Indole-3-Acetic Acid Is Synthesized by the Endophyte Cyanodermella asteris via a Tryptophan-Dependent and -Independent Way and Mediates the Interaction with a Non-Host Plant
Abstract
1. Introduction
2. Results
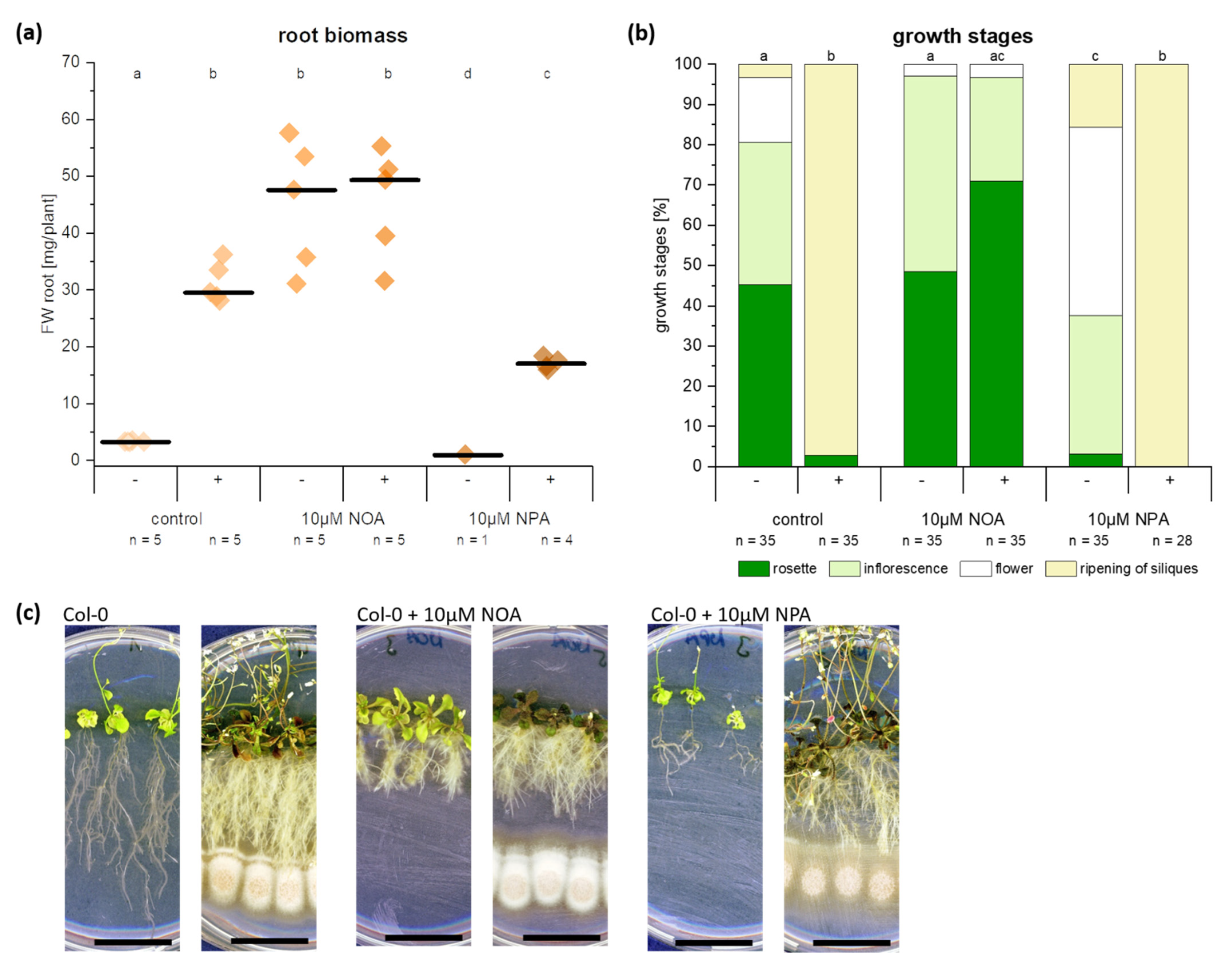
2.1. The Endophyte C. asteris Induces a Root Phenotype on the Non-Host Plant A. thaliana that Is Reminiscent of High Auxin
2.2. The Fungal Genome of C. asteris Reveals Several Putative Genes Involved in IAA Biosynthesis
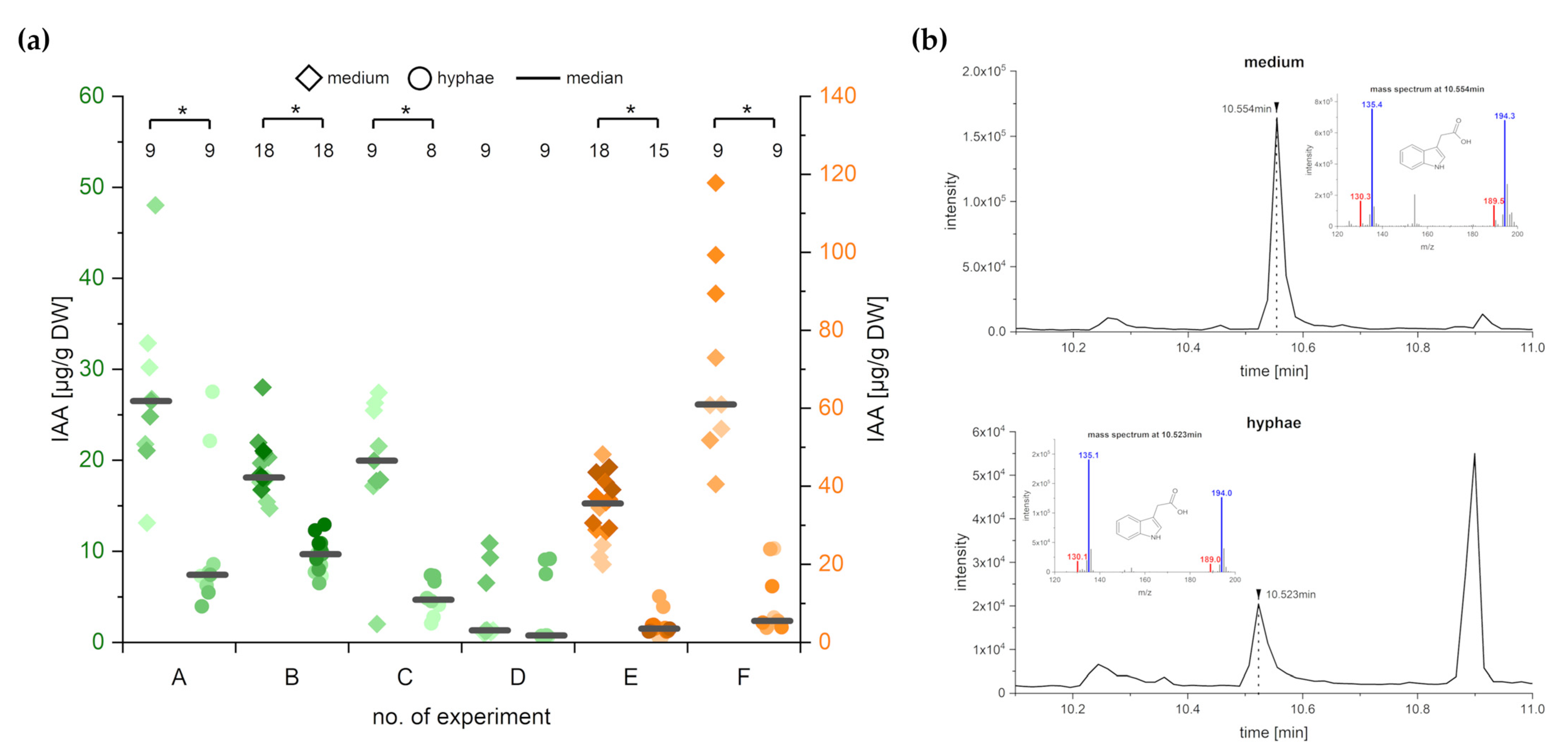
2.3. C. asteris Secretes the Synthesized IAA into the Liquid Medium
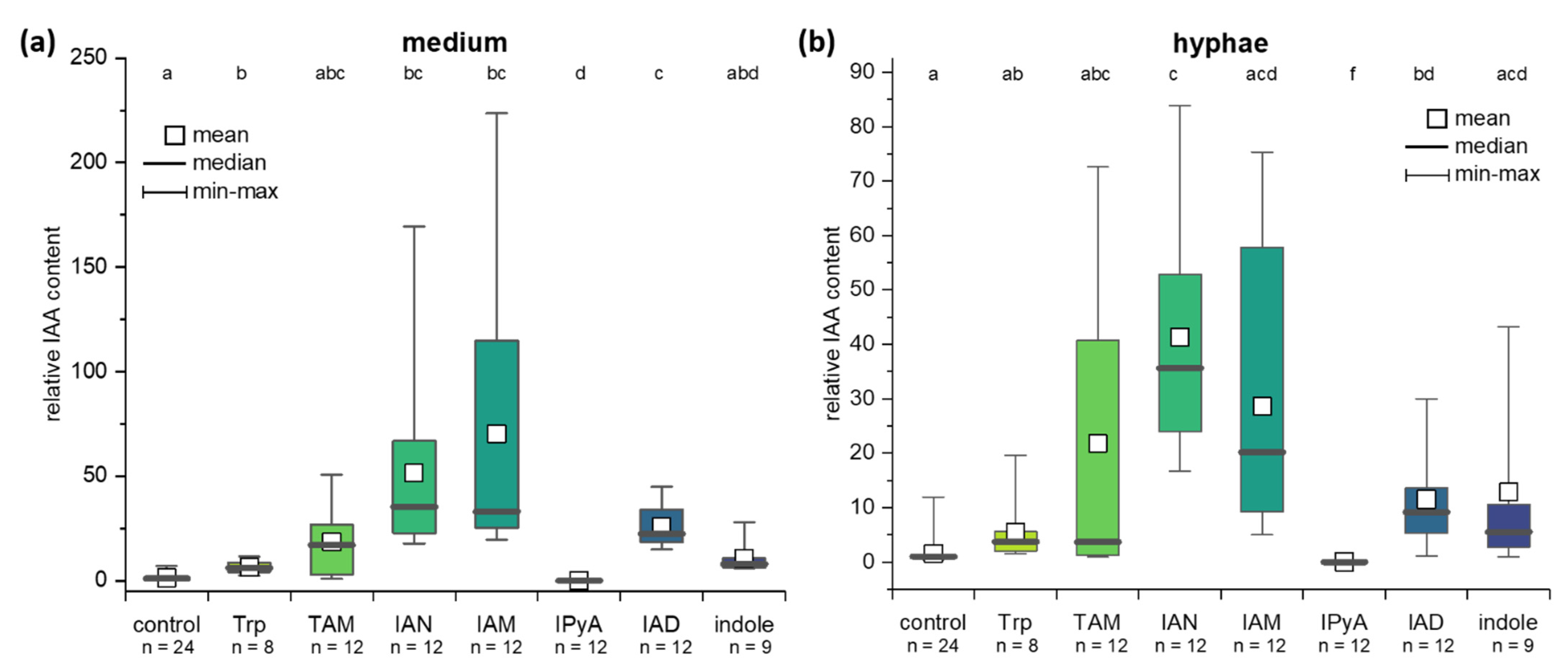
2.4. IAA Biosynthesis in C. asteris
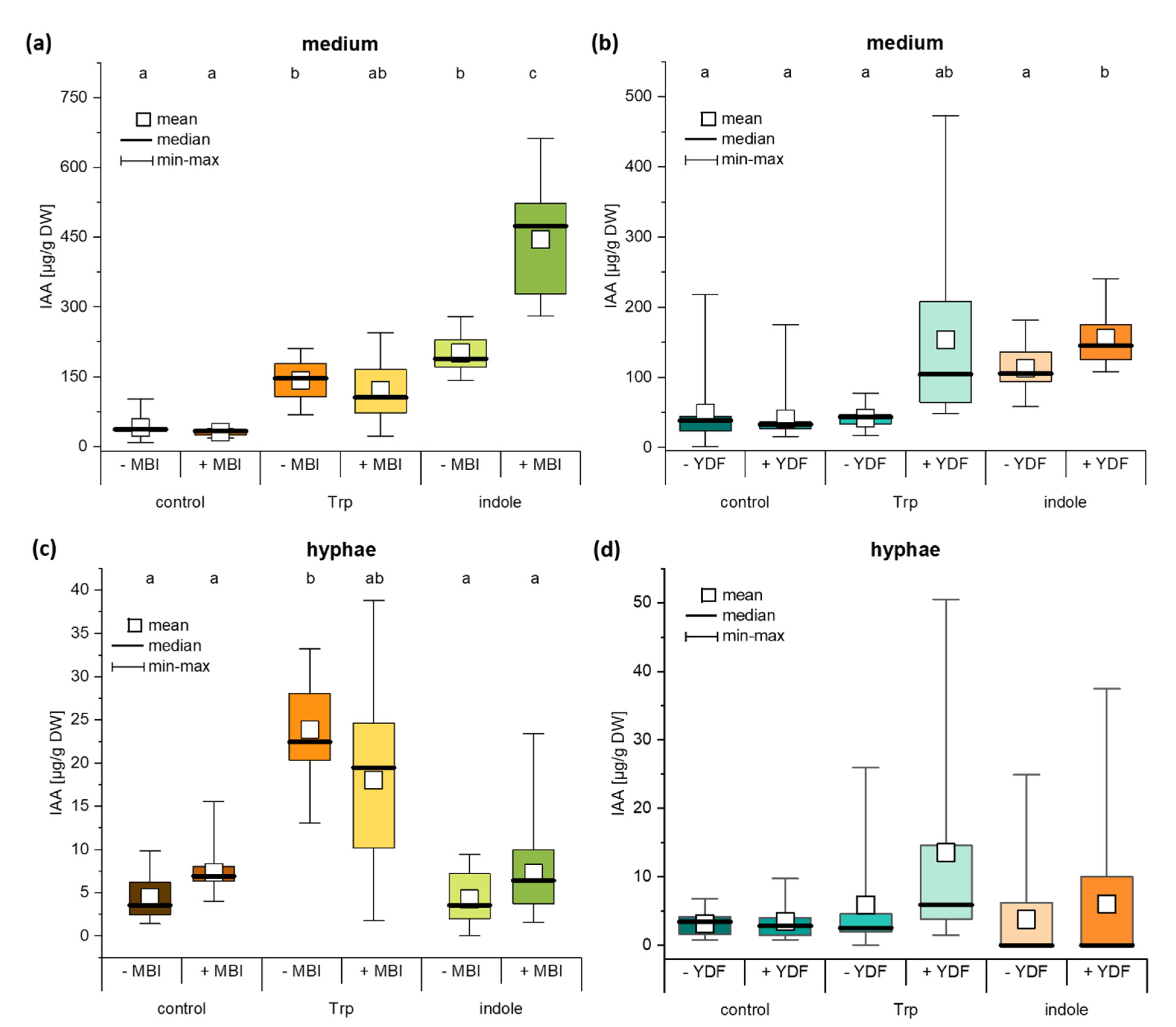
2.5. The IAA Biosynthesis Inhibitor MBI Reveals a Trp-Independent Biosynthesis Pathway in C. asteris
2.6. Auxin Synthesis by C. asteris and Uptake into the Host Plant Are Important for the Root Phenotype
3. Discussion
4. Materials and Methods
4.1. Plant and Fungal Material
4.2. Co-Cultivation of A. thaliana with C. asteris
4.3. GUS Staining of A. thaliana DR5::GUS
4.4. Incubation of C. asteris with IAA Precursors and/or Biosynthesis Inhibitors
4.5. Extraction and Detection of IAA
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a Plant Beneficial Fungus, Enhances Biomass Production and Promotes Lateral Root Growth Through an Auxin-dependent Mechanism in Arabidopsis. Plant. Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Castro, R.; Díaz-Pérez, C.; Martínez-Trujillo, M.; del Río, R.E.; Campos-García, J.; López-Bucio, J. Transkingdom Signaling Based on Bacterial Cyclodipeptides with Auxin Activity in Plants. Proc. Natl. Acad. Sci. USA 2011, 108, 7253–7258. [Google Scholar] [CrossRef]
- Rai, M.; Varma, A. Arbuscular Mycorrhiza-like Biotechnological Potential of Piriformospora indica, Which Promotes the Growth of Adhatoda Vasica Nees. Electron. J. Biotechnol. 2005, 8, 1–6. [Google Scholar] [CrossRef]
- Suebrasri, T.; Harada, H.; Jogloy, S.; Ekprasert, J.; Boonlue, S. Auxin-producing Fungal Endophytes Promote Growth of Sunchoke. Rhizosphere 2020, 16, 100271. [Google Scholar] [CrossRef]
- Jahn, L.; Mucha, S.; Bergmann, S.; Horn, C.; Staswick, P.; Steffens, B.; Siemens, J.; Ludwig-Müller, J. The Clubroot Pathogen (Plasmodiophora brassicae) Influences Auxin Signaling to Regulate Auxin Homeostasis in Arabidopsis. Plants 2013, 2, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Leontovycová, H.; Trdá, L.; Dobrev, P.I.; Šašek, V.; Gay, E.; Balesdent, M.-H.; Burketová, L. Auxin Biosynthesis in the Phytopathogenic Fungus Leptosphaeria maculans Is Associated with Enhanced Transcription of Indole-3-pyruvate Decarboxylase LmIPDC2 and Tryptophan Aminotransferase LmTAM1. Res. Microbiol. 2020, 171, 174–184. [Google Scholar] [CrossRef]
- Vandeputte, O.; Oden, S.; Mol, A.; Vereecke, D.; Goethals, K.; El Jaziri, M.; Prinsen, E. Biosynthesis of Auxin by the Gram-positive Phytopathogen Rhodococcus fascians Is Controlled by Compounds Specific to Infected Plant Tissues. Appl. Environ. Microbiol. 2005, 71, 1169–1177. [Google Scholar] [CrossRef]
- Davies, P.J. The Plant Hormones: Their Nature, Occurrence, and Functions. In Plant Hormones: Biosynthesis, Signal Transduction, Action! Davies, P.J., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 1–15. ISBN 9781402026867. [Google Scholar]
- Ludwig-Müller, J. Auxin Conjugates: Their Role for Plant Development and in the Evolution of Land Plants. J. Exp. Bot. 2011, 62, 1757–1773. [Google Scholar] [CrossRef]
