Abstract
Background: Perioperative cisplatin-based chemotherapy (CBC) can improve the outcome of patients with muscle-invasive bladder cancer (MIBC), but it is still to be defined which patients benefit. Mutations in DNA damage response genes (DDRG) can predict the response to CBC. The value of DDRG expression as a marker of CBC treatment effect remains unclear. Material and methods: RNA expression of the nine key DDRG (BCL2, BRCA1, BRCA2, ERCC2, ERCC6, FOXM1, RAD50, RAD51, and RAD52) was assessed by qRT-PCR in a cohort of 61 MICB patients (median age 66 y, 48 males, 13 females) who underwent radical cystectomy in a tertiary care center. The results were validated in the The Cancer Genome Atlas (TCGA) cohort of MIBC (n = 383). Gene expression was correlated with disease-free survival (DFS) and overall survival (OS). Subgroup analyses were performed in patients who received adjuvant cisplatin-based chemotherapy (ACBC) (Mannheim n = 20 and TCGA n = 75). Results: Low expression of RAD52 was associated with low DFS in both the Mannheim and the TCGA cohorts (Mannheim: p = 0.039; TCGA: p = 0.017). This was especially apparent in subgroups treated with ACBC (Mannheim: p = 0.0059; TCGA: p = 0.012). Several other genes showed an influence on DFS in the Mannheim cohort (BRCA2, ERCC2, FOXM1) where low expression was associated with poor DFS (p < 0.05 for all). This finding was not fully supported by the data in the TCGA cohort, where high expression of FOXM1 and BRCA2 correlated with poor DFS. Conclusion: Low expression of RAD52 correlated with decreased DFS in the Mannheim and the TCGA cohort. This effect was especially pronounced in the subset of patients who received ACBC, making it a promising indicator for response to ACBC on the level of gene expression.
Keywords:
muscle-invasive bladder cancer; DNA-damage response; BCL2; BRCA1; BRCA2; ERCC2; ERCC6; FOXM1; RAD50; RAD51; RAD52; cisplatin; adjuvant chemotherapy 1. Introduction
Bladder cancer (BC) is the ninth most commonly diagnosed cancer worldwide [1]. Radical cystectomy (RC) with optional perioperative chemotherapy is the standard of care in muscle-invasive bladder cancer (MIBC) [2]. The five-year, disease-specific survival rate with RC alone is only 50–67% [3,4]. Perioperative cisplatin-based chemotherapy (CBC) may improve long-term survival and has been used since the 1980s [5]. However, only 30–40% of MIBC are responding to CBC [6]. Responders show improved survival rates [5,7,8]. Selection for perioperative CBC currently relies on clinicopathological features associated with a high risk of recurrence [2]. Concerns about overtreatment and the limited response rates cause poor implementation of perioperative CBC in clinical practice. Despite guideline recommendation and robust data [2,5], neoadjuvant CBC is administered only in around 30% of the applicable patients in the USA and in even less in many European countries [9].
Development of biomarkers to accurately identify responders could improve patient selection on the path to a more personalized approach to perioperative CBC.
Several targets have been investigated. It has been shown that different molecular subtypes of BC have shown differences in CBC response [10]. In addition, mutations in DNA damage response genes (DDRG) have been associated with increased sensitivity to CBC [11,12,13,14]. A correlation between DDRG and molecular subtypes has not been investigated.
A major problem in the clinical application of DDRG alterations as a marker of CBC response is the fact that mutations in the particular genes are seldom seen and profiling of the genes with relevance for DNA-damage response makes panel sequencing necessary. In addition, as gene expression is quantitatively obtainable in all samples, RNA expression could give information not only for those patients carrying a specific mutation. Furthermore, it can easily be assessed using standardized qRT-PCR assays.
Therefore, we aimed to evaluate the relevance of RNA expression of several DDRG in a MIBC cohort from a high-volume tertiary care center and The Cancer Genome Atlas (TCGA) MIBC dataset.
2. Results
2.1. Basic Characteristics of Patients
Patient characteristics of the Mannheim cohort are given in Table 1.

Table 1.
Patient characteristics of the Mannheim cohort.
Patient characteristics of the TCGA cohort are given in Table 2.

Table 2.
Patient characteristics of the The Cancer Genome Atlas (TCGA) cohort.
