Streptomyces sp.—A Treasure Trove of Weapons to Combat Methicillin-Resistant Staphylococcus aureus Biofilm Associated with Biomedical Devices
Abstract
1. Introduction
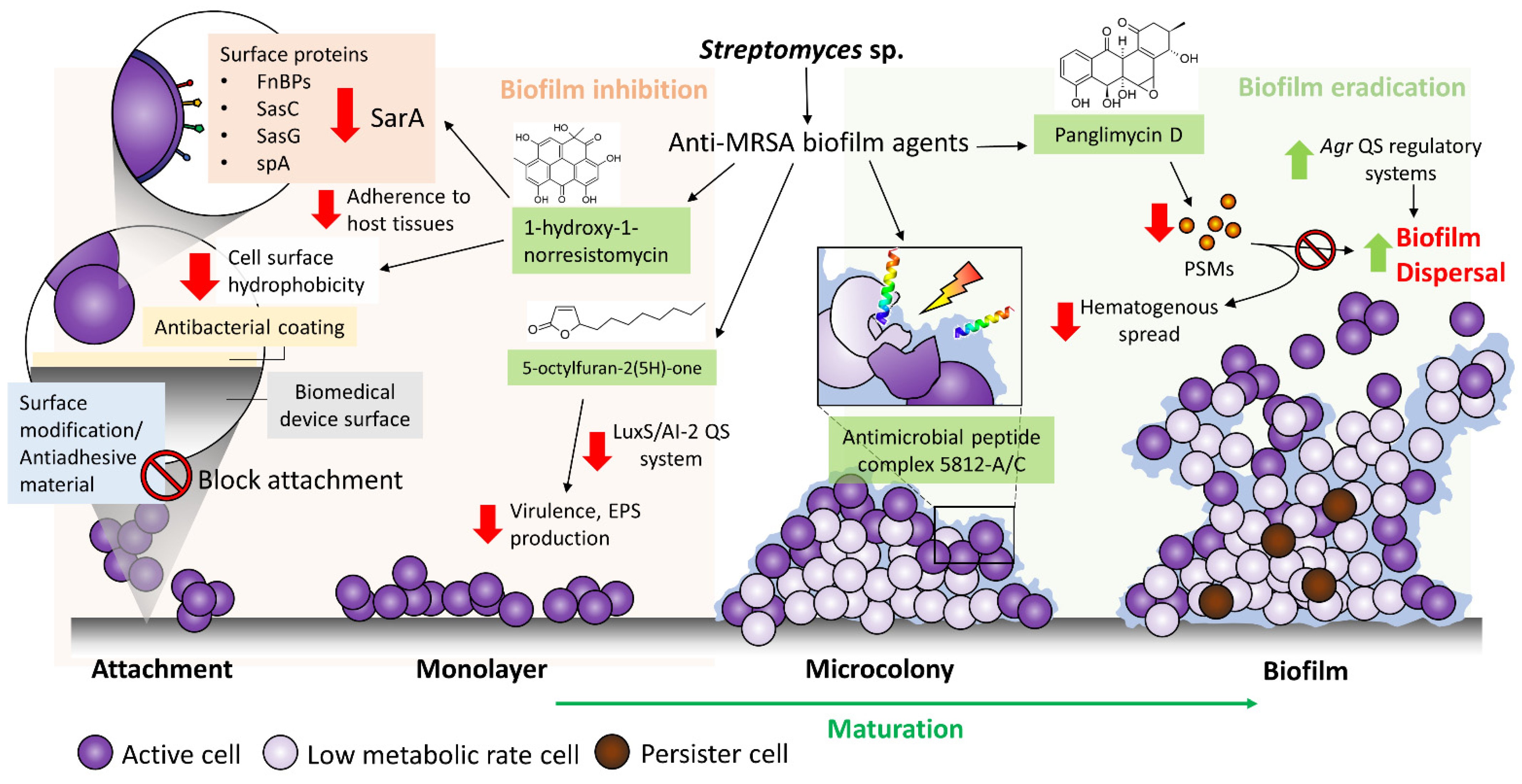
2. MRSA and Biofilm Formation on Biomedical Devices
3. Streptomyces sp. a Valuable Source for Anti-MRSA Biofilm Agents
Newly Reported Anti-MRSA Biofilm Compounds Synthesized by Streptomyces Bacteria
4. Clinically Used Antibiotics Derived from Streptomyces Bacteria for Medical Device-Related MRSA Biofilm Infections
5. Targeting MRSA Biofilm in the Treatment of Biomedical Device-Related Infections
6. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRSA | methicillin-resistant Staphylococcus aureus |
| MSCRAMM | microbial surface components recognizing adhesive matrix molecules |
| FnBP | fibronectin-binding protein |
| Clf | clumping factor |
| Sdr | serine-aspartate repeat protein |
| Cna | collagen binding protein |
| EPS | extracellular polymeric substance |
| PIA | polysaccharide intercellular adhesion |
| SasG | S. aureus protein G |
| Aap | accumulation-associated protein |
| eDNA | extracellular DNA |
| PNAG | poly-N-acetyl-glucosamine |
| PSM | phenol-soluble modulin |
| agr | accessory gene regulator |
| NGS | Next-Generation Sequencing |
| BIC90 | 90% biofilm inhibitory concentration |
| MSSA | methicillin-susceptible S. aureus |
| AMP | antimicrobial peptide |
| IC50 | 50% inhibitory concentration |
| PLGA | poly(lactic-co-glycolic acid) |
| spA | protein A |
| SarA | staphylococcal accessory regulatory protein |
| QS | quorum sensing |
| AHL | acyl-homoserine lactone |
| AIs | autoinducers |
| AI2 | autoinducer 2 |
| AIP | auto-inducing peptide |
References
- Kemung, H.M.; Tan, L.T.; Khan, T.M.; Chan, K.G.; Pusparajah, P.; Goh, B.H.; Lee, L.H. Streptomyces as a prominent resource of future anti-MRSA drugs. Front. Microbiol. 2018, 9, 2221. [Google Scholar] [CrossRef]
- Tarai, B.; Das, P.; Kumar, D. Recurrent challenges for clinicians: Emergence of methicillin-resistant Staphylococcus aureus, vancomycin resistance, and current treatment options. J. Lab. Physicians 2013, 5, 071–078. [Google Scholar] [CrossRef]
- Low, C.X.; Tan, L.T.-H.; Ab Mutalib, N.-S.; Pusparajah, P.; Goh, B.-H.; Chan, K.-G.; Letchumanan, V.; Lee, L.-H. Unveiling the impact of antibiotics and alternative methods for animal husbandry: A review. Antibiotics 2021, 10, 578. [Google Scholar] [CrossRef]
- Stewart, P.S.; William Costerton, J. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Gominet, M.; Compain, F.; Beloin, C.; Lebeaux, D. Central venous catheters and biofilms: Where do we stand in 2017? APMIS 2017, 125, 365–375. [Google Scholar] [CrossRef]
- Esposito, S.; Purrello, S.; Bonnet, E.; Novelli, A.; Tripodi, F.; Pascale, R.; Unal, S.; Milkovich, G. Central venous catheter-related biofilm infections: An up-to-date focus on meticillin-resistant Staphylococcus aureus. J. Glob. Antimicrob. Resist. 2013, 1, 71–78. [Google Scholar] [CrossRef]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.j.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef]
- Hui, M.L.-Y.; Tan, L.T.-H.; Letchumanan, V.; He, Y.-W.; Fang, C.-M.; Chan, K.-G.; Law, J.W.-F.; Lee, L.-H. The extremophilic Actinobacteria: From microbes to medicine. Antibiotics 2021, 10, 682. [Google Scholar] [CrossRef]
- Chew, S.-S.; Tan, L.T.-H.; Law, J.W.-F.; Pusparajah, P.; Goh, B.-H.; Ab Mutalib, N.S.; Lee, L.-H. Targeting gut microbial biofilms—A key to hinder colon carcinogenesis? Cancers 2020, 12, 2272. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Moumbock, A.F.A.; Gao, M.; Qaseem, A.; Li, J.; Kirchner, P.A.; Ndingkokhar, B.; Bekono, B.D.; Simoben, C.V.; Babiaka, S.B.; Malange, Y.I.; et al. StreptomeDB 3.0: An updated compendium of streptomycetes natural products. Nucleic Acids Res. 2020, 49, D600–D604. [Google Scholar] [CrossRef]
- Ab Mutalib, N.-S.; Wong, S.H.; Ser, H.-L.; Duangjai, A.; Law, J.W.-F.; Ratnakomala, S.; Tan, L.T.-H.; Letchumanan, V. Bioprospecting of microbes for valuable compounds to mankind. Prog. Microbes Mol. Biol. 2020, 3, a0000088. [Google Scholar] [CrossRef]
- Tan, L.T.-H.; Lee, L.-H.; Goh, B.-H. The bioprospecting of anti-Vibrio Streptomyces species: Prevalence and applications. Prog. Microbes Mol. Biol. 2019, 2, a0000034. [Google Scholar] [CrossRef]
- Kemung, H.M.; Tan, L.T.-H.; Chan, K.-G.; Ser, H.-L.; Law, J.W.-F.; Goh, B.H. Streptomyces sp. strain MUSC 5 from mangrove forest in Malaysia: Identification, antioxidant potential and chemical profiling of its methanolic extract. Prog. Microbes Mol. Biol. 2020, 3, a0000087. [Google Scholar] [CrossRef]
