Optogenetic Activation of Astrocytes—Effects on Neuronal Network Function
Abstract
:1. Introduction
2. Results
2.1. Specificity of Expression of AAV2/5 GfaABC1D_ChR2(H134R)-mCherry and AAV2/5 GfaABC1D_Opto-a1AR-EYFP
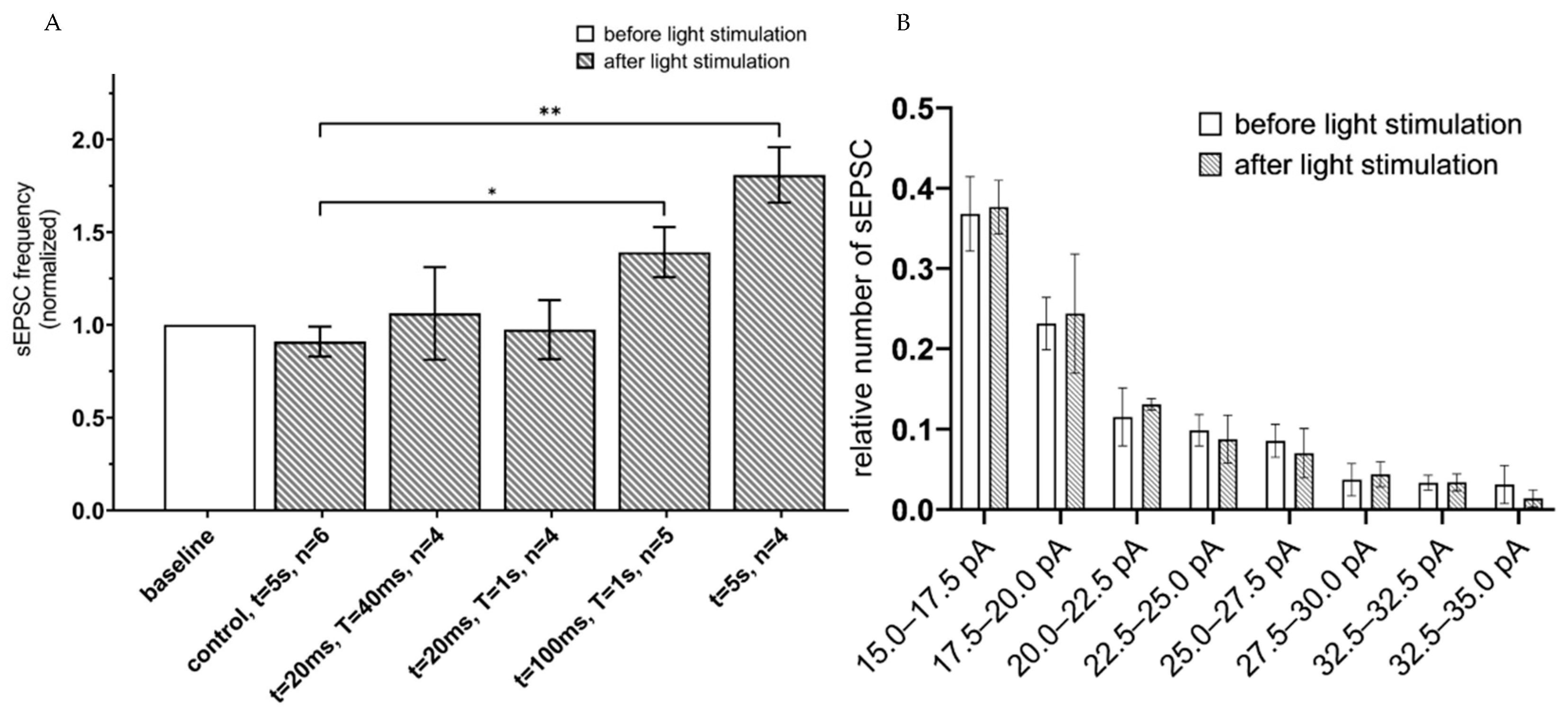
2.2. Activation of Astrocytes Expressing ChR2(H134R) or Opto-α1AR Leads to Enhancement of Pyramidal Neuron’s Activity
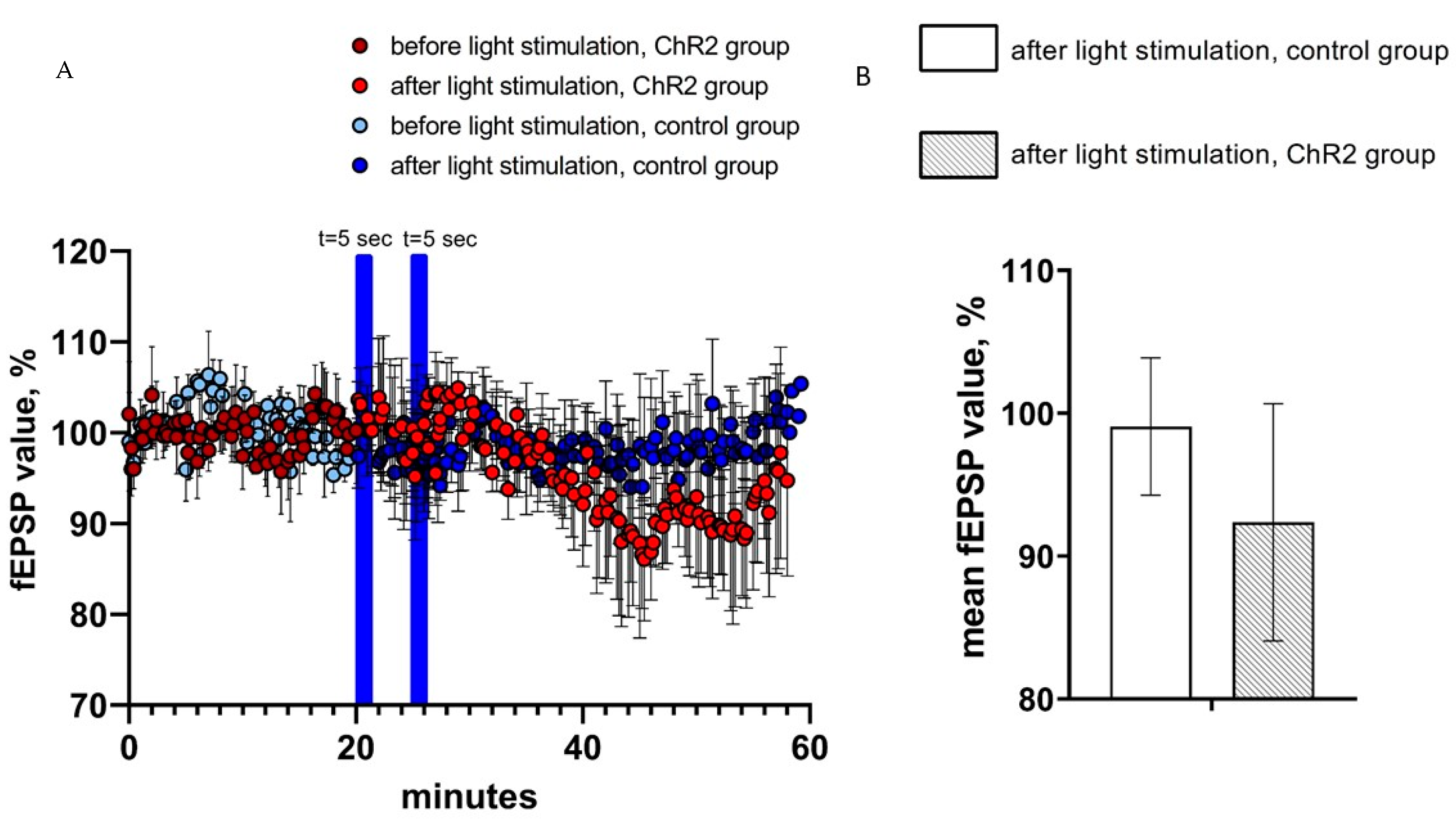
2.3. Activation of Astrocytes Expressing ChR2(H134R)-mCherry Had No Effect on the Field Excitatory Postsynaptic Potentials
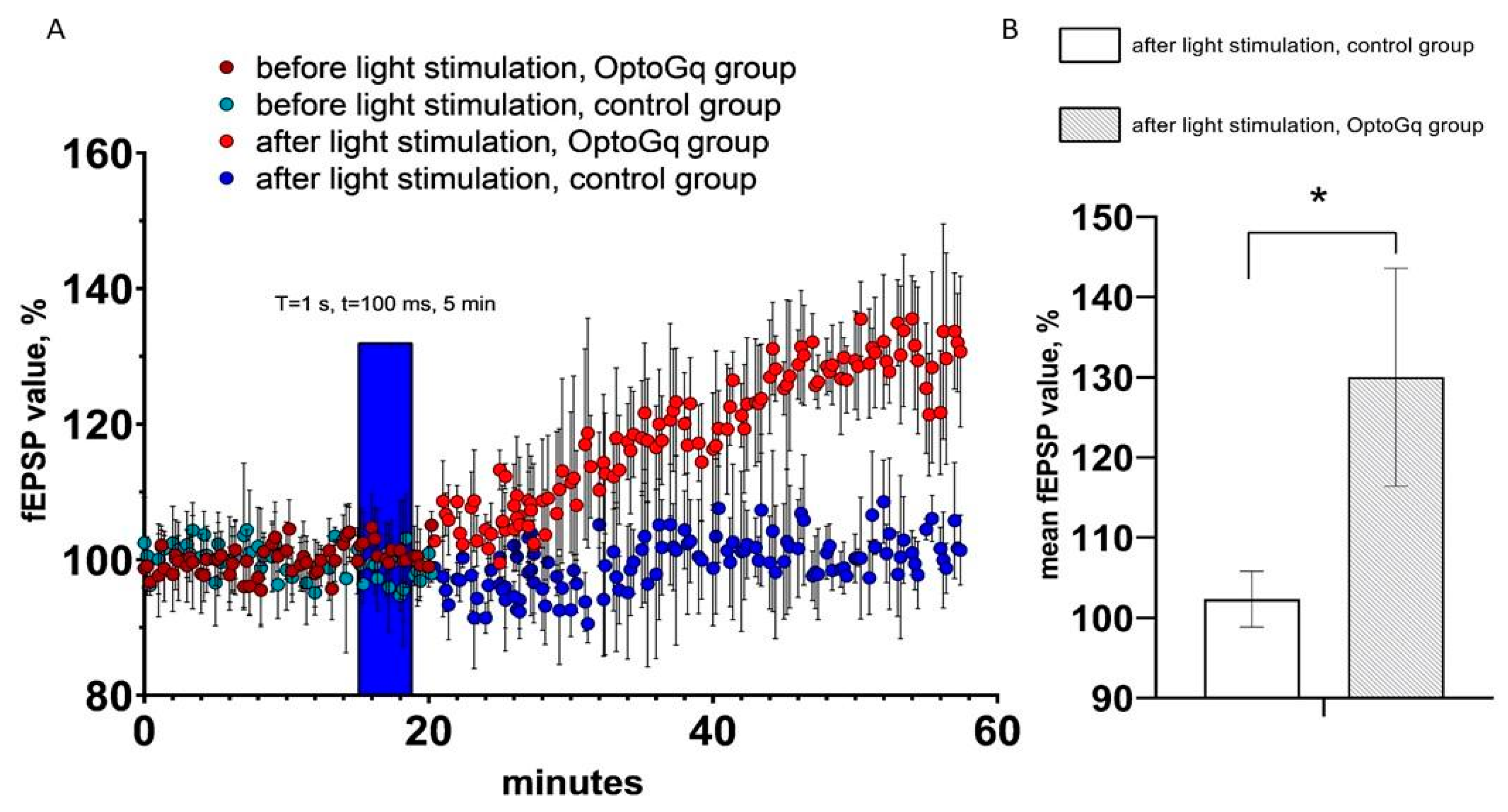
2.4. Activation of Opto-α1AR-EYFP-Expressing Astrocytes Leads to Increase of Field Excitatory Postsynaptic Potential
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Plasmids and Production of Viral Constructs
4.3. Viral Constructs Delivery via Stereotaxic Surgery
4.4. Immunohistochemistry
4.5. Slice Electrophysiology
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.H.; Bergles, D.E. Glutamate transporters bring competition to the synapse. Curr. Opin. Neurobiol. 2004, 14, 346–352. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Schousboe, A. Pharmacological and Functional Characterization of Astrocytic GABa Transport: A Short Review. Neurochem. Res. 2000, 25, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Egawa, K.; Fukuda, A. Diverse actions of astrocytes in GABAergic signaling. Int. J. Mol. Sci. 2019, 20, 2964. [Google Scholar] [CrossRef] [Green Version]
- Shibasaki, K.; Hosoi, N.; Kaneko, R.; Tominaga, M.; Yamada, K. Glycine release from astrocytes via functional reversal of GlyT1. J. Neurochem. 2017, 140, 395–403. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuga, N.; Sasaki, T.; Takahara, Y.; Matsuki, N.; Ikegaya, Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J. Neurosci. 2011, 31, 2607–2614. [Google Scholar] [CrossRef] [Green Version]
