Fulkerson, P.C.; Schollaert, K.L.; Bouffi, C.; Rothenberg, M.E. IL-5 triggers a cooperative cytokine network that promotes eosinophil precursor maturation. J. Immunol. 2014, 193, 4043–4052. [Google Scholar] [CrossRef]
- Roufosse, F. Targeting the Interleukin-5 Pathway for Treatment of Eosinophilic Conditions Other than Asthma. Front. Med. 2018, 5, 49. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kubatzky, K.F.; Mitra, D.K. An Update on Interleukin-9: From Its Cellular Source and Signal Transduction to Its Role in Immunopathogenesis. Int. J. Mol. Sci. 2019, 20, 2113. [Google Scholar] [CrossRef]
- Angkasekwinai, P.; Dong, C. IL-9-producing T cells: Potential players in allergy and cancer. Nat. Rev. Immunol. 2021, 21, 37–48. [Google Scholar] [CrossRef]
- Ingram, J.L.; Kraft, M. IL-13 in asthma and allergic disease: Asthma phenotypes and targeted therapies. J. Allergy Clin. Immunol. 2012, 130, 829–842. [Google Scholar] [CrossRef]
- Marone, G.; Granata, F.; Pucino, V.; Pecoraro, A.; Heffler, E.; Loffredo, S.; Scadding, G.W.; Varricchi, G. The Intriguing Role of Interleukin 13 in the Pathophysiology of Asthma. Front. Pharmacol. 2019, 10, 1387. [Google Scholar] [CrossRef]
- Zhu, Z.; Homer, R.J.; Wang, Z.; Chen, Q.; Geba, G.P.; Wang, J.; Zhang, Y.; Elias, J.A. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Investig. 1999, 103, 779–788. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef]
- Peters, S.P. Asthma phenotypes: Nonallergic (intrinsic) asthma. J. Allergy Clin. Immunol. Pract. 2014, 2, 650–652. [Google Scholar] [CrossRef]
- Corren, J. Asthma phenotypes and endotypes: An evolving paradigm for classification. Discov. Med. 2013, 15, 243–249. [Google Scholar]
- James, D.R.; Lyttle, M.D. British guideline on the management of asthma: SIGN Clinical Guideline 141, 2014. Arch. Dis. Child. Educ. Pract. Ed. 2016, 101, 319–322. [Google Scholar] [CrossRef]
- Robinson, D.; Humbert, M.; Buhl, R.; Cruz, A.A.; Inoue, H.; Korom, S.; Hanania, N.A.; Nair, P. Revisiting Type 2-high and Type 2-low airway inflammation in asthma: Current knowledge and therapeutic implications. Clin. Exp. Allergy 2017, 47, 161–175. [Google Scholar] [CrossRef]
- Baines, K.J.; Simpson, J.L.; Wood, L.G.; Scott, R.J.; Gibson, P.G. Transcriptional phenotypes of asthma defined by gene expression profiling of induced sputum samples. J. Allergy Clin. Immunol. 2011, 127, 153–160, 160.e151–159. [Google Scholar] [CrossRef]
- D’Silva, L.; Hassan, N.; Wang, H.Y.; Kjarsgaard, M.; Efthimiadis, A.; Hargreave, F.E.; Nair, P. Heterogeneity of bronchitis in airway diseases in tertiary care clinical practice. Can. Respir. J. 2011, 18, 144–148. [Google Scholar] [CrossRef]
- Simpson, J.L.; Scott, R.; Boyle, M.J.; Gibson, P.G. Inflammatory subtypes in asthma: Assessment and identification using induced sputum. Respirology 2006, 11, 54–61. [Google Scholar] [CrossRef]
- Crimi, E.; Spanevello, A.; Neri, M.; Ind, P.W.; Rossi, G.A.; Brusasco, V. Dissociation between airway inflammation and airway hyperresponsiveness in allergic asthma. Am. J. Respir. Crit. Care Med. 1998, 157, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.M.; James, A.; Uasuf, C.; Payne, D.N.; Hablas, H.; Agrofioti, C.; Bush, A. Asthma severity and inflammation markers in children. Pediatr. Allergy Immunol. 2001, 12, 125–132. [Google Scholar] [CrossRef]
- Djukanovic, R.; Wilson, S.J.; Kraft, M.; Jarjour, N.N.; Steel, M.; Chung, K.F.; Bao, W.; Fowler-Taylor, A.; Matthews, J.; Busse, W.W.; et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am. J. Respir. Crit. Care Med. 2004, 170, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Leckie, M.J.; Brinke, A.t.; Khan, J.; Diamant, Z.; O’Connor, B.J.; Walls, C.M.; Mathur, A.K.; Cowley, H.C.; Chung, K.F.; Djukanovic, R.; et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsìveness, and the late asthmatic response. Lancet 2000, 356, 2144–2148. [Google Scholar] [CrossRef]
