The Regulatory Cross-Talk between microRNAs and Novel Members of the B7 Family in Human Diseases: A Scoping Review
Abstract
1. Introduction
2. Methods
2.1. Identifying the Research Question
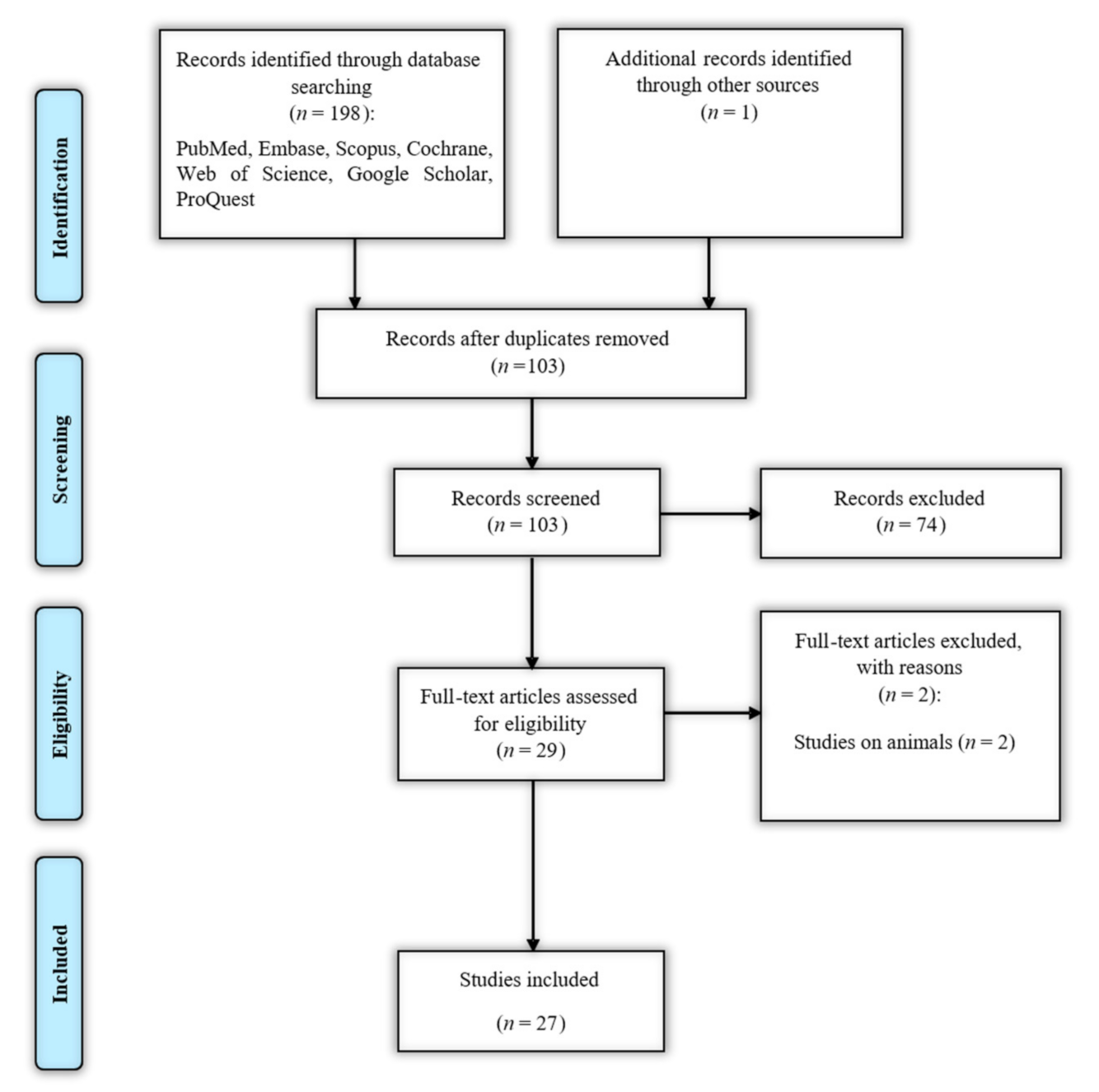
2.2. Identifying Relevant Studies
2.3. Selecting Studies
2.4. Charting the Data
2.5. Collating, Summarizing, and Reporting the Results
2.6. Bioinformatics Analysis
3. Results
3.1. B7-H3
3.2. B7-H4
3.3. B7-H5
3.4. B7-H6
3.5. B7-H7
3.6. ILDR2
3.7. BTNL2
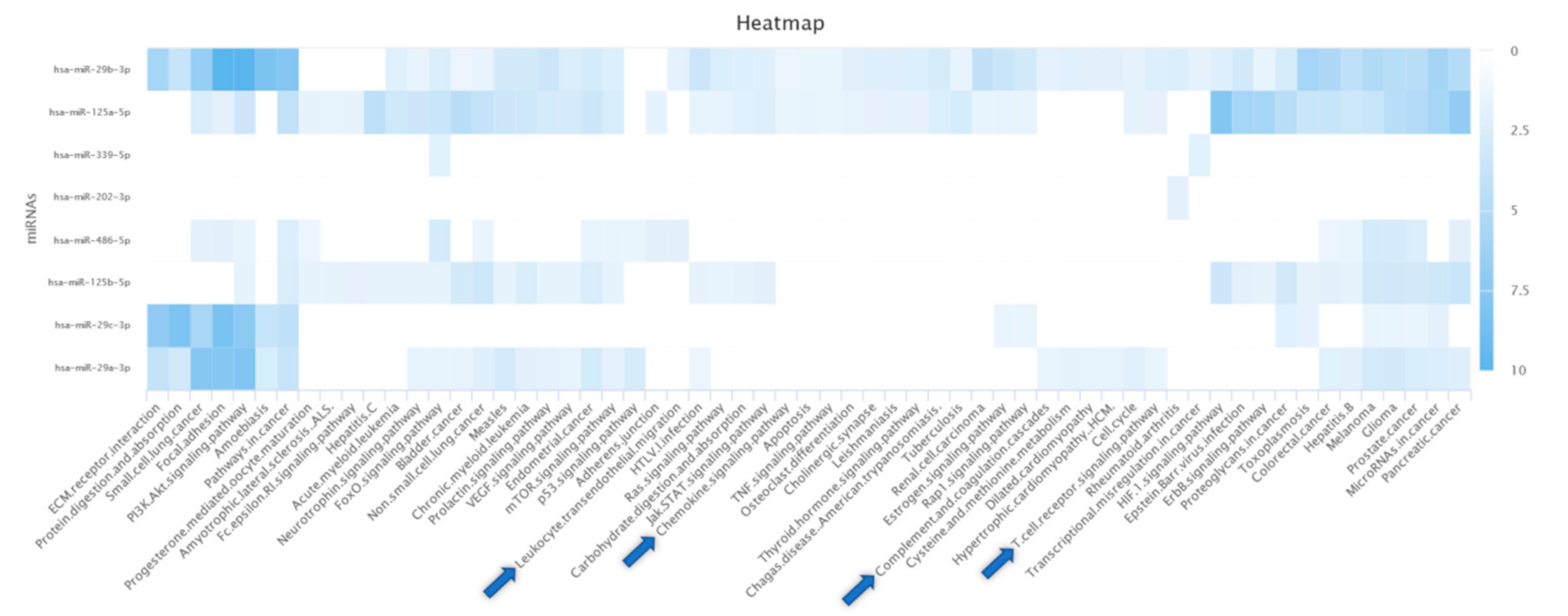
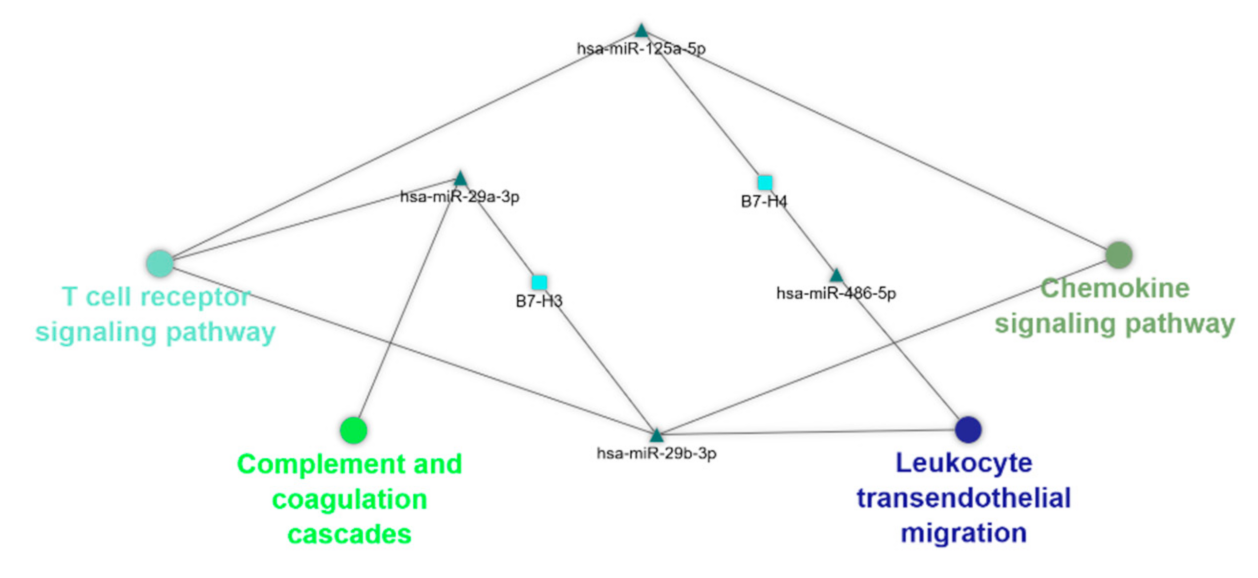
3.8. Bioinformatics Analysis
4. Discussion
4.1. B7-H3
4.2. B7-H4
4.3. B7-H5
4.4. B7-H6
4.5. B7-H7
4.6. Bioinformatics Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Zhu, H.-X.; Yao, Y.; Bian, Z.-H.; Zheng, Y.-J.; Li, L.; Moutsopoulos, H.M.; Gershwin, M.E.; Lian, Z.-X. Immune checkpoint molecules. Possible future therapeutic implications in autoimmune diseases. J. Autoimmun. 2019, 104, 102333. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Dong, C. New B7 Family Checkpoints in Human Cancers. Mol. Cancer Ther. 2017, 16, 1203–1211. [Google Scholar] [CrossRef]
- Collins, M.; Ling, V.; Carreno, B.M. The B7 family of immune-regulatory ligands. Genome Biol. 2005, 6, 223. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Zheng, Q.; Jin, L. The Role of B7 Family Molecules in Maternal–Fetal Immunity. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Chapoval, A.I.; Chapoval, S.P.; Shcherbakova, N.S.; Shcherbakov, D.N. Immune Checkpoints of the B7 Family. Part 2. Representatives of the B7 Family B7-H3, B7-H4, B7-H5, B7-H6, B7-H7, and ILDR2 and Their Receptors. Russ. J. Bioorgan. Chem. 2019, 45, 321–334. [Google Scholar] [CrossRef]
- Janakiram, M.; Shah, U.A.; Liu, W.; Zhao, A.; Schoenberg, M.P.; Zang, X. The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3. Immunol. Rev. 2017, 276, 26–39. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Shirafkan, N.; Shomali, N.; Kazemi, T.; Shanehbandi, D.; Ghasabi, M.; Baghbani, E.; Ganji, M.; Khaze, V.; Mansoori, B.; Baradaran, B. microRNA-193a-5p inhibits migration of human HT-29 colon cancer cells via suppression of metastasis pathway. J. Cell. Biochem. 2018. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Christopher, A.F.; Kaur, R.P.; Kaur, G.; Kaur, A.; Gupta, V.; Bansal, P. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect. Clin. Res. 2016, 7, 68–74. [Google Scholar] [CrossRef]
- Sadeghiyeh, N.; Sehati, N.; Mansoori, B.; Mohammadi, A.; Shanehbandi, D.; Khaze, V.; Baradaran, B. MicroRNA-145 replacement effect on growth and migration inhibition in lung cancer cell line. Biomed. Pharmacother. 2019, 111, 460–467. [Google Scholar] [CrossRef]
- Yang, Y.; Alderman, C.; Sehlaoui, A.; Xiao, Y.; Wang, W. MicroRNAs as Immunotherapy Targets for Treating Gastroenterological Cancers. Can. J. Gastroenterol. Hepatol. 2018, 2018, 9740357. [Google Scholar] [CrossRef]
- Argentiero, A.; De Summa, S.; Di Fonte, R.; Iacobazzi, R.M.; Porcelli, L.; Da Vià, M.; Brunetti, O.; Azzariti, A.; Silvestris, N.; Solimando, A.G. Gene Expression Comparison between the Lymph Node-Positive and -Negative Reveals a Peculiar Immune Microenvironment Signature and a Theranostic Role for WNT Targeting in Pancreatic Ductal Adenocarcinoma: A Pilot Study. Cancers 2019, 11, 942. [Google Scholar] [CrossRef]
- Porcelli, L.; Iacobazzi, R.M.; Di Fonte, R.; Serratì, S.; Intini, A.; Solimando, A.G.; Brunetti, O.; Calabrese, A.; Leonetti, F.; Azzariti, A.; et al. CAFs and TGF-β Signaling Activation by Mast Cells Contribute to Resistance to Gemcitabine/Nabpaclitaxel in Pancreatic Cancer. Cancers 2019, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Brunetti, O.; Gnoni, A.; Cascinu, S.; Gasparini, G.; Lorusso, V.; Ribatti, D.; Silvestris, N. Angiogenesis in pancreatic ductal adenocarcinoma: A controversial issue. Oncotarget 2016, 7, 58649–58658. [Google Scholar] [CrossRef] [PubMed]
- Holla, S.; Stephen-Victor, E.; Prakhar, P.; Sharma, M.; Saha, C.; Udupa, V.; Kaveri, S.V.; Bayry, J.; Balaji, K.N. Mycobacteria-responsive sonic hedgehog signaling mediates programmed death-ligand 1- and prostaglandin E2-induced regulatory T cell expansion. Sci. Rep. 2016, 6, 24193. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Zhao, W.; Sun, Z.; Yan, H.; Zhu, J. Oxidized low-density lipoprotein upregulates microRNA-146a via JNK and NF-κB signaling. Mol. Med. Rep. 2016, 13, 1709–1716. [Google Scholar] [CrossRef]
- Kwok, G.T.; Zhao, J.T.; Weiss, J.; Mugridge, N.; Brahmbhatt, H.; MacDiarmid, J.A.; Robinson, B.G.; Sidhu, S.B. Translational applications of microRNAs in cancer, and therapeutic implications. Non-Coding RNA Res. 2017, 2, 143–150. [Google Scholar] [CrossRef]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The Potential for microRNA Therapeutics and Clinical Research. Front. Genet. 2019, 10. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2019, 48, D127–D131. [Google Scholar] [CrossRef]
- Kehl, T.; Kern, F.; Backes, C.; Fehlmann, T.; Stöckel, D.; Meese, E.; Lenhof, H.-P.; Keller, A. miRPathDB 2.0: A novel release of the miRNA Pathway Dictionary Database. Nucleic Acids Res. 2019, 48, D142–D147. [Google Scholar] [CrossRef]