- Kemung, H.M.; Tan, L.T.-H.; Chan, K.-G.; Ser, H.-L.; Law, J.W.-F.; Lee, L.-H.; Goh, B.-H. Antioxidant activities of Streptomyces sp. strain MUSC 14 from mangrove forest soil in Malaysia. BioMed Res. Int. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Law, J.W.-F.; Law, L.N.-S.; Letchumanan, V.; Tan, L.T.-H.; Wong, S.H.; Chan, K.-G.; Ab Mutalib, N.-S.; Lee, L.-H. Anticancer drug discovery from microbial sources: The unique mangrove streptomycetes. Molecules 2020, 25, 5365. [Google Scholar] [CrossRef]
- Tan, L.T.-H.; Chan, C.-K.; Chan, K.-G.; Pusparajah, P.; Khan, T.M.; Ser, H.-L.; Lee, L.-H.; Goh, B.-H. Streptomyces sp. MUM256: A source for apoptosis inducing and cell cycle-arresting bioactive compounds against colon cancer cells. Cancers 2019, 11, 1742. [Google Scholar] [CrossRef]
- Tan, L.T.-H.; Ser, H.-L.; Yin, W.-F.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H. Investigation of antioxidative and anticancer potentials of Streptomyces sp. MUM256 isolated from Malaysia mangrove soil. Front. Microbiol. 2015, 6, 1316. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Frank, D.N.; Feazel, L.M.; Bessesen, M.T.; Price, C.S.; Janoff, E.N.; Pace, N.R. The human nasal microbiota and Staphylococcus aureus carriage. PLoS ONE 2010, 5, e10598. [Google Scholar] [CrossRef]
- Sollid, J.U.E.; Furberg, A.S.; Hanssen, A.M.; Johannessen, M. Staphylococcus aureus: Determinants of human carriage. Infect. Genet. Evol. 2014, 21, 531–541. [Google Scholar] [CrossRef]
- Creech, C.B.; Al-Zubeidi, D.N.; Fritz, S.A. Prevention of recurrent staphylococcal skin infections. Infect. Dis. Clin. N. Am. 2015, 29, 429–464. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-resistant Staphylococcus aureus (MRSA): Antibiotic-resistance and the biofilm phenotype. MedChemComm 2019, 10, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Götz, F. Staphylococcus and biofilms. Mol. Microbiol. 2002, 43, 1367–1378. [Google Scholar] [CrossRef]
- Zheng, Y.; He, L.; Asiamah, T.K.; Otto, M. Colonization of medical devices by staphylococci. Environ. Microbiol. 2018, 20, 3141–3153. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Carbone, D.; Parrino, B.; Pecoraro, C.; Giovannetti, E.; Cirrincione, G.; Diana, P. Therapeutic strategies to counteract antibiotic resistance in MRSA biofilm-associated infections. ChemMedChem 2021, 16, 65–80. [Google Scholar] [CrossRef]
- van Hal, S.J.; Fowler, V.G., Jr. Is it time to replace vancomycin in the treatment of methicillin-resistant Staphylococcus aureus infections? Clin. Infect. Dis. 2013, 56, 1779–1788. [Google Scholar] [CrossRef][Green Version]
- Heilmann, C.; Hussain, M.; Peters, G.; Götz, F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 1997, 24, 1013–1024. [Google Scholar] [CrossRef]
- Gross, M.; Cramton, S.E.; Götz, F.; Peschel, A. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 2001, 69, 3423–3426. [Google Scholar] [CrossRef]
- Clarke, S.R.; Foster, S.J. Surface adhesins of Staphylococcus aureus. Adv. Microb. Physiol. 2006, 51, 187–224. [Google Scholar] [CrossRef]
- Geoghegan, J.A.; Foster, T.J. Cell wall-anchored surface proteins of Staphylococcus aureus: Many proteins, multiple functions. In Staphylococcus aureus; Springer: Cham, Switzerland, 2015; pp. 95–120. [Google Scholar]
- Herman-Bausier, P.; Formosa-Dague, C.; Feuillie, C.; Valotteau, C.; Dufrene, Y.F. Forces guiding staphylococcal adhesion. J. Struct. Biol. 2017, 197, 65–69. [Google Scholar] [CrossRef]
- Farnsworth, C.W.; Schott, E.M.; Jensen, S.E.; Zukoski, J.; Benvie, A.M.; Refaai, M.A.; Kates, S.L.; Schwarz, E.M.; Zuscik, M.J.; Gill, S.R. Adaptive upregulation of clumping factor A (ClfA) by Staphylococcus aureus in the obese, type 2 diabetic host mediates increased virulence. Infect. Immun. 2017, 85, e01005–e01016. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Cramton, S.E.; Gerke, C.; Schnell, N.F.; Nichols, W.W.; Götz, F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 1999, 67, 5427–5433. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal infections: Mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu. Rev. Med. 2013, 64, 175–188. [Google Scholar] [CrossRef]
- Kogan, G.; Sadovskaya, I.; Chaignon, P.; Chokr, A.; Jabbouri, S. Biofilms of clinical strains of Staphylococcus that do not contain polysaccharide intercellular adhesin. FEMS Microbiol. Lett. 2006, 255, 11–16. [Google Scholar] [CrossRef]
