Abstract
5-hydroxytryptamine type 3 (5-HT3) receptors are ligand gated ion channels, which clearly distinguish their mode of action from the other G-protein coupled 5-HT or serotonin receptors. 5-HT3 receptors are well established targets for emesis and gastrointestinal mobility and are used as adjunct targets in treating schizophrenia. However, the distribution of these receptors is wider than the nervous system and there is potential that these additional sites can be targeted to modulate inflammatory and/or metabolic conditions. Recent progress in structural biology and pharmacology of 5-HT3 receptors have provided profound insights into mechanisms of their action. These advances, combined with insights into clinical relevance of mutations in genes encoding 5-HT3 subunits and increasing understanding of their implications in patient’s predisposition to diseases and response to the treatment, open new avenues for personalized precision medicine. In this review, we recap on the current status of 5-HT3 receptor-based therapies using a biochemical and physiological perspective. We assess the potential for targeting 5-HT3 receptors in conditions involving metabolic or inflammatory disorders based on recent findings, underscoring the challenges and limitations of this approach.
1. Introduction
Isolation of serotonin (sero = serum and tonin = vasoconstrictive) by Rapport [1] and the confirmation that the compound “enteramine” isolated by Erspamer was the same compound and actually 5-hydroxytryptamine (5-HT) [2] opened up the field of serotonin research. Initially two different types of 5-HT receptors were characterized as D and M receptors in guinea pig ileum, where D refers to receptors blocked by dibenzyline and M refers to those blocked by morphine found mainly in muscle and nervous tissue, respectively [3]. Radioligand binding studies, over the next 20 years or so, revealed that the situation was more complex and that further subtypes of 5-HT receptors existed. During the mid-1980s, 5-HT receptors were classified into three categories, 5-HT1 receptors containing some D receptors and 5-HT1 binding sites, 5-HT2 receptors mainly D receptors and 5-HT3 receptors being equivalent to M receptors [4] and then the 5-HT3 receptor was found to be an ion channel [5].
Now it is known that there are seven classes of 5-HT receptors and that M or 5-HT3 receptor are unique as ion channels and are distinct from the others, which are G protein coupled receptors [6,7,8]. The 5-HT3 receptor is a member of the cysteine loop ligand gated ion channel family. The receptor is arranged as an assembly of five subunits around a central pore [9] and carries cations (e.g., sodium, potassium, and calcium ions) into cells [5]. More recent studies have revealed that several different 5-HT3 receptor subunits (A, B, C, D and E) are expressed in humans [10,11,12,13]. Since the differential subunit composition of a 5-HT3 receptor can alter its properties, we refer to the 5-HT3 receptor of undefined subunit permutation as 5-HT3 receptors for simplicity. However, the presence of the A subunit is essential for the formation of functional plasma membrane ion channels [10,14], and only 5-HT3A subunits can form a functional homopentamer. A schematic outline of several major events in the study of 5-HT3 receptors is shown in Figure 1. Here, we first briefly review 5-HT3 receptor distribution, function and 5-HT3 receptor antagonists currently used in the clinic. The focus of this review is to discuss how recent structural insights combined with enhanced understanding of the role of 5-HT3 receptors, contribute to potential novel therapeutic directions in inflammation and metabolic disorders.
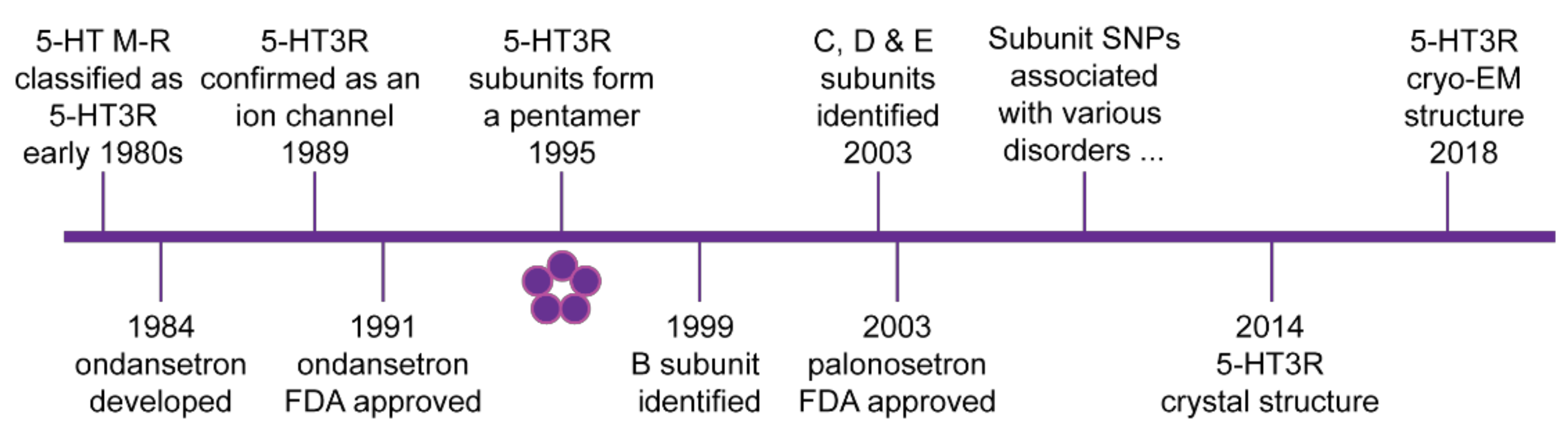
Figure 1.
Schematic timeline of major discoveries in 5-HT3 receptor biology. 5-HT3 receptors (5-HT3R) are cysteine loop ligand gated ion channels formed by a pentamer arrangement of subunits as shown by the five circles. Initial clinical approval by the US Food and Drug Administration (FDA) of the first generation 5-HT3 receptor antagonist ondansetron and the second-generation antagonist palonosetron are also indicated. From the early 2000s, several single nucleotide polymorphisms (SNPs) and other polymorphisms have been associated with various clinical disorders. Further details are outlined in the text.
2. Distribution of 5-HT3 Receptors
Since this is a well-documented and reviewed area (e.g., [8,15,16,17,18,19,20,21,22]), we will briefly describe the distribution of 5-HT3 receptors throughout the body focusing on known and potential target regions and the potential contribution of the different subunits. As only the A subunit was initially identified, earlier studies merely report its expression. Discovery of the different subunits revealed their distribution across human body organ systems [11,13,23]. Some selective subunit localization is evident in humans and other species. Most notably, rodents only contain A and B subunits [14] and differ in other aspects of their cellular function and physiology to humans [24,25,26].
Autoradiography mapping studies using radiolabeled 5-HT3 receptor antagonists such as granisetron revealed high density of 5-HT3 receptors in human brainstem including nucleus tractus solitarius, area postrema, and dorsal motor nucleus of the vagus nerve [27,28,29]. All these areas are important for starting and coordinating the vomiting reflex and are thus likely to contribute to the anti-emetic effects of 5-HT3 receptor antagonists [15,30]. 5-HT3 receptors also occur in the medulla oblongata, hippocampus, caudate and putamen [31,32,33]. Heteromeric expression of 5-HT3 receptors [34,35] is likely throughout the human brain correlating with the widespread distribution of transcripts of the different subunits reported in brain regions [13,14]. Postsynaptic 5-HT3 receptors modulate fast excitatory synaptic transmission when activated while the presynaptic 5-HT3 receptors modulate neurotransmitter release indicating an interconnected and complex network [15] that, at least in the mouse model, modulates cerebellum synaptic plasticity [36] in cellular events involved in cortical construction [37]. Distribution patterns of 5-HT3 receptors have encouraged the consideration of 5-HT3 receptor antagonists generally as adjunct therapies in treating various neurological and psychiatric disorders [18,38]. Of note, mutations in genes encoding different 5-HT3 subunits have been associated with the occurrence of bipolar affective disorder, depression, and schizophrenia, and may be of clinical relevance in predicting the effectiveness of their treatment (Table 1).

Table 1.
Summary of disorders related to mutations of HTR3 genes encoding 5-HT3 receptor subunits 1.
5-HT3 receptor subunit expression occurs in the enteric nervous system and other regions of the gut, and they are particularly known for their involvement in brain-gut circuitry. Gene transcripts encoding 5-HT3 receptor subunits have been detected in the human colon [13,14,105,106,107,108] and the stomach [109]. Unexpectedly, there was a spatial differential in the tissue distribution of the E subunit being only present in the mucosal layers [108] and a single nucleotide polymorphism in the gene encoding E subunit is associated with diarrhea predominant irritable bowel syndrome [42]. Other polymorphisms in the gene encoding C subunit are also useful in predicting treatment response [97] (Table 1). Interestingly, the expression level of 5-HT3 receptors in intestinal mucosa of patients with diarrhea predominant irritable bowel syndrome is significantly higher than those of healthy subjects, thus 5-HT3 receptors may be involved in the pathogenesis of this disease and act as a potential target for intervention [97,110].
5-HT3 receptors in the gastrointestinal tract modulate physiological responses such as gastric emptying, colonic peristalsis and transit [111,112,113]. Thus, 5-HT3 receptor antagonists are used to treat diarrhea predominant irritable bowel syndrome [22,114,115] in addition to chemotherapy-induced nausea and vomiting (CINV) or post-operative vomiting (POV) [30,116,117]. Genetic polymorphisms in genes for 5-HT3B, 5-HT3C, and 5-HT3D, predominantly expressed in the gastrointestinal tract and various brain regions [13,14], may contribute to facilitating individual risk predictions (Table 1). 5-HT3 receptor expression has been detected in myenteric and submucosal plexus of human colon and rectum by autoradiography [118] and immunohistochemical [119,120] methods.
As this is discussed in detail in a later section, we only highlight here that 5-HT3 receptors are expressed in various cells of the immune system. Mainly, the 5-HT3 receptor A subunit has been reported in monocytes, chondrocytes, T-cells, B-cells, synovial tissue, platelets, dendritic cells [121,122,123,124,125,126,127] and is overexpressed in lymphomas [127]. The presence of 5-HT3 receptors is likely to reflect immune challenge as there is a significant increase of the A receptor subunit in peripheral blood mononuclear cells from asthmatic patients and those exposed to air pollution [128] leading to inflammatory responses that can potentially be down regulated with 5-HT3 receptor antagonists [121,124]. Similarly, the lack of or reduced expression of 5-HT3 receptors in psoriatic epidermis implies a role for these receptors in proliferation and differentiation of keratinocytes [129], one of the first lines of immune defense. Since 5-HT3 receptors are expressed in basal epidermal cells, particularly in acrosyringium and in the epithelium of hair follicles [130], they may be targeted for the treatment of chronic skin diseases and pruritus. 5-HT3 antagonists reduce the severity of serotonin-induced skin itch [131] and neuraxial opioid-induced pruritus [132]. Direct irradiation cell death evoked by serotonin in keratinocytes is mediated through 5-HT3 receptors, thus identifying these as a potential target for ameliorating ionizing radiation damage [133].
5-HT3 receptors are widely distributed in other human body organ systems highlighting the interconnectedness between physiological processes regulated via these receptors. 5-HT3 receptor subunits are found in respiratory, urogenital, renal, and cardiovascular systems [13,14,23]. It is highly likely that further interconnections will be established between systems involving those occurring between gastrointestinal regulation and emetic events [134,135,136], inflammatory bowel disease as well as pain and inflammation in various sites [8,16,17,18,135,137,138].
3. Structural Insights into 5-HT3 Receptor Action
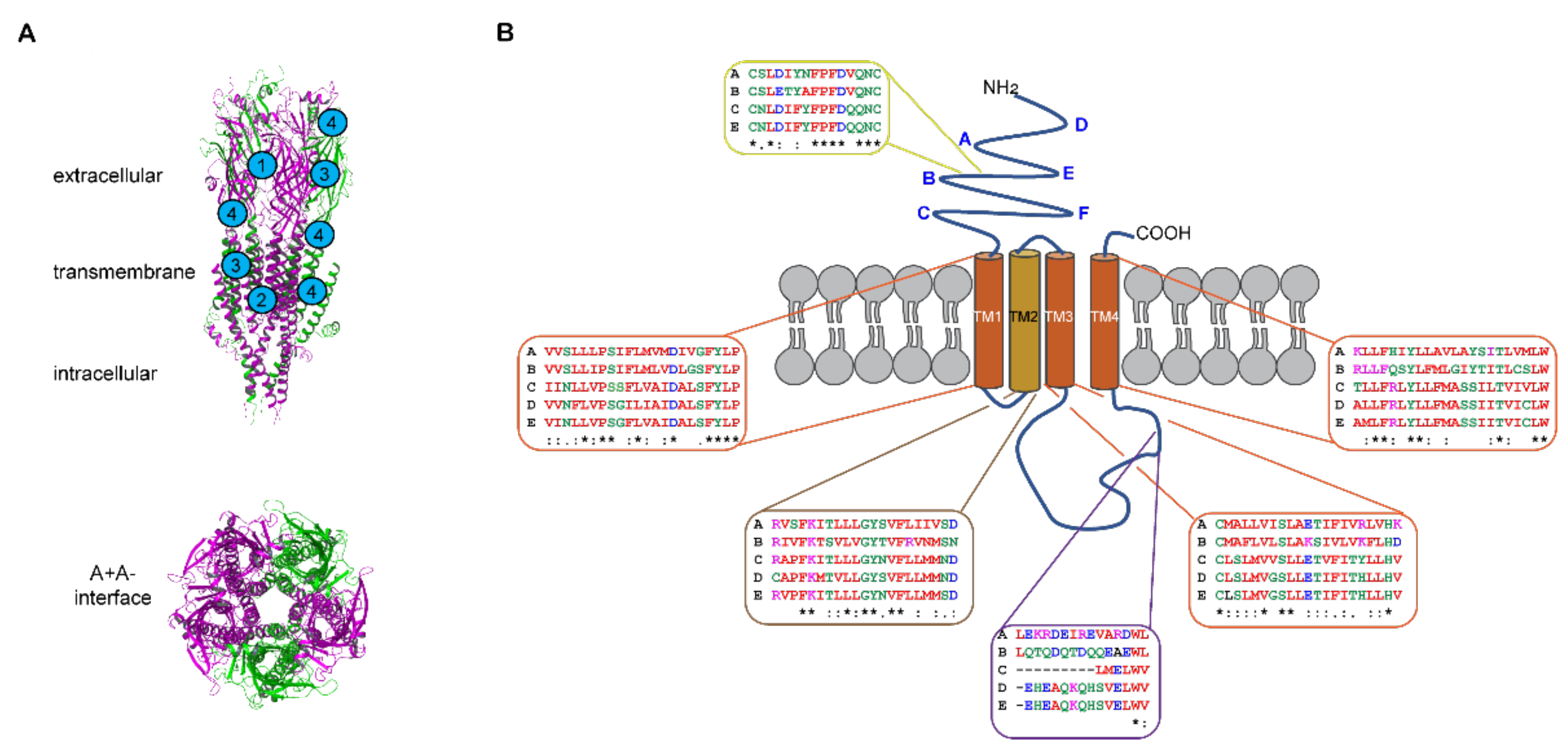
The structure of the mouse homologous 5-HT3A receptor revealed through crystallography [139] and cryo-electron micrography [140,141,142,143,144,145,146] provides considerable insight into 5-HT3 receptors and is reviewed [20,147]. The mouse A subunit shares 95% homology with the human A subunit, so these studies are readily translatable. The 5-HT3 receptor subunits each share a similar architecture and arrange in a pentamer with a central pore (Figure 2). Each subunit contains extracellular N- and C-termini, four transmembrane (TM) spanning α-helical-domains and an intracellular domain. The Cys-loop is located in the N-terminal region of the extracellular domain where cysteine and proline residues contribute to receptor conformation [148,149]. High-resolution structural studies have been particularly revealing about the extracellular and transmembrane domains of the homomeric receptor [140,141,142,143,144,145,146]. The extracellular N-terminal region contains the orthosteric ligand binding site that is created between two A subunits asymmetrically arranged to form the A+A- interface [141,142,143,144,145,146,147] (Figure 2A). The structural studies have, to a large degree, refined and elaborated on the molecular understanding of the orthosteric sites initially characterized through mutagenesis and ligand binding approaches [15,19,150,151]. The three-dimensional structures open up many opportunities to employ additional techniques such as molecular dynamics simulations [141,142,145,146] in conjunction with photo-crosslinking and mass spectrometry to delve further into the dynamics of ligand receptor interactions [152]. The transmembrane domains are arranged asymmetrically so transmembrane domain 2 (TM2) faces the pore and contributes most to ion interactions therein (Figure 2). The pore widens when 5-HT binds at the orthosteric site due to conformational changes twisting transmembrane domain 2 outwards [142,144,146,147].
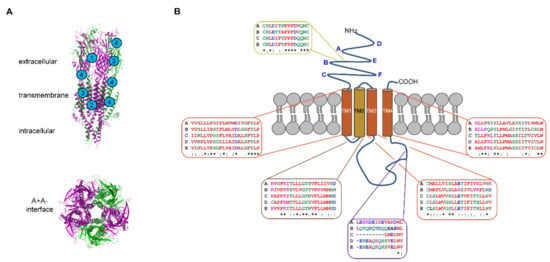
Figure 2.
Architecture of 5-HT3 receptors. (A) Homology model of the human 5-HT3AC receptor assembled in the A3C2 stoichiometry showing membrane spanning side and central pore views (maroon = A and green = C subunits). Examples of proposed binding sites for competitive, non-competitive, and dual acting antagonists (labeled 1–3), and allosteric modulators (indicated with 4). The model is from [153] where A and C subunits were created from the mouse 5-HT3A receptor structure [139] as they have 95% and 58% homology with the mouse A subunit, respectively. (B) Schematic diagram of a single 5-HT3 receptor subunit showing the different domains and extracellular A–F loops (bolded in blue). A Clustal omega alignment was done on the human 5-HT3 receptor subunit protein sequences obtained from NCBI [154] and this is shown for the Cys-loop (yellow box), membrane-spanning α-helices of transmembrane domains 1 to 4 (TM1-TM4) (brown and orange boxes), the triple R region of the intracellular loop involved in pentamer assembly [155] and the low conductance associated with A homomer receptors [156] (purple box). Accession numbers: NP_000860 (A subunit), NP_006019 (B subunit), NP_570126 (C subunit), NP_001157118 (D subunit), and NP_872395 (E subunit). Identical residues are indicated with an asterisk (*), conserved residues with a colon (:), and semi-conserved residues with a full stop (.). Image drawn in Adobe Illustrator.
The large intracellular loop between transmembrane domains 3 and 4 contains an intrinsically disordered domain refractory to structural studies. However, this intracellular domain is critically important in assembling 5-HT3A receptor pentamers [155,157]. This region has greater diversity in amino acid sequences between the different subunits (Figure 2B). The A subunit contains a triplet of arginine residues that are important in regulating conductance [156] and these residues form salt bridges that contribute to pentamer formation [155] possibly forming part of the explanation for the necessity of A subunits in heteromers. The intracellular domain is also necessary for direct interactions with acetylcholine receptor chaperone (RIC3) that enables post-translational trafficking to the plasma membrane [158,159,160,161]. A specific region of the intracellular domain in the A subunit directly interacts with RIC3 [158] but RIC3 binding sites have not yet been identified for the other subunits [161].
5-HT3 receptors are subject to post-translational modifications, including the disulfide bond in the Cys-loop present in all human 5-HT3 subunits, except 5-HT3D (Figure 2B), which impact their structure and function. These modifications include N-glycosylation in the extracellular N-terminus of human 5-HT3A and 5-HT3B subunits (at four and five asparagine residues, respectively) [162] important for cell surface trafficking and stability of the receptors [163,164,165]. Inhibiting glycosylation at any of the N-glycosylation sites reduced or prevented membrane expression of 5-HT3A subunits and interfered with the formation of 5-HT3 receptor binding site. N-glycosylation at N5 is less critical, probably due to its distance from the ligand binding loops (Figure 2B) than modification of N81, N147, and N163 and notably these human 5-HT3A subunit canonical motifs are conserved across species, likely reflecting their significance [163]. Site-directed mutagenesis studies confirmed that the disruption of any N-glycosylation site on human 5-HT3B subunit (N31, N75, N117, N147, and N182) resulted in reduced expression of the subunit in cell membranes, presumably incorporated within heteromeric 5-HT3 receptors [165]. Although N-glycosylation at N145, residing within the Cys-loop (Figure 2B), is conserved in both 5-HT3A and 5-HT3B subunits, and many other ligand-gated ion channels, it seems less important in facilitating 5-HT3B subunit membrane incorporation [165].
