Abstract
The members of the B7 family, as immune checkpoint molecules, can substantially regulate immune responses. Since microRNAs (miRs) can regulate gene expression post-transcriptionally, we conducted a scoping review to summarize and discuss the regulatory cross-talk between miRs and new B7 family immune checkpoint molecules, i.e., B7-H3, B7-H4, B7-H5, butyrophilin like 2 (BTNL2), B7-H6, B7-H7, and immunoglobulin like domain containing receptor 2 (ILDR2). The current study was performed using a six-stage methodology structure and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. PubMed, Embase, Scopus, Cochrane, ProQuest, and Google Scholar were systematically searched to obtain the relevant records to 5 November 2020. Two authors independently reviewed the obtained records and extracted the desired data. After quantitative and qualitative analyses, we used bioinformatics approaches to extend our knowledge about the regulatory cross-talk between miRs and the abovementioned B7 family members. Twenty-seven articles were identified that fulfilled the inclusion criteria. Studies with different designs reported gene–miR regulatory axes in various cancer and non-cancer diseases. The regulatory cross-talk between the aforementioned B7 family molecules and miRs might provide valuable insights into the pathogenesis of various human diseases.
1. Introduction
Immune checkpoints can considerably regulate immune responses [1]. These molecules are critical for maintaining self-tolerance and preventing the stimulation of immune responses against normal peripheral tissues. Indeed, suppressing inhibitory axes, e.g., the immune checkpoint axis of cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed death-ligand 1 (PD-L1), has revolutionized cancer immunotherapy [2].
The B7 family is a group of immune checkpoints commonly expressed in different immune cells, such as antigen-presenting cells, T cells, B cells, natural killer cells, and various tissues. They play a crucial role in immune response; for example, they have substantial roles in directing the fate of T cells by binding their receptors. Various members of the B7 family have been identified, e.g., B7.1 (CD80), B7.2 (CD86), B7-H1 (PD-L1, or CD274), B7-DC (PD-L2, PDC1LG2, or CD273), B7-H2 (ICOSL: inducible T-cell co-stimulator ligand, or CD275), B7-H3 (CD276), B7-H4 (VTCN1), B7-H5 (VISTA: V-domain Ig suppressor of T cell activation, Dies1: differentiation of embryonic stem cells 1, or C10orf54), butyrophilin like 2 (BTNL2), B7-H6 (NCR3LG1: natural killer cell cytotoxicity receptor 3 ligand 1), B7-H7 (HHLA2: human endogenous retro virus–H long repeat-associating 2), and immunoglobulin like domain containing receptor 2 (ILDR2) [2,3,4,5,6]. Indeed, BTNL2 and ILDR2 are introduced as B7-like molecules, and further investigation is needed. B7 family genes have been linked with various pathological conditions, e.g., cancers, infections, autoimmune diseases, and transplantation complications [6]. Thus, a better understanding of their biology might pave the way for introducing novel strategies to treat the abovementioned diseases and complications.
MicroRNAs (miRs), as small, non-coding RNAs, can bind to their complementary sequences, which are often the mRNA 3′-untranslated regions (3′UTR) of their targets. Since miRs can cleave their target mRNAs by guiding the RNA-induced silencing complex (RISC) to target mRNAs, in order to direct the cleavage of mRNA through Argonaute (AGO) endonuclease activity [7], destabilize their target mRNAs via cutting their poly(A) tail, and make the translation of their target mRNA less effective, they are considered potent post-transcriptional gene regulators [8,9,10]. In higher eukaryotes, miRs can regulate the expression of approximately 60% of genes. It is well-established that miRs can contribute to many biological processes, e.g., cell growth, differentiation, metabolism, and immune response regulation [11,12]. Indeed, miRs can modulate the function of immune cells and regulate the expression of immune checkpoints [13,14,15]. Thus, alteration in miR expression is involved in the pathogenesis of various human diseases, like cancers [11,13,16]. Moreover, miRs can regulate the expression of B7 family members in various diseases; thus, there is a need to properly understand the scope and effect of this regulation in human diseases [17,18].
This scoping review focuses on novel B7 family members, i.e., B7-H3, B7-H4, B7-H5, BTNL2, B7-H6, B7-H7, and ILDR2. This study aimed to map the current regulatory cross-talk between the above-mentioned members of the B7 family and miRs. Moreover, we used bioinformatics approaches to identify other potential miRs targeting these genes. Since miRs are promising targets for therapeutic applications [19,20], the results of this review might provide valuable insights for a better understanding the regulatory cross-talk between novel B7 immune checkpoint members and miRs in the development of various human diseases, as well as provide new molecular targets for use in therapeutic and clinical applications.
2. Methods
The methodology for the current scoping review was according to the framework recommended by Arksey and O’Malley [21] and later enhanced by Levac et al. [22]. It consists of five distinctive phases: (1) identifying the research question; (2) identifying relevant studies; (3) study selection; (4) charting the data; and (5) collating, summarizing, and reporting results. An optional sixth phase, consultation, was not a part of our scoping review. This study was also carefully guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) Checklist [23].
2.1. Identifying the Research Question
This study aimed to map the current literature on new B7 family members and miRs’ regulatory axis. To address this aim, we sought to answer the upcoming question: precisely what is known from existing literature about the regulation of new B7 family molecules in human diseases by miRs?
2.2. Identifying Relevant Studies
PubMed, Embase, Scopus, Cochrane, ProQuest, and Google Scholar were searched based on the specific search tips of each database without any restriction, in order to recognize all potentially eligible publications. MeSH and Emtree terms were used if available. The search strategy for PubMed and Embase databases are described in Supplementary Materials. The last search was conducted on 5 November 2020. We also included articles identified from hand-searching or reference lists of the selected studies.
2.3. Selecting Studies
The included studies fulfilled the following criteria: (1) explicitly discussing the regulatory axes between the new B7 family members and miRs, (2) evaluating human specimens or cell lines, and (3) written in English. The exclusion criteria were (1) studies on animals and (2) studies that were not primary/original research. First, publication titles and abstracts were independently screened by two investigators (N.K.A. and H.S.) for eligibility, according to the above-mentioned criteria. In the second phase, the full-text assessment of the selected studies was conducted, and studies that met the eligibility criteria were included in the final data analysis. Any disagreements were solved by involving a third reviewer (B.B.), if required.
2.4. Charting the Data
Two authors (N.K.A. and H.S.) independently extracted data into a predefined charting form in Microsoft Excel. It provided information about regulatory axes, samples, methods, diseases, and key findings of each study.
2.5. Collating, Summarizing, and Reporting the Results
Both quantitative and qualitative analyses were carried out. We presented a descriptive numerical summary of the chosen studies’ features for the quantitative portion. We prepared a narrative review, addressing our abovementioned research question for the qualitative assessment, considering the importance of findings in the broader context proposed by Levac et al. [22].
2.6. Bioinformatics Analysis
The miRDB database (accessed on 15 February 2021) [24] was used to predict other possible new B7 family members and miRs’ interaction. Only functional human miRs with a target score >80 were included. According to miRDB, a predicted target with a score >80 is most likely to be real. Also, miRs with >2000 predicted targets in the genome were excluded. The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was performed by miRPathDB v2.0 to obtain insights on the pathways that are targeted by predicted miRs [25]. Specifically, the focus was on the “Immune system” in the KEGG database. Selection of data based on strong experimental evidence and with a minimum of two significant miRs per pathway was used as criteria for the miRPathDB query.
3. Results
The flowchart of literature identification, inclusion, and exclusion is demonstrated in Figure 1.