- Rohde, H.; Burandt, E.C.; Siemssen, N.; Frommelt, L.; Burdelski, C.; Wurster, S.; Scherpe, S.; Davies, A.P.; Harris, L.G.; Horstkotte, M.A.; et al. Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials 2007, 28, 1711–1720. [Google Scholar] [CrossRef]
- Boles, B.R.; Horswill, A.R. Staphylococcal biofilm disassembly. Trends Microbiol. 2011, 19, 449–455. [Google Scholar] [CrossRef]
- Law, J.W.-F.; Letchumanan, V.; Tan, L.T.-H.; Ser, H.-L.; Goh, B.-H.; Lee, L.-H. The rising of “modern actinobacteria” era. Prog. Microbes Mol. Biol. 2020, 3, a0000064. [Google Scholar] [CrossRef][Green Version]
- Seipke, R.F.; Kaltenpoth, M.; Hutchings, M.I. Streptomyces as symbionts: An emerging and widespread theme? FEMS Microbiol. Rev. 2012, 36, 862–876. [Google Scholar] [CrossRef]
- Manteca, Á.; Yagüe, P. Streptomyces as a source of antimicrobials: Novel approaches to activate cryptic secondary metabolite pathways. In Antimicrobials, Antibiotic Resistance, Antibiofilm Strategies and Activity Methods; IntechOpen: London, UK, 2019; p. 119. [Google Scholar]
- Lyddiard, D.; Jones, G.L.; Greatrex, B.W. Keeping it simple: Lessons from the golden era of antibiotic discovery. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Li, D.; Chen, Y.; Dou, Q.P. Are we seeing a resurgence in the use of natural products for new drug discovery? Expert Opin. Drug Discov. 2019, 14, 417–420. [Google Scholar] [CrossRef]
- Niu, G. Genomics-driven natural product discovery in actinomycetes. Trends Biotechnol. 2018, 36, 238–241. [Google Scholar] [CrossRef]
- Zhang, M.M.; Qiao, Y.; Ang, E.L.; Zhao, H. Using natural products for drug discovery: The impact of the genomics era. Expert Opin. Drug Discov. 2017, 12, 475–487. [Google Scholar] [CrossRef]
- Lee, N.; Kim, W.; Hwang, S.; Lee, Y.; Cho, S.; Palsson, B.; Cho, B.-K. Thirty complete Streptomyces genome sequences for mining novel secondary metabolite biosynthetic gene clusters. Sci. Data 2020, 7, 55. [Google Scholar] [CrossRef]
- Ser, H.-L.; Tan, L.T.-H.; Tan, W.-S.; Yin, W.-F.; Chan, K.-G. Whole-genome sequence of bioactive streptomycete derived from mangrove forest in Malaysia, Streptomyces sp. MUSC 14. Prog. Microbes Mol. Biol. 2021, 4, a0000195. [Google Scholar] [CrossRef]
- Tan, L.T.-H.; Lee, L.-H.; Goh, B.-H. Critical review of fermentation and extraction of anti-Vibrio compounds from Streptomyces. Prog. Microbes Mol. Biol. 2020, 3, a0000051. [Google Scholar] [CrossRef]
- Mangzira Kemung, H.; Tan, L.T.-H.; Chan, K.-G.; Ser, H.-L.; Law, J.W.-F.; Lee, L.-H.; Goh, B.-H. Streptomyces sp. strain MUSC 125 from mangrove soil in Malaysia with anti-MRSA, anti-biofilm and antioxidant activities. Molecules 2020, 25, 3545. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Dubey, A.K. Isolation and characterization of a new endophytic Actinobacterium Streptomyces californicus strain ADR1 as a promising source of anti-bacterial, anti-biofilm and antioxidant metabolites. Microorganisms 2020, 8, 929. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.-H.; Chan, K.-G.; Pusparajah, P.; Yin, W.-F.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Mangrove derived Streptomyces sp. MUM265 as a potential source of antioxidant and anticolon-cancer agents. BMC Microbiol. 2019, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.H.; Mahendra, C.K.; Yow, Y.Y.; Chan, K.G.; Khan, T.M.; Lee, L.H.; Goh, B.H. Streptomyces sp. MUM273b: A mangrove-derived potential source for antioxidant and UVB radiation protectants. MicrobiologyOpen 2019, 8, e859. [Google Scholar] [CrossRef] [PubMed]
- Kemung, H.M.; Tan, L.T.-H.; Chan, K.-G.; Ser, H.-L.; Law, J.W.-F.; Lee, L.-H.; Goh, B.-H. Investigating the antioxidant potential of Streptomyces sp. MUSC 11 from mangrove soil in Malaysia. Prog. Drug Discov. Biomed. Sci. 2019, 2, a0000033. [Google Scholar] [CrossRef]
- Tan, L.T.-H.; Chan, K.-G.; Chan, C.K.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Antioxidative potential of a Streptomyces sp. MUM292 isolated from mangrove soil. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Bakkiyaraj, D.; Karutha Pandian, S.T. In vitro and in vivo antibiofilm activity of a coral associated actinomycete against drug resistant Staphylococcus aureus biofilms. Biofouling 2010, 26, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Skaf, J.; Holzgrabe, U.; Bharti, R.; Förstner, K.U.; Ziebuhr, W.; Humeida, U.H.; Abdelmohsen, U.R.; Oelschlaeger, T.A. A new bioactive compound from the marine sponge-derived Streptomyces sp. SBT348 inhibits staphylococcal growth and biofilm formation. Front. Microbiol. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Othman, E.M.; Kampik, D.; Stopper, H.; Hentschel, U.; Ziebuhr, W.; Oelschlaeger, T.A.; Abdelmohsen, U.R. Marine sponge-derived Streptomyces sp. SBT343 Extract Inhibits staphylococcal biofilm formation. Front. Microbiol. 2017, 8, 236. [Google Scholar] [CrossRef]