- Battista, M.; Musto, A.; Navarra, A.; Minopoli, G.; Russo, T.; Parisi, S. miR-125b regulates the early steps of ESC differentiation through dies1 in a TGF-independent manner. Int. J. Mol. Sci. 2013, 14, 13482–13496. [Google Scholar] [CrossRef]
- Parisi, S.; Battista, M.; Musto, A.; Navarra, A.; Tarantino, C.; Russo, T. A regulatory loop involving Dies1 and miR-125a controls BMP4 signaling in mouse embryonic stem cells. FASEB J. 2012, 26, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Cheung, I.Y.; Farazi, T.A.; Ostrovnaya, I.; Xu, H.; Tran, H.; Mihailovic, A.; Tuschl, T.; Cheung, N.K. Deep MicroRNA sequencing reveals downregulation of miR-29a in neuroblastoma central nervous system metastasis. Genes Chromosomes Cancer 2014, 53, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Zhang, C.; Liu, X.; Li, G.; Liu, S.; Sun, L.; Liang, J.; Hu, H.; Liu, Y.; et al. Genetic and clinical characterization of B7-H3 (CD276) expression and epigenetic regulation in diffuse brain glioma. Cancer Sci. 2018, 109, 2697–2705. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, P.; Li, W.J.; Zhang, J.; Wang, G.P.; Jiang, D.F.; Chen, F.P. LncRNA NEAT1 sponges miR-214 to regulate M2 macrophage polarization by regulation of B7-H3 in multiple myeloma. Mol. Immunol. 2020, 117, 20–28. [Google Scholar] [CrossRef]
- Huang, L. The expression and clinical significance of B7-H3 an miR-145 in lung cancer patients with malignant pleural effusion. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6759–6766. [Google Scholar]
- Kanchan, R.K.; Perumal, N.; Atri, P.; Chirravuri Venkata, R.; Thapa, I.; Klinkebiel, D.L.; Donson, A.M.; Perry, D.; Punsoni, M.; Talmon, G.A.; et al. MiR-1253 exerts tumor-suppressive effects in medulloblastoma via inhibition of CDK6 and CD276 (B7-H3). Brain Pathol. 2020, 30, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Wu, Y.Y.; Sun, H.M.; Gu, W.J.; Zhang, X.X.; Wang, M.J.; Yan, Y.D.; Hao, C.L.; Ji, W.; Chen, Z.R. The role of miR-29c/B7-H3/Th17 axis in children with Mycoplasma pneumoniae pneumonia. Ital. J. Pediatr. 2019, 45, 61. [Google Scholar] [CrossRef]
- Li, R.G.; Gao, Z.; Jiang, Y. B7-H3 repression by miR-539 suppresses cell proliferation in human gliomas. Int. J. Clin. Exp. Pathol. 2017, 10, 4363–4369. [Google Scholar]
- Nygren, M.K.; Tekle, C.; Ingebrigtsen, V.A.; Makela, R.; Krohn, M.; Aure, M.R.; Nunes-Xavier, C.E.; Perala, M.; Tramm, T.; Alsner, J.; et al. Identifying microRNAs regulating B7-H3 in breast cancer: The clinical impact of microRNA-29c. Br. J. Cancer 2014, 110, 2072–2080. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.; Carvalho, J.; Rocha, S.; Azevedo, M.; Reis, I.; Camilo, V.; Sousa, B.; Valente, S.; Paredes, J.; Almeida, R.; et al. Dies1/VISTA expression loss is a recurrent event in gastric cancer due to epigenetic regulation. Sci. Rep. 2016, 6, 34860. [Google Scholar] [CrossRef]
- Purvis, I.J.; Avilala, J.; Guda, M.R.; Venkataraman, S.; Vibhakar, R.; Tsung, A.J.; Velpula, K.K.; Asuthkar, S. Role of MYC-miR-29-B7-H3 in Medulloblastoma Growth and Angiogenesis. J. Clin. Med. 2019, 8, 1158. [Google Scholar] [CrossRef]
- Qian, Y.; Feng, L.; Wu, W.; Weng, T.; Hu, C.; Hong, B.; Wang, F.X.C.; Shen, L.; Wang, Q.; Jin, X.; et al. MicroRNA Expression Profiling of Pancreatic Cancer Cell Line L3.6p1 Following B7-H4 Knockdown. Cell. Physiol. Biochem. 2017, 44, 494–504. [Google Scholar] [CrossRef]
- Tang, L.; He, S.; Zhu, Y.; Feng, B.; Su, Z.; Liu, B.; Xu, F.; Wang, X.; Liu, H.; Li, C.; et al. Downregulated miR-187 contributes to the keratinocytes hyperproliferation in psoriasis. J. Cell. Physiol. 2019, 234, 3661–3674. [Google Scholar] [CrossRef]
- Wang, B.; Ran, Z.; Liu, M.; Ou, Y. Prognostic Significance of Potential Immune Checkpoint Member HHLA2 in Human Tumors: A Comprehensive Analysis. Front. Immunol. 2019, 10, 1573. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chong, K.K.; Nakamura, Y.; Nguyen, L.; Huang, S.K.; Kuo, C.; Zhang, W.; Yu, H.; Morton, D.L.; Hoon, D.S.B. B7-H3 associated with tumor progression and epigenetic regulatory activity in cutaneous melanoma. J. Investig. Dermatol. 2013, 133, 2050–2058. [Google Scholar] [CrossRef]
- Wang, L.; Kang, F.B.; Sun, N.; Wang, J.; Chen, W.; Li, D.; Shan, B.E. The tumor suppressor miR-124 inhibits cell proliferation and invasion by targeting B7-H3 in osteosarcoma. Tumour Biol. 2016, 37, 14939–14947. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Xu, M.; Zhou, F.; Yan, J. Serum miR-1301-3p, miR-335-5p, miR-28-5p, and their target B7-H3 may serve as novel biomarkers for colorectal cancer. J. Balk. Union Oncol. 2019, 24, 1120–1127. [Google Scholar]
- Wang, S.; Mou, J.; Cui, L.; Wang, X.; Zhang, Z. Astragaloside IV inhibits cell proliferation of colorectal cancer cell lines through down-regulation of B7-H3. Biomed. Pharmacother. 2018, 102, 1037–1044. [Google Scholar] [CrossRef]
