Abstract
Cancer is a major cause of death, affecting human life in both developed and developing countries. Numerous antitumor agents exist but their toxicity and low efficacy limits their utility. Furthermore, the complex pathophysiological mechanisms of cancer, serious side effects and poor prognosis restrict the administration of available cancer therapies. Thus, developing novel therapeutic agents are required towards a simultaneous targeting of major dysregulated signaling mediators in cancer etiology, while possessing lower side effects. In this line, the plant kingdom is introduced as a rich source of active phytochemicals. The secondary metabolites produced by plants could potentially regulate several dysregulated pathways in cancer. Among the secondary metabolites, flavonoids are hopeful phytochemicals with established biological activities and minimal side effects. Flavonoids inhibit B-cell lymphoma 2 (Bcl-2) via the p53 signaling pathway, which is a significant apoptotic target in many cancer types, hence suppressing a major dysregulated pathway in cancer. To date, there have been no studies reported which extensively highlight the role of flavonoids and especially the different classes of flavonoids in the modulation of Bcl-2 in the P53 signaling pathway. Herein, we discuss the modulation of Bcl-2 in the p53 signaling pathway by different classes of flavonoids and highlight different mechanisms through which this modulation can occur. This study will provide a rationale for the use of flavonoids against different cancers paving a new mechanistic-based approach to cancer therapy.
1. Introduction
Cancer is the primary cause of death all over the world and millions of individuals are diagnosed with cancer annually, foremost at later life stages [1]. Worldwide, an estimated 19.3 million new cancer cases (18.1 million excluding nonmelanoma skin cancer) and almost 10.0 million cancer deaths (9.9 million excluding nonmelanoma skin cancer) occurred in 2020. The global cancer burden is expected to be 28.4 million cases in 2040, a 47% rise from 2020, with a larger increase in transitioning (64% to 95%) versus transitioned (32% to 56%) countries due to demographic changes, although this may be further exacerbated by increasing risk factors associated with globalization and a growing economy [2].
Numerous antitumor agents are being used to combat cancer; however, they cause toxicity which limits their administration [3,4]. Due to serious side effects and poor prognosis of available cancer therapies, such as radiotherapy, surgery, and chemotherapy, their therapeutic effect is limited [5]. Medical plants are notable sources for novel anticancer drug discovery. Approximately 50% of anticancer drugs approved between the 1940s and 2006 are derived from natural compounds [6]. Among them, flavonoids are natural compounds with promising biological activities and health benefits. These plant-derived secondary metabolites have been shown to target multiple dysregulated pathways in cancer [7,8].
Apoptosis plays a critical role in preventing the progression of cancer. Growing evidence has established a major role for B-cell lymphoma 2 (Bcl-2) through the p53 pathway in apoptosis and cancer phases [9,10,11]. The Bcl-2 family of proteins is comprised of Bcl-2, Bcl-xl, and Mcl-1 [12]. The initiators Bcl-2 Homology 3 (BH3) only proteins induce apoptosis by either interacting with Bax, Bak, and Bok or by binding to the anti-apoptotic proteins to liberate Bax and bak [13]. Therefore, anti- and pro-apoptotic proteins of the Bcl-2 family will drive cell survival or death. Overexpression of the Bcl-2 protein has been reported in prostate cancer, breast cancer, B-cell lymphomas, and colorectal cancer [14]. Overexpression of the Bcl-2 protein promotes cell survival and proliferation. In recent years, several BCL-2 protein inhibitors have been developed (Oblimersen, ABT-737, ABT-263, obatoclax mesylate, AT-101, and S55746), to specifically target BCL-2 protein for cancer treatments [15,16,17]. Therefore, Bcl-2 is a validated drug target, and inhibitors targeting Bcl-2 have a wide range of clinical applications.
Dietary flavonoids in p53-mediated immune dysfunctions have been linked to cancer prevention [18,19]. The current study is the first review regarding targeting Bcl-2 through the p53 pathway by flavonoids in cancer.
2. The Role of Ethnopharmacology in Cancer Therapy
The majority of natural products used for nutritional or therapeutic uses are derived from higher plants [20], including plant-derived phytochemicals [21,22]. Amongst these therapeutic agents, >200,000 metabolites have been isolated from the plant kingdom [23]. Plant-derived phytochemicals have been used for the treatment of various diseases for thousands of years [24]. The U.S. National Cancer Institute (NCI) first reported on the efficacy of natural products as anticancer agents in the 1950s [3]. Presently, >60% of cancer patients use the medicinal plant as suitable alternative therapies [25]. In addition, despite the increased recognition of natural products in cancer therapy presented in Table 1, only about one-fifth to one-sixth of plant species have been evaluated for medical purposes [26].

Table 1.
The in vitro and in vivo activities of flavonoids in different cancer cell lines and their mechanism of action.
3. Flavonoids: Structure, Biological Activities and Health Benefits
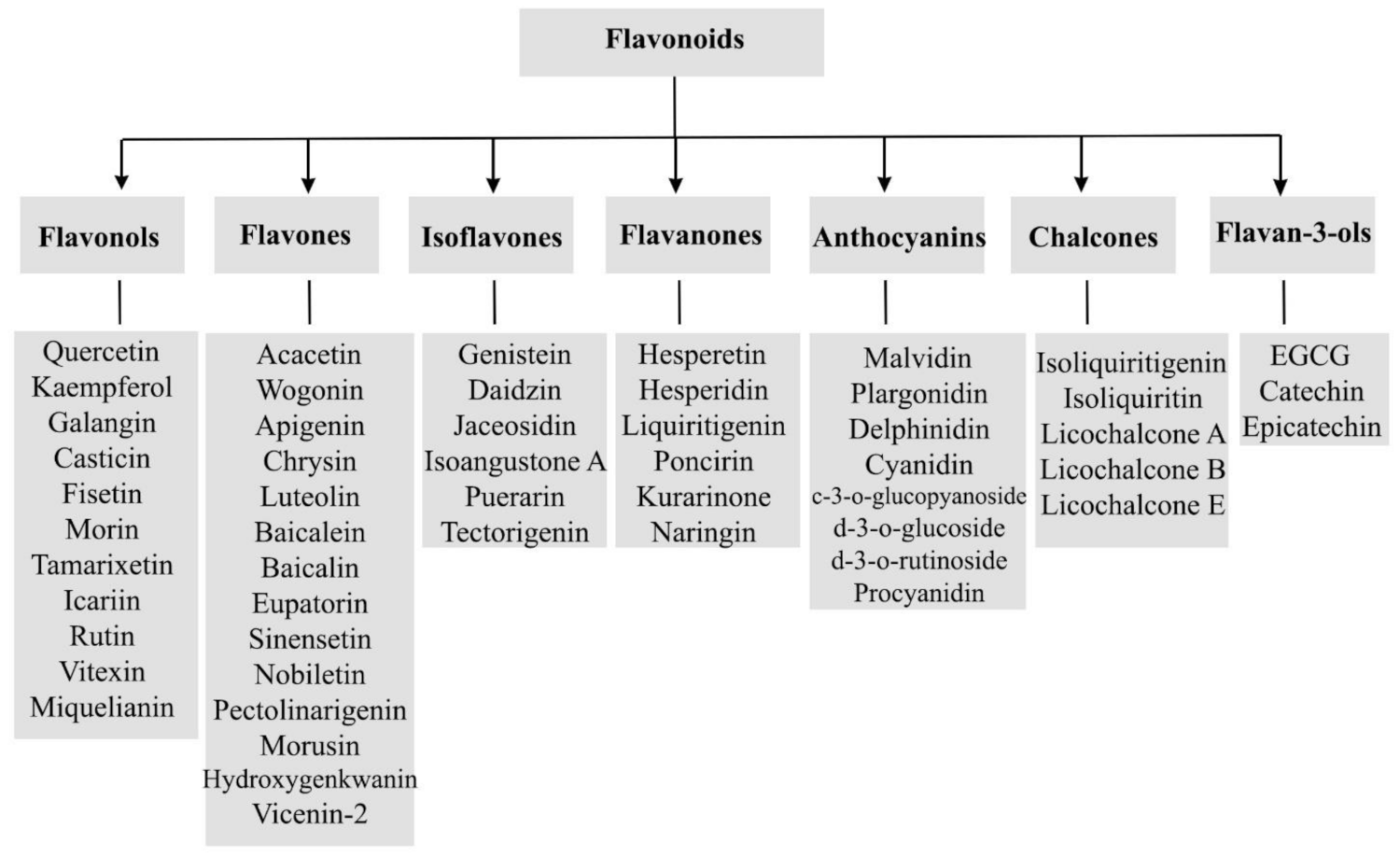
Generally, flavonoids are divided into various sub-classes including flavonols, flavones isoflavones, flavanones, anthocyanins, chalcones, and flavan-3-ols as presented in Figure 1. The chemical structures of flavonoids possess a fifteen-carbon based skeleton, including two benzene rings (A and B) connected via a heterocyclic pyran ring (C) as presented in the structure of Quercetin in Figure 2. Differences in the substitution of the C ring generate different classes of flavonoids, while those differences in A and B rings result in individual compounds within a flavonoid class. [85,86,87].
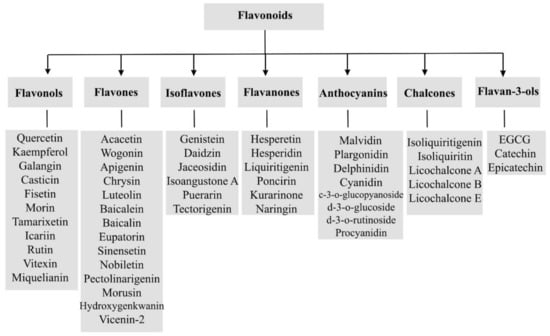
Figure 1.
A general scheme of the classification of flavonoids.

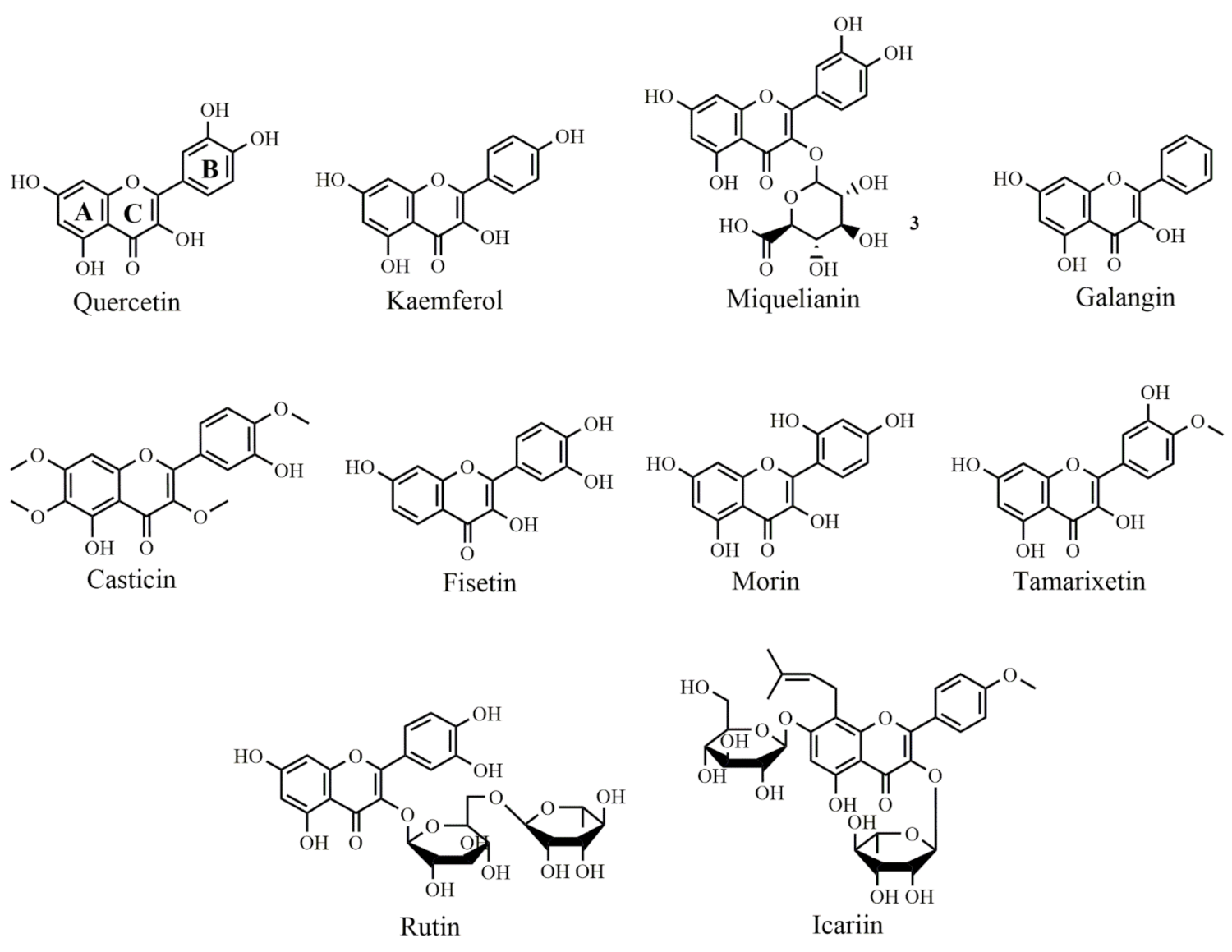
Figure 2.
