Early Bone Healing on Hydroxyapatite-Coated and Chemically-Modified Hydrophilic Implant Surfaces in an Ovine Model
Abstract
:1. Introduction
2. Results
2.1. Implant Surfaces
2.2. Resonance Frequency Analysis
2.3. Torque Testing
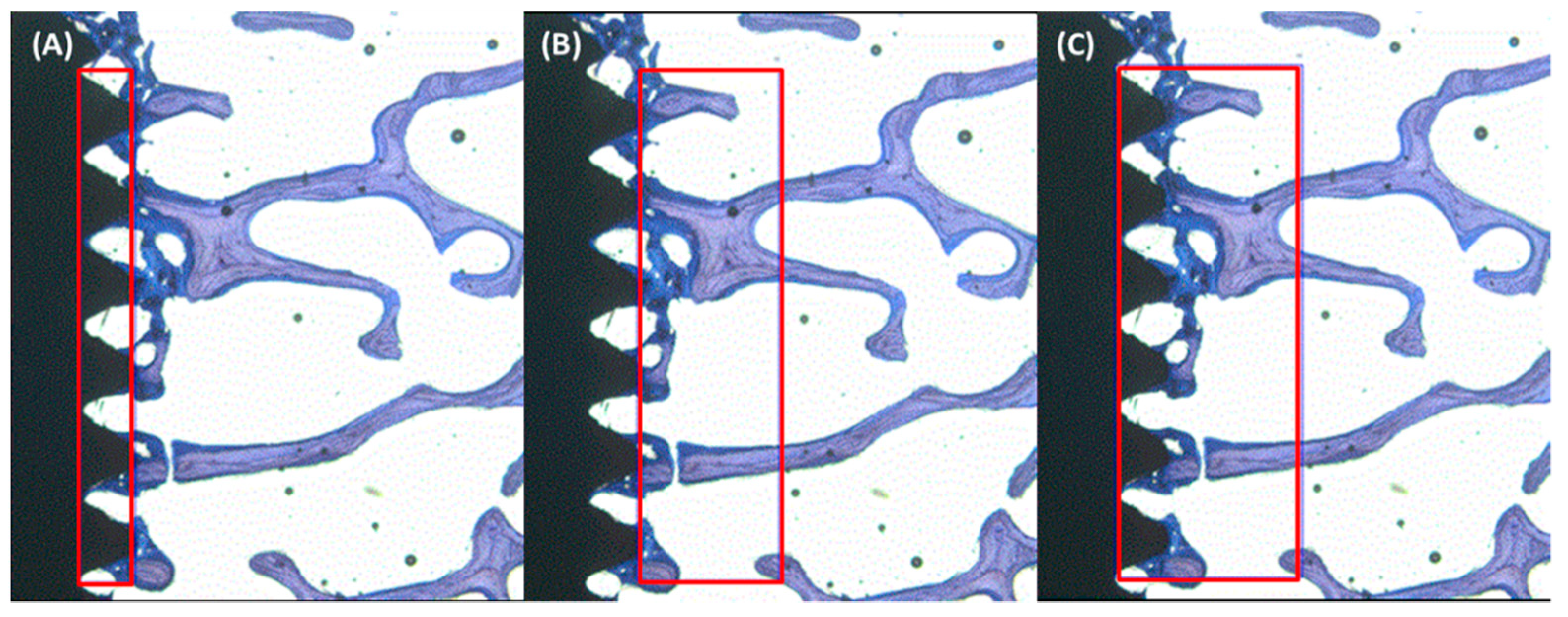
2.4. Histological Evaluation
2.5. Histomorphometry
3. Discussion
4. Materials and Methods
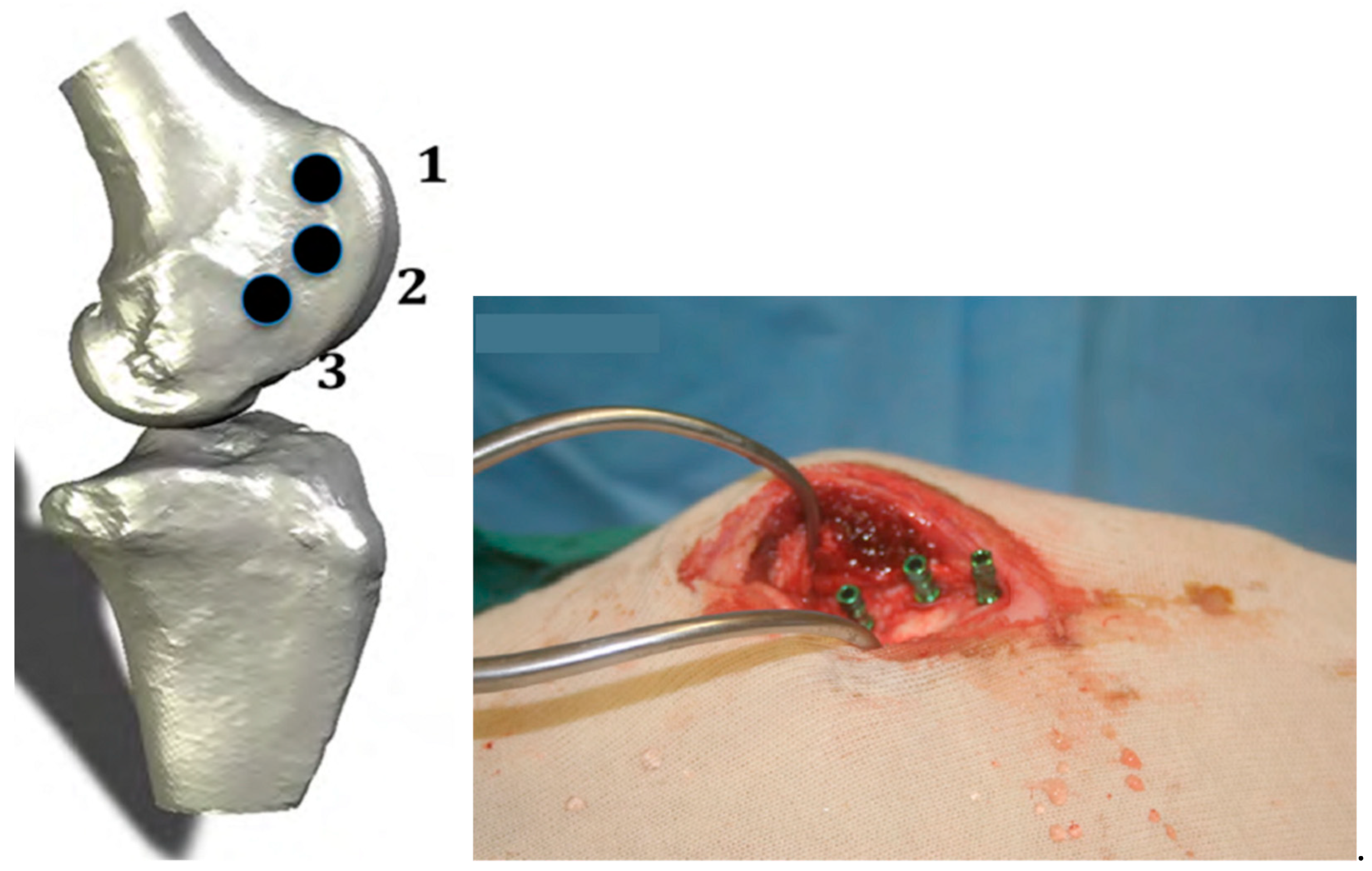
4.1. Animal Model
4.2. Implant Systems
4.3. Surgical Procedure and Euthanasia
4.4. Resonance Frequency Analysis
4.5. Torque Measurement
4.6. Histology and Histomorphometry
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puleo, D.A.; Nanci, A. Understanding and controlling the bone-implant interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar] [CrossRef]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef]
- Pattanaik, B.; Pawar, S.; Pattanaik, S. Biocompatible implant surface treatments. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2012, 23, 398–406. [Google Scholar] [CrossRef]
- Le Guehennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Yeo, I.S.; Han, J.S.; Yang, J.H. Biomechanical and histomorphometric study of dental implants with different surface characteristics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Shayganpour, A.; Rebaudi, A.; Cortella, P.; Diaspro, A.; Salerno, M. Electrochemical coating of dental implants with anodic porous titania for enhanced osteointegration. Beilstein J. Nanotechnol. 2015, 6, 2183–2192. [Google Scholar] [CrossRef]
- Binahmed, A.; Stoykewych, A.; Hussain, A.; Love, B.; Pruthi, V. Long-term follow-up of hydroxyapatite-coated dental implants--a clinical trial. Int. J. Oral Maxillofac. Implant. 2007, 22, 963–968. [Google Scholar]