Phosphorylation is also a critical post-translational reversible protein modification. Phosphorylation of the large intracellular loop (Figure 2B) likely involves protein kinase A or C effects on ion conductance and rate of desensitization and depends on particular isoforms of 5-HT3A subunits present [166,167,168,169,170]. Effects of agents affecting the actin cytoskeleton together with point mutations of the potential phosphorylation sites suggested that protein kinase C modulates 5-HT3A receptor function and trafficking indirectly, likely via an F-actin-dependent mechanism [169]. Moreover, protein kinase CK2 enhances current flux through 5-HT3 receptor channels [171] while calcineurin—a calcium/calmodulin-dependent phosphatase—does not modulate the fast desensitization of 5-HT3 receptor, it may regulate channel activity via the modulation of the steady-state desensitization [172].
The lipid membrane itself is also involved with 5-HT3 receptors accumulating in lipid rafts—highly dynamic cell membrane microdomains enriched in cholesterol and glycosphingolipids [173]. The impairment of lipid rafts diminishes 5-HT3 receptor currents although only a minor proportion of the 5-HT3 receptors appeared to be constantly present in lipid rafts [174]. Interestingly, removal of cholesterol from the bilayer of large unilamellar vesicles containing homogenously distributed 5-HT3 receptors resulted in the formation of tightly packed patches [175]. Cholesterol binds to and regulates some ligand gated ion channels [176]. Molecular dynamics simulations have revealed sustained interaction between cholesterol and TM domain of 5-HT3A receptors, where the lipid penetrates a preactivated receptor monomer through binding between M1 and M4 helices, with hydrogen bonding between cholesterol and TM4 helix [148]. A lipid-binding pocket lined by TM3 of one subunit and TM1 and TM4 helices of the neighboring subunit, where R309 in TM3 and R435 in TM4 residues interact with the phospholipid has been revealed in 5-HT3 receptors in the absence of the ligand [140]. A further structural basis for the receptor modulation by lipids, together with a model for allosterically lipid-modulated asymmetric activation of the homopentameric 5-HT3 receptor reconstituted into saposin-based lipid bilayer discs, were recently proposed [146]. Apart from enabling the receptor to adopt an asymmetric conformation in the absence of the ligand, the lipid moieties were shown to facilitate a more compact, ‘coupled’ conformation of the receptor, with transmembrane domains compressed along the central axis, allowing free diffusion of sodium ions.
Arrangement of the 5-HT3 receptor heteromers has generated some controversy with earlier atomic force microscopy studies using antisera to tagged subunits suggesting that the AB heteromer had a B-B-A-B-A (A2B3) stoichiometry [177], whereas the consensus of multiple mutagenesis studies indicated that at least one A+A- interface is necessary in the 5-HT3AB receptors [151,178,179], favoring an A-A-B-B-A or A-A-B-A-B (A3B2) conformation. Confirmation of the A3B2 stoichiometry with separated B subunits was obtained using fluorescent proteins inserted into the intracellular loop between transmembrane domains 3 and 4 to obtain fluorescence intensity ratio (FIR) and FRET efficiency measurements [180]. The conformation of heteromers involving the other subunits is unknown but pharmacological studies involving agonist and antagonist binding strongly indicate that an A+A- interface is present like that which occurs with the AB heteromer [178]. Thus, 5-HT3A receptor homomers are likely to have more orthosteric binding sites than heteromers, possibly underscoring pharmacological differences in ligand binding between homomers and heteromers. Sequential ligand binding is proposed for A homomers especially in a lipid environment where cholesterol can contribute allosterically to receptor conformational changes [146,148].
4. Insights from Pharmacology and Electrophysiology Studies
Electrophysiological and pharmacological approaches have provided many insights into the action of the 5-HT3 receptor. The majority of studies use 5-HT3A or 5-HT3AB receptors as the C, D and E subunits in humans have been more recently discovered and are not expressed in all organisms [11,13,14]. 5-HT is the endogenous agonist and several synthetic 5-HT3 receptor agonists incorporating a basic amine, an aromatic ring and hydrogen bond acceptors are mainly used as experimental tools. The potent agonists commonly used in experiments include 2-methyl-5-HT, phenylbiguanide, and meta-chlorophenylbiguanide (m-CPBG) [8,181]. Differences in functional responses to agonists are evident between species [8]. An example involves the functional neural 5-HT3 receptors present along the length of the mouse, rat and guinea pig intestinal tracts that show differences in agonist efficacies between species and in separate regions of the tissue [182,183]. Interest has been generated in partial agonists as potential therapeutic agents and CSTI-300 is one such compound that has recently been characterized [184].
Electrophysiological studies reveal that at least part of the functional differences between species lie in the molecular structure and potential stoichiometry of the channel. 5-HT3 receptors mainly carry sodium and potassium ions following activation by 5-HT to mediate a rapidly activating and desensitizing inward rectifying current, although ion substitution experiments have revealed that 5-HT3 receptors also carry calcium ions [15]. 5-HT3 receptors are homomeric (all A subunits) or heteromeric (mixture of A and either B, C, D, or E subunits) ion channels [8,15,18,19,21,22]. There are variations in the number of subunits between species, as rodents do not express the C, D or E subunits unlike humans and many other species including cats, dogs, and cattle [14]. Therefore, most work has focused on the 5-HT3A or 5-HT3AB receptors [8]. Heterologous system studies have revealed several differences between 5-HT3A and 5-HT3AB receptors [185]. The marked difference in single channel conductance (5-HT3A is <1 pS versus 5-HT3AB at 16–30 pS) is due to the triplet arginine residues present in the portal region of the intracellular loop between the transmembrane domains 3 and 4 of the A subunit that are lacking in the B subunit [156]. The corresponding residues present in the C, D and E subunits differ from either the A or B subunits (Figure 2B). Few studies have been made with the C, D or E subunit and these subunits appear to contribute subtle changes to 5-HT or various agonists relative to the B subunit [14,161,186,187,188]. Distinctive albeit subtly different pharmacological profiles for 5-HT3AC, 5-HT3AD and 5-HT3AE receptors were revealed using partial agonists [187].
All known agonists and competitive antagonists bind at the orthosteric binding site in the extracellular N-terminal region, at the interface between two A subunits [15,19,141,142,143,145,151]. The competitive antagonists tend to be larger than agonists and are mainly represented by the setron family but include morphine and cocaine [3,8,18,22,151]. The first generation setrons including bemestron, tropisetron, granisetron and ondansetron have strong antiemetic activities and generally nanomolar affinity for 5-HT3A receptors [189,190,191,192,193]. Tropisetron also exhibits selective potent partial agonist activity at α7 nicotinic receptors not seen with other 5-HT3 receptor antagonists like ondansetron [194,195] while ondansetron at micromolar concentrations can attenuate solute carrier family 1 member 1 (SLC1A1 formerly known as excitatory amino acid transporter type 3 (EAAT3)) activity [196]. Ondansetron has more recently been shown to activate the ATP binding cassette subfamily A member 1 (ABCA1) activating transcription through the nuclear receptor subfamily 1 group H member 3 pathway (NR1H3) to stimulate apoliproprotein E (ApoE) secretion from astrocytes and liver cells [197]. Picrotoxin and related compounds, such as ginkgolides, block the 5-HT3 receptor channel [179,198]. Palonosetron is a second generation setron that has both competitive and non-competitive antagonistic properties [185,199]. Several ligands have allosteric properties acting at sites spatially distinct from the orthosteric ligand binding site and include anesthetics, n-alcohols, cannabinoids, steroids, as well as terpenes and pungent substances frequently occurring in plants [8,151,200,201,202,203]. Many of these positive or negative allosteric modulators bind to transmembrane sites of 5-HT3 receptors [202,204,205].
Therefore, the ligands can be classified by the site they bind: 1. competitive antagonists (e.g., ondansetron); 2. non-competitive antagonists (e.g., picrotoxin) acting via locations other than the orthosteric binding site; 3. dual acting antagonists (e.g., palonosetron [206,207]), which bind at orthosteric or transmembrane sites although structural studies indicate palonosetron only binds at the orthosteric site [145]; and 4. allosteric modulators that bind at sites distinct from the orthosteric site such as the interface of the extracellular and transmembrane domains [15,19,141,142,143,145,150,151] (Figure 2A). Importantly from an experimental and potentially clinical view (see below), ligands can have different potencies at 5-HT3A versus 5-HT3AB receptors [89,184,185,208,209,210]. For instance, the agonist m-CPBG can bind at all five interfaces of 5-HT3AB distinguishing it from 5-HT, which only binds at the orthosteric binding site at the A+A- interface within the 5-HT3AB receptor enabling allosteric modulation [208]. While VUF10166 has a high affinity for 5-HT3A receptors binding at the A+A- site, its affinity for 5-HT3AB receptors is lower and its effects are thought to be mediated by binding to a secondary A+B- site [211]. Various anticancer drugs inhibit or potentiate current in 5-HT3 receptors, and the degree differs with topotecan, irinotecan and imatinib depending on whether it is a receptor homomer of A subunits or heteromer of A and B subunits [89,212]. Whole-cell patch clamp recordings revealed that the dual acting antagonist palonosetron distinguishes 5-HT3ACE receptors from 5-HT3A, 5-HT3AC and 5-HT3AE receptors [188]. Most studies using C, D or E subunits use diheteromeric receptors in heterologous systems generally showing that these subunits only contribute subtle changes to agonists or antagonists compared to homomeric 5-HT3A receptors [14,153,161,186,187,188].
Since the discovery of additional 5-HT3 receptor subunits and various clinically relevant polymorphisms such as the single nucleotide polymorphism in the B subunit that alters electrophysiological characteristics of the 5-HT3AB receptors [86], there has been an interest in targeting specific 5-HT3 receptor subunits (Table 1). Despite this interest, current progress in this area has been limited in part due to the difficulties involved in selectively targeting the active site of heteromeric receptors. Specific allosteric modulators and improved characterization of the receptor heteromers provide some hope that personalized approaches will eventuate. Future refinement of ligand design potentially can lead to drugs that target homomeric or heteromeric receptors with a degree of specificity that will enable targeted approaches for patients expressing specific polymorphisms (e.g., subunit B variants associated with bipolar affective disorder [49,79], Table 1) or those who have alternate loads of the different 5-HT3 receptor subunits (e.g., patients expressing more C or E subunits).
5. Current Clinically Used 5-HT3 Receptor-Based Therapies
As noted above, both 5-HT3 receptor small molecule antagonists and agonists have been identified, but currently 5-HT3 receptor antagonists dominate since 5-HT3 receptor agonists have major emetogenic effects handicapping their clinical use (Figure 3). 5-HT3 receptor antagonists were initially established for clinical use to treat chemotherapy-induced nausea and vomiting (CINV) in the 1980s as ondansetron outshone the commonly used antiemetic, metoclopramide [213,214,215]. Ondansetron is still a common component in chemotherapy-induced nausea and vomiting therapies [22,30,116,216]. The evolutionary development of this 5-HT3 receptor antagonist class has generated many structural analogs designed to improve selectivity, reduce side effects, and improve ligand pharmacodynamics for delayed emesis compared to acute emesis, bringing additional ‘setron’ ligands, such as granisetron, ramosetron, dolasetron, tropisetron, and the second-generation ligand, palonosetron. These advances have been thoroughly reviewed [8,19,21,22,151,217,218,219]. This drug class possesses excellent antiemetic treatment and prophylaxis effects due to their ability to inhibit serotonin activity on both the central nervous system in the chemoreceptor trigger zone and peripherally on gastrointestinal vagal nerve terminals [22,30,217,218,220]. Over time, their clinical usage has expanded beyond chemotherapy induced emesis to nausea and vomiting provoked by radiation therapy and post-operative procedures in adult and pediatric patients [30,116,136,217] and also pregnancy induced emesis [217].
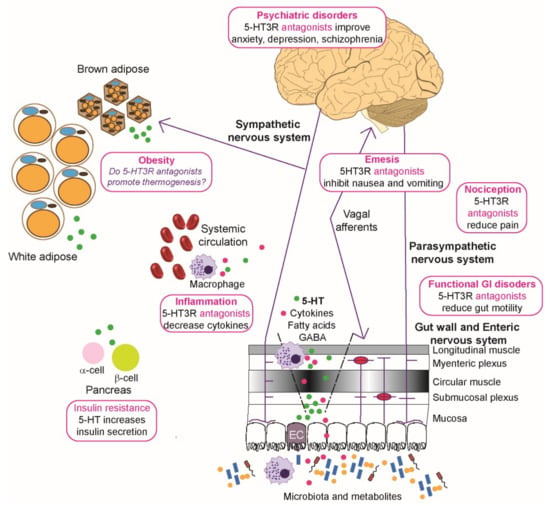
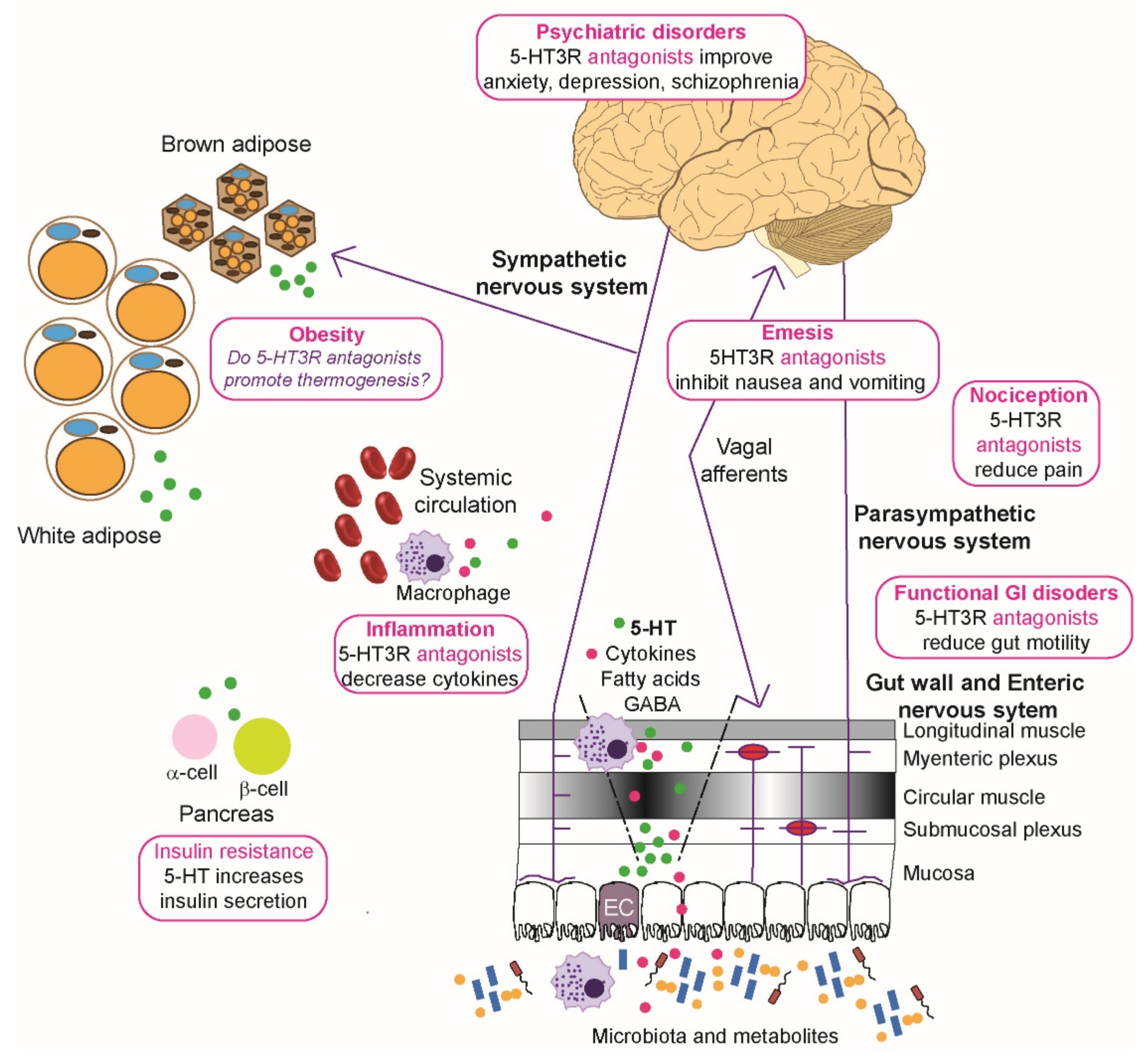
Figure 3.
Roles of 5-HT3 receptors in diseases and metabolic disorders. Microbiota and their metabolites present in the lumen of the gut can affect the epithelial cells and any dendritic cells to modulate local immune function and induce enterochromaffin (EC) cells to secrete serotonin (5-HT, shown as green circles) that enters the circulation. Visceral stimulation via the sympathetic nervous system induces production of serotonin by EC cells. The enteric nervous system, consisting of submucosal plexus underneath the epithelial cells lining, interacts directly with layers of the gut wall and parasympathetic, spinal, and vagal nerves to enable bidirectional communication with the central nervous system. 5-HT release from the ECs activates 5-HT3 receptors on enteric neurons, vagal and spinal afferents, and these in turn relay input to the dorsal root ganglia and the brain stem. Overactivation of the chemoreceptor complex in the brainstem can lead to emesis and the processes also contribute to functional gastrointestinal (GI) disorders, such as irritable bowel syndrome, and nociception. Neuropsychiatric disorders like depression and schizophrenia are affected by disturbed 5-HT3 receptor signaling in the limbic region of the brain. Increased circulatory 5-HT is associated with increases in production of cytokines (shown as magenta circles), and sometimes nociception. In addition, these circulatory changes in 5-HT can potentially modify adipose function where brown adipose cells decrease thermogenesis normally raised by sympathetic activation, although this has mainly been studied using rodent models. By activating 5-HT2 and 5-HT3 receptors, 5-HT in the pancreas enhances ß cells to secrete more insulin, which acts on α cells to inhibit glucagon secretion. Physiological levels of 5-HT released by ß cells in response to glucose also lead to decrease in glucagon secretion and this effect is mediated by 5-HT1 receptor on α cells. For further details see the text (Section 5 and Section 6). Image drawn in Adobe Illustrator with brain image created by Hugh Guiney (CC BY-SA 3.0 https://creativecommons.org/licenses/by-sa/3.0, accessed on 25 October 2021), via Wikimedia Commons (https://commons.wikimedia.org/wiki/File:Human-brain.SVG, accessed on 25 October 2021) and red blood cells (https://commons.wikimedia.org/wiki/File:Blausen_0761_RedBloodCells.png, accessed on 25 October 2021) created by [248].
5-HT and its interaction with multiple types of 5-HT receptors are a critical component of the healthy functional gastrointestinal tract (Figure 3). Increases in intestinal and serum 5-HT levels are associated with the functional disorder, irritable bowel syndrome (IBS) [135,221]. 5-HT3 receptor antagonists (e.g., ondansetron, alosetron) are effective at treating motility problems and abdominal pain associated with diarrhea predominant irritable bowel syndrome but can lead to potential constipation [222]. Since 5-HT levels rise in diarrhea conditions, a new rationale involving use of 5-HT3 receptor partial agonists has been suggested. Selective partial 5-HT3 receptor agonists (e.g., CSTI-300) compete with high levels of endogenous 5-HT but do not completely inhibit the receptor and pre-clinical studies in animal models did not evoke constipation or emesis [184]. Therapies involving CSTI-300 or its analogs are also likely to be beneficial for patients suffering chronic diarrhea and increased circulating 5-HT such as occurs in carcinoid syndrome or diabetes induced diarrhea currently treated with 5-HT3 receptor antagonists [184,223,224,225].
The association of irritable bowel disease with depression and a central nervous component may in part contribute to the effectiveness of 5-HT3 receptor antagonists in treating the diarrhea predominant version [18,226,227,228,229,230]. The relatively few adverse effects of 5-HT3 receptor antagonists combined with their efficacy for treating conditions with a central component prompted investigations into their use in neurological and psychiatric disorders and are extensively reviewed [18,231,232]. 5-HT3 receptor antagonists show most promise in adjunct therapy for treating schizophrenia, perhaps reflective of the 5-HT3 receptor antagonist activity of clozapine [233,234]. Patients with schizophrenia receiving ondansetron in addition to their primary treatment of either haloperidol or risperidone showed improvement of their primary symptoms [232,235,236]. Other neurological conditions, such as obsessive compulsive disorder, may benefit from 5-HT3 receptor antagonist therapy but this requires further exploration [237].
5-HT3 receptor antagonists are also useful in treating alcohol dependence [22,41,238,239,240] but show little clinical usefulness in treating dependencies on other drugs of abuse [18,241]. Since 5-HT3 receptors are present in the reward center [242], this differential is unexpected. One possible contributing factor to the improved effect of 5-HT3 receptor antagonists on alcohol dependence may be due to ethanol acting as an allosteric modulator of 5-HT3 receptors [19,151,243,244,245,246,247].