- Kamarudheen, N.; Rao, K.V.B. Fatty acyl compounds from marine Streptomyces griseoincarnatus strain HK12 against two major bio-film forming nosocomial pathogens; an in vitro and in silico approach. Microb. Pathog. 2019, 127, 121–130. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, J.-H.; Kim, C.-J.; Lee, J.-C.; Cho, M.H.; Lee, J. Extracellular protease in Actinomycetes culture supernatants inhibits and detaches Staphylococcusaureus biofilm formation. Biotechnol. Lett. 2012, 34, 655–661. [Google Scholar] [CrossRef]
- Oja, T.; San Martin Galindo, P.; Taguchi, T.; Manner, S.; Vuorela, P.M.; Ichinose, K.; Metsä-Ketelä, M.; Fallarero, A. Effective antibiofilm polyketides against Staphylococcus aureus from the pyranonaphthoquinone biosynthetic pathways of Streptomyces species. Antimicrob. Agents Chemother. 2015, 59, 6046–6052. [Google Scholar] [CrossRef]
- Driche, E.H.; Sabaou, N.; Bijani, C.; Zitouni, A.; Pont, F.; Mathieu, F.; Badji, B. Streptomyces sp. AT37 isolated from a Saharan soil produces a furanone derivative active against multidrug-resistant Staphylococcus aureus. World J. Microbiol. Biotechnol. 2017, 33, 105. [Google Scholar] [CrossRef] [PubMed]
- Bauermeister, A.; Pereira, F.; Grilo, I.R.; Godinho, C.C.; Paulino, M.; Almeida, V.; Gobbo-Neto, L.; Prieto-Davó, A.; Sobral, R.G.; Lopes, N.P.; et al. Intra-clade metabolomic profiling of MAR4 Streptomyces from the Macaronesia Atlantic region reveals a source of anti-biofilm metabolites. Environ. Microbiol. 2019, 21, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nong, X.H.; Amin, M.; Qi, S.H. Hygrocin C from marine-derived Streptomyces sp. SCSGAA 0027 inhibits biofilm formation in Bacillus amyloliquefaciens SCSGAB0082 isolated from South China Sea gorgonian. Appl. Microbiol. Biotechnol. 2018, 102, 1417–1427. [Google Scholar] [CrossRef]
- Jabila Mary, T.R.; Kannan, R.R.; Iniyan, A.M.; Ramachandran, D.; Prakash Vincent, S.G. Cell wall distraction and biofilm inhibition of marine Streptomyces derived angucycline in methicillin resistant Staphylococcus aureus. Microb. Pathog. 2021, 150, 104712. [Google Scholar] [CrossRef]
- Vasilchenko, A.S.; Julian, W.T.; Lapchinskaya, O.A.; Katrukha, G.S.; Sadykova, V.S.; Rogozhin, E.A. A novel peptide antibiotic produced by Streptomyces roseoflavus strain INA-Ac-5812 with directed activity against Gram-positive bacteria. Front. Microbiol. 2020, 11, 556063. [Google Scholar] [CrossRef]
- Kim, J.W.; Kwon, Y.; Bang, S.; Kwon, H.E.; Park, S.; Lee, Y.; Deyrup, S.T.; Song, G.; Lee, D.; Joo, H.S.; et al. Unusual bridged angucyclinones and potent anticancer compounds from Streptomyces bulli GJA1. Org. Biomol. Chem. 2020, 18, 8443–8449. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Ohtaguro, N.; Yoshida, Y.; Hirai, M.; Matsuo, H.; Yamada, Y.; Imamura, N.; Tsuchiya, T. A compound inhibits biofilm formation of Staphylococcus aureus from Streptomyces. Biol. Pharm. Bull. 2015, 38, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, E.; Choi, H.; Lee, J. Collismycin C from the Micronesian marine bacterium Streptomyces sp. MC025 inhibits Staphylococcus aureus biofilm formation. Mar. Drugs 2017, 15, 387. [Google Scholar] [CrossRef]
- Lakshmi, S.A.; Bhaskar, J.P.; Krishnan, V.; Sethupathy, S.; Pandipriya, S.; Aruni, W.; Pandian, S.K. Inhibition of biofilm and biofilm-associated virulence factor production in methicillin-resistant Staphylococcus aureus by docosanol. J. Biotechnol. 2020, 317, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Liang, J.; Zhang, W.; Zhang, L.; Hu, Z.L.; Zhang, Y.; Xu, Y. Butenolide, a marine-derived broad-spectrum antibiofilm agent against both Gram-positive and Gram-negative pathogenic bacteria. Mar. Biotechnol. 2019, 21, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, V.; Mahamuni, D.; Rajaram, R. In vitro and in silico approaches of antibiofilm activity of 1-hydroxy-1-norresistomycin against human clinical pathogens. Microb. Pathog. 2019, 132, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Chee, P.Y.; Mang, M.; Lau, E.S.; Tan, L.T.-H.; He, Y.-W.; Lee, W.-L.; Pusparajah, P.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H. Epinecidin-1, an antimicrobial peptide derived from grouper (Epinephelus coioides): Pharmacological activities and applications. Front. Microbiol. 2019, 10, 2631. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Executive summary: Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1–10. [Google Scholar] [CrossRef]