- Wang, Z.S.; Zhong, M.; Bian, Y.H.; Mu, Y.F.; Qin, S.L.; Yu, M.H.; Qin, J. MicroRNA-187 inhibits tumor growth and invasion by directly targeting CD276 in colorectal cancer. Oncotarget 2016, 7, 44266–44276. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Tang, R.; Qi, Q.; Zhou, X.; Zhou, H.; Mao, Y.; Li, R.; Liu, C.; Wang, W.; Hua, D.; et al. Five functional polymorphisms of B7/CD28 co-signaling molecules alter susceptibility to colorectal cancer. Cell. Immunol. 2015, 293, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Ma, X.P.; Shi, Y.; Bao, C.H.; Jin, X.M.; Lu, Y.; Zhao, J.M.; Zhou, C.L.; Chen, D.; Liu, H.R. Alterations in microRNA expression profiles in inflamed and noninflamed ascending colon mucosae of patients with active Crohn’s disease. J. Gastroenterol. Hepatol. 2017, 32, 1706–1715. [Google Scholar] [CrossRef][Green Version]
- Xu, H.; Cheung, I.Y.; Guo, H.F.; Cheung, N.K.V. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: Potential implications for immune based therapy of human solid tumors. Cancer Res. 2009, 69, 6275–6281. [Google Scholar] [CrossRef]
- Yang, L.; Cai, Y.; Zhang, D.; Sun, J.; Xu, C.; Zhao, W.; Jiang, W.; Pan, C. miR-195/miR-497 Regulate CD274 Expression of Immune Regulatory Ligands in Triple-Negative Breast Cancer. J. Breast Cancer 2018, 21, 371–381. [Google Scholar] [CrossRef]
- Yang, X.; Feng, K.X.; Li, H.; Wang, L.; Xia, H. MicroRNA-199a Inhibits Cell Proliferation, Migration, and Invasion and Activates AKT/mTOR Signaling Pathway by Targeting B7-H3 in Cervical Cancer. Technol. Cancer Res. Treat. 2020, 19, 1533033820942245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.; Sun, H.; Yan, Y.; Huang, L.; Gu, W.; Jiang, W.; Wang, Y.; Zhu, C.; Ji, W.; et al. The role of miR-29c/B7-H3 axis in children with allergic asthma. J. Transl. Med. 2018, 16, 218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lei, T.; Xu, C.; Li, H.; Ma, W.; Yang, Y.; Fan, S.; Liu, Y. MicroRNA-187, down-regulated in clear cell renal cell carcinoma and associated with lower survival, inhibits cell growth and migration though targeting B7-H3. Biochem. Biophys. Res. Commun. 2013, 438, 439–444. [Google Scholar] [CrossRef]
- Zhou, X.; Mao, Y.; Zhu, J.; Meng, F.; Chen, Q.; Tao, L.; Li, R.; Fu, F.; Liu, C.; Hu, Y.; et al. TGF-β1 promotes colorectal cancer immune escape by elevating B7-H3 and B7-H4 via the miR-155/miR-143 axis. Oncotarget 2016, 7, 67196–67211. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.W.; Wang, J.; Zhu, M.X.; Wang, Y.F.; Yang, S.Y.; Ke, X.Y. MicroRNA-506 inhibits the proliferation and invasion of mantle cell lymphoma cells by targeting B7H3. Biochem. Biophys. Res. Commun. 2019, 508, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Flies, D.B.; Liu, D.; Dong, H.; Sica, G.L.; Zhu, G.; Tamada, K.; et al. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Leitner, J.; Klauser, C.; Pickl, W.F.; Stöckl, J.; Majdic, O.; Bardet, A.F.; Kreil, D.P.; Dong, C.; Yamazaki, T.; Zlabinger, G.; et al. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur. J. Immunol. 2009, 39, 1754–1764. [Google Scholar] [CrossRef]
- Veenstra, R.G.; Flynn, R.; Kreymborg, K.; McDonald-Hyman, C.; Saha, A.; Taylor, P.A.; Osborn, M.J.; Panoskaltsis-Mortari, A.; Schmitt-Graeff, A.; Lieberknecht, E.; et al. B7-H3 expression in donor T cells and host cells negatively regulates acute graft-versus-host disease lethality. Blood 2015, 125, 3335–3346. [Google Scholar] [CrossRef]
- Prasad, D.V.; Nguyen, T.; Li, Z.; Yang, Y.; Duong, J.; Wang, Y.; Dong, C. Murine B7-H3 is a negative regulator of T cells. J. Immunol. 2004, 173, 2500–2506. [Google Scholar] [CrossRef]
- Suh, W.K.; Gajewska, B.U.; Okada, H.; Gronski, M.A.; Bertram, E.M.; Dawicki, W.; Duncan, G.S.; Bukczynski, J.; Plyte, S.; Elia, A.; et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat. Immunol. 2003, 4, 899–906. [Google Scholar] [CrossRef]
- Cong, F.; Yu, H.; Gao, X. Expression of CD24 and B7-H3 in breast cancer and the clinical significance. Oncol. Lett. 2017, 14, 7185–7190. [Google Scholar] [CrossRef][Green Version]
- Sun, Y.; Wang, Y.; Zhao, J.; Gu, M.; Giscombe, R.; Lefvert, A.K.; Wang, X. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer 2006, 53, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Thompson, R.H.; Al-Ahmadie, H.A.; Serio, A.M.; Reuter, V.E.; Eastham, J.A.; Scardino, P.T.; Sharma, P.; Allison, J.P. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc. Natl. Acad. Sci. USA 2007, 104, 19458–19463. [Google Scholar] [CrossRef] [PubMed]
- Fauci, J.M.; Straughn, J.M., Jr.; Ferrone, S.; Buchsbaum, D.J. A review of B7-H3 and B7-H4 immune molecules and their role in ovarian cancer. Gynecol. Oncol. 2012, 127, 420–425. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.; Qing, G. Targeting oncogenic Myc as a strategy for cancer treatment. Signal Transduct. Target. Ther. 2018, 3, 5. [Google Scholar] [CrossRef]
- Shchors, K.; Shchors, E.; Rostker, F.; Lawlor, E.R.; Brown-Swigart, L.; Evan, G.I. The Myc-dependent angiogenic switch in tumors is mediated by interleukin 1beta. Genes Dev. 2006, 20, 2527–2538. [Google Scholar] [CrossRef]
- Li, Y.; Guo, G.; Song, J.; Cai, Z.; Yang, J.; Chen, Z.; Wang, Y.; Huang, Y.; Gao, Q. B7-H3 Promotes the Migration and Invasion of Human Bladder Cancer Cells via the PI3K/Akt/STAT3 Signaling Pathway. J. Cancer 2017, 8, 816–824. [Google Scholar] [CrossRef]