Since 5-HT is an effective nociceptive inflammatory mediator, interactions between the different receptor classes are complex and this is further complicated by 5-HT3 receptors having a mixed nociceptive responses [249]. Both nociceptive events contributing to chronic pain and antinociceptive effects are mediated by 5-HT3 receptors and influence neuropathic pain in particular [249,250,251]. 5-HT3 receptor antagonists are used in some clinical situations to alleviate chronic pain like fibromyalgia [21,252,253]. The role of 5-HT3 receptor antagonists in the treatment of headaches is more controversial, with some studies reporting efficacy and others recommending against their use [254,255], although they may have roles in combatting nausea and emesis associated with some headaches. Irritable bowel syndrome is associated with abdominal pain and 5-HT3 receptor antagonists moderate the pain relative to placebo [256].
The efficacy of 5-HT3 receptor ligands is complicated by the natural heterogeneity of 5-HT3 receptor complexes and various polymorphisms, which may account for discrepancies in patient responses to treatments. Multiple genomic studies have revealed differences in responses to 5-HT3 ligands and/or clinical disorders that can be related to polymorphisms in individual subunits. Importantly, these findings indicate that there are subtle contributions of the different subunits that can have impacts on function so that, when disturbed via polymorphisms, can lead to different outcomes. For instance, single nucleotide polymorphisms in genes encoding A and B subunits are associated with bipolar affective disorder involving modifications of receptor expression and electrical properties [49,86,257,258]. Relationships between function or disorder and polymorphisms in genes encoding 5-HT3 receptor subunits were previously reviewed [19,21]. Advances since these reviews have continued the strong themes involving different subunits associated with clinical conditions (Table 1) highlighting the potential to target 5-HT3 receptors in neurological and psychiatric disorders as discussed by Fakhouri et al. [18]. Studies of considerable statistical power are required to unlock several of these associations as many polymorphisms are relatively rare and confounding interactions exist. Meta-analyses of separate studies have revealed associations that were previously obscured [44,99], enhancing our understanding of effects of polymorphisms on receptor function that will continue as genomic data becomes a more routine aspect of medicine.
6. 5-HT3 Receptors in Whole Body Metabolism
It should be kept in mind that 5-HT activates several serotonin receptors and there are recent reviews capturing interactions between 5-HT receptors in metabolism [259,260]. This review focuses on the roles of 5-HT3 receptors in energy homeostasis that are being unmasked. Several early studies focused on the interaction between glucose metabolism and centrally located 5-HT3 receptors in the brain. The effects of 5-HT3 receptors on peripheral glucose metabolism have been the subject of some controversy. Early findings using 5-HT3 receptor agonists, such as 2-methyl-5-HT, indicated that 5-HT3 receptors were not involved in glucose metabolism as no change in glucose, glucagon or insulin levels were detected [261]. However, administration of the 5-HT3 receptor agonist m-CPBG to rat brains raised blood glucose levels that could be blocked by ondansetron [262], implicating 5-HT3 receptors in centrally regulated whole body glucose metabolism. Now a consensus is being reached that the role of the 5-HT3 receptors is influenced by the metabolic state and sites of stimuli. The 5-HT3 receptor agonist SR-57227 that acts both centrally and peripherally [263] inhibits food intake by fasting but not fed mice [264], while in rats the effect of 5-HT3 receptor agonists depends on the brain region stimulated [265]. The 5-HT2C receptor particularly, but also the 5-HT1B, 5-HT6 and 5-HT3 receptors, are all involved in regulating satiety at a central level where 5-HT itself interacts with peripheral leptin and cholecystokinin (for reviews see [8,266,267]).
In the gut, where ~95% of the body’s serotonin resides, most 5-HT is produced by a specialized subtype of endocrine cells, called enterochromaffin (EC) cells, residing alongside the epithelium lining the gut lumen. Enterochromaffin cells release 5-HT into the lamina propria, where it can signal via 5-HT receptors expressed on nerves or be inactivated by the serotonin reuptake transporter (SERT)-mediated uptake into enterocytes where it is catabolized by monoamine oxidase [268]. Indigenous spore-forming bacteria promote biosynthesis of 5-HT in enterochromaffin cells [269] and the release of 5-HT is controlled by luminal glucose status. For instance, high post-prandial glucose levels stimulate the rapid secretion of 5-HT, whereas enterochromaffin cells adapt to chronic low glucose levels by increasing transcripts of Tph1 involved in synthesizing 5-HT [270]. The 5-HT then activates 5-HT3 receptors on mucosal vagal afferent terminals [271,272] (Figure 3) and this is augmented by glucose [273]. Extracellular glucose levels alter rat gastric vagal afferent 5-HT3 receptor density rapidly and probably by receptor trafficking as increased glucose rapidly results in more functional surface receptors [273]. Similarly, in healthy older subjects, local duodenal motor effects are modulated by 5-HT3 receptor mediated responses to glucose present in the small intestine [274]. However, short exposures to high fat diets compromise the ability of glucose to amplify 5-HT3 mediated responses in gastric vagal afferent neurons [275].
Tropisetron treatment reduced weight gain in mice with access to glucose infused water but increased their ketone body production implicating 5-HT3 receptors in hepatic carbohydrate and fat metabolism [276]. There also appears to be a relationship with the microbiome and inflammation and the gut brain axis with 5-HT3 receptors playing a role [260,277,278]. Tropisetron and palonosetron reduce endotoxin escape into the body in the ob/ob (leptin deficient) genetic obese model mouse and this subsequently reduces liver inflammation and fat accumulation [279]. These effects could be in part because tropisetron can reverse 5-HT inhibition of insulin release [280]. Polymorphisms in 5-HT3 receptor B subunits have also been related to type 2 diabetes mellitus [85], further highlighting the roles of these receptors in modulating metabolism. Exercise, which utilizes plasma glucose and fatty acids, also stimulates increases in hippocampal 5-HT levels. 5-HT3 receptors are critical for mediating exercise enhanced hippocampal neurogenesis, and interestingly also in the anti-depressant effects induced by exercise but not learning, as demonstrated by behavioral studies [281].
Knock out mice studies provide a powerful tool to investigate the roles of peripheral and central 5-HT in addition to the receptors mediating its action. Mice on a high fat diet are not only heavier but have increased insulin resistance and non-alcoholic fatty liver disease that can be reduced pharmacologically with inhibitors of Tph1 or genetically in mice deficient in Tph1 [282]. Increased peripheral 5-HT is important in pregnancy to drive pancreatic beta cell expansion in mice via 5-HT2B receptors [283]. Beta cells also contain 5-HT that co-locates in the vesicles with insulin [284], where it is co-secreted. 5-HT2B, 5-HT1D, 5-HT4 and 5-HT3 receptors are found on beta cells and have autocrine responses to secreted 5-HT [259,285] regulating insulin production and glucose levels. 5-HT3 receptors are required for glucose-induced insulin secretion and this is more important in mice fed high fat diets where an obesity induced insulin-resistant state is developed [286] and in pregnant mice also suffering insulin resistance [287]. Both tropisetron and granisetron reduced plasma glucose levels [288,289,290], however, only tropisetron exhibited positive effects on diabetic nephropathy in rats with streptozotocin induced diabetes [288] and also lowers damage to liver tissue [289] possibly due to extra actions of tropisetron [195]. These findings implicate 5-HT3 receptor antagonists in improving glucose tolerance but should be treated cautiously before applying to human subjects. Ondansetron and tropisetron inhibit multidrug and toxin extrusion (MATE) and organic cation transporters (OCT) [291,292]. Although ondansetron lowered glucose levels in metformin treated healthy subjects relative to placebo, its affect alone on glucose tolerance is unclear and it is likely that pharmacodynamic interactions of the two drugs occur [293].
Polymorphisms in Thp1 [294] and Thp2 [295] are implicated in obesity. These findings are supported by higher Thp1 expression in duodenum of obese subjects with an increased ability to secrete 5-HT [296]. Links between 5-HT and adipose tissue and metabolism have been reviewed [259,278,297,298] where mouse studies have been particularly informative. White adipose tissue is the major site of lipid storage whereas brown adipose tissue is involved in whole body thermogenic regulation as it contains mitochondrial uncoupling protein 1 (UCP1). In rodents, brown adipose tissue is sympathetically innervated [299,300,301,302], while it is only recently that a nerve has been associated with brown-like adipose tissue deposits in humans [303]. High fat diet induced obesity is reduced by inhibiting Thp1 or in Thp1 deficient mice and this correlates with activation of uncoupling protein 1 (UCP1) mediated thermogenesis [282]. Mice on high fat diets have increased 5-HT levels that promote lipogenesis in white adipose tissue by activating 5-HT2 receptors while augmenting the suppression of thermogenesis in brown adipocytes by activating 5-HT3 receptors [304]. Central thermoregulatory neural circuits activating serotonergic neurons have been further implicated using fiber photometry and electrophysiology studies. Body weight and energy expenditure are modified through sympathetic regulation of brown adipose tissue that is directed through neurons in the dorsal raphe nucleus expressing melanocortin 4 receptors that in turn innervate 5-HT neurons [305]. A high fat diet is also associated with anxiety and depression that correlates with desensitization of GABAergic AgRP neurons projecting onto melanocortin 4 receptor neurons in the dorsal bed of nucleus of the stria terminus containing α5-GABAA receptors and afferent serotonergic neurons with 5-HT3 receptors [306]. Pharmacologically or genetically subduing 5-HT3 receptors or enhancing α5-GABAA receptors suppressed food intake and removed the high fat diet induced anxiety or depression [306]. In fact, altered food intake preferences favoring a low fat diet were seen in combined pharmacotherapies of granisetron and zonisamide [306]. Zonisamide is a sulfonamide anticonvulsant that has been considered as a clinical treatment for obesity [307,308] but not in combination with 5-HT3 receptor antagonists. It should be noted that 5-HT3 receptors are expressed widely in epileptogenic neural networks and 5-HT3 receptor antagonists have been associated with seizures (for a discussion see [18]), so this combination of pharmacotherapy may be effective. However, the 5-HT3 receptor antagonist ondansetron reduces meal evoked satiety in subjects taking a satiation nutrient drink test [309], highlighting influences of central and peripheral 5-HT3 receptors. Yet at this stage, links between 5-HT3 receptors and poor metabolic control are not properly established although some tenuous connections are indicated in Figure 3 based on rodent and human studies as discussed above.
7. 5-HT3 Receptors in Inflammation
5-HT has long been associated with inflammatory responses in part because of the extensive release of 5-HT from platelet stores upon platelet activation and stimulation of 5-HT release from enterochromaffin cells during inflammatory bowel conditions [135,310,311,312]. In addition, pro-inflammatory white blood cells, such as mast and T cells, can synthesize and selectively release 5-HT [313,314,315,316,317]. 5-HT receptors are expressed by many inflammatory cells including monocytes, macrophages, dendritic cells, T cells, B cells and mast cells (reviewed by [310,311,312]). In fact, monocytes contain several types of 5-HT receptors [311,318,319] and 5-HT modifies macrophage polarization principally via 5-HT2B and 5-HT7 receptors [320].
Initially, a role for 5-HT3 receptors in regulating lymphocyte ion currents was implicated in a patch clamp study using 5-HT and the 5-HT3 receptor agonist 2-methyl-5-HT, where the inactivation of lymphocyte potassium ion conductance was inhibited by tropisetron (ICS-205-930) [321]. Sodium ion influx into human lymphocytes was stimulated by 5-HT3 receptor specific agonists further confirming the presence of 5-HT3 receptors [123]. However, studies into the effects of 5-HT3 receptors in inflammatory conditions have been confounded by various off-target effects of the commonly used 5-HT3 receptor antagonists such as tropiseton [38,194,195]. Tropisetron has been implicated in modulating calcineurin regulated nuclear factor of activated T cells pathways independently of 5-HT3 receptors [18,322,323] which is of interest as calcineurin is likely to affect sensitivity of 5-HT3 receptors to 5-HT [172]. Studies using cell lines have also been complicated by the presence of 5-HT in serum [311,315].
A complex interplay is likely to exist between signaling mediated by toll-like receptors (TLRs) recognizing molecules associated with microbial infection, such as lipopolysaccharide, and 5-HT receptors. For instance, TLR2 and TLR4 knock out studies in mice have shown changes in 5-HT2, 5-HT3, 5-HT4 and 5-HT7 receptor transcript expression and, importantly, this correlates with changes in function in the ileum and colon [324,325]. In addition, 5-HT modulates the lipopolysaccharide-induced release of various pro-inflammatory cytokines such as interleukin-1β (IL-1β), IL-6, IL-8/CXCL8, IL-12p40, tumor necrosis factor α (TNFα), but not IL-18 and interferon γ (IFNγ), from human monocytes [318]. A 5-HT3 receptor knockout study reveals its role in mediating IL-6 production and intestinal mobility contributing to rotavirus-induced diarrhea [326].
Expression levels of 5-HT3A receptors are increased in asthmatic patients or those exposed to air pollution [128] suggesting a response to immune challenge. The activation of 5-HT3 receptors forms part of the allergen-induced inflammation responses in mast cells within nodose ganglia [327]. Further evidence for a role for 5-HT3 receptors comes from a study showing that tropisetron dose dependently inhibited lipopolysaccharide-induced TNFα and IL-1β secretion in human monocytes [121,124]. However, neither mRNA levels of these cytokines nor the transcriptional activity of TNFα promoter were affected by tropisetron in lipopolysaccharide-activated human peripheral monocytes. Instead, the pro-inflammatory cytokines are selectively inhibited at post-translational level, and the anti-inflammatory effects of tropisetron involve inhibition of p38 mitogen-associated protein kinase [328].
Several other immune related cells contain functional 5-HT3 receptors. For instance, dolasetron was found to be the more effective than tropisetron or granisetron in reducing prostaglandins and IL-6 secreted by human primary chondrocytes induced by IL-1β [329]. Alveolar epithelial type II cells are regulators of immune function in the lung and contain several functional 5-HT receptors including 5-HT3 receptors that stimulate release of IL-8 [330]. Ondansetron, palonosetron, and ramosetron blocked A549 human lung adenocarcinoma cell proliferation in a dose-dependent manner and promoted autophagy via the extracellular signal-regulated kinase pathway [331], indicating the anti-tumor potential of 5-HT3 receptor antagonists. Similarly, dendritic cells also contain several 5-HT receptors including 5-HT3 receptors that stimulate release of pro-inflammatory cytokines [332,333]. Interestingly, dendritic cells matured in the presence of 5-HT switch to secreting the anti-inflammatory cytokine, IL-10 [333]. Notably, IL-10 can reprogram macrophage metabolism by enhancing selective degradation of mitochondria via autophagy (mitophagy), thus preventing accumulation of dysfunctional and ROS-producing mitochondria, promoting macrophages with fewer dysfunctional mitochondria [334].
Macrophages will contribute to any inflammation present in functional digestive tract disorders where 5-HT3 receptors are the target, in part because they are widely found in the enteric nervous system. Various studies have revealed that the microbiome alters with inflammatory bowel conditions [277,278,298] and this is probably associated with influx of host macrophages. Macrophages in the small intestine lamina propria express the greatest proportion of 5-HT3 receptors [335,336] and peritoneal macrophages express 5-HT3 receptors in mouse models [337]. A mouse model of post-operative ileus supports the use of 5-HT3 receptor antagonists to reduce inflammation by preferentially targeting peritoneal macrophages expressing 5-HT3 receptors [337]. 5-HT upregulates pro-inflammatory mediators (e.g., inducible nitric oxide synthase (iNOS), TNFα, IFNγ and IL-17A) via 5-HT3 receptors as this was inhibited by ondansetron or ramosetron in a dextran sulfate sodium-induced colitis mouse model [335]. Post-inflammatory visceral hypersensitivity in male rats that had recovered from acetic acid-induced colitis was relieved by 5-HT3 receptor antagonists, alosetron and granisetron [338]. Granisetron also alleviates colonic levels of cytokines and histological inflammatory appearance in acetic acid-induced colitis [339].
5-HT3 receptor antagonists thus have the potential to suppress pro-inflammatory cytokine production and so reduce inflammation. No studies have investigated how the different subunits contribute to 5-HT3 receptor function in immune cells. Microarray studies suggested that only very low levels of genes encoding 5-HT3 receptor subunits were found in white blood cells [127] despite the several lines of evidence for receptor function discussed above. More recent RNAseq studies on human lymphoblastoid cell lines have revealed that the A, B and C subunit transcripts are all equally distributed [23,340]. This finding suggests that 5-HT3 receptors containing A homomers, and/or AB or AC heteromers are likely to be present in white blood cells.
Rats with diabetes induced by streptozotocin and treated with tropisetron had decreased levels of oxidative stress and TNFα as well as reduced urinary cytokine secretion [288] and showed renoprotective effects in early-stage diabetic nephropathy by blocking calcineurin/nuclear factor of activated T-cell pathway [322]. This pathway was inhibited by tropisetron to halt the antigen-induced proliferation of human peripheral T cells and the generation of IL-2 [323]. In a recent cohort study, ondansetron usage was associated with reduced risk-adjusted in-hospital mortality in critically ill patients suffering from acute kidney injury [341]. Anti-inflammatory effects of 5-HT3 receptor antagonists underscore the potential of 5-HT3 receptors as immunomodulatory targets in autoimmune diseases, including multiple sclerosis and rheumatoid arthritis [310].
8. Conclusions
Although 5-HT3 receptors play roles in the regulation of metabolism and inflammation, further studies are required to elucidate the complex interplay of the large number of functional 5-HT3 receptors, their contribution to energy homeostasis, and their detailed biochemical and pharmacological properties. Despite recent advances in the field, the comprehension of 5-HT3 receptor physiological functions is hindered due to complex expression patterns, numerous isoforms, and subunit types. This is further complicated by difficulties in studying different types of heteromers, whose assembly, composition, stoichiometry and expression in diverse cell types and tissues appears to have significant physiological implications but is poorly understood. The function of most available drugs has been studied using 5-HT3A homomeric receptors and, to a lesser extent, the AB heteromer. However, rare genetic variations in genes encoding other 5-HT3 receptor subunits also contribute to a number of disorders (Table 1). Therefore, a thorough characterization of heteromeric receptors and determination of their pharmacology is required, as subunit arrangements influence ligand binding kinetics and the subsequent physiological function of the receptor. Further challenges are imposed by the fact that rodents lack the 5-HT3 receptor C, D and E subunits, complicating the practicality of in vivo genetic studies. Finally, although most attention has been focused on 5-HT3 receptors present at the cell surface, the receptors are also distributed on intracellular organelles. However, the roles and subunit composition of organelle-located 5-HT3 receptors are still to be investigated. Interestingly, 5-HT3 receptors are present in mitochondrial membranes, where they augment calcium ion uptake in hypoxia and increase the respiration control ratio in mice [342]. Furthermore, emerging studies revealed that blood in the normal physiological state contains whole functional cell-free mitochondria [343,344]. This observation may have profound implications for the field of inflammation and clinical applications [343], potentially involving signaling via 5-HT3 receptors present in these mitochondrial membranes.
In conclusion, emerging studies suggest there is much potential for therapeutic intervention in areas beyond those for which 5-HT3 receptors are currently used. Collectively, genetic animal models combined with the identification of genetic variants and the increasing availability of human genotyping will be of benefit in gaining insight into metabolic signaling. Targeting 5-HT3 receptors may form a new prospect for the personalized treatment of immune and metabolic diseases.