- Parry, G.; Delbarre, A.; Marchant, A.; Swarup, R.; Napier, R.; Perrot-Rechenmann, C.; Bennett, M.J. Novel Auxin Transport Inhibitors Phenocopy Auxin Influx Carrier Mutation aux1. Plant. J. 2001, 25, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alonso, M.-M.; Guerrero-Galán, C.; Scholz, S.S.; Kiba, T.; Sakakibara, H.; Ludwig-Müller, J.; Krapp, A.; Oelmüller, R.; Vicente-Carbajosa, J.; Pollmann, S. Harnessing Symbiotic Plant–Fungus Interactions Unleash Hidden Forces from Extreme Plant Ecosystems. J. Exp. Bot. 2020, 71, 3865–3877. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Auxin Biosynthesis. Arab. Book Am. Soc. Plant Biol. 2014, 12. [Google Scholar] [CrossRef]
- Mashiguchi, K.; Tanaka, K.; Sakai, T.; Sugawara, S.; Kawaide, H.; Natsume, M.; Hanada, A.; Yaeno, T.; Shirasu, K.; Yao, H.; et al. The Main Auxin Biosynthesis Pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 18512–18517. [Google Scholar] [CrossRef]
- Lehmann, T.; Hoffmann, M.; Hentrich, M.; Pollmann, S. Indole-3-Acetamide-Dependent Auxin Biosynthesis: A Widely Distributed Way of Indole-3-Acetic Acid Production? Eur. J. Cell Biol. 2010, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Kriechbaumer, V.; Glawischnig, E. Auxin Biosynthesis within the Network of Tryptophan Metabolism. JNBT 2005, 2, 55–58. [Google Scholar]
- Wright, A.D.; Moehlenkamp, C.A.; Perrot, G.H.; Neuffer, M.G.; Cone, K.C. The Maize Auxotrophic Mutant Orange Pericarp Is Defective in Duplicate Genes for Tryptophan Synthase β. Plant Cell 1992, 4, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; Neuffer, M.G. Orange Pericarp in Maize: Filial Expression in a Maternal Tissue. J. Hered. 1989, 80, 229–233. [Google Scholar] [CrossRef]
- Ouyang, J.; Shao, X.; Li, J. Indole-3-glycerol Phosphate, a Branchpoint of Indole-3-acetic Acid Biosynthesis from the Tryptophan Biosynthetic Pathway in Arabidopsis thaliana. Plant J. 2000, 24, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Patten, C.L.; Glick, B.R. Bacterial Biosynthesis of Indole-3-acetic Acid. Can. J. Microbiol. 1996, 42, 207–220. [Google Scholar] [CrossRef]
- Hull, A.K.; Vij, R.; Celenza, J.L. Arabidopsis Cytochrome P450s That Catalyze the First Step of Tryptophan-dependent Indole-3-acetic Acid Biosynthesis. Proc. Natl. Acad. Sci. USA 2000, 97, 2379–2384. [Google Scholar] [CrossRef]
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-acetic Acid in Plant–microbe Interactions. Antonie Van Leeuwenhoek 2014, 106, 85–125. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-F.; Wei, J.-Y.; Chen, H.-W.; Liu, Y.-Y.; Lu, H.-Y.; Chou, J.-Y. Indole-3-Acetic Acid: A Widespread Physiological Code in Interactions of Fungi with Other Organisms. Plant Signal. Behav. 2015, 10, e1048052. [Google Scholar] [CrossRef]
- Chanclud, E.; Morel, J.-B. Plant Hormones: A Fungal Point of View. Mol. Plant Pathol 2016, 17, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Reineke, G.; Heinze, B.; Schirawski, J.; Buettner, H.; Kahmann, R.; Basse, C.W. Indole-3-acetic Acid (IAA) Biosynthesis in the Smut Fungus Ustilago maydis and Its Relevance for Increased IAA Levels in Infected Tissue and Host Tumour Formation. Mol. Plant Pathol 2008, 9, 339–355. [Google Scholar] [CrossRef]
- Chung, K.R.; Tzeng, D.D. Biosynthesis of Indole-3-Acetic Acid by the Gall-Inducing Fungus Ustilago esculenta. J. Biol. Sci. 2004, 4, 744–750. [Google Scholar]
- Yamada, T.; Tsukamoto, H.; Shiraishi, T.; Nomura, T.; Oku, H. Detection of Indoleacetic Acid Biosynthesis in Some Species of Taphrina Causing Hyperplastic Diseases in Plants. Jpn. J. Phytopathol. 1990, 56, 532–540. [Google Scholar] [CrossRef]
- Tsavkelova, E.; Oeser, B.; Oren-Young, L.; Israeli, M.; Sasson, Y.; Tudzynski, B.; Sharon, A. Identification and Functional Characterization of Indole-3-acetamide-mediated IAA Biosynthesis in Plant-associated Fusarium Species. Fungal Genet. Biol. 2012, 49, 48–57. [Google Scholar] [CrossRef]