- Heimann, R.B. Osseoconductive and Corrosion-Inhibiting Plasma-Sprayed Calcium Phosphate Coatings for Metallic Medical Implants. Metals 2017, 7, 468. [Google Scholar] [CrossRef] [Green Version]
- Cook, S.D.; Baffes, G.C.; Wolfe, M.W.; Palafox, A.J. A comparison of femoral and mandibular animal models for the evaluation of HA-coated implants. J. Oral Implantol. 1992, 18, 359–365. [Google Scholar]
- Eom, T.G.; Jeon, G.R.; Jeong, C.M.; Kim, Y.K.; Kim, S.G.; Cho, I.H.; Cho, Y.S.; Oh, J.S. Experimental study of bone response to hydroxyapatite coating implants: Bone-implant contact and removal torque test. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Weinlaender, M.; Kenney, E.B.; Lekovic, V.; Beumer, J., 3rd; Moy, P.K.; Lewis, S. Histomorphometry of bone apposition around three types of endosseous dental implants. Int. J. Oral Maxillofac. Implant. 1992, 7, 491–496. [Google Scholar]
- Orenstein, I.H.; Tarnow, D.P.; Morris, H.F.; Ochi, S. Three-year post-placement survival of implants mobile at placement. Ann. Periodontol. 2000, 5, 32–41. [Google Scholar] [CrossRef]
- Lee, J.J.; Rouhfar, L.; Beirne, O.R. Survival of hydroxyapatite-coated implants: A meta-analytic review. J. Oral Maxillofac. Surg. 2000, 58, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoat, M.; McGlumphy, E.; Reddy, M.; Geurs, N.; M Proskin, H. A comparison of hydroxyapatite (HA)-coated threaded, HA-coated cylindric, and titanium threaded endosseous dental implants. Int. J. Oral Maxillofac. Implant. 2003, 18, 406–410. [Google Scholar] [CrossRef]
- Yang, C.Y.; Lee, T.M.; Yang, C.W.; Chen, L.R.; Wu, M.C.; Lui, T.S. In vitro and in vivo biological responses of plasma-sprayed hydroxyapatite coatings with posthydrothermal treatment. J. Biomed. Mater. Res. Part A 2007, 83, 263–271. [Google Scholar] [CrossRef]