- Timsit, J.; L‘Hériteau, F.; Lepape, A.; Francais, A.; Ruckly, S.; Venier, A.; Jarno, P.; Boussat, S.; Coignard, B.; Savey, A. A multicentre analysis of catheter-related infection based on a hierarchical model. Intensive Care Med. 2012, 38, 1662–1672. [Google Scholar] [CrossRef]
- Boudjemaa, R.; Briandet, R.; Revest, M.; Jacqueline, C.; Caillon, J.; Fontaine-Aupart, M.-P.; Steenkeste, K. New Insight into daptomycin bioavailability and localization in Staphylococcus aureus biofilms by dynamic fluorescence imaging. Antimicrob. Agents Chemother. 2016, 60, 4983–4990. [Google Scholar] [CrossRef][Green Version]
- Abdelhady, W.; Bayer, A.S.; Seidl, K.; Nast, C.C.; Kiedrowski, M.R.; Horswill, A.R.; Yeaman, M.R.; Xiong, Y.Q. Reduced vancomycin susceptibility in an in vitro catheter-related biofilm model correlates with poor therapeutic outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 1447–1454. [Google Scholar] [CrossRef]
- Dombrowski, J.C.; Winston, L.G. Clinical failures of appropriately-treated methicillin-resistant Staphylococcus aureus infections. J. Infect. 2008, 57, 110–115. [Google Scholar] [CrossRef]
- Zimmerli, W.; Sendi, P. Role of rifampin against Staphylococcal biofilm infections in vitro, in animal models, and in orthopedic-device-related infections. Antimicrob. Agents Chemother. 2019, 63, e01746-18. [Google Scholar] [CrossRef] [PubMed]
- Saginur, R.; Stdenis, M.; Ferris, W.; Aaron, S.D.; Chan, F.; Lee, C.; Ramotar, K. Multiple combination bactericidal testing of staphylococcal biofilms from implant-associated infections. Antimicrob. Agents Chemother. 2006, 50, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mirani, Z.A.; Jamil, N. Effect of sub-lethal doses of vancomycin and oxacillin on biofilm formation by vancomycin intermediate resistant Staphylococcus aureus. J. Basic Microbiol. 2011, 51, 191–195. [Google Scholar] [CrossRef]
- Kaplan, J.B. Antibiotic-induced biofilm formation. Int. J. Artif. Organs 2011, 34, 737–751. [Google Scholar] [CrossRef]
- El-Azizi, M.; Rao, S.; Kanchanapoom, T.; Khardori, N. In vitro activity of vancomycin, quinupristin/dalfopristin, and linezolid against intact and disrupted biofilms of staphylococci. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Sahore, S.; Kaur, P.; Rani, A.; Ray, P. Penetration barrier contributes to bacterial biofilm-associated resistance against only select antibiotics, and exhibits genus-, strain-and antibiotic-specific differences. Pathog. Dis. 2016, 74, ftw056. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.; Wang, H.; Ding, T.; Zhu, W.; Zhuang, X.; Chu, W. Azithromycin reduces the production of α-hemolysin and biofilm formation in Staphylococcus aureus. Indian J. Microbiol. 2014, 54, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-X.; Tu, H.-P.; Sun, X.; Xu, G.-J.; Chen, J.-W.; Deng, Q.-W.; Yu, Z.-J.; Qu, D. In vitro activities of telithromycin against Staphylococcus aureus biofilms compared with azithromycin, clindamycin, vancomycin and daptomycin. J. Med. Microbiol. 2020, 69, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Nakaminami, H.; Ozawa, K.; Wajima, T.; Noguchi, N. In vitro anti-biofilm effect of anti-methicillin-resistant Staphylococcus aureus (anti-MRSA) agents against the USA300 clone. J. Glob. Antimicrob. Resist. 2021, 24, 63–71. [Google Scholar] [CrossRef]
- Hogan, S.; Zapotoczna, M.; Stevens, N.T.; Humphreys, H.; O’Gara, J.P.; O’Neill, E. In vitro approach for identification of the most effective agents for antimicrobial lock therapy in the treatment of intravascular catheter-related infections caused by Staphylococcus aureus. Antimicrob. Agents Chemother. 2016, 60, 2923–2931. [Google Scholar] [CrossRef]
- Chopra, S.; Harjai, K.; Chhibber, S. Antibiotic susceptibility of ica-positive and ica-negative MRSA in different phases of biofilm growth. J. Antibiot. 2015, 68, 15–22. [Google Scholar] [CrossRef]
- Hall Snyder, A.D.; Vidaillac, C.; Rose, W.; McRoberts, J.P.; Rybak, M.J. Evaluation of high-dose daptomycin versus vancomycin alone or combined with clarithromycin or rifampin against Staphylococcus aureus and S. epidermidis in a novel in vitro PK/PD model of bacterial biofilm. Infect. Dis. Ther. 2015, 4, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Mao, N.-F.; Wang, L.; Zhang, H.-B.; Chen, Q.; Liu, H.; Tang, X.; Jin, T.; Zhu, C.-T.; Li, F.-B.; et al. Efficacy of combined vancomycin and fosfomycin against methicillin-resistant Staphylococcus aureus in biofilms in vivo. PLoS ONE 2014, 9, e113133. [Google Scholar] [CrossRef]