- Flood-Page, P.T.; Menzies-Gow, A.N.; Kay, A.B.; Robinson, D.S. Eosinophil’s role remains uncertain as anti–Interleukin-5 only partially depletes numbers in asthmatic airway. Am. J. Respir. Crit. Care Med. 2003, 167, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.A.; Hargadon, B.; Shelley, M.; Parker, D.; Shaw, D.E.; Green, R.H.; Bradding, P.; Brightling, C.E.; Wardlaw, A.J.; Pavord, I.D. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N. Engl. J. Med. 2006, 354, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Price, D.B.; Rigazio, A.; Campbell, J.D.; Bleecker, E.R.; Corrigan, C.J.; Thomas, M.; Wenzel, S.E.; Wilson, A.M.; Small, M.B.; Gopalan, G.; et al. Blood eosinophil count and prospective annual asthma disease burden: A UK cohort study. Lancet Respir. Med. 2015, 3, 849–858. [Google Scholar] [CrossRef]
- Price, D.; Wilson, A.M.; Chisholm, A.; Rigazio, A.; Burden, A.; Thomas, M.; King, C. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J. Asthma Allergy 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Woodruff, P.G.; Modrek, B.; Choy, D.F.; Jia, G.; Abbas, A.R.; Ellwanger, A.; Arron, J.R.; Koth, L.L.; Fahy, J.V. T-helper type 2–driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 388–395. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 inflammation in asthma—present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef]
- Stokes, J.R.; Casale, T.B. Characterization of asthma endotypes: Implications for therapy. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2016, 117, 121–125. [Google Scholar] [CrossRef]
- Boulet, L.P.; Reddel, H.K.; Bateman, E.; Pedersen, S.; FitzGerald, J.M.; O’Byrne, P.M. The Global Initiative for Asthma (GINA): 25 years later. Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef]
- Wenzel, S.E. Asthma: Defining of the persistent adult phenotypes. Lancet 2006, 368, 804–813. [Google Scholar] [CrossRef]
- Smith, A.D.; Cowan, J.O.; Brassett, K.P.; Herbison, G.P.; Taylor, D.R. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N. Engl. J. Med. 2005, 352, 2163–2173. [Google Scholar] [CrossRef]
- Pifferi, M.; Bush, A.; Pioggia, G.; Di Cicco, M.; Chinellato, I.; Bodini, A.; Macchia, P.; Boner, A.L. Monitoring asthma control in children with allergies by soft computing of lung function and exhaled nitric oxide. Chest 2011, 139, 319–327. [Google Scholar] [CrossRef]
- Dweik, R.A.; Sorkness, R.L.; Wenzel, S.; Hammel, J.; Curran-Everett, D.; Comhair, S.A.; Bleecker, E.; Busse, W.; Calhoun, W.J.; Castro, M.; et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am. J. Respir. Crit. Care Med. 2010, 181, 1033–1041. [Google Scholar] [CrossRef]
- Kuo, C.-H.S.; Pavlidis, S.; Loza, M.; Baribaud, F.; Rowe, A.; Pandis, I.; Hoda, U.; Rossios, C.; Sousa, A.; Wilson, S.J.; et al. A Transcriptome-driven analysis of epithelial brushings and bronchial biopsies to define asthma phenotypes in U-BIOPRED. Am. J. Respir. Crit. Care Med. 2017, 195, 443–455. [Google Scholar] [CrossRef]
- Sur, S.; Crotty, T.B.; Kephart, G.M.; Hyma, B.A.; Colby, T.V.; Reed, C.E.; Hunt, L.W.; Gleich, G.J. Sudden-onset fatal asthma. A distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am. Rev. Respir. Dis. 1993, 148, 713–719. [Google Scholar] [CrossRef]
- Wenzel, S.E.; Szefler, S.J.; Leung, D.Y.; Sloan, S.I.; Rex, M.D.; Martin, R.J. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am. J. Respir. Crit. Care Med. 1997, 156, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-T.; Chen, Y.-L.; Lien, C.-I.; Liu, W.-L.; Hsu, L.-C.; Yagita, H.; Chiang, B.-L. Notch ligand DLL4 alleviates allergic airway inflammation via induction of a homeostatic regulatory pathway. Sci. Rep. 2017, 7, 43535. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-T.; Chiu, C.-J.; Chiang, B.-L. Multi-faceted Notch in allergic airway inflammation. Int. J. Mol. Sci. 2019, 20, 3508. [Google Scholar] [CrossRef]
- Raita, Y.; Camargo, C.A.; Bochkov, Y.A.; Celedón, J.C.; Gern, J.E.; Mansbach, J.M.; Rhee, E.P.; Freishtat, R.J.; Hasegawa, K. Integrated-omics endotyping of infants with rhinovirus bronchiolitis and risk of childhood asthma. J. Allergy Clin. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tyler, S.R.; Bunyavanich, S. Leveraging-omics for asthma endotyping. J. Allergy Clin. Immunol. 2019, 144, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention; Global Initiative for Asthma—GINA: Fontana, WI, USA, 2020. [Google Scholar]
