Abstract
Scilla species are used as medicinal plants and contain lanosterol-type triterpene glycosides. The phytochemical investigation of the bulbs of Scilla peruviana led to the isolation of 17 compounds, including three new rearranged pentacyclic-lanosterol glycosides (1–3) and two new homoisoflavanone glycosides (12 and 13). The structures of the undescribed compounds were determined by extensive spectroscopic analyses, including two-dimensional (2D) NMR. Among the triterpene glycosides, 2, 3, and 6 showed significant pancreatic lipase inhibitory activity in a concentration-dependent manner in vitro. The oral administration of scillascilloside D-2 (6) reduced serum triglyceride levels in a dose-dependent manner in soybean oil-loaded mice.
1. Introduction
Scilla, Eucomis, Chionodoxa, and Muscari species belong to the Asparagaceae (formerly Liliaceae) family and are rich sources of lanosterol-type triterpene glycosides [1,2,3,4]. Although the bulbs of Scilla scilloides (Lindl.) Druces, which contain homoisoflavones and triterpenes, has been used as a traditional medicine for the treatment of dermal inflammation [5,6]. S. peruviana L. are widely used as an ornamental plant. Previously, we conducted phytochemical examinations of the bulbs of S. peruviana and characterized two rearranged pentacyclic-lanosterol glycosides, peruvianosides A and B [7,8], a pentacyclic-norlanosterol glycoside, scillascilloside E-1 [9], and four hexacyclic-lanosterol glycosides with a modified spirocyclic side chain, including scillascilloside D-2 and scillasaponin B [10,11]. Peruvianoside A and scillasaponin B were found to show cyclic adenosine monophosphatase (cAMP) phosphodiesterase inhibitory activity [10,11]. We performed further phytochemical analyses of the bulbs of S. peruviana that resulted in the isolation of 17 compounds (1–17), including three undescribed rearranged pentacyclic-lanosterol glycosides (1–3) and two undescribed homoisoflavanone glycosides (12 and 13). The structures of the undescribed compounds were determined by extensive spectroscopic analyses, including two-dimensional (2D) NMR. Recently, some triterpenes and triterpene glycosides have been reported to exhibit potent pancreatic lipase inhibitory activity and reduce serum triglyceride (TG) levels in mice via pancreatic lipase inhibition, such as oleanane-type triterpene bisdesmosides (IC50 81.4–223 μM). Pancreatic lipase plays a key role in the digestion of TG and is one of the targets for anti-obesity treatment [12,13,14]. Our previous study revealed that oral administration of cymbopogonol, a lupine-type triterpene, from Cymbopogon citratus reduced serum TG levels in mice via pancreatic lipase inhibitory activity [15]. On the other hand, homoisoflavanones have not been reported previously as pancreatic lipase inhibitors in natural products polyphenols [16]. In this study, we evaluated the pancreatic lipase inhibitory activity of the isolated lanosterol-type triterpene glycosides (1–11) and the effect of 6 on serum TG levels in soybean oil-loaded mice.
2. Results and Discussion
2.1. Structural Elucidation
The bulbs of S. peruviana (11.2 kg dry weight) were treated with MeOH. After removing the solvent, the MeOH extract (981 g) was applied to a porous-polymer polystyrene resin (Diaion HP-20) column. The MeOH-eluted fraction was passed through column chromatography (CC) on silica gel and octadecylsilanized (ODS) silica gel, producing 4–11 and 14–17. Compounds 4–11 and 14–17 were identified as peruvianoside A (4) [7], peruvianoside B (5) [7], (23S,25R)-3β,31-dihydroxy-17α,23-epoxy-5α-lanost-8-en-23,26-olactone3β-O-[O-α-l-rhamnopyranosyl-(1→2)-O-β-d-gluco-pyranosyl-(1→2)-O-α-l-arabinopyranosyl-(1→6)-O-β-d-glucopyranoside] (scillascilloside D-2) (6) [10], (23S,25R)-3β,30,31-trihydroxy-17α,23-epoxy-5α-lanost-5-en-23,26-olactone3-O-[O-α-l-rhamno-pyranosyl-(1→2)-O-β-d-glucopyranosyl-(1→2)-O-α-l-arabinopyranosyl-(1→6)-O-β-d-glucopyranoside] (7) [10], (23S,25R)-3β,31-dihydroxy-17α,23-epoxy-5α-lanost-8-en-23,26-olactone 3β-O-[O-β-d-glucopyranosyl-(1→3)-O-[α-l-rhamnopyranosyl-(1→2)]-O-β-d-glucopyranosyl-(1→2)-O-α-l-arabinopyranosyl-(1→6)-O-β-d-glucopyranoside] (scillanoside L-2) (8) [17], 15-deoxy-eucosterol3β-[O-β-d-glucopyranosyl-(1→3)-O-[α-l-rhamnopyranosyl-(1→2)]-β-d-glucopyranosyl-(1→2)-O-α-l-arabinopyranosyl-(1→6)-O-β-d-glucopyranoside] (scillascilloside E-1) (9) [9], 15-deoxy-30-hydroxyeucosterol3β-[O-β-d-glucopyranosyl-(1→3)-O-[α-l-rhamnopyranosyl-(1→2)]-β-d-glucopyranosyl-(1→2)-O-α-l-arabinopyranosyl-(1→6)-O-β-d-glucopyranoside] (scillanoside L-1) (10) [17], scillanostaside G (11) [18], (3R)-5,7-dihydroxy-6-methoxy-3-(4′-methoxybenzyl)-chroman-4-one (14) [19], (3R)-5,7-dihydroxy-3-(4′-hydroxybenzyl)-6-methoxy-chroman-4-one (15) [20], and (3S)-6,7-dimethoxy-5-hydroxy-3-(4′-hydroxybenzyl)-chroman-4-one (16) [21], and (3R)-5,7-dihydroxy-3-(4′-methoxybenzyl)-chroman-4-one (17) [22] (Figure 1). This is the first report on the isolation of 8, 10, 11, and 14–17 from S. peruviana.