- Perlroth, J.; Kuo, M.; Tan, J.; Bayer, A.S.; Miller, L.G. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: A systematic review of the literature. Arch. Intern. Med. 2008, 168, 805–819. [Google Scholar] [CrossRef]
- Oliveira, I.M.; Borges, A.; Simões, M. Chapter 14—The potential of drug repurposing to face bacterial and fungal biofilm infections. In Recent Trends in Biofilm Science and Technology; Simoes, M., Borges, A., Chaves Simoes, L., Eds.; Academic Press: London, UK, 2020; pp. 307–328. [Google Scholar]
- Pham, J.V.; Yilma, M.A.; Feliz, A.; Majid, M.T.; Maffetone, N.; Walker, J.R.; Kim, E.; Cho, H.J.; Reynolds, J.M.; Song, M.C.; et al. A review of the microbial production of bioactive natural products and biologics. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Lee, K.; Kim, C.J.; Park, D.J.; Ju, Y.; Lee, J.C.; Wood, T.K.; Lee, J. Streptomyces-derived actinomycin D inhibits biofilm formation by Staphylococcus aureus and its hemolytic activity. Biofouling 2016, 32, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Chaudhry, U.; Raza, A.; Ghosh, D.; Zhao, X. In vitro activity of ivermectin against Staphylococcus aureus clinical isolates. Antimicrob. Resist. Infect. Control 2018, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Xie, H.; Zhang, B.; Zhou, J.; Dou, Z.; Wang, X.; Wang, N. A novel ivermectin-derived compound D4 and its antimicrobial/biofilm properties against MRSA. Antibiotics 2021, 10, 208. [Google Scholar] [CrossRef]
- Torres, N.S.; Abercrombie, J.J.; Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K.; Leung, K.P. Screening a commercial library of pharmacologically active small molecules against Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2016, 60, 5663–5672. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef]
- Kruszewski, K.M.; Nistico, L.; Longwell, M.J.; Hynes, M.J.; Maurer, J.A.; Hall-Stoodley, L.; Gawalt, E.S. Reducing Staphylococcus aureus biofilm formation on stainless steel 316L using functionalized self-assembled monolayers. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2059–2069. [Google Scholar] [CrossRef]
- Stevens, K.N.; Crespo-Biel, O.; van den Bosch, E.E.; Dias, A.A.; Knetsch, M.L.; Aldenhoff, Y.B.; van der Veen, F.H.; Maessen, J.G.; Stobberingh, E.E.; Koole, L.H. The relationship between the antimicrobial effect of catheter coatings containing silver nanoparticles and the coagulation of contacting blood. Biomaterials 2009, 30, 3682–3690. [Google Scholar] [CrossRef]
- Windolf, C.D.; Lögters, T.; Scholz, M.; Windolf, J.; Flohé, S. Lysostaphin-coated titan-implants preventing localized osteitis by Staphylococcus aureus in a mouse model. PLoS ONE 2014, 9, e115940. [Google Scholar] [CrossRef][Green Version]
- Jena, P.; Mohanty, S.; Mallick, R.; Jacob, B.; Sonawane, A. Toxicity and antibacterial assessment of chitosan-coated silver nanoparticles on human pathogens and macrophage cells. Int. J. Nanomed. 2012, 7, 1805–1818. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Wozniak, D.J.; Stoodley, P.; Hall-Stoodley, L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev. Anti Infect. Ther. 2015, 13, 1499–1516. [Google Scholar] [CrossRef]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef]
- Chien, Y.-t.; Manna, A.C.; Projan, S.J.; Cheung, A.L. SarA, a global regulator of virulence determinants in Staphylococcus aureus, binds to a conserved motif essential for sar-dependent gene regulation. J. Biol. Chem. 1999, 274, 37169–37176. [Google Scholar] [CrossRef]
- Chan, P.F.; Foster, S.J. Role of SarA in virulence determinant production and environmental signal transduction in Staphylococcus aureus. J. Bacteriol. 1998, 180, 6232–6241. [Google Scholar] [CrossRef]
- Kiedrowski, M.R.; Horswill, A.R. New approaches for treating staphylococcal biofilm infections. Ann. N. Y. Acad. Sci. 2011, 1241, 104–121. [Google Scholar] [CrossRef]
- Al-Wahaibi, A.S.M.; Lapinska, E.; Rajarajan, N.; Dobretsov, S.; Upstill-Goddard, R.; Burgess, J.G. Secretion of DNases by marine bacteria: A culture based and bioinformatics approach. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Periasamy, S.; Joo, H.-S.; Duong, A.C.; Bach, T.-H.L.; Tan, V.Y.; Chatterjee, S.S.; Cheung, G.Y.C.; Otto, M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.; Syed, A.K.; Stephenson, R.E.; Rickard, A.H.; Boles, B.R. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog. 2012, 8, e1002744. [Google Scholar] [CrossRef]