Author Contributions
Conceptualization, H.I.; investigation, H.I., N.Y. and I.T.; writing—original draft preparation, H.I.; writing—review and editing, H.I., I.T., C.K. and N.Y.; visualization, H.I. and I.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank Amy Li (La Trobe), Ian Coupar (Monash), David Manallack (Monash), Betty Exintaris (Monash), Katie Leach (Monash) and Santosh Tata (La Trobe) for helpful discussions over the years relating to various aspects of this review. We also thank David Manallack for providing images of the original homology models used in Figure 2A.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rapport, M.M.; Green, A.A.; Page, I.H. Serum vasoconstrictor (serotonin) IV. Isolation and characterization. J. Biol. Chem. 1948, 176, 1243–1251. [Google Scholar] [CrossRef]
- Erspamer, V.; Asero, B. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature 1952, 169, 800–801. [Google Scholar] [CrossRef] [PubMed]
- Gaddum, J.H.; Picarelli, Z.P. Two kinds of tryptamine receptor. Brit. J. Pharmacol. 1957, 12, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.B.; Engel, G.; Feniuk, W.; Fozard, J.R.; Humphrey, P.P.A.; Middlemiss, D.N.; Mylecharane, E.J.; Richardson, B.P.; Saxena, P. Proposals for the classification and nomenclature of functional receptors for 5-hyroxytryptamine. Neuropharmacology 1986, 25, 563–576. [Google Scholar] [CrossRef]
- Derkach, V.; Surprenant, A.; North, R.A. 5-HT3 receptors are membrane ion channels. Nature 1989, 339, 706–709. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Davenport, A.P.; Kelly, E.; Marrion, N.; Peters, J.A.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Southan, C.; et al. The concise guide to pharmacology 2015/2016: G protein-coupled receptors. Brit. J. Pharmacol. 2015, 172, 5744–5869. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Peters, J.A.; Kelly, E.; Marrion, N.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Southan, C.; Davies, J.A.; et al. The concise guide to pharmacology 2015/2016: Ligand-gated ion channels. Brit. J. Pharmacol. 2015, 172, 5870–5903. [Google Scholar] [CrossRef]
- Barnes, N.M.; Ahern, G.P.; Becamel, C.; Bockaert, J.; Camilleri, M.; Chaumont-Dubel, S.; Claeysen, S.; Cunningham, K.A.; Fone, K.C.; Gershon, M.; et al. International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function. Pharmacol. Rev. 2021, 73, 310. [Google Scholar] [CrossRef]
- Boess, F.G.; Beroukhim, R.; Martin, I.L. Ultrastructure of the 5-hydroxytryptamine3 receptor. J. Neurochem. 1995, 64, 1401–1405. [Google Scholar] [CrossRef]
- Davies, P.A.; Pistis, M.; Hanna, M.C.; Peters, J.A.; Lambert, J.J.; Hales, T.G.; Kirkness, E.F. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature 1999, 397, 359–363. [Google Scholar] [CrossRef]
- Karnovsky, A.M.; Gotow, L.F.; McKinley, D.D.; Piechan, J.L.; Ruble, C.L.; Mills, C.J.; Schellin, K.A.; Slightom, J.L.; Fitzgerald, L.R.; Benjamin, C.W.; et al. A cluster of novel serotonin receptor 3-like genes on human chromosome 3. Gene 2003, 319, 137–148. [Google Scholar] [CrossRef]
- Maricq, A.V.; Peterson, A.S.; Brake, A.J.; Myers, R.M.; Julius, D. Primary structure and functional expression of the 5-HT3 receptor, a serotonin-gated ion channel. Science 1991, 254, 432–437. [Google Scholar] [CrossRef]
- Niesler, B.; Frank, B.; Kapeller, J.; Rappold, G.A. Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D and HTR3E. Gene 2003, 310, 101–111. [Google Scholar] [CrossRef]
- Holbrook, J.D.; Gill, C.H.; Zebda, N.; Spencer, J.P.; Leyland, R.; Rance, K.H.; Trinh, H.; Balmer, G.; Kelly, F.M.; Yusaf, S.P.; et al. Characterisation of 5-HT3C, 5-HT3D and 5-HT3E receptor subunits: Evolution, distribution and function. J. Neurochem. 2009, 108, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.M.; Hales, T.G.; Lummis, S.C.; Peters, J.A. The 5-HT3 receptor—The relationship between structure and function. Neuropharmacology 2009, 56, 273–284. [Google Scholar] [CrossRef]
- Faerber, L.; Drechsler, S.; Ladenburger, S.; Gschaidmeier, H.; Fischer, W. The neuronal 5-HT3 receptor network after 20 years of research—Evolving concepts in management of pain and inflammation. Eur. J. Pharmacol. 2007, 560, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fakhfouri, G.; Mousavizadeh, K.; Mehr, S.E.; Dehpour, A.R.; Zirak, M.R.; Ghia, J.E.; Rahimian, R. From chemotherapy-induced emesis to neuroprotection: Therapeutic opportunities for 5-HT3 receptor antagonists. Mol. Neurobiol. 2015, 52, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Fakhfouri, G.; Rahimian, R.; Dyhrfjeld-Johnsen, J.; Zirak, M.R.; Beaulieu, J.-M. 5-HT3 receptor antagonists in neurologic and neuropsychiatric disorders: The iceberg still lies beneath the surface. Pharmacol. Rev. 2019, 71, 383–412. [Google Scholar] [CrossRef]
- Walstab, J.; Rappold, G.; Niesler, B. 5-HT3 receptors: Role in disease and target of drugs. Pharmacol. Therap. 2010, 128, 146–169. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Cheng, H.; Jiang, Y.; Melcher, K.; Xu, H.E. Ion channels gated by acetylcholine and serotonin: Structures, biology, and drug discovery. Acta Pharmacol. Sin. 2015, 36, 895–907. [Google Scholar] [CrossRef]
- Yaakob, N.; Malone, D.T.; Exintaris, B.; Irving, H.R. Heterogeneity amongst 5 HT3 receptor subunits: Is this significant? Curr. Mol. Med. 2011, 11, 57–68. [Google Scholar] [CrossRef]
- Juza, R.; Vlcek, P.; Mezeiova, E.; Musilek, K.; Soukup, O.; Korabecny, J. Recent advances with 5-HT3 modulators for neuropsychiatric and gastrointestinal disorders. Med. Res. Rev. 2020, 40, 1593–1678. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, R.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Iny Stein, T.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From gene data mining to disease genome sequence analysis. Curr. Protocols Bioinform. 2016, 54, 1.30.31–31.30.33. [Google Scholar] [CrossRef]
- Hodge, R.D.; Bakken, T.E.; Miller, J.A.; Smith, K.A.; Barkan, E.R.; Graybuck, L.T.; Close, J.L.; Long, B.; Johansen, N.; Penn, O.; et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 2019, 573, 61–68. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.M.; Farrugia, G.; Schemann, M. 5-HT receptors on interstitial cells of Cajal, smooth muscle and enteric nerves. Neurogastroenterol. Motil. 2007, 19, 5–12. [Google Scholar] [CrossRef]
- Barnes, J.M.; Barnes, N.M.; Costall, B.; Deakin, J.F.W.; Ironside, J.W.; Kilpatrick, G.J.; Naylor, R.J.; Rudd, J.A.; Simpson, M.D.C.; Slater, P.; et al. Identification and distribution of 5-HT3 recognition sites within the human brain-stem. Neurosci. Lett. 1990, 111, 80–86. [Google Scholar] [CrossRef]
- Barnes, N.M.; Costall, B.; Ironside, J.W.; Naylor, R.J. Identification of 5-HT3 recognition sites in human brain tissue using (H-3) zacopride. J. Pharm. Pharmacol. 1988, 40, 668. [Google Scholar] [CrossRef]
- Parker, R.M.C.; Barnes, J.M.; Ge, J.; Barber, P.C.; Barnes, N.M. Autoradiographic distribution of [3H]-(S)-zacopride-labelled 5-HT3 receptors in human brain. J. Neurol. Sci. 1996, 144, 119–127. [Google Scholar] [CrossRef]
- Navari, R.M. 5-HT3 receptors as important mediators of nausea and vomiting due to chemotherapy. Biochim. Biophys. Acta 2015, 1848, 2738–2746. [Google Scholar] [CrossRef]
- Abi-Dargham, A.; Laruelle, M.; Wong, D.T.; Robertson, D.W.; Weinberger, D.R.; Kleinman, J.E. Pharmacological and regional characterization of [3H]LY278584 binding sites in human brain. J. Neurochem. 1993, 60, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Bufton, K.E.; Steward, L.J.; Barber, P.C.; Barnes, N.M. Distribution and characterization of the [3H]granisetron-labelled 5-HT3 receptor in the human forebrain. Neuropharmacology 1993, 32, 1325–1331. [Google Scholar] [CrossRef]
- Waeber, C.; Hoyer, D.; Palacios, J.M. 5-Hydroxytryptamine3 receptors in the human brain: Autoradiographic visualization using [3H] ICS 205-930. Neuroscience 1989, 31, 393–400. [Google Scholar] [CrossRef]
- Brady, C.A.; Dover, T.J.; Massoura, A.N.; Princivalle, A.P.; Hope, A.G.; Barnes, N.M. Identification of 5-HT3A and 5-HT3B receptor subunits in human hippocampus. Neuropharmacology 2007, 52, 1284–1290. [Google Scholar] [CrossRef]
- Massoura, A.N.; Brady, C.A.; Dover, T.J.; Princivalle, A.P.; Hope, A.G.; Barnes, N.M. The human hippocampus displays the potential to express both homomeric and heteromeric 5-HT3 receptors. J. Pharmacol. Sci. 2006, 101, 121. [Google Scholar]
- Oostland, M.; Sellmeijer, J.; van Hooft, J.A. Transient expression of functional serotonin 5-HT3 receptors by glutamatergic granule cells in the early postnatal mouse cerebellum. J. Physiol. 2011, 589, 4837–4846. [Google Scholar] [CrossRef] [PubMed]
- Vitalis, T.; Ansorge, M.S.; Dayer, A.G. Serotonin homeostasis and serotonin receptors as actors of cortical construction: Special attention to the 5-HT3A and 5-HT6 receptor subtypes. Front. Cell. Neurosci. 2013. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, M.H.; Viswenaden, P.; Jasamai, M.; Azmi, N.; Yaakob, N.S. Potential roles of 5-HT3 receptor (5-HT3R) antagonists in modulating the effects of nicotine. Biomed. Pharmacother. 2019, 112, 108630. [Google Scholar] [CrossRef]
- Windemuth, A.; Calhoun, V.D.; Pearlson, G.D.; Kocherla, M.; Jagannathan, K.; Ruaño, G. Physiogenomic analysis of localized fMRI brain activity in schizophrenia. Ann. Biomed. Eng. 2008, 36, 877–888. [Google Scholar] [CrossRef]
- Yang, J.; Li, M.D. Association and interaction analyses of 5-HT3 receptor and serotonin transporter genes with alcohol, cocaine and nicotine dependence using the SAGE data. Hum. Gen. 2014, 133, 905–918. [Google Scholar] [CrossRef]
- Johnson, B.A.; Seneviratne, C.; Wang, X.-Q.; Ait-Daoud, N.; Li, M.D. Determination of genotype combinations that can predict the outcome of the treatment of alcohol dependence using the 5-HT3 antagonist ondansetron. Am. J. Psych. 2013, 170, 1020–1031. [Google Scholar] [CrossRef]
- Kapeller, J.; Houghton, L.A.; Mönnikes, H.; Walstab, J.; Möller, D.; Bönisch, H.; Burwinkel, B.; Autschbach, F.; Funke, B.; Lasitschka, F.; et al. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum. Mol. Gen. 2008, 17, 2967–2977. [Google Scholar] [CrossRef]
- Kilpatrick, L.A.; Labus, J.S.; Coveleskie, K.; Hammer, C.; Rappold, G.; Tillisch, K.; Bueller, J.A.; Suyenobu, B.; Jarcho, J.M.; McRoberts, J.A.; et al. The HTR3A polymorphism c. -42C > T is associated with amygdala responsiveness in patients with irritable bowel syndrome. Gastroenterology 2011, 140, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Li, T.; Cai, W.; Huang, D.; Ouyang, P.; Wang, Y.M.; Chen, H.-Y.; Wu, K.; Ma, X. HTR3A and HTR3E gene polymorphisms and diarrhea predominant irritiable bowel syndrome risk: Evidence from a meta-analysis. Oncotarget 2017, 8, 100459–100468. [Google Scholar] [CrossRef] [PubMed]
- Mujakovic, S.; Ter Linde, J.J.M.; de Wit, N.J.; van Marrewijk, C.J.; Fransen, G.A.J.; Onland-Moret, N.C.; Laheif, R.J.F.; Muris, J.W.M.; Grobbee, D.E.; Samson, M.; et al. Serotonin receptor 3A polymorphism c.-42C > T is associated with severe dysepsia. BMC Med. Gen. 2011, 12, 140. [Google Scholar] [CrossRef]
- Jounger, S.L.; Christidis, N.; Hedenberg-Magnusson, B.; List, T.; Svensson, P.; Schalling, M.; Ernberg, M. Influence of polymorphisms in the HTR3A and HTR3B genes on experimental pain and the effect of the 5-HT3 antagonist granisetron. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Ledermann, K.; Hasler, G.; Jenewein, J.; Sprott, H.; Schnyder, U.; Martin-Soelch, C.J.S. 5’UTR polymorphism in the serotonergic receptor HTR3A gene is differently associated with striatal Dopamine D2/D3 receptor availability in the right putamen in Fibromyalgia patients and healthy controls–preliminary evidence. Synapse 2020, 74, e22147. [Google Scholar] [CrossRef]
- Niesler, B.; Flohr, T.; Nöthen, M.M.; Fischer, C.; Rietschel, M.; Franzek, E.; Albus, M.; Propping, P.; Rappoid, G. Association between the 5′-UTR variant C178T of the the serotonin receptor gene HTR3A and bipolar affective disorder. Pharmacogenetics 2001, 11, 471–475. [Google Scholar] [CrossRef]
- Hammer, C.; Cichon, S.; Mühleisen, T.W.; Haenisch, B.; Degenhardt, F.; Mattheisen, M.; Breuer, R.; Witt, S.H.; Strohmaier, J.; Oruc, L.; et al. Replication of functional serotonin receptor type 3A and B variants in bipolar affective disorder: A European multicenter study. Translat. Psych. 2012, 2, e103. [Google Scholar] [CrossRef]
- Perroud, N.; Zewdie, S.; Stenz, L.; Adouan, W.; Bavamian, S.; Prada, P.; Nicastro, R.; Hasler, R.; Nallet, A.; Piguet, C.; et al. Methylation of serotonin receptor 3A in ADHD, borderline personality, and bipolar disorders: Link with the severity of the disorders and childhood maltreatment. Depress. Anxiety 2016, 33, 45–55. [Google Scholar] [CrossRef]
- Kato, M.; Fukuda, T.; Wakeno, M.; Fukuda, K.; Okugawa, G.; Ikenaga, Y.; Yamashita, M.; Takekita, Y.; Nobuhara, K.; Azuma, J.; et al. Effects of the serotonin type 2A, 3A and 3B receptor and the seotonin transporter genes on paroxetine and fluvoxamine efficiacy and adverse drug reactions in depressed Japanese patients. Neuropschyhobiology 2006, 53, 186–195. [Google Scholar] [CrossRef]
- Gatt, J.M.; Nemeroff, C.B.; Schofield, P.R.; Paul, R.H.; Clark, C.R.; Gordon, E.; Williams, C. Early life stress combined with serotonin 3A receptor and brain-derived growth factor valine 66 to methionine genotypes impacts emotional brain and arousal correlates of risk for depression. Biol. Psych. 2011, 68, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Gatt, J.M.; Williams, L.M.; Schofield, P.R.; Dobson-Stone, C.; Paul, R.H.; Grieve, S.M.; Clark, C.R.; Gordon, E.; Nemeroff, C.B. Impact of the HTR3A gene with early life trauma on emotional brain networks and depressed mood. Depress. Anxiety 2010, 27, 752–759. [Google Scholar] [CrossRef]
- Jang, K.-I.; Lee, S.-H.; Huh, H.J.; Chae, J.-H. Influence of the 5-HT3A Receptor Gene Polymorphism and Childhood Sexual Trauma on Central Serotonin Activity. PLoS ONE 2015, 10, e0145269. [Google Scholar] [CrossRef]
- Ji, X.; Takahashi, M.; Saito, S.; Ishihara, R.; Maeno, N.; Inada, T.; Ozaki, N. Relationship between three serotonin receptor subtypes (HTR3A, HTR2A and HTR4) and treatment-resistant schizohrenia in the Japanese population. Neurosci. Lett. 2008, 435, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Jajodia, A.; Kaur, H.; Kumari, K.; Gupta, M.; Baghel, R.; Srivastava, A.; Sood, M.; Chadda, R.K.; Jain, S.; Kukreti, R. Evidence for schizophrenia susceptibility alleles in the Indian population: An association of neurodevelopmental genes in case-control and familial samples. Schiz. Res. 2015, 162, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, B.; Arranz, M.J.; Huezo-Diaz, P.; Dempster, D.; Matthiasson, P.; Travis, M.; Munro, J.; Osborne, S.; Kerwin, R.W. Novel mutations in 5-HT3A and 5-HT3B receptor genes not associate with clozapine response. Schiz. Res. 2002, 58, 93–97. [Google Scholar] [CrossRef]
- Rajkumar, A.P.; Poonkuzhali, B.; Kuruvilla, A.; Srivastava, A.; Jacob, M.; Jacob, K.S. Outcome definitions and clinical predictors influence pharmacogenetic associations between HTR3A gene polymorphisms and response to clozapine in patients with schizophrenia. Psychopharmacology 2012, 224, 441–449. [Google Scholar] [CrossRef]
- Souza, R.; de Luca, V.; Meltzer, H.; Lieberman, J.; Kennedy, J. Influence of serotonin 3A and 3B receptor genes on clozapine treatment response in schizophrenia. Pharmacogen. Genom. 2010, 20, 274–276. [Google Scholar] [CrossRef]
- Han, H.; Liu, Q.; Yang, Z.; Wang, M.; Ma, Y.; Cao, L.; Cui, W.; Yuan, W.; Payne, T.J.; Li, L.; et al. Association and cis-mQTL analysis of variants in serotonergic genes associated with nicotine dependence in Chinese Han smokers. Translat. Psych. 2018, 8, 243. [Google Scholar] [CrossRef]
- Schechter, D.S.; Moser, D.A.; Pointet, V.C.; Aue, T.; Stenz, L.; Paoloni-Giacobino, A.; Adouan, W.; Manini, A.; Suardi, F.; Vital, M.; et al. The association of serotonin receptor 3A methylation with maternal violence exposure, neural activity, and child aggression. Behav. Brain Res. 2017, 325, 268–277. [Google Scholar] [CrossRef]
- Melke, J.; Westberg, L.; Nilsson, S.; Landen, M.; Soderstrom, H.; Baghaeri, F.; Rosmond, R.; Hom, G.; Bjorntorp, P.; Nilsson, L.-G.; et al. A polymorphism in the serotonin 3A (HTR3A) gene and its association with harm avoidance in women. Arch. Gen. Psych. 2003, 60, 1017–1023. [Google Scholar] [CrossRef]
- Anderson, B.M.; Schnetz-Boutaud, N.C.; Bartlett, J.; Wotawa, A.M.; Wright, H.H.; Abramson, R.K.; Cuccaro, M.L.; Gilbert, J.R.; Pericak-Vance, M.A.; Haines, J.L. Examination of association of genes in the serotonin system to autism. Neurogenetics 2009, 10, 209–216. [Google Scholar] [CrossRef]
- Seneviratne, C.; Franklin, J.; Beckett, K.; Ma, J.Z.; Ait-Daoud, N.; Payne, T.J.; Johnson, B.A.; Li, M.D. Association, interaction, and replication analysis of genes encoding serotonin transporter and 5-HT3 receptor subunits A and B in alcohol dependence. Hum. Gen. 2013, 132, 1165–1176. [Google Scholar] [CrossRef]
- Gu, B.; Wang, L.; Zhang, A.-P.; Ma, G.; Zhao, X.-Z.; Li, H.-F.; Feng, G.-Y.; He, L.; Xing, Q.-H. Association between a polymorphism of the HTR3A gene and therapeutic response to riseridone tretment in drug-naive Chinese schizophrenia patients. Pharmacogen. Genom. 2008, 18, 721–727. [Google Scholar] [CrossRef]