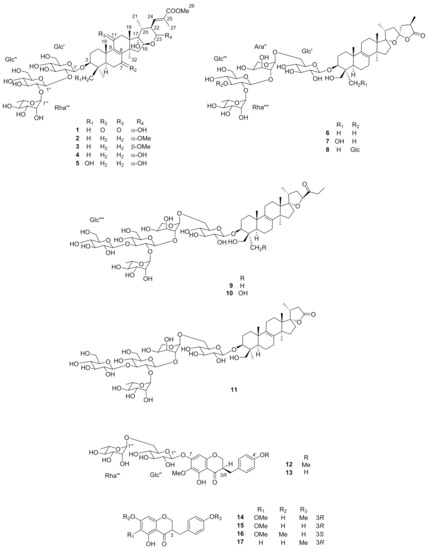
Figure 1.
Isolated compounds from Scilla peruviana.
Compound 1 was obtained as an amorphous solid having the molecular formula C49H74O22, which was determined by high-resolution electrospray ionization time-of-flight mass spectrometry (HR-ESI-TOF-MS, m/z 1037.4563 [M + Na]+, calculated for C49H74NaO22: 1037.4569) and 13C NMR (49 carbon signals) data. The IR spectrum of 1 showed absorption bands of hydroxy groups at 3398 cm−1 and carbonyl groups at 1699, 1670, and 1587 cm−1. The 1H and 13C NMR spectra showed signals assignable to seven methyl groups [δH 1.99 (d, J = 1.2 Hz, Me-27), 1.84 (s, Me-32), 1.37 (s, Me-19), 1.30 (s, Me-30), 1.28 (s, Me-18), 1.16 (s, Me-31), and 1.07 (d, J = 6.9 Hz, Me-21); δC 30.7 (Me-32), 27.9 (Me-30), 21.1 (Me-18), 17.4 (Me-19), 16.5 (Me-31), 13.3 (Me-27), and 11.9 (Me-21)], two olefinic groups [δC 151.2 (C-8) and 151.6 (C-9) and δH 6.96 (dd, J = 10.6, 1.2 Hz, H-24); δC 143.5 (C-24) and 130.3 (C-25)], two oxymethine groups [δH 3.35 (dd, J = 11.8, 4.7 Hz, H-3); δC 88.4 (C-3), and δH 5.10 (dd, J = 7.4, 6.8 Hz, H-16); δC 79.4 (C-16)], an hemiacetalic proton and carbon [δH 5.24 (d, J = 6.5 Hz, H-23); δC 97.6 (C-23)], a tertiary carbon bearing a hydroxy group [δC 82.1 (C-17)], a methyl ester group [δC 168.3 (C-26) and δH 3.71(3H, s); δC 51.6], and three anomeric protons and carbons [δH 6.38 (br s, H-1′′′), 5.83 (d, J = 6.9 Hz, H-1′′), and 4.91 (d, J = 7.7 Hz, H-1′); δC 105.2 (C-1′), 102.1 (C-1′′′), and 101.9 (C-1′′)] (Table 1 and Table 2). These 1H and 13C NMR spectral features of 1 were similar to those of 4 [5], except for the signals arising from the B and C-rings parts of the aglycone moiety. The two methylene signals at δC 26.5 (C-7) and 21.1 (C-11) observed in the 13C NMR spectrum of 4 were displaced by two conjugated carbonyl signals at δC 201.9 (C-7) and 203.0 (C-11) in 1. Furthermore, an olefinic carbon signals of 4 at δC 135.9 (C-8) and 134.6 (C-9) were shifted downfield by 15.3 and 17.0 ppm and observed at δC 151.2 (C-8) and 151.6 (C-9) in 1, respectively. These data indicated that 1 was a 7,11-dioxo derivate of 4. This was confirmed by long-range correlations between H2-12 at δH 3.75 and 2.77 and C-11 at δC 203.0, and between H-5 at δH 1.69/H2-6 at δH 2.60 and 2.30 and C-7 at δC 201.9 in the heteronuclear multiple bond correlation (HMBC) spectrum of 1 (Figure 2). The configuration of the C-23 hydroxy group was confirmed to be α based on the spin-coupling constants of 3JH-20,H-22 = 11.8 Hz, 3JH-22,H-23 = 6.5 Hz, and 3JH-22,H-24 = 10.6 Hz, and NOE correlations between H-23 and H-24/H-20 [10]. Enzymatic hydrolysis of 1 gave d-glucose (Glc) and l-rhamnose (Rha), which were identified by comparison of their refractive index and optical rotations with those of authentic samples using HPLC. The anomeric conformation of the Glc and Rha groups was ascertained by the J values of their anomeric protons, respectively. In the HMBC spectrum of 1, long-range correlations were observed between H-1′′′ of Rha at δH 6.38 and C-2′′ of Glc at δC 78.7, H-1′′ of Glc at δH 5.83 and C-2′ of Glc at δC 78.7, and between H-1′ of Glc at δH 4.91 and C-3 of the aglycone at δC 88.4. Accordingly, 1 was determined to be 7,11-dioxo-peruvianoside A.

Table 1.
1H and 13C NMR (500 and 125 MHz, C5D5N) spectroscopic assignments of the aglycone moieties of 1–3.

Table 2.
1H and 13C NMR (500 and 125 MHz, C5D5N) spectroscopic assignments of the sugar moieties of 1–3.