- Presneau, N.; Eskandarpour, M.; Shemais, T.; Henderson, S.; Halai, D.; Tirabosco, R.; Flanagan, A.M. MicroRNA profiling of peripheral nerve sheath tumours identifies miR-29c as a tumour suppressor gene involved in tumour progression. Br. J. Cancer 2013, 108, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Fang, J.H.; Yun, J.P.; Yang, J.; Zhang, Y.; Jia, W.H.; Zhuang, S.M. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 2010, 51, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-J.; Lin, J.; Lwin, T.; Yang, H.; Guo, J.; Kong, W.; Dessureault, S.; Moscinski, L.C.; Rezania, D.; Dalton, W.S.; et al. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood 2010, 115, 2630–2639. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Qian, D.; Wang, F.-W.; Liao, D.-Z.; Wei, J.-H.; Tong, Z.-T.; Fu, J.; Huang, X.-X.; Liao, Y.-J.; Deng, H.-X.; et al. MicroRNA-29c enhances the sensitivities of human nasopharyngeal carcinoma to cisplatin-based chemotherapy and radiotherapy. Cancer Lett. 2013, 329, 91–98. [Google Scholar] [CrossRef]
- Sun, D.-M.; Tang, B.-F.; Li, Z.-X.; Guo, H.-B.; Cheng, J.-L.; Song, P.-P.; Zhao, X. MiR-29c reduces the cisplatin resistance of non-small cell lung cancer cells by negatively regulating the PI3K/Akt pathway. Sci. Rep. 2018, 8, 8007. [Google Scholar] [CrossRef]
- Li, J.; Ju, J.; Ni, B.; Wang, H. The emerging role of miR-506 in cancer. Oncotarget 2016, 7, 62778–62788. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Hamilton, R.; Mandal, C.C. miR-214: A potential biomarker and therapeutic for different cancers. Future Oncol. 2015, 11, 349–363. [Google Scholar] [CrossRef]
- Prasad, D.V.; Richards, S.; Mai, X.M.; Dong, C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity 2003, 18, 863–873. [Google Scholar] [CrossRef]
- Zang, X.; Loke, P.; Kim, J.; Murphy, K.; Waitz, R.; Allison, J.P. B7x: A widely expressed B7 family member that inhibits T cell activation. Proc. Natl. Acad. Sci. USA 2003, 100, 10388–10392. [Google Scholar] [CrossRef]
- MacGregor, H.L.; Ohashi, P.S. Molecular Pathways: Evaluating the Potential for B7-H4 as an Immunoregulatory Target. Clin. Cancer Res. 2017, 23, 2934–2941. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Z. B7-H4 is Predictive of Poor Prognosis in Patients with Gastric Cancer. Med. Sci. Monit. 2016, 22, 4233–4237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yao, Y.; Ye, H.; Qi, Z.; Mo, L.; Yue, Q.; Baral, A.; Hoon, D.S.B.; Vera, J.C.; Heiss, J.D.; Chen, C.C.; et al. B7-H4(B7x)-Mediated Cross-talk between Glioma-Initiating Cells and Macrophages via the IL6/JAK/STAT3 Pathway Lead to Poor Prognosis in Glioma Patients. Clin. Cancer Res. 2016, 22, 2778–2790. [Google Scholar] [CrossRef]
- Wang, L.; Cao, N.N.; Wang, S.; Man, H.W.; Li, P.F.; Shan, B.E. Roles of coinhibitory molecules B7-H3 and B7-H4 in esophageal squamous cell carcinoma. Tumour Biol. 2016, 37, 2961–2971. [Google Scholar] [CrossRef] [PubMed]
- Krambeck, A.E.; Thompson, R.H.; Dong, H.; Lohse, C.M.; Park, E.S.; Kuntz, S.M.; Leibovich, B.C.; Blute, M.L.; Cheville, J.C.; Kwon, E.D. B7-H4 expression in renal cell carcinoma and tumor vasculature: Associations with cancer progression and survival. Proc. Natl. Acad. Sci. USA 2006, 103, 10391–10396. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tao, L.; Yuan, C.; Xiu, D. The prognostic value of B7-H4 in pancreatic cancer: Systematic review and meta-analysis. Medicine 2018, 97, e0088. [Google Scholar] [CrossRef]
- Liang, L.; Jiang, Y.; Chen, J.-S.; Niu, N.; Piao, J.; Ning, J.; Zu, Y.; Zhang, J.; Liu, J. B7-H4 expression in ovarian serous carcinoma: A study of 306 cases. Hum. Pathol. 2016, 57, 1–6. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, L.; Zhang, G.; Shen, Y.; Huang, J. B7-H4 promotes tumor growth and metastatic progression in lung cancer by impacting cell proliferation and survival. Oncotarget 2017, 8, 18861–18871. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rasheed, Z.; Rasheed, N.; Abdulmonem, W.A.; Khan, M.I. MicroRNA-125b-5p regulates IL-1β induced inflammatory genes via targeting TRAF6-mediated MAPKs and NF-κB signaling in human osteoarthritic chondrocytes. Sci. Rep. 2019, 9, 6882. [Google Scholar] [CrossRef]
- Diao, W.; Lu, L.; Li, S.; Chen, J.; Zen, K.; Li, L. MicroRNA-125b-5p modulates the inflammatory state of macrophages via targeting B7-H4. Biochem. Biophys. Res. Commun. 2017, 491, 912–918. [Google Scholar] [CrossRef]
- Liang, M.; Li, J.; Wang, D.; Li, S.; Sun, Y.; Sun, T.; Zhang, J.; Chen, X.; Li, Q.; Sun, S. T-cell infiltration and expressions of T lymphocyte co-inhibitory B7-H1 and B7-H4 molecules among colorectal cancer patients in northeast China’s Heilongjiang province. Tumor. Biol. 2014, 35, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Aloia, L.; Parisi, S.; Fusco, L.; Pastore, L.; Russo, T. Differentiation of embryonic stem cells 1 (Dies1) is a component of bone morphogenetic protein 4 (BMP4) signaling pathway required for proper differentiation of mouse embryonic stem cells. J. Biol. Chem. 2010, 285, 7776–7783. [Google Scholar] [CrossRef]
- Ren, G.; Beech, C.; Smas, C.M. The immunoglobulin superfamily protein differentiation of embryonic stem cells 1 (dies1) has a regulatory role in preadipocyte to adipocyte conversion. PLoS ONE 2013, 8, e65531. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.; Le Mercier, I.; Putra, J.; Chen, W.; Liu, J.; Schenk, A.D.; Nowak, E.C.; Suriawinata, A.A.; Li, J.; Noelle, R.J. Disruption of the immune-checkpoint VISTA gene imparts a proinflammatory phenotype with predisposition to the development of autoimmunity. Proc. Natl. Acad. Sci. USA 2014, 111, 14846–14851. [Google Scholar] [CrossRef]