Selected chemical structures of flavonols targeting Bcl-2 in the p53 pathway. Two benzene rings (A and B) connected via a heterocyclic pyran ring (C).
In terms of their anticancer effects, flavonoids target several signaling mediators to exert their therapeutic effects. Down-regulation of mutant p53, arresting the cell cycle, inhibition of tyrosine kinase/heat shock proteins, Ras protein inhibition, and binding to estrogen receptor are the common mechanisms of flavonoids in combating cancer [88]. Among the aforementioned mediators, Bcl-2 and p53 seem to play crucial roles in suppressing cancer.
4. Bcl-2 in Cancer Etiology
The Bcl-2 protein is a member of the Bcl-2 family and an oncogene resulting from chromosome translocation, which causes malignant lymphomagenesis [89]. In the early 1990 s, Bcl-2 was identified as an anti-apoptotic protein that prevents cell death. On the other hand, Bcl-2 Associated X (Bax) protein, which has similar sequence and structure homology to Bcl-2 and heterodimerizes with it, induces apoptosis. Accordingly, Bcl-2 family proteins are classified into pro and anti-apoptotic proteins [90]. These proteins affect the mitochondrial outer membrane permeabilization, allowing for the release of cytochrome C and DIABLO (second mitochondria-derived activator of caspases or SMAC) from the intermembrane mitochondrial space into the cytosol. The released cytochrome C binds to apoptosis protease–activating factor 1 (Apaf-1) and caspases [91]. Cytochrome C and Apaf-1 also stimulate caspase-3, c-Jun N-terminal Kinase (JNK), and p53 to inhibit cyclin-D in turn activating apoptotic pathways [92].
It has been earlier reported that the Bcl-2 expression of malignant/normal myeloid lineage cells produced significant effects [93]. Overexpression of the Bcl-2 gene is inherent to various cancers, for example, 90% of colorectal adenocarcinomas, 80% of undifferentiated nasopharyngeal cancers, 70% of breast adenocarcinomas and chronic lymphocytic leukaemias, 60% of gastric cancers, 30–60% of prostate cancers, and in varying percentages of melanomas, blastomas, and kidney cancers [94].
5. p53 in Cancer: Its Association with Apoptosis and Bcl-2
Approximately half of the cancers are associated with inactivated p53 [95]. Major biological functions of p53 include apoptosis, senescence, angiogenesis, cell cycle regulation, cellular differentiation, and DNA metabolism [96]. p53 acts as a sensor and restricts cell propagation under destructive conditions, including oncogene signals, hypoxia, ribosome dysfunction, DNA damage, and nutrient deprivation [97]. Accordingly, during low-level stress, p53 affords pro-survival and protective responses, including antioxidant responses, cell-cycle arrest, and DNA repair, to maintain genome integrity/viability [97]. In contrast, following cell exposure to potent stress signals, p53 provides irreversible programs of senescence or apoptosis [98,99]. p53 induces apoptosis by activating the Bax gene (a key member of the Bcl-2 family) [100]. In turn, Bax binds to Bcl-2, thereby activating the production of apoptotic mediators (e.g., caspase 3/9 and cytochrome C). Thus, targeting Bcl-2 through p53 offers efficient means for combating cancer.
6. Bcl-2 Inhibition Passes through the p53 Pathway by Flavonoids
A linkage exists between the activity of Bcl-2 and p53. Considering the potential role of flavonoids (e.g., flavonols, flavones, isoflavones, flavanones, chalcones, anthocyanins, and catechins) in the modulation of Bcl-2 through the p53 pathway, they could be promising agents in the treatment of cancer (Figure 1).
6.1. Flavonols
Quercetin is a bioflavonoid found in abundance in grapes, citrus fruits, berries, and onions. In human breast cancer (MCF-7) cells, quercetin (Figure 2) treatment effectively suppressed the cell proliferation in both the dose and time-dependent manner. Quercetin also significantly reduced Bcl-2 expression levels while increased Bax, resulting in the induction of apoptosis [27]. Quercetin treatment of prostate cancer-induced rats significantly increased the levels of antioxidant enzymes. The expression levels of Akt and anti-apoptotic protein Bcl-2 were downregulated and caspase-3 protein expression was upregulated. Moreover, quercetin down-regulated cell proliferation, viability and thus acted as a chemopreventive agent against prostate cancer in the rat model [28]. Granado-Serrano et al., 2006 reported quercetin-induced apoptosis in HepG2 cell line and evaluated the modulation and expression of Bcl-x and Bax. Bcl-xL has been identified as a caspase substrate and the product of Bcl-xL cleavage, Bcl-xS, has a pro-apoptotic function. The level of Bcl-xS was elevated at all quercetin concentrations compared with controls after 18 h of incubation. Quercetin reduced the Bcl-xL:Bcl-xS ratio, which reached a minimum at 50 µmol/L. Bax has been demonstrated to translocate from the cytoplasm to the outer mitochondrial membrane, where it forms holes and mediates apoptosis. Western blot analysis of mitochondrial and cytoplasmatic fractions revealed that after 18 h of quercetin treatment, translocation of Bax to the mitochondria increased to its greatest level at 50 mmol/L, decreased at 75 mmol/L, and returned to control levels at 100 mmol/L [101].
The kaempferol (Figure 2) caused a marked anti-cancer effect in MCF-7 breast cancer cell lines mediated by down-regulation of Bcl-2 expression, accompanied by the overexpression of Bax protein and thus produced apoptosis [29]. Kaempferol therapy slowed the progression of tumor xenografts by downregulating the proteins cyclin B1 and Cdk1. Furthermore, kaempferol treatment reduced the level of Bcl-2 while upregulated Bax expression consequently acting as chemopreventive agent [30]. Miquelianin (Figure 2) (Quercetin-3-O-glucuronide) has a protective role against 1-methyl-4-phenylpyridinium-induced neurotoxicity. MTT assay indicated miquelianin significantly inhibited apoptosis, which was accompanied by a decrease in PARP cleavage. Additionally, it attenuated MPP-induced intracellular ROS with the decrease in Bax/ Bcl-2 ratio [83].
Galangin (Figure 2) was isolated from the rhizome of Alpinia officinarum has anticancer effects against many cancer cells such as liver, lung, breast, and esophageal cancer. It inhibited cell proliferation, induced apoptosis evident from the reduced Bcl-2 and higher cleaved caspase-3 expressions [4].
Casticin (Figure 2) induced apoptotic cell death in human lung cancer cells and caused the activation of multiple apoptotic proteins such as procaspase-9 and procaspase-3. Additionally, casticin down-regulated Bcl-XL and upregulated Bax, and also increased death receptor 5 (DR5) expression levels [31]. In another study, casticin caused cycle arrest at G0/G1 phase and induced apoptosis by increasing the expression of Bax and p27 proteins and down-regulating Bcl-2 expression in human gallbladder cancer cells [102].
Fisetin (Figure 2) treatment of plasma cancer cells (U266) [103] and human non-small cell lung cancer cell lines (NCI-H460) [32] promoted the activation of caspase-3, upregulated Bax, Bim and Bad proteins expression while down-regulated Mcl-1L and Bcl-2 expression. Consistently, morin, a bioflavonoid found in the mulberry that acts as an apoptotic inducing agent in multiple human cancers. Morin (Figure 2) induced the upregulation of the Fas receptor and activated caspase-8, caspase-9, and caspase-3 in human colon cancer (HCT-116) cells. It additionally caused a mitochondrial potential loss, activated Bax protein, inhibited Bcl-2 and enhanced the generation of reactive oxygen species (ROS) [33]. Morin exhibited a protective effect in myocardial ischemia-reperfusion injury (MIRI) by increasing cell viability and enhanced the regaining of heart function in rats. Moreover, morin treatment prevented the decrease of mitochondrial membrane potential and reduced the levels of cytochrome c, caspase-9, and caspase-3. Furthermore, morin treatment significantly down-regulated the expression of Bax while upregulated the expression of Bcl-2 [34].
Tamarixetin (Figure 2) showed cytotoxicity towards leukemic cells, inhibiting cancer cells proliferation by enhancing apoptotic activity and blocking cell cycle progression accompanied by the increase in p21 and cyclin B1. In addition, tamarixetin induced an increase in Bax expression and decreased Bid expression. Apoptosis was caused due to the release of cytochrome C, activation of caspases and cleavage of poly ADP-ribose polymerase (PARP) [35]. From another point of view, oxidative stress causes mitochondrial membrane loss and may cause myocardial damage. H2O2 is a Reactive Oxygen Species (ROS) that activates caspase-3 and increases Bax expression while decreasing Bcl-2 expression and causing cell death in rat H9c2 cell lines. In this line, rutin treatment reversed this process by inhibiting Bax and caspase-3 while increases Bcl-2 expression and act as an anti-apoptotic agent preventing myocardial damage caused by oxidative stress [36].
Icariin (Figure 2) inhibited the growth of many tumor cells [104]. In human hepatoma (SMMC-7721) cell lines, icariin initiated the mitochondrial-dependent apoptotic pathway by increasing the Bax/Bcl-2 ratio, dysfunctioning of mitochondrial membrane potential to make the release of cytochrome C and activating caspase cascade. Icariin also triggered ROS generation in SMMC-7721 cells [37].
6.2. Flavones
Acacetin (Figure 3) activated apoptosis in human breast cells (MCF-7) by activating caspase-7 and mitochondrial-mediated death signaling. Acacetin treatment significantly inhibited Bcl-2 expression while increased the expression levels of Bax. Acacetin increased the release of apoptosis-inducing factor (AIF) into the cytoplasm leading to ROS generation and subsequently induces apoptosis in hepatoma cell lines (SMMC-7721) [38]. Amongst other flavones, wogonin (Figure 3) treatment increased the apoptotic activity of SMMC-7721 by modulating the expression of Bcl-2 protein and Bax protein in a time and dose-dependent manner. The expression of Bcl-2 protein was significantly reduced while the expression of Bax protein was increased [39].