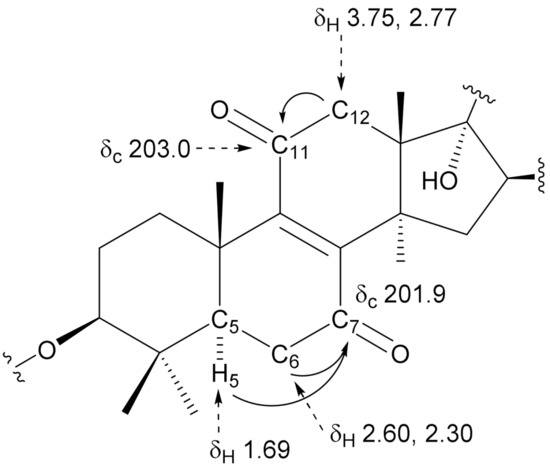
Figure 2.
Key HMBC correlations of the aglycone moiety of 1.
The 1H and 13C NMR spectral features of 2 (C50H80O20) and 3 (C50H80O20) were closely related to those of 4. However, when the 13C NMR spectra of 2 and 3 were compared with that of 4, the signal assignable to the hemiacetal carbon at δC 97.7 (C-23) in 4 was shifted downfield by 12.7 ppm in 2 and 6.2 ppm in 3, and observed at δC 110.4 in 2 and at δC 103.9 in 3. The 1H NMR spectra of 2 and 3 displayed a deshielded methyl singlet signal at δH 3.49 and δH 3.42, respectively (Table 1). In the HMBC spectrum of 2, the methyl singlet at δH 3.49 showed a long-range correlation with C-23 at δC 110.4. Additionally, the methyl singlet at δH 3.42 exhibited an HMBC correlation with C-23 at δC 103.9 in 3. These data indicated that the hemiacetal moiety of 4 was changed to the methyl acetal in 2 and 3. In the NOESY spectrum of 2, NOE correlations between H-23 and H-24 and between H-22 and C23-OMe/Me-27, and a spin-coupling constant of 3JH-22,H-23 = 6.3 Hz showed the configuration at C-23 to be α in 2. On the other hand, NOE correlations between C23-OMe and H-20/H-24 and between H-23 and H-22/Me-27, and a spin-coupling constant of 3JH-22,H-23 = 5.8 Hz gave evidence that 3 was the C-23 epimer of 2. The triglycoside moiety attached to C-3 of the aglycone of 2 and 3 was ascertained to be the same as that of 4 by the analysis of the HMBC spectra of 2 and 3. Therefore, 2 and 3 were identified as 23α-methyl-peruvianoside A and 23β-methyl-peruvianoside A. The 1H NMR spectrum of 12 (C30H38O15) was essentially analogous to that of the homoisoflavanone (14), showing signals for two methylene groups at δH 4.36 (dd, J = 11.5, 4.0 Hz, H-2a) and 4.19 (dd, J = 11.5, 6.5 Hz, H-2b), and δH 3.13 (dd, J = 14.0, 9.0 Hz, H-9a) and 2.72 (dd, J = 14.0, 7.0 Hz, H-9b), a methine proton at δH 2.96 (m, H-3), 1,4-disubstituted aromatic ring at δH 7.16 (d, J = 8.5 Hz, H-2′ and H-6′) and 6.86 (d, J = 8.5 Hz, H-3′ and H-5′), and an aromatic proton at δH 6.31 (s, H-8), and two methoxy groups at δH 3.77 (s) and 3.75 (s) (Table 3). Furthermore, signals for two anomeric protons and carbons were observed at δH 4.96 (d, J = 7.5 Hz); δC 101.7 and δH 4.68 (br s); δC 102.2. The enzymatic hydrolysis of 12 gave 5,7-dihydroxy-6-methoxy-3-(4′-methoxybenzyl)-chroman-4-one (12a) [19] and d-glucose (Glc) and l-rhamnose (Rha) as the carbohydrate moieties. The absolute configuration at C-3 was confirmed to be R because of the CD spectrum [(MeOH, ∆ε): 284 (−1.98) nm], and the specific rotation of 12a almost agreed with that of 14. In the HMBC spectrum of 12, long-range correlations were observed between the anomeric proton (H-1′′′) of Rha at δH 4.68 and C-6′′ of Glc at δC 67.6, and between H-1′′ of Glc at δH 4.96 and C-7 of the aglycone at δC 160.8. Thus, 12 was identified as (3R)-5-hydroxy-6-methoxy-3-(4′-methoxybenzyl)-7-[(O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranosyl)oxy]-chroman-4-one.

Table 3.
1H and 13C NMR (500 and 125 MHz, CD3OD) spectroscopic assignments of 12 and 13.
The 1H and 13C NMR spectra of 13 (C29H36O15) showed a close similarity to those of 12, including the signals attributed to the sugar moiety attached to C-7. However, 13 differed from 12 in the lack of the 1H and 13C signals for the methoxy group at C-4′ [δH 3.77 (s); δC 55.7] (Table 3). Furthermore, the C-4′ signal of 13 was shifted upfield by 1.7 ppm when compared with that of 12. These data implied that the aglycone of 13 corresponded to 15 and that 13 had an O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranosyl group at C-7. Thus, 13 was identified as (3R)-5-hydroxy-6-methoxy-3-(4′-hydroxybenzyl)-7-[(O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranosyl)oxy]-chroman-4-one.
Structures of known compounds 14–17, including their absolute configurations, were determined based on a spectroscopic analysis [19,20,21,22].