- Wenzel, S.E.; Busse, W.W. Severe asthma: Lessons from the Severe Asthma Research Program. J. Allergy Clin. Immunol. 2007, 119, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Allegra, L.; Cremonesi, G.; Girbino, G.; Ingrassia, E.; Marsico, S.; Nicolini, G.; Terzano, C. Real-life prospective study on asthma control in Italy: Cross-sectional phase results. Respir. Med. 2012, 106, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Beasley, R.; Harper, J.; Bird, G.; Maijers, I.; Weatherall, M.; Pavord, I.D. Inhaled Corticosteroid Therapy in Adult Asthma. Time for a New Therapeutic Dose Terminology. Am. J. Respir. Crit. Care Med. 2019, 199, 1471–1477. [Google Scholar] [CrossRef]
- Jackson, D.J.; Bacharier, L.B.; Mauger, D.T.; Boehmer, S.; Beigelman, A.; Chmiel, J.F.; Fitzpatrick, A.M.; Gaffin, J.M.; Morgan, W.J.; Peters, S.P.; et al. Quintupling Inhaled Glucocorticoids to Prevent Childhood Asthma Exacerbations. N. Engl. J. Med. 2018, 378, 891–901. [Google Scholar] [CrossRef]
- Lemanske, R.F., Jr.; Mauger, D.T.; Sorkness, C.A.; Jackson, D.J.; Boehmer, S.J.; Martinez, F.D.; Strunk, R.C.; Szefler, S.J.; Zeiger, R.S.; Bacharier, L.B.; et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N. Engl. J. Med. 2010, 362, 975–985. [Google Scholar] [CrossRef]
- Schulman, E.S. Development of a monoclonal anti-immunoglobulin E antibody (omalizumab) for the treatment of allergic respiratory disorders. Am. J. Respir. Crit. Care Med. 2001, 164, S6–S11. [Google Scholar] [CrossRef]
- Chang, T.W.; Wu, P.C.; Hsu, C.L.; Hung, A.F. Anti-IgE antibodies for the treatment of IgE-mediated allergic diseases. Adv. Immunol. 2007, 93, 63–119. [Google Scholar] [CrossRef]
- Johansson, M.W. Eosinophil Activation Status in Separate Compartments and Association with Asthma. Front. Med. 2017, 4, 75. [Google Scholar] [CrossRef]
- Walsh, G.M. Reslizumab, a humanized anti-IL-5 mAb for the treatment of eosinophil-mediated inflammatory conditions. Curr. Opin. Mol. Ther. 2009, 11, 329–336. [Google Scholar]
- Castro, M.; Mathur, S.; Hargreave, F.; Boulet, L.P.; Xie, F.; Young, J.; Wilkins, H.J.; Henkel, T.; Nair, P. Reslizumab for poorly controlled, eosinophilic asthma: A randomized, placebo-controlled study. Am. J. Respir. Crit. Care Med. 2011, 184, 1125–1132. [Google Scholar] [CrossRef]
- Pham, T.H.; Damera, G.; Newbold, P.; Ranade, K. Reductions in eosinophil biomarkers by benralizumab in patients with asthma. Respir. Med. 2016, 111, 21–29. [Google Scholar] [CrossRef]
- Fitzgerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- O’Quinn, S.; Xu, X.; Hirsch, I. Rescue medication use reduction with Benralizumab for patients with severe, uncontrolled eosinophilic asthma. Ann. Allergy Asthma Immunol. 2018, 121, S18. [Google Scholar] [CrossRef]
- Deeks, E.D. Dupilumab: A Review in Moderate to Severe Asthma. Drugs 2019, 79, 1885–1895. [Google Scholar] [CrossRef]
- Varricchi, G.; Pecoraro, A.; Marone, G.; Criscuolo, G.; Spadaro, G.; Genovese, A.; Marone, G. Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders, and Cancer. Front. Immunol. 2018, 9, 1595. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Colice, G.; Griffiths, J.M.; Almqvist, G.; Ponnarambil, S.; Kaur, P.; Ruberto, G.; Bowen, K.; Hellqvist, A.; Mo, M.; et al. NAVIGATOR: A phase 3 multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the efficacy and safety of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir. Res. 2020, 21, 266. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Parnes, J.R.; Wang, L.; Mo, M.; Roseti, S.L.; Griffiths, J.M.; van der Merwe, R. Tezepelumab in Adults with Uncontrolled Asthma. N. Engl. J. Med. 2019, 380, 2082. [Google Scholar] [CrossRef]
- Emson, C.; Diver, S.; Chachi, L.; Megally, A.; Small, C.; Downie, J.; Parnes, J.R.; Bowen, K.; Colice, G.; Brightling, C.E. CASCADE: A phase 2, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the effect of tezepelumab on airway inflammation in patients with uncontrolled asthma. Respir. Res. 2020, 21, 265. [Google Scholar] [CrossRef] [PubMed]