2.2. Mannheim Cohort: All Patients
To investigate the impact of nine genes known to code for proteins relevant for DNA damage response on MIBC outcome we analyzed an unselected cohort of 61 patients who underwent radical cystectomy in the Department of Urology and Urosurgery of the University Medical Centre Mannheim. The expression of none of the genes differed between male and female, younger and older (≤70 years vs. >70 years), tumor stage T2 vs. tumor stage T3/4, and negative lymph nodes (N0) vs. positive lymph nodes (N+) at the time of cystectomy. Spearman correlation analyses revealed highly significant positive correlations among all combinations of genes, except for FOXM1 and BCL2. Correlation coefficients (ρ) ranged from 0.4303 (p = 0.0032) for BCL2 and RAD51 to 0.8746 (p < 0.0001) for BRCA1 and BRCA2 (Supplementary Table S1).
After cutoff determination using the partition test, the expression of none of the nine selected genes showed a significant association with overall survival (OS). Yet, for disease-free survival (DFS) a significant association could be seen for a lowered expression of BRCA2 (p = 0.0361, median DFS 7 months (mo) vs. not reached (n.r.)), ERCC2 (p = 0.0476, 8 vs. 27 mo), FOXM1 (p = 0.0268, 10 mo vs. n.r.), and RAD52 (p = 0.0392, 8 mo vs. n.r.) (Figure 1).
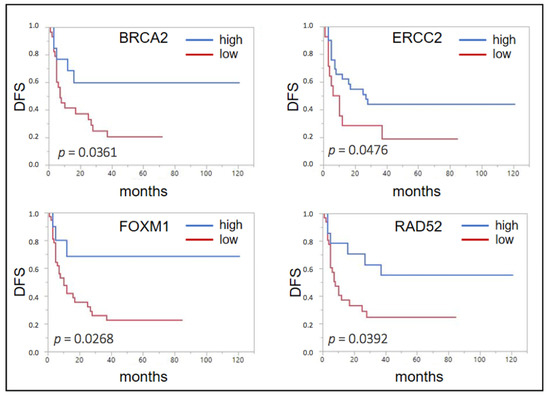
Figure 1.
Forrest plots of the correlation between RNA expression and disease free survival (DFS) in the unselected Mannheim cohort.
2.3. Mannheim Cohort: Patients Treated with ACBC
Using the same cutoff determination method for the subcohort of 20 patients who received ACBC, a low expression of BRCA1 (p = 0.0062, median OS 19 mo vs. n.r.) and BRCA2 (p = 0.0195, 36 mo vs. n.r.) was associated with a shorter OS (Figure 2A). For BRCA1 (p = 0.0026, median DFS 5 mo vs. n.r.), ERCC2 (p = 0.0274, 7 mo vs. n.r.), FOXM1 (p = 0.0143, 8 mo vs. n.r.), RAD50 (p = 0.0392, 7.5 mo vs. n.r.), RAD51 (p = 0.0250, 7 mo vs. n.r.), and RAD52 (p = 0.0059, 5 mo vs. n.r.), this was also seen for DFS (Figure 2B). BRCA2 almost reached significance (p = 0.0644, 7 mo vs. n.r.).
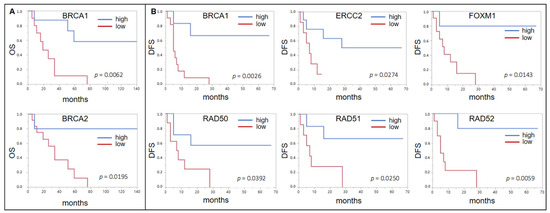
Figure 2.
(A) Forrest plots of the correlation between RNA expression and overall survival (OS) in the Mannheim adjuvant cisplatin based chemotherapy (ACBC) cohort. (B) Forrest plots of the correlation between RNA expression and DFS in the Mannheim ACBC cohort.
2.4. TCGA Cohort: All Patients
To validate these findings, we performed the same analyses in the TCGA cohort. After correlation with patient- and tumor-derived parameters, the expression of none of the genes differed significantly according to gender, tumor (T) stage (T2 vs. T3/T4), and presence of lymph node metastases. ERCC6 was significantly more highly expressed in younger patients (<70 years, p = 0.0006). All other genes did not show differential expression according to patient age.