2.2. Lipase Inhibitory Activity
Various types of triterpenes and triterpene glycosides have been reported as pancreatic lipase inhibitors and expected to be promising treatments for obesity [12,13,14,16,23]. The pancreatic lipase inhibitory activity of the isolated lanosterol-type triterpene glycosides (1–11) was measured using a commercially available kit (Figure 3 and Table 4). Cetilistat was used as a positive control (IC50 1.43 ± 0.052 μM). As a result, 2, 3, and 6 showed the inhibitory activity in a dose-dependent manner (IC50 0.84 ± 0.029, 0.59 ± 0.020, and 0.81 ± 0.029 mM). Interestingly, 11, a tetranorlanosterol glycoside, inhibited the pancreatic lipase activity in an inverted dose-dependent manner. Of the compounds with the peruvianoside A derivatives (1–5), the modification of the hemiacetal group to the corresponding methyl acetal group (2 and 3) enhanced the lipase inhibitory activity. In the lanosterol derivatives with a spiro-lactone group (6–8), 6 had the most potent lipase inhibitory activity. Next, the effects of 6 on TG levels in ddY mice fed a single-dose administration of a high-fat diet (intralipid) were examined (Figure 4). Compound 6 at 150 or 300 mg/kg was orally administrated to mice, respectively, 15 min before intralipid loading. Serum TG levels were determined at 120 min after intralipid oral treatment. In the control group, serum TG levels increased from 48.3 ± 8.2 to 264.9 ± 32.2 mg/dL following administration of intralipid compared to those of the untreated group. When the mice were treated with cetilistat as a positive control, serum TG levels were held at 130.2 ± 23.4 mg/dL. Although inhibition potency of cetilistat in vivo was weaker than that of the lipase inhibitory activity in vitro (IC50 1.43 μM), the same range was reported in previous works [24,25]. The single oral administration of 6 at 300 mg/kg significantly suppressed the elevation in serum TG levels, with values held at 90.9 ± 16.2 mg/dL in mice.
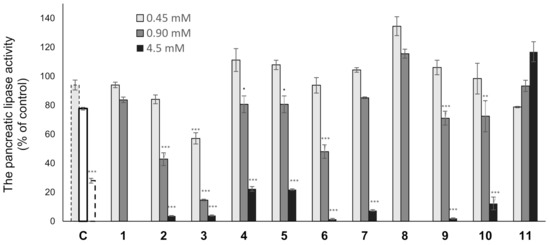
Figure 3.
The pancreatic lipase activity of 1–11. Compounds 1 and 8 (0.45 and 0.90 mM), 2–7 and 9–11 (0.45, 0.90, and 4.5 mM), and cetilistat (c) (0.1 [ ], 1.0 [ ], and 10 [ ] μM); Data are represented as the mean ± S.E.M. of three experiments performed in triplicate; *** p < 0.0001 vs. control, ** p < 0.001 vs. control, ˙ p < 0.05 vs. control.

Table 4.
The pancreatic lipase inhibitory activity of 1–11.
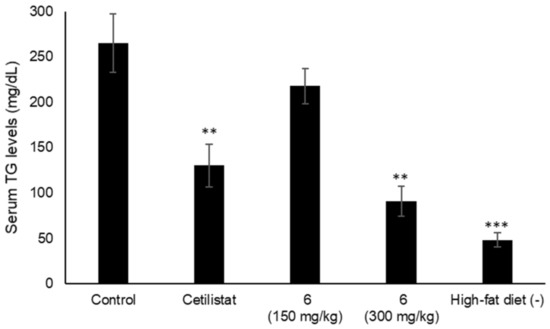
Figure 4.
Effects of 6 on the serum TG levels in mice fed a single-dose administration of a high-fat diet. Compound 6 (150 or 300 mg/kg) and cetilistat (180 mg/kg); Data are represented as the mean ± S.E.M. (n = 5); *** p < 0.0001 vs. control, ** p < 0.001 vs. control.
3β-Hydroxylanosta 9,24-dien-21-oic acid, a lanostane-type triterpene, isolated from Protorhus longifolia, was reported to inhibit the pancreatic lipase activity [26]. However, no previous reports have shown the effect of purified lanostane-type triterpene glycoside on both lipase inhibition in vitro and TG reduction in vivo [27]. This is the first report of the lipase inhibitory activity of lanosterol glycosides. The inhibition of lipase activity might be only one of the possible mechanisms inhibiting triglycerides absorption.
In conclusion, five rearranged pentacyclic-lanosterol glycosides (1–5), three hexacyclic-lanosterol glycosides with a modified spirocyclic side chain (6–8), two pentacyclic-norlanosterol glycosides (9 and 10), a pentacyclic-tetranorlanosterol glycoside (11), a homoisoflavanone glycosides (12 and 13), and four homoisoflavanones (14–17) were isolated from the MeOH extract of S. peruviana bulbs. Their structures were determined by extensive spectroscopic analysis and the results of hydrolytic cleavage. Compounds 1–3, 12, and 13 are undescribed glycosides, and 8, 10, 11, and 14–17 were isolated from S. peruviana for the first time. The pancreatic lipase inhibitory activity of the isolated triterpene glycosides (1–11) was evaluated. Compounds 2, 3, and 6 showed significant lipase inhibitory activity in a dose-dependent manner (IC50 0.84 ± 0.029, 0.59 ± 0.020, and 0.81 ± 0.029 mM) and a single oral dose of 6 at 300 mg/kg reduced serum TG levels in mice loaded with intralipid. It is concluded that 6 suppress elevated serum TG levels partially via lipase inhibition. Further studies are needed to reveal the mechanism of the effects of 6 on the serum TG reduction.