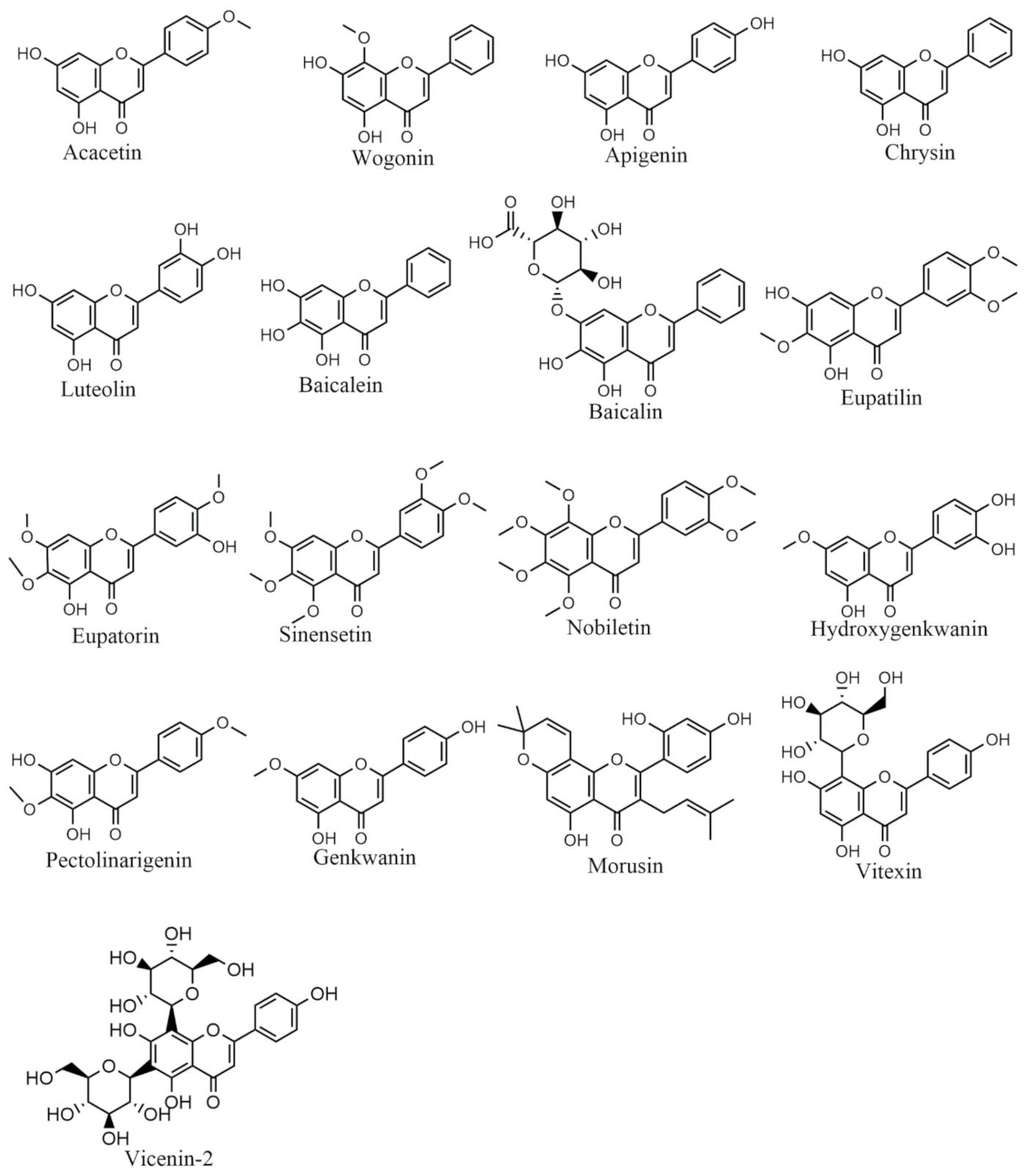
Figure 3.
Selected chemical structures of flavones targeting Bcl-2 in the p53 pathway.
In human lung cancer (A549) cell lines, apigenin (Figure 3) treatment caused cytotoxicity by inducing DNA damage and decreasing cell viability in a dose-dependent manner. Apigenin induced apoptosis accompanied by the increase in the expression of Bax protein and inhibited Bcl-2 protein level leading to the disruption of mitochondrial membrane, which caused the release of cytochrome C and Endo G and induced the activation of caspases [40]. In hepatocellular carcinoma cells (HCC), chrysin (Figure 3) treatment increased the expression of pro-apoptotic agents such as p53, Bad, Bax and Bak proteins and reduced the expression levels of anti-apoptotic agents such as Bcl-2 protein which resulted in the induction of apoptosis. Chrysin also suppressed the viability of hepatocellular carcinoma cells dose-dependently [41].
Luteolin (Figure 3) exhibited anticancer and antitumor effects against many types of cancers such as brain, lung, breast, prostate and pancreatic cancers [105]. The pro-apoptotic and anti-proliferative activities of luteolin were analyzed in vitro in colon adenocarcinoma (HCT-15) cells. Luteolin treatment inhibited Wnt/β-catenin signaling pathway and induced cell cycle arrest at the G2/M phase. It suppressed Bcl-2 expression levels and increased the expression of Bax and caspase-3 [42]. In human breast cancer lines MCF-7, a combination of baicalin and baicalein (Figure 3) showed remarkable anti-proliferative activity in a time and dose-dependently. This combinatorial treatment activated the caspase cascade, upregulated p53 and Bax expression while down-regulated Bcl-2 expression which is associated with the activation of ERK/p38 mitogen-activated protein kinase (MAPK) pathway [43]. In HeLa cell lines, eupatorin (Figure 3) treatment caused cell cycle arrest at the G2/M phase and then induced cell death by inhibiting cyclin D1, initiated the cleavage of caspase cascade, enhanced p53, p21 and Bax expression levels through activating both p53 dependent and independent pathways [44].
Sinensetin (Figure 3) increased caspases and PARP expression levels and induced autophagy in human T-cell lymphoma cells by activating ROS/terminal kinase and inhibiting Akt/mTOR signaling pathways [45]. Nobiletin (Figure 3) induced cell death in human breast cancer MCF-7 cells by modulating the expression of Bax and Bcl-2 proteins. Nobiletin upregulates the apoptotic inducing proteins Bax and p53 while down-regulate the antiapoptotic Bcl-2 protein in the breast cancer cell line (MCF-7). It also blocked cell migration by inhibiting MMP-2 and MMP-9 proteins [46].
The eupatilin (Figure 3) treatment has reversed the apoptosis in PC12 cells by enhancing Bcl-2 expression, inhibiting Bax protein and inactivating caspase-3 to prevent oxidative stress-induced neuronal injury in PC12 cell lines [47]. Similarly, eupatilin pretreatment attenuated myocardial ischemia/reperfusion injury by inhibiting apoptosis and reducing oxidative stress through the activation of the Akt/glycogen synthase kinase-3β (GSK-3β) pathway [48].
Vitexin (Figure 3) protected against heart failure in rats by inhibiting oxidative stress-induced myocardial apoptosis through decreasing Bax and increasing Bcl-2 protein expression [50]. Similarly, it has been found that vitexin induced apoptotic activity and decreased Bcl-2/Bax expression ratio while increased the expression of cleaved caspase-3 in human non-small cell lung cancer A549 cells. Additionally, it induced the release of cytochrome C into the cytosol from the mitochondria leading towards the loss of mitochondrial membrane potential [49].
Pectolinarigenin (PG) (Figure 3) blocked osteosarcoma cells proliferation, induced cell death and decreased the level of cyclin D1, Survivin, Bcl-2 and Bcl-xL proteins [51]. Wu et al. investigated the protective activity of PG in a rat model of spinal cord injury, PG effectively enhanced functional recovery and inhibited apoptosis in neuronal cells by down-regulating the activated caspase proteins and PARP, decreasing Bax expression, and increasing Bcl2 expression [52].
In human prostate cancer, morusin (Figure 3) inactivated STAT3 signaling and caused apoptosis by inhibiting Bcl-2, Bcl-xL and surviving. Furthermore, it has been found that morusin exerted growth inhibitory effects on HCC cells both in vitro and in vivo. Additionally, it induced apoptosis accompanied by the increased active caspase-3 and decreased Bcl-2 expression [53].
Vicenin-2 (Figure 3) reversed the diethyl nitrosamine-induced liver carcinoma in rats by potentially inhibiting the production of ROS, decreasing the liver weight, and reducing cellular changes in the liver which were previously induced by the diethylnitrosamine in rats. Furthermore, vicenin-2 downregulated the expression of anti-apoptotic proteins Bcl-xL and Bcl-2 while upregulated the expression of pro-apoptotic protein Bax and cleaved caspase-3 [54]. It has been reported that vicenin-2 stimulated significant cell cycle arrest at the G2/M phase and also induced apoptotic cell death in HT-29 cells [55]. Moreover, vicenin-2 treatment upregulated the expression of caspase-3 and Bax, increased the dysfunction of the mitochondrial membrane potential whereas down-regulated the Bcl-2 expression.
Hydroxygenkwanin (Figure 3) in combination with apigenin inhibited brain tumor cells proliferation through upregulating TNF-α levels, activating caspase-3, caspase-8, and down-regulating Bcl-2 [55]. It has been investigated that hydroxygenkwanin along with kaempferol showed both cytotoxic and antioxidative potential against HepG2 cell lines [106]. This may be due to the fact that hydroxygenkwanin has multiple hydroxyls that may donate hydrogen and act as an antioxidant in forming phenoxyl radicals, therefore induce cytotoxicity.
6.3. Isoflavones
In this class of phytochemicals, puerarin (Figure 4A) act as a protective agent against ROS-induced apoptosis by decreasing Bax/Bcl-2 ratio and apoptosis, as well as preventing neuronal disorders such as Alzheimer’s disease [107] and PC12 cancer cells [56]. Puerarin treatment of HCC cell lines increased the phosphorylation and activation of MAPK and act as an anticancer agent by exhibiting pro-apoptotic activities [108]. Combinatorial treatment of genistein and hypericin (Figure 4A) in human breast cancer cells resulted in the reduction of Bcl-2 expression while an increase in Bax expression suppressed Akt and ERK1/2 phosphorylation [57].


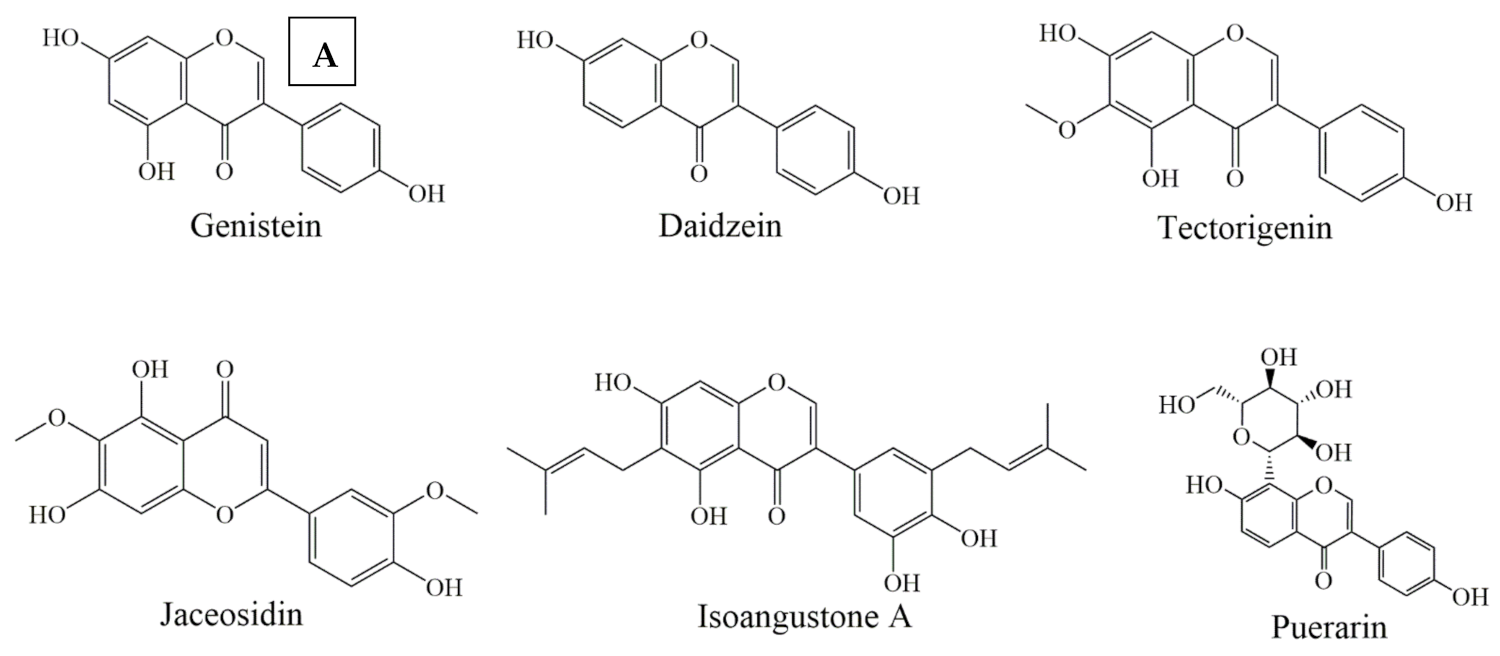
Figure 4.