- Queck, S.Y.; Khan, B.A.; Wang, R.; Bach, T.-H.L.; Kretschmer, D.; Chen, L.; Kreiswirth, B.N.; Peschel, A.; Deleo, F.R.; Otto, M. Mobile genetic element-encoded cytolysin connects virulence to methicillin resistance in MRSA. PLoS Pathog. 2009, 5, e1000533. [Google Scholar] [CrossRef]
- Wang, R.; Khan, B.A.; Cheung, G.Y.C.; Bach, T.-H.L.; Jameson-Lee, M.; Kong, K.-F.; Queck, S.Y.; Otto, M. Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. J. Clin. Investig. 2011, 121, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2. [Google Scholar] [CrossRef]
- Tan, W.-S.; Law, J.W.-F.; Law, L.N.-S.; Letchumanan, V.; Chan, K.-G. Insights into quorum sensing (QS): QS-regulated biofilm and inhibitors. Prog. Microbes Mol. Biol. 2020, 3, a0000141. [Google Scholar] [CrossRef]
- Ma, R.; Qiu, S.; Jiang, Q.; Sun, H.; Xue, T.; Cai, G.; Sun, B. AI-2 quorum sensing negatively regulates rbf expression and biofilm formation in Staphylococcus aureus. Int. J. Med. Microbiol. 2017, 307, 257–267. [Google Scholar] [CrossRef]
- Le, K.Y.; Otto, M. Quorum-sensing regulation in staphylococci—An overview. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Zhao, L.; Xue, T.; Shang, F.; Sun, H.; Sun, B. Staphylococcus aureus AI-2 quorum sensing associates with the KdpDE two-component system to regulate capsular polysaccharide synthesis and virulence. Infect. Immun. 2010, 78, 3506–3515. [Google Scholar] [CrossRef]
- Yu, D.; Zhao, L.; Xue, T.; Sun, B. Staphylococcus aureus autoinducer-2 quorum sensing decreases biofilm formation in an icaR-dependent manner. BMC Microbiol. 2012, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, R.; Al-Bataineh, S.; Gordon, O.; Luginbuehl, R.; Otto, M.; Textor, M.; Landmann, R. Furanone at subinhibitory concentrations enhances staphylococcal biofilm formation by luxS repression. Antimicrob. Agents Chemother. 2009, 53, 4159–4166. [Google Scholar] [CrossRef]
- Kayumov, A.R.; Sharafutdinov, I.S.; Trizna, E.Y.; Bogachev, M.I. Chapter 6—Antistaphylococcal activity of 2(5H)-furanone derivatives. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Biofilms; Yadav, M.K., Singh, B.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 77–89. [Google Scholar]
- Zang, T.; Lee, B.W.K.; Cannon, L.M.; Ritter, K.A.; Dai, S.; Ren, D.; Wood, T.K.; Zhou, Z.S. A naturally occurring brominated furanone covalently modifies and inactivates LuxS. Bioorg. Med. Chem. Lett. 2009, 19, 6200–6204. [Google Scholar] [CrossRef] [PubMed]
- Supramaniam, J.; Low, D.Y.S.; Wong, S.K.; Tan, L.T.H.; Leo, B.F.; Goh, B.H.; Darji, D.; Mohd Rasdi, F.R.; Chan, K.G.; Lee, L.H. Facile synthesis and characterization of palm CNF-ZnO nanocomposites with antibacterial and reinforcing properties. Int. J. Mol. Sci. 2021, 22, 5781. [Google Scholar] [CrossRef] [PubMed]
- Kemung, H.M.; Tan, L.T.-H.; Khaw, K.Y.; Ong, Y.S.; Chan, C.K.; Low, D.Y.S.; Tang, S.Y.; Goh, B.-H. An optimized anti-adherence and anti-biofilm assay: Case study of zinc oxide nanoparticles versus MRSA biofilm. Prog. Microbes Mol. Biol. 2020, 3, a0000091. [Google Scholar] [CrossRef]
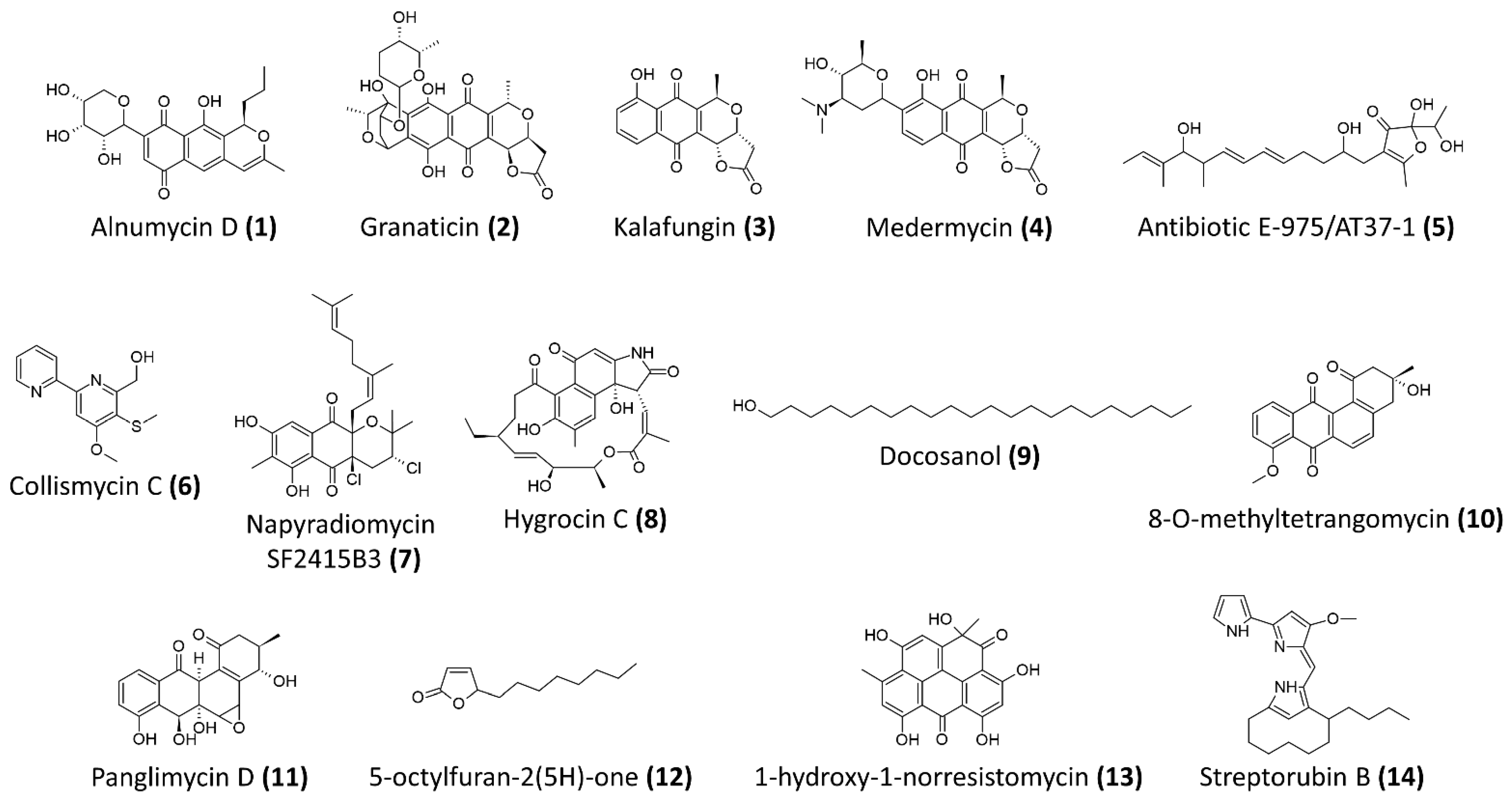
| Compound Name | Chemical Class | Streptomyces Producer and Isolation Source | Anti-Staphylococcal/MRSA Biofilm Activity | Reference |
|---|---|---|---|---|
| Alnumycin D (1) | polyketides | Streptomyces albus (pAlnuoriΔaln6) | Resazurin-based viability assay Preexposure IC50 against planktonic cells of S. aureus ATCC 25,923 = 2.66 μM Preexpsoure IC50 against biofilm cells of S. aureus ATCC 25,923 = 1.75 μM Postexposure IC50 against preformed ATCC 25,923 biofilms = 4.02 μM | [63] |
| Granaticin B (2) | polyketides | Streptomyces violaceoruber Tü22 | Resazurin-based viability assay Preexposure IC50 against planktonic cells of S. aureus ATCC 25,923 = 2.61 μM Preexpsoure IC50 against biofilm cells of S. aureus ATCC 25,923 = 2.76 μM Postexposure IC50 against preformed ATCC 25,923 biofilms = 3.72 μM | [63] |
| Kalafungin (3) | polyketides | Streptomyces tanashiensis Kala | Resazurin-based viability assay Preexposure IC50 against planktonic cells of S. aureus ATCC 25,923 = 1.11 μM Preexpsoure IC50 against biofilm cells of S. aureus ATCC 25,923 = 3.87 μM Postexposure IC50 against preformed ATCC 25,923 biofilms = 27.8 μM | [63] |
| Medermycin (4) | polyketides | Streptomyces coelicolor CH999/pIK340 | Resazurin-based viability assay Preexposure IC50 against planktonic cells of S. aureus ATCC 25,923 = 2.81 μM Preexpsoure IC50 against biofilm cells of S. aureus ATCC 25,923 = 2.5 μM Postexposure IC50 against preformed ATCC 25,923 biofilms = 24.6 μM | [63] |