- Salmina, A.B.; Gorina, Y.V.; Erofeev, A.I.; Balaban, P.M.; Bezprozvanny, I.B.; Vlasova, O.L. Optogenetic and chemogenetic modulation of astroglial secretory phenotype. Rev. Neurosci. 2021, 32, 459–479. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Haydon, P.G. Integrated brain circuits: Astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 2009, 72, 335–355. [Google Scholar] [CrossRef] [Green Version]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Perea, G.; Araque, A. Glial calcium signaling and neuron-glia communication. Cell Calcium 2005, 38, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Santello, M.; Calì, C.; Bezzi, P. Gliotransmission and the tripartite synapse. Adv. Exp. Med. Biol. 2012, 970, 307–331. [Google Scholar] [CrossRef] [PubMed]
- Rappold, P.M.; Tieu, K. Astrocytes and Therapeutics for Parkinson’s Disease. Neurotherapeutics 2010, 7, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palpagama, T.H.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. The Role of Microglia and Astrocytes in Huntington’s Disease. Front. Mol. Neurosci. 2019, 12, 258. [Google Scholar] [CrossRef] [Green Version]
- Frost, G.R.; Li, Y.M. The role of astrocytes in amyloid production and Alzheimer’s disease. Open Biol. 2017, 7, 170228. [Google Scholar] [CrossRef] [Green Version]
- Perez-Nievas, B.G.; Serrano-Pozo, A. Deciphering the astrocyte reaction in Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sánchez, K.; Ariza-Salamanca, D.; Mora-Muñoz, L. Involvement of astrocytes in Alzheimer’s disease from a neuroinflammatory and oxidative stress perspective. Front. Mol. Neurosci. 2017, 10, 427. [Google Scholar] [CrossRef] [Green Version]
- Prà, I.D.; Armato, U.; Chiarini, A. Astrocytes’ Role in Alzheimer’s Disease Neurodegeneration. Astrocyte Physiol. Pathol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, A.; Shah, Z.A. Neurodegenerative disease. Diet Exerc. Chronic Dis. Biol. Basis Prev. 2014, 339–390. [Google Scholar] [CrossRef]