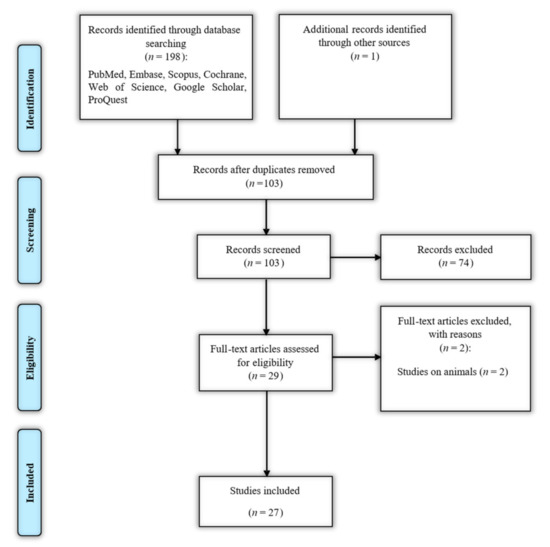
Figure 1.
Search strategy flow chart based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
A total of 199 articles were identified through database searching and hand-searching, of which 96 were duplicates. Seventy-four articles were excluded during the title and abstract review, based on irrelevance to the inclusion criteria. The full-text assessment of the remaining 29 records was conducted, and two more records were excluded because they were not human studies [26,27]. Finally, a total of 27 eligible papers remained [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. The characteristics of the included studies are summarized in Table 1.

Table 1.
The characteristics of the included studies.
The included articles were published between 2009 and 2020. Twenty studies were performed in China [29,30,31,33,34,38,39,40,42,43,44,45,46,47,49,50,51,52,53,54], five in the United States [28,32,37,41,48], one in Canada [36], and one in Norway [35]. Clinical and experimental data from different human specimens, i.e., tissue, blood, serum, bone marrow, nasopharyngeal secretion, skin, and human cell lines, were evaluated. Different approaches like bioinformatics analysis were utilized to predict interactions between miRs and the desired genes. Furthermore, various molecular techniques and cell-based assays, e.g., real-time PCR, Western blotting, protein lysate microarray, miR array, gene silencing techniques, sequencing, cell viability assays, cell proliferation assays, cytotoxicity assays, and cell death assays were used to assess these interactions. Luciferase activity assay and transfection technique were also used to validate the regulatory mechanisms.
3.1. B7-H3
Twenty-one papers studied B7-H3 regulatory axes [28,29,30,31,32,33,34,35,37,39,41,42,43,44,45,48,50,51,52,53,54]. Thirty-three miRs involved in the regulatory axes and diseases pathogenesis, including miR-29 (in Burkitt lymphoma, adenocarcinoma, brain tumor, hepatoblastoma, neuroblastoma, sarcomas, Wilms’ tumor, cutaneous melanoma, breast cancer, central nervous system (CNS) neuroblastoma, allergic asthma, diffuse brain glioma, colorectal cancer, medulloblastoma, Mycoplasma pneumoniae pneumonia), miR-539 (in glioma), miR-124 (in osteosarcoma), miR-187 (in psoriasis, colorectal cancer, and clear-cell renal cell carcinoma), miR-380–5p, miR-125b-2, miR-363, miR-940, miR-214, miR-34b, miR-665, miR-593, miR-555, miR-885–3p, miR-567, miR-297, miR-187–3p, miR-124a-1, miR-326, miR-601, miR-506 and miR-708 (in breast cancer), miR-155, miR-143, miR-192, miR-378, miR-1301–3p, miR-335–5p and miR-28–5p (in colorectal cancer), miR-506 (in mantle cell lymphoma), miR-214 (in multiple myeloma), miR-1253 (in medulloblastoma), miR-145 (in lung cancer and colorectal cancer), and miR-199a (in cervical cancer).
3.2. B7-H4
Three studies investigated the regulatory axes between B7-H4 and miRs in human diseases [38,46,53]. The studied miRs and diseases were as follows: miR-155, miR-143, miR-1207 (in colorectal cancer), hsa-miR-299–5p, hsa-miR-2115–3p, hsa-miR-1284, hsa-miR-1265, hsa-miR-3178, hsa-miR-204–3p, hsa-miR-3646, hsa-miR-3686, hsa-miR-4279, hsa-miR-302e, hsa-miR-138–1-3p, hsa-miR-31–5p, hsa-miR-4290, hsa-miR-4306, hsa-miR-483–3p, hsa-miR-744–5p, hsa-miR-183–3p, hsa-miR-24–1-5p, hsa-miR-4324, hsa-miR-519e-3p, hsa-miR-3202, hsa-miRPlus-G1246–3p, hsa-miR-361–5p, hsa-miR-2116–5p, hsa-miR-335–3p, hsa-miR-4258, hsa-miR-4284, hsa-miR-3685, hsa-miR-302a-3p, hsa-miR-361–3p, hsa-miR-33a-5p, hsa-miR-642a-5p, hsa-miR-33b-5p, hsa-miR-642b-5p, hsa-miR-3651, hsa-miR-7–5p, hsa-miR-1260a, hsa-miR-21–5p, hsa-miR-149–3p, hsa-miR-130b-3p, hsa-miR-525–5p, hsa-miR-4288, hsa-miR-513a-5p, hsa-let-7f-5p, hsa-miR-937, hsa-miR-31–3p, hsa-miR-1290, hsa-miR-196a-5p, hsa-miR-1246, hsa-miR-374c-5p, hsa-miR-615–3p, hsa-miR-27b-3p, hsa-miR-1973, hsa-miR-17–3p, hsa-miR-186–5p, hsa-miR-3676–3p, hsa-let-7c, hsa-miR-335–5p, hsa-miR-122–3p, hsa-miR-148a-3p, hsa-miR-520d-5p, and hsa-miR-21–3p (in pancreatic cancer).
3.3. B7-H5
Two studies investigated the miR-16/B7-H5 and miR-125a-5p/B7-H5 regulatory axes in active Crohn’s disease and gastric cancer, respectively [36,47].
3.4. B7-H6
One study reported that the miR-93/B7-H6, miR-195/B7-H6, and miR-340/B7-H6 regulatory axes might contribute to regulating immune evasion in breast cancer [49].
3.5. B7-H7
One study indicated that the regulatory axes of hsa-miR-3116/B7-H7 and hsa-miR-6870–5p/B7-H7 contribute to the regulatory mechanisms of human tumors [40].
3.6. ILDR2
There were no studies investigating the interaction of miR with this gene.
3.7. BTNL2
There were no papers investigating the interaction of miR with this gene.
3.8. Bioinformatics Analysis
Predictions of possible gene–miR interactions and KEGG pathway enrichment analysis revealed that four miRs are annotated for KEGG pathways in the category immune system (Figure 2): hsa-miR-29b-3p was annotated for “Chemokine signaling pathway”, “T cell receptor signaling pathway”, and “Leukocyte transendothelial migration”; hsa-miR-29a-3p was annotated for “T cell receptor signaling pathway” and “Complement and coagulation cascades”; hsa-miR-125a-5p was annotated for “T cell receptor signaling pathway” and “Chemokine signaling pathway”; and hsa-miR-486-5p was annotated for “Leukocyte transendothelial migration”.
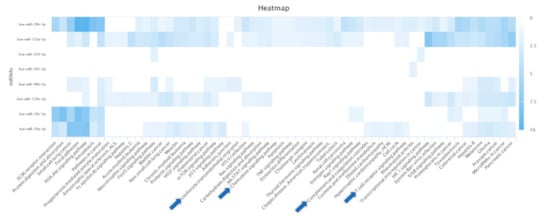
Figure 2.
Enriched KEGG pathways of predicted miRs obtained from the miRDB database. Enriched pathways with at least two associated miRs were shown. The color of individual fields represents the −log10-transformed p-value of the respective enrichment results. Darker colors indicate more significant associations between an miR and the target pathway. Enriched KEGG pathways in the category of immune system are indicated by blue arrows.