- Luo, K.; Rocheleau, H.; Qi, P.-F.; Zheng, Y.-L.; Zhao, H.-Y.; Ouellet, T. Indole-3-Acetic Acid in Fusarium graminearum: Identification of Biosynthetic Pathways and Characterization of Physiological Effects. Fungal Biol. 2016, 120, 1135–1145. [Google Scholar] [CrossRef]
- Maor, R.; Haskin, S.; Levi-Kedmi, H.; Sharon, A. In Planta Production of Indole-3-Acetic Acid by Colletotrichum gloeosporioides f.sp. aeschynomene. Appl. Environ. Microbiol. 2004, 70, 1852–1854. [Google Scholar] [CrossRef]
- Yin, C.; Park, J.-J.; Gang, D.R.; Hulbert, S.H. Characterization of a Tryptophan 2-Monooxygenase Gene from Puccinia graminis f.sp. tritici Involved in Auxin Biosynthesis and Rust Pathogenicity. Mol. Plant Microbe Interact. 2014, 27, 227–235. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Minic, Z.; Thongbam, P.D.; Bhaskar, V.; Montaut, S. Indolyl-3-Acetaldoxime Dehydratase from the Phytopathogenic Fungus Sclerotinia sclerotiorum: Purification, Characterization, and Substrate Specificity. Phytochemistry 2010, 71, 1952–1962. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Hussain, A.; Irshad, M.; Hamayun, M.; Iqbal, A.; Khan, N. In Vitro Production of IAA by Endophytic Fungus Aspergillus awamori and Its Growth Promoting Activities in Zea mays. Symbiosis 2019, 77, 225–235. [Google Scholar] [CrossRef]
- Mehmood, A.; Hussain, A.; Irshad, M.; Hamayun, M.; Iqbal, A.; Tawab, A.; Khan, N. Yucasin and Cinnamic Acid Inhibit IAA and Flavonoids Biosynthesis Minimizing Interaction Between Maize and Endophyte Aspergillus nomius. Symbiosis 2020, 81, 149–160. [Google Scholar] [CrossRef]
- Pons, S.; Fournier, S.; Chervin, C.; Bécard, G.; Rochange, S.; Frey, N.F.D.; Pagès, V.P. Phytohormone Production by the Arbuscular Mycorrhizal Fungus Rhizophagus irregularis. PLoS ONE 2020, 15, e0240886. [Google Scholar] [CrossRef] [PubMed]
- Vadassery, J.; Ritter, C.; Venus, Y.; Camehl, I.; Varma, A.; Shahollari, B.; Novák, O.; Strnad, M.; Ludwig-Müller, J.; Oelmüller, R. The Role of Auxins andCytokinins in the Mutualistic Interaction of Arabidopsis and Piriformospora indica. Mol. Plant Microbe Interact. 2008, 21, 1371–1383. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Kanehisa, M. Toward Understanding the Origin and Evolution of Cellular Organisms. Protein Sci. A Publ. Protein Soc. 2019, 28, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating Viruses and Cellular Organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef] [PubMed]
- Oberhänsli, T.; Défago, G.; Haas, D. Indole-3-acetic Acid (IAA) Synthesis in the Biocontrol Strain CHA0 of Pseudomonas fluorescens: Role of Tryptophan Side Chain Oxidase. Microbiology 1991, 137, 2273–2279. [Google Scholar] [CrossRef] [PubMed]
- Magie, A.R.; Wilson, E.E.; Kosuge, T. Indoleacetamide as an Intermediate in the Synthesis of Indoleacetic Acid in Pseudomonas savastanoi. Science 1963, 141, 1281–1282. [Google Scholar] [CrossRef]
- Kosuge, T.; Heskett, M.G.; Wilson, E.E. Microbial Synthesis and Degradation of Indole-3-acetic Acid I.The Conversion of L-tryptophan to Indole-3-acetamide by an Enzyme System from Pseudomonas savastoi. J. Biol. Chem. 1966, 241, 3738–3744. [Google Scholar] [CrossRef]
- Hutzinger, O.; Kosuge, T. Microbial Synthesis and Degradation of Indole-3-acetic Acid II. The Source of Oxygen in the Conversion of L-tryptophan to Indole-3-acetamide. Biochim. Biophys. Acta (BBA) Gen. Subj. 1967, 136, 389–391. [Google Scholar] [CrossRef]