- Murphy, M.P.; Levine, H. Alzheimer’s disease and the amyloid-β peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popugaeva, E.; Pchitskaya, E.; Bezprozvanny, I. Dysregulation of Intracellular Calcium Signaling in Alzheimer’s Disease. Antioxid. Redox Signal. 2018, 29, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Binder, L.I.; Guillozet-Bongaarts, A.L.; Garcia-Sierra, F.; Berry, R.W. Tau, tangles, and Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1739, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Eichler, S.A.; Meier, J.C. E-I balance and human diseases—From molecules to networking. Front. Mol. Neurosci. 2008, 1, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vico Varela, E.; Etter, G.; Williams, S. Excitatory-inhibitory imbalance in Alzheimer’s disease and therapeutic significance. Neurobiol. Dis. 2019, 127, 605–615. [Google Scholar] [CrossRef]
- Toniolo, S.; Sen, A.; Husain, M. Modulation of brain hyperexcitability: Potential new therapeutic approaches in alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 9318. [Google Scholar] [CrossRef] [PubMed]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef]
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 2003, 100, 13940–13945. [Google Scholar] [CrossRef] [Green Version]
- Octeau, J.C.; Gangwani, M.R.; Allam, S.L.; Tran, D.; Huang, S.; Hoang-Trong, T.M.; Golshani, P.; Rumbell, T.H.; Kozloski, J.R.; Khakh, B.S. Transient, Consequential Increases in Extracellular Potassium Ions Accompany Channelrhodopsin2 Excitation. Cell Rep. 2019, 27, 2249–2261.e7. [Google Scholar] [CrossRef] [Green Version]
- Airan, R.D.; Thompson, K.R.; Fenno, L.E.; Bernstein, H.; Deisseroth, K. Temporally precise in vivo control of intracellular signalling. Nature 2009, 458, 1025–1029. [Google Scholar] [CrossRef]
- Griffin, J.M.; Fackelmeier, B.; Fong, D.M.; Mouravlev, A.; Young, D.; O’Carroll, S.J. Astrocyte-selective AAV gene therapy through the endogenous GFAP promoter results in robust transduction in the rat spinal cord following injury. Gene Ther. 2019, 26, 198–210. [Google Scholar] [CrossRef]