- Roy, M.; Bandyopadhyay, A.; Bose, S. Induction Plasma Sprayed Nano Hydroxyapatite Coatings on Titanium for Orthopaedic and Dental Implants. Surf. Coat. Technol. 2011, 205, 2785–2792. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, S.J.; Broggini, N.; Wieland, M.; de Wild, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Buser, D. Biomechanical evaluation of the interfacial strength of a chemically modified sandblasted and acid-etched titanium surface. J. Biomed. Mater. Res. Part A 2006, 78, 291–297. [Google Scholar] [CrossRef]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef]
- Sammons, R.L.; Lumbikanonda, N.; Gross, M.; Cantzler, P. Comparison of osteoblast spreading on microstructured dental implant surfaces and cell behaviour in an explant model of osseointegration. A scanning electron microscopic study. Clin. Oral Implant. Res. 2005, 16, 657–666. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Valderrama, P.; Jones, A.A.; Wilson, T.G.; Seibl, R.; Cochran, D.L. Bone apposition around two different sandblasted and acid-etched titanium implant surfaces: A histomorphometric study in canine mandibles. Clin. Oral Implant. Res. 2008, 19, 233–241. [Google Scholar] [CrossRef]
- Schwarz, F.; Herten, M.; Sager, M.; Wieland, M.; Dard, M.; Becker, J. Histological and immunohistochemical analysis of initial and early osseous integration at chemically modified and conventional SLA titanium implants: Preliminary results of a pilot study in dogs. Clin. Oral Implant. Res. 2007, 18, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haq, J.; Karabuda, C.Z.; Arisan, V.; Mutlu, Z.; Kurkcu, M. Osseointegration and stability of a modified sand-blasted acid-etched implant: An experimental pilot study in sheep. Clin. Oral Implant. Res. 2011, 22, 265–274. [Google Scholar] [CrossRef]