3. Materials and Methods
3.1. General Experimental Procedures
The instruments and experimental conditions were the same as those described in a previous paper [28]. Optical rotations were measured on a JASCO P-1030 automatic digital polarimeter (Jasco). IR spectra were obtained using a JASCO FT-IR 620 spectrophotometer (Jasco). NMR spectra were recorded on a Bruker DRX-500 or AV-600 spectrometer (Bruker) using standard Bruker pulse programs. Chemical shifts (δ) were given with reference to tetramethylsilane (TMS) as an internal standard. HR-ESI-TOF-MS data were obtained using a Water-Micromass LCT mass spectrometer (Waters-Micromass). CC was conducted by Diaion HP-20 (Mitsubishi-chemical), silica gel Chromatorex BW-300 (Fuji-Silysia Chemical), and ODS silica gel COSMOSIL 75C18-OPN (Nacalai Tesque). Analytical TLC was performed on precoated silica gel 60 F254 or RP18 F254S plates (0.25 mm thick) (Merck). The spots were detected by spraying the plates with 10% H2SO4 aqueous solution and then heating. HPLC was conducted using a system consisting of a CCPM pump (Shimadzu), an RI-8021 (Tosoh) or a Shodex OR-2 (Showa-Denko) detector, and a Rheodyne injection port (Rohnert Park). A TSK gel ODS-100Z column (10 mm i.d. × 250 mm, 5 µm) (Tosoh) was used for the preparative HPLC. Enzymatic hydrolysis was carried out using naringinase (EC 232-962-4, Sigma), acetic acid (AcOH) and potassium acetate (AcOK) (Wako).
3.2. Plant Material
The bulbs of Scilla peruviana. (11.2 kg dry weight) were obtained from Takii (Kyoto, Japan) in 2014. The bulbs were cultivated, and the morphological characteristics of the flowering plants allowed us to identify the plant material. A voucher specimen was deposited at the Herbarium of the Tokyo University of Pharmacy and Life Sciences (KS-2014-017).
3.3. Extraction and Isolation
The bulbs of S. peruviana (11.2 kg) were treated with MeOH (50 L) at 50 °C for 2 h. After evaporating the solvent in vacuo, the MeOH extract (981 g) was subjected to a Diaion HP-20 column (2200 g, 85 mm i.d. × 600 mm) and successively partitioned by eluting with MeOH–H2O (3:7; 1:1), MeOH, EtOH, and EtOAc (each 10 L) in order of decreasing polarity. The MeOH-eluted fraction (221 g) was passed through a silica gel CC (2400 g, 42 mm i.d. × 430 mm) eluted with gradient mixtures of CHCl3–MeOH–H2O (20:10:1; 7:4:1) and MeOH, to give 8 fractions (Frs. A-H). Fr. A was separated by ODS silica gel CC (930 g, 32 mm i.d. × 300 mm) eluted with MeOH–H2O (2:1) and silica gel CC (580 g, 22 mm i.d. × 380 mm) eluted with CHCl3–MeOH–H2O (40:10:1) to give 13 (13.1 mg). Fr. C was separated by silica gel CC (1800 g, 80 mm i.d. × 400 mm) eluted with EtOAc–MeOH (2:1; 1:1) and sequentially ODS silica gel CC (700 g, 30 mm i.d. × 250 mm) eluted with MeOH–H2O (2:1; 3:1) and MeCN–H2O (1:2; 2:3) to yield 7 (22.1 mg). Fr. E was fractionated by ODS silica gel CC (930 g, 35 mm i.d. × 300 mm) using MeOH–H2O (2:1; 3:1; 4:1) and MeCN–H2O (1:2; 2:3; 1:1), and preparative TLC using CHCl3–MeOH–H2O (20:10:1) to give 1 (15.5 mg), 4 (16.0 mg), and 10 (43.8 mg). Fr. E-1 was separated by ODS silica gel CC (930 g, 35 mm i.d. × 300 mm) eluted with MeOH–H2O (2:1; 3:1; 4:1) and silica gel CC (430 g, 22 mm i.d. × 280 mm) eluted with CHCl3–MeOH–H2O (70:40:1; 10:10:1) to give 12 (21.8 mg). Fr. F was purified by ODS silica gel CC (930 g, 32 mm i.d. × 320 mm) using MeOH–H2O (2:1; 3:1; 4:1) and MeCN–H2O (1:2; 2:3; 1:1), and preparative HPLC using MeCN–H2O (2:3) to yield 5 (16.8 mg), 6 (59.0 mg) and 8 (15.6 mg). Fr. G was fractionated by ODS silica gel CC (500 g, 22 mm i.d. × 330 mm) using MeCN–H2O (1:2; 2:3) and silica gel CC (450 g, 22 mm i.d. × 280 mm) eluted with CHCl3–MeOH–H2O (10:10:1) to give 2 (69.3 mg), 3 (56.1 mg), 9 (25.0 mg) and 11 (50.8 mg). The EtOH-eluted fraction (5.4 g) passed through a silica gel CC (1000 g, 32 mm i.d. × 320 mm) eluted with gradient mixtures of hexane–EtOAc (3:1; 1:1) and MeOH, to give 7 fractions (Frs. a–g). Fr. d was fractionated by ODS silica gel CC (280 g, 17 mm i.d. × 300 mm) using MeOH–H2O (5:2; 7:1) and preparative HPLC using MeOH–H2O (4:1) to give 14 (7.4 mg), 15 (42.3 mg), 16 (74.2 mg), and 17 (9.7 mg).
3.4. Structural Characterization
Compound 1: An amorphous solid; [α]D25 −10.1 (c 0.27, MeOH); IR νmax (film) cm−1: 3398 (OH), 2963 (CH), 1699 (C=O ester), 1670 (C=O ketone and C=C), 1587 (C=O ketone); UV λmax (MeOH) nm (log ε): 264 (3.98) (C=O ketone), 204 (4.26) (C=O ester). 1H and 13C NMR (500 and 125 MHz, C5D5N), see Table 1 and Table 2; HR-ESI-TOF-MS (m/z: 1037.4563 [M + Na]+, calculated for C49H74NaO22: 1037.4569).