Spearman correlation analyses again revealed numerous strong positive correlations between several genes: BRCA1 correlated with BRCA2 (ρ = 0.6432, p < 0.0001), ERCC6 (ρ = 0.1751, p = 0.0006), FOXM1 (ρ = 0.6806, p < 0.0001), RAD50 (ρ = 0.2389, p < 0.0001), and RAD51 (ρ = 0.5626, p < 0.0001) and BRCA2 correlated with ERCC6 (ρ = 0.2911, p < 0.0001), FOXM1 (ρ = 0.5119, p < 0.0001), RAD50 (ρ = 0.4313, ρ < 0.0001), and RAD51 (ρ = 0.4197, p < 0.0001). Furthermore, ERCC2 correlated with RAD52 (ρ = 0.1371, p = 0.0072), ERCC6 correlated with RAD50 (ρ = 0.3522, p < 0.0001), and RAD51 (ρ = 0.1542, p = 0.0025) and FOXM1 correlated with RAD51 (ρ = 0.6294, p < 0.0001). Weak to moderate negative correlations were seen between BCL2 and RAD51, BRCA2 and ERCC2, and ERCC2 and ERCC6. Detailed information is given in Table S2.
In the whole cohort (n = 383), only for a low expression of RAD52 (p = 0.0178, median OS low 31.4 mo vs. high “not reached” (n.r.)), a significantly shorter OS was seen and ERCC6 almost reached significance (p = 0.0528, 28.3 vs. 33.6 mo). Interestingly, for BCL2 (p = 0.0169, 33.6 vs. 21.4 mo), BRCA1 (p = 0.0322, 35.9 vs. 19.9 mo), FOXM1 (p = 0.0006, 35.9 vs. 17.1 mo), RAD50 (p = 0.0177, 35.5 vs. 16.7 mo), and RAD51 (p = 0.0375, 47.3 vs. 26.5 mo) a high expression predicted a shorter OS (Figure 3A).
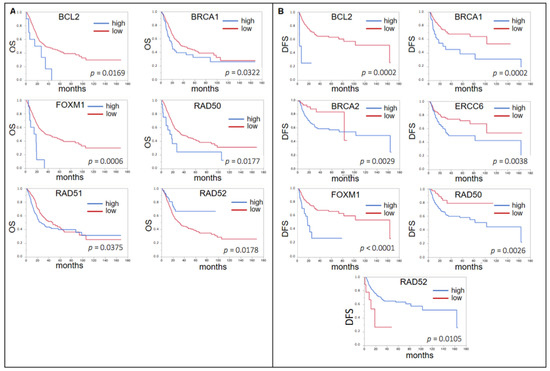
Figure 3.
(A) Forrest plots of the correlation between RNA expression of DNA damage response genes (DDRG) and OS in the TCGA unselected cohort. (B) Forrest plots of the correlation between RNA expression of DDRG and DFS in the TCGA unselected cohort.
With regard to DFS, again only for RAD52 (p = 0.0105, median DFS 18.6 vs. 163.7 mo), a low expression was associated with a worse prognosis, whereas the same was found for a high expression of BCL2 (p = 0.0002, 163.6 vs. 5.4 mo), BRCA1 (p = 0.0002, n.r. vs. 25.6 mo), ERCC6 (p = 0.0038, n.r. vs. 35.5 mo), FOXM1 (p < 0.0001, 163.6 vs. 18.5 mo), and RAD50 (p = 0.0026, n.r. vs. 102,9 mo) (Figure 3B). BRCA2 also showed a significant result (p = 0.0029) with a shorter DFS for low expression (82.4 vs. 102.9 mo). However, the curves crossed not before the last event in the low expression group.
2.5. TCGA Cohort: Patients Treated with ACBC
In those patients who received ACBC (n = 76) a high expression of ERCC2 indicated a shorter OS (p = 0.0170, median OS n.r. vs. 24.0 mo). Similar to our own cohort, a low expression of BCL2 (p = 0.0029, 18.1 vs. n.r.), ERCC6 (p = 0.0394, 28.6 vs. n.r.), FOXM1 (p = 0.0213, 28.8 vs. n.r.), RAD50 (p = 0.0227, 28.6 vs. n.r.), and RAD52 (p = 0.0226, 23.7 vs. n.r.) was indicative of a shorter OS (Figure 4A). A high expression of FOXM1 (p = 0.0005, median DFS 163.6 vs. 12.6 mo) and a low expression of RAD52 (p = 0.0129, 13.0 vs. 163.6 mo) indicated a shorter DFS (Figure 4B).
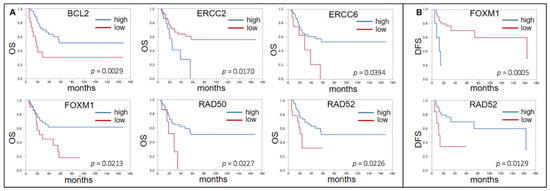
Figure 4.
(A) Forrest plots of the correlation between RNA expression of DDRG and OS in the TCGA ACBC cohort. (B) Forrest plots of the correlation between RNA expression of DDRG and DFS in the TCGA ACBC cohort.
2.6. Uni- and Multivariable Analysis of the Whole TCGA Cohort Regarding Risk Factors for OS and DFS
Univariable analyses of clinical factors and DNA damage response genes in the whole cohort identified age, T stage, nodal (N) stage, BCL2, BRCA1, FOXM1, RAD50, RAD51, and RAD52 as risk factors for a shorter OS. Yet, in multivariable analyses only N stage, FOXM1, RAD50, and RAD52 remained to be independent risk factors in multivariable analyses (Table S3).
For DFS T stage, N stage, BCL2, BRCA1, BRCA2, ERCC6, FOXM1, RAD50, and RAD52 were significant in univariable and N stage FOXM1, RAD50, and RAD52 proved to be independent risk factors (Table S4).
2.7. Uni- and Multivariable Analysis of the TCGA Cohort Treated with ACBC Regarding Risk Factors for OS and DFS
In the subgroup of patients with adjuvant chemotherapy N stage, BCL2, ERCC2, FOXM1, and RAD52 showed significant association with OS in univariable analyses, with N stage, BCL2, ERCC2, and FOXM1 remaining independent (Table S5).
For DFS only N stage, FOXM1, RAD50, and RAD52 were significant in univariable analyses. FOXM1, RAD50, and RAD52 but not N stage remained independent risk factors (Table S6).
3. Discussion
In MIBC, CBC can improve outcome in responders in a neoadjuvant, as well as in an adjuvant, setting [5,15]. However, the burden of this treatment is considerable and only a fraction of patients responds and, thus, benefits.
We examined the gene expression of nine DDRG with known relevance in cisplatin resistance in a cohort of 61 patients with MIBC in an effort to identify responders. Genes were selected after literature review and in silico analysis of the TCGA set. The bcl-2 (b cell lymphoma 2) protein encoded by BCL2 is a major apoptosis regulator and has mainly antiapoptotic effects in many different cancer entities [16]. BRCA1 and BRCA2 code for the correspondent breast cancer type 1/2 susceptibility proteins. They both take direct action in the repair of DNA double-strand breaks. Typically both proteins form a complex together with Palb2 (partner an localizer of BRCA2), which then recruits the recombinase Rad51 (encoded by RAD51) and the BCDX2 complex (among others consisting of several Rad51 paralogs), which is able to bind to the DNA for homologous recombinational repair [17,18]. Similar to Rad51, Rad50 (as a part of the MRN complex, encoded by RAD50) is also involved in the repair of DNA double-strand breaks. Yet, this complex has its main role earlier in the cascade by detecting double-strand breaks and recruiting and activating the serine-protein kinase ATM [19,20]. Rad52 (encoded by RAD52) is both a mediator of Rad51 function and, together with ERCC1 (DNA excision repair protein ERCC-1, encoded by ERCC1), is a major component in DNA repair of the single-strand annealing pathway of homologous recombination [21,22]. ERCC6 encodes for DNA excision repair protein ERCC-6, also called CS-B protein, which has both helicase and ATPase activity, is involved both in base excision repair and nucleotide excision repair, and facilitates homologous recombination repair [23,24]. Forkhead Box M1 (encoded by FOXM1) is a transcription factor and has master regulatory effects of the transcription of multiple genes coding for proteins relevant in processes in DNA damage repair, e.g., DNA damage recognition, excision of damaged DNA, DNA unwinding, chromatin remodeling, and DNA synthesis and ligation [25].
In the unselected Mannheim cohort, irrespective of systemic treatment, BRCA2, ERCC2, FOXM1, and RAD52 were associated with a shorter DFS. Regarding OS, none of the investigated genes reached statistical significance with RAD52 showing a promising trend. Low expression correlated with poor outcome in all four genes. For FOXM1 this is partially controversial to recent findings regarding the prognostic role of this gene in both NMIBC and MIBC [26,27].
In the TCGA set, we found that the expression of six genes correlated with OS and of seven genes correlated with DFS, in the unselected cohort. Interestingly, low expression correlated with a better outcome in six out of the seven genes, differing from our results. For RAD52 the findings were consistent between cohorts.
Looking at the patients who received ACBC, six genes showed correlations in the Mannheim cohort (BRCA1 and BRCA2 for OS; BRCA1, ERCC2, FOXM1, RAD50, RAD51, and RAD52 for DFS). This is remarkable as only 20 patients were analyzed. RAD52 had the strongest correlation, surpassing the significant correlation in the unselected cohort (p = 0.0059). Low expression correlated with poor outcome in all genes.
In the TCGA cohort, low expression of BCL2, ERCC6, FOXM1, RAD50, and RAD52 correlated with decreased OS. In RAD52 this finding was validated also in DFS. Interestingly, low expression of FOXM1 was associated with poor OS but an improvement in DFS.
Overall, the association of low RAD52 expression and poor outcome was consistent throughout both cohorts and also subgroups that received ACBC with DFS as an endpoint. The other way around a high expression of RAD52 could be an indicator for a favorable response to ACBC.
Bellmunt et al. examined RNA expression of several DDRG in a cohort of 57 patients with MIBC in a similar design as the present study [28]. ERCC1 was the only gene that could show a significant influence on OS. Low expression correlated with better outcome, differing from our cohort for this particular gene.
The reason for the partially conflicting results in several genes remains unclear. We believe that differences in the methods of analysis, next generation sequencing (NGS) for TCGA data and qRT-PCR for our own data, as well as differences in the cohort composition and ethnical and regional aspects may contribute to these differences, as we also observed this for other target genes [29].
To further investigate if this prognostic role of RAD52 expression is independent of other risk factors we performed a multivariate analysis using logistic regression in the TCGA dataset. Here we could demonstrate that RAD52 was highly prognostic in the whole cohort (for OS) as well as in patients treated with ACBC (for both OS and DFS).
Although there have been several studies showing the influence of mutations in DDRG on outcome and response to CBC, we chose to examine RNA expression in selected genes. Detection of mutations in DDRG requires panel sequencing and the proportion of defective genes is relatively low [11,14]. Therefore, we chose to examine the possibility to detect also functional gene alterations on the level of RNA expression, which does not necessarily need to be the result of a mutation in the respective gene.
Limitatons
Our study has several weaknesses. The number of patients and the proportion of patients who received ACBC were limited. Furthermore, the patients were selected retrospectively. Therefore, the comparison has to be interpreted with caution. Although the effect of DDRG alterations appears to be more pronounced in patients who received ACBC, we could not differentiate between the prognostic role and the role as markers of cisplatin sensitivity. Moreover, patients who received ACBC were lymph node positive in a higher proportion, suggesting some extent of selection bias. The same applies to the TCGA data set, however.
Furthermore, our study was limited to only a subset of genes with relevance in DNA damage response. Other genes or their correspondent proteins, either directly involved in the repair of DNA damages, e.g., like phosphorylated histone family member X (γH2AX) [30] or circumvention of apoptosis, could potentially also be valuable markers for cisplatin sensitivity in MIBC.
4. Materials and Methods
4.1. Patients
After approval by the institutional ethics committee (medical faculty Mannheim, Medizinische Ethikkommission II, registration number 2015-549N-MA), a cohort of 61 patients (median age 66 years, ranging from 40–86 years, 48 males, 13 females) with MIBC who underwent radical cystectomy in the Department of Urology and Urosurgery of the University Medical Centre Mannheim was retrospectively identified (Mannheim cohort). Of these, 20 received adjuvant cisplatin-based chemotherapy (ACBC). Clinical data were extracted from medical records. All analyses were approved by the institutional review board (2016-814R-MA).
4.2. Selection of Genes
Candidates were selected after extensive literature review. Genes that have shown correlation to CBC response in MIBC (e.g., ERCC1, BCL2, and FOXM1) as well as genes that are associated to CBC response in other cancers were included (BRCA1, BRCA 2, RAD 51, and RAD 52). Afterwards, in silico analyses were performed with the TCGA data set. Finally, we selected nine DDRG that appeared most promising.
4.3. RNA Extraction and qPCR
Hematoxylin-eosin-stained (HE) 3-µm slides and subsequent 10-µm unstained slides were obtained from formalin-fixed, paraffin-embedded tissue samples. On the HE slides, areas with a high tumor content were marked and dissected from subsequent unstained slides to achieve a tumor content of at least 50% for subsequent RNA extraction, which was performed with the nucleic acid XTRACT FFPE kit (STRATIFYER Molecular Pathology GmbH, Cologne, Germany). RNA concentration and quality were measured with a Nanodrop (Thermo Fisher, Waltham, MA, USA) spectral photometer. Next, cDNA synthesis with an optimized protocol for analysis for formalin fixed paraffin embedded (FFPE) -derived samples, using a pool of sequence-specific reverse PCR primers (reference genes RPL37A and target genes BCL2, BRCA1, BRCA2, ERCC2, ERCC6, FOXM1, RAD50, RAD51, and RAD52), was performed. As reverse transcriptase, Superscript III (Thermo Fisher Scientific, Waltham, MA, USA), was used at 55 °C for 120 min, followed by an enzyme inactivation step at 70 °C for 15 min. The cDNA was stored at −20 °C or immediately used for qPCR. Forty cycles of amplification with 3 s of 95 °C and 30 s of 60 °C were conducted on a StepOnePlus qRT-PCR cycler (Applied Biosystems, Waltham, MA, USA). RPL37A served as housekeeping gene for normalization according to the 40-(∆Ct) method. Sequences of primers and fluorescent probes are given in Table S7. The workflow is summarized in supplementary Figure S1.
4.4. In Silico Analyses
The TCGA cohort for urinary bladder cancer was used for validation [31]. Clinical data and mRNA expression data were obtained from CBioPortal (https://www.cbioportal.org, accessed on 5 October 2019) [32]. Patients with non-muscle invasive bladder cancer (NMIBC) and patients with follow-up of less than three months were excluded from the subsequent analyses. Analyses with cutoff definition and Kaplan–Meier analyses were performed in analogy to the analyses in the Mannheim cohort for both the whole cohort of MIBC (n = 383) and those patients receiving ACBC chemotherapy (n = 75). Patient characteristics are given in Table 2.
4.5. Statistical Analyses
For statistical analyses, non-parametric two-sided t-test, Spearman correlation, partition test, and Kaplan–Meier analyses were used. Statistics were performed with SAS JMP 14 (SAS Institute, Cary, NC, USA) and Prism 7 (GraphPad software Inc., San Diego, CA, USA).
All methods were carried out in accordance with the relevant guidelines and regulations. Informed consent was obtained from all patients in the Mannheim cohort, prior to cystectomy.
5. Conclusions
RNA expression of several DDRG is correlated with DFS and OS of MIBC patients. This was also the case in the subset of patients who received ACBC, where the correlation was especially pronounced. Of the investigated genes, RAD52 showed consistent results in both the Mannheim and the TCGA cohorts, making it a potential marker to identify patients who will likely benefit from ACBC. Although our sample size was not sufficient to draw conclusions for clinical practice, we demonstrated that RNA expression of DDRG is methodically feasible and relevant. Further investigation and validation in larger, controlled cohorts are warranted.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/article/10.3390/ijms22084188/s1.
Author Contributions
J.H.: concept and design, data analysis and interpretation, drafting of the manuscript; H.S.: data collection, laboratory experiments, in silico analysis; K.N.: supervision of laboratory experiments, critical revision of the manuscript; C.-A.W.: critical revision of the manuscript; P.N.: critical revision of the manuscript, supervision; J.v.H.: data analysis and interpretation, critical revision of the manuscript; M.S.M.: critical revision of the manuscript, supervision, P.E.: concept and design, data analysis and interpretation, critical revision of the manuscript, supervision; T.S.W.: concept and design, statistical analysis, in silico analysis, drafting of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by the Monika Kutzner Foundation, Berlin, Germany.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of the Medical Faculty Mannheim (Medizinische Ethik-Komission II) registration number 2015-549N-MA (for tissue analyses, approved 10 June 2015) and 2016-814R-MA (for clinical data and follow-up, approved 5 April 2016).
Informed Consent Statement
Patient consent of tissue analysis was obtained prior to cystectomy from all patients in the Mannheim cohort.
Data Availability Statement
Data from the TCGA cohort are available at https://www.cbioportal.org (accessed on 5 October 2019).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACBC | adjuvant cisplatin-based chemotherapy |
| BC | bladder cancer |
| CBC | cisplatin-based chemotherapy |
| DDRG | DNA-damage response genes |
| DFS | disease-free survival |
| MIBC | muscle invasive bladder cancer |
| NACBC | non-muscle invasive bladder cancer |
| OS | overall survival |
| RC | radical cystectomy |
| TCGA | The Cancer Genome Atlas |
References
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Witjes, J.A.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernández, V.; Espinós, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef]
- Dalbagni, G.; Genega, E.; Hashibe Mi Zhang Zf Russo, P.; Herr, H.; Reuter, V. Cystectomy for bladder cancer: A contemporary series. J. Urol. 2001, 165, 1111–1116. [Google Scholar] [CrossRef]
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.C.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M.; et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1054 patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef]
- Yin, M.; Joshi, M.; Meijer, R.P.; Glantz, M.; Holder, S.; Harvey, H.A.; Kaag, M.; Van De Putte, E.E.; Horenblas, S.; Drabick, J.J. Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. Oncologist 2016, 21, 708–715. [Google Scholar] [CrossRef]
- Shah, J.B.; McConkey, D.J.; Dinney, C.P.N. New strategies in muscle-invasive bladder cancer: On the road to personalized medicine. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 2608–2612. [Google Scholar] [CrossRef]
- Grossman, H.B.; Natale, R.B.; Tangen, C.M.; Speights, V.O.; Vogelzang, N.J.; Trump, D.L.; White, R.W.; Sarosdy, M.F.; Wood, D.P., Jr.; Raghavan, D.; et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 2003, 349, 859–866. [Google Scholar] [CrossRef] [PubMed]
- EORTC Genito-Urinary Group; Australian Bladder Cancer Study Group; National Cancer Institute of Canada Clinical Trials Group; Finnbladder, Norwegian Bladder Cancer Study Group; Club Urologico Espanol de Tratamiento Oncologico (CUETO) group. Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: A randomised controlled trial. International collaboration of trialists. Lancet Lond. Engl. 1999, 354, 533–540. [Google Scholar] [CrossRef]
- Raj, G.V.; Karavadia, S.; Schlomer, B.; Arriaga, Y.; Lotan, Y.; Sagalowsky, A.; Frenkel, E. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer 2011, 117, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.L.; et al. Identification of Distinct Basal and Luminal Subtypes of Muscle-Invasive Bladder Cancer with Different Sensitivities to Frontline Chemotherapy. Cancer Cell 2014, 25, 152–165. [Google Scholar] [CrossRef]
- Plimack, E.R.; Dunbrack, R.L.; Brennan, T.A.; Andrake, M.D.; Zhou, Y.; Serebriiskii, I.G.; Slifker, M.; Alpaugh, K.; Dulaimi, E.; Palma, N.; et al. Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder Cancer. Eur. Urol. 2015, 68, 959–967. [Google Scholar] [CrossRef]
- Yoshida, T.; Kates, M.; Fujita, K.; Bivalacqua, T.J.; McConkey, D.J. Predictive biomarkers for drug response in bladder cancer. Int. J. Urol. 2019, 26, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Van Allen, E.M.; Mouw, K.W.; Kim, P.; Iyer, G.; Wagle, N.; Al-Ahmadie, H.; Zhu, C.; Ostrovnaya, I.; Kryukov, G.V.; O’Connor, K.W.; et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014, 4, 1140–1153. [Google Scholar] [CrossRef]
- Teo, M.Y.; Bambury, R.M.; Zabor, E.C.; Jordan, E.; Al-Ahmadie, H.; Boyd, M.E.; Bouvier, N.; Mullane, S.A.; Cha, E.K.; Roper, N.; et al. DNA Damage Response and Repair Gene Alterations Are Associated with Improved Survival in Patients with Platinum-Treated Advanced Urothelial Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 3610–3618. [Google Scholar] [CrossRef]
- Leow, J.J.; Martin-Doyle, W.; Rajagopal, P.S.; Patel, C.G.; Anderson, E.M.; Rothman, A.T.; Cote, R.J.; Urun, Y.; Chang, S.L.; Choueiri, T.K.; et al. Adjuvant chemotherapy for invasive bladder cancer: A 2013 updated systematic review and meta-analysis of randomized trials. Eur. Urol. 2014, 66, 42–54. [Google Scholar] [CrossRef]