- Bacchelli, B.; Giavaresi, G.; Franchi, M.; Martini, D.; De Pasquale, V.; Trire, A.; Fini, M.; Giardino, R.; Ruggeri, A. Influence of a zirconia sandblasting treated surface on peri-implant bone healing: An experimental study in sheep. Acta Biomater. 2009, 5, 2246–2257. [Google Scholar] [CrossRef]
- Franchi, M.; Bacchelli, B.; Giavaresi, G.; De Pasquale, V.; Martini, D.; Fini, M.; Giardino, R.; Ruggeri, A. Influence of different implant surfaces on peri-implant osteogenesis: Histomorphometric analysis in sheep. J. Periodontol. 2007, 78, 879–888. [Google Scholar] [CrossRef]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cell Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nafei, A.; Danielsen, C.C.; Linde, F.; Hvid, I. Properties of growing trabecular ovine bone. Part I: Mechanical and physical properties. J. Bone Jt. Surg. Br. Vol. 2000, 82, 910–920. [Google Scholar] [CrossRef]
- Willie, B.M.; Bloebaum, R.D.; Bireley, W.R.; Bachus, K.N.; Hofmann, A.A. Determining relevance of a weight-bearing ovine model for bone ingrowth assessment. J. Biomed. Mater. Res. Part A 2004, 69, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Huffer, W.E.; Benedict, J.J.; Turner, A.S.; Briest, A.; Rettenmaier, R.; Springer, M.; Walboomers, X.F. Repair of sheep long bone cortical defects filled with COLLOSS, COLLOSS E, OSSAPLAST, and fresh iliac crest autograft. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2007, 82, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, D.B.; Jee, W.S. A quantitative histologic study of bone turnover in young adult beagles. Anat. Rec. 1982, 203, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Chugh, T.; Jain, A.K.; Jaiswal, R.K.; Mehrotra, P.; Mehrotra, R. Bone density and its importance in orthodontics. J. Oral Biol. Craniofac Res. 2013, 3, 92–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, L.A.; Simpson, A.H. In