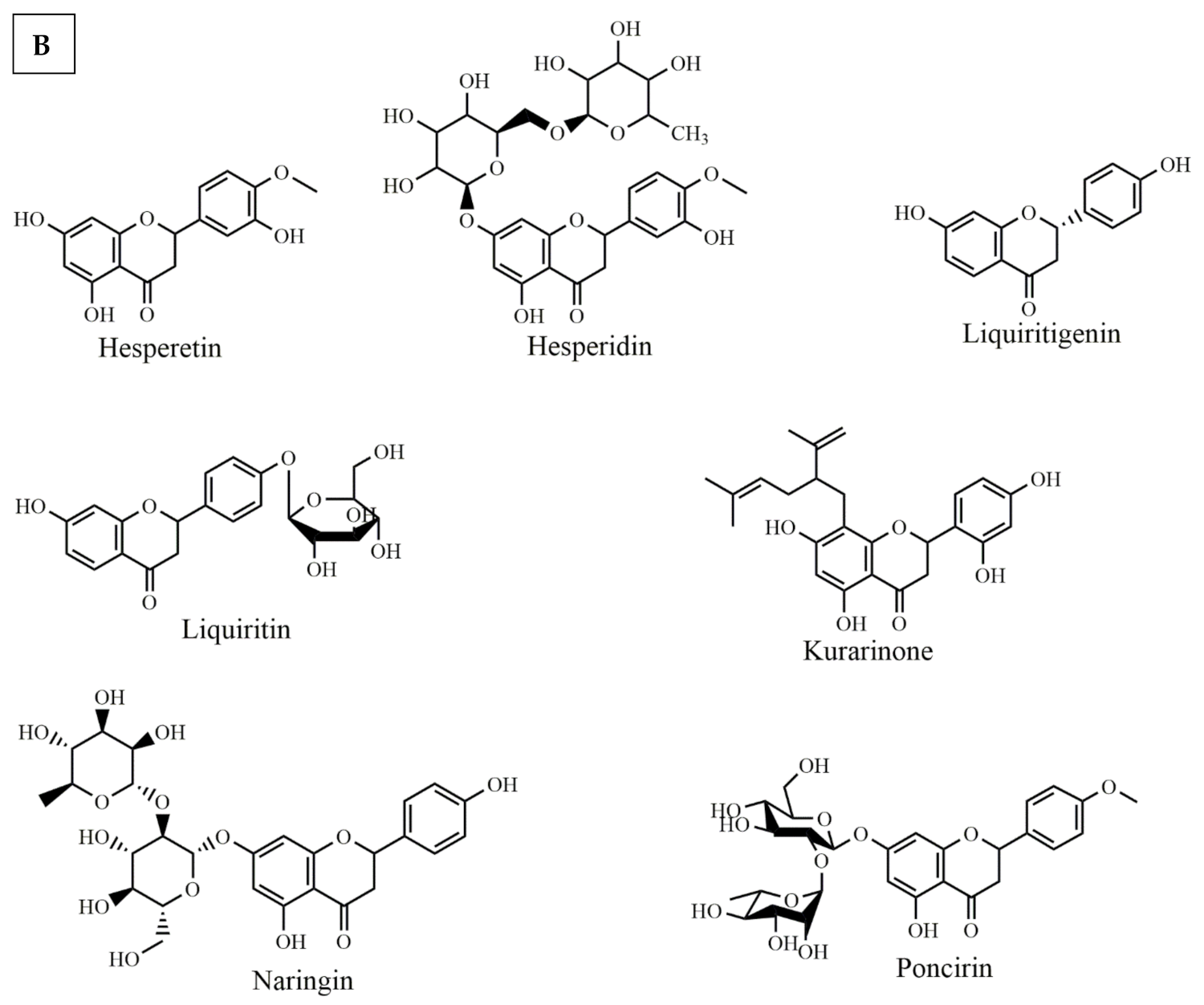
Selected chemical structures of isoflavones (A), and flavanones (B) targeting Bcl-2 in the p53 pathway.
Daidzein (Figure 4A) initiated cell death in SK-HEP-1 cells by enhancing Bak expression and inhibiting Bcl-2 and Bcl-xL proteins through mitochondrial-mediated apoptosis [58]. While ultraviolet radiation damages the skin extracellular matrix and induces cell death, tectorigenin (Figure 4A) treatment decreased the levels of ROS, increased anti-apoptotic Bcl-2 protein expression, overexpressed glutathione and catalase and inhibited skin cells death [59]. Jaceosidin (Figure 4A) induced cell cycle arrest at the G2/M phase, upregulated the expression levels of p53 and Bax proteins and caused the loss of mitochondrial membrane potential by releasing cytochrome C and activating caspase-3 in U87 cell lines [109]. Isoangustone A (Figure 4A) induced apoptosis and activated the caspase cascade cleavage while down-regulated Bcl-2 protein in human colorectal adenocarcinoma (SW480) cells [60]. The Biochanin A was initially found in the Trifolium pretense L.(clover) plant and was extracted from the stems and leaves. Biochanin A exhibits significant anticancer and antioxidant activities. It induced apoptosis, block metastasis, and caused cell cycle arrest by targeting multiple signaling pathways of cancer [110].
6.4. Flavanones
Several flavanones have also shown potential anticancer effects through Bcl-2 modulation in the p53 signaling pathway. Among them, hesperetin (Figure 4B) treatment causes cell cycle arrest at G1-phase in MCF-7 cell lines, through downregulating the cyclins and upregulating p21. Additionally, hesperetin enhances the binding of CDK4 with p21, which suggests the fact that hesperitin is involved in anticancer pathways [111]. In addition, hesperetin induced apoptosis in gastric cancer cells by activating the mitochondrial signaling pathway which caused the upregulation of Bax protein expression and down-regulating Bcl-2 expression [112]. Hesperidin (Figure 4B) also caused cell death in human colon cancer cells through caspase-3 activation. It significantly enhanced Bax expression and reduced the expression of Bcl-2 [61]. Liquiritigenin (Figure 4B) effectively enhanced cell viability and inhibited palmitate-induced apoptosis by decreasing the cleavage of caspases and PARP while upregulating Bcl-2 expression [62].
Naringin (Figure 4B) treatment prevented gentamicin-induced nephrotoxicity, through a potential reduction of caspase-3, p53 and Bax as well as enhancement of the Bcl-2 protein expression [63]. Similarly, Saralamma et al. showed anticancer effects of poncirin (Figure 4B). They reported that poncirin treatment increased the expression of death receptors Fas Ligand (FasL) protein in human gastric cancer cells. Additionally, it induced the activation of caspase-8 and caspase-3 and cleavage of PARP [64]. Kuarinone (Figure 4B), norkurarinol and 2′ methoxy kurarinone are kushen flavonoids that inhibit the growth of many cancers cell lines such as A549, SPC-A-1 and NCI-H46, respectively [113]. Kurarinone inhibited the proliferation of A549 cell lines and decreased Bcl-2/Bax levels while activating the caspase-9 and caspase-3 [65].
6.5. Chalcones
The isoliquiritigenin (Figure 5) have shown a significant anticancer effect through decreasing the production of prostaglandin E2 (PGE2) and nitric oxide (NO) in mice macrophages. This decrease in PGE2 was influenced by the downregulation of cyclooxygenase-2 expression, Bcl-2 and decreasing in NO which was influenced by the low expression of inducible nitric oxide synthase (iNOS) [114,115]. Isoliquiritigenin treatment suppressed the growth of abnormal cells and induced apoptotic activity in mouse and human colon carcinoma cells [66]. In Ca Ski cells, isoliquiritigenin down-regulated the expression of HPV16 E6 which is associated with the increased levels of p53 and p21, enhanced Bax expressions and decreased Bcl-2 expressions and sequentially activated caspase cascade by cleaving caspase-9, caspase-3 and PARP [116].
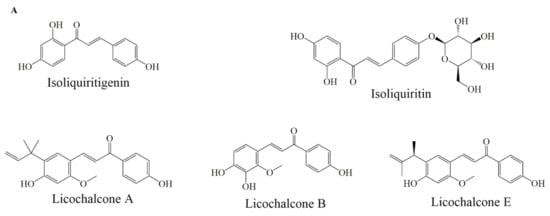
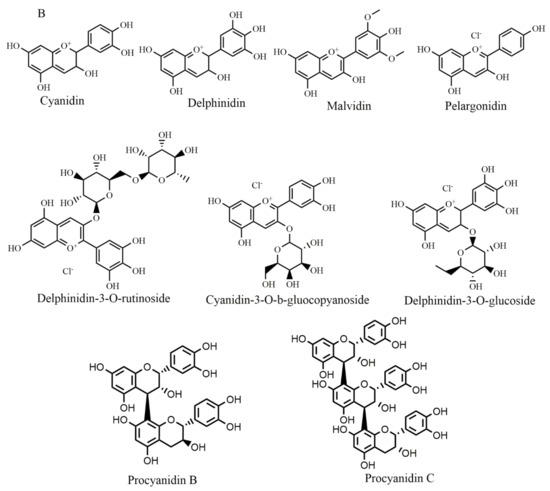
Figure 5.