- Lines, J.L.; Pantazi, E.; Mak, J.; Sempere, L.F.; Wang, L.; O’Connell, S.; Ceeraz, S.; Suriawinata, A.A.; Yan, S.; Ernstoff, M.S.; et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014, 74, 1924–1932. [Google Scholar] [CrossRef]
- Wang, L.; Rubinstein, R.; Lines, J.L.; Wasiuk, A.; Ahonen, C.; Guo, Y.; Lu, L.F.; Gondek, D.; Wang, Y.; Fava, R.A.; et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011, 208, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Flies, D.B.; Han, X.; Higuchi, T.; Zheng, L.; Sun, J.; Ye, J.J.; Chen, L. Coinhibitory receptor PD-1H preferentially suppresses CD4+ T cell-mediated immunity. J. Clin. Investig. 2014, 124, 1966–1975. [Google Scholar] [CrossRef]
- Ohno, T.; Zhang, C.; Kondo, Y.; Kang, S.; Furusawa, E.; Tsuchiya, K.; Miyazaki, Y.; Azuma, M. The immune checkpoint molecule VISTA regulates allergen-specific Th2-mediated immune responses. Int. Immunol. 2018, 30, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Flajnik, M.F.; Tlapakova, T.; Criscitiello, M.F.; Krylov, V.; Ohta, Y. Evolution of the B7 family: Co-evolution of B7H6 and NKp30, identification of a new B7 family member, B7H7, and of B7’s historical relationship with the MHC. Immunogenetics 2012, 64, 571–590. [Google Scholar] [CrossRef]
- Brandt, C.S.; Baratin, M.; Yi, E.C.; Kennedy, J.; Gao, Z.; Fox, B.; Haldeman, B.; Ostrander, C.D.; Kaifu, T.; Chabannon, C.; et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J. Exp. Med. 2009, 206, 1495–1503. [Google Scholar] [CrossRef]
- Chen, Y.; Mo, J.; Jia, X.; He, Y. The B7 Family Member B7-H6: A New Bane of Tumor. Pathol. Oncol. Res. 2018, 24, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.; Baratin, M.; Chiche, L.; Forel, J.M.; Cognet, C.; Thomas, G.; Farnarier, C.; Piperoglou, C.; Papazian, L.; Chaussabel, D.; et al. Induction of B7-H6, a ligand for the natural killer cell-activating receptor NKp30, in inflammatory conditions. Blood 2013, 122, 394–404. [Google Scholar] [CrossRef]
- Schlecker, E.; Fiegler, N.; Arnold, A.; Altevogt, P.; Rose-John, S.; Moldenhauer, G.; Sucker, A.; Paschen, A.; von Strandmann, E.P.; Textor, S.; et al. Metalloprotease-mediated tumor cell shedding of B7-H6, the ligand of the natural killer cell-activating receptor NKp30. Cancer Res. 2014, 74, 3429–3440. [Google Scholar] [CrossRef]
- Wang, C.; Feng, H.; Cheng, X.; Liu, K.; Cai, D.; Zhao, R. Potential Therapeutic Targets of B7 Family in Colorectal Cancer. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, S.; Iliopoulou, B.P.; Han, X.; Augustine, M.M.; Xu, H.; Phennicie, R.T.; Flies, S.J.; Broadwater, M.; Ruff, W.; et al. B7-H5 costimulates human T cells via CD28H. Nat. Commun. 2013, 4, 2043. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, M.; Chinai, J.M.; Zhao, A.; Sparano, J.A.; Zang, X. HHLA2 and TMIGD2: New immunotherapeutic targets of the B7 and CD28 families. Oncoimmunology 2015, 4, e1026534. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Freeman, G.J. A New B7:CD28 Family Checkpoint Target for Cancer Immunotherapy: HHLA2. Clin. Cancer Res. 2015, 21, 2201–2203. [Google Scholar] [CrossRef] [PubMed]
- Rieder, S.A.; Wang, J.; White, N.; Qadri, A.; Menard, C.; Stephens, G.; Karnell, J.L.; Rudd, C.E.; Kolbeck, R. B7-H7 (HHLA2) inhibits T-cell activation and proliferation in the presence of TCR and CD28 signaling. Cell. Mol. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Cui, H.; Xie, N.; Tan, Z.; Yang, S.; Icyuz, M.; Thannickal, V.J.; Abraham, E.; Liu, G. miR-125a-5p regulates differential activation of macrophages and inflammation. J. Biol. Chem. 2013, 288, 35428–35436. [Google Scholar] [CrossRef]
- ElKhouly, A.M.; Youness, R.A.; Gad, M.Z. MicroRNA-486-5p and microRNA-486-3p: Multifaceted pleiotropic mediators in oncological and non-oncological conditions. Non-Coding RNA Res. 2020, 5, 11–21. [Google Scholar] [CrossRef]
| Regulatory Axis | Samples | Methods | Disease | Key Findings | References |
|---|---|---|---|---|---|
| miR-29/B7-H3 | Human Burkitt lymphoma cell line Daudi, HeLa, LAN-1,NB1691, solid tumor samples (15 brain tumors, 5 hepatoblastomas, 41 neuroblastomas, 11 sarcomas, and 5 Wilms’ tumors) and 18 normal tissues | Monoclonal antibodies, whole-cell lysates, Western blot, subcellular fractionation, 8H9 antigen affinity purification, (q)RT-PCR, immunofluorescence, and luciferase reporter assay | Human cancers | Downregulation of miR-29 isoforms. Inverse regulation of B7-H3 by miR-29. Immune escape in solid tumors. | [48] |
| miR-187/B7-H3 | Blood and tissue | qRT-PCR, luciferase reporter assay, dimethylthiazol-diphenyltetrazolium bromide (MTT) assay, tumorigenicity assay, and scratch assay | Clear cell renal cell carcinoma | Downregulation of miR-187 in clear-cell renal cell carcinoma. Association with lower survival in patients. Inverse regulation of B7-H3 by miR-187. Overexpression of miR-187 inhibits cell growth and migration. | [52] |