| Antibiotic E-975 (5) | Heterocyclic furanone | Streptomyces sp. AT37 | Minimum concentration for 50% inhibition of biofilm formation of S. aureus ATCC 25,923 and MRSA ATCC 43,300 = 15 μg/mL and 10 μg/mL, respectively | [64] |
| Collismycin C (6) | Polyketides-nonribosomal peptides | Streptomyces sp. MC025 | Significant inhibition of biofilm formation by MRSA, ATCC 33,591 at concentration >5 μg/mL At 10 μg/mL, more 50% inhibition against biofilm formation and no antibacterial activity against the bacterial growth | [71] |
| Napyradiomycin SF2415B3 (7) | Hybrid isoprenoids | Streptomyces sp. MAR4, marine sediments from Madeira Archipelago | Minimum biofilm inhibitory concentration of 15.6 μg/mL—inhibits biofilm formation of S. aureus NCTC8325-4 | [65] |
| Hygrocin C (8) | Ansamycin, lipopeptides | Streptomyces sp. SCSGAA 0027, South China Sea gorgonian Subergorgia suberosa | Minimum concentration for 80% inhibition of biofilm formation of S. aureus ATCC 6538 = 25 μg/mL | [66] |
| Docosanol (9) | Aliphatic alcohol | Streptomyces griseus TBG19NRA1 | Around 80% reduction in biofilm formation at concentration >500 μg/mL | [72] |
| Antibiotic 5812-A/C | Antimicrobial peptide complex | Streptomyces roseoflavus INA-Ac-5812 | More than 50% reduction of preformed biofilms of S. aureus 209P at 1.8 μg/mL Penetrate and inhibit the metabolic activity of S. aureus 209P in preformed biofilms | [68] |
| 8-O-metyltetrangomycin (10) | Angucycline, aromatic polyketides | Streptomyces sp. SBRK-2, marine sponge Spirostella sp. | At 2 μg/mL, 70% inhibition of biofilm formation by S. aureus ATCC 25923 Membrane damaging and increased cell surface hydrophobicity | [67] |
| Panglimycin D (11) | Angucyclinones, aromatic polyketides | Streptomyces bulli GJA1, endophyte of Gardenia jasminoides | At 5 μg/mL, biofilm formation of MRSA USA300 was inhibited by 40% Inhibited the production of PSMα2, PSMα3, PSMα4, and δ-toxin of MRSA USA300 | [69] |
| 5-octylfuran-2(5H)-one (12) | Butenolides, furanones | Marine-derived Streptomyces sp. | 100% inhibition of biofilm formation and eradication of preformed biofilm of MRSA ATCC43300 at 200 μg/mL, while minimum inhibitory concentration of >1200 μg/mL Inhibition of autoinducer-2 and acyl-homoserine lactone, suggested it could be a non-specific quorum-sensing inhibitor | [73] |
| 1-hydroxy-1-norresistomycin (13) | Pentacyclic polyketides | Streptomyces variabilis, Scleractinia coral Acropora Formosa | 93% inhibition of biofilm formation by S. aureus at 200 μg/mL Reduced S. aureus cell surface hydrophobicity Docking study showed good affinity towards SarA and ScpA protein of S. aureus | [74] |
| Streptorubin B (14) | Prodiginine, bacterial alkaloids | Streptomyces sp. strain MC11024, soil sample from Suita, Osaka, Japan | IC50 of biofilm inhibition against MRSA N315 = 0.22 μg/mL (0.56 μM), minimum inhibitory concentration of growth = 32 μg/mL | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pusparajah, P.; Letchumanan, V.; Law, J.W.-F.; Ab Mutalib, N.-S.; Ong, Y.S.; Goh, B.-H.; Tan, L.T.-H.; Lee, L.-H. Streptomyces sp.—A Treasure Trove of Weapons to Combat Methicillin-Resistant Staphylococcus aureus Biofilm Associated with Biomedical Devices. Int. J. Mol. Sci. 2021, 22, 9360. https://doi.org/10.3390/ijms22179360
Pusparajah P, Letchumanan V, Law JW-F, Ab Mutalib N-S, Ong YS, Goh B-H, Tan LT-H, Lee L-H. Streptomyces sp.—A Treasure Trove of Weapons to Combat Methicillin-Resistant Staphylococcus aureus Biofilm Associated with Biomedical Devices. International Journal of Molecular Sciences. 2021; 22(17):9360. https://doi.org/10.3390/ijms22179360
Chicago/Turabian StylePusparajah, Priyia, Vengadesh Letchumanan, Jodi Woan-Fei Law, Nurul-Syakima Ab Mutalib, Yong Sze Ong, Bey-Hing Goh, Loh Teng-Hern Tan, and Learn-Han Lee. 2021. "Streptomyces sp.—A Treasure Trove of Weapons to Combat Methicillin-Resistant Staphylococcus aureus Biofilm Associated with Biomedical Devices" International Journal of Molecular Sciences 22, no. 17: 9360. https://doi.org/10.3390/ijms22179360
APA StylePusparajah, P., Letchumanan, V., Law, J. W.-F., Ab Mutalib, N.-S., Ong, Y. S., Goh, B.-H., Tan, L. T.-H., & Lee, L.-H. (2021). Streptomyces sp.—A Treasure Trove of Weapons to Combat Methicillin-Resistant Staphylococcus aureus Biofilm Associated with Biomedical Devices. International Journal of Molecular Sciences, 22(17), 9360. https://doi.org/10.3390/ijms22179360