Enzymatic hydrolysis of 1: Compound 1 (4.9 mg) was treated with naringinase (80 mg) in AcOH/AcOK buffer (pH 4.3, 4.0 mL) for 24 h. The crude hydrolysate was subjected to Diaion HP-20 CC (6 mm i.d. × 200 mm) eluted with MeOH–H2O (3:7), Me2CO–EtOH (1:1), and MeOH to yield the sugar fraction (0.31 mg). The aglycone moiety of 1 was decomposed. The sugar fraction of 1 was analyzed by HPLC under the following conditions: Capcell Pak NH2 UG80 column (4.6 mm i.d.× 250 mm, 5 μm, Shiseido), mobile phase of MeCN–H2O (7:3), detection by refractive index and optical rotation, and flow rate of 1.0 mL/min. d-Glucose and l-rhamnose were identified by comparing their retention times and optical rotation with those of authentic samples. tR (min): 16.85 (d-glucose, positive optical rotation) and 7.97 (l-rhamnose, negative optical rotation).
Compound 2: An amorphous solid; [α]D25 +10.4 (c 0.17, MeOH); IR νmax (film) cm−1: 3390 (OH), 2935 (CH), 1699 (C=O ester), 1648 (C=C); UV λmax (MeOH) nm (log ε): 219 (4.37) (C=C), 205 (4.28)(C=O ester); 1H and 13C NMR (500 and 125 MHz, C5D5N), see Table 1 and Table 2; HR-ESI-TOF-MS (m/z: 1023.5141 [M + Na]+, calculated for C50H80NaO20: 1023.5141).
Compound 3: An amorphous solid; [α]D25 −31.1 (c 0.36, MeOH): IR νmax (film) cm−1: 3382 (OH), 2927 (CH), 1715 (C=C), 1643 (C=O ester); UV λmax (MeOH) nm (log ε): 220 (4.42) (C=C), 206 (4.42) (C=O ester); 1H and 13C NMR (500 and 125 MHz, C5D5N), see Table 1 and Table 2; HR-ESI-TOF-MS (m/z: 1023.5140 [M + Na]+, calculated for C50H80NaO20: 1023.5141).
Compound 12: An amorphous solid; [α]D25 −13.1 (c 0.05, MeOH): IR νmax (film) cm−1: 3413 (OH), 2922 (CH), 1730(C=O), 1590, 1456 (aromatic rings); UV λmax (MeOH) nm (log ε): 284 (3.72), 205 (C=O ester); CD λmax (MeOH) nm (∆ε): 282 (−1.80); 1H and 13C NMR (500 and 125 MHz, CD3OD), see Table 3; HR-ESI-TOF-MS (m/z: 661.2119 [M + Na]+, calculated for C30H38NaO15: 661.2108).
Enzymatic hydrolysis of 12: Compound 12 (4.0 mg) was subjected to enzymatic hydrolysis with naringinase (80 mg) as described for 1 to give 12a (2.0 mg) and a sugar fraction (1.2 mg). The HPLC analysis of the sugar fraction under the same conditions as in the case of 1 showed the presence of d-glucose and l-rhamnose. tR (min): 12.71 (d-glucose, positive optical rotation) and 7.83 (l-rhamnose, negative optical rotation).
Compound 12a: An amorphous solid; [α]D25 −32.8 (c 0.05, MeOH): CD λmax (MeOH) nm (∆ε): 284 (−1.98); 1H NMR (500 MHz, CD3OD) δH 7.15 and 6.86 (each 2H, d, J = 8.2 Hz), 5.92 (s), 4.30 (dd, J = 11.4, 4.2 Hz), 4.15 (dd, J = 11.4, 7.0 Hz), 3.76 (s), 3.71 (s), 3.12 (dd, J = 13.8, 4.3 Hz), 2.83 (m), 2.70 (dd, J = 13.8, 10.5 Hz); HR-ESI-TOF-MS (m/z: 353.1005 [M + Na]+, calculated for C18H18NaO6: 353.1001).
Compound 13: An amorphous solid; [α]D25 −1.2 (c 0.20, MeOH): IR νmax (film) cm−1: 3411 (OH), 2924 (CH), 1740(C=O),1626, 1539 (aromatic rings); UV λmax (MeOH) nm (log ε): 285 (3.64), 205 (C=O ester); CD λmax (MeOH) nm (∆ε): 271 (−0.01); 1H and 13C NMR (500 and 125 MHz, CD3OD), see Table 3; HR-ESI-TOF-MS (m/z: 647.1954 [M + Na]+, calculated for C29H36NaO15: 647.1952).
3.5. Lipase-Inhibitory Activity
The pancreatic lipase activity of each test sample was measured by a modified method using a commercially available kit (Lipase Kit S, Dainippon Pharmaceutical). The purities of test samples were confirmed by HPLC analysis (Figure S6 in Supplementary Materials). Cetilistat was used as a positive control at 0.1, 1.0, and 10 μM. Test sample was dissolved in EtOH–H2O (1:1) and diluted to concentrations of 0.45, 0.90, and 4.5 mM, respectively. Lipase (lipase from porcine pancreas, TYPE II, 100–500 units/mg) was purchased from Sigma-Aldrich. Briefly, test sample was mixed with enzyme buffer, and incubated for 5 min at 30 °C. After incubation, 2,3-dimercapto-1-propanol tributyrate (BALB) as substrate was added and the enzyme reaction was proceeded for 30 min at 30 °C. Lipase activity was determined by measuring the absorbance of the resulting 5-thio-2-nitrobenzoate anion at 412 nm using a microplate reader. The inhibition rate was calculated by the following formula: Inhibition rate (%) = [1 − (ΔAsample − ΔAblank)/(ΔAcontrol − ΔAblank)] × 100. The inhibitory concentration (IC50) was calculated by log-Probit analysis.