- Chinthrajah, S.; Cao, S.; Liu, C.; Lyu, S.C.; Sindher, S.B.; Long, A.; Sampath, V.; Petroni, D.; Londei, M.; Nadeau, K.C. Phase 2a randomized, placebo-controlled study of anti-IL-33 in peanut allergy. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Lemanske, R.F.; Hanania, N.A.; Korenblat, P.E.; Parsey, M.V.; Arron, J.R.; Harris, J.M.; Scheerens, H.; Wu, L.C.; Su, Z.; et al. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 2011, 365, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Ultsch, M.; Bevers, J.; Nakamura, G.; Vandlen, R.; Kelley, R.F.; Wu, L.C.; Eigenbrot, C. Structural basis of signaling blockade by anti-IL-13 antibody Lebrikizumab. J. Mol. Biol. 2013, 425, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Korenblat, P.; Kerwin, E.; Leshchenko, I.; Yen, K.; Holweg, C.T.J.; Anzures-Cabrera, J.; Martin, C.; Putnam, W.S.; Governale, L.; Olsson, J.; et al. Efficacy and safety of lebrikizumab in adult patients with mild-to-moderate asthma not receiving inhaled corticosteroids. Respir. Med. 2018, 134, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Casale, T.B.; Chipps, B.E.; Rosen, K.; Trzaskoma, B.; Haselkorn, T.; Omachi, T.A.; Greenberg, S.; Hanania, N.A. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy 2018, 73, 490–497. [Google Scholar] [CrossRef]
- Hanania, N.A.; Alpan, O.; Hamilos, D.L.; Condemi, J.J.; Reyes-Rivera, I.; Zhu, J.; Rosen, K.E.; Eisner, M.D.; Wong, D.A.; Busse, W. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: A randomized trial. Ann. Intern. Med. 2011, 154, 573–582. [Google Scholar] [CrossRef]
- MacDonald, K.M.; Kavati, A.; Ortiz, B.; Alhossan, A.; Lee, C.S.; Abraham, I. Short- and long-term real-world effectiveness of omalizumab in severe allergic asthma: Systematic review of 42 studies published 2008–2018. Expert Rev. Clin. Immunol. 2019, 15, 553–569. [Google Scholar] [CrossRef]
- Yancey, S.W.; Ortega, H.G.; Keene, O.N.; Mayer, B.; Gunsoy, N.B.; Brightling, C.E.; Bleecker, E.R.; Haldar, P.; Pavord, I.D. Meta-analysis of asthma-related hospitalization in mepolizumab studies of severe eosinophilic asthma. J. Allergy Clin. Immunol. 2017, 139, 1167–1175.e1162. [Google Scholar] [CrossRef]
- Gupta, A.; Ikeda, M.; Geng, B.; Azmi, J.; Price, R.G.; Bradford, E.S.; Yancey, S.W.; Steinfeld, J. Long-term safety and pharmacodynamics of mepolizumab in children with severe asthma with an eosinophilic phenotype. J. Allergy Clin. Immunol. 2019, 144, 1336–1342.e1337. [Google Scholar] [CrossRef]
- Gupta, A.; Pouliquen, I.; Austin, D.; Price, R.G.; Kempsford, R.; Steinfeld, J.; Bradford, E.S.; Yancey, S.W. Subcutaneous mepolizumab in children aged 6 to 11 years with severe eosinophilic asthma. Pediatric Pulmonol. 2019, 54, 1957–1967. [Google Scholar] [CrossRef]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D.; Investigators, S. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef]
- Corren, J.; Weinstein, S.; Janka, L.; Zangrilli, J.; Garin, M. Phase 3 Study of Reslizumab in Patients With Poorly Controlled Asthma: Effects Across a Broad Range of Eosinophil Counts. Chest 2016, 150, 799–810. [Google Scholar] [CrossRef]
- Bjermer, L.; Lemiere, C.; Maspero, J.; Weiss, S.; Zangrilli, J.; Germinaro, M. Reslizumab for Inadequately Controlled Asthma With Elevated Blood Eosinophil Levels: A Randomized Phase 3 Study. Chest 2016, 150, 789–798. [Google Scholar] [CrossRef]
- Rogliani, P.; Calzetta, L.; Matera, M.G.; Laitano, R.; Ritondo, B.L.; Hanania, N.A.; Cazzola, M. Severe asthma and biological therapy: When, which, and for whom. Pulm. Ther. 2020, 6, 47–66. [Google Scholar] [CrossRef]
- Casale, T.B.; Pacou, M.; Mesana, L.; Farge, G.; Sun, S.X.; Castro, M. Reslizumab Compared with Benralizumab in Patients with Eosinophilic Asthma: A Systematic Literature Review and Network Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2019, 7, 122–130.e121. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Nogueira, M.; Torres, T. Dupilumab for atopic dermatitis: Evidence to date. G Ital. Dermatol. Venereol. 2019, 154, 696–713. [Google Scholar] [CrossRef]
- Zayed, Y.; Kheiri, B.; Banifadel, M.; Hicks, M.; Aburahma, A.; Hamid, K.; Bachuwa, G.; Chandran, A. Dupilumab safety and efficacy in uncontrolled asthma: A systematic review and meta-analysis of randomized clinical trials. J. Asthma 2019, 56, 1110–1119. [Google Scholar] [CrossRef]
- Brooks, G.D. Updated Evaluation of Dupilumab in the Treatment of Asthma: Patient Selection and Reported Outcomes. Ther. Clin. Risk Manag. 2020, 16, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Just, J.; Deschildre, A.; Lejeune, S.; Amat, F. New perspectives of childhood asthma treatment with biologics. Pediatric Allergy Immunol. 2019, 30, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. The airway epithelium in asthma. Nat. Med. 2012, 18, 684–692. [Google Scholar] [CrossRef]
- Viana, F. TRPA1 channels: Molecular sentinels of cellular stress and tissue damage. J. Physiol. 2016, 594, 4151–4169. [Google Scholar] [CrossRef]