- Perea, G.; Yang, A.; Boyden, E.S.; Sur, M. Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 2018, 174, 59–71.e14. [Google Scholar] [CrossRef] [Green Version]
- Henneberger, C.; Papouin, T.; Oliet, S.H.R.; Rusakov, D.A. Long-term potentiation depends on release of d-serine from astrocytes. Nature 2010, 463, 232–236. [Google Scholar] [CrossRef]
- Chen, J.; Tan, Z.; Zeng, L.; Zhang, X.; He, Y.; Gao, W.; Wu, X.; Li, Y.; Bu, B.; Wang, W.; et al. Heterosynaptic long-term depression mediated by ATP released from astrocytes. Glia 2013, 61, 178–191. [Google Scholar] [CrossRef]
- Tan, Z.; Liu, Y.; Xi, W.; Lou, H.; Zhu, L.; Guo, Z.; Mei, L.; Duan, S. Glia-derived ATP inversely regulates excitability of pyramidal and CCK-positive neurons. Nat. Commun. 2017, 8, 13772. [Google Scholar] [CrossRef] [Green Version]
- Mederos, S.; Hernández-Vivanco, A.; Ramírez-Franco, J.; Martín-Fernández, M.; Navarrete, M.; Yang, A.; Boyden, E.S.; Perea, G. Melanopsin for precise optogenetic activation of astrocyte-neuron networks. Glia 2019, 67, 915–934. [Google Scholar] [CrossRef]
- Courtney, C.D.; Sobieski, C.; Ramakrishnan, C.; Ingram, R.J.; Wojnowski, N.M.; DeFazio, R.A.; Deisseroth, K.; Christian-Hinman, C.A. Optogenetic activation of Gq signaling in astrocytes yields stimulation-specific effects on basal hippocampal synaptic excitation and inhibition. bioRxiv 2021, 105825, 1–32. [Google Scholar] [CrossRef]
- Covelo, A.; Araque, A. Neuronal activity determines distinct gliotransmitter release from a single astrocyte. Elife 2018, 7, e32237. [Google Scholar] [CrossRef]
- McNeill, J.; Rudyk, C.; Hildebrand, M.E.; Salmaso, N. Ion Channels and Electrophysiological Properties of Astrocytes: Implications for Emergent Stimulation Technologies. Front. Cell. Neurosci. 2021, 15, 1–22. [Google Scholar] [CrossRef]