- Ducci, F.; Enoch, M.-A.; Yuan, Q.; Shen, P.-H.; White, K.V.; Hodkinson, C.; Albaugh, B.; Virkkunen, M.; Goldman, D. HTR3B is associated with alcoholism with antisocial behavior and alpha EEG power-an intermediate phenotype for alcoholism and co-morbid behaviors. Alcohol 2009, 43, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Rueffert, H.; Thieme, V.; Wallenborn, J.; Lemnitz, N.; Bergmann, A.; Rudlof, K.; Wehner, M.; Olthoff, D.; Kaisers, U.X. Do variations in the 5-HT3A and 5-HT3B serotonin receptor genes (HTR3A and HTR3B) influence the occurrence of postoperative vomiting? Anesth. Analg. 2009, 109, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Li, C.; Xu, J.; Qiao, D.; Mi, G.; Chen, X.; Tang, M. Associations of serotonin receptor gene HTR3A, HTR3B, and HTR3A haplotypes with bipolar disorder in Chinese patients. Genet. Mol. Res. 2016, 15, gmr.15038671. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, Z.J.; Shi, Y.; Pu, M.; Yuan, Y.; Zhang, X.; Li, L.; Reynolds, G.P. Influence and interaction of genetic polymorphisms in the serootonin system and life stress on antidepressant drug response. J. Psychopharmacol. 2012, 26, 349–359. [Google Scholar] [CrossRef]
- Lesurtel, M.; Soll, C.; Graf, R.; Clavien, P.A. Role of serotonin in the hepato-gastroIntestinal tract: An old molecule for new perspectives. Cell. Mol. Life Sci. 2008, 65, 940–952. [Google Scholar] [CrossRef]
- Kim, H.W.; Kang, J.I.; Lee, S.-H.; An, S.K.; Sohn, S.Y.; Hwang, E.H.; Lee, S.Y.; Kim, S.J. Common variants of HTR3 genes are associated with obsessive-compulsive disorder and its phenotypic expression. Sci. Rep. 2016, 6, 32564. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.X.; Chen, Q.X.; Wu, S.J.; Hu, Y.; Fang, X.M. Polymorphisms of the HTR3B gene are associated with post-surgery emesis in a Chinese Han population. J. Clin. Pharmacy Therap. 2013, 38, 150–155. [Google Scholar] [CrossRef]
- Kang, G.; Kim, K.-R.; Shim, H.-J.; Hwang, J.-E.; Bae, W.-K.; Chung, I.-J.; Kim, H.-N.; Lee, J.; Choi, K.; Shin, H.-Y.; et al. Effect of the allelic variants of ABCB1, CYP2D6 and HTR3B on response of ramosetron to prevent chemotherapy-induced nausea and vomiting in Korean cancer patients. Asia-Pacific J. Clin. Oncol. 2017, 13, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Lee, J.-R.; Choi, E.-M.; Kim, E.H.; Choi, S.H. Association of 5-HT3B Receptor Gene Polymorphisms with the Efficacy of Ondansetron for Postoperative Nausea and Vomiting. Yonsei Med. J. 2015, 56, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kobayashi, D.; Murakami, Y.; Ozaki, N.; Suzuki, T.; Iwata, N.; Haraguchi, K.; Ieiri, I.; Kinukawa, N.; Hosoi, M.; et al. Genetic polymorphisms in the 5-hydroxytryptamine type 3B receptor gene and paroxetine-induced nausea. Int. J. Neuropsychopharmacol. 2008, 11, 261–267. [Google Scholar] [CrossRef]
- Tremblay, P.-B.; Kaiser, R.; Sezer, O.; Rosler, N.; Schelenz, C.; Possinger, K.; Roots, I.; Brockmoller, J. Variations in the 5-Hydroxytryptamine type 3B receptor gene as predictors of the efficacy of antiemetic treatment in cancer patients. J. Clin. Oncol. 2003, 21, 2147–2155. [Google Scholar] [CrossRef]
- Ji, X.; Takahashi, N.; Branko, A.; Ishihara, R.; Nagai, T.; Mouri, A.; Saito, S.; Maeno, N.; Inada, T.; Ozaki, N. An association between serotonin receptor 3B gene (HTR3B) and treatment-resistant schizophrenia (TRS) in a Japanese population. Nagoya J. Med. Sci. 2008, 70, 11–17. [Google Scholar]
- Yamada, K.; Hattori, E.; Iwayama, Y.; Ohnishi, T.; Ohba, H.; Toyota, T.; Takao, H.; Minabe, Y.; Nakatani, N.; Higuchi, T.; et al. Distinguishable haplotype blocks in the HTR3A and HTR3B region in the Japanese reveal evidence of association of HTR3B with female major depression. Biol. Psych. 2006, 60, 192–201. [Google Scholar] [CrossRef]
- Frank, B.; Niesler, B.; Nothen, M.M.; Neidt, H.; Propping, P.; Bondy, B.; Rietschel, M.; Maier, W.; Albus, M.; Rappoid, G. Investigation of the human serotonin receptor gene HTR3B in bipolar affective and schizohrenic patients. Am. J. Med. Gen. Part B Neuropsych. Gen. 2004, 131B, 1–5. [Google Scholar] [CrossRef]
- Sugai, T.; Suzuki, Y.; Sawamura, K.; Fukui, N.; Inoue, Y.; Someya, T. The effect of 5-hydroxytrptamine 3A and 3B receptor genes on nausea induced by paroxetine. Pharmacogen. J. 2006, 6, 351–356. [Google Scholar] [CrossRef]
- Laugsand, E.A.; Flavvad, T.; Skorpen, F.; Maltoni, M.; Kaasa, S.; Fayers, P.; Klepstad, P. Clinical and geentic factors associated with nausea and vomiting in cancer patients receiving opioids. Eur. J. Cancer 2011, 47, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Hammer, C.; Kapellar, J.; Endele, M.; Fischer, C.; Hebebrand, J.; Hinney, A.; Friedel, S.; Gratacòs, M.; Estivill, X.; Fichter, M.; et al. Functional variants of the serotonin receptor type 3A and B gene are associated with eating disorders. Pharmacogen. Genom. 2009, 19, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Enoch, M.-A.; Gorodetsky, E.; Hodgkinson, C.; Roy, A.; Goldman, D. Functional genetic variants that increase synaptic serotonin and 5-HT3 receptor sensitivity additively predict alcohol and drug dependence. Mol. Psych. 2011, 16, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Horjales-Araujo, E.; Demontis, D.; Lund, E.K.; Finnerup, N.B.; Borglum, A.D.; Jensen, T.S.; Svensson, P.; Vase, L. Polymorphism in serotonin receptor 3B is associated with pain catastrophizing. PLoS ONE 2013, 8, e78889. [Google Scholar] [CrossRef]
- Kwon, Y.-J.; Hong, K.-W.; Park, B.J.; Jung, D.-H. Serotonin receptor 3B polymorphisms are associated with type 2 diabetes: The Korean Genome and Epidemiology Study. Diabetes Res. Clin. Pract. 2019, 153, 76–85. [Google Scholar] [CrossRef]
- Krzywkowski, K.; Davies, P.A.; Feinberg-Zadek, P.L.; Bräuner-Osborne, H.; Jensen, A.A. High-frequency HTR3B variant asssociated with major depression dramatically augments the signalling of the human 5-HT3AB receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Walstab, J.; Hammer, C.; Bonisch, H.; Rappoid, G.; Niesler, B. Naturally occurring variants in the HTR3B gene significantly alter properties of human heteromeric 5-hydroxytrptamine-3A/B receptors. Pharmacogen. Genom. 2008, 18, 793–802. [Google Scholar] [CrossRef]
- Gupta, M.; Jain, S.; Moily, N.S.; Kaur, H.; Jajodia, A.; Purushottam, M.; Kukreti, R. Genetic studies indicate a potential target 5-HT3B for drug therapy in schizoprenia patients. Am. J. Med. Gen. Part B Neuropsych. Gen. 2012, 159B, 1006–1008. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ishida, Y.; Yamada, T.; Kiondo, M.; Shimada, S. Subunit-dependent inhibition and potentiation of 5-HT3 receptor by the anticancer drug, toptecan. J. Neurochem. 2013, 125, 7–15. [Google Scholar] [CrossRef]
- Oades, R.D.; Lasky-Su, J.; Christiansen, H.; Faraone, S.V.; Sonuga-Barke, E.J.S.; Banaschewski, T.; Chen, W.; Anney, R.J.L.; Buitelaar, J.K.; Ebstein, R.P.; et al. The influence of serotonin- and other genes on impulsive behavioral aggression and cognitive impulsivity in children with attention-deficit/hyperactivity disorder (ADHD): Findings from a family-based association test (FBAT) analysis. Behav. Brain Funct. 2008, 4, 48. [Google Scholar] [CrossRef]
- Fasching, P.A.; Kollmannsberger, B.; Strissel, P.L.; Niesler, B.; Engel, J.; Kreis, H.; Lux, M.P.; Weihbrecht, S.; Lausen, B.; Bani, M.R.; et al. Polymorphisms in the novel serotonin receptor subunit gene HTR3C show different risks for acute chemotherapy-induced vomiting after anthracycline chemotherapy. J. Can. Res. Clin. Oncol. 2008, 134, 1079–1086. [Google Scholar] [CrossRef]
- Pud, D.; Har-Zahav, G.; Laitman, Y.; Rubinek, T.; Yeheske, A.; Ben-Ami, S.; Kaufman, B.; Friedman, E.; Symon, Z.; Wolf, I. Association between variants of 5-hydroxytryptamine receptor 3C (HTR3C) and chemotherapy-induced symptoms in women receiving adjuvant treatment for breast cancer. Breast Can. Res. Treat. 2014, 144, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Mukoyama, N.; Yoshimi, A.; Goto, A.; Kotani, H.; Ishikawa, K.; Miyazaki, N.; Miyazaki, M.; Yamada, K.; Kikkawa, F.; Hasegawa, Y.; et al. An Analysis of Behavioral and Genetic Risk Factors for Chemotherapy-Induced Nausea and Vomiting in Japanese Subjects. Biol. Pharmaceut. Bull. 2016, 39, 1852–1858. [Google Scholar] [CrossRef][Green Version]
- Ward, M.B.; Kotasek, D.; McKinnon, R.A. Investigation of HTR3C mutations for association with 5-HT3 receptor antagonist anti-emetic efficacy. Pharmacogenomics 2008, 9, 1027–1033. [Google Scholar] [CrossRef]
- Rehnström, K.; Ylisaukko-oja, T.; Nummela, I.; Ellonen, P.; Kempas, E.; Vanhala, R.; Wendt, L.; Järvelä, I.; Peltonen, L. Allelic variants in HTR3C show association with autism. Am. J. Med. Genet. B Neuopsych. Genet. 2009, 150B, 741–746. [Google Scholar] [CrossRef]
- Lennertz, L.; Wagner, M.; Grabbe, C.; Franke, P.E.; Guttenthaler, V.; Rampacher, F.; Schulze-Rauschenbach, S.; Vogeley, A.; Benninghoff, J.; Ruhrmann, S.; et al. 5-HT3 receptor influences the washing phenotype and visual organization in obsessive-compulsive disorder supporting 5-HT3 receptor antagonists as novel treatment option. Eur. Neuropsychopharmacol. 2014, 24, 86–94. [Google Scholar] [CrossRef]
- Gunn, D.; Garsed, K.; Lam, C.; Singh, G.; Lingaya, M.; Wahl, V.; Niesler, B.; Henry, A.; Hall, I.P.; Whorwell, P.; et al. Abnormalities of mucosal serotonin metabolism and 5-HT3 receptor subunit 3C polymorphism in irritable bowel syndrome with diarrhoea predict responsiveness to ondansetron. Alim. Pharmacol. Therapeut. 2019, 50, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Goecke, T.W.; Ekici, A.B.; Niesler, B.; Loehberg, C.R.; Hammer, C.; Rappold, G.; Schanze, D.; Straub, V.; Altmann, H.H.; Strissel, P.; et al. Two naturally occurring variants of the serotonin receptor gene HTR3C are associated with nausea in pregnancy. Acta Obstet. Gynecolg. Scand. 2010, 89, 7–14. [Google Scholar] [CrossRef]
- Eliasen, A.; Dalhoff, K.; Mathiasen, R.; Schmiegelow, K.; Rechnitzer, C.; Schelde, A.B.; Perwitasari, D.A.; Tsuji, D.; Brok, J. Pharmacogenetics of antiemetics for chemotherapy-induced nausea and vomiting: A systematic review and meta-analysis. Crit. Rev. Oncol/Hematol. 2020, 149, 102939. [Google Scholar] [CrossRef] [PubMed]
- Hammer, C.; Fasching, P.A.; Loehberg, C.R.; Rauh, C.; Ekici, A.B.; Jud, S.M.; Bani, M.R.; Beckmann, M.W.; Strick, R.; Niesler, B. Polymorphism in HTR3D shows different risks for acute chemotherapy-induced vomiting after anthracycline chemotherapy. Pharmacogenomics 2010, 11, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.Y.; Ma, L.; Tam, P.O.S.; Pang, C.P.; Tham, C.C.Y.; Chen, L.J. Genetic association of the PARL-ABCC5-HTR3D-HTR3C locus with primary angle-closure glaucoma in Chinese. Invest. Opthalmol. Vis. Sci. 2017, 58, 4104–4109. [Google Scholar] [CrossRef]
- Schuhmacher, A.; Mössner, R.; Quednow, B.B.; Kühn, K.-U.; Wagner, M.; Cvetanovska, G.; Rujescu, D.; Zill, P.; Möller, H.-J.; Rietschel, M.; et al. Influence of 5-HT3 receptor subunit genes HTR3A, HTR3B, HTR3C, HTR3D and HTR3E on treatment response to antipsychotics in schizophrenia. Pharmacogen. Genom. 2009, 19, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Lennertz, L.; Wagner, M.; Frommann, I.; Schulze-Rauschenbach, S.; Schuhmacher, A.; Kühn, K.U.; Pukrop, R.; Klosterkötter, J.; Wölwer, W.; Gaebel, W.; et al. A coding variant of the novel serotonin receptor subunit 5-HT3E influences sustained attention in schizophrenia patients. Eur. Neuropsychopharmacol. 2010, 20, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Alemany-Navarro, M.; Cruz, R.; Real, E.; Segalàs, C.; Bertolín, S.; Rabionet, R.; Carracedo, Á.; Menchón, J.M.; Alonso, P. Looking into the genetic bases of OCD dimensions: A pilot genome-wide association study. Translat. Psych. 2020, 10, 151. [Google Scholar] [CrossRef]
- Chetty, N.; Coupar, I.M.; Tan, Y.Y.; Desmond, P.V.; Irving, H.R. Distribution of serotonin receptors and interacting proteins in the human sigmoid colon. Neurogastroenterol. Motil. 2009, 21, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Irving, H.R.; Tan, Y.Y.; Tochon-Danguy, N.; Liu, H.; Chetty, N.; Desmond, P.V.; Pouton, C.W.; Coupar, I.M. Comparison of 5-HT4 and 5-HT7 receptor expression and function in the circular muscle of the human colon. Life Sci. 2007, 80, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Mochizuki, S.; Takemoto, Y.; Akuzawa, S. Molecular cloning of human 5-hydroxytryptamine3 receptor: Heterogeneity in distribution and function among species. Mol. Pharmacol. 1995, 48, 407–416. [Google Scholar] [PubMed]
- Yaakob, N.S.; Chinkwo, K.A.; Chetty, N.; Coupar, I.M.; Irving, H.R. Distribution of 5-HT3, 5-HT4 and 5-HT7 receptors along the human colon. J. Neurogastroenerol. Mot. 2015, 21, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Van Lelyveld, N.; Ter Linde, J.; Schipper, M.E.I.; Samson, M. Regional differences in expression of TPH-1, SERT, 5-HT3 and 5-HT4 receptors in the human stomach and duodenum. Neurogastroenterol. Motil. 2007, 19, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Chen, M.; Chen, Y.; Mao, G.; Liu, S. Expression and clinical significance of 5-HT and 5-HT3R in the intestinal mucosa of patient with diarrhea-type irritable bowel syndrome. Exper. Therap. Med. 2019, 17, 3077–3082. [Google Scholar] [CrossRef]
- Foxx-Orenstein, A.E.; Kuemmerle, J.F.; Grider, J.R. Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology 1996, 111, 1281–1290. [Google Scholar] [CrossRef]
- Raybould, H.E.; Glatzle, J.; Robin, C.; Meyer, J.H.; Phan, T.; Wong, H.; Sternini, C. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G367–G372. [Google Scholar] [CrossRef]
- Talley, N.J.; Phillips, S.F.; Haddad, A.; Miller, L.J.; Twomey, C.; Zinsmeister, A.R.; MacCarty, R.L.; Ciociola, A. GR 38032F (ondansetron), a selective 5HT3 receptor antagonist, slows colonic transit in healthy man. Dig. Dis. Sci. 1990, 35, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S.; Okumura, T.; Inamori, M.; Okuyama, Y.; Kanazawa, M.; Kamiya, T.; Sato, K.; Shiotani, A.; Naito, Y.; Fujikawa, Y.; et al. Evidence-based clinical practice guidelines for irritable bowel syndrome 2020. J. Gastroent. 2021, 56, 193–217. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.M.; Sayuk, G.S. Current US Food and Drug Administration-Approved Pharmacologic Therapies for the Treatment of Irritable Bowel Syndrome with Diarrhea. Adv. Therap. 2020, 37, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Navari, R.M. Management of Chemotherapy-Induced Nausea and Vomiting in Pediatric Patients. Pediatric Drugs 2017, 19, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.; Slusher, B.S. Pharmacological mechanisms of 5-HT3 and tachykinin NK1 receptor antagonism to prevent chemotherapy-induced nausea and vomiting. Eur. J. Pharmacol. 2012, 684, 1–7. [Google Scholar] [CrossRef]
- Sakurai-Yamashita, Y.; Yamashita, K.; Yoshimura, M.; Taniyama, K. Differential localization of 5-hydroxytryptamine3 and 5-hydroxytryptamine4 receptors in the human rectum. Life Sci. 2000, 66, 31–34. [Google Scholar] [CrossRef]
- Kapeller, J.; Möller, D.; Lasitschka, F.; Autschbach, F.; Hovius, R.; Rappoid, G.; Brüss, M.; Gershon, M.D.; Niesler, B. Serotonin receptor diversity in the human colon: Expression of serotonin type 3 receptor subunits 5-HT32, 5-HT3D, and 5-HT3E. J. Comp. Neurol. 2011, 519, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Michel, K.; Zeller, F.; Langer, R.; Nekarda, H.; Kruger, D.; Dover, T.J.; Brady, C.A.; Barnes, N.M.; Schemann, M. Serotonin excites neurons in the human submucous plexus via 5-HT3 receptors. Gastroenterology 2005, 128, 1317–1326. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Akundi, R.S.; Lieb, K.; Candelario-Jalil, E.; Gmeiner, D.; Haus, U.; Müller, W.; Stratz, T.; Muñoz, E. Antiinflammatory effects of 5-HT3 receptor antagonists in lipopolysaccharide-stimulated primary human monocytes. Scand. J. Rheum. Supp. 2004, 33, 28–32. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Akundi, R.S.; Seidel, M.; Geyer, V.; Haus, U.; Müller, W.; Stratz, T.; Candelario-Jalil, E. Expression of 5-HT3A receptors in cells of the immune system. Scand. J. Rheum. Supp. 2004, 33, 9–11. [Google Scholar] [CrossRef]
- Khan, N.A.; Poisson, J.P. 5-HT3 receptor-channels coupled with Na+ influx in human T cells: Role in T cell activation. J. Neuroimmunol. 1999, 99, 53–60. [Google Scholar] [CrossRef]
- Schneider, E.M.; Ma, X.; Stratz, T.; Müller, W.; Lorenz, I.; Seeling, W.D. Immunomodulatory function of the 5-HT3 receptor antagonist tropisetron. Scand. J. Rheum. Supp. 2004, 33, 34–40. [Google Scholar] [CrossRef]
- Seidel, M.F.; Fiebich, B.L.; Ulrich-Merzenich, G.; Candelario-Jalil, E.; Koch, F.W.; Vetter, H. Serotonin mediates PGE2 overexpression through 5-HT2A and 5-HT3 receptor subtypes in serum-free tissue culture of macrophage-like synovial cells. Rheum. Int. 2008, 28, 1017–1022. [Google Scholar] [CrossRef]
- Stratz, C.; Trenk, D.; Bhatia, H.S.; Valina, C.; Neumann, F.J.; Fiebich, B.L. Identification of 5-HT3 receptors on human platelets: Increased surface immunoreactivity after activation with adenosine diphosphate (ADP) and thrombin receptor-activating peptide (TRAP). Thromb. Haemost. 2008, 99, 784–786. [Google Scholar] [CrossRef]