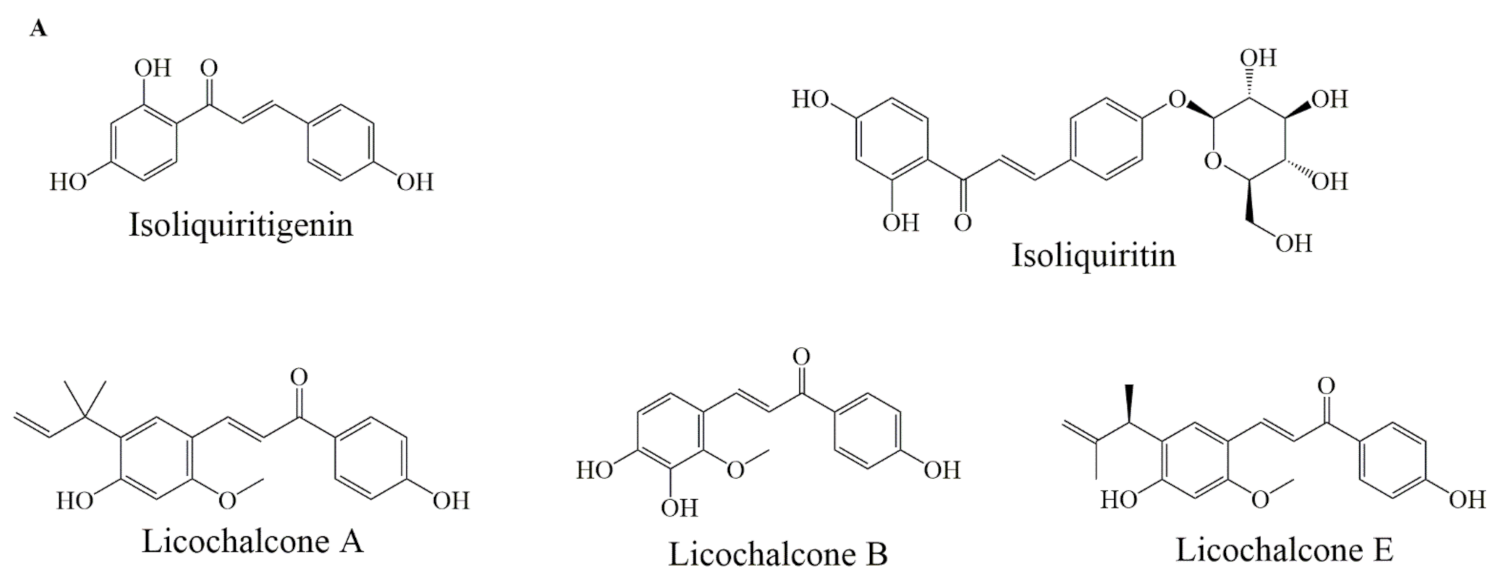
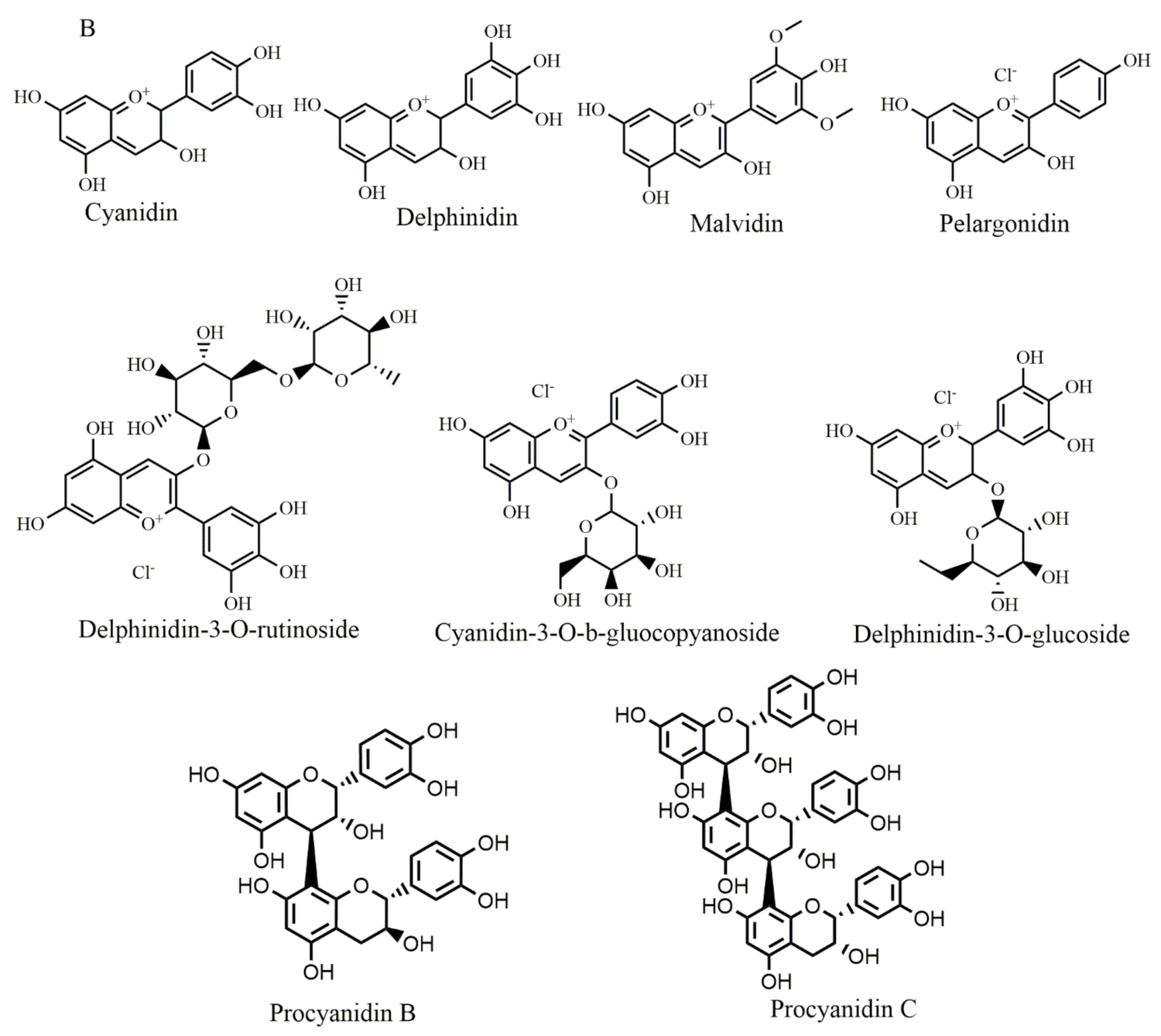
Selected chemical structures of chalcones (A), anthocyaninsx and catechins (B) that target Bcl-2 in the p53 pathway.
Liquiritin, isoliquirigenin and isoliquiritin (Figure 5A) when applied in combination, against lung cancer cells, increased cytotoxic capacity and upregulated the p53 and p21 proteins, also downregulated the expression of MDM2, Bcl-2, p-Akt proteins through p53 dependent signaling pathway. Additionally, it inhibited cell cycle arrest at the G2/M phase [67]. Licochalcone A (Figure 5A) induced apoptosis by modulating Bcl-2 protein expression and decreased Bcl-2/Bax ratio in MCF-7 and HL-60 cell lines [68]. Licochalcone B (Figure 5A) treatment reduced Bcl-2 and survivin levels, increased Bax expression, also activated caspase-3 and cleaved PARP protein [69]. Licochalcone E (Figure 5A) treatment increased the Fas ligand expression levels and increased caspase-8 proteins in FaDu cells. Moreover, Lico-E treatment increased apoptotic activity by upregulating Bax, caspase-9 and onco-suppressor p53 whereas decreases Bcl-2, consequently, induced apoptosis by both intrinsic and extrinsic signaling pathways [70].
6.6. Anthocyanin
A recent study has introduced anthocyanins as promising anticancer agents [8]. In this regard, malvidin (Figure 5B) upregulated p21 expression levels in human colorectal cancer [71] and its combined use with blueberry induced cell death through Bax-mediated intrinsic pathway in SCC131 cells by inhibiting Bcl-2 with an increase in Bax expression and initiating cleavage of caspases [72]. Cyanidin-3-O-β-glucopyranoside (Figure 5B) treatment of leukemia cell lines caused cell death and significantly upregulated p53 and Bax expression also down-regulated Bcl-2 expression in a time-dependent manner [73]. Pelargonidin (Figure 5B) treatment reduced the expression of Bcl-xL and Bcl-2 and increased the expression of Bid and Bax. Additionally, it enhanced p53 and p21 expression levels in human colorectal cell lines [74].
In human breast cancer cells, delphinidin (Figure 5B) treatment inhibited cell proliferation by blocking the Akt signaling pathway and inducing apoptosis by increasing Bcl-2 expression along with increasing Bax expression in a dose-dependent manner [75]. Bilberry extract (Figure 5B) (which contain delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, delphinidin-3-O-rutinoside, cyanidin-3-O-galactoside, and cyanidin-3-O-rutinoside flavonoids) was applied to chronic lymphocytic leukemia which activated caspase-3, de-phosphorylated Akt and inhibited Bcl-2 and resulted in the induction of apoptosis [108]. Procyanidins B and procyanidin C (Figure 5B) are formed from the oligomers of catechin and epicatechin. When they are depolymerized in an oxidative environment, cyanidins are formed. Procyanidins extracted from Pinus koraiensis bark promoted apoptosis in the HeLa cell line by raising Bax protein expression and inhibiting Bcl-2 and survivin protein expression [117].
6.7. Flavan-3-ols
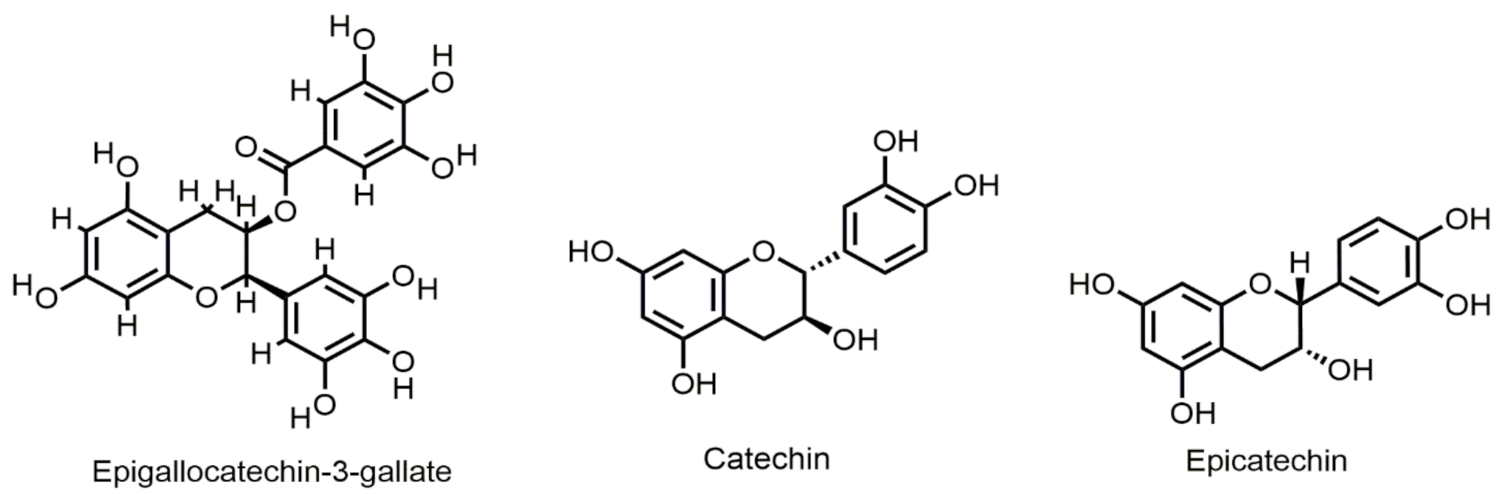
Epigallocatechin-3-gallate (EGCG) (Figure 6) is found in green tea to inhibit growth and induce apoptosis in various types of human cancer cells. EGCG inhibited gastric and hepatocarcinoma cell growth and reduced Bcl-2 expression in a time-dependent manner [77]. EGCG when applied to colon cancer cells, inhibited cyclooxygenase-2 (COX-2) and activated AMP-activated protein kinase (AMPK) accompanied by a decrease in vascular endothelial growth factor (VEGF) and Glut-1 levels [60,78]. Chemical stress damages the lens epithelium of rats and causes apoptosis through increasing Bax/Bcl-2 ratio, which could be regulated by catechin [118]. Catechin (Figure 6) and gemigliptin have anti-apoptotic effects on tacrolimus-induced renal injury in mice [119]. Their study confirmed that combination use of catechin and gemigleptin exerts anti-apoptotic effects by increasing the expression of anti-apoptotic protein Bcl-2 in tacrolimus-induced nephropathic mice. Iranian green tea extract (IGTE) contains active flavonoid catechin. Safari et al., reported that treatment of A549, PC3, and MCF-7 cell lines with IGTE induced apoptosis by increasing the levels of Bax and decreasing the expression of Bcl-2 [80].

Figure 6.
Selected chemical structures of flavan-3-ols targeting Bcl-2 in the p53 pathway.
Epicatechin (Figure 6) is one of the most abundant flavonoids found in apples, grapes, blackberries, etc. A research study suggested that Epicatechin (EC) extracted from Euonymus alatus had a protective effect against acute liver injury of mice by inhibiting apoptosis in hepatocytes. Western blot analysis revealed reduced expression of cleaved Caspase-3 and Bax. Their results revealed it increased the expression of Bcl-2, confirming its protective effect in liver injury [82].
7. Challenges and Possible Solutions to Flavonoids Therapy
The low water solubility of most flavonoids coupled with their shorter intestinal residence time as well as their lower absorption refrain humans to suffer acute toxic effects from flavonoids consumption. Although most flavonoids/phenolics are considered safe, flavonoid/phenolic therapy or chemopreventive use should be evaluated because there have been reports of toxic flavonoid-drug interactions, liver failure, hemolytic anaemia, contact dermatitis, and estrogenic-related concerns such as male reproductive health and breast cancer linked to dietary flavonoid/phenolic consumption or exposure [120]. However, the low water solubility and bioavailability of flavonoids present a potential problem for its medicinal applications [121]. Nanotechnology can serve as an efficient tool in eradicating the limitations stated above. By reducing the size of the flavonoids based nano-medicine and modifying their surface properties, the aqueous solubility and permeability through the biological membrane can be potentially increased [122]. Several novel nanotechnology-based drug delivery systems have been reported (such as niosomes, liposomes, phytosomes, and nanospheres) to potentially improve the bioavailability and therapeutic efficacy of flavonoids. The incorporation of phytomedicines (e.g., flavonoids) in these delivery systems also aid in increasing the solubility, enhancing stability and therapeutic efficacy, minimizing toxicity, improving tissue macrophage distribution, sustained delivery and protection from chemical and physical degradation [123].