- Caceres, A.I.; Brackmann, M.; Elia, M.D.; Bessac, B.F.; del Camino, D.; D’Amours, M.; Witek, J.S.; Fanger, C.M.; Chong, J.A.; Hayward, N.J.; et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc. Natl. Acad. Sci. USA 2009, 106, 9099–9104. [Google Scholar] [CrossRef]
- Balestrini, A.; Joseph, V.; Dourado, M.; Reese, R.M.; Shields, S.D.; Rouge, L.; Bravo, D.D.; Chernov-Rogan, T.; Austin, C.D.; Chen, H.; et al. A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment. J. Exp. Med. 2021, 218, e20201637. [Google Scholar] [CrossRef]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef]
- Rautava, S.; Ruuskanen, O.; Ouwehand, A.; Salminen, S.; Isolauri, E. The hygiene hypothesis of atopic disease-an extended version. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 378–388. [Google Scholar] [CrossRef]
- Wold, A.E. The hygiene hypothesis revised: Is the rising frequency of allergy due to changes in the intestinal flora? Allergy 1998, 53, 20–25. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Lynch, S.V. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host. Microbe 2015, 17, 592–602. [Google Scholar] [CrossRef]
- Kirjavainen, P.V.; Karvonen, A.M.; Adams, R.I.; Täubel, M.; Roponen, M.; Tuoresmäki, P.; Loss, G.; Jayaprakash, B.; Depner, M.; Ege, M.J.; et al. Farm-like indoor microbiota in non-farm homes protects children from asthma development. Nat. Med. 2019, 25, 1089–1095. [Google Scholar] [CrossRef]
- Kozik, A.; Huang, Y.J. Ecological interactions in asthma: From environment to microbiota and immune responses. Curr. Opin. Pulm. Med. 2020, 26, 27–32. [Google Scholar] [CrossRef]
- Karvonen, A.M.; Kirjavainen, P.V.; Täubel, M.; Jayaprakash, B.; Adams, R.I.; Sordillo, J.E.; Gold, D.R.; Hyvärinen, A.; Remes, S.; von Mutius, E.; et al. Indoor bacterial microbiota and development of asthma by 10.5 years of age. J. Allergy Clin. Immunol. 2019, 144, 1402–1410. [Google Scholar] [CrossRef]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; Fitzgerald, M.G.; Fulton, R.S.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar]
- Velasquez-Manoff, M. Gut Microbiome: The peacekeepers. Nature 2015, 518, S3–S11. [Google Scholar] [CrossRef]
- Frati, F.; Salvatori, C.; Incorvaia, C.; Bellucci, A.; Di Cara, G.; Marcucci, F.; Esposito, S. The role of the microbiome in asthma: The gut-lung axis. Int. J. Mol. Sci. 2018, 20, 123. [Google Scholar] [CrossRef]
- Dzidic, M.; Abrahamsson, T.R.; Artacho, A.; Björkstén, B.; Collado, M.C.; Mira, A.; Jenmalm, M.C. Aberrant IgA responses to the gut microbiota during infancy precede asthma and allergy development. J. Allergy Clin. Immunol. 2017, 139, 1017–1025.e1014. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2010, 331, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Strauch, U.G.; Obermeier, F.; Grunwald, N.; Gurster, S.; Dunger, N.; Schultz, M.; Griese, D.P.; Maehler, M.; Scholmerich, J.; Rath, H.C. Influence of intestinal bacteria on induction of regulatory T cells: Lessons from a transfer model of colitis. Gut 2005, 54, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Ostman, S.; Rask, C.; Wold, A.E.; Hultkrantz, S.; Telemo, E. Impaired regulatory T cell function in germ-free mice. Eur. J. Immunol. 2006, 36, 2336–2346. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef]
- Tsuda, M.; Hosono, A.; Yanagibashi, T.; Kihara-Fujioka, M.; Hachimura, S.; Itoh, K.; Hirayama, K.; Takahashi, K.; Kaminogawa, S. Intestinal commensal bacteria promote T cell hyporesponsiveness and down-regulate the serum antibody responses induced by dietary antigen. Immunol. Lett. 2010, 132, 45–52. [Google Scholar] [CrossRef]
- Chinen, T.; Volchkov, P.Y.; Chervonsky, A.V.; Rudensky, A.Y. A critical role for regulatory T cell-mediated control of inflammation in the absence of commensal microbiota. J. Exp. Med. 2010, 207, 2323–2330. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; de Roos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Zeng, H.; Chi, H. Metabolic control of regulatory T cell development and function. Trends Immunol. 2015, 36, 3–12. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Lewis, G.; Wang, B.; Shafiei Jahani, P.; Hurrell, B.P.; Banie, H.; Aleman Muench, G.R.; Maazi, H.; Helou, D.G.; Howard, E.; Galle-Treger, L.; et al. Dietary fiber-induced microbial short chain fatty acids suppress ILC2-dependent airway inflammation. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef]