- Jahn, L.; Schafhauser, T.; Pan, S.; Weber, T.; Wohlleben, W.; Fewer, D.; Sivonen, K.; Flor, L.; van Pée, K.-H.; Caradec, T.; et al. Cyanodermella Asteris Sp. Nov. (Ostropales) from the Inflorescence Axis of Aster Tataricus. Mycotaxon 2017, 132, 107–123. [Google Scholar] [CrossRef]
- Shao, Y.; Ho, C.-T.; Chin, C.-K.; Rosen, R.T.; Hu, B.; Qin, G.-W. Triterpenoid Saponins from Aster auriculatus. Phytochemistry 1997, 44, 337–340. [Google Scholar] [CrossRef]
- Shirota, O.; Morita, H.; Takeya, K.; Itokawa, H.; Iitaka, Y. Cytotoxic Triterpenes from Aster tataricus. Nat. Med. 1997, 51, 170–172. [Google Scholar]
- Shao, Y.; Ho, C.T.; Chin, C.K.; Poobrasert, O.; Yang, S.W.; Cordell, G. Asterlingulatosides C and D, Cytotoxic Triterpenoid Saponins from Aster lingulatus. J. Nat. Prod. 1997, 60, 743–746. [Google Scholar] [CrossRef]
- Xu, H.-M.; Zeng, G.-Z.; Zhou, W.-B.; He, W.-J.; Tan, N.-H. Astins K–P, Six New Chlorinated Cyclopentapeptides from Aster tataricus. Tetrahedron 2013, 69, 7964–7969. [Google Scholar] [CrossRef]
- Morita, H.; Nagashima, S.; Uchiumi, Y.; Kuroki, O.; Takeda, K.; Itokawa, H. Cyclic Peptides from Higher Plants. XXVIII. Antitumor Activity and Hepatic Microsomal Biotransformation of Cyclic Pentapeptides, Astins, from Aster tataricus. Chem. Pharm. Bull. 1996. [Google Scholar] [CrossRef]
- Schafhauser, T.; Jahn, L.; Kirchner, N.; Kulik, A.; Flor, L.; Lang, A.; Caradec, T.; Fewer, D.P.; Sivonen, K.; Berkel, W.J.H.; et al. Antitumor Astins Originate from the Fungal Endophyte Cyanodermella asteris Living Within the Medicinal Plant Aster tataricus. Proc. Natl. Acad. Sci. USA 2019, 116, 26909–26917. [Google Scholar] [CrossRef]
- Shi, C.-L.; Park, H.-B.; Lee, J.S.; Ryu, S.; Ryu, C.-M. Inhibition of Primary Roots and Stimulation of Lateral Root Development in Arabidopsis thaliana by the Rhizobacterium Serratia marcescens 90-166 Is Through Both Auxin-dependent and -independent Signaling Pathways. Mol. Cells 2010, 29, 251–258. [Google Scholar] [CrossRef]
- López-Bucio, J.; Campos-Cuevas, J.C.; Hernández-Calderón, E.; Velásquez-Becerra, C.; Farías-Rodríguez, R.; Macías-Rodríguez, L.I.; Valencia-Cantero, E. Bacillus megaterium Rhizobacteria Promote Growth and Alter Root-system Architecture Through an Auxin- and Ethylene-independent Signaling Mechanism in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2007, 20, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Splivallo, R.; Fischer, U.; Göbel, C.; Feussner, I.; Karlovsky, P. Truffles Regulate Plant Root Morphogenesis via the Production of Auxin and Ethylene. Plant Physiol. 2009, 150, 2018–2029. [Google Scholar] [CrossRef] [PubMed]
- Ilic, N.; Östin, A.; Cohen, J.D. Differential Inhibition of Indole-3-acetic Acid and Tryptophan Biosynthesis by Indole Analogues. I. Tryptophan Dependent IAA Biosynthesis. Plant Growth Regul 1999, 27, 57–62. [Google Scholar] [CrossRef]
- Tsugafune, S.; Mashiguchi, K.; Fukui, K.; Takebayashi, Y.; Nishimura, T.; Sakai, T.; Shimada, Y.; Kasahara, H.; Koshiba, T.; Hayashi, K. Yucasin DF, a Potent and Persistent Inhibitor of Auxin Biosynthesis in Plants. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Nishimura, T.; Hayashi, K.; Suzuki, H.; Gyohda, A.; Takaoka, C.; Sakaguchi, Y.; Matsumoto, S.; Kasahara, H.; Sakai, T.; Kato, J.; et al. Yucasin Is a Potent Inhibitor of YUCCA, a Key Enzyme in Auxin Biosynthesis. Plant J. 2014, 77, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Lanková, M.; Smith, R.S.; Pešek, B.; Kubeš, M.; Zažímalová, E.; Petrášek, J.; Hoyerová, K. Auxin Influx Inhibitors 1-NOA, 2-NOA, and CHPAA Interfere with Membrane Dynamics in Tobacco Cells. J. Exp. Bot 2010, 61, 3589–3598. [Google Scholar] [CrossRef]