vivo models of bone repair. J. Bone Jt. Surg. Br. Vol. 2012, 94, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Juodzbalys, G.; Kubilius, M. Clinical and radiological classification of the jawbone anatomy in endosseous dental implant treatment. J. Oral Maxillofac. Res. 2013, 4, e2. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.I.; Duncan, W.J. The Effect of a Keratin Hydrogel Coating on Osseointegration: An Histological Comparison of Coated and Non-coated Dental Titanium Implants in an Ovine Model. J. Maxillofac. Oral Surg. 2014, 13, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Velasco-Ortega, E.; Ortiz-Garcia, I.; Jimenez-Guerra, A.; Monsalve-Guil, L.; Munoz-Guzon, F.; Perez, R.A.; Gil, F.J. Comparison between Sandblasted Acid-Etched and Oxidized Titanium Dental Implants: In Vivo Study. Int J. Mol. Sci. 2019, 20, 3267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.H.; Kim, H.W.; Lee, S.H.; Kong, Y.M.; Kim, H.E. Biocompatibility of titanium implants modified by microarc oxidation and hydroxyapatite coating. J. Biomed. Mater. Res. Part A 2005, 73, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ergun, C.; Liu, H.; Webster, T.J.; Olcay, E.; Yilmaz, S.; Sahin, F.C. Increased osteoblast adhesion on nanoparticulate calcium phosphates with higher Ca/P ratios. J. Biomed. Mater. Res. Part A 2008, 85, 236–241. [Google Scholar] [CrossRef]
- Sohn, S.H.; Jun, H.K.; Kim, C.S.; Kim, K.N.; Chung, S.M.; Shin, S.W.; Ryu, J.J.; Kim, M.K. Biological responses in osteoblast-like cell line according to thin layer hydroxyapatite coatings on anodized titanium. J. Oral Rehabil. 2006, 33, 898–911. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.; Romanos, G. Potential Causes of Titanium Particle and Ion Release in Implant Dentistry: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 3585. [Google Scholar] [CrossRef] [Green Version]