To further understand and visualize the interactions, a gene–miR pathway network was constructed (Figure 3).
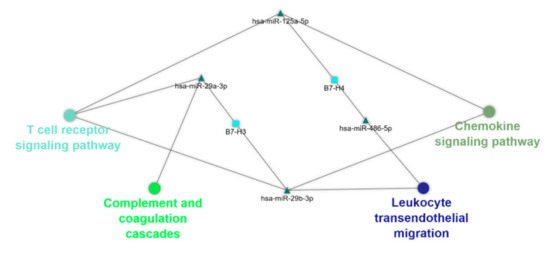
Figure 3.
The predicted gene-miR-pathway network. Cytoscape v3.8.0 software was used for evaluating gene–miRNA interaction, and their corresponding immune-related pathways via their visualization as a network. Ellipse, triangle, and round rectangle represent pathway, miR, and gene, respectively.
Based the interactions, we identified potential new interactions between the B7 family members and miRs: (1) hsa-miR-29b-3p/B7-H3, (2) hsa-miR-29a-3p/B7-H3, (3) hsa-miR-125a-5p/B7-H4, and (4) hsa-miR-486-5p/B7-H4. The association of different isoforms of miR-29 with B7-H3 was supported by the reviewed literature.
4. Discussion
4.1. B7-H3
B7–H3, also referred to as CD276, can regulate the stimulation and inhibition of T cells [55,56]. A variety of cells, e.g., natural killer cells, activated T-cells, dendritic cells, macrophages, and non-hematopoietic cells, can express B7-H3 [57]. Preliminary findings reported that B7-H3 could promote CD4+ and CD8+ T cell proliferation by T cell receptor (TCR) stimulation using immobilized Ig fusion protein [55]. However, it is well-established that B7–H3 can suppress the activation of CD4+ T-cell and the release of effector cytokines [58,59]. This suppression might facilitate the function of transcription factors like nuclear factor of activated T cells (NF-AT), nuclear factor kappa B (NF-κB), and activator protein 1 (AP-1), playing significant roles in T cell function [58]. Moreover, B7-H3 overexpression has been identified in various cancers, e.g., breast [60], lung [61], kidney, prostate [62], and ovarian cancer [63]. Furthermore, the inhibition of B7-H3 has decreased angiogenesis in medulloblastoma, indicating its essential role in tumor angiogenesis [37]. As an overexpressed oncogene in various cancers, MYC has a critical role in cancer development, e.g., angiogenesis, apoptosis, proliferation [64,65]. Since MYC inhibition has been associated with the suppressed expression of B7-H3 in medulloblastoma cells, the MYC-B7-H3 regulatory axis can play an essential role in regulating angiogenesis [37]. It has been indicated that B7-H3 knockdown can repress the PI3K/Akt pathway, resulting in decreased STAT3 activity. Since STAT3 can promote the expression of matrix metalloproteinase 2 (MMP2) and matrix metalloproteinase 9 (MMP9), B7-H3 can regulate the expression of MMP2 and MMP9 [66]. Moreover, B7-H3 can be involved in inflammatory conditions, e.g., sepsis and bacterial meningitis [51]. Since the mRNA expression of B7–H3 is not as remarkable as its protein expression, the post-transcriptional regulating process might have a considerable effect [57].
The expression of B7-H3 is controlled by various factors, e.g., cytokines, oncogenes, long non-coding RNAs (lncRNAs), and miRs [30,34,37,53]. Although several miRs can directly target the mRNA of B7-H3, several miRs can indirectly regulate B7-H3 expression by targeting an intermediate mRNA [13]. It has been indicated that isoforms of mir-29 can inversely regulate B7-H3 expression in various cancers, e.g., neuroblastoma [28], cutaneous melanoma [41], breast cancer [35], diffuse brain glioma [29], colorectal cancer [44], and medulloblastoma [37]. Growing evidence highlights the crucial role of dysregulated miR-29c in tumor development and the prognosis of affected patients [67,68,69]. The low expression of miR-29c has been associated with TP53 mutation in breast cancer patients. Indeed, the tumors with low miR-29c expression lack TP53 tumor suppressor activity [35]. Of interest, miR-29 can repress STAT3, integrin, vascular endothelial growth factor (VEGF), NF-κB, and the PI3K/AKT signaling pathway, and upregulate the expression of phosphatase and tensin homolog (PTEN) and p53 [37]. A study has found that 13 miRs, i.e., miR-363, miR-326, miR-214, miR-29c, miR-665, miR-940, miR-708, miR-601, miR-34b, miR-380–5p, miR-124a, miR-593, and miR-885–3p, can directly target B7-H3. Indeed, miR-29c overexpression has been indicative of the improved prognosis of breast cancer patients [35]. This correlation between miR-29c and B7-H3 has also been identified in other diseases. For instance, there is a negative association between miR-29c expression and B7-H3 expression in children with asthma deterioration. Indeed, miR-29c can target B7-H3 in macrophages and alter the differentiation of T helper cells [51]. One study has demonstrated a negative correlation between miR-29c and B7-H3 in children with Mycoplasma pneumoniae pneumonia [33]. Moreover, Astragaloside IV, which can suppress cell proliferation in colorectal cancer, can downregulate B7-H3 via miR-29c and enhance the chemosensitivity of tumor cells [44]. In line with this, miR-29c can enhance the sensitivity to cisplatin in nasopharyngeal carcinoma cells [70]. In addition, miR-29c can improve the chemosensitivity of non-small-cell lung cancer cells to cisplatin by regulating the PI3K/Akt pathway [71].
Furthermore, the downregulation of miR-335–5p, miR-1301–3p, and miR-28–5p can upregulate B7-H3 expression in advanced colorectal cancer [43]. Besides, there is a negative association between B7-H3 and miR-187 in clear-cell renal cell carcinoma and colorectal cancer [52]. Also, miR-187, which is downregulated in cytokine-stimulated keratinocytes and psoriasis, can suppress keratinocyte hyperproliferation via negatively regulating B7-H3 expression [39]. In mantle cell lymphoma, miR-506 downregulation and B7-H3 overexpression have been associated with increased cell proliferation and tumor migration [54]. The altered expression of miR-506 in cancers has resulted from the methylation of the promoter and modifications in upstream transcription factors [72]. In glioma, miR-539 upregulation, which can suppress B7-H3 expression, can repress cancer development by targeting caspase recruitment domain-containing, membrane-associated, guanylate kinase protein-1 (CARMA1), sperm-associated antigen 5 (SPAG5), and the gene encoding matrix metalloproteinase 8 (MMP8) [34]. In addition, the upregulation of miR-214 can result in the proliferation and migration of tumor cells in ovarian and gastric cancers [73]. In multiple myeloma, lncRNA NEAT1, by targeting miR-214, upregulates B7-H3, promoting M2 macrophage polarization and tumor development. Indeed, miR-214 and B7-H3 can serve as valuable prognostic factors and be used as promising targets for treating multiple myeloma patients [30].