8. Conclusions and Future Direction
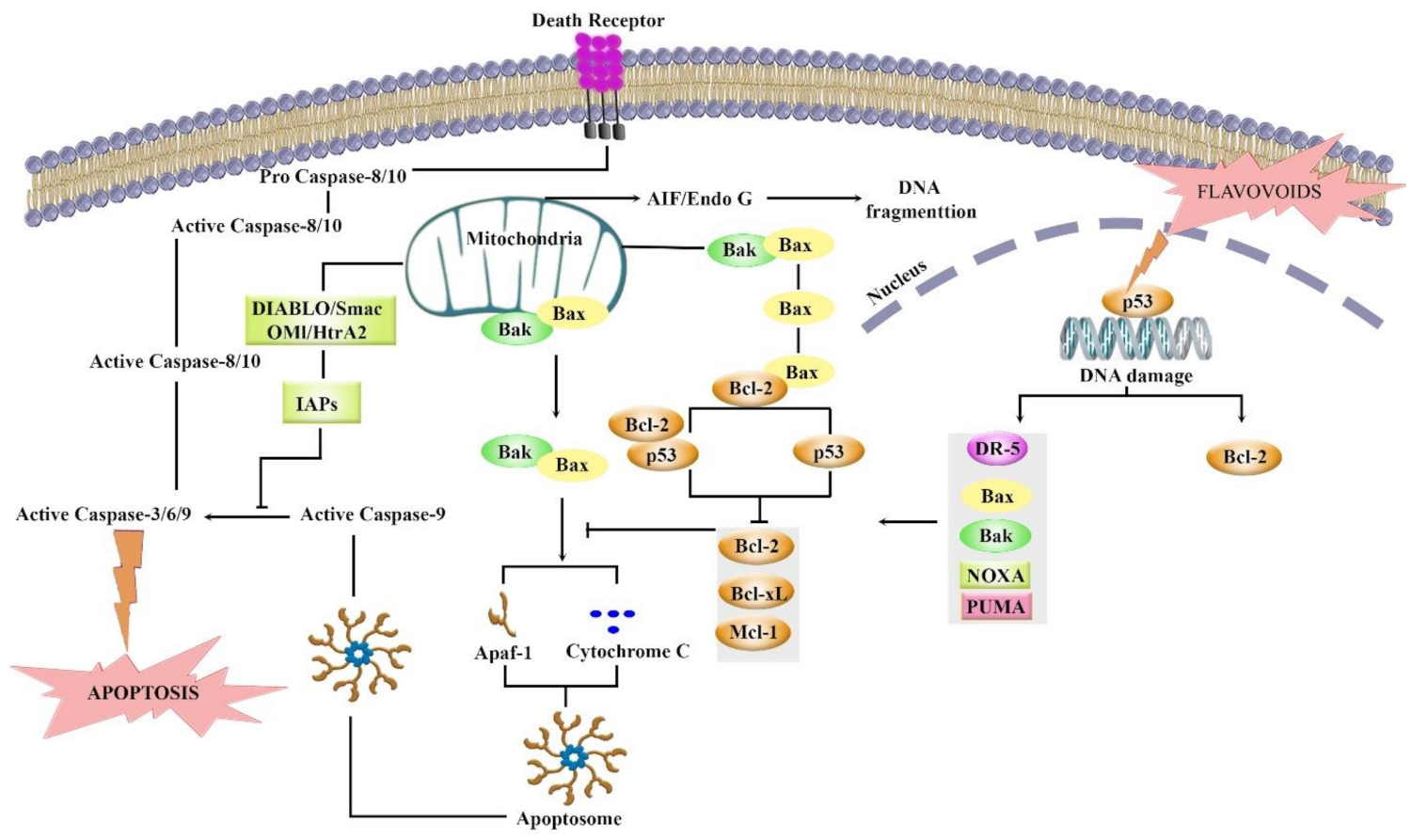
Flavonoids are phytochemicals with potential biological activities and health benefits. They regulate several signaling pathways to target apoptosis, inflammation, and oxidative stress, thereby exerting potential anticancer effects. Among those signaling pathways, modulation of Bcl-2 through the p53 pathway seems to be of great importance. Flavonoids have been found to effectively modulate Bcl-2 through the p53 pathway in cancer (Figure 7).

Figure 7.
Targeting Bcl-2 in the p53 pathway by different flavonoids. It shows details of various events including up and downregulation, or expression that leads to apoptosis.
Flavonoids are degraded by intestinal microorganisms/enzymes after being administered orally. Thus, developing innovative flavonoid delivery strategies may enhance their anticancer properties [8]. Future studies should characterize signaling pathways responsive to flavonoids and provide insight into their antitumor potential, addressing their efficacy in the treatment and management of cancers.
Author Contributions
Conceptualization, N.R. and A.Z.; methodology, N.R. and A.Z.; formal analysis, N.R. and S.F.; data curation, N.R. A.Z. and K.G.; writing—original draft preparation, N.R. A.Z. and K.G.; writing—review and editing, N.R. A.K. and S.F.; visualization, S.F. and N.R.; Reviewed and Edited, M.A. and H.K.; supervision, H.K. and L.S.; project administration, H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
No special grant has been received for this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Fouché, G.; Cragg, G.; Pillay, P.; Kolesnikova, N.; Maharaj, V.; Senabe, J. In vitro anticancer screening of South African plants. J. Ethnopharmacol. 2008, 119, 455–461. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Jorjani, M.; Pourgholami, M.H. The effects of anticancer medicinal herbs on vascular endothelial growth factor based on pharmacological aspects: A review study. Nutr. Cancer 2021, 73, 1–15. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Moradi, S.Z.; Farzaei, M.H.; Bishayee, A. Modulation of Dysregulated Cancer Metabolism by Plant Secondary Metabolites: A Mechanistic Review; Seminars in cancer biology; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Fakhri, S.; Khodamorady, M.; Naseri, M.; Farzaei, M.H.; Khan, H. The ameliorating effects of anthocyanins on the cross-linked signaling pathways of cancer dysregulated metabolism. Pharmacol. Res. 2020, 159, 104895. [Google Scholar] [CrossRef]
- Zhang, H.-W.; Hu, J.-J.; Fu, R.-Q.; Liu, X.; Zhang, Y.-H.; Li, J.; Liu, L.; Li, Y.-N.; Deng, Q.; Luo, Q.-S. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in cancer and apoptosis. Cancers 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting multiple signaling pathways in cancer: The rutin therapeutic approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef]
- Oltersdorf, T.; Elmore, S.W.; Shoemaker, A.R.; Armstrong, R.C.; Augeri, D.J.; Belli, B.A.; Bruncko, M.; Deckwerth, T.L.; Dinges, J.; Hajduk, P.J. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005, 435, 677–681. [Google Scholar] [CrossRef]
- Martin, L.-A.; Dowsett, M. BCL-2: A new therapeutic target in estrogen receptor-positive breast cancer? Cancer Cell 2013, 24, 7–9. [Google Scholar] [CrossRef]
- Kirkin, V.; Joos, S.; Zörnig, M. The role of Bcl-2 family members in tumorigenesis. Biochim. Biophys. Acta BBA Mol. Cell Res. 2004, 1644, 229–249. [Google Scholar] [CrossRef]
- Han, Z.; Liang, J.; Li, Y.; He, J. Drugs and clinical approaches targeting the antiapoptotic protein: A review. BioMed Res. Int. 2019, 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Schoenwaelder, S.M.; Jarman, K.E.; Gardiner, E.E.; Hua, M.; Qiao, J.; White, M.J.; Josefsson, E.C.; Alwis, I.; Ono, A.; Willcox, A. Bcl-xL–inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood 2011, 118, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.S.; Rahman, S.; Rupasinghe, H.; Vazhappilly, C.G. Dietary Flavonoids in p53—Mediated Immune Dysfunctions Linking to Cancer Prevention. Biomedicines 2020, 8, 286. [Google Scholar] [CrossRef]
- Muhammad, I.; Rahman, N.; Nayab, G.E.; Niaz, S.; Shah, M.; Afridi, S.G.; Khan, H.; Daglia, M.; Capanoglu, E. The Molecular Docking of Flavonoids Isolated from Daucus carota as a Dual Inhibitor of MDM2 and MDMX. Recent Pat. Anti-Cancer Drug Discov. 2020, 15, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Siahsar, B.; Rahimi, M.; Tavassoli, A.; Raissi, A. Application of biotechnology in production of medicinal plants. Am Eurasian J Agric Env. Sci 2011, 11, 439–444. [Google Scholar]
- Benavente-Garcia, O.; Castillo, J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Bhise, K.; Kashaw, S.K.; Sau, S.; Iyer, A.K. Nanostructured lipid carriers employing polyphenols as promising anticancer agents: Quality by design (QbD) approach. Int. J. Pharm. 2017, 526, 506–515. [Google Scholar] [CrossRef]
- Dixon, R.A. Phytochemistry meets genome analysis, and beyond. Phytochemistry 2003, 62, 815–816. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, X.; Tan, M.; Gong, J.; Tan, W.; Bian, B.; Chen, M.; Wang, Y. Fighting fire with fire: Poisonous Chinese herbal medicine for cancer therapy. J. Ethnopharmacol. 2012, 140, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Duo, J.; Ying, G.-G.; Wang, G.-W.; Zhang, L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol. Med. Rep. 2012, 5, 1453–1456. [Google Scholar]
- Sharmila, G.; Bhat, F.; Arunkumar, R.; Elumalai, P.; Singh, P.R.; Senthilkumar, K.; Arunakaran, J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin. Nutr. 2014, 33, 718–726. [Google Scholar] [CrossRef]
- Yi, X.; Zuo, J.; Tan, C.; Xian, S.; Luo, C.; Chen, S.; Yu, L.; Luo, Y. Kaempferol, a flavonoid compound from gynura medica induced apoptosis and growth inhibition in mcf-7 breast cancer cell. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 210–215. [Google Scholar] [CrossRef]
- Song, H.; Bao, J.; Wei, Y.; Chen, Y.; Mao, X.; Li, J.; Yang, Z.; Xue, Y. Kaempferol inhibits gastric cancer tumor growth: An in vitro and in vivo study. Oncol. Rep. 2015, 33, 868–874. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Y.; Mao, Q.-Q.; Li, X.; Chen, M.-W.; Su, J.; Tian, L.; Mao, N.-Q.; Long, L.-Z.; Quan, M.-F. Casticin induces caspase-mediated apoptosis via activation of mitochondrial pathway and upregulation of DR5 in human lung cancer cells. Asian Pac. J. Trop. Med. 2013, 6, 372–378. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Hyun, J.W. Fisetin induces apoptosis in human nonsmall lung cancer cells via a mitochondria-mediated pathway. Vitr. Cell. Dev. Biol. Anim. 2015, 51, 300–309. [Google Scholar] [CrossRef]
- Hyun, H.-B.; Lee, W.S.; Go, S.-I.; Nagappan, A.; Park, C.; Han, M.H.; Hong, S.H.; Kim, G.; Kim, G.Y.; Cheong, J. The flavonoid morin from Moraceae induces apoptosis by modulation of Bcl-2 family members and Fas receptor in HCT 116 cells. Int. J. Oncol. 2015, 46, 2670–2678. [Google Scholar] [CrossRef]
- Liu, S.; Wu, N.; Miao, J.; Huang, Z.; Li, X.; Jia, P.; Guo, Y.; Jia, D. Protective effect of morin on myocardial ischemia-reperfusion injury in rats. Int. J. Mol. Med. 2018, 42, 1379–1390. [Google Scholar] [CrossRef]
- Nicolini, F.; Burmistrova, O.; Marrero, M.T.; Torres, F.; Hernández, C.; Quintana, J.; Estevez, F. Induction of G2/M phase arrest and apoptosis by the flavonoid tamarixetin on human leukemia cells. Mol. Carcinog. 2014, 53, 939–950. [Google Scholar] [CrossRef]
- Jeong, J.J.; Ha, Y.M.; Jin, Y.C.; Lee, E.J.; Kim, J.S.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Kang, S.S.; Kim, Y.S. Rutin from Lonicera japonica inhibits myocardial ischemia/reperfusion-induced apoptosis in vivo and protects H9c2 cells against hydrogen peroxide-mediated injury via ERK1/2 and PI3K/Akt signals in vitro. Food Chem. Toxicol. 2009, 47, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, P.; Wang, J.; Zhang, J.; Gu, J.; Wu, X.; Wu, W.; Fei, X.; Zhang, Z.; Wang, Y. Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via a ROS/JNK-dependent mitochondrial pathway. Cancer Lett. 2010, 298, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.-Y.; Park, J.-H.; Paik, H.-D.; Nah, S.-Y.; Kim, D.S.; Han, Y.S. Acacetin-induced apoptosis of human breast cancer MCF-7 cells involves caspase cascade, mitochondria-mediated death signaling and SAPK/JNK1/2-c-Jun activation. Mol. Cells 2007, 24, 95–104. [Google Scholar] [PubMed]
- Wang, W.; Guo, Q.; You, Q.; Zhang, K.; Yang, Y.; Yu, J.; Liu, W.; Zhao, L.; Gu, H.; Hu, Y. Involvement of bax/bcl-2 in wogonin-induced apoptosis of human hepatoma cell line SMMC-7721. Anti-Cancer Drugs 2006, 17, 797–805. [Google Scholar] [CrossRef]
- Lu, H.-F.; Chie, Y.-J.; Yang, M.-S.; Lee, C.-S.; Fu, J.-J.; Yang, J.-S.; Tan, T.-W.; Wu, S.-H.; Ma, Y.-S.; Ip, S.-W. Apigenin induces caspase-dependent apoptosis in human lung cancer A549 cells through Bax-and Bcl-2-triggered mitochondrial pathway. Int. J. Oncol. 2010, 36, 1477–1484. [Google Scholar]
- Zhang, Q.; Ma, S.; Liu, B.; Liu, J.; Zhu, R.; Li, M. Chrysin induces cell apoptosis via activation of the p53/Bcl-2/caspase-9 pathway in hepatocellular carcinoma cells. Exp. Ther. Med. 2016, 12, 469–474. [Google Scholar] [CrossRef]