- Thorsen, J.; Rasmussen, M.A.; Waage, J.; Mortensen, M.; Brejnrod, A.; Bønnelykke, K.; Chawes, B.L.; Brix, S.; Sørensen, S.J.; Stokholm, J.; et al. Infant airway microbiota and topical immune perturbations in the origins of childhood asthma. Nat. Commun. 2019, 10, 5001. [Google Scholar] [CrossRef]
- Hilty, M.; Burke, C.; Pedro, H.; Cardenas, P.; Bush, A.; Bossley, C.; Davies, J.; Ervine, A.; Poulter, L.; Pachter, L.; et al. Disordered microbial communities in asthmatic airways. PLoS ONE 2010, 5, e8578. [Google Scholar] [CrossRef]
- Huang, Y.J.; Nariya, S.; Harris, J.M.; Lynch, S.V.; Choy, D.F.; Arron, J.R.; Boushey, H. The airway microbiome in patients with severe asthma: Associations with disease features and severity. J. Allergy Clin. Immunol. 2015, 136, 874–884. [Google Scholar] [CrossRef]
- Zhou, Y.; Jackson, D.; Bacharier, L.B.; Mauger, D.; Boushey, H.; Castro, M.; Durack, J.; Huang, Y.; Lemanske, R.F., Jr.; Storch, G.A.; et al. The upper-airway microbiota and loss of asthma control among asthmatic children. Nat. Commun. 2019, 10, 5714. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Arévalo, A.; Stiemsma, L.; Dimitriu, P.; Chico, M.E.; Loor, S.; Vaca, M.; Boutin, R.C.T.; Morien, E.; Jin, M.; et al. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J. Allergy Clin. Immunol. 2018, 142, 424–434.e410. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Chua, H.-H.; Chou, H.-C.; Tung, Y.-L.; Chiang, B.-L.; Liao, C.-C.; Liu, H.-H.; Ni, Y.-H. Intestinal dysbiosis featuring abundance of Ruminococcus gnavus associates with allergic diseases in infants. Gastroenterology 2018, 154, 154–167. [Google Scholar] [CrossRef]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.A.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut–lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wopereis, H.; Oozeer, R.; Knipping, K.; Belzer, C.; Knol, J. The first thousand days—intestinal microbiology of early life: Establishing a symbiosis. Pediatric Allergy Immunol. 2014, 25, 428–438. [Google Scholar] [CrossRef]
- Johnson, C.C.; Ownby, D.R. The infant gut bacterial microbiota and risk of pediatric asthma and allergic diseases. Transl. Res. 2017, 179, 60–70. [Google Scholar] [CrossRef]
- Zimmermann, P.; Messina, N.; Mohn, W.W.; Finlay, B.B.; Curtis, N. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: A systematic review. J. Allergy Clin. Immunol. 2019, 143, 467–485. [Google Scholar] [CrossRef]
- Galazzo, G.; van Best, N.; Bervoets, L.; Dapaah, I.O.; Savelkoul, P.H.; Hornef, M.W.; Lau, S.; Hamelmann, E.; Penders, J. Development of the microbiota and associations with birth mode, diet, and atopic disorders in a longitudinal analysis of stool samples, collected from infancy through early childhood. Gastroenterology 2020, 158, 1584–1596. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef]
- Goleva, E.; Jackson, L.P.; Harris, J.K.; Robertson, C.E.; Sutherland, E.R.; Hall, C.F.; Good, J.T., Jr.; Gelfand, E.W.; Martin, R.J.; Leung, D.Y. The effects of airway microbiome on corticosteroid responsiveness in asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 1193–1201. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V.; Nariya, S.; Bhakta, N.R.; Beigelman, A.; Castro, M.; Dyer, A.-M.; Israel, E.; Kraft, M.; Martin, R.J.; et al. Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J. Allergy Clin. Immunol. 2017, 140, 63–75. [Google Scholar] [CrossRef]
- Song, X.; Sun, X.; Oh, S.F.; Wu, M.; Zhang, Y.; Zheng, W.; Geva-Zatorsky, N.; Jupp, R.; Mathis, D.; Benoist, C.; et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 2020, 577, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.; McKenney, P.T.; Konstantinovsky, D.; Isaeva, O.I.; Schizas, M.; Verter, J.; Mai, C.; Jin, W.-B.; Guo, C.-J.; Violante, S.; et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 2020, 581, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Barcik, W.; Boutin, R.C.T.; Sokolowska, M.; Finlay, B.B. The role of lung and gut microbiota in the pathology of asthma. Immunity 2020, 52, 241–255. [Google Scholar] [CrossRef]
- Azad, M.B.; Coneys, J.G.; Kozyrskyj, A.L.; Field, C.J.; Ramsey, C.D.; Becker, A.B.; Friesen, C.; Abou-Setta, A.M.; Zarychanski, R. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: Systematic review and meta-analysis. BMJ 2013, 347, f6471. [Google Scholar] [CrossRef]