- Marenzi, G.; Impero, F.; Scherillo, F.; Sammartino, J.C.; Squillace, A.; Spagnuolo, G. Effect of Different Surface Treatments on Titanium Dental Implant Micro-Morphology. Materials 2019, 12, 733. [Google Scholar] [CrossRef] [Green Version]
- Rosenlicht, J. Advancements in soft-bone implant stability. West. Indian Dent. J. 2002, 6, 2–7. [Google Scholar]
- Trisi, P.; Todisco, M.; Consolo, U.; Travaglini, D. High Versus Low Implant Insertion Torque: A Histologic, Histomorphometric, and Biomechanical Study in the Sheep Mandible. Int. J. Oral Maxillofac. Implant. 2011, 26, 837–849. [Google Scholar]
- Sanz-Martin, I.; Paeng, K.; Park, H.; Cha, J.K.; Jung, U.W.; Sanz, M. Significance of implant design on the efficacy of different peri-implantitis decontamination protocols. Clin. Oral Investig. 2021, 25, 3589–3597. [Google Scholar] [CrossRef]
- Duncan, W.J.; Lee, M.H.; Dovban, A.S.; Hendra, N.; Ershadi, S.; Rumende, H. Anodization increases early integration of Osstem implants in sheep femurs. Ann. R. Australas. Coll. Dent. Surg. 2008, 19, 152–156. [Google Scholar] [PubMed]
- Gottlow, J.; Barkarmo, S.; Sennerby, L. An experimental comparison of two different clinically used implant designs and surfaces. Clin. Implant Dent. Relat. Res. 2012, 14 (Suppl. 1), e204–e212. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, R.; Kuhlisch, E.; Schulz, M.C.; Eckelt, U.; Stadlinger, B. Comparison of bone-implant contact and bone-implant volume between 2D-histological sections and 3D-SRmicroCT slices. Eur. Cell Mater. 2012, 23, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamdan, K.; Al-Moaber, S.H.; Junker, R.; Jansen, J.A. Effect of implant surface properties on peri-implant bone healing: A histological and histomorphometric study in dogs. Clin. Oral Implant. Res. 2011, 22, 399–405. [Google Scholar] [CrossRef] [PubMed]