- Rinaldi, A.; Chiaravalli, A.M.; Mian, M.; Zucca, E.; Tibiletti, M.G.; Capella, C.; Bertoni, F. Serotonin receptor 3A expression in normal and neoplastic B cells. Pathobiol. J. Immunol. Mol. Cell. Biol. 2010, 77, 129–135. [Google Scholar] [CrossRef]
- Ahangari, G.; Amirabad, L.M.; Mozafari, S.; Majeidi, A.; Deilami, G.D. Investigation of 5-HT3A receptor gene expression in peripheral blood mononuclear cells of individuals who had been exposed to air pollution. Inflamm. Allergy Drug Targ. 2013, 12, 433–438. [Google Scholar] [CrossRef]
- Nordlind, K.; Thorslund, K.; Lonne-Rahm, S.; Mohabbati, S.; Berki, T.; Morales, M.; Azmitia, E.C. Expression of serotonergic receptors in psoriatic skin. Arch. Dermatol. Res. 2006, 298, 99. [Google Scholar] [CrossRef]
- Lundeberg, L.; El-Nour, H.; Mohabbati, S.; Morales, M.; Azmitia, E.; Nordlind, K. Expression of serotonin receptors in allergic contact eczematous human skin. Arch. Dermatol. Res. 2002, 294, 393–398. [Google Scholar] [CrossRef]
- Weisshaar, E.; Ziethen, B.; Gollnick, H. Can a serotonin type 3 (5-HT3) receptor antagonist reduce experimentally-induced itch? Inflamm. Res. 1997, 46, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.-P.; Marret, E.; Josserand, J.; Mercier, F.J. Effect of prophylactic 5-HT3 receptor antagonists on pruritus induced by neuraxial opioids: A quantitative systematic review. Brit. J. Anaesth. 2008, 101, 311–319. [Google Scholar] [CrossRef]
- Curtis, J.J.; Vo, N.T.K.; Seymour, C.B.; Mothersill, C.E. Serotonin and 5-HT3 receptors sensitize human skin cells to direct irradiation cell death but not to soluble radiation-induced bystander signals. Environ. Res. 2020, 180, 108807. [Google Scholar] [CrossRef]
- Ando, K.; Takagi, K. Solid gastric emptying mediated by the serotonin 5-HT3 receptor in mice is a simple marker to predict emesis. J. Toxicol. Sci. 2011, 36, 23–29. [Google Scholar] [CrossRef][Green Version]
- Spiller, R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: Alterations in 5-HT signalling and metabolism in human disease. Neurogastroenterol. Motil. 2007, 19, 25–31. [Google Scholar] [CrossRef]
- Sanger, G.J.; Andrews, P.L.R. A History of Drug Discovery for Treatment of Nausea and Vomiting and the Implications for Future Research. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Kato, S. Role of serotonin 5-HT3 receptors in intestinal inflammation. Biol. Pharmaceut. Bull. 2013, 36, 1406–1409. [Google Scholar] [CrossRef]
- Spiller, R.; Major, G. IBS and IBD-separate entities or on a spectrum? Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 613–621. [Google Scholar] [CrossRef]
- Hassaine, G.; Deluz, C.; Grasso, L.; Wyss, R.; Tol, M.B.; Hovius, R.; Graff, A.; Stahlberg, H.; Tomizaki, T.; Desmyter, A.; et al. X-ray structure of the mouse serotonin 5-HT3 receptor. Nature 2014, 512, 276–281. [Google Scholar] [CrossRef]
- Basak, S.; Gicheru, Y.; Samanta, A.; Molugu, S.K.; Huang, W.; la de Fuente, M.; Hughes, T.; Taylor, D.J.; Nieman, M.T.; Moiseenkova-Bell, V.; et al. Cryo-EM structure of 5-HT3A receptor in its resting conformation. Nat. Commun. 2018, 9, 514. [Google Scholar] [CrossRef]
- Basak, S.; Kumar, A.; Ramsey, S.; Gibbs, E.; Kapoor, A.; Filizola, M.; Chakrapani, S. High-resolution structures of multiple 5-HT3AR-setron complexes reveal a novel mechanism of competitive inhibition. eLife 2020, 9, e57870. [Google Scholar] [CrossRef]
- Polovinkin, L.; Hassaine, G.; Perot, J.; Neumann, E.; Jensen, A.A.; Lefebvre, S.N.; Corringer, P.-J.; Neyton, J.; Chipot, C.; Dehez, F.; et al. Conformational transitions of the serotonin 5-HT3 receptor. Nature 2018, 563, 275–279. [Google Scholar] [CrossRef]
- Basak, S.; Gicheru, Y.; Kapoor, A.; Mayer, M.L.; Filizola, M.; Chakrapani, S. Molecular mechanism of setron-mediated inhibition of full-length 5-HT3A receptor. Nat. Commun. 2019, 10, 3225. [Google Scholar] [CrossRef]
- Basak, S.; Gicheru, Y.; Rao, S.; Sansom, M.S.P.; Chakrapani, S. Cryo-EM reveals two distinct serotonin-bound conformations of full-length 5-HT3A receptor. Nature 2018, 563, 270–274. [Google Scholar] [CrossRef]
- Zarkadas, E.; Zhang, H.; Cai, W.; Effantin, G.; Perot, J.; Neyton, J.; Chipot, C.; Schoehn, G.; Dehez, F.; Nury, H. The Binding of Palonosetron and Other Antiemetic Drugs to the Serotonin 5-HT3 Receptor. Structure 2020, 28, 1131–1140.e1134. [Google Scholar] [CrossRef]
- Zhang, Y.; Dijkman, P.M.; Zou, R.; Zandl-Lang, M.; Sanchez, R.M.; Eckhardt-Strelau, L.; Köfeler, H.; Vogel, H.; Yuan, S.; Kudryashev, M. Asymmetric opening of the homopentameric 5-HT3A serotonin receptor in lipid bilayers. Nat. Commun. 2021, 12, 1074. [Google Scholar] [CrossRef]
- Gibbs, E.; Chakrapani, S. Structure, Function and Physiology of 5-Hydroxytryptamine Receptors Subtype 3. In Macromolecular Protein Complexes III: Structure and Function; Harris, J.R., Marles-Wright, J., Eds.; Springer: Cham, Switzerland, 2021; pp. 373–408. [Google Scholar] [CrossRef]
- Guros, N.B.; Balijepalli, A.; Klauda, J.B. Microsecond-timescale simulations suggest 5-HT–mediated preactivation of the 5-HT3A serotonin receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 405. [Google Scholar] [CrossRef]
- Lummis, S.C.R. 5-HT3 receptors. J. Biol. Chem. 2012, 287, 40239–40245. [Google Scholar] [CrossRef]
- Del Cadia, M.; Di Rienzo, F.; Weston, D.A.; Thompson, A.J.; Menziani, M.C.; Lummis, S.C. Exploring a potential palonsetron allosteric binding site in the 5-HT3 receptor. Bioorg. Med. Chem. 2013, 21, 7523–7528. [Google Scholar] [CrossRef]
- Thompson, A.J. Recent developments in 5-HT3 receptor pharmacology. Trends Pharmacol. Sci. 2013, 34, 100–109. [Google Scholar] [CrossRef]
- Jack, T.; Leuenberger, M.; Ruepp, M.-D.; Vernekar, S.K.V.; Thompson, A.J.; Braga-Lagache, S.; Heller, M.; Lochner, M. Mapping the Orthosteric Binding Site of the Human 5-HT3 Receptor Using Photo-cross-linking Antagonists. ACS Chem. Neurosci. 2019, 10, 438–450. [Google Scholar] [CrossRef]
- Abad, I.P.L.; Fam, R.L.; Nguyen, D.-T.; Nowell, C.J.; Trinh, P.N.H.; Manallack, D.T.; Freihat, L.A.; Chakrabarti, J.; Jamil, A.; Exintaris, B.; et al. Visualising functional 5-HT3 receptors containing A and C subunits at or near the cell surface. Biomed. Pharmacother. 2020, 132, 110860. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nuc. Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed]
- Pandhare, A.; Pirayesh, E.; Stuebler, A.G.; Jansen, M. Triple arginines as molecular determinants for pentameric assembly of the intracellular domain of 5-HT3A receptors. J. Gen. Physiol. 2019, 151, 1135–1145. [Google Scholar] [CrossRef]
- Kelley, S.P.; Dunlop, J.I.; Kirkness, E.F.; Lambert, J.J.; Peters, J.A. A cytoplasmic region determines single-channel conductance in 5-HT3 receptors. Nature 2003, 424, 321–324. [Google Scholar] [CrossRef]
- Pandhare, A.; Grozdanov, P.N.; Jansen, M. Pentameric quaternary structure of the intracellular domain of serotonin type 3A receptors. Sci. Rep. 2016, 6, 23921. [Google Scholar] [CrossRef]
- Pirayesh, E.; Stuebler, A.G.; Pandhare, A.; Jansen, M. Delineating the Site of Interaction of the 5-HT3A Receptor with the Chaperone Protein RIC-3. Biophys. J. 2020, 118, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Bollan, K.A.; Greenwood, S.M.; Irving, A.J.; Connolly, C.N. Differential subcellular localization of RIC-3 isoforms and their role in determining 5-HT3 receptor composition. J. Biol. Chem. 2007, 282, 26158–26166. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; McDonald, N.A.; Connolly, C.N. Cell surface expression of 5-hydroxytryptamine type 3 receptors is promoted by RIC-3. J. Biol. Chem. 2005, 280, 22502–22507. [Google Scholar] [CrossRef]
- Walstab, J.; Hammer, C.; Lasitschka, F.; Moller, D.; Connolly, C.N.; Rappold, G.; Bruss, M.; Bonisch, H.; Niesler, B. RIC-3 exclusively enhances the surface expression of human homomeric 5-hydroxytryptamine type 3A (5-HT3A) receptors despite direct interactions with 5-HT3A, -C, -D and -E subunits. J. Biol. Chem. 2010, 285, 26956–26965. [Google Scholar] [CrossRef]
- Boyd, G.W.; Low, P.; Dunlop, J.I.; Robertson, L.A.; Vardy, A.; Lambert, J.J.; Peters, J.A.; Connolly, C.N. Assembly and cell surface expression of homomeric and heteromeric 5-HT3 receptors: The role of oligomerization and chaperone proteins. Mol. Cell. Neurosci. 2002, 21, 38–50. [Google Scholar] [CrossRef]
- Monk, S.A.; Williams, J.M.; Hope, A.G.; Barnes, N.M. Identification and importance of N-glycosylation of the human 5-hydroxytryptamine 3A receptor subunit. Biochem. Pharmacol. 2004, 68, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Quirk, P.L.; Rao, S.; Roth, B.L.; Siegel, R.E. Three putative N-glycosylation sites within the murine 5-HT3A receptor sequence affect plasma membrane targeting, ligand binding, and calcium influx in heterologous mammalian cells. J. Neurosci. Res. 2004, 77, 498–506. [Google Scholar] [CrossRef]
- Massoura, A.N.; Dover, T.J.; Newman, A.S.; Barnes, N.M. The identification of N-glycosylated residues of the human 5-HT3B receptor subunit: Importance for cell membrane expression. J. Neurochem. 2011, 116, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Van Hooft, J.A.; Vijverberg, H.P. Phosphorylation controls conductance of 5-HT3 receptor ligand-gated ion channels. Recept. Channels 1995, 3, 7–12. [Google Scholar]
- Hubbard, P.C.; Thompson, A.J.; Lummis, S.C.R. Functional differences between splice variants of the murine 5-HT3A receptor: Possible role for phosphorylation. Mol. Brain Res. 2000, 81, 101–108. [Google Scholar] [CrossRef]
- Yakel, J.L.; Shao, X.M.; Jackson, M.B. Activation and desensitization of the 5-HT3 receptor in a rat glioma x mouse neuroblastoma hybrid cell. J. Physiol. 1991, 436, 293–308. [Google Scholar] [CrossRef]
- Sun, H.; Hu, X.-Q.; Moradel, E.M.; Weight, F.F.; Zhang, L. Modulation of 5-HT3 Receptor-mediated Response and Trafficking by Activation of Protein Kinase C. J. Biol. Chem. 2003, 278, 34150–34157. [Google Scholar] [CrossRef]
- Zhang, L.; Oz, M.; Weight, F.F. Potentiation of 5-HT3 receptor-mediated responses by protein kinase C activation. Neuroreports 1995, 6, 1464–1468. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Yakel, J.L. Casein kinase II (protein kinase ck2) regulates serotonin 5-HT3 receptor channel function in NG108-15 cells. Neuroscience 2003, 119, 629–634. [Google Scholar] [CrossRef]
- Boddeke, H.W.G.M.; Meigel, I.; Boeijinga, P.; Arbuckle, J.; Docherty, R.J. Modulation by calcineurin of 5-HT3 receptor function in NG108-15 neuroblastoma x glioma cells. Brit. J. Pharmacol. 1996, 118, 1836–1840. [Google Scholar] [CrossRef]
- Eisensamer, B.; Uhr, M.; Meyr, S.; Gimpl, G.; Deiml, T.; Rammes, G.; Lambert, J.J.; Zieglgänsberger, W.; Holsboer, F.; Rupprecht, R. Antidepressants and Antipsychotic Drugs Colocalize with 5-HT3 Receptors in Raft-Like Domains. J. Neurosci. 2005, 25, 10198. [Google Scholar] [CrossRef] [PubMed]
- Nothdurfter, C.; Tanasic, S.; Di Benedetto, B.; Rammes, G.; Wagner, E.-M.; Kirmeier, T.; Ganal, V.; Kessler, J.S.; Rein, T.; Holsboer, F.; et al. Impact of Lipid Raft Integrity on 5-HT3 Receptor Function and its Modulation by Antidepressants. Neuropsychopharmacology 2010, 35, 1510–1519. [Google Scholar] [CrossRef]
- Kudryashev, M.; Castaño-Díez, D.; Deluz, C.; Hassaine, G.; Grasso, L.; Graf-Meyer, A.; Vogel, H.; Stahlberg, H. The Structure of the Mouse Serotonin 5-HT3 Receptor in Lipid Vesicles. Structure 2016, 24, 165–170. [Google Scholar] [CrossRef][Green Version]
- Fantini, J.; Barrantes, F.J. Sphingolipid/cholesterol regulation of neurotransmitter receptor conformation and function. Biochim. Biophys. Acta Biomembr. 2009, 1788, 2345–2361. [Google Scholar] [CrossRef]
- Barrera, N.P.; Herbert, P.; Henderson, R.M.; Martin, I.L.; Edwardson, J.M. Atomic force microscopy reveals the stoichiometry and subunit arrangement of 5-HT3 receptors. Proc. Natl. Acad. Sci. USA 2005, 102, 12595–12600. [Google Scholar] [CrossRef]
- Lochner, M.; Lummis, S.C.R. Agonists and antagonists bind to an A-A interface in the heteromeric 5-HT3AB Receptor. Biophys. J. 2010, 98, 1494–1502. [Google Scholar] [CrossRef]
- Thompson, A.J.; Price, K.L.; Lummis, S.C.R. Cysteine modification reveals which subunits form the ligand binding site in human heteromeric 5-HT3AB receptors. J. Physiol. 2011, 589, 4243–4257. [Google Scholar] [CrossRef][Green Version]
- Miles, T.F.; Dougherty, D.A.; Lester, H.A. The 5-HT3AB receptor shows an A3B2 stoichometry at the plasma membrane. Biophys. J. 2013, 105, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, G.J.; Butler, A.; Burridge, J.; Oxford, A.W. 1-(m-chlorophenyl)-biguanide, a potent high affinity 5-HT3 receptor agonist. Eur. J. Pharmacol. 1990, 182, 193–197. [Google Scholar] [CrossRef]
- Chetty, N.; Irving, H.R.; Coupar, I.M. Activation of 5-HT3 receptors in the rat and mouse intestinal tract: A comparative study. Brit. J. Pharmacol. 2006, 148, 1012–1021. [Google Scholar] [CrossRef][Green Version]
- Chetty, N.; Irving, H.R.; Coupar, I.M. Effects of the novel 5-HT3 agonist MKC-733 on the rat, mouse and guinea-pig digestive tract. Pharmacology 2008, 81, 104–109. [Google Scholar] [CrossRef]
- Roberts, A.; Grafton, G.; Powell, A.D.; Brock, K.; Chen, C.; Xie, D.; Huang, J.; Liu, S.; Cooper, A.J.; Brady, C.A.; et al. CSTI-300 (SMP-100); a Novel 5-HT3 Receptor Partial Agonist with Potential to Treat Patients with Irritable Bowel Syndrome or Carcinoid Syndrome. J. Pharmacol. Exp. Therap. 2020, 373, 122. [Google Scholar] [CrossRef]
- Thompson, A.; Lummis, S. Discriminating between 5-HT3A and 5-HT3AB receptors. Brit. J. Pharmacol. 2013, 169, 736–747. [Google Scholar] [CrossRef]
- Niesler, B.; Walstab, J.; Combrink, S.; Möller, D.; Kapeller, J.; Rietdorf, J.; Bönisch, H.; Göthert, M.; Rappoid, G.; Brüss, M. Characterization of the novel human serotonin receptor subunits 5-HT3C, 5-HT3D, and 5-HT3E. Mol. Pharmacol. 2007, 72, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Price, K.L.; Hirayama, Y.; Lummis, S.C.R. Subtle Differences among 5-HT3AC, 5-HT3AD, and 5-HT3AE Receptors Are Revealed by Partial Agonists. ACS Chem. Neurosci. 2017, 8, 1085–1091. [Google Scholar] [CrossRef]
- Yaakob, N.S.; Nguyen, D.-T.; Exintaris, B.; Irving, H.R. The C and E subunits of the serotonin 5-HT3 receptor subtly modulate the electrical properties of the receptor. Biomed. Pharmacother. 2018, 97, 1701–1709. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Mathie, A.; Peters, J.A.; Veale, E.L.; Striessnig, J.; Kelly, E.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; et al. The Concise Guide to Pharmacology 2019/20: Ion channels. Brit. J. Pharmacol. 2019, 176, S142–S228. [Google Scholar] [CrossRef]
- Costall, B.; Domeney, A.M.; Naylor, R.J.; Tattersall, F.D. 5-Hydroxytryptamine M-receptor antagonism to prevent cisplatin-induced emesis. Neuropharmacology 1986, 25, 959–961. [Google Scholar] [CrossRef]
- Fozard, J.R. MDL 72222: A potent and highly selective antagonist at neuronal 5-hydroxytryptamine receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1984, 326, 36–44. [Google Scholar] [CrossRef]
- Richardson, B.P.; Engel, G.; Donatsch, P.; Stadler, P.A. Identification of serotonin M-receptor subtypes and their specific blockade by a new class of drugs. Nature 1985, 316, 126–131. [Google Scholar] [CrossRef]
- Wong, E.H.F.; Clark, G.; Leung, E.; Loury, D.; Bonhaus, D.W.; Jakeman, L.; Parnes, H.; Whiting, R.L.; Eglen, R.M. The interaction of RS 25259-197, a potent and selective antagonist, with 5-HT3 receptors, in vitro. Brit. J. Pharmacol. 1995, 114, 851–859. [Google Scholar] [CrossRef]
- Ishikawa, M.; Sakata, M.; Toyohara, J.; Oda, K.; Ishii, K.; Wu, J.; Yoshida, T.; Iyo, M.; Ishiwata, K.; Hashimoto, K. Occupancy of α7 nicotinic acetylcholine receptors in the brain by tropisetron: A positron emission tomography study using [(11)C]CHIBA-1001 in healthy human subjects. Clin. Psychopharmacol. Neurosci. 2011, 9, 111–116. [Google Scholar] [CrossRef][Green Version]
- Macor, J.E.; Gurley, D.; Lanthorn, T.; Loch, J.; Mack, R.A.; Mullen, G.; Tran, O.; Wright, N.; Gordon, J.C. The 5-HT3 antagonist tropisetron (ICS 205-930) is a potent and selective alpha7 nicotinic receptor partial agonist. Bioinorg. Med. Chem. Lett. 2001, 11, 319–321. [Google Scholar] [CrossRef]
- Hur, W.; Lee, M.K.; Park, H.-P.; Kim, C.-S.; Yoon, H.-J.; Zuo, Z.; Do, S.-H. Ondansetron attenuates the activity of excitatory amino acid transporter type 3 expressed in Xenopus oocytes. Eur. J. Pharmacol. 2014, 733, 7–12. [Google Scholar] [CrossRef]
- Shinohara, M.; Shinohara, M.; Zhao, J.; Fu, Y.; Liu, C.-C.; Kanekiyo, T.; Bu, G. 5-HT3 Antagonist Ondansetron Increases apoE Secretion by Modulating the LXR-ABCA1 Pathway. Int. J. Mol. Sci. 2019, 20, 1488. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Dillon, G.H. The 5-HT3B subunit confers reduced sensitivity to picrotoxin when co-expressed with the 5-HT3A receptor. Mol. Brain Res. 2003, 119, 207–212. [Google Scholar] [CrossRef]
- Eglen, R.M.; Bonhaus, D.W.; Johnson, L.G.; Leung, E.; Clark, R.D. Pharmacological characterization of two novel and potent 5-HT4 receptor agonists, RS 67333 and RS 67506, in vitro and in vivo. Brit. J. Pharmacol. 1995, 115, 1387–1392. [Google Scholar] [CrossRef]