- Elazab, N.; Mendy, A.; Gasana, J.; Vieira, E.R.; Quizon, A.; Forno, E. Probiotic administration in early life, atopy, and asthma: A meta-analysis of clinical trials. Pediatrics 2013, 132, e666–e676. [Google Scholar] [CrossRef]
- Zuccotti, G.; Meneghin, F.; Aceti, A.; Barone, G.; Callegari, M.L.; Di Mauro, A.; Fantini, M.P.; Gori, D.; Indrio, F.; Maggio, L.; et al. Probiotics for prevention of atopic diseases in infants: Systematic review and meta-analysis. Allergy 2015, 70, 1356–1371. [Google Scholar] [CrossRef]
- Wei, X.; Jiang, P.; Liu, J.; Sun, R.; Zhu, L. Association between probiotic supplementation and asthma incidence in infants: A meta-analysis of randomized controlled trials. J. Asthma Off. J. Assoc. Care Asthma 2020, 57, 167–178. [Google Scholar] [CrossRef]
- Wickens, K.; Black, P.; Stanley, T.V.; Mitchell, E.; Barthow, C.; Fitzharris, P.; Purdie, G. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin. Exp. Allergy 2012, 42, 1071–1079. [Google Scholar] [CrossRef]
- Wickens, K.; Stanley, T.V.; Mitchell, E.A.; Barthow, C.; Fitzharris, P.; Purdie, G.; Siebers, R. Early supplementation with Lactobacillus rhamnosus HN001 reduces eczema prevalence to 6 years: Does it also reduce atopic sensitization? Clin. Exp. Allergy 2013, 43, 1048–1057. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Salminen, S.; Poussa, T.; Isolauri, E. Probiotics during the first 7 years of life: A cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2007, 119, 1019–1021. [Google Scholar] [CrossRef]
- Kuitunen, M.; Kukkonen, K.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; Tuure, T.; Haahtela, T. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J. Allergy Clin. Immunol. 2009, 123, 335–341. [Google Scholar] [CrossRef]
- Dotterud, C.K.; Storrø, O.; Johnsen, R.; Oien, T. Probiotics in pregnant women to prevent allergic disease: A randomized, double-blind trial. Br. J. Dermatol. 2010, 163, 616–623. [Google Scholar] [CrossRef]
- Boyle, R.J.; Ismail, I.H.; Kivivuori, S.; Licciardi, P.V.; Robins-Browne, R.M.; Mah, L.J.; Axelrad, C. Lactobacillus GG treatment during pregnancy for the prevention of eczema: A randomized controlled trial. Allergy 2011, 66, 509–516. [Google Scholar] [CrossRef]
- Jensen, M.P.; Meldrum, S.; Taylor, A.L.; Dunstan, J.A.; Prescott, S.L. Early probiotic supplementation for allergy prevention: Long-term outcomes. J. Allergy Clin. Immunol. 2012, 130, 1209–1211. [Google Scholar] [CrossRef]
- West, C.E.; Hammarström, M.L.; Hernell, O. Probiotics in primary prevention of allergic disease-follow-up at 8–9 years of age. Allergy 2013, 68, 1015–1020. [Google Scholar] [CrossRef]
- Abrahamsson, T.R.; Jakobsson, T.; Björkstén, B.; Oldaeus, G.; Jenmalm, M.C. No effect of probiotics on respiratory allergies: A seven-year follow-up of a randomized controlled trial in infancy. Pediatr. Allergy Immunol. 2013, 24, 556–561. [Google Scholar] [CrossRef]
- Kauppi, P.K.; Kuokkanen, M.K.; Kukkonen, K.K.; Laitinen, T.H.; Kuitunen, M.K. Interaction of NPSR1 genotypes and probiotics in the manifestation of atopic eczema in early childhood. Allergol. Immunopathol. 2014, 42, 560–567. [Google Scholar]
- Loo, E.X.; Llanora, G.V.; Lu, Q.; Aw, M.M.; Lee, B.W.; Shek, L.P. Supplementation with probiotics in the first 6 months of life did not protect against eczema and allergy in at-risk Asian infants: A 5-year follow-up. Int. Arch. Allergy Immunol. 2014, 163, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, D.M.W.; Rutten, N.; Oostermeijer, C.M.J.; Niers, L.E.M.; Hoekstra, M.O.; Rijkers, G.T.; van der Ent, C.K. Preventive effects of selected probiotic strains on the development of asthma and allergic rhinitis in childhood. Clin. Exp. Allergy 2014, 44, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Di Costanzo, M.; Bedogni, G.; Amoroso, A.; Cosenza, L.; Di Scala, C.; Granata, V. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial. J. Allergy Clin. Immunol. 2017, 139, 1906–1913.e1904. [Google Scholar] [CrossRef] [PubMed]
- Cabana, M.D.; McKean, M.; Caughey, A.B.; Fong, L.; Lynch, S.; Wong, A. Early probiotic supplementation for eczema and asthma prevention: A randomized controlled trial. Pediatrics 2017, 140, 1–9. [Google Scholar] [CrossRef]