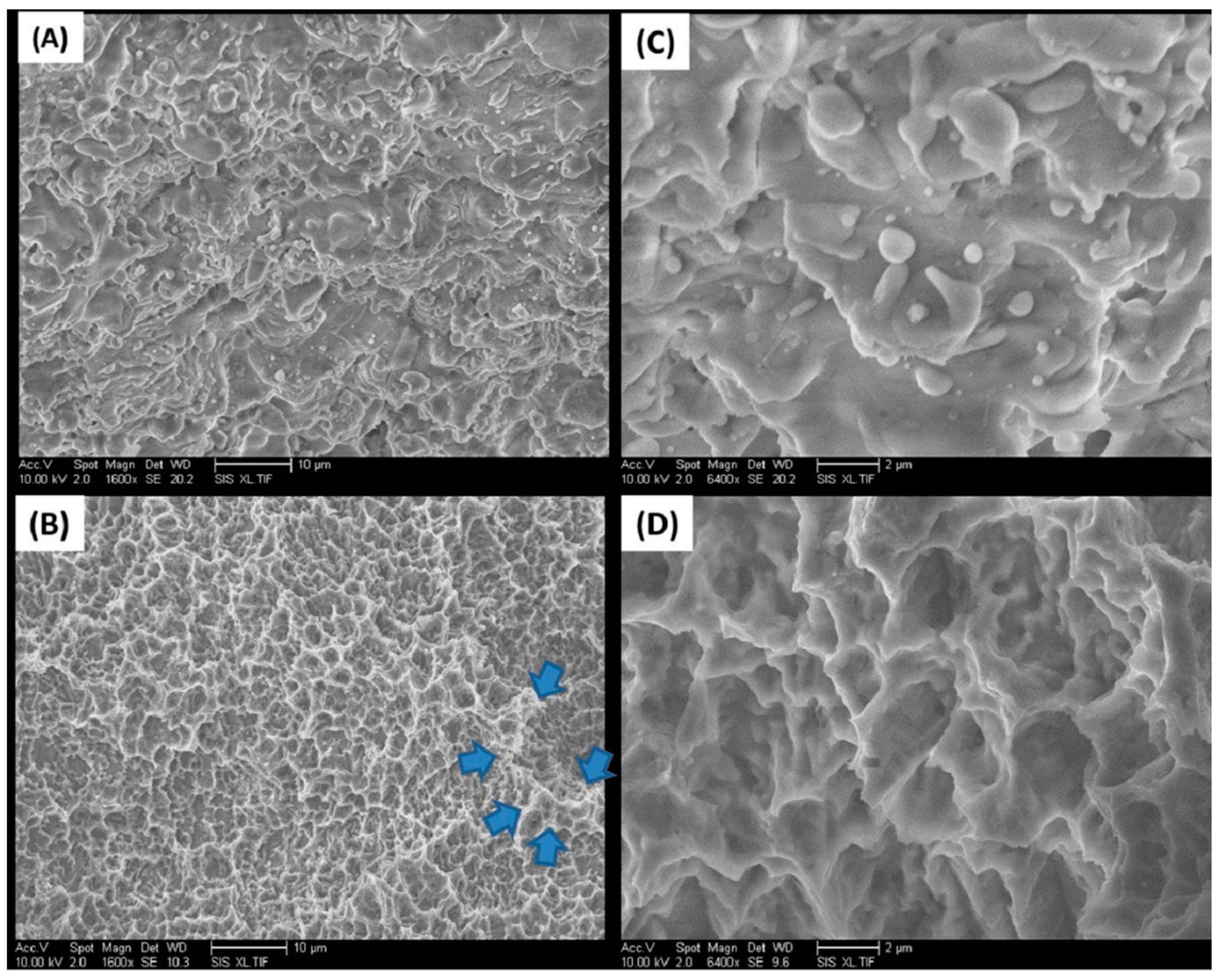
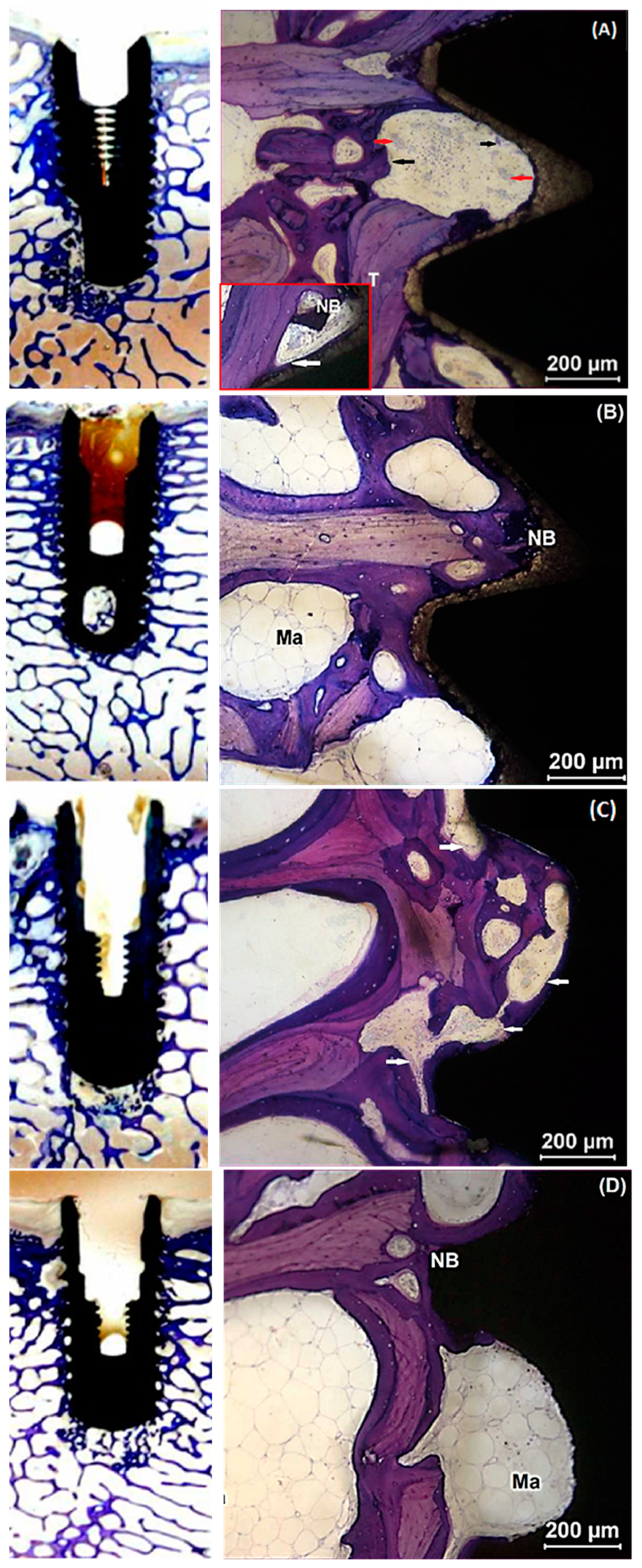
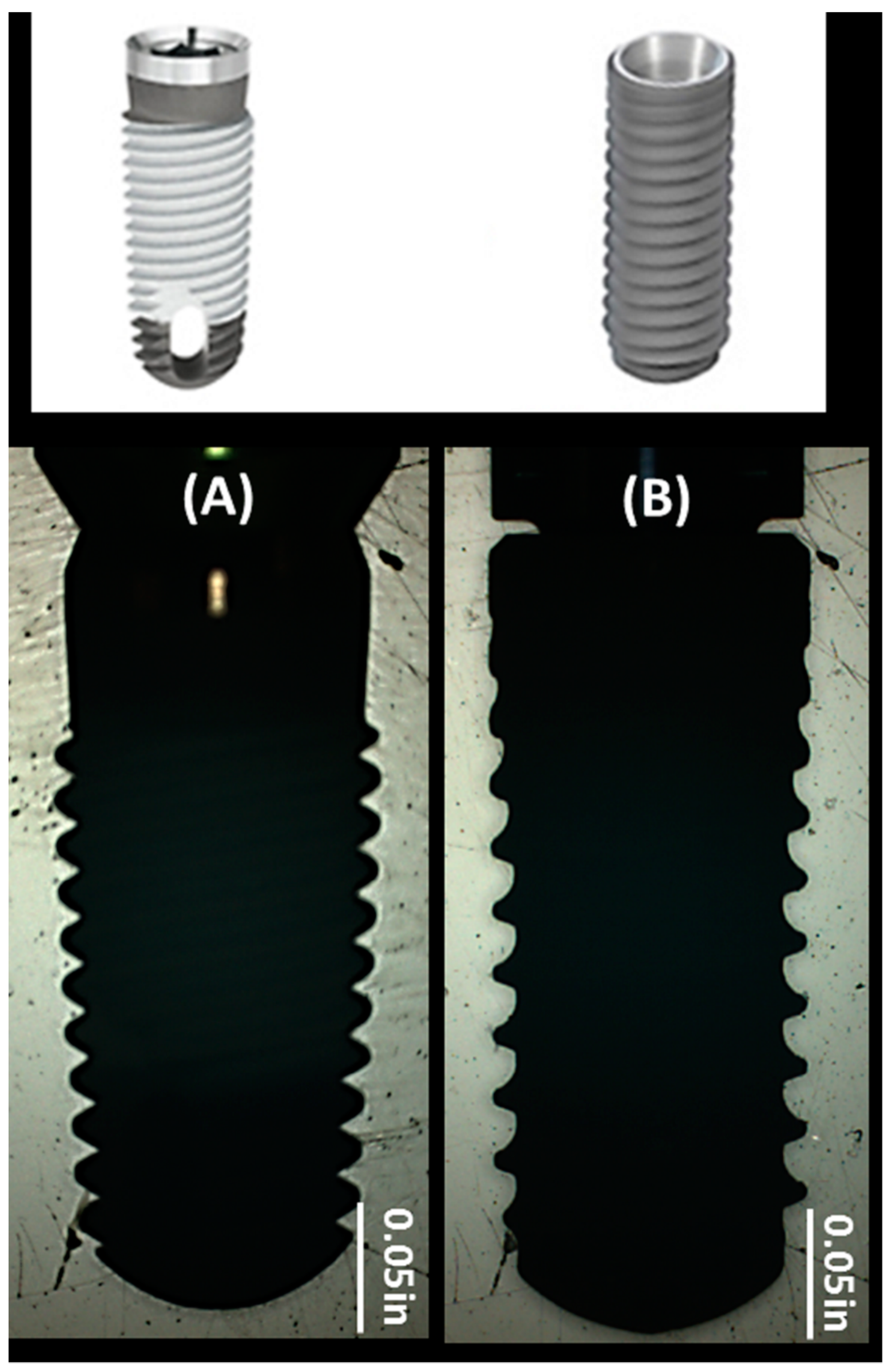
| Implant Type | Element Analysis (Atomic %) | Surface Roughness (Ra) (µm) |
|---|---|---|
| TSV MP-1 HA | C (1.67), O (37.34), P (18.84), Ca (42.15) | 4.63 ± 0.83 |
| cmSLA | O (2.57), F (0.44), Na (14.74), Cl (21.73), Ti (60.52) | 1.57 ± 0.24 |
| (A) ISQ Median (IQR) | |||
| TSV MP-1 HA | cmSLA | Between Groups | |
| Group A (Week 3) | |||
| Week 0 | 69.2 (2.8) | 70.0 (6.9) | p = 0.24 |
| Week 3 | 71.5 (9.1) | 72.5 (5.1) | p = 0.01 |
| Difference (Week 3 − 0) | 0.1 (8.2) | 2.2 (2.8) | p = 0.08 |
| Week 3 (Within Group) | p = 0.65 | p = 0.02 | -- |
| Group B (Week 6) | |||
| Week 0 | 66.5 (5.6) | 68.4 (8.7) | p = 0.37 |
| Week 6 | 70.8 (4.4) | 72.4 (7.2) | p = 0.15 |
| Difference (Week 6 − 0) | 6.2 (8.8) | 2.6 (8.7) | p = 0.75 |
| Week 6 (Within Group) | p = 0.03 | p = 0.05 | -- |
| (B) Torque Median (IQR) | |||
| TSV MP-1 HA | cmSLA | Between Groups | |
| Group A (Week 3) | |||
| Week 0 (ITV) | 102.7 (61.2) | 33.7 (39.5) | p = 0.01 |
| Week 3 (RTV) | 158.5 (36.6) | 120.5 (82.4) | p = 0.02 |
| Difference (Week 3 − 0) | 74.6 (66.8) | 63.5 (56.3) | p = 0.97 |
| Week 3 (Within Group) | p < 0.001 | p < 0.001 | -- |
| Group B (Week 6) | |||
| Week 0 (ITV) | 86.7 (45.8) | 35.4 (19.8) | p = 0.04 |
| Week 6 (RTV) | 267.9 (44.6) | 212.2 (62.0) | p = 0.03 |
| Difference (Week 6 − 0) | 181.6 (42.5) | 165.1 (99.0) | p = 0.45 |
| Week 6 (Within Group) | p < 0.001 | p < 0.001 | -- |
| Table 3. BIC% & BAF% | Week 3 Median (IQR) | Week 6 Median (IQR) | Within Groups |
| BIC% | |||
| TSV MP-1 HA | 92 (10) | 98 (5) | p = 0.15 |