- Wu, H.; Huang, M.; Liu, Y.; Shu, Y.; Liu, P. Luteolin induces apoptosis by up-regulating miR-34a in human gastric cancer cells. Technol. Cancer Res. Treat. 2015, 14, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-m.; Wang, S.; Zhang, H.; Lu, Y.-y.; Wang, X.-f.; Motoo, Y.; Su, S.-b. The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol. Sin. 2009, 30, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Da Lee, H.; Jung, Y.J.; Shin, S.Y.; Lee, Y.H. The natural flavone eupatorin induces cell cycle arrest at the G2/M phase and apoptosis in HeLa cells. Appl. Biol. Chem. 2016, 59, 193–199. [Google Scholar] [CrossRef]
- Tan, K.-T.; Lin, M.-X.; Lin, S.-C.; Tung, Y.-T.; Lin, S.-H.; Lin, C.-C. Sinensetin induces apoptosis and autophagy in the treatment of human T-cell lymphoma. Anti-Cancer Drugs 2019, 30, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, S.; Tian, S.; He, Y.; Lou, H.; Yang, Z.; Kong, Y.; Cao, X. Nobiletin inhibits breast cancer via p38 mitogen-activated protein kinase, nuclear transcription factor-κB, and nuclear factor erythroid 2-related factor 2 pathways in MCF-7 cells. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hua, C.; Pan, X.; Fu, X.; Wu, W. Eupatilin inhibits OGD/R-induced neuronal injury in PC12 cells. Int. J. Clin. Exp. Med. 2017, 10, 6728–6734. [Google Scholar]
- Qiao, Z.; Xu, Y.-w.; Yang, J. Eupatilin inhibits the apoptosis in H9c2 cardiomyocytes via the Akt/GSK-3β pathway following hypoxia/reoxygenation injury. Biomed. Pharmacother. 2016, 82, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, Q.; Liu, H.; Luo, S. Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non-small cell lung cancer A549 cells. Biol. Res. 2019, 52, 1–7. [Google Scholar] [CrossRef]
- Tian, F.; Mao, Y.; Sun, X. Vitexin prevents myocardial infarction in rats via inhibiting oxidative stress and myocardial apoptosis. Int. J. Clin. Exp. Med. 2019, 12, 8971–8977. [Google Scholar]
- Zhang, T.; Li, S.; Li, J.; Yin, F.; Hua, Y.; Wang, Z.; Lin, B.; Wang, H.; Zou, D.; Zhou, Z. Natural product pectolinarigenin inhibits osteosarcoma growth and metastasis via SHP-1-mediated STAT3 signaling inhibition. Cell Death Dis. 2016, 7, e2421. [Google Scholar] [CrossRef]
- Wu, B.; Liang, J. Pectolinarigenin promotes functional recovery and inhibits apoptosis in rats following spinal cord injuries. Exp. Ther. Med. 2019, 17, 3877–3882. [Google Scholar] [CrossRef]
- Lim, S.-L.; Park, S.-Y.; Kang, S.; Park, D.; Kim, S.-H.; Um, J.-Y.; Jang, H.-J.; Lee, J.-H.; Jeong, C.-H.; Jang, J.-H. Morusin induces cell death through inactivating STAT3 signaling in prostate cancer cells. Am. J. Cancer Res. 2015, 5, 289. [Google Scholar]
- Zhang, C.; Chen, Y.; Zhang, M.; Xu, C.; Gong, G.; Veeraraghavan, V.P.; Bolla, S.R.; Li, Y. Vicenin-2 Treatment Attenuated the Diethylnitrosamine-Induced Liver Carcinoma and Oxidative Stress through Increased Apoptotic Protein Expression in Experimental Rats. J. Environ. Pathol. Toxicol. Oncol. 2020, 39, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, S.Y.; Yin, H.L.; Xu, N.L.; Peng, Y.J.; Zhou, H.; Kang, W. Synergistic anti-glioma effect of Hydroxygenkwanin and Apigenin in vitro. Chem. Biol. Interact. 2013, 206, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Liu, J.; Bao, Y.; An, L. Hydrogen peroxide-induced apoptosis in pc12 cells and the protective effect of puerarin. Cell Biol. Int. 2003, 27, 1025–1031. [Google Scholar] [CrossRef]
- Ferenc, P.; Solár, P.; Kleban, J.; Mikeš, J.; Fedoročko, P. Down-regulation of Bcl-2 and Akt induced by combination of photoactivated hypericin and genistein in human breast cancer cells. J. Photochem. Photobiol. B Biol. 2010, 98, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Jeon, Y.K.; You, D.H.; Nam, M.J. Daidzein causes cytochrome c-mediated apoptosis via the Bcl-2 family in human hepatic cancer cells. Food Chem. Toxicol. 2013, 60, 542–549. [Google Scholar] [CrossRef]
- Noh, D.; Choi, J.G.; Huh, E.; Oh, M.S. Tectorigenin, a flavonoid-based compound of leopard lily rhizome, attenuates UV-B-induced apoptosis and collagen degradation by inhibiting oxidative stress in human keratinocytes. Nutrients 2018, 10, 1998. [Google Scholar] [CrossRef]
- Huang, W.; Tang, S.; Qiao, X.; Ma, W.; Ji, S.; Wang, K.; Ye, M.; Yu, S. Isoangustone A induces apoptosis in SW480 human colorectal adenocarcinoma cells by disrupting mitochondrial functions. Fitoterapia 2014, 94, 36–47. [Google Scholar] [CrossRef]
- Park, H.; Kim, M.-J.; Ha, E.; Chung, J.-H. Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedicine 2008, 15, 147–151. [Google Scholar] [CrossRef]
- Bae, G.D.; Park, E.-Y.; Baek, D.J.; Jun, H.-S.; Oh, Y.S. Liquiritigenin prevents palmitate-induced beta-cell apoptosis via estrogen receptor-mediated AKT activation. Biomed. Pharmacother. 2018, 101, 348–354. [Google Scholar] [CrossRef]
- Sahu, B.D.; Tatireddy, S.; Koneru, M.; Borkar, R.M.; Kumar, J.M.; Kuncha, M.; Srinivas, R.; Sistla, R. Naringin ameliorates gentamicin-induced nephrotoxicity and associated mitochondrial dysfunction, apoptosis and inflammation in rats: Possible mechanism of nephroprotection. Toxicol. Appl. Pharmacol. 2014, 277, 8–20. [Google Scholar] [CrossRef]
- Saralamma, V.V.G.; Nagappan, A.; Hong, G.E.; Lee, H.J.; Yumnam, S.; Raha, S.; Heo, J.D.; Lee, S.J.; Lee, W.S.; Kim, E.H. Poncirin induces apoptosis in AGS human gastric cancer cells through extrinsic apoptotic pathway by up-regulation of fas ligand. Int. J. Mol. Sci. 2015, 16, 22676–22691. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, H.; Wang, Q.; Deng, S.; Huang, M.; Ma, X.; Song, P.; Du, J.; Huang, Y.; Wen, Y. Inhibitory effect of kurarinone on growth of human non-small cell lung cancer: An experimental study both in vitro and in vivo studies. Front. Pharmacol. 2018, 9, 252. [Google Scholar] [CrossRef]
- Yoshida, T.; Horinaka, M.; Takara, M.; Tsuchihashi, M.; Mukai, N.; Wakada, M.; Sakai, T. Combination of isoliquiritigenin and tumor necrosis factor-related apoptosis-inducing ligand induces apoptosis in colon cancer HT29 cells. Environ. Health Prev. Med. 2008, 13, 281–287. [Google Scholar] [CrossRef]
- Zhou, Y.; Ho, W.S. Combination of liquiritin, isoliquiritin and isoliquirigenin induce apoptotic cell death through upregulating p53 and p21 in the A549 non-small cell lung cancer cells. Oncol. Rep. 2014, 31, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Rafi, M.M.; Rosen, R.T.; Vassil, A.; Ho, C.-T.; Zhang, H.; Ghai, G.; Lambert, G.; DiPaola, R.S. Modulation of bcl-2 and cytotoxicity by licochalcone-A, a novel estrogenic flavonoid. Anticancer Res. 2000, 20, 2653–2658. [Google Scholar]
- Yuan, X.; Li, T.; Xiao, E.; Zhao, H.; Li, Y.; Fu, S.; Gan, L.; Wang, Z.; Zheng, Q.; Wang, Z. Licochalcone B inhibits growth of bladder cancer cells by arresting cell cycle progression and inducing apoptosis. Food Chem. Toxicol. 2014, 65, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Cho, I.A.; Kang, K.R.; Jung, Y.R.; Cho, S.S.; Yoon, G.; Oh, J.S.; You, J.S.; Seo, Y.S.; Lee, G.J. Licochalcone-E induces caspase-dependent death of human pharyngeal squamous carcinoma cells through the extrinsic and intrinsic apoptotic signaling pathways. Oncol. Lett. 2017, 13, 3662–3668. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, H.; Zhang, J.; Huang, H.; Liu, L.; Sun, Y. Malvidin induced anticancer activity in human colorectal HCT-116 cancer cells involves apoptosis, G2/M cell cycle arrest and upregulation of p21WAFI. Int. J. Clin. Exp. Med. 2018, 11, 1734–1741. [Google Scholar]
- Baba, A.B.; Nivetha, R.; Chattopadhyay, I.; Nagini, S. Blueberry and malvidin inhibit cell cycle progression and induce mitochondrial-mediated apoptosis by abrogating the JAK/STAT-3 signalling pathway. Food Chem. Toxicol. 2017, 109, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Fimognari, C.; Berti, F.; Nüsse, M.; Cantelli-Forti, G.; Hrelia, P. Induction of apoptosis in two human leukemia cell lines as well as differentiation in human promyelocytic cells by cyanidin-3-O-β-glucopyranoside. Biochem. Pharmacol. 2004, 67, 2047–2056. [Google Scholar] [CrossRef]
- Karthi, N.; Kalaiyarasu, T.; Kandakumar, S.; Mariyappan, P.; Manju, V. Pelargonidin induces apoptosis and cell cycle arrest via a mitochondria mediated intrinsic apoptotic pathway in HT29 cells. RSC Adv. 2016, 6, 45064–45076. [Google Scholar] [CrossRef]
- Seo, E.Y. Delphinidin inhibits cell proliferation and induces apoptosis in MDA-MB-231 human breast cancer cell lines. J. Nutr. Health 2013, 46, 503–510. [Google Scholar] [CrossRef]
- Alhosin, M.; León-González, A.J.; Dandache, I.; Lelay, A.; Rashid, S.K.; Kevers, C.; Pincemail, J.; Fornecker, L.-M.; Mauvieux, L.; Herbrecht, R. Bilberry extract (Antho 50) selectively induces redox-sensitive caspase 3-related apoptosis in chronic lymphocytic leukemia cells by targeting the Bcl-2/Bad pathway. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Tan, X.-H.; Zhang, Y.-L.; Zhou, D.-Y. EGCG induced apoptosis and expression change of bcl-2 protein in gastric and hepatic carcinoma cells. Chin. J. Cancer 2000, 7. [Google Scholar]
- Du, G.-J.; Zhang, Z.; Wen, X.-D.; Yu, C.; Calway, T.; Yuan, C.-S.; Wang, C.-Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef]
- Hwang, J.-T.; Ha, J.; Park, I.-J.; Lee, S.-K.; Baik, H.W.; Kim, Y.M.; Park, O.J. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007, 247, 115–121. [Google Scholar] [CrossRef]