| miR-29c/B7-H3 | Melanoma tissue and cell lines, i.e., M-1, M-101, M-111, M-12, M-14, JK-0346 Mel-B, JH-1173, and Wm266–4 | IHC analysis, immunofluorescence, RT-qPCR assay, Western blotting, development of B7-H3-knockdown cells, development of B7-H3-overexpressing cells, colony formation, invasion assay, and cell migration | Cutaneous melanoma | Tumor suppressor function of miR-29c. miR-29c directly targets B7-H3. Overexpression of B7-H3 increased cell migration and invasion. | [41] |
| miR-29c/B7-H3, miR-892a/B7-H3, miR-363/B7-H3, miR-940/B7-H3, miR-214/B7-H3, miR-34b/B7-H3, miR-665/B7-H3, miR-593/B7-H3, miR-885–3p/B7-H3, miR-124a/B7-H3, miR-326/B7-H3, miR-601/B7-H3, and miR-708/B7-H3 | JIMT-1 cell line, KPL-4 cell line, and breast cancer tumor | Microarray screening and data analysis, immunoblotting, luciferase assays, qRT-PCR | Breast cancer | Western immunoblotting validated the 20 most effective miRs ((hsa-miR-892a, hsa-miR-380–5p, hsa-miR-125b-2, hsa-miR-363, hsa-miR-940, hsa-miR-214, hsa-miR-34b, hsa-miR-665, hsa-miR-593, hsa-miR29c, hsa-miR-555, hsa-miR-885–3p, hsa-miR-567, hsa-miR-297, hsa-miR-187–3p, hsa-miR-124a-1, hsa-miR-326, hsa-miR-601, hsa-miR-506, and hsa-miR-708) that can downregulate B7-H3 expression in the JIMT-1 cells. Thirteen miRs directly targeted B7-H3. miR-29c levels were low, whereas B7-H3 had a relatively high expression. The high miR-29c expression is associated with increased survival. | [35] |
| miR-29a/B7-H3 | CL021, CL043, CL044, CL013, and tissue samples. | Sequencing, statistical analysis of differential miR expression, and qRT-PCR | Central nervous system (CNS) neuroblastoma | Low expression of miR-29a among pre-CNS primaries and CNS metastasis compared to non-CNS. B7-H3 expression was targeted by miR-29a. | [28] |
| miR-124/B7-H3 | Tumor samples and mg-63 cell line | Dual-luciferase reporter assay qRT-PCR and Western blotting analysis | Osteosarcoma (OS) | Downregulation of miR-124 in clinical OS specimens associated with advanced clinical stage and pulmonary metastasis. miR-124 directly targets B7-H3. Overexpression of B7-H3 abolished the reduction of cell growth and invasion. | [42] |
| miR-155/CEBPB/miR-143/B7-H3, miR-145/B7-H3, miR-192/B7-H3, miR-378/B7-H3 miR-155/CEBPB/miR-143/B7-H4 | tissue samples, including cancer and adenoma tissues; Caco-2, HCT-116, LoVo, Jurkat, SW480, SW620, and CHO cell lines | Gene silencing, microRNA array, construction of miR–miR functional synergistic network, KEGG pathway enrichment analysis, qRT-PCR, immunohistochemistry, cell lysates and cell fractionations, Western blot, immunofluorescence, and ELISA and MTT assay | Colorectal cancer (CRC) | The miR-155 node was the largest in CRC. Elevated the B7-H4 and B7-H3 expression in adenoma tissues. miR-155 abated miR-143 expression through the transcription factor CCAAT enhancer binding protein beta (CEBPB). MiR-143 inhibited the growth of CRC cells. The lowly-expressed miR-192, miR-378, and miR-145 contributed to the B7-H3 over-expression in colorectal cancer, consequently leading to cancer immune evasion. | [53] |
| miR-187/B7-H3 | A total of 32 pairs of colorectal tumor and matched nontumor tissues and 80 CRC tissues. Human CRC cell lines SW1116, SW480, SW620, HT29, LOVO, and the normal colonic epithelial cell line NCM460. | Real-time PCR, cell proliferation assay, transwell cell migration, invasion assay, apoptosis assay, miR target prediction, Western blotting, plasmid construction, and luciferase reporter assays | Colorectal cancer | Decreased miR-187 expression shorter overall survival and relapse-free survival of patients with CRC. B7-H3, is negatively correlated miR-187 level in CRC cells. | [45] |
| miR-539/B7-H3 | Cell lines: U87 and U251, normal human astrocytes | Cell viability assay, real-time PCR, colony formation, Western blotting, and luciferase assays | Human gliomas | Increased B7-H3 expression and downregulation of miR-539 glioma cell lines. B7-H3 repression by miR-539 suppresses cell proliferation in human gliomas. | [34] |
| miR-29c/B7-H3 | Peripheral blood samples, human monocyte cell line THP-1 | Microarray analysis of miRs, luciferase reporter assay, immunofluorescence staining, qRT-PCR, and plasma B7-H3 detected by ELISA | Allergic asthma | The lower level of miR-29c and a higher level of plasma B7-H3 in children with asthma exacerbation. The function of miR-29c on macrophage in regulating T cell differentiation. miR-29c is correlated to its target gene B7-H3. | [51] |
| miR-29/B7-H3 | From the CGGA dataset, we collected RNA-Seq data for 325 samples, ranging from WHO grade II to grade IV. In the TCGA dataset, RNA-Seq data were available for 669 samples. | Detection of isocitrate dehydrogenase mutation and defining immune pathways | Diffuse brain glioma | Regulation of B7-H3 by methylation and miR-29 family at different stages, respectively. Association of B7-H3 with higher malignancy and development and the progression of gliomas | [29] |
| miR-29c/B7-H3 | Human CRC cell lines SW620 and HCT116 and human colonic epithelial cell line FHC | Cell viability assay, cell cycle assay, RT-PCR, Western blot, cycloheximide chase assay, and dual-luciferase reporter assay | Colorectal cancer | Downregulation of miR-29c in many cancer types; miR-29c directly binds to B7-H3 mRNA and suppresses B7-H3 expression. Astragaloside IV treatment downregulated B7-H3 via the elevation of miR-29c. | [44] |
| miR-506/B7-H3 | Bone marrow mononuclear cells were isolated from 12 de novo mantle cell lymphoma (MCL) patients with bone marrow involvement. Human MCL cell lines Maver and Z138. | qRT-PCR, Western blotting, transfection and lentivirus infection, dual-luciferase assay, cell proliferation assays, cell cycle assays, cell migration, and transwell invasion assays | Mantle cell lymphoma | Overexpression of B7-H3 and downregulation of miR-506 in MCL patients. miR-506 inhibits the proliferation and invasion of MCL cells by targeting B7-H3. miR-506 induced MCL cell cycle arrest in the G0/G1 phase and repressed MCL cell migration. | [54] |
| miR-187/B7-H3 | HaCaT cell line and psoriatic skin samples. | qRT-PCR, cell viability assay, Western blot analysis, RNA transfection, luciferase reporter assays, B7-H3 overexpression (vector), histological analysis, immunohistochemistry, and cell cycle analysis | Psoriasis | Downregulation of miR-187 in the cytokine-stimulated keratinocytes compared with corresponding normal controls. Decreased miR-187 level in psoriatic skin compared with adjacent, uninvolved psoriatic skin. B7-H3 is a direct molecular target of miR-187. Upregulation of B7-H3 level in psoriatic skin. | [39] |
| MYC/miR-29/B7-H3 | Five human medulloblastoma (MB) tumor tissues; D283 Med, D425 Med, D458 med cell line, and human umbilical vein endothelial cells (HUVECs). | In silico analysis, ELISA, human angiogenesis array, immunoblotting, gelatin zymography, F-actin staining and immunostaining, RT-PCR and RNA-Seq, immuno-paired antibody detection analysis, chromatin immunoprecipitation and DNA sequencing, fluorescence-activated cell sorting analysis, in vitro angiogenesis assay, and chick chorioallantoic membrane assay | Medulloblastoma | Association of B7-H3 high expression with poor survival in MB patients. B7-H3 promotes angiogenesis in MB cells. miR-29 exhibits global anti-tumor functions and promotes STAT1 activation. Induces apoptosis via miR-29 in combination with MYC inhibition. | [37] |
| miR-1301–3p/B7-H3, miR-335–5p/B7-H3 miR-28–5p/B7-H3 | Serum samples from patients with colorectal cancer | ELISA, analysis of predicted putative miRs, and RT-PCR | Colorectal cancer | Upregulation of serum B7-H3 expressions in CRC patients. B7-H3 was predicted to be a target of miR-1301–3p, miR-335–5p, and miR-28–5p. Decrease serum miR-28–5p, miR-1301–3p, and miR-335–5p expressions in stage III and IV disease compared to stage I and II. These miRs are negatively related to the advanced TNM stages. | [43] |
| lncRNA NEAT1/miR-214/B7-H3 | Bone marrow samples from 30 multiple myeloma patients and multiple myeloma cells, i.e., line RPMI 8226, and human monocyte cell line THP-1. | Dual-luciferase reporter assay, RNA immunoprecipitation assay, ELISA assay, Western blotting, and RT-qPCR | Multiple myeloma (MM) | Increase the level of long non-coding RNA (lncRNA) NEAT1 and B7-H3. Downregulation of miR-214 expression occurred in multiple myeloma tissues. lncRNA NEAT1 directly targeted miR-214 to promote M2 macrophage polarization by upregulating B7-H3 in MM. | [30] |
| miR-29c/B7-H3/Th17 | Peripheral blood and nasopharyngeal secretions samples, and human monocytic cell line THP-1 | Quantitative ELISA-specific M. pneumoniae immunoglobulin G (IgG) and immunoglobulin M (IgM), real-time PCR for M. pneumoniae detection, multiple pathogen detection, examination of soluble B7-H3 and IL-17 in plasma, immunofluorescence staining, and luciferase assay | Mycoplasma pneumoniae pneumonia (MPP) | The lower level of miR-29c and a higher level of B7-H3 and IL-17 in children with MPP. B7-H3 is the direct target of miR-29c, and miR-29c silencing or overexpression could up- or downregulate the expression of B7-H3 in THP-1 cells. | [33] |
| miR-1253/B7-H3 | All tumor samples were obtained from patients in the pediatric age group, with DAOY, D283, D341, D425, D556, D458, and HDMB03 cell lines. | PCR, cell proliferation assay, colony formation assay, cell migration and invasion, wound healing, annexin V-FITC/PI analysis, Western blotting, dual-luciferase reporter assay, target prediction in situ hybridization, immunohistochemistry, DNA methylation profiling and tumor classification, and de-methylation studies | Medulloblastoma | Deregulation of miR-1253 expression in medulloblastoma. miR-1253 inhibits mediators of cellular proliferation and promotes tumor cell apoptosis. B7-H3 is oncogenic target of miR-1253. High expression of B7-H3 in tumor samples. Both miR-1253 restoration and B7-H3 silencing reduce the migratory and invasive medulloblastoma cells. | [32] |
| miR-145/B7-H3 | Pleural effusion | Observational indexes and qRT-PCR | Lung cancer | The higher expression level of B7-H3 in lung cancer. Lower expression level of miR-145 in the pleural effusion of the study group than in the control group. Relationship with lymphatic metastasis, differentiation degree, and TNM stage. | [31] |
| miR-199a/B7-H3 | HeLa, C4–1, SiHa, CaSki, and C-33A | Cell proliferation assay, luciferase reporter assays, enzyme-linked immunosorbent assay, Western blot assays, qRT-PCR, cell migration, invasion assay, and immunohistochemical staining | Cervical cancer (CC) | miR-199a was expressed at lower levels in CC tissues than in adjacent normal tissues. miR-199a inhibits cell proliferation, migration and the invasion of CC cells by targeting B7-H3. The expression level of B7-H3 was significantly upregulated in CC tissues. | [50] |
| miR-1207–5p/B7-H4 | Whole blood samples | Selection of miR single-nucleotide polymorphisms (SNPs), SNP genotyping, selection of miR SNPs from published databases, and luciferase reporter assay | Colorectal cancer | Upregulation of miR-1207–5p in CRC tissues. miR-1207–5p can suppress the expression of B7-H4 molecule by binding with the rs13505 G-allele-specific 3’-UTR of B7-H4 gene, which is impacted by the rs13505. | [46] |
| 62 different miRs * | L3.6p1 cells, which were derived from a human pancreatic carcinoma | Bioinformatics analysis and miR microarray analysis | Pancreatic cancer | miRs participate in the B7-H4-mediated regulation of oncogenicity and pathogenesis of pancreatic cancer. | [38] |
| miR-125a-5p/B7-H5 | Cell lines MCF10A, MDA-MB-468, MDA-MB-231, MCF7, MKN28, SNU1, MKN45, AGS, SW480, HCT116, HT29, and RKO, as well as gastric tumors | RNA extraction, expression quantification; DNA extraction, methylation analysis; short-interference-RNA experiments; BMP4 treatment; and antimiR experiments | Gastric cancer (GC) | B7-H5 expression loss is a recurrent event in GC, caused by promoter methylation and/or miR-125a-5p overexpression, and GC-microenvironment myofibroblasts overexpress B7-H5. B7-H5 expression is under the control of miR-125a-5p, as its targeted inhibition led to an overexpression of B7-H5. | [36] |
| miR-16/B7-H5 | Colon tissue | miR microarray analysis, gene microarray analysis, qRT-PCR, miR target gene prediction, and luciferase reporter assays | Active Crohn’s disease | Upregulation of miR-16 in the inflammatory areas of the ascending colon mucosa. Inhibition of B7-H5 expression. Immune inflammatory responses in the ascending colon. Reduction in miR-16 expression suggests the possibility of canceration of the inflammatory colon. hsa-miR-16–1 directly regulated the human B7-H5 gene. | [47] |
| miR-93/B7-H6, miR-340/B7-H6, miR-195/B7-H6 | The data were from TCGA data from 1092 patients with breast cancer, including gene expression, miR expression, and survival data. Human breast cancer cell lines, i.e., MCF7, MDA-MB-231, SK-BR-3, and human fibrocystic disease epithelium cell line MCF10A. | TCGA breast cancer transcriptome profiling analysis, sorting analysis of the most likely miRs targeting the B7 family, and qRT-PCR | Triple-negative breast cancer | Upregulation of B7-H6 in breast cancer based on an analysis of the TCGA database. A high level of B7-H6 suggested a worse prognosis. Bioinformatic analysis predicted that miR-93, miR-195, and miR-340 are potential regulators of the immune evasion of breast cancer cells, and they exert this function by targeting B7-H6. | [49] |
| miR-3116/B7-H7, miR-6870–5p/B7-H7 | Clinical and experimental data from multiple databases, including cBioPortal, TCGA, Cistrome, TIMER, Oncomine, Kaplan–Meier, GeneXplain. | Expression profiling of B7-H7 in human cancers, pan-cancer survival analysis, and bioinformatics analysis for understanding the regulatory mechanism of B7-H7 | Clear cell carcinoma | The bioinformatic view showed that basic leucine zipper ATF-like transcription factor (BATF) in B lymphocyte and SMAD in monocytes might be responsible for the dysregulation of B7-H7 in kidney renal clear cell carcinoma (KIRC). miR-3116 and miR-6870–5p may have a role in the regulation of B7-H7. | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahangar, N.K.; Hemmat, N.; Khalaj-Kondori, M.; Shadbad, M.A.; Sabaie, H.; Mokhtarzadeh, A.; Alizadeh, N.; Derakhshani, A.; Baghbanzadeh, A.; Dolatkhah, K.; et al. The Regulatory Cross-Talk between microRNAs and Novel Members of the B7 Family in Human Diseases: A Scoping Review. Int. J. Mol. Sci. 2021, 22, 2652. https://doi.org/10.3390/ijms22052652
Ahangar NK, Hemmat N, Khalaj-Kondori M, Shadbad MA, Sabaie H, Mokhtarzadeh A, Alizadeh N, Derakhshani A, Baghbanzadeh A, Dolatkhah K, et al. The Regulatory Cross-Talk between microRNAs and Novel Members of the B7 Family in Human Diseases: A Scoping Review. International Journal of Molecular Sciences. 2021; 22(5):2652. https://doi.org/10.3390/ijms22052652
Chicago/Turabian StyleAhangar, Noora Karim, Nima Hemmat, Mohammad Khalaj-Kondori, Mahdi Abdoli Shadbad, Hani Sabaie, Ahad Mokhtarzadeh, Nazila Alizadeh, Afshin Derakhshani, Amir Baghbanzadeh, Katayoun Dolatkhah, and et al. 2021. "The Regulatory Cross-Talk between microRNAs and Novel Members of the B7 Family in Human Diseases: A Scoping Review" International Journal of Molecular Sciences 22, no. 5: 2652. https://doi.org/10.3390/ijms22052652
APA StyleAhangar, N. K., Hemmat, N., Khalaj-Kondori, M., Shadbad, M. A., Sabaie, H., Mokhtarzadeh, A., Alizadeh, N., Derakhshani, A., Baghbanzadeh, A., Dolatkhah, K., Silvestris, N., & Baradaran, B. (2021). The Regulatory Cross-Talk between microRNAs and Novel Members of the B7 Family in Human Diseases: A Scoping Review. International Journal of Molecular Sciences, 22(5), 2652. https://doi.org/10.3390/ijms22052652