Transforming growth factor-beta 1 (TGF-β1) can upregulate the B7-H3 expression and pave the way for immune evasion of colorectal cancer cells via the miR-155/miR-143 axis. Indeed, miR-143 can inhibit B7-H3 expression in colorectal cancer cells [53]. The downregulation of miR-124 can increase the expression of B7-H3 in osteosarcoma. It has been reported that miR-124 might decrease proliferating cell nuclear antigen (PCNA) and cyclin D1, and elevate apoptotic protein poly-ADP ribose polymerase (PARP). Also, miR-124 mimics might increase the therapeutic efficacy of monoclonal antibodies targeting B7-H3 [42]. Furthermore, miR-1253 can inhibit cell proliferation and promotes the apoptosis of tumor cells in medulloblastoma. Indeed, miR-1253 restoration and B7-H3 silencing can substantially decrease the migration of medulloblastoma cells [32]. Zhou et al. found that the low expression of miR-192, miR-378, and miR-145 can result in B7-H3 overexpression and immune evasion in colorectal cancer [53]. Consistent with this, the downregulation of miR-145 has been associated with increased B7-H3 expression, facilitating lymph node metastasis in lung cancer patients [31]. In cervical cancer, miR-199a, which targets B7-H3, can inhibit cell proliferation, invasion, and migration [50].
4.2. B7-H4
B7-H4, also known as B7x, B7S1, and VTCN1, can inhibit cytokine production, proliferation, cell cycle progression, and the stimulation of CD4+ and CD8+ T cells [74,75]. Although its transcripts can be found in various tissues, its protein has low expression in most human normal tissues [76]. B7-H4 expression is positively correlated with cancer development in patients with gastric cancer [77], glioma [78], squamous cell esophageal carcinoma [79], renal cell carcinoma [80], pancreatic cancer [81], cholangiocarcinoma [18], ovarian cancer [82], and lung cancer [83]. Since B7-H4 has been associated with cancer development, it can be an appealing target for treating cancer patients [76].
It has been shown that miR-125b-5p has an anti-inflammatory role and can regulate interleukin (IL)-1β-induced inflammatory genes by targeting the TNF receptor associated factor (TRAF6)/mitogen-activated protein kinase (MAPK)/NF-κB pathway in human osteoarthritic chondrocytes [84]. However, miR-125b-5p overexpression in macrophages can increase IL-2 secretion and the proliferation of CD8+ T cells. Indeed, miR-125b-5p can target B7-H4 and facilitate inflammation [85]. In line with this, B7-H4 overexpression has been associated with poor prognosis in colorectal cancer patients [86]. In 24.4% of colorectal cancer patients, single-nucleotide polymorphism (SNP) rs13505 GG of B7-H4 can confer an alternate binding site for miR-1207–5p, which might result in downregulation of this gene [46]. Furthermore, TGF-β1 can upregulate B7-H4 and facilitate immune escape via the miR-155/miR-143 axis in colorectal cancer [53].
In 2017, 62 hsa-miRs were identified as regulating B7-H4 in pancreatic cancer [38]. These miRs were mentioned above in the Results section.
4.3. B7-H5
B7-H5, also known as VISTA, C10orf54, Dies1, and PD-1H, is a type-I membrane protein that can stimulate terminal differentiation of embryonic stem cells (ESCs) into cardiomyocytes/neurons via the bone morphogenetic protein (BMP) signaling pathway [87]. It has been reported that miR-125a-5p can directly repress the transcription of B7-H5 and inhibit ESC differentiation [26]. B7-H5 also plays a pivotal regulatory function in adipocyte differentiation independently from BMP signaling. In particular, the elevated level of B7-H5 has been shown exclusively in differentiated fat cells and blocked adipocyte differentiation [88]. In B7-H5 knockout mice, the elevation of inflammatory cytokines can result in chronic multi-organ inflammation, indicating the critical role of B7-H5 in suppressing inflammation [89]. In Crohn’s disease, there is a negative association between B7-H5 expression and hsa-miR-16–1 [47]. B7-H5 can serve as a ligand and receptor on T cells, suppressing the activation of naïve and memory T cells [90,91]. The presence of two PKC binding sites in the cytoplasmic region of B7-H5 might indicate that B7-H5 is a receptor [92,93]. B7-H5 can be overexpressed in cancer-associated/cancer-adjacent gastric myofibroblasts. However, B7-H5 expression is generally downregulated in epithelial gastric cancer cells. This can be explained by B7-H5 promoter methylation, the overexpression of miR-125a-5p, or a combination of both, and even the existence of mutant p53 [36]. Indeed, the downregulation of B7-H5 has been associated with de-differentiation and triggered epithelial–mesenchymal transition (EMT) in epithelial cells.
4.4. B7-H6
B7-H6, also known as NCR3LG1, is a ligand for the NKp30 [94]. B7-H6 sequence is functionally similar to the other B7 family members. Although B7-H6 is not found in normal human tissues, it is highly expressed in cancers, e.g., renal cell carcinoma, leukemia, breast cancer, ovarian cancer, and sarcomas [95]. Various factors can regulate B7-H6 expressions, e.g., protease inhibitors, proinflammatory cytokines, natural killer cells, and miRs. Histone deacetylase inhibitors (HDACi) and metalloprotease inhibitors can regulate the B7-H6 expression at transcription and post-transcriptional levels, respectively [96]. Following stimulation of CD14+CD16+ neutrophils and monocytes, B7-H6 can be expressed on these proinflammatory immune cells [97]. Tumoral B7-H6 can be recognized and eliminated via natural killer cells. However, metalloproteases can cleave B7-H6 and shield tumor cells from natural killer-mediated immune responses [98]. Bioinformatics analysis has predicted that miR-93, miR-195, and miR-340 can regulate immune responses by targeting B7-H6 in breast cancer cells [49].
4.5. B7-H7
B7-H7, which has previously been referred to as B7-H5, is known as the human endogenous retro virus–H long repeat-associating 2 (HHLA2) [99,100]. Its receptors can be found on various immune cells, e.g., monocytes, T cells, B cells, and dendritic cells. TMIGD2, which is referred to as CD28 homolog, is one of the identified B7-H7 receptors [101]. In antigen-presenting cells, B7-H7 co-stimulates the proliferation of naïve T cell and cytokine production across TMIGD2 by serine–threonine kinase AKT phosphorylation. However, the second B7-H7 receptor on activated T cells can exert a coinhibitory role, because activated T cells do not express TMIGD2. The identification of the second receptor might clarify the role of B7-H7 in T cell activation and the tumor microenvironment [102]. It has been reported that B7-H7 is upregulated in lung cancer, osteosarcoma, and breast cancer, and its elevated expression is correlated with a poor prognosis in affected patients [103]. BATF in B lymphocytes and SMAD in monocytes might be involved in the dysregulation of B7-H7 in kidney clear-cell carcinoma. It has been indicated that hsa-miR-6870–5p and hsa-miR-3116 might have a role in this modulatory mechanism [40].
4.6. Bioinformatics Analysis
Based on our results, four potential new interactions between B7 family members and miRs have been identified: (1) the hsa-miR-29b-3p/B7-H3 axis, (2) the hsa-miR-29a-3p/B7-H3 axis, (3) the hsa-miR-125a-5p/B7-H4 axis, and (4) the hsa-miR-486-5p/B7-H4 axis. Of these four interactions, the association of different isoforms of miR-29 with B7-H3 has been investigated in previous studies (see above). As mentioned earlier, hsa-miR-125a-5p regulates B7-H5 expression in gastric cancer, but its association with B7-H4 has not been studied. Recent findings have shown that miR-125a-5p plays a pivotal role in suppressing the classical activation of macrophages (M1-type) induced by lipopolysaccharide (LPS) stimulation, while promoting IL-4-induced expression of the alternative M2 macrophages by targeting KLF13, a transcriptional factor that is involved in T lymphocyte activation and inflammation [104]. In addition, miR-486-5p is an immunomodulatory tumor suppressor miR that has been reported to have key roles in various oncological and non-oncological disorders [105]. Although our knowledge about the role of miR-125a-5p/B7-H4 and miR-486-5p/B7-H4 axes in the immune pathways and the pathogenesis of various diseases is still preliminary, our in silico analysis can pave the way for further investigations.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/22/5/2652/s1.