- Teale, W.; Palme, K. Naphthylphthalamic Acid and the Mechanism of Polar Auxin Transport. J. Exp. Bot 2017, 69, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Jahn, L.; Schafhauser, T.; Wibberg, D.; Rückert, C.; Winkler, A.; Kulik, A.; Weber, T.; Flor, L.; van Pée, K.-H.; Kalinowski, J.; et al. Linking Secondary Metabolites to Biosynthesis Genes in the Fungal Endophyte Cyanodermella asteris: The Anti-cancer Bisanthraquinone Skyrin. J. Biotechnol. 2017, 257, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Costacurta, A.; Keijers, V.; Vanderleyden, J. Molecular Cloning and Sequence Analysis of an Azospirilium brasilense Indole-3-pyruvate Decarboxylase Gene. Mol. Gen. Genet. 1994, 243, 463–472. [Google Scholar] [CrossRef]
- Prinsen, E.; Costacurta, A.; Michiels, K.; Vanderleyden, J.; Van Onckelen, H. Azospirillum brasilense Indole-3-acetic Acid Biosynthesis: Evidence for a Non-tryptophan Dependent Pathway. Mol. Plant Microbe Interact. 1993, 6, 609. [Google Scholar] [CrossRef]
- Pedraza, R.O.; Ramírez-Mata, A.; Xiqui, M.L.; Baca, B.E. Aromatic Amino Acid Aminotransferase Activity and Indole-3-acetic Acid Production by Associative Nitrogen-fixing Bacteria. Fems Microbiol. Lett. 2004, 233, 15–21. [Google Scholar] [CrossRef]
- Pollmann, S.; Neu, D.; Weiler, E.W. Molecular Cloning and Characterization of an Amidase from Arabidopsis thaliana Capable of Converting Indole-3-Acetamide into the Plant Growth Hormone, Indole-3-Acetic Acid. Phytochemistry 2003, 62, 293–300. [Google Scholar] [CrossRef]
- Numponsak, T.; Kumla, J.; Suwannarach, N.; Matsui, K.; Lumyong, S. Biosynthetic Pathway and Optimal Conditions for the Production of Indole-3-acetic Acid by an Endophytic Fungus, Colletotrichum fructicola CMU-A109. PLoS ONE 2018, 13, e0205070. [Google Scholar] [CrossRef]
- Robinson, M.; Riov, J.; Sharon, A. Indole-3-acetic Acid Biosynthesis in Colletotrichum gloeosporioides f. sp. aeschynomene. Appl. Environ. Microbiol. 1998, 64, 5030–5032. [Google Scholar] [CrossRef] [PubMed]
- Follin, A.; Inzé, D.; Budar, F.; Genetello, C.; Van Montagu, M.; Schell, J. Genetic Evidence That the Tryptophan 2-mono-oxygenase Gene of Pseudomonas savastonoi Is Functionally Equivalent to One of the T-DNA Genes Involved in Plant Tumour Formation by Agrobacterium tumefaciens. Mol. Gen. Genet. 1985, 201, 178–185. [Google Scholar] [CrossRef]
- Manulis, S.; Shafrir, H.; Epstein, E.; Lichter, A.; Barash, I. Biosynthesis of Indole-3-acetic Acid via the Indole-3-acetamide Pathway in Streptomyces spp. Microbiology 1994, 140, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Pace, H.C.; Brenner, C. The Nitrilase Superfamily: Classification, Structure and Function. Genome Biol. 2001, 2, reviews0001.1. [Google Scholar] [CrossRef]
- Feil, H.; Feil, W.S.; Chain, P.; Larimer, F.; DiBartolo, G.; Copeland, A.; Lykidis, A.; Trong, S.; Nolan, M.; Goltsman, E.; et al. Comparison of the Complete Genome Sequences of Pseudomonas syringae pv. syringae B728a and Pv. Tomato DC3000. Proc. Natl. Acad. Sci. USA 2005, 102, 11064–11069. [Google Scholar] [CrossRef]
- Kiziak, C.; Conradt, D.; Stolz, A.; Mattes, R.; Klein, J. Nitrilase from Pseudomonas fluorescens EBC191: Cloning and Heterologous Expression of the Gene and Biochemical Characterization of the Recombinant Enzyme. Microbiology 2005, 151, 3639–3648. [Google Scholar] [CrossRef]
- Kobayashi, M.; Izui, H.; Nagasawa, T.; Yamada, H. Nitrilase in Biosynthesis of the Plant Hormone Indole-3-acetic Acid from Indole-3-acetonitrile: Cloning of the Alcaligenes Gene and Site-directed Mutagenesis of Cysteine Residues. Proc. Natl. Acad. Sci. USA 1993, 90, 247–251. [Google Scholar] [CrossRef]
- Nagasawa, T.; Mauger, J.; Yamada, H. A Novel Nitrilase, Arylacetonitrilase, of Alcaligenes faecalis JM3. Eur. J. Biochem. 1990, 194, 765–772. [Google Scholar] [CrossRef]