- Hol, J.; van Leer, E.H.G.; Elink Schuurman, B.E.E.; de Ruiter, L.F.; Samsom, J.N.; Hop, W.; Neijens, H.J. The acquisition of tolerance toward cow’s milk through probiotic supplementation: A randomized, controlled trial. J. Allergy Clin. Immunol. 2008, 121, 1448–1454. [Google Scholar] [CrossRef]
- Kopp, M.V.; Hennemuth, I.; Heinzmann, A.; Urbanek, R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: No clinical effects of Lactobacillus GG supplementation. Pediatrics 2008, 121, e850. [Google Scholar] [CrossRef]
- Wickens, K.; Black, P.N.; Stanley, T.V.; Mitchell, E.; Fitzharris, P.; Tannock, G.W.; Purdie, G. A differential effect of 2 probiotics in the prevention of eczema and atopy: A double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2008, 122, 788–794. [Google Scholar] [CrossRef]
- West, C.E.; Hammarström, M.L.; Hernell, O. Probiotics during weaning reduce the incidence of eczema. Pediatr. Allergy Immunol. 2009, 20, 430–437. [Google Scholar] [CrossRef]
- Van der Aa, L.B.; van Aalderen, W.M.C.; Heymans, H.S.A.; Henk Sillevis Smitt, J.; Nauta, A.J.; Knippels, L.M.J.; Ben Amor, K. Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy 2011, 66, 170–177. [Google Scholar] [CrossRef]
- Gore, C.; Custovic, A.; Tannock, G.W.; Munro, K.; Kerry, G.; Johnson, K.; Peterson, C. Treatment and secondary prevention effects of the probiotics Lactobacillus paracasei or Bifidobacterium lactis on early infant eczema: Randomized controlled trial with follow-up until age 3 years. Clin. Exp. Allergy 2012, 42, 112–122. [Google Scholar] [CrossRef]
- Ou, C.Y.; Kuo, H.C.; Wang, L.; Hsu, T.Y.; Chuang, H.; Liu, C.A.; Chang, J.C. Prenatal and postnatal probiotics reduces maternal but not childhood allergic diseases: A randomized, double-blind, placebo-controlled trial. Clin. Exp. Allergy 2012, 42, 1386–1396. [Google Scholar] [CrossRef]
- Simpson, M.R.; Dotterud, C.K.; Storrø, O.; Johnsen, R.; Øien, T. Perinatal probiotic supplementation in the prevention of allergy related disease: 6 year follow up of a randomised controlled trial. BMC Dermatol. 2015, 15, 13. [Google Scholar] [CrossRef]
- Wickens, K.; Barthow, C.; Mitchell, E.A.; Stanley, T.V.; Purdie, G.; Rowden, J.; Kang, J.; Hood, F.; van den Elsen, L.; Forbes-Blom, E.; et al. Maternal supplementation alone with Lactobacillus rhamnosus HN001 during pregnancy and breastfeeding does not reduce infant eczema. Pediatr. Allergy Immunol. 2018, 29, 296–302. [Google Scholar] [CrossRef]
- Patnode, M.L.; Beller, Z.W.; Han, N.D.; Cheng, J.; Peters, S.L.; Terrapon, N.; Henrissat, B.; Le Gall, S.; Saulnier, L.; Hayashi, D.K.; et al. Interspecies competition impacts targeted manipulation of human gut bacteria by fiber-derived glycans. Cell 2019, 179, 59–73.e13. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Bindels, L.B. Food for thought about manipulating gut bacteria. Nature 2020, 577, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe 2020. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Laxman, B.; Naureckas, E.T.; Hogarth, D.K.; Sperling, A.I.; Solway, J.; Ober, C.; Gilbert, J.A.; White, S.R. Associations between fungal and bacterial microbiota of airways and asthma endotypes. J. Allergy Clin. Immunol. 2019, 144, 1214–1227.e1217. [Google Scholar] [CrossRef] [PubMed]
- Vandenborght, L.-E.; Enaud, R.; Urien, C.; Coron, N.; Girodet, P.-O.; Ferreira, S.; Berger, P.; Delhaes, L. Type 2–high asthma is associated with a specific indoor mycobiome and microbiome. J. Allergy Clin. Immunol. 2021, 147, 1296–1305.e1296. [Google Scholar] [CrossRef] [PubMed]
- Denner, D.R.; Sangwan, N.; Becker, J.B.; Hogarth, D.K.; Oldham, J.; Castillo, J.; Sperling, A.I.; Solway, J.; Naureckas, E.T.; Gilbert, J.A.; et al. Corticosteroid therapy and airflow obstruction influence the bronchial microbiome, which is distinct from that of bronchoalveolar lavage in asthmatic airways. J. Allergy Clin. Immunol. 2016, 137, 1398–1405.e1393. [Google Scholar] [CrossRef]
- McCauley, K.; Durack, J.; Valladares, R.; Fadrosh, D.W.; Lin, D.L.; Calatroni, A.; LeBeau, P.K.; Tran, H.T.; Fujimura, K.E.; LaMere, B.; et al. Distinct nasal airway bacterial microbiotas differentially relate to exacerbation in pediatric patients with asthma. J. Allergy Clin. Immunol. 2019, 144, 1187–1197. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).