| cmSLA | 87 (27) | 79 (17) | p = 1 |
| Between Groups | p = 0.15 | p = 0.01 | -- |
| BAF% at intra-threads | |||
| TSV MP-1 HA | 51 (16) | 50 (12) | p = 0.87 |
| cmSLA | 47 (11) | 47 (14) | p = 0.75 |
| Between Groups | p = 0.20 | p = 0.42 | -- |
| BAF% at adjacent 1 mm host bone | |||
| TSV MP-1 HA | 44 (14) | 43.9 (21) | p = 1 |
| cmSLA | 38 (14) | 42.0 (17) | p = 0.63 |
| Between Groups | p = 0.15 | p = 0.08 | -- |
| BAF% total of intra-threads and adjacent 1 mm host bone | |||
| TSV MP-1 HA | 46 (13) | 45 (21) | p = 1 |
| cmSLA | 41 (8) | 43 (19) | p = 1 |
| Between Groups | p = 0.42 | p = 0.75 | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajami, E.; Fu, C.; Wen, H.B.; Bassett, J.; Park, S.J.; Pollard, M. Early Bone Healing on Hydroxyapatite-Coated and Chemically-Modified Hydrophilic Implant Surfaces in an Ovine Model. Int. J. Mol. Sci. 2021, 22, 9361. https://doi.org/10.3390/ijms22179361
Ajami E, Fu C, Wen HB, Bassett J, Park SJ, Pollard M. Early Bone Healing on Hydroxyapatite-Coated and Chemically-Modified Hydrophilic Implant Surfaces in an Ovine Model. International Journal of Molecular Sciences. 2021; 22(17):9361. https://doi.org/10.3390/ijms22179361
Chicago/Turabian StyleAjami, Elnaz, Cong Fu, Hai Bo Wen, Jeffrey Bassett, Sun Jin Park, and Marie Pollard. 2021. "Early Bone Healing on Hydroxyapatite-Coated and Chemically-Modified Hydrophilic Implant Surfaces in an Ovine Model" International Journal of Molecular Sciences 22, no. 17: 9361. https://doi.org/10.3390/ijms22179361
APA StyleAjami, E., Fu, C., Wen, H. B., Bassett, J., Park, S. J., & Pollard, M. (2021). Early Bone Healing on Hydroxyapatite-Coated and Chemically-Modified Hydrophilic Implant Surfaces in an Ovine Model. International Journal of Molecular Sciences, 22(17), 9361. https://doi.org/10.3390/ijms22179361