- Kobayashi, M.; Suzuki, T.; Fujita, T.; Masuda, M.; Shimizu, S. Occurrence of Enzymes Involved in Biosynthesis of Indole-3-acetic Acid from Indole-3-acetonitrile in Plant-associated Bacteria, Agrobacterium and Rhizobium. Proc. Natl. Acad. Sci. USA 1995, 92, 714–718. [Google Scholar] [CrossRef]
- Perley, J.E.; Stowe, B.B. On the Ability of Taphrina deformans to Produce Indoleacetic Acid from Tryptophan by Way of Tryptamine. Plant Physiol. 1966, 41, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Lovett, B.; Fang, W.; St Leger, R.J. Metarhizium robertsii Produces Indole-3-acetic Acid, Which Promotes Root Growth in Arabidopsis and Enhances Virulence to Insects. Microbiology 2017, 163, 980–991. [Google Scholar] [CrossRef]
- Hartmann, A.; Singh, M.; Klingmüller, W. Isolation and Characterization of Azospirillum Mutants Excreting High Amounts of Indoleacetic Acid. Can. J. Microbiol. 1983, 29, 916–923. [Google Scholar] [CrossRef]
- Basse, C.W.; Lottspeich, F.; Steglich, W.; Kahmann, R. Two Potential Indole-3-acetaldehyde Dehydrogenases in the Phytopathogenic Fungus Ustilago maydis. Eur. J. Biochem. 1996, 242, 648–656. [Google Scholar] [CrossRef]
- Rao, R.P.; Hunter, A.; Kashpur, O.; Normanly, J. Aberrant Synthesis of Indole-3-acetic Acid in Saccharomyces cerevisiae Triggers Morphogenic Transition, a Virulence Trait of Pathogenic Fungi. Genetics 2010, 185, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Müller, J.; Denk, K.; Cohen, J.D.; Quint, M. An Inhibitor of Tryptophan-dependent Biosynthesis of Indole-3-acetic Acid Alters Seedling Development in Arabidopsis. J. Plant Growth Regul. 2010, 29, 242–248. [Google Scholar] [CrossRef]
- Wright, A.D.; Sampson, M.B.; Neuffer, M.G.; Michalczuk, L.; Slovin, J.P.; Cohen, J.D. Indole-3-acetic Acid Biosynthesis in the Mutant Maize Orange Pericarp, a Tryptophan Auxotroph. Science 1991, 254, 998–1000. [Google Scholar] [CrossRef]
- Normanly, J.; Cohen, J.D.; Fink, G.R. Arabidopsis thaliana Auxotrophs Reveal a Tryptophan-independent Biosynthetic Pathway for Indole-3-acetic Acid. Proc. Natl. Acad. Sci. USA 1993, 90, 10355–10359. [Google Scholar] [CrossRef]
- Goldsmith, M.H.M. The Polar Transport of Auxin. Ann. Rev. Plant Physiol. 1977, 28, 439–478. [Google Scholar] [CrossRef]
- Geisler, M.; Blakeslee, J.J.; Bouchard, R.; Lee, O.R.; Vincenzetti, V.; Bandyopadhyay, A.; Titapiwatanakun, B.; Peer, W.A.; Bailly, A.; Richards, E.L.; et al. Cellular Efflux of Auxin Catalyzed by the Arabidopsis MDR/PGP Transporter AtPGP1. Plant J. 2005, 44, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Pierce, M.; Titapiwatanakun, B.; Sohn, E.J.; Fang, F.; Larive, C.K.; Blakeslee, J.; Cheng, Y.; Cuttler, S.; Peer, W.A.; Murphy, A.S.; et al. Arabidopsis P-Glycoprotein 19 Participates in the Inhibition of Gravitropism by Gravacin. Chem. Biol. 2007, 14, 1366–1376. [Google Scholar] [CrossRef]
- Nagashima, A.; Uehara, Y.; Sakai, T. The ABC Subfamily B Auxin Transporter AtABCB19 Is Involved in the Inhibitory Effects of N-1-naphthyphthalamic Acid on the Phototropic and Gravitropic Responses of Arabidopsis Hypocotyls. Plant Cell Physiol. 2008, 49, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Ueda, J.; Komaki, M.K.; Bell, C.J.; Shimura, Y. Requirement of the Auxin Polar Transport System in Early Stages of Arabidopsis Floral Bud Formation. Plant Cell 1991, 3, 677–684. [Google Scholar] [CrossRef]