Author Contributions
Conceptualization and study design, N.K.A., B.B., and N.S.; literature search and data extraction: N.K.A. and H.S.; Data analysis and interpretation of results: N.K.A. with input from N.H., M.K.-K., M.A.S., A.M., N.A., A.D., A.B., and K.D.; visualization, N.K.A. and H.S.; writing—original draft preparation, N.K.A., N.H., and M.K.-K.; writing—review and editing, N.K.A. and M.A.S. with input from all authors; supervision, B.B. and N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in this review.
Acknowledgments
The authors would like to thank the Immunology Research Centre, Tabriz University of Medical Sciences for their support.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Huang, C.; Zhu, H.-X.; Yao, Y.; Bian, Z.-H.; Zheng, Y.-J.; Li, L.; Moutsopoulos, H.M.; Gershwin, M.E.; Lian, Z.-X. Immune checkpoint molecules. Possible future therapeutic implications in autoimmune diseases. J. Autoimmun. 2019, 104, 102333. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Dong, C. New B7 Family Checkpoints in Human Cancers. Mol. Cancer Ther. 2017, 16, 1203–1211. [Google Scholar] [CrossRef]
- Collins, M.; Ling, V.; Carreno, B.M. The B7 family of immune-regulatory ligands. Genome Biol. 2005, 6, 223. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Q.; Jin, L. The Role of B7 Family Molecules in Maternal–Fetal Immunity. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Chapoval, A.I.; Chapoval, S.P.; Shcherbakova, N.S.; Shcherbakov, D.N. Immune Checkpoints of the B7 Family. Part 2. Representatives of the B7 Family B7-H3, B7-H4, B7-H5, B7-H6, B7-H7, and ILDR2 and Their Receptors. Russ. J. Bioorgan. Chem. 2019, 45, 321–334. [Google Scholar] [CrossRef]
- Janakiram, M.; Shah, U.A.; Liu, W.; Zhao, A.; Schoenberg, M.P.; Zang, X. The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3. Immunol. Rev. 2017, 276, 26–39. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Shirafkan, N.; Shomali, N.; Kazemi, T.; Shanehbandi, D.; Ghasabi, M.; Baghbani, E.; Ganji, M.; Khaze, V.; Mansoori, B.; Baradaran, B. microRNA-193a-5p inhibits migration of human HT-29 colon cancer cells via suppression of metastasis pathway. J. Cell. Biochem. 2018. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Christopher, A.F.; Kaur, R.P.; Kaur, G.; Kaur, A.; Gupta, V.; Bansal, P. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect. Clin. Res. 2016, 7, 68–74. [Google Scholar] [CrossRef]
- Sadeghiyeh, N.; Sehati, N.; Mansoori, B.; Mohammadi, A.; Shanehbandi, D.; Khaze, V.; Baradaran, B. MicroRNA-145 replacement effect on growth and migration inhibition in lung cancer cell line. Biomed. Pharmacother. 2019, 111, 460–467. [Google Scholar] [CrossRef]
- Yang, Y.; Alderman, C.; Sehlaoui, A.; Xiao, Y.; Wang, W. MicroRNAs as Immunotherapy Targets for Treating Gastroenterological Cancers. Can. J. Gastroenterol. Hepatol. 2018, 2018, 9740357. [Google Scholar] [CrossRef]
- Argentiero, A.; De Summa, S.; Di Fonte, R.; Iacobazzi, R.M.; Porcelli, L.; Da Vià, M.; Brunetti, O.; Azzariti, A.; Silvestris, N.; Solimando, A.G. Gene Expression Comparison between the Lymph Node-Positive and -Negative Reveals a Peculiar Immune Microenvironment Signature and a Theranostic Role for WNT Targeting in Pancreatic Ductal Adenocarcinoma: A Pilot Study. Cancers 2019, 11, 942. [Google Scholar] [CrossRef]
- Porcelli, L.; Iacobazzi, R.M.; Di Fonte, R.; Serratì, S.; Intini, A.; Solimando, A.G.; Brunetti, O.; Calabrese, A.; Leonetti, F.; Azzariti, A.; et al. CAFs and TGF-β Signaling Activation by Mast Cells Contribute to Resistance to Gemcitabine/Nabpaclitaxel in Pancreatic Cancer. Cancers 2019, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Brunetti, O.; Gnoni, A.; Cascinu, S.; Gasparini, G.; Lorusso, V.; Ribatti, D.; Silvestris, N. Angiogenesis in pancreatic ductal adenocarcinoma: A controversial issue. Oncotarget 2016, 7, 58649–58658. [Google Scholar] [CrossRef] [PubMed]
- Holla, S.; Stephen-Victor, E.; Prakhar, P.; Sharma, M.; Saha, C.; Udupa, V.; Kaveri, S.V.; Bayry, J.; Balaji, K.N. Mycobacteria-responsive sonic hedgehog signaling mediates programmed death-ligand 1- and prostaglandin E2-induced regulatory T cell expansion. Sci. Rep. 2016, 6, 24193. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Zhao, W.; Sun, Z.; Yan, H.; Zhu, J. Oxidized low-density lipoprotein upregulates microRNA-146a via JNK and NF-κB signaling. Mol. Med. Rep. 2016, 13, 1709–1716. [Google Scholar] [CrossRef]
- Kwok, G.T.; Zhao, J.T.; Weiss, J.; Mugridge, N.; Brahmbhatt, H.; MacDiarmid, J.A.; Robinson, B.G.; Sidhu, S.B. Translational applications of microRNAs in cancer, and therapeutic implications. Non-Coding RNA Res. 2017, 2, 143–150. [Google Scholar] [CrossRef]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The Potential for microRNA Therapeutics and Clinical Research. Front. Genet. 2019, 10. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2019, 48, D127–D131. [Google Scholar] [CrossRef]
- Kehl, T.; Kern, F.; Backes, C.; Fehlmann, T.; Stöckel, D.; Meese, E.; Lenhof, H.-P.; Keller, A. miRPathDB 2.0: A novel release of the miRNA Pathway Dictionary Database. Nucleic Acids Res. 2019, 48, D142–D147. [Google Scholar] [CrossRef]
- Battista, M.; Musto, A.; Navarra, A.; Minopoli, G.; Russo, T.; Parisi, S. miR-125b regulates the early steps of ESC differentiation through dies1 in a TGF-independent manner. Int. J. Mol. Sci. 2013, 14, 13482–13496. [Google Scholar] [CrossRef]
- Parisi, S.; Battista, M.; Musto, A.; Navarra, A.; Tarantino, C.; Russo, T. A regulatory loop involving Dies1 and miR-125a controls BMP4 signaling in mouse embryonic stem cells. FASEB J. 2012, 26, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Cheung, I.Y.; Farazi, T.A.; Ostrovnaya, I.; Xu, H.; Tran, H.; Mihailovic, A.; Tuschl, T.; Cheung, N.K. Deep MicroRNA sequencing reveals downregulation of miR-29a in neuroblastoma central nervous system metastasis. Genes Chromosomes Cancer 2014, 53, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Zhang, C.; Liu, X.; Li, G.; Liu, S.; Sun, L.; Liang, J.; Hu, H.; Liu, Y.; et al. Genetic and clinical characterization of B7-H3 (CD276) expression and epigenetic regulation in diffuse brain glioma. Cancer Sci. 2018, 109, 2697–2705. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, P.; Li, W.J.; Zhang, J.; Wang, G.P.; Jiang, D.F.; Chen, F.P. LncRNA NEAT1 sponges miR-214 to regulate M2 macrophage polarization by regulation of B7-H3 in multiple myeloma. Mol. Immunol. 2020, 117, 20–28. [Google Scholar] [CrossRef]