3.6. Animals
Male ddY mice, 5 weeks old, were purchased from Japan Laboratory Animals and maintained in the animal facility of the Tokyo University of Pharmacy and Life Sciences. The animals were housed in cages under controlled temperature (23 ± 2 °C) and a 12 h light-dark cycle. The mice were allowed free access to breeding food and water. All experiments were approved by the Tokyo University of Pharmacy and Life Sciences Animal Use Committee (approval number: P17-84, date of approval: 24 April 2017).
3.7. Measurement of Triglyceride in Animals
After 5 weeks, mice were fasted overnight and randomly divided into five groups (n = 5). The body weight of each mouse was measured (24.0–30.7 g/body). 0.5% Carboxymethyl cellulose (CMC; Wako Pure Chemical Industries), cetilistat (Tokyo Kasei, purity > 98.0%; 180 mg/kg), or compound 6 (150 or 300 mg/kg) were prepared for test samples and the intralipid (soybean oil 20% emulsion, 20 mL/kg, Sigma-Aldrich) was used for the high-fat diet. Single dose of 0.5% CMC, cetilistat, and compound 6 (150 or 300 mg/kg) were orally administered to mice in each group (n = 5) 15 min before the intralipid loading. Blood samples were collected from the abdominal vena cava 120 min after the intralipid-loaded treatment and centrifuged (700× g for 10 min) to obtain serum samples. The TG levels in serum were measured using a commercially available kit (Wako Triglyceride E-Test kit, Wako Pure Chemical Industries).
3.8. Statistical Analysis
Data are represented as the mean ± standard error of the mean (S.E.M.) of three experiments performed in triplicate. Dunnett’s test was used for statistical analysis, and the level of significance is indicated by p values. All statistical analyses were performed with R (R version 3.2.4).
Supplementary Materials
The supplementary materials can be found online at https://www.mdpi.com/1422-0067/22/3/1262/s1.
Author Contributions
Conceptualization, Y.M. (Yukiko Matsuo), N.I., and Y.M. (Yoshihiro Mimaki); methodology, Y.M. (Yukiko Matsuo). N.I. and Y.M. (Yoshihiro Mimaki); software, Y.M. (Yukiko Matsuo); formal analysis, N.I.; investigation, A.Y., K.O., M.N., and H.T.; resources, Y.M. (Yoshihiro Mimaki); data curation, Y.M. (Yukiko Matsuo). and N.I.; writing—original draft preparation, Y.M. (Yukiko Matsuo) and N.I.; writing—review and editing, Y.M. (Yukiko Matsuo), N.I., and Y.M.; visualization, N.I.; supervision, N.I.; project administration, N.I. and Y.M. (Yoshihiro Mimaki); funding acquisition, Y.M. (Yukiko Matsuo). All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported in part by the Japan Society for the Promotion of Sciences (JSPS) KAKENHI Grant Numbers JP16K18901.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ori, K.; Kuroda, M.; Mimaki, Y.; Sakagami, H.; Sashida, Y. Norlanostane and lanostane glycosides from the bulbs of Chionodoxa luciliae and their cytotoxic activity. Chem. Pharm. Bull. 2003, 51, 92–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuroda, M.; Mimaki, Y.; Ori, K.; Koshino, H.; Nukada, T.; Sakagami, H.; Sashida, Y. Lucilianosides A and B, two novel tetranor-lanostane hexaglycosides from the bulbs of Chionodoxa luciliae. Tetrahedron 2002, 58, 6735–6740. [Google Scholar] [CrossRef]
- Ori, K.; Kuroda, M.; Mimaki, Y.; Sakagami, H.; Sashida, Y. Lanosterol and tetranorlanosterol glycosides from the bulbs of Muscari paradoxum. Phytochemistry 2003, 64, 1351–1359. [Google Scholar] [CrossRef]
- Lee, H.B.; Lee, S.M. Antimicrobial activity of eucosterol oligosaccharides isolated from bulb of squill (Scilla scilloides). Pharmacol. Pharm. 2013, 4, 110–114. [Google Scholar] [CrossRef]
- Ono, M.; Toyohisa, D.; Morishita, T.; Horita, H.; Yasuda, S.; Nishida, Y.; Nohara, T. Three new nortriterpene glycosides and two new triterpene glycosides from the bulbs of Scilla scilloides. Chem. Pharm. Bull. 2011, 59, 1348–1354. [Google Scholar] [CrossRef][Green Version]
- Nishida, Y.; Sugahara, S.; Wada, K.; Toyohisa, D.; Tanaka, T.; Ono, M.; Yasuda, S. Inhibitory effects of the ethyl acetate extract from bulbs of Scilla scilloides on lipoxygenase and hyaluronidase activities. Pharm. Biol. 2014, 52, 1351–1357. [Google Scholar] [CrossRef]
- Mimaki, Y.; Ori, K.; Sashida, Y.; Nikaido, T.; Song, L.G.; Ohmoto, T. Peruvianosides A and B, novel triterpene glycosides from the bulbs of Scilla peruviana. Bull. Chem. Soc. Jpn. 1993, 66, 1182–1186. [Google Scholar] [CrossRef]
- Mimaki, Y.; Ori, K.; Sashida, Y.; Nikaido, T.; Song, L.G. Peruvianoside A, a novel migrated lanostane trisaccharide from Scilla peruviana. Chem. Lett. 1992, 10, 1999–2000. [Google Scholar] [CrossRef]