- Reinhardt, D.; Mandel, T.; Kuhlemeier, C. Auxin Regulates the Initiation and Radial Position of Plant Lateral Organs. Plant Cell 2000, 12, 507–518. [Google Scholar] [CrossRef]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN Auxin Efflux Facilitator Network Controls Growth and Patterning in Arabidopsis Roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, J.; Sung, Z.R.; Berleth, T. Responses of Plant Vascular Systems to Auxin Transport Inhibition. Development 1999, 126, 2979–2991. [Google Scholar]
- Bailly, A.; Sovero, V.; Vincenzetti, V.; Santelia, D.; Bartnik, D.; Koenig, B.W.; Mancuso, S.; Martinoia, E.; Geisler, M. Modulation of P-Glycoproteins by Auxin Transport Inhibitors Is Mediated by Interaction with Immunophilins. J. Biol. Chem. 2008, 283, 21817–21826. [Google Scholar] [CrossRef]
- Rincón, A.; Priha, O.; Sotta, B.; Bonnet, M.; Le Tacon, F. Comparative Effects of Auxin Transport Inhibitors on Rhizogenesis and Mycorrhizal Establishment of Spruce Seedlings Inoculated with Laccaria bicolor. Tree Physiol. 2003, 23, 785–791. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Felten, J.; Kohler, A.; Morin, E.; Bhalerao, R.P.; Palme, K.; Martin, F.; Ditengou, F.A.; Legué, V. The Ectomycorrhizal Fungus Laccaria bicolor Stimulates Lateral Root Formation in Poplar and Arabidopsis Through Auxin Transport and Signaling. Plant Physiol. 2009, 151, 1991–2005. [Google Scholar] [CrossRef] [PubMed]
- Niemi, K.; Vuorinen, T.; Ernstsen, A. Ectomycorrhizal Fungi and Exogenous Auxins Influence Root and Mycorrhiza Formation of Scots Pine Hypocotyl Cuttings In Vitro. Tree Physiol. 2002, 22, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Fitze, D.; Wiepning, A.; Kaldorf, M.; Ludwig-Müller, J. Auxins in the Development of an Arbuscular Mycorrhizal Symbiosis in Maize. J. Plant Physiol. 2005, 162, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Salas-Marina, M.A.; Silva-Flores, M.A.; Cervantes-Badillo, M.G.; Rosales-Saavedra, M.T.; Islas-Osuna, M.A.; Casas-Flores, S. The Plant Growth-promoting Fungus Aspergillus ustus Promotes Growth and Induces Resistance Against Different Lifestyle Pathogens in Arabidopsis thaliana. J. Microbiol. Biotechnol. 2011, 21, 686–696. [Google Scholar] [CrossRef]
- Sukumar, P.; Legué, V.; Vayssières, A.; Martin, F.; Tuskan, G.A.; Kalluri, U.C. Involvement of Auxin Pathways in Modulating Root Architecture During Beneficial Plant–microorganism Interactions. Plant Cell Environ. 2013, 36, 909–919. [Google Scholar] [CrossRef]
- Contesto, C.; Milesi, S.; Mantelin, S.; Zancarini, A.; Desbrosses, G.; Varoquaux, F.; Bellini, C.; Kowalczyk, M.; Touraine, B. The Auxin-signaling Pathway Is Required for the Lateral Root Response of Arabidopsis to the Rhizobacterium Phyllobacterium brassicacearum. Planta 2010, 232, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of Plant Growth by ACC Deaminase-producing Soil Bacteria. Eur. J. Plant Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Marschner, H.; Dell, B. Nutrient Uptake in Mycorrhizal Symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar] [CrossRef]
- Slankis, V. Soil Factors Influencing Formation of Mycorrhizae. Annu Rev. Phytopathol 1974, 12, 437–457. [Google Scholar] [CrossRef]
- Hutner, S.H.; Provasoli, L.; Schatz, A.; Haskins, C.P. Some Approaches to the Study of the Role of Metals in the Metabolism of Microorganisms. PAPHS 1950, 94, 152–170. [Google Scholar]
- Ilic, N.; Normanly, J.; Cohen, J.D. Quantification of Free Plus Conjugated Indoleacetic Acid in Arabidopsis Requires Correction for the Nonenzymatic Conversion of Indolic Nitriles. Plant Physiol. 1996, 111, 781–788. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lenhard, W.; Lenhard, A. Calculation of Effect Sizes. Dettelbach (Germany): Psychometrica. 2016. Available online: https://www.psychometrica.de/effect_size.html (accessed on 12 January 2021).