- Huang, L. The expression and clinical significance of B7-H3 an miR-145 in lung cancer patients with malignant pleural effusion. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6759–6766. [Google Scholar]
- Kanchan, R.K.; Perumal, N.; Atri, P.; Chirravuri Venkata, R.; Thapa, I.; Klinkebiel, D.L.; Donson, A.M.; Perry, D.; Punsoni, M.; Talmon, G.A.; et al. MiR-1253 exerts tumor-suppressive effects in medulloblastoma via inhibition of CDK6 and CD276 (B7-H3). Brain Pathol. 2020, 30, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Wu, Y.Y.; Sun, H.M.; Gu, W.J.; Zhang, X.X.; Wang, M.J.; Yan, Y.D.; Hao, C.L.; Ji, W.; Chen, Z.R. The role of miR-29c/B7-H3/Th17 axis in children with Mycoplasma pneumoniae pneumonia. Ital. J. Pediatr. 2019, 45, 61. [Google Scholar] [CrossRef]
- Li, R.G.; Gao, Z.; Jiang, Y. B7-H3 repression by miR-539 suppresses cell proliferation in human gliomas. Int. J. Clin. Exp. Pathol. 2017, 10, 4363–4369. [Google Scholar]
- Nygren, M.K.; Tekle, C.; Ingebrigtsen, V.A.; Makela, R.; Krohn, M.; Aure, M.R.; Nunes-Xavier, C.E.; Perala, M.; Tramm, T.; Alsner, J.; et al. Identifying microRNAs regulating B7-H3 in breast cancer: The clinical impact of microRNA-29c. Br. J. Cancer 2014, 110, 2072–2080. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.; Carvalho, J.; Rocha, S.; Azevedo, M.; Reis, I.; Camilo, V.; Sousa, B.; Valente, S.; Paredes, J.; Almeida, R.; et al. Dies1/VISTA expression loss is a recurrent event in gastric cancer due to epigenetic regulation. Sci. Rep. 2016, 6, 34860. [Google Scholar] [CrossRef]
- Purvis, I.J.; Avilala, J.; Guda, M.R.; Venkataraman, S.; Vibhakar, R.; Tsung, A.J.; Velpula, K.K.; Asuthkar, S. Role of MYC-miR-29-B7-H3 in Medulloblastoma Growth and Angiogenesis. J. Clin. Med. 2019, 8, 1158. [Google Scholar] [CrossRef]
- Qian, Y.; Feng, L.; Wu, W.; Weng, T.; Hu, C.; Hong, B.; Wang, F.X.C.; Shen, L.; Wang, Q.; Jin, X.; et al. MicroRNA Expression Profiling of Pancreatic Cancer Cell Line L3.6p1 Following B7-H4 Knockdown. Cell. Physiol. Biochem. 2017, 44, 494–504. [Google Scholar] [CrossRef]
- Tang, L.; He, S.; Zhu, Y.; Feng, B.; Su, Z.; Liu, B.; Xu, F.; Wang, X.; Liu, H.; Li, C.; et al. Downregulated miR-187 contributes to the keratinocytes hyperproliferation in psoriasis. J. Cell. Physiol. 2019, 234, 3661–3674. [Google Scholar] [CrossRef]
- Wang, B.; Ran, Z.; Liu, M.; Ou, Y. Prognostic Significance of Potential Immune Checkpoint Member HHLA2 in Human Tumors: A Comprehensive Analysis. Front. Immunol. 2019, 10, 1573. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chong, K.K.; Nakamura, Y.; Nguyen, L.; Huang, S.K.; Kuo, C.; Zhang, W.; Yu, H.; Morton, D.L.; Hoon, D.S.B. B7-H3 associated with tumor progression and epigenetic regulatory activity in cutaneous melanoma. J. Investig. Dermatol. 2013, 133, 2050–2058. [Google Scholar] [CrossRef]
- Wang, L.; Kang, F.B.; Sun, N.; Wang, J.; Chen, W.; Li, D.; Shan, B.E. The tumor suppressor miR-124 inhibits cell proliferation and invasion by targeting B7-H3 in osteosarcoma. Tumour Biol. 2016, 37, 14939–14947. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Xu, M.; Zhou, F.; Yan, J. Serum miR-1301-3p, miR-335-5p, miR-28-5p, and their target B7-H3 may serve as novel biomarkers for colorectal cancer. J. Balk. Union Oncol. 2019, 24, 1120–1127. [Google Scholar]
- Wang, S.; Mou, J.; Cui, L.; Wang, X.; Zhang, Z. Astragaloside IV inhibits cell proliferation of colorectal cancer cell lines through down-regulation of B7-H3. Biomed. Pharmacother. 2018, 102, 1037–1044. [Google Scholar] [CrossRef]
- Wang, Z.S.; Zhong, M.; Bian, Y.H.; Mu, Y.F.; Qin, S.L.; Yu, M.H.; Qin, J. MicroRNA-187 inhibits tumor growth and invasion by directly targeting CD276 in colorectal cancer. Oncotarget 2016, 7, 44266–44276. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Tang, R.; Qi, Q.; Zhou, X.; Zhou, H.; Mao, Y.; Li, R.; Liu, C.; Wang, W.; Hua, D.; et al. Five functional polymorphisms of B7/CD28 co-signaling molecules alter susceptibility to colorectal cancer. Cell. Immunol. 2015, 293, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Ma, X.P.; Shi, Y.; Bao, C.H.; Jin, X.M.; Lu, Y.; Zhao, J.M.; Zhou, C.L.; Chen, D.; Liu, H.R. Alterations in microRNA expression profiles in inflamed and noninflamed ascending colon mucosae of patients with active Crohn’s disease. J. Gastroenterol. Hepatol. 2017, 32, 1706–1715. [Google Scholar] [CrossRef][Green Version]
- Xu, H.; Cheung, I.Y.; Guo, H.F.; Cheung, N.K.V. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: Potential implications for immune based therapy of human solid tumors. Cancer Res. 2009, 69, 6275–6281. [Google Scholar] [CrossRef]
- Yang, L.; Cai, Y.; Zhang, D.; Sun, J.; Xu, C.; Zhao, W.; Jiang, W.; Pan, C. miR-195/miR-497 Regulate CD274 Expression of Immune Regulatory Ligands in Triple-Negative Breast Cancer. J. Breast Cancer 2018, 21, 371–381. [Google Scholar] [CrossRef]
- Yang, X.; Feng, K.X.; Li, H.; Wang, L.; Xia, H. MicroRNA-199a Inhibits Cell Proliferation, Migration, and Invasion and Activates AKT/mTOR Signaling Pathway by Targeting B7-H3 in Cervical Cancer. Technol. Cancer Res. Treat. 2020, 19, 1533033820942245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.; Sun, H.; Yan, Y.; Huang, L.; Gu, W.; Jiang, W.; Wang, Y.; Zhu, C.; Ji, W.; et al. The role of miR-29c/B7-H3 axis in children with allergic asthma. J. Transl. Med. 2018, 16, 218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lei, T.; Xu, C.; Li, H.; Ma, W.; Yang, Y.; Fan, S.; Liu, Y. MicroRNA-187, down-regulated in clear cell renal cell carcinoma and associated with lower survival, inhibits cell growth and migration though targeting B7-H3. Biochem. Biophys. Res. Commun. 2013, 438, 439–444. [Google Scholar] [CrossRef]