- Martineau, M.; Parpura, V.; Mothet, J.P. Cell-type specific mechanisms of D-serine uptake and release in the brain. Front. Synaptic Neurosci. 2014, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, M.; Lane, S.; Stout, R.F.; Liu, B.; Parpura, V.; Teschemacher, A.G.; Kasparov, S. Comparative analysis of optogenetic actuators in cultured astrocytes. Cell Calcium 2014, 56, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Borodinova, A.A.; Balaban, P.M.; Bezprozvanny, I.B.; Salmina, A.B.; Vlasova, O.L. Genetic Constructs for the Control of Astrocytes’ Activity. Cells 2021, 10, 1600. [Google Scholar] [CrossRef]
- Osten, P.; Cetin, A.; Komai, S.; Eliava, M.; Seeburg, P.H. Stereotaxic gene delivery in the rodent brain. Nat. Protoc. 2007, 1, 3166–3173. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, H.; Liu, J.; Popugaeva, E.; Xu, N.-J.; Feske, S.; White, C.L.; Bezprozvanny, I. Reduced Synaptic STIM2 Expression and Impaired Store-Operated Calcium Entry Cause Destabilization of Mature Spines in Mutant Presenilin Mice. Neuron 2014, 82, 79–93. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimov, E.; Erofeev, A.; Borodinova, A.; Bolshakova, A.; Balaban, P.; Bezprozvanny, I.; Vlasova, O.L. Optogenetic Activation of Astrocytes—Effects on Neuronal Network Function. Int. J. Mol. Sci. 2021, 22, 9613. https://doi.org/10.3390/ijms22179613
Gerasimov E, Erofeev A, Borodinova A, Bolshakova A, Balaban P, Bezprozvanny I, Vlasova OL. Optogenetic Activation of Astrocytes—Effects on Neuronal Network Function. International Journal of Molecular Sciences. 2021; 22(17):9613. https://doi.org/10.3390/ijms22179613
Chicago/Turabian StyleGerasimov, Evgenii, Alexander Erofeev, Anastasia Borodinova, Anastasia Bolshakova, Pavel Balaban, Ilya Bezprozvanny, and Olga L. Vlasova. 2021. "Optogenetic Activation of Astrocytes—Effects on Neuronal Network Function" International Journal of Molecular Sciences 22, no. 17: 9613. https://doi.org/10.3390/ijms22179613
APA StyleGerasimov, E., Erofeev, A., Borodinova, A., Bolshakova, A., Balaban, P., Bezprozvanny, I., & Vlasova, O. L. (2021). Optogenetic Activation of Astrocytes—Effects on Neuronal Network Function. International Journal of Molecular Sciences, 22(17), 9613. https://doi.org/10.3390/ijms22179613