- Sholichin, M.; Miyahara, K.; Kawasaki, T. Oligoglycosides of spirocyclic nortriterpenoids related to eucosterol. Chem. Pharm. Bull. 1985, 33, 1756–1759. [Google Scholar] [CrossRef][Green Version]
- Mimaki, Y.; Nishino, H.; Ori, K.; Kuroda, M.; Matsui, T.; Sashida, Y. Lanosterol oligosaccharides from the plants of the subfamily Scilloideae and their antitumor-promoter activity. Chem. Pharm. Bull. 1994, 42, 327–332. [Google Scholar] [CrossRef]
- Mimaki, Y.; Ori, K.; Kubo, S.; Sashida, Y.; Nikaido, T.; Song, L.G.; Ohmoto, T. Scillasaponins A, B, and C, new triterpenoid oligosaccharides from the plants of the subfamily Scilloideae. Chem. Lett. 1992, 21, 1863–1866. [Google Scholar] [CrossRef]
- Morikawa, T.; Xuezheng, L.; Nishida, E.; Nakamura, S.; Ninomiya, K.; Matsuda, H.; Yoshikawa, M. Acylated oleanane-type triterpene bisdesmosides: Perennisaponins G, H, I, J, K, L, and M with pancreatic lipase inhibitory activity from the flowers of Bellis perennis. Helv. Chim. Acta 2010, 93, 573–586. [Google Scholar] [CrossRef]
- Jang, D.S.; Lee, G.Y.; Kim, J.; Lee, Y.M.; Kim, J.M.; Kim, Y.S.; Kim, J.S. A new pancreatic lipase inhibitor isolated from the roots of Actinidia arguta. Arch. Pharmacal Res. 2008, 31, 666–670. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, W.; Han, L.; Koike, K. Pancreatic lipase-inhibiting triterpenoid saponins from Gypsophila oldhamiana. Chem. Pharm. Bull. 2007, 55, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Kubota, A.; Masimo, R.; Fukaya, H.; Inaba, N.; Mimaki, Y. Cymbopogonol from Cymbopogon citratus and its biological activity. Shoyakugaku Zasshi 2017, 71, 98–99. [Google Scholar]
- de la Garza, A.L.; Milagro-Yoldi, F.I.; Boque, N.; Campión-Zabalza, J.; Martinez, J.A. Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Med. 2011, 77, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Chun, H.K.; Lee, C.H.; Min, B.S.; Lee, E.S.; Kho, Y.H. Eucosterol oligoglycosides isolated from Scilla scilloides and their anti-tumor activity. Chem. Pharm. Bull. 2002, 50, 1245–1249. [Google Scholar] [CrossRef]
- Ono, M.; Takatsu, Y.; Ochiai, T.; Yasuda, S.; Nishida, Y.; Tanaka, T.; Okawa, M.; Kinjo, J.; Yoshimitsu, H.; Nohara, T. Two new nortriterpenoid glycosides and a new phenylpropanoid glycoside from the bulbs of Scilla scilloides. Chem. Pharm. Bull. 2012, 60, 1314–1319. [Google Scholar] [CrossRef][Green Version]
- Crouch, N.R.; Bangani, V.; Mulholland, D.A. Homoisoflavanones from three South African: Scilla species. Phytochemistry 1999, 51, 943–946. [Google Scholar] [CrossRef]
- Adinolfi, M.; Lanzetta, R.; Laonigro, G.; Parrilli, M.; Breitmaier, E. 1H and 13C chemical shift assignments of homoisoflavanones. Magn. Reson. Chem. 1986, 24, 663–666. [Google Scholar] [CrossRef]
- Mutanyatta, J.; Matapa, B.G.; Shushu, D.D.; Abegaz, B.M. Homoisoflavonoids and xanthones from the tubers of wild and in vitro regenerated Ledebouria graminifolia and cytotoxic activities of some of the homoisoflavonoids. Phytochemistry 2003, 62, 797–804. [Google Scholar] [CrossRef]
- Morales-Serna, J.A.; Jiménez, A.; Estrada-Reyes, R.; Marquez, C.; Cárdenas, J.; Salmón, M. Homoisoflavanones from Agave tequilana weber. Molecules 2010, 15, 3295–3301. [Google Scholar] [CrossRef] [PubMed]
- Lunagariya, N.A.; Patel, N.K.; Jagtap, S.C.; Bhutani, K.K. Inhibitors of pancreatic lipase: State of the art and clinical perspectives. EXCLI J. 2014, 13, 897–921. [Google Scholar] [PubMed]
- Karu, N.; Reifen, R.; Kerem, Z. Weight gain reduction in mice fed Panax ginseng saponin, a pancreatic lipase inhibitor. J. Agric. Food Chem. 2007, 55, 2824–2828. [Google Scholar] [CrossRef]
- Yamada, Y.; Kato, T.; Ogino, H.; Ashina, S.; Kato, K. Cetilistat (ATL-962), a novel pancreatic lipase inhibitor, ameliorates body weight gain and improves lipid profiles in rats. Horm. Metab. Res. 2008, 40, 539–543. [Google Scholar] [CrossRef]
- Mosa, R.A.; Naidoo, J.J.; Nkomo, F.S.; Mazibuko, S.E.; Muller, C.J.F.; Opoku, A.R. In vitro anti-hyperlipidemic potential of triterpenes from stem bark of Protorhus longifolia. Planta Med. 2014, 80, 1685–1691. [Google Scholar]
- Jeepipalli, S.P.; Du, B.; Sabitaliyevich, U.Y.; Xu, B. New insights into potential nutritional effects of dietary saponins in protecting against the development of obesity. Food Chem. 2020, 318, 126474. [Google Scholar] [CrossRef]
- Matsuo, Y.; Takaku, R.; Mimaki, Y. Novel steroidal glycosides from the bulbs of Lilium pumilum. Molecules 2015, 20, 16255–16265. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).