- Trattnig, S.M.; Harpsoe, K.; Thygesen, S.B.; Rahr, L.M.; Ahring, P.K.; Balle, T.; Jensen, A.A. Discovery of a novel allosteric modulator of 5-HT3 receptors: Inhibition and potentiation of Cys-loop receptor signaling through a conserved transmembrane intersubunit site. J. Biol. Chem. 2012, 287, 25241–25254. [Google Scholar] [CrossRef]
- Walstab, J.; Krüger, D.; Stark, T.; Hofmann, T.; Demir, I.E.; Ceyhan, G.O.; Feistel, B.; Schemann, M.; Niesler, B. Ginger and its pungent constituents non-competitively inhibit activation of human recombinant and native 5-HT3 receptors of enteric neurons. Neurogastroenterol. Motil. 2013, 25, 439–447. [Google Scholar] [CrossRef]
- Nebrisi, E.E.; Prytkova, T.; Lorke, D.E.; Howarth, L.; Alzaabi, A.H.; Yang, K.S.; Howarth, F.C.; Oz, M. Capsaicin is a negative allosteric modulator of the 5-HT3 receptor. Front. Pharmacol. 2020, 11, 1274. [Google Scholar] [CrossRef] [PubMed]
- Ziemba, P.M.; Schreiner, B.S.P.; Flegel, C.; Herbrechter, R.; Stark, T.D.; Hofmann, T.; Hatt, H.; Werner, M.; Gisselmann, G. Activation and modulation of recombinantly expressed serotonin receptor type 3A by terpenes and pungent substances. Biochem. Biophys. Res. Commun. 2015, 467, 1090–1096. [Google Scholar] [CrossRef]
- Lohning, A.E.; Marx, W.; Isenring, L. In silico investigation into the interactions between murine 5-HT3 receptor and the principle active compounds of ginger (Zingiber officinale). J. Mol. Graph. Model. 2016, 70, 315–327. [Google Scholar] [CrossRef]
- Lansdell, S.J.; Sathyaprakash, C.; Doward, A.; Millar, N.S. Activation of Human 5-Hydroxytryptamine Type 3 Receptors via an Allosteric Transmembrane Site. Mol. Pharmacol. 2015, 87, 87. [Google Scholar] [CrossRef]
- Rojas, C.; Stathis, M.; Thomas, A.G.; Massuda, E.B.; Alt, J.; Zhang, J.; Rubenstein, E.; Sebastiani, S.; Cantoreggi, S.; Snyder, S.H.; et al. Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth. Analg. 2008, 107, 469–478. [Google Scholar] [CrossRef]
- Rojas, C.; Thomas, A.G.; Alt, J.; Stathis, M.; Zhang, J.; Rubenstein, E.B.; Sebastiani, S.; Cantoreggi, S.; Slusher, B.S. Palonosetron triggers 5-HT3 receptor internalization and causes prolonged inhibition of receptor function. Eur. J. Pharmacol. 2010, 626, 193–199. [Google Scholar] [CrossRef]
- Miles, T.F.; Lester, H.A.; Dougherty, D.A. Allosteric activation of the 5-HT3AB receptor by mCPBG. Neuropharmacology 2015, 91, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Solt, K.; Stevens, R.J.; Davies, P.A.; Raines, D.E. General anesthetic-induced channel gating enhancement of 5-hydroxytryptamine type 3 receptors depends on receptor subunit composition. J. Pharmacol. Exp. Ther. 2005, 315, 771–776. [Google Scholar] [CrossRef]
- Walstab, J.; Wohlfarth, C.; Hovius, R.; Schmitteckert, S.; Röth, R.; Lasitschka, F.; Wink, M.; Bönisch, H.; Niesler, B. Natural compounds boldine and menthol are antagonists of human 5-HT3 receptors: Implications for treating gastrointestinal disorders. Neurogastroenterol. Motil. 2014, 26, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Verheij, M.H.P.; de Esch, I.J.P.; Lummis, S.C.R. VUF10166, a novel compound with differing activities at 5-HT₃A and 5-HT₃AB receptors. J. Pharmacol. Exp. Therap. 2012, 341, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ishida, Y.; Kondo, M.; Shimada, S. Direct modification of the 5-HT3 receptor current by some anticancer drugs. Eur. J. Pharmacol. 2018, 821, 21–28. [Google Scholar] [CrossRef]
- Blackwell, C.P.; Harding, S.M. The clinical pharmacology of ondansetron. Eur. J. Cancer. Clin. Oncol. 1989, 25 (Suppl. 1), S21–S24, discussion S25–S27. [Google Scholar]
- Navari, R.M.; Madajewicz, S.; Anderson, N.; Tchekmedyian, N.S.; Whaley, W.; Garewal, H.; Beck, T.M.; Chang, A.Y.; Greenberg, B.; Caldwell, K.C. Oral ondansetron for the control of cisplatin-induced delayed emesis: A large, multicenter, double-blind, randomized comparative trial of ondansetron versus placebo. J. Clin. Oncol. 1995, 13, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Tyers, M.B.; Bunce, K.T.; Humphrey, P.P. Pharmacological and anti-emetic properties of ondansetron. Eur. J. Cancer. Clin. Oncol. 1989, 25 (Suppl. 1), S15–S19. [Google Scholar]
- Aapro, M.; Scotté, F.; Escobar, Y.; Celio, L.; Berman, R.; Franceschetti, A.; Bell, D.; Jordan, K. Practice Patterns for Prevention of Chemotherapy-Induced Nausea and Vomiting and Antiemetic Guideline Adherence Based on Real-World Prescribing Data. Oncologist 2021, 26, e1073–e1082. [Google Scholar] [CrossRef]
- Theriot, J.; Wermuth, H.R.; Ashurst, J.V. Antiemetic Serotonin-5-HT3 Receptor Blockers. [Updated 2020 Dec 23]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Zhong, W.; Shahbaz, O.; Teskey, G.; Beever, A.; Kachour, N.; Venketaraman, V.; Darmani, N.A. Mechanisms of Nausea and Vomiting: Current Knowledge and Recent Advances in Intracellular Emetic Signaling Systems. Int. J. Mol. Sci. 2021, 22, 5797. [Google Scholar] [CrossRef] [PubMed]
- Chow, R.; Aapro, M.; Navari, R.M.; Gralla, R.; Chiu, N.; Chiu, L.; Chan, S.; Popovic, M.; Lam, H.; Lock, M.; et al. Do we still need to study palonosetron for chemotherapy-induced nausea and vomiting? A cumulative meta-analysis. Crit. Rev. Oncol/Hematol. 2019, 142, 164–186. [Google Scholar] [CrossRef]
- Workman, E.A. Neuropharmacology. In Encyclopedia of the Human Brain; Ramachandran, V.S., Ed.; Academic Press: New York, NY, USA, 2002; pp. 541–560. [Google Scholar] [CrossRef]
- Camilleri, M.; Halawi, H.; Oduyebo, I. Biomarkers as a diagnostic tool for irritable bowel syndrome: Where are we? Exp. Rev. Gastroenterol. Hepatol. 2017, 11, 303–316. [Google Scholar] [CrossRef]
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable bowel syndrome. New Eng. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef]
- Hofland, J.; Herrera-Martinez, A.D.; Zandee, W.T.; de Herder, W.W. Management of carcinoid syndrome: A systematic review and meta-analysis. Endocr. Relat. Cancer 2019, 26, R145–R156. [Google Scholar] [CrossRef] [PubMed]
- Kiesewetter, B.; Duan, H.; Lamm, W.; Haug, A.; Riss, P.; Selberherr, A.; Scheuba, C.; Raderer, M. Oral ondansetron offers effective antidiarrheal activity for carcinoid syndrome refractory to somatostatin analogs. Oncologist 2019, 24, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Selby, A.; Reichenbach, Z.W.; Piech, G.; Friedenberg, F.K. Pathophysiology, Differential Diagnosis, and Treatment of Diabetic Diarrhea. Dig. Dis. Sci. 2019, 64, 3385–3393. [Google Scholar] [CrossRef] [PubMed]
- Fichna, J.; Storr, M.A. Brain-gut interactions in IBS. Front. Pharmacol. 2012, 3, 127. [Google Scholar] [CrossRef]
- Fukudo, S. Irritable bowel syndrome, emotion regulation, and gut microbiota. Brain Nerve 2016, 68, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S.; Kinoshita, Y.; Okumura, T.; Ida, M.; Akiho, H.; Nakashima, Y.; Nishida, A.; Haruma, K. Ramosetron Reduces Symptoms of Irritable Bowel Syndrome with Diarrhea and Improves Quality of Life in Women. Gastroenterology 2016, 150, 258–366e258. [Google Scholar] [CrossRef] [PubMed]
- Koloski, N.A.; Jones, M.; Kalantar, J.; Weltman, M.; Zaguire, J.; Talley, N.J. The brain-gut pathway in functional gastrointestinal disordes is bidirectional: A 12-year prospective population-based study. Gut 2012, 61, 1284–1290. [Google Scholar] [CrossRef]
- Rokkas, T.; Ekmektzoglou, K.; Niv, Y. Comparative effectiveness of 5-hydroxytryptamine 3 receptor antagonists in irritable bowel syndrome: A network meta-analysis of randomized controlled studies. Ann. Gastroenterol. 2021, 34, 535–546. [Google Scholar] [CrossRef]
- Greenwald, M.K. Anti-stress neuropharmacological mechanisms and targets for addiction treatment: A translational framework. Neurobiol. Stress 2018, 9, 84–104. [Google Scholar] [CrossRef]
- Zheng, W.; Cai, D.-B.; Zhang, Q.-E.; He, J.; Zhong, L.-Y.; Sim, K.; Ungvari, G.S.; Ning, Y.-P.; Xiang, Y.-T. Adjunctive ondansetron for schizophrenia: A systematic review and meta-analysis of randomized controlled trials. J. Psych. Res. 2019, 113, 27–33. [Google Scholar] [CrossRef]
- Hermann, B.; Wetzel, C.H.R.; Pestel, E.; Zieglgänsberger, W.; Holsboer, F.; Rupprecht, R. Functional antagonistic properties of clozapine at the 5-HT3 receptor. Biochem. Biophys. Res. Commun. 1996, 225, 957–960. [Google Scholar] [CrossRef]
- Watling, K.J.; Beer, M.S.; Stanton, J.A.; Newberry, N.R. Interaction of the atypical neuroleptic clozapine with 5-HT3 receptors in the cerebral cortex and superior cervical ganglion of the rat. Eur. J. Pharmacol. 1990, 182, 465–472. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Mohammadi, N.; Noroozian, M.; Karamghadiri, N.; Ghoreishi, A.; Jamshidi, A.H.; Forghani, S. Added ondansetron for stable schizophrenia: A double blind, placebo controlled trial. Schiz. Res. 2009, 107, 206–212. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Kang, W.H.; Li, Q.; Wang, X.Y.; Yao, S.M.; Ma, A.Q. Beneficial effects of ondansetron as an adjunct to haloperidol for chronic, treatment-resistant schizophrenia: A double-blind, randomized, placebo-controlled study. Schiz. Res. 2006, 88, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Serata, D.; Kotzalidis, G.D.; Rapinesi, C.; Janiri, D.; Di Pietro, S.; Callovini, G.; Piacentino, D.; Gasperoni, C.; Brugnoli, R.; Ferri, V.R.; et al. Are 5-HT3 antagonists effective in obsessive–compulsive disorder? A systematic review of literature. Hum. Psychopharmacol. Clin. Exp. 2015, 30, 70–84. [Google Scholar] [CrossRef]
- Johnson, B.A.; Campling, G.M.; Griffiths, P.; Cowen, P.J. Attenuation of some alcohol-induced mood changes and the desire to drink by 5-HT(3) receptor blockade—A preliminary-study in healthy male-volunteers. Psychopharmacology 1993, 112, 142–144. [Google Scholar] [CrossRef]
- Johnson, B.A.; Roache, J.D.; Javors, M.A.; DiClemente, C.C.; Cloninger, C.R.; Prihoda, T.J.; Bordnick, P.S.; Ait-Daoud, N.; Hensler, J. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: A randomized controlled trial. J. Am. Med. Assoc. 2000, 284, 963–971. [Google Scholar] [CrossRef]
- Myrick, H.; Anton, R.F.; Li, X.; Henderson, S.; Randall, P.K.; Voronin, K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch. Gen. Psych. 2008, 65, 466–475. [Google Scholar] [CrossRef]
- Chu, L.F.; Sun, J.; Clemenson, A.; Erlendson, M.J.; Rico, T.; Cornell, E.; Obasi, H.; Sayyid, Z.N.; Encisco, E.M.; Yu, J.; et al. Ondansetron Does Not Reduce Withdrawal in Patients With Physical Dependence on Chronic Opioid Therapy. J. Addiction Med. 2017, 11. [Google Scholar] [CrossRef]
- Jensen, A.A.; Davies, P.A.; Bräuner-Osborne, H.; Krzywkowski, K. 3B but which 3B? And that’s just one of the questions: The heterogeneity of human 5-HT3 receptors. Trends Pharmacol. Sci. 2008, 29, 437–444. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davies, P.A. Allosteric modulation of the 5-HT3 receptor. Curr. Opin. Phamacol. 2011, 11, 75–80. [Google Scholar] [CrossRef]
- Lovinger, D.M.; Sung, K.W.; Zhou, Q. Ethanol and trichloroethanol alter gating of 5-HT3 receptor-channels in NCB-20 neuroblastoma cells. Neuropharmacology 2000, 39, 561–570. [Google Scholar] [CrossRef]
- Lovinger, D.M.; Zhou, Q. Alcohols potentiate ion current mediated by recombinant 5-HT3RA receptors expressed in a mammalian cell line. Neuropharmacology 1994, 33, 1567–1572. [Google Scholar] [CrossRef]
- Machu, T.K. Therapeutics of 5-HT3 receptor antagonists: Current uses and future directions. Pharmacol. Therap. 2011, 130, 338–347. [Google Scholar] [CrossRef]
- Zhang, L.; Hosoi, M.; Fukuzawa, M.; Sun, H.; Rawlings, R.R.; Weight, F.F. Distinct molecular basis for differential sensitivity of the serotonin type 3A receptor to ethanol in the absence and presence of agonist. J. Biol. Chem. 2002, 277, 46256–46264. [Google Scholar] [CrossRef]
- Blausen com Staff. Medical gallery of Blausen Medical 2014. WikiJ. Med. 2014, 1. [Google Scholar] [CrossRef]
- Neugebauer, V. Chapter 17—Serotonin—pain modulation. In Handbook of Behavioral Neuroscience; Müller, C.P., Cunningham, K.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 309–320. [Google Scholar]
- Zeitz, K.P.; Guy, N.; Malmberg, A.B.; Dirajlal, S.; Martin, W.J.; Sun, L.; Bonhaus, D.W.; Stucky, C.L.; Julius, D.; Basbaum, A.I. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J. Neurosci. 2002, 22, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Ernberg, M.; Wieslander Fältmars, A.; Hajizadeh Kopayeh, M.; Arzt Wallén, S.; Cankalp, T.; Christidis, N. The Effect of Granisetron on Sensory Detection and Pain Thresholds in Facial Skin of Healthy Young Males. Front. Neurol. 2020, 11. [Google Scholar] [CrossRef]
- Späth, M.; Stratz, T.; Neeck, G.; Kötter, I.; Hammel, B.; Amberger, C.C.; Haus, U.; Färber, L.; Pongratz, D.; Müller, W. Efficacy and tolerability of intravenous tropisetron in the treatment of fibromyalgia. Scand. J. Rheum. 2004, 33, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Stratz, T.; Farber, L.; Varga, B.; Baumgartner, C.; Haus, U.; Müller, W. Fibromyalgia treatment with intravenous tropisetron administration. Drugs Exp. Clin. Res. 2001, 27, 113–118. [Google Scholar] [PubMed]
- Naeem, F.; Schramm, C.; Friedman, B.W. Emergent management of primary headache: A review of current literature. Curr. Opin. Neurol. 2018, 31. [Google Scholar] [CrossRef]
- Talai, A.; Heilbrunn, B. Ondansetron for Acute Migraine in the Pediatric Emergency Department. Pediatric Neurol. 2020, 103, 52–56. [Google Scholar] [CrossRef]
- Camilleri, M.; Boeckxstaens, G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 2017, 66, 966–974. [Google Scholar] [CrossRef]
- Krzywkowski, K.; Davies, P.A.; Irving, A.J.; Brauner-Osborne, H.; Jensen, A.A. Characterization of the effects of four HTR3B polymorphisms on human 5-HT3AB receptor expression and signalling. Pharmacogen. Genom. 2008, 18, 1027–1040. [Google Scholar] [CrossRef]
- Krzywkowski, K.; Jensen, A.A.; Connolly, C.N.; Brauner-Osborne, H. Naturally occuring variations in the human 3-HT3A gene profoundly impact 5-HT3 receptor function and expression. Pharmacogen. Genom. 2007, 17, 255–266. [Google Scholar] [CrossRef]
- Oh, C.M.; Park, S.; Kim, H. Serotonin as a new therapeutic target for diabetes mellitus and obesity. Diabetes Metab. J. 2016, 40, 89–98. [Google Scholar] [CrossRef]
- Wyler, S.C.; Lord, C.C.; Lee, S.M.; Elmquist, J.K.; Liu, C. Serotonergic control of metabolic homeostasis. Front. Cell. Neurosci. 2017, 11, 277. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Yamada, J.; Yoshikawa, T.; Horisaka, K. The effects of peripheral serotonin2 (5-HT2) and serotonin3 (5-HT3) receptor agonists on blood glucose levels in rats. Biol. Pharmaceut. Bull. 1996, 19, 1384–1386. [Google Scholar] [CrossRef][Green Version]
- Carvalho, F.; Macedo, D.; Bandeira, I.; Maldonado, I.; Salles, L.; Azevedo, M.F.; Rocha Jr, M.A.; Fregoneze, J.B.; Castro-e-Silva, E. Central 5-HT3 receptor stimulation by m-CPBG increases blood glucose in rats. Horm. Metab. Res. 2002, 34, 55–61. [Google Scholar] [CrossRef]
- Bachy, A.; Héaulme, M.; Giudice, A.; Michaud, J.-C.; Lefevre, I.A.; Souilhac, J.; Manara, L.; Emerit, M.B.; Gozlan, H.; Hamon, M.; et al. SR 57227A: A potent and selective agonist at central and peripheral 5-HT3 receptors in vitro and in vivo. Eur. J. Pharmacol. 1993, 237, 299–309. [Google Scholar] [CrossRef]
- Li, B.; Shao, D.; Luo, Y.; Wang, P.; Liu, C.; Zhang, X.; Cui, R. Role of 5-HT3 receptor on food intake in fed and fasted mice. PLoS ONE 2015, 10, e0121473. [Google Scholar] [CrossRef]
- Pratt, W.E.; Lin, P.; Pierce-Messick, Z.; Ilesanmi, A.O.; Clissold, K.A. Contrasting effects of 5-HT3 receptor stimulation of the nucleus accumbens or ventral tegmentum on food intake in the rat. Behav. Brain Res. 2017, 323, 15–23. [Google Scholar] [CrossRef]
- Voight, J.-P.; Fink, H. Serotonin controlling feeding and satiety. Behav. Brain Res. 2015, 277, 14–31. [Google Scholar] [CrossRef]
- Lee, M.D.; Clifton, P.G. Chapter 27—Role of the serotonergic system in appetite and ingestion control. In Handbook of Behavioral Neuroscience; Müller, C.P., Cunningham, K.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 469–487. [Google Scholar]
- Gershon, M.D.; Tack, J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Zelkas, L.; Raghupathi, R.; Lumsden, A.L.; Martin, A.M.; Sun, E.; Spencer, N.J.; Young, R.L.; Keating, D.J. Serotonin-secreting enteroendocrine cells respond via diverse mechanisms to acute and chronic changes in glucose availability. Nut. Metab. 2015, 12, 55. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.Y.; Zhu, J.X.; Owyang, C. Intestinal serotonin acts as a paracrine substance to mediate pancreatic secretion stimulated by luminal factors. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G916–G923. [Google Scholar] [CrossRef]
- Zhu, J.X.; Wu, X.Y.; Owyang, C.; Li, Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J. Physiol. 2001, 530, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Babic, T.; Troy, A.E.; Fortna, S.R.; Browning, K.N. Glucose-dependent trafficking of 5-HT3 receptors in rat gastrointestinal vagal affererent neurons. Neurogastroenterol. Motil. 2012, 24, e476–e488. [Google Scholar] [CrossRef] [PubMed]