- Safari, F.; Rabieepor, M.; Jamalomidi, F.; Baghaeifar, Z.; Khodaei, L. Evaluation of Anti-cancer and Pro-apoptotic Activities of Iranian Green Tea Extract Against A549, PC3, and MCF-7 Cancer Cell Lines. Int. J. Basic Sci. Med. 2019, 4, 113–118. [Google Scholar] [CrossRef]
- Li, K.; Li, Q.; Zhang, T.; Han, Z.; Li, J.; Liu, Z.; Zheng, F. Procyanidins from Pinus koraiensis bark inhibits HeLa cell growth by inducing apoptosis and reducing survivin protein expression. Afr. J. Biotechnol. 2011, 10, 7766–7771. [Google Scholar]
- Wu, H.; Xie, Y.; Xu, Y.; Hu, Z.; Wan, X.; Huang, H.; Huang, D. Protective effect of epicatechin on APAP-induced acute liver injury of mice through anti-inflammation and apoptosis inhibition. Nat. Prod. Res. 2020, 34, 855–858. [Google Scholar] [CrossRef]
- Pariyar, R.; Bastola, T.; Seo, J. Protective Effects of Quercetin-3-O-glucuronide against 1-methyl-4-phenylpyridinium-induced Neurotoxicity. J. Life Sci. 2019, 29, 191–197. [Google Scholar]
- Liang, X.; Wang, P.; Yang, C.; Huang, F.; Wu, H.; Shi, H.; Wu, X. Galangin Inhibits Gastric Cancer Growth Through Enhancing STAT3 Mediated ROS Production. Front. Pharmacol. 2021, 12, 908. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids Nanoparticles in Cancer: Treatment, Prevention and Clinical Prospects; Seminars in cancer biology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 200–211. [Google Scholar]
- Khan, H.; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef]
- Li, Y.-R.; Li, G.-H.; Zhou, M.-X.; Xiang, L.; Ren, D.-M.; Lou, H.-X.; Wang, X.-N.; Shen, T. Discovery of natural flavonoids as activators of Nrf2-mediated defense system: Structure-activity relationship and inhibition of intracellular oxidative insults. Bioorganic Med. Chem. 2018, 26, 5140–5150. [Google Scholar] [CrossRef]
- Chen, K.-C.; Hsu, W.-H.; Ho, J.-Y.; Lin, C.-W.; Chu, C.-Y.; Kandaswami, C.C.; Lee, M.-T.; Cheng, C.-H. Flavonoids Luteolin and Quercetin Inhibit RPS19 and contributes to metastasis of cancer cells through c-Myc reduction. J. Food Drug Anal. 2018, 26, 1180–1191. [Google Scholar] [CrossRef]
- Correia, C.; Lee, S.-H.; Meng, X.W.; Vincelette, N.D.; Knorr, K.L.; Ding, H.; Nowakowski, G.S.; Dai, H.; Kaufmann, S.H. Emerging understanding of Bcl-2 biology: Implications for neoplastic progression and treatment. Biochim. Biophys. Acta BBA Mol. Cell Res. 2015, 1853, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Deng, J.; Seymour, J.F.; Tam, C.; Kim, S.Y.; Fein, J.; Yu, L.; Brown, J.R.; Westerman, D.; Si, E.G. The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism. Blood J. Am. Soc. Hematol. 2016, 127, 3215–3224. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ren, J.S.; Masuyer, E.; Ferlay, J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int. J. Cancer 2013, 132, 1133–1145. [Google Scholar] [CrossRef]
- Sung, Y.-J.; Kao, T.-Y.; Kuo, C.-L.; Fan, C.-C.; Cheng, A.N.; Fang, W.-C.; Chou, H.-Y.; Lo, Y.-K.; Chen, C.-H.; Jiang, S.S. Mitochondrial Lon sequesters and stabilizes p53 in the matrix to restrain apoptosis under oxidative stress via its chaperone activity. Cell Death Dis. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Delia, D.; Aiello, A.; Soligo, D.; Fontanella, E.; Melani, C.; Pezzella, F.; Pierotti, M.A.; Della Porta, G. bcl-2 proto-oncogene expression in normal and neoplastic human myeloid cells. Blood 1992, 79, 1291–1298. [Google Scholar] [CrossRef]
- Berghella, A.M.; Pellegrini, P.; Contasta, I.; Del Beato, T.; Adorno, D. Bcl-2 and drugs used in the treatment of cancer: New strategies of biotherapy which should not be underestimated. Cancer Biother. Radiopharm. 1998, 13, 225–237. [Google Scholar] [CrossRef]
- Soussi, T.; Béroud, C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat. Rev. Cancer 2001, 1, 233–239. [Google Scholar] [CrossRef]
- Suzuki, K.; Matsubara, H. Recent advances in p53 research and cancer treatment. J. Biomed. Biotechnol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.A.; Attardi, L.D. p53 at a glance. J. Cell Sci. 2010, 123, 2527–2532. [Google Scholar] [CrossRef]
- Vousden, K.H.; Ryan, K.M. p53 and metabolism. Nat. Rev. Cancer 2009, 9, 691–700. [Google Scholar] [CrossRef]
- Vousden, K.H.; Prives, C. Blinded by the light: The growing complexity of p53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef]
- Burns, T.F.; Fei, P.; Scata, K.A.; Dicker, D.T.; El-Deiry, W.S. Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (taxol)-exposed cells. Mol. Cell. Biol. 2003, 23, 5556–5571. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2). J. Nutr. 2006, 136, 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-L.; Zhang, Y.-J.; Wang, X.-F.; Zhang, W.-J.; Wang, Z.; Zhang, F.; Zhang, Y.-J.; Lu, J.-H.; Mei, J.-W.; Hu, Y.-P. Casticin induces apoptosis and G0/G1 cell cycle arrest in gallbladder cancer cells. Cancer Cell Int. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Jang, K.Y.; Jeong, S.-J.; Kim, S.-H.; Jung, J.H.; Kim, J.-H.; Koh, W.; Chen, C.-Y.; Kim, S.-H. Activation of reactive oxygen species/AMP activated protein kinase signaling mediates fisetin-induced apoptosis in multiple myeloma U266 cells. Cancer Lett. 2012, 319, 197–202. [Google Scholar] [CrossRef]
- Tan, H.-L.; Chan, K.-G.; Pusparajah, P.; Saokaew, S.; Duangjai, A.; Lee, L.-H.; Goh, B.-H. Anti-cancer properties of the naturally occurring aphrodisiacs: Icariin and its derivatives. Front. Pharmacol. 2016, 7, 191. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.-H.; Zhang, J.; Yu, B.-Y. Comparative evaluation of cytotoxicity and antioxidative activity of 20 flavonoids. J. Agric. Food Chem. 2008, 56, 3876–3883. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Lao, M.; Ma, Z.; Yi, X. Puerarin protects Alzheimer’s disease neuronal cybrids from oxidant-stress induced apoptosis by inhibiting pro-death signaling pathways. Exp. Gerontol. 2011, 46, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-G.; Yin, X.-C.; Liu, X.-F.; Meng, K.-W.; Tang, K.; Huang, F.-L.; Xu, G.; Gao, J. Puerarin induces hepatocellular carcinoma cell apoptosis modulated by MAPK signaling pathways in a dose-dependent manner. Anticancer Res. 2017, 37, 4425–4431. [Google Scholar]
- Khan, M.; Yu, B.; Rasul, A.; Al Shawi, A.; Yi, F.; Yang, H.; Ma, T. Jaceosidin induces apoptosis in U87 glioblastoma cells through G2/M phase arrest. Evid. Based Complementary Altern. Med. 2012, 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, A.; Javeed, M.; Shah, M.A.; Hussain, G.; Shafiq, N.; Sarfraz, I.; Riaz, A.; Sadiqa, A.; Zara, R.; Zafar, S. Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total Environ. 2020, 722, 137907. [Google Scholar] [CrossRef]
- Choi, E.J. Hesperetin induced G1-phase cell cycle arrest in human breast cancer MCF-7 cells: Involvement of CDK4 and p21. Nutr. Cancer 2007, 59, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, D.; Song, J.; Wang, J.; Yi, J.; Dong, W. Hesperetin induces the apoptosis of gastric cancer cells via activating mitochondrial pathway by increasing reactive oxygen species. Dig. Dis. Sci. 2015, 60, 2985–2995. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Zuo, J.; Duan, J.-F.; Han, J.; Fan, S.-M.; Zhang, W.; Zhu, L.-F.; Yao, M.-H. Antitumor activities of kushen flavonoids in vivo and in vitro. Zhong Xi Yi Jie He Xue Bao J. Chin. Integr. Med. 2008, 6, 51–59. [Google Scholar] [CrossRef]
- Ambs, S.; Merriam, W.G.; Bennett, W.P.; Felley-Bosco, E.; Ogunfusika, M.O.; Oser, S.M.; Klein, S.; Shields, P.G.; Billiar, T.R.; Harris, C.C. Frequent nitric oxide synthase-2 expression in human colon adenomas: Implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998, 58, 334–341. [Google Scholar]
- Eberhart, C.E.; Coffey, R.J.; Radhika, A.; Giardiello, F.M.; Ferrenbach, S.; Dubois, R.N. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1994, 107, 1183–1188. [Google Scholar] [CrossRef]
- Hirchaud, F.; Hermetet, F.; Ablise, M.; Fauconnet, S.; Vuitton, D.A.; Prétet, J.-L.; Mougin, C. Isoliquiritigenin induces caspase-dependent apoptosis via downregulation of HPV16 E6 expression in cervical cancer Ca Ski cells. Planta Med. 2013, 79, 1628–1635. [Google Scholar] [CrossRef]
- Shah, U.; Shah, R.; Acharya, S.; Acharya, N. Novel anticancer agents from plant sources. Chin. J. Nat. Med. 2013, 11, 16–23. [Google Scholar] [CrossRef]
- Lee, S.M.; Ko, I.-G.; Kim, S.-E.; Kim, D.H.; Kang, B.N. Protective effect of catechin on apoptosis of the lens epithelium in rats with N-methyl-N-nitrosourea-induced cataracts. Korean J. Ophthalmol. 2010, 24, 101–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, H.L.; SHIN, B.C.; Chung, J.H. P1614 Antiapoptotic effecs of catechin and gemigliptin on tacrolimus-induced renal injury in mice. Nephrol. Dial. Transplant. 2020, 35 3, gfaa142.P1614. [Google Scholar] [CrossRef]
- Galati, G.; O’brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 16. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: An effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed. 2014, 4, S1–S7. [Google Scholar] [CrossRef]
- Jain, D.; Raturi, R.; Jain, V.; Bansal, P.; Singh, R. Recent technologies in pulsatile drug delivery systems. Biomatter 2011, 1, 57–65. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).