- Zhou, X.; Mao, Y.; Zhu, J.; Meng, F.; Chen, Q.; Tao, L.; Li, R.; Fu, F.; Liu, C.; Hu, Y.; et al. TGF-β1 promotes colorectal cancer immune escape by elevating B7-H3 and B7-H4 via the miR-155/miR-143 axis. Oncotarget 2016, 7, 67196–67211. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.W.; Wang, J.; Zhu, M.X.; Wang, Y.F.; Yang, S.Y.; Ke, X.Y. MicroRNA-506 inhibits the proliferation and invasion of mantle cell lymphoma cells by targeting B7H3. Biochem. Biophys. Res. Commun. 2019, 508, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Flies, D.B.; Liu, D.; Dong, H.; Sica, G.L.; Zhu, G.; Tamada, K.; et al. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Leitner, J.; Klauser, C.; Pickl, W.F.; Stöckl, J.; Majdic, O.; Bardet, A.F.; Kreil, D.P.; Dong, C.; Yamazaki, T.; Zlabinger, G.; et al. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur. J. Immunol. 2009, 39, 1754–1764. [Google Scholar] [CrossRef]
- Veenstra, R.G.; Flynn, R.; Kreymborg, K.; McDonald-Hyman, C.; Saha, A.; Taylor, P.A.; Osborn, M.J.; Panoskaltsis-Mortari, A.; Schmitt-Graeff, A.; Lieberknecht, E.; et al. B7-H3 expression in donor T cells and host cells negatively regulates acute graft-versus-host disease lethality. Blood 2015, 125, 3335–3346. [Google Scholar] [CrossRef]
- Prasad, D.V.; Nguyen, T.; Li, Z.; Yang, Y.; Duong, J.; Wang, Y.; Dong, C. Murine B7-H3 is a negative regulator of T cells. J. Immunol. 2004, 173, 2500–2506. [Google Scholar] [CrossRef]
- Suh, W.K.; Gajewska, B.U.; Okada, H.; Gronski, M.A.; Bertram, E.M.; Dawicki, W.; Duncan, G.S.; Bukczynski, J.; Plyte, S.; Elia, A.; et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat. Immunol. 2003, 4, 899–906. [Google Scholar] [CrossRef]
- Cong, F.; Yu, H.; Gao, X. Expression of CD24 and B7-H3 in breast cancer and the clinical significance. Oncol. Lett. 2017, 14, 7185–7190. [Google Scholar] [CrossRef][Green Version]
- Sun, Y.; Wang, Y.; Zhao, J.; Gu, M.; Giscombe, R.; Lefvert, A.K.; Wang, X. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer 2006, 53, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Thompson, R.H.; Al-Ahmadie, H.A.; Serio, A.M.; Reuter, V.E.; Eastham, J.A.; Scardino, P.T.; Sharma, P.; Allison, J.P. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc. Natl. Acad. Sci. USA 2007, 104, 19458–19463. [Google Scholar] [CrossRef] [PubMed]
- Fauci, J.M.; Straughn, J.M., Jr.; Ferrone, S.; Buchsbaum, D.J. A review of B7-H3 and B7-H4 immune molecules and their role in ovarian cancer. Gynecol. Oncol. 2012, 127, 420–425. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.; Qing, G. Targeting oncogenic Myc as a strategy for cancer treatment. Signal Transduct. Target. Ther. 2018, 3, 5. [Google Scholar] [CrossRef]
- Shchors, K.; Shchors, E.; Rostker, F.; Lawlor, E.R.; Brown-Swigart, L.; Evan, G.I. The Myc-dependent angiogenic switch in tumors is mediated by interleukin 1beta. Genes Dev. 2006, 20, 2527–2538. [Google Scholar] [CrossRef]
- Li, Y.; Guo, G.; Song, J.; Cai, Z.; Yang, J.; Chen, Z.; Wang, Y.; Huang, Y.; Gao, Q. B7-H3 Promotes the Migration and Invasion of Human Bladder Cancer Cells via the PI3K/Akt/STAT3 Signaling Pathway. J. Cancer 2017, 8, 816–824. [Google Scholar] [CrossRef]
- Presneau, N.; Eskandarpour, M.; Shemais, T.; Henderson, S.; Halai, D.; Tirabosco, R.; Flanagan, A.M. MicroRNA profiling of peripheral nerve sheath tumours identifies miR-29c as a tumour suppressor gene involved in tumour progression. Br. J. Cancer 2013, 108, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Fang, J.H.; Yun, J.P.; Yang, J.; Zhang, Y.; Jia, W.H.; Zhuang, S.M. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 2010, 51, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-J.; Lin, J.; Lwin, T.; Yang, H.; Guo, J.; Kong, W.; Dessureault, S.; Moscinski, L.C.; Rezania, D.; Dalton, W.S.; et al. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood 2010, 115, 2630–2639. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Qian, D.; Wang, F.-W.; Liao, D.-Z.; Wei, J.-H.; Tong, Z.-T.; Fu, J.; Huang, X.-X.; Liao, Y.-J.; Deng, H.-X.; et al. MicroRNA-29c enhances the sensitivities of human nasopharyngeal carcinoma to cisplatin-based chemotherapy and radiotherapy. Cancer Lett. 2013, 329, 91–98. [Google Scholar] [CrossRef]
- Sun, D.-M.; Tang, B.-F.; Li, Z.-X.; Guo, H.-B.; Cheng, J.-L.; Song, P.-P.; Zhao, X. MiR-29c reduces the cisplatin resistance of non-small cell lung cancer cells by negatively regulating the PI3K/Akt pathway. Sci. Rep. 2018, 8, 8007. [Google Scholar] [CrossRef]
- Li, J.; Ju, J.; Ni, B.; Wang, H. The emerging role of miR-506 in cancer. Oncotarget 2016, 7, 62778–62788. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Hamilton, R.; Mandal, C.C. miR-214: A potential biomarker and therapeutic for different cancers. Future Oncol. 2015, 11, 349–363. [Google Scholar] [CrossRef]
- Prasad, D.V.; Richards, S.; Mai, X.M.; Dong, C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity 2003, 18, 863–873. [Google Scholar] [CrossRef]
- Zang, X.; Loke, P.; Kim, J.; Murphy, K.; Waitz, R.; Allison, J.P. B7x: A widely expressed B7 family member that inhibits T cell activation. Proc. Natl. Acad. Sci. USA 2003, 100, 10388–10392. [Google Scholar] [CrossRef]
- MacGregor, H.L.; Ohashi, P.S. Molecular Pathways: Evaluating the Potential for B7-H4 as an Immunoregulatory Target. Clin. Cancer Res. 2017, 23, 2934–2941. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Z. B7-H4 is Predictive of Poor Prognosis in Patients with Gastric Cancer. Med. Sci. Monit. 2016, 22, 4233–4237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yao, Y.; Ye, H.; Qi, Z.; Mo, L.; Yue, Q.; Baral, A.; Hoon, D.S.B.; Vera, J.C.; Heiss, J.D.; Chen, C.C.; et al. B7-H4(B7x)-Mediated Cross-talk between Glioma-Initiating Cells and Macrophages via the IL6/JAK/STAT3 Pathway Lead to Poor Prognosis in Glioma Patients. Clin. Cancer Res. 2016, 22, 2778–2790. [Google Scholar] [CrossRef]
- Wang, L.; Cao, N.N.; Wang, S.; Man, H.W.; Li, P.F.; Shan, B.E. Roles of coinhibitory molecules B7-H3 and B7-H4 in esophageal squamous cell carcinoma. Tumour Biol. 2016, 37, 2961–2971. [Google Scholar] [CrossRef] [PubMed]
- Krambeck, A.E.; Thompson, R.H.; Dong, H.; Lohse, C.M.; Park, E.S.; Kuntz, S.M.; Leibovich, B.C.; Blute, M.L.; Cheville, J.C.; Kwon, E.D. B7-H4 expression in renal cell carcinoma and tumor vasculature: Associations with cancer progression and survival. Proc. Natl. Acad. Sci. USA 2006, 103, 10391–10396. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tao, L.; Yuan, C.; Xiu, D. The prognostic value of B7-H4 in pancreatic cancer: Systematic review and meta-analysis. Medicine 2018, 97, e0088. [Google Scholar] [CrossRef]