- Gentilcore, D.; Little, T.J.; Feinle-Bisset, C.; Samsom, M.; Snmout, A.J.P.M.; Horowitz, M.; Jones, K.L. Role of 5-hydroxytryptamine mechanisms in mediating the effects of small intestinal glucose on blood pressure and antropyloroduodenal motility in older subjects. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G692–G698. [Google Scholar] [CrossRef]
- Troy, A.E.; Simmonds, S.S.; Stocker, S.D.; Browning, K.N. High fat diet attenuates glucose-dependent facilitation of 5-HT3-mediated responses in rat gastric vagal afferents. J. Physiol. 2016, 594, 99–114. [Google Scholar] [CrossRef]
- Weber, S.; Volynets, V.; Kanuri, G.; Bergheim, I.; Bischoff, S.C. Treatment with 5-HT3 antagonist tropisetron modulates glucose-induced obesity in mice. Int. J. Obes. 2009, 33, 1339–1347. [Google Scholar] [CrossRef]
- Bliss, E.S.; Whiteside, E. The Gut-Brain Axis, the Human gut microbiota and their integration in the development of obesity. Front. Physiol. 2018, 9, 900. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The microbiota-gut-brain axis: From motility to mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Haub, S.; Ritze, Y.; Ladel, I.; Saum, K.; Hubert, A.; Spruss, A.; Trautwein, C.; Bischoff, S.C. Serotonin receptor type 3 antagonists improve obesity-associated fatty liver disease in mice. J. Pharmacol. Exp. Therap. 2011, 339, 790–798. [Google Scholar] [CrossRef]
- Heimes, K.; Feistel, B.; Verspohl, E.J. Impact of 5-HT3 receptor channel system for insulin secretion and interaction of ginger extracts. Eur. J. Pharmacol. 2009, 624, 58–65. [Google Scholar] [CrossRef]
- Kondo, M.; Nakamura, Y.; Ishida, Y.; Shimada, S. The 5-HT3 receptor is essential for exercise-induced hippocampal neurogenesis and antidepressant effects. Mol. Psych. 2015, 20, 1428–1437. [Google Scholar] [CrossRef]
- Crane, J.D.; Palanivel, R.; Mottillo, E.P.; Bujak, A.L.; Wang, H.; Ford, R.J.; Collins, A.; Blümer, R.M.; Fullerton, M.D.; Yabut, J.M.; et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat. Med. 2015, 21, 166–172. [Google Scholar] [CrossRef]
- Kim, H.; Toyofuku, Y.; Lynn, F.C.; Chak, E.; Uchida, T.; Mizukami, H.; Fujitani, Y.; Kawamori, R.; Miyatsuka, T.; Kosaka, Y.; et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat. Med. 2010, 16, 804–808. [Google Scholar] [CrossRef]
- Gershon, M.D.; Ross, L.L. Location of sites of 5-hydroxytryptamine storage and metabolism by autoradiography. J. Physiol. 1966, 186, 477–492. [Google Scholar] [CrossRef]
- Chen, H.; Hong, F.; Chen, Y.; Li, J.; Yao, Y.-S.; Zhang, Y.; Zheng, L.-F.; Zhu, J.-X. Activation of islet 5-HT4 receptor regulates glycemic control through promoting insulin secretion. Eur. J. Pharmacol. 2016, 789, 354–361. [Google Scholar] [CrossRef]
- Kim, K.; Oh, C.-M.; Ohara-Imaizumi, M.; Park, S.; Namkung, J.; Yadav, V.K.; Tamarina, N.A.; Roe, M.W.; Philipson, L.H.; Karsenty, G.; et al. Functional Role of Serotonin in Insulin Secretion in a Diet-Induced Insulin-Resistant State. Endocrinology 2015, 156, 444–452. [Google Scholar] [CrossRef]
- Ohara-Imaizumi, M.; Kim, H.; Yoshida, M.; Fujiwara, T.; Aoyagi, K.; Toyofuku, Y.; Nakamichi, Y.; Nishiwaki, C.; Okamura, T.; Uchida, T.; et al. Serotonin regulates glucose-stimulated insulin secretion from pancreatic β cells during pregnancy. Proc. Natl. Acad. Sci. USA 2013, 110, 19420–19425. [Google Scholar] [CrossRef]
- Barzegar-Fallah, A.; Alimoradi, H.; Asadi, F.; Dehpour, A.R.; Asgari, M.; Shafiei, M. Tropisetron ameliorates early diabetic nephropathy in streptozotocin-induced diabetic rats. Clin. Exper Pharmacol. Physiol. 2015, 42, 361–368. [Google Scholar] [CrossRef]
- Naderi, R.; Pourheydar, B.; Ghiasi, R.; Shafiei, F. Modulatory effect of tropisetron in the liver of streptozotocin-induced diabetes in rats: Biochemical and histological evidence. Horm. Mol. Biol. Clin. Investig. 2020, 41. [Google Scholar] [CrossRef] [PubMed]
- Naderi, R.; Shirpoor, A.; Samadi, M.; Pourheydar, B.; Moslehi, A. Tropisetron improves pancreas function and increases insulin synthesis and secretion in the STZ-induced diabetic rats: Involvement of UCP2/ZnT8 pathway. J. Pharm. Pharmacol. 2020, 72, 1082–1091. [Google Scholar] [CrossRef]
- Li, Q.; Guo, D.; Dong, Z.; Zhang, W.; Zhang, L.; Huang, S.-M.; Polli, J.E.; Shu, Y. Ondansetron can enhance cisplatin-induced nephrotoxicity via inhibition of multiple toxin and extrusion proteins (MATEs). Toxicol. Appl. Pharmacol. 2013, 273, 100–109. [Google Scholar] [CrossRef]
- Tzvetkov, M.V.; Saadatmand, A.R.; Bokelmann, K.; Meineke, I.; Kaiser, R.; Brockmöller, J. Effects of OCT1 polymorphisms on the cellular uptake, plasma concentrations and efficacy of the 5-HT3 antagonists tropisetron and ondansetron. Pharmacogen. J. 2012, 12, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, H.; Guo, D.; Zhang, T.; Polli, J.E.; Zhou, H.; Shu, Y. Effect of Ondansetron on Metformin Pharmacokinetics and Response in Healthy Subjects. Drug Metab. Disposit. 2016, 44, 489. [Google Scholar] [CrossRef]
- Kwak, S.H.; Park, B.L.; Kim, H.; German, M.S.; Go, M.J.; Jung, H.S.; Koo, B.K.; Cho, Y.M.; Choi, S.H.; Cho, Y.S.; et al. Association of Variations in TPH1 and HTR2B with Gestational Weight Gain and Measures of Obesity. Obesity 2012, 20, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Groth, S.W.; Hodgkinson, C.A.; Mariani, T.J. Serotonin system genes contribute to the susceptibility to obesity in Black adolescents. Obes. Sci. Pract. 2021. [Google Scholar] [CrossRef] [PubMed]
- Young, R.L.; Lumsden, A.L.; Martin, A.M.; Schober, G.; Pezos, N.; Thazhath, S.S.; Isaacs, N.J.; Cvijanovic, N.; Sun, E.W.L.; Wu, T.; et al. Augmented capacity for peripheral serotonin release in human obesity. Int. J. Obes. 2018, 42, 1880–1889. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocrine Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Fried, S.; Wemelle, E.; Cani, P.D.; Knauf, C. Interactions between the microbiota and enteric nervous system during gut-brain disorders. Neuropharmacology 2021, 197, 108721. [Google Scholar] [CrossRef]
- Bartness, T.J.; Vaughan, C.H.; Song, C.K. Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. 2010, 34, S36–S42. [Google Scholar] [CrossRef]
- Morrison, S.F.; Madden, C.J.; Tupone, D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 2014, 19, 741–756. [Google Scholar] [CrossRef]
- Rathner, J.A.; Morrison, S.F. Rostral ventromedial periaqueductal gray: A source of inhibition of the sympathetic outflow to brown adipose tissue. Brain Res. 2006, 1077, 99–107. [Google Scholar] [CrossRef]
- Van Schaik, L.; Kettle, C.; Green, R.; Irving, H.R.; Rathner, J.A. Effects of Caffeine on Brown Adipose Tissue Thermogenesis and Metabolic Homeostasis: A Review. Front. Neurosci. 2021, 15, 54. [Google Scholar] [CrossRef]
- Sievers, W.; Rathner, J.A.; Green, R.A.; Kettle, C.; Irving, H.R.; Whelan, D.R.; Fernandez, R.G.D.; Zacharias, A. Innervation of supraclavicular adipose tissue: A human cadaveric study. PLoS ONE 2020, 15, 0236286. [Google Scholar] [CrossRef]
- Oh, C.M.; Namkung, J.; Go, Y.; Shong, K.E.; Kim, K.; Kim, H.; Park, B.Y.; Lee, H.W.; Jeon, Y.H.; Song, J.; et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat. Commun. 2015, 6, 7794. [Google Scholar] [CrossRef]
- Han, Y.; Xia, G.; Srisai, D.; Meng, F.; He, Y.; Ran, Y.; He, Y.; Farias, M.; Hoang, G.; Tóth, I.; et al. Deciphering an AgRP-serotoninergic neural circuit in distinct control of energy metabolism from feeding. Nat. Commun. 2021, 12, 3525. [Google Scholar] [CrossRef]
- Xia, G.; Han, Y.; Meng, F.; He, Y.; Srisai, D.; Farias, M.; Dang, M.; Palmiter, R.D.; Xu, Y.; Wu, Q. Reciprocal control of obesity and anxiety–depressive disorder via a GABA and serotonin neural circuit. Mol. Psych. 2021. [Google Scholar] [CrossRef]
- Gadde, K.M.; Kopping, M.F.; Wagner, H.R.; Yonish, G.M.; Allison, D.B.; Bray, G.A. Zonisamide for weight reduction in obese adults: A 1-year randomized controlled trial. Arch. Intern. Med. 2012, 172, 1557–1564. [Google Scholar] [CrossRef]
- McElroy, S.L.; Kotwal, R.; Guerdjikova, A.I.; Welge, J.A.; Nelson, E.B.; Lake, K.A.; D’Alessio, D.A.; Keck, P.E.; Hudson, J.I. Zonisamide in the treatment of binge eating disorder with obesity: A randomized controlled trial. J. Clin. Psych. 2006, 67, 1897–1906. [Google Scholar] [CrossRef]
- Janssen, P.; Vos, R.; van Oudenhove, L.; Tack, J. Influence of the 5-HT3 receptor antagonist ondansetron on gastric sensorimotor function and nutrient tolerance in healthy volunteers. Neurogastroenterol. Motil. 2011, 23, 444.e175. [Google Scholar] [CrossRef]
- Wan, M.; Ding, L.; Wang, D.; Han, J.; Gao, P. Serotonin: A Potent Immune Cell Modulator in Autoimmune Diseases. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Ahern, G.P. 5-HT and the immune system. Curr. Opin. Phamacol. 2011, 11. [Google Scholar] [CrossRef]
- Shajib, M.S.; Knan, W. The role of serotonin and its receptors in activation of immune response and inflammation. Acta Physiol. 2015, 213, 561–574. [Google Scholar] [CrossRef]
- Chen, Y.; Leon-Ponte, M.; Pingle, C.; O’Connell, P.J.; Ahern, G.P. T lymphocytes possess the machinery for 5-HT synthesis, storage, degradation and release. Acta Physiol. 2015, 215, 860–867. [Google Scholar] [CrossRef]
- Kushnir-Sukhov, N.M.; Gilfillan, A.M.; Coleman, J.W.; Brown, J.M.; Bruening, S.; Toth, M.; Metcalfe, D.D. 5-Hydroxytryptamine induces mast cell adhesion and migration. J. Immunol. 2006, 177, 6422–6432. [Google Scholar] [CrossRef]
- Leon-Ponte, M.; Ahern, G.P.; O’Connell, P.J. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood 2007, 109, 3139–3146. [Google Scholar] [CrossRef]
- Nowak, E.C.; de Vries, V.C.; Wasiuk, A.; Ahonen, C.; Bennett, K.A.; Le Mercier, I.; Ha, D.-G.; Noelle, R.J. Tryptophan hydroxylase-1 regulates immune tolerance and inflammation. J. Exp. Med. 2012, 209, 2127–2135. [Google Scholar] [CrossRef]
- O’Connell, P.J.; Wang, X.; Leon-Ponte, M.; Griffiths, C.; Pingle, S.C.; Ahern, G.P. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood 2006, 107, 1010–1017. [Google Scholar] [CrossRef]
- Dürk, T.; Panther, E.; Müller, T.; Sorichter, S.; Ferrari, D.; Pizzirani, C.; Di Virgilio, F.; Myrtek, D.; Norgauer, J.; Idzko, M. 5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes. Int. Immunol. 2005, 17, 599–606. [Google Scholar] [CrossRef]
- Stefulj, J.; Jernej, B.; Cicin-Sain, L.; Rinner, I.; Schauenstein, K. mRNA expression of serotonin recpetors in cells of the immune tissues of the rat. Brain Behav. Immun. 2000, 14, 219–224. [Google Scholar] [CrossRef]
- Casa-Engel, M.d.I.; Dominguez-Soto, A.; Sierra-Filardi, E.; Bragado, R.; Nieto, C.; Puig-Kroger, A.; Samaniego, R.; Loza, M.; Corcuera, M.T.; Gomez-Aguado, F.; et al. Serotonin skews human macrophage polarization through HTR2B and HTR7. J. Immunol. 2013, 190, 2301–2310. [Google Scholar] [CrossRef]
- Choquet, D.; Korn, H. Dual effects of serotonin on a voltage-gated conductance in lymphocytes. Proc. Natl. Acad. Sci. USA 1988, 85, 4557–4561. [Google Scholar] [CrossRef]
- Barzegar-Fallah, A.; Alimoradi, H.; Razmi, A.; Dehpour, A.R.; Asgari, M.; Shafiei, M. Inhibition of calcineurin/NFAT pathway plays an essential role in renoprotective effect of tropisetron in early stage of diabetic nephropathy. Eur. J. Pharmacol. 2015, 767, 152–159. [Google Scholar] [CrossRef]
- De la Vega, L.M.; Muñoz, E.; Calzado, M.A.; Lieb, K.; Candelario-Jalil, E.; Gschaidmeir, H.; Färber, L.; Mueller, W.; Stratz, T.; Fiebich, B.L. The 5-HT3 receptor antagonist tropisetron inhibits T cell activation by targeting the calcineurin pathway. Biochem. Pharmacol. 2005, 70, 369–380. [Google Scholar] [CrossRef]
- Forcen, R.; Latorre, E.; Pardo, J.; Alcaide, A.I.; Murillo, M.D.; Grasa, L. Toll-like receptors 2 and 4 exert opposite effects on the contractile response induced by serotonin in the mouse colon: Role of serotonin receptors. Exp. Physiol. 2016, 101, 1064–1074. [Google Scholar] [CrossRef]
- Forcén, R.; Latorre, E.; Pardo, J.; Alcalde, A.I.; Murillo, M.D.; Grasa, L. Toll-like receptors 2 and 4 modulate the contractile response induced by serotonin in mouse ileum: Analysis of the serotonin receptors involved. Neurogastroenterol. Motil. 2015, 27, 1258–1266. [Google Scholar] [CrossRef]
- Hagbom, M.; Hellysaz, A.; Istrate, C.; Nordgren, J.; Sharma, S.; Meira de-Faria, F.; Magnusson, K.-E.; Svensson, L. The 5-HT3 Receptor Affects Rotavirus-Induced Motility. J. Virol. 2021, 95, 15. [Google Scholar] [CrossRef]
- Moore, K.A.; Oh, E.J.; Weinreich, D. 5-HT3 receptors mediate inflammation-induced unmasking of functional tachykinin responses in vitro. J. Appl. Physiol. 2002, 92, 2529–2534. [Google Scholar] [CrossRef] [PubMed]
- Stratz, C.; Bhatia, H.S.; Akundi, R.S.; Nührenberg, T.; Trenk, D.; Muñoz, E.; Fiebich, B.L. The anti-inflammatory effects of the 5-HT3 receptor antagonist tropisetron are mediated by the inhibition of p38 MAPK activation in primary human monocytes. Int. Immunopharmacol. 2012, 13, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Stratz, C.; Anakwue, J.; Bhatia, H.S.; Pitz, S.; Fiebich, B.L. Anti-inflammatory effects of 5-HT3 receptor antagonists on interleukin-1beta stimulated primary human chondrocytes. Int. Immunol. 2014, 22, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Bayer, H.; Müller, T.; Myrtek, D.; Sorichter, S.; Ziegenhagen, M.; Norgauer, J.; Zissel, C.; Idzko, M. Serotoninergic receptors on human airway epithelial cells. Am. J. Resp. Cell Mol. Biol. 2007, 36, 85–93. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, S.Y.; Kim, N.Y.; Kim, D.W.; Oh, J.E.; Heo, E.; Lee, J.S.; Yoo, Y.C. Anti-Tumor Potential of a 5-HT3 Receptor Antagonist as a Novel Autophagy Inducer in Lung Cancer: A Retrospective Clinical Study with In Vitro Confirmation. J. Clin. Med. 2019, 8, 1380. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Panther, E.; Stratz, C.; Müller, T.; Bayer, H.; Zissel, G.; Dürk, T.; Sorichter, S.; Di Virgilio, F.; Geissler, M.; et al. The serotoninergic receptors of human dendritic cells: Identification and coupling to cytokine release. J. Immunol. 2004, 172, 6011–6019. [Google Scholar] [CrossRef]
- Müller, T.; Dürk, T.; Blumenthal, B.; Grimm, M.; Cicko, S.; Panther, E.; Sorichter, S.; Herouy, Y.; Di Vigilio, F.; Ferrari, D.; et al. 5-Hydroxytryptamine modulates migration, cytokine and chemokine release and T-cell priming capacity of dendritic cells in vitro and in vivo. PLoS ONE 2009, 4, e6453. [Google Scholar] [CrossRef] [PubMed]
- Ip, W.K.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef]
- Utsumi, D.; Matsumoto, K.; Amagase, K.; Horie, S.; Kato, S. 5-HT3 receptors promote colonic inflammation via activation of substance P/neurokinin-1 receptors in dextran sulphate sodium-induced murine colitis. Brit. J. Pharmacol. 2016, 173, 1835–1849. [Google Scholar] [CrossRef]
- Yasuda, M.; Kato, S.; Yamanaka, N.; Iimori, M.; Matsumoto, K.; Utsumi, D.; Kitahara, Y.; Amagase, K.; Horie, S.; Takeuchi, K. 5-HT3 receptor antagonists ameliorate 5-fluorouracil-induced intestinal mucositis by suppression of apoptosis in murine intestinal crypt cells. Brit. J. Pharmacol. 2013, 168, 1388–1400. [Google Scholar] [CrossRef]
- Maehara, T.; Matsumoto, K.; Horiguchi, K.; Kondo, M.; Iino, S.; Horie, S.; Murata, T.; Tsubone, H.; Shimada, S.; Ozaki, H.; et al. Therapeutic action of 5-HT3 receptor antagonists targeting peritoneal macrophages in post-operative ileus. Brit. J. Pharmacol. 2015, 172, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-D.; Sung, T.-S.; Kim, H.-J.; Kim, T.-W.; Yang, I.-S. Increased 5-hydroxytryptamine mediates post-inflammatory visceral hypersensitivity via the 5-hydroxytryptamine 3 receptor in rats. Dig. Dis. Sci. 2008, 53, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Fakhfouri, G.; Rahimian, R.; Daneshmand, A.; Bahremand, A.; Rasouli, M.R.; Dehpour, A.R.; Mehr, S.E.; Mousavizadeh, K. Granisetron ameliorates acetic acid-induced colitis in rats. Hum. Exp. Toxicol. 2010, 29, 321–328. [Google Scholar] [CrossRef]
- Lappalainen, T.; Sammeth, M.; Friedlander, M.R.; ‘t Hoen, P.A.C.; Monlong, J.; Rivas, M.A.; Gonzalez-Porta, M.; Kurbatova, N.; Griebel, T.; Ferreira, P.G.; et al. Transcriptome and genome sequencing uncovers functional variation in humans. Nature 2012, 501, 506–511. [Google Scholar] [CrossRef]
- Tao, L.; Zhou, S.; Chang, P.; An, S. Effects of ondansetron use on outcomes of acute kidney injury in critically ill patients: An analysis based on the MIMIC-IV database. J. Crit. Care 2021, 66, 117–122. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Xu, H.; Guo, D.; Shi, H.; Li, Y.; Zhang, W.; Gu, Y. 5-HTR3 and 5-HTR4 located on the mitochondrial membrane and functionally regulated mitochondrial functions. Sci. Rep. 2016, 6, 37336. [Google Scholar] [CrossRef]
- Trumpff, C.; Michelson, J.; Lagranha, C.J.; Taleon, V.; Karan, K.R.; Sturm, G.; Lindqvist, D.; Fernström, J.; Moser, D.; Kaufman, B.A.; et al. Stress and circulating cell-free mitochondrial DNA: A systematic review of human studies, physiological considerations, and technical recommendations. Mitochondrion 2021, 59, 225–245. [Google Scholar] [CrossRef]
- Al Amir Dache, Z.; Otandault, A.; Tanos, R.; Pastor, B.; Meddeb, R.; Sanchez, C.; Arena, G.; Lasorsa, L.; Bennett, A.; Grange, T.; et al. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 2020, 34, 3616–3630. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).