- Hafezi, S.; Rahmani, M. Targeting BCL-2 in Cancer: Advances, Challenges, and Perspectives. Cancers 2021, 13, 1292. [Google Scholar] [CrossRef]
- Jensen, R.B.; Carreira, A.; Kowalczykowski, S.C. Purified human BRCA2 stimulates RAD51-mediated recombination. Nature 2010, 467, 678–683. [Google Scholar] [CrossRef]
- Chun, J.; Buechelmaier, E.S.; Powell, S.N. Rad51 paralog complexes BCDX2 and CX3 act at different stages in the BRCA1-BRCA2-dependent homologous recombination pathway. Mol. Cell Biol. 2013, 33, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Meng, Y.; Zhang, M.; Li, D. MRE11-RAD50-NBS1 complex alterations and DNA damage response: Implications for cancer treatment. Mol. Cancer 2019, 18, 169. [Google Scholar] [CrossRef] [PubMed]
- Situ, Y.; Chung, L.; Lee, C.S.; Ho, V. MRN (MRE11-RAD50-NBS1) Complex in Human Cancer and Prognostic Implications in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 816. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.M.; Pierce, A.J.; Oh, J.; Pastink, A.; Jasin, M. Genetic steps of mammalian homologous repair with distinct mutagenic consequences. Mol. Cell Biol. 2004, 24, 9305–9316. [Google Scholar] [CrossRef] [PubMed]
- Singleton, M.R.; Wentzell, L.M.; Liu, Y.; West, S.C.; Wigley, D.B. Structure of the single-strand annealing domain of human RAD52 protein. Proc. Natl. Acad. Sci. USA 2002, 99, 13492–13497. [Google Scholar] [CrossRef] [PubMed]
- van Hoffen, A.; Natarajan, A.T.; Mayne, L.V.; van Zeeland, A.A.; Mullenders, L.H.; Venema, J. Deficient repair of the transcribed strand of active genes in Cockayne’s syndrome cells. Nucleic Acids Res. 1993, 21, 5890–5895. [Google Scholar] [CrossRef][Green Version]
- Batenburg, N.L.; Thompson, E.L.; Hendrickson, E.A.; Zhu, X.-D. Cockayne syndrome group B protein regulates DNA double-strand break repair and checkpoint activation. EMBO J. 2015, 34, 1399–1416. [Google Scholar] [CrossRef]
- Zona, S.; Bella, L.; Burton, M.J.; Nestal de Moraes, G.; Lam, E.W.-F. FOXM1: An emerging master regulator of DNA damage response and genotoxic agent resistance. Biochim. Biophys. Acta 2014, 1839, 1316–1322. [Google Scholar] [CrossRef]
- Breyer, J.; Wirtz, R.M.; Erben, P.; Rinaldetti, S.; Worst, T.S.; Stoehr, R.; Eckstein, M.; Sikic, D.; Denzinger, S.; Burger, M.; et al. FOXM1 overexpression is associated with adverse outcome and predicts response to intravesical instillation therapy in stage pT1 non-muscle-invasive bladder cancer (NMIBC). BJU Int. 2019, 123, 187–196. [Google Scholar] [CrossRef]
- Rinaldetti, S.; Wirtz, R.M.; Worst, T.S.; Eckstein, M.; Weiss, C.A.; Breyer, J.; Otto, W.; Bolenz, C.; Hartmann, A.; Erben, P. FOXM1 predicts overall and disease specific survival in muscle-invasive urothelial carcinoma and presents a differential expression between bladder cancer subtypes. Oncotarget 2017, 8, 47595. [Google Scholar] [CrossRef]
- Bellmunt, J.; Paz-Ares, L.; Cuello, M.; Cecere, F.L.; Albiol, S.; Guillem, V.; Gallardo, E.; Carles, J.; Mendez, P.; De la Cruz, J.J.; et al. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2007, 18, 522–528. [Google Scholar] [CrossRef]
- Rinaldetti, S.; Rempel, E.; Worst, T.S.; Eckstein, M.; Steidler, A.; Weiss, C.A.; Bolenz, C.; Hartmann, A.; Erben, P. Subclassification, survival prediction and drug target analyses of chemotherapy-naïve muscle-invasive bladder cancer with a molecular screening. Oncotarget 2018, 9, 25935–25945. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, F.; Graziani, G.; Antinozzi, C.; Feldman, D.R.; Houldsworth, J.; Bosl, G.J.; Chaganti, R.S.; Moynahan, M.E.; Jasin, M.; Barchi, M. Reduced Proficiency in Homologous Recombination Underlies the High Sensitivity of Embryonal Carcinoma Testicular Germ Cell Tumors to Cisplatin and Poly (ADP-Ribose) Polymerase Inhibition. PLoS ONE 2012, 7, e51563. [Google Scholar] [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).