- Liang, L.; Jiang, Y.; Chen, J.-S.; Niu, N.; Piao, J.; Ning, J.; Zu, Y.; Zhang, J.; Liu, J. B7-H4 expression in ovarian serous carcinoma: A study of 306 cases. Hum. Pathol. 2016, 57, 1–6. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, L.; Zhang, G.; Shen, Y.; Huang, J. B7-H4 promotes tumor growth and metastatic progression in lung cancer by impacting cell proliferation and survival. Oncotarget 2017, 8, 18861–18871. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rasheed, Z.; Rasheed, N.; Abdulmonem, W.A.; Khan, M.I. MicroRNA-125b-5p regulates IL-1β induced inflammatory genes via targeting TRAF6-mediated MAPKs and NF-κB signaling in human osteoarthritic chondrocytes. Sci. Rep. 2019, 9, 6882. [Google Scholar] [CrossRef]
- Diao, W.; Lu, L.; Li, S.; Chen, J.; Zen, K.; Li, L. MicroRNA-125b-5p modulates the inflammatory state of macrophages via targeting B7-H4. Biochem. Biophys. Res. Commun. 2017, 491, 912–918. [Google Scholar] [CrossRef]
- Liang, M.; Li, J.; Wang, D.; Li, S.; Sun, Y.; Sun, T.; Zhang, J.; Chen, X.; Li, Q.; Sun, S. T-cell infiltration and expressions of T lymphocyte co-inhibitory B7-H1 and B7-H4 molecules among colorectal cancer patients in northeast China’s Heilongjiang province. Tumor. Biol. 2014, 35, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Aloia, L.; Parisi, S.; Fusco, L.; Pastore, L.; Russo, T. Differentiation of embryonic stem cells 1 (Dies1) is a component of bone morphogenetic protein 4 (BMP4) signaling pathway required for proper differentiation of mouse embryonic stem cells. J. Biol. Chem. 2010, 285, 7776–7783. [Google Scholar] [CrossRef]
- Ren, G.; Beech, C.; Smas, C.M. The immunoglobulin superfamily protein differentiation of embryonic stem cells 1 (dies1) has a regulatory role in preadipocyte to adipocyte conversion. PLoS ONE 2013, 8, e65531. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Le Mercier, I.; Putra, J.; Chen, W.; Liu, J.; Schenk, A.D.; Nowak, E.C.; Suriawinata, A.A.; Li, J.; Noelle, R.J. Disruption of the immune-checkpoint VISTA gene imparts a proinflammatory phenotype with predisposition to the development of autoimmunity. Proc. Natl. Acad. Sci. USA 2014, 111, 14846–14851. [Google Scholar] [CrossRef]
- Lines, J.L.; Pantazi, E.; Mak, J.; Sempere, L.F.; Wang, L.; O’Connell, S.; Ceeraz, S.; Suriawinata, A.A.; Yan, S.; Ernstoff, M.S.; et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014, 74, 1924–1932. [Google Scholar] [CrossRef]
- Wang, L.; Rubinstein, R.; Lines, J.L.; Wasiuk, A.; Ahonen, C.; Guo, Y.; Lu, L.F.; Gondek, D.; Wang, Y.; Fava, R.A.; et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011, 208, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Flies, D.B.; Han, X.; Higuchi, T.; Zheng, L.; Sun, J.; Ye, J.J.; Chen, L. Coinhibitory receptor PD-1H preferentially suppresses CD4+ T cell-mediated immunity. J. Clin. Investig. 2014, 124, 1966–1975. [Google Scholar] [CrossRef]
- Ohno, T.; Zhang, C.; Kondo, Y.; Kang, S.; Furusawa, E.; Tsuchiya, K.; Miyazaki, Y.; Azuma, M. The immune checkpoint molecule VISTA regulates allergen-specific Th2-mediated immune responses. Int. Immunol. 2018, 30, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Flajnik, M.F.; Tlapakova, T.; Criscitiello, M.F.; Krylov, V.; Ohta, Y. Evolution of the B7 family: Co-evolution of B7H6 and NKp30, identification of a new B7 family member, B7H7, and of B7’s historical relationship with the MHC. Immunogenetics 2012, 64, 571–590. [Google Scholar] [CrossRef]
- Brandt, C.S.; Baratin, M.; Yi, E.C.; Kennedy, J.; Gao, Z.; Fox, B.; Haldeman, B.; Ostrander, C.D.; Kaifu, T.; Chabannon, C.; et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J. Exp. Med. 2009, 206, 1495–1503. [Google Scholar] [CrossRef]
- Chen, Y.; Mo, J.; Jia, X.; He, Y. The B7 Family Member B7-H6: A New Bane of Tumor. Pathol. Oncol. Res. 2018, 24, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.; Baratin, M.; Chiche, L.; Forel, J.M.; Cognet, C.; Thomas, G.; Farnarier, C.; Piperoglou, C.; Papazian, L.; Chaussabel, D.; et al. Induction of B7-H6, a ligand for the natural killer cell-activating receptor NKp30, in inflammatory conditions. Blood 2013, 122, 394–404. [Google Scholar] [CrossRef]
- Schlecker, E.; Fiegler, N.; Arnold, A.; Altevogt, P.; Rose-John, S.; Moldenhauer, G.; Sucker, A.; Paschen, A.; von Strandmann, E.P.; Textor, S.; et al. Metalloprotease-mediated tumor cell shedding of B7-H6, the ligand of the natural killer cell-activating receptor NKp30. Cancer Res. 2014, 74, 3429–3440. [Google Scholar] [CrossRef]
- Wang, C.; Feng, H.; Cheng, X.; Liu, K.; Cai, D.; Zhao, R. Potential Therapeutic Targets of B7 Family in Colorectal Cancer. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, S.; Iliopoulou, B.P.; Han, X.; Augustine, M.M.; Xu, H.; Phennicie, R.T.; Flies, S.J.; Broadwater, M.; Ruff, W.; et al. B7-H5 costimulates human T cells via CD28H. Nat. Commun. 2013, 4, 2043. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, M.; Chinai, J.M.; Zhao, A.; Sparano, J.A.; Zang, X. HHLA2 and TMIGD2: New immunotherapeutic targets of the B7 and CD28 families. Oncoimmunology 2015, 4, e1026534. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Freeman, G.J. A New B7:CD28 Family Checkpoint Target for Cancer Immunotherapy: HHLA2. Clin. Cancer Res. 2015, 21, 2201–2203. [Google Scholar] [CrossRef] [PubMed]
- Rieder, S.A.; Wang, J.; White, N.; Qadri, A.; Menard, C.; Stephens, G.; Karnell, J.L.; Rudd, C.E.; Kolbeck, R. B7-H7 (HHLA2) inhibits T-cell activation and proliferation in the presence of TCR and CD28 signaling. Cell. Mol. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Cui, H.; Xie, N.; Tan, Z.; Yang, S.; Icyuz, M.; Thannickal, V.J.; Abraham, E.; Liu, G. miR-125a-5p regulates differential activation of macrophages and inflammation. J. Biol. Chem. 2013, 288, 35428–35436. [Google Scholar] [CrossRef]
- ElKhouly, A.M.; Youness, R.A.; Gad, M.Z. MicroRNA-486-5p and microRNA-486-3p: Multifaceted pleiotropic mediators in oncological and non-oncological conditions. Non-Coding RNA Res. 2020, 5, 11–21. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).