- Lakens, D. Calculating and Reporting Effect Sizes to Facilitate Cumulative Science: A Practical Primer for T-Tests and ANOVAs. Front. Psychol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
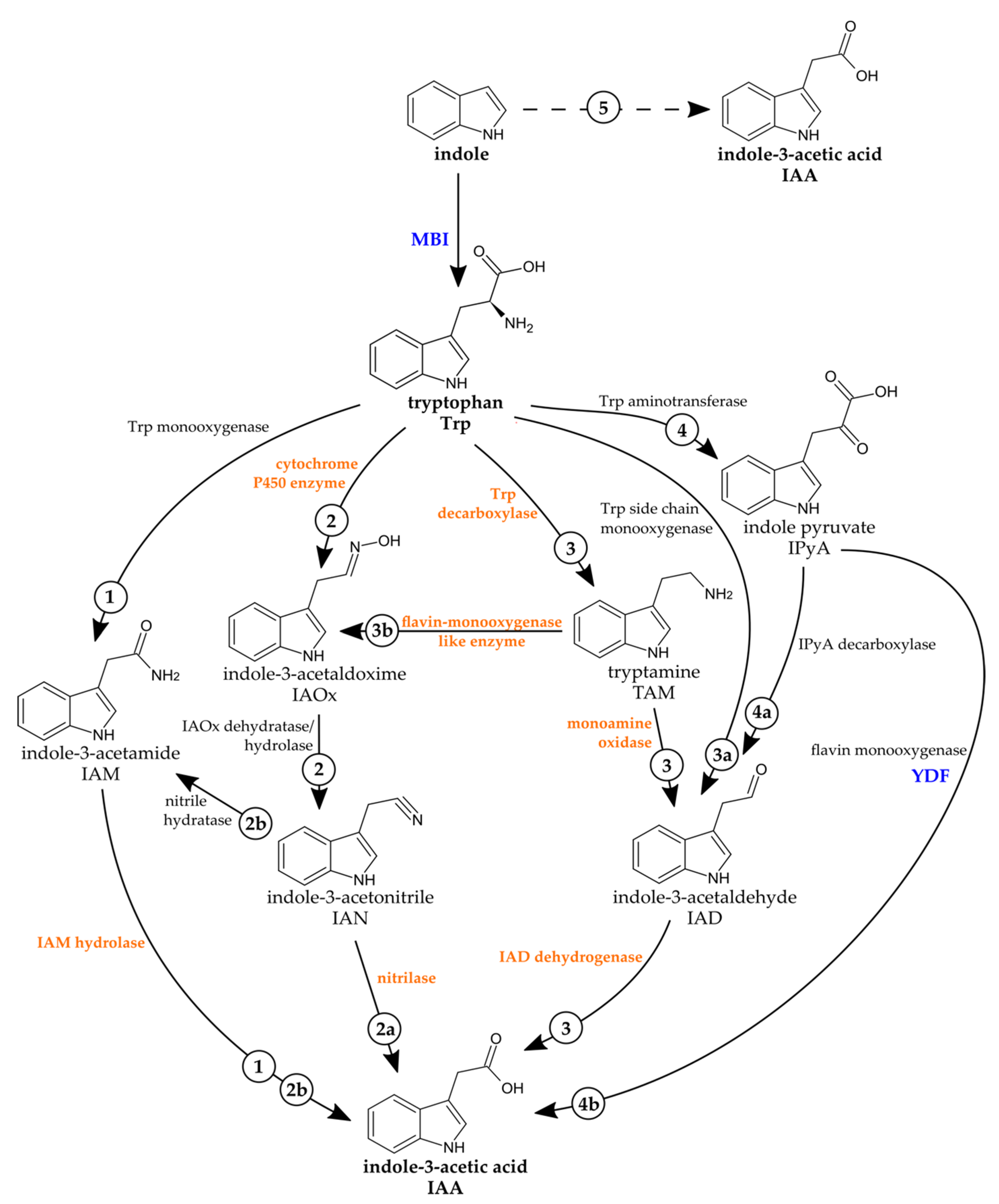


| Reaction | Enzyme | Candidate Genes (Gene ID) |
|---|---|---|
| Trp → TAM | Trp decarboxylase | 1234 |
| TAM → IAOx | flavin monooxygenase enzyme * | 17, 2090, 4995, 5792, 7832 2451, 3001, 6023, 9811 |
| TAM → IAD | monoamine oxidase | 3466, 3467, 8359 |
| IAD → IAA | IAD dehydrogenase | 10233, 6228, 7132, 8151, 9123 |
| Trp → IAOx | cytochrome P450 | 17, 2090, 4995, 5792, 7832 |
| IAOx → IAN | IAOx dehydratase/hydrolase | - |
| IAN → IAA | nitrilase | 1223, 5809 |
| IAN → IAM | nitrile hydratase | - |
| Trp → IAM | Trp monooxygenase | - |
| IAM → IAA | IAM hydrolase | 1862, 6419 |
| Trp → IPyA | Trp aminotransferase | - |
| IPyA → IAD | IPyA decarboxylase | - |
| indole → IAA | - | 2451, 3001, 6023, 9811 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahn, L.; Hofmann, U.; Ludwig-Müller, J. Indole-3-Acetic Acid Is Synthesized by the Endophyte Cyanodermella asteris via a Tryptophan-Dependent and -Independent Way and Mediates the Interaction with a Non-Host Plant. Int. J. Mol. Sci. 2021, 22, 2651. https://doi.org/10.3390/ijms22052651
Jahn L, Hofmann U, Ludwig-Müller J. Indole-3-Acetic Acid Is Synthesized by the Endophyte Cyanodermella asteris via a Tryptophan-Dependent and -Independent Way and Mediates the Interaction with a Non-Host Plant. International Journal of Molecular Sciences. 2021; 22(5):2651. https://doi.org/10.3390/ijms22052651
Chicago/Turabian StyleJahn, Linda, Uta Hofmann, and Jutta Ludwig-Müller. 2021. "Indole-3-Acetic Acid Is Synthesized by the Endophyte Cyanodermella asteris via a Tryptophan-Dependent and -Independent Way and Mediates the Interaction with a Non-Host Plant" International Journal of Molecular Sciences 22, no. 5: 2651. https://doi.org/10.3390/ijms22052651
APA StyleJahn, L., Hofmann, U., & Ludwig-Müller, J. (2021). Indole-3-Acetic Acid Is Synthesized by the Endophyte Cyanodermella asteris via a Tryptophan-Dependent and -Independent Way and Mediates the Interaction with a Non-Host Plant. International Journal of Molecular Sciences, 22(5), 2651. https://doi.org/10.3390/